 Found 3045 hits with Last Name = 'mori' and Initial = 'm'
Found 3045 hits with Last Name = 'mori' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50295693
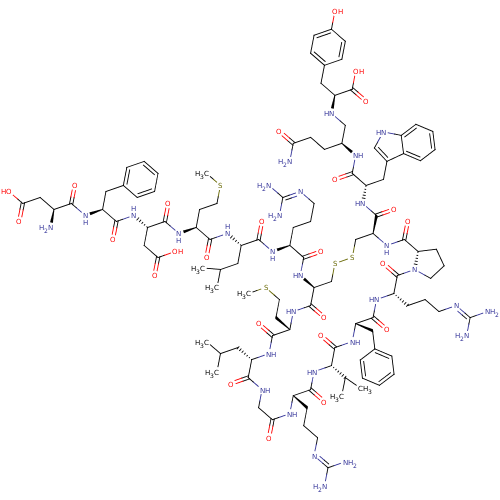
(CHEMBL557629)Show SMILES CSCC[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](N)CC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@@H]2CCCN2C(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)CNC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCSC)NC1=O)C(C)C)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCC(N)=O)CN[C@@H](Cc1ccc(O)cc1)C(O)=O |r,wU:27.28,36.36,71.73,148.153,55.55,60.134,8.12,wD:112.115,4.4,97.100,93.131,82.84,16.24,134.137,44.44,64.69,156.161,120.123,(5.27,-20.83,;6.6,-20.06,;6.6,-18.52,;5.27,-17.75,;5.27,-16.21,;3.93,-15.44,;2.6,-16.21,;2.6,-17.75,;1.27,-15.44,;1.27,-13.9,;2.6,-13.13,;3.93,-13.9,;2.6,-11.59,;-.07,-16.21,;-1.4,-15.44,;-1.4,-13.9,;-2.73,-16.21,;-2.73,-17.75,;-1.4,-18.52,;-.07,-17.74,;1.27,-18.51,;1.27,-20.05,;-.08,-20.82,;-1.4,-20.05,;-4.07,-15.44,;-5.4,-16.21,;-5.4,-17.75,;-6.74,-15.44,;-8.07,-16.21,;-6.74,-13.9,;-8.07,-13.13,;-8.07,-11.59,;-9.4,-13.9,;6.6,-15.44,;6.6,-13.9,;7.93,-16.21,;9.27,-15.44,;9.27,-13.9,;10.6,-13.13,;10.6,-11.59,;11.94,-13.9,;10.6,-16.21,;10.6,-17.75,;11.94,-15.44,;13.27,-16.21,;13.27,-17.75,;11.94,-18.52,;11.94,-20.06,;10.6,-20.83,;10.6,-22.37,;9.27,-23.14,;11.94,-23.14,;14.6,-15.44,;14.6,-13.9,;15.94,-16.21,;17.27,-15.44,;17.27,-13.9,;15.94,-13.13,;15.71,-11.29,;14.63,-10.2,;15.04,-8.71,;16.59,-8.74,;17.38,-7.41,;18.92,-7.43,;16.62,-6.07,;15.22,-6.04,;14.79,-4.38,;16.14,-3.62,;17.27,-4.66,;18.6,-3.89,;18.6,-2.35,;19.94,-4.66,;19.94,-6.2,;21.27,-6.97,;21.27,-8.51,;19.94,-9.28,;19.94,-10.82,;18.6,-11.59,;21.27,-11.59,;21.27,-3.89,;22.61,-4.66,;22.61,-6.2,;23.94,-3.89,;23.94,-2.35,;22.61,-1.58,;22.61,-.04,;21.28,.73,;19.95,-.04,;19.95,-1.59,;21.27,-2.35,;25.27,-4.66,;26.61,-3.89,;26.61,-2.35,;27.94,-4.66,;27.94,-6.2,;26.61,-6.97,;25.27,-6.2,;26.61,-8.51,;27.94,-9.28,;27.94,-10.82,;29.27,-11.59,;29.27,-13.13,;30.61,-13.9,;31.94,-13.13,;30.61,-15.44,;25.27,-9.28,;25.27,-10.82,;23.94,-11.59,;26.61,-11.59,;26.61,-13.13,;25.27,-13.9,;23.94,-13.13,;25.27,-15.44,;26.61,-16.21,;26.61,-17.75,;27.94,-18.52,;25.27,-18.52,;23.94,-16.21,;22.61,-15.44,;22.61,-13.9,;21.27,-16.21,;21.27,-17.75,;19.94,-18.52,;19.94,-20.06,;18.6,-20.83,;19.94,-15.44,;18.6,-16.21,;18.6,-17.75,;29.27,-3.89,;30.61,-4.66,;29.27,-2.35,;13.71,-7.94,;13.71,-6.4,;12.38,-8.71,;11.04,-7.94,;11.05,-6.4,;9.71,-5.63,;8.29,-6.29,;7.28,-5.11,;8.05,-3.78,;7.58,-2.33,;8.6,-1.2,;10.11,-1.52,;10.57,-2.98,;9.54,-4.11,;9.71,-8.71,;9.71,-10.25,;8.38,-7.93,;7.04,-8.7,;7.04,-10.24,;5.71,-11.01,;5.7,-12.55,;7.19,-12.94,;5.3,-14.03,;5.71,-7.93,;4.38,-8.7,;3.04,-7.93,;3.04,-6.39,;1.71,-5.62,;1.72,-4.07,;.39,-3.3,;-.95,-4.07,;-2.28,-3.3,;-.94,-5.62,;.39,-6.38,;1.71,-8.7,;.38,-7.92,;1.71,-10.24,)| Show InChI InChI=1S/C109H162N30O25S4/c1-58(2)45-75-91(148)123-55-86(142)125-70(27-17-39-118-107(112)113)95(152)138-89(60(5)6)104(161)135-78(48-62-23-13-10-14-24-62)98(155)129-74(29-19-41-120-109(116)117)105(162)139-42-20-30-84(139)103(160)137-83(102(159)133-79(50-64-53-121-69-26-16-15-25-67(64)69)96(153)124-65(33-36-85(111)141)54-122-81(106(163)164)49-63-31-34-66(140)35-32-63)57-168-167-56-82(101(158)128-73(38-44-166-8)93(150)131-75)136-92(149)71(28-18-40-119-108(114)115)126-97(154)76(46-59(3)4)132-94(151)72(37-43-165-7)127-100(157)80(52-88(145)146)134-99(156)77(47-61-21-11-9-12-22-61)130-90(147)68(110)51-87(143)144/h9-16,21-26,31-32,34-35,53,58-60,65,68,70-84,89,121-122,140H,17-20,27-30,33,36-52,54-57,110H2,1-8H3,(H2,111,141)(H,123,148)(H,124,153)(H,125,142)(H,126,154)(H,127,157)(H,128,158)(H,129,155)(H,130,147)(H,131,150)(H,132,151)(H,133,159)(H,134,156)(H,135,161)(H,136,149)(H,137,160)(H,138,152)(H,143,144)(H,145,146)(H,163,164)(H4,112,113,118)(H4,114,115,119)(H4,116,117,120)/t65-,68-,70-,71-,72-,73-,74-,75-,76-,77-,78-,79-,80-,81-,82-,83-,84-,89-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [35S] 4-(1-(3,4-difluorophenyl)-2-(ethyl(3-(6-fluoro-3H-spiro[isobenzofuran-1,4'-piperidine]-1'-yl)propyl)amino)-2-oxoethyl)-3-oxopip... |
Bioorg Med Chem Lett 19: 2835-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.102
BindingDB Entry DOI: 10.7270/Q2R49QS7 |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Mus musculus) | BDBM50295693

(CHEMBL557629)Show SMILES CSCC[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](N)CC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@@H]2CCCN2C(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)CNC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCSC)NC1=O)C(C)C)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCC(N)=O)CN[C@@H](Cc1ccc(O)cc1)C(O)=O |r,wU:27.28,36.36,71.73,148.153,55.55,60.134,8.12,wD:112.115,4.4,97.100,93.131,82.84,16.24,134.137,44.44,64.69,156.161,120.123,(5.27,-20.83,;6.6,-20.06,;6.6,-18.52,;5.27,-17.75,;5.27,-16.21,;3.93,-15.44,;2.6,-16.21,;2.6,-17.75,;1.27,-15.44,;1.27,-13.9,;2.6,-13.13,;3.93,-13.9,;2.6,-11.59,;-.07,-16.21,;-1.4,-15.44,;-1.4,-13.9,;-2.73,-16.21,;-2.73,-17.75,;-1.4,-18.52,;-.07,-17.74,;1.27,-18.51,;1.27,-20.05,;-.08,-20.82,;-1.4,-20.05,;-4.07,-15.44,;-5.4,-16.21,;-5.4,-17.75,;-6.74,-15.44,;-8.07,-16.21,;-6.74,-13.9,;-8.07,-13.13,;-8.07,-11.59,;-9.4,-13.9,;6.6,-15.44,;6.6,-13.9,;7.93,-16.21,;9.27,-15.44,;9.27,-13.9,;10.6,-13.13,;10.6,-11.59,;11.94,-13.9,;10.6,-16.21,;10.6,-17.75,;11.94,-15.44,;13.27,-16.21,;13.27,-17.75,;11.94,-18.52,;11.94,-20.06,;10.6,-20.83,;10.6,-22.37,;9.27,-23.14,;11.94,-23.14,;14.6,-15.44,;14.6,-13.9,;15.94,-16.21,;17.27,-15.44,;17.27,-13.9,;15.94,-13.13,;15.71,-11.29,;14.63,-10.2,;15.04,-8.71,;16.59,-8.74,;17.38,-7.41,;18.92,-7.43,;16.62,-6.07,;15.22,-6.04,;14.79,-4.38,;16.14,-3.62,;17.27,-4.66,;18.6,-3.89,;18.6,-2.35,;19.94,-4.66,;19.94,-6.2,;21.27,-6.97,;21.27,-8.51,;19.94,-9.28,;19.94,-10.82,;18.6,-11.59,;21.27,-11.59,;21.27,-3.89,;22.61,-4.66,;22.61,-6.2,;23.94,-3.89,;23.94,-2.35,;22.61,-1.58,;22.61,-.04,;21.28,.73,;19.95,-.04,;19.95,-1.59,;21.27,-2.35,;25.27,-4.66,;26.61,-3.89,;26.61,-2.35,;27.94,-4.66,;27.94,-6.2,;26.61,-6.97,;25.27,-6.2,;26.61,-8.51,;27.94,-9.28,;27.94,-10.82,;29.27,-11.59,;29.27,-13.13,;30.61,-13.9,;31.94,-13.13,;30.61,-15.44,;25.27,-9.28,;25.27,-10.82,;23.94,-11.59,;26.61,-11.59,;26.61,-13.13,;25.27,-13.9,;23.94,-13.13,;25.27,-15.44,;26.61,-16.21,;26.61,-17.75,;27.94,-18.52,;25.27,-18.52,;23.94,-16.21,;22.61,-15.44,;22.61,-13.9,;21.27,-16.21,;21.27,-17.75,;19.94,-18.52,;19.94,-20.06,;18.6,-20.83,;19.94,-15.44,;18.6,-16.21,;18.6,-17.75,;29.27,-3.89,;30.61,-4.66,;29.27,-2.35,;13.71,-7.94,;13.71,-6.4,;12.38,-8.71,;11.04,-7.94,;11.05,-6.4,;9.71,-5.63,;8.29,-6.29,;7.28,-5.11,;8.05,-3.78,;7.58,-2.33,;8.6,-1.2,;10.11,-1.52,;10.57,-2.98,;9.54,-4.11,;9.71,-8.71,;9.71,-10.25,;8.38,-7.93,;7.04,-8.7,;7.04,-10.24,;5.71,-11.01,;5.7,-12.55,;7.19,-12.94,;5.3,-14.03,;5.71,-7.93,;4.38,-8.7,;3.04,-7.93,;3.04,-6.39,;1.71,-5.62,;1.72,-4.07,;.39,-3.3,;-.95,-4.07,;-2.28,-3.3,;-.94,-5.62,;.39,-6.38,;1.71,-8.7,;.38,-7.92,;1.71,-10.24,)| Show InChI InChI=1S/C109H162N30O25S4/c1-58(2)45-75-91(148)123-55-86(142)125-70(27-17-39-118-107(112)113)95(152)138-89(60(5)6)104(161)135-78(48-62-23-13-10-14-24-62)98(155)129-74(29-19-41-120-109(116)117)105(162)139-42-20-30-84(139)103(160)137-83(102(159)133-79(50-64-53-121-69-26-16-15-25-67(64)69)96(153)124-65(33-36-85(111)141)54-122-81(106(163)164)49-63-31-34-66(140)35-32-63)57-168-167-56-82(101(158)128-73(38-44-166-8)93(150)131-75)136-92(149)71(28-18-40-119-108(114)115)126-97(154)76(46-59(3)4)132-94(151)72(37-43-165-7)127-100(157)80(52-88(145)146)134-99(156)77(47-61-21-11-9-12-22-61)130-90(147)68(110)51-87(143)144/h9-16,21-26,31-32,34-35,53,58-60,65,68,70-84,89,121-122,140H,17-20,27-30,33,36-52,54-57,110H2,1-8H3,(H2,111,141)(H,123,148)(H,124,153)(H,125,142)(H,126,154)(H,127,157)(H,128,158)(H,129,155)(H,130,147)(H,131,150)(H,132,151)(H,133,159)(H,134,156)(H,135,161)(H,136,149)(H,137,160)(H,138,152)(H,143,144)(H,145,146)(H,163,164)(H4,112,113,118)(H4,114,115,119)(H4,116,117,120)/t65-,68-,70-,71-,72-,73-,74-,75-,76-,77-,78-,79-,80-,81-,82-,83-,84-,89-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [35S] 4-(1-(3,4-difluorophenyl)-2-(ethyl(3-(6-fluoro-3H-spiro[isobenzofuran-1,4'-piperidine]-1'-yl)propyl)amino)-2-oxoethyl)-3-oxopip... |
Bioorg Med Chem Lett 19: 2835-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.102
BindingDB Entry DOI: 10.7270/Q2R49QS7 |
More data for this
Ligand-Target Pair | |
Alpha-1B adrenergic receptor
(Rattus norvegicus (rat)) | BDBM29568

(CHEMBL2 | PRAZOSIN | PRAZOSIN HYDROCHLORIDE | [3H]...)Show SMILES COc1cc2nc(nc(N)c2cc1OC)N1CCN(CC1)C(=O)c1ccco1 Show InChI InChI=1S/C19H21N5O4/c1-26-15-10-12-13(11-16(15)27-2)21-19(22-17(12)20)24-7-5-23(6-8-24)18(25)14-4-3-9-28-14/h3-4,9-11H,5-8H2,1-2H3,(H2,20,21,22) | Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]prazosin from adrenergic alpha-1B receptor in rat liver membranes |
Bioorg Med Chem Lett 16: 4045-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.002
BindingDB Entry DOI: 10.7270/Q2514001 |
More data for this
Ligand-Target Pair | |
Protein kinase C eta type
(Homo sapiens (Human)) | BDBM50391386

(CHEMBL2148106)Show SMILES CO[C@@H](CC[C@H](C)[C@H]1O[C@@]23C[C@H](OC(=O)C[C@@H](OC(=O)C[C@](O)(O2)[C@H](C)CC3(C)C)[C@@H](C)O)[C@@H]1C)c1cccc(O)c1 |r| Show InChI InChI=1S/C32H48O10/c1-18(11-12-24(38-7)22-9-8-10-23(34)13-22)29-20(3)26-16-32(41-29)30(5,6)15-19(2)31(37,42-32)17-28(36)39-25(21(4)33)14-27(35)40-26/h8-10,13,18-21,24-26,29,33-34,37H,11-12,14-17H2,1-7H3/t18-,19+,20-,21+,24-,25+,26?,29+,31-,32-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]PDBu binding to PKCeta C1B domain |
J Med Chem 55: 5614-26 (2012)
Article DOI: 10.1021/jm300566h
BindingDB Entry DOI: 10.7270/Q200036J |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50391386

(CHEMBL2148106)Show SMILES CO[C@@H](CC[C@H](C)[C@H]1O[C@@]23C[C@H](OC(=O)C[C@@H](OC(=O)C[C@](O)(O2)[C@H](C)CC3(C)C)[C@@H](C)O)[C@@H]1C)c1cccc(O)c1 |r| Show InChI InChI=1S/C32H48O10/c1-18(11-12-24(38-7)22-9-8-10-23(34)13-22)29-20(3)26-16-32(41-29)30(5,6)15-19(2)31(37,42-32)17-28(36)39-25(21(4)33)14-27(35)40-26/h8-10,13,18-21,24-26,29,33-34,37H,11-12,14-17H2,1-7H3/t18-,19+,20-,21+,24-,25+,26?,29+,31-,32-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]PDBu binding to PKCtheta C1B domain |
J Med Chem 55: 5614-26 (2012)
Article DOI: 10.1021/jm300566h
BindingDB Entry DOI: 10.7270/Q200036J |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50391386

(CHEMBL2148106)Show SMILES CO[C@@H](CC[C@H](C)[C@H]1O[C@@]23C[C@H](OC(=O)C[C@@H](OC(=O)C[C@](O)(O2)[C@H](C)CC3(C)C)[C@@H](C)O)[C@@H]1C)c1cccc(O)c1 |r| Show InChI InChI=1S/C32H48O10/c1-18(11-12-24(38-7)22-9-8-10-23(34)13-22)29-20(3)26-16-32(41-29)30(5,6)15-19(2)31(37,42-32)17-28(36)39-25(21(4)33)14-27(35)40-26/h8-10,13,18-21,24-26,29,33-34,37H,11-12,14-17H2,1-7H3/t18-,19+,20-,21+,24-,25+,26?,29+,31-,32-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]PDBu binding to PKCbeta C1A domain |
J Med Chem 55: 5614-26 (2012)
Article DOI: 10.1021/jm300566h
BindingDB Entry DOI: 10.7270/Q200036J |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(RAT) | BDBM50000066
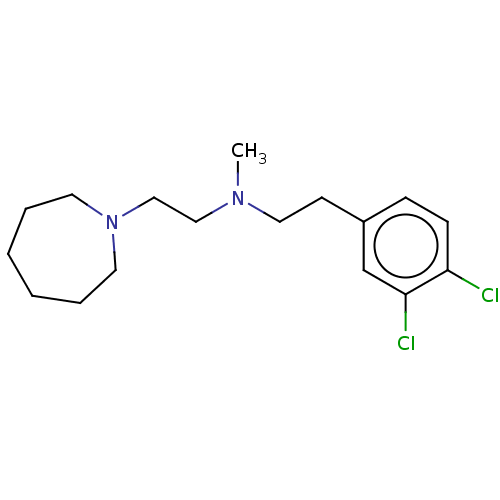
((2-Azepan-1-yl-ethyl)-[2-(3,4-dichloro-phenyl)-eth...)Show InChI InChI=1S/C17H26Cl2N2/c1-20(12-13-21-9-4-2-3-5-10-21)11-8-15-6-7-16(18)17(19)14-15/h6-7,14H,2-5,8-13H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago 60607-7061
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-NANM binding to sigma receptor obtained from tissue homogenate preparation of fresh whole rat brain minus cerebellum |
J Med Chem 41: 468-77 (1998)
Article DOI: 10.1021/jm970059p
BindingDB Entry DOI: 10.7270/Q27W6CW7 |
More data for this
Ligand-Target Pair | |
Protein kinase C eta type
(Homo sapiens (Human)) | BDBM50493217

(DEMETHOXYDEBROMOAPLYSIATOXIN)Show SMILES [H][C@]12C[C@]3(O[C@]([H])([C@@H](C)CCCc4cccc(O)c4)[C@H]1C)O[C@@](O)(CC(=O)O[C@]([H])(CC(=O)O2)[C@@H](C)O)[C@H](C)CC3(C)C |r| Show InChI InChI=1S/C31H46O9/c1-18(9-7-10-22-11-8-12-23(33)13-22)28-20(3)25-16-31(39-28)29(5,6)15-19(2)30(36,40-31)17-27(35)37-24(21(4)32)14-26(34)38-25/h8,11-13,18-21,24-25,28,32-33,36H,7,9-10,14-17H2,1-6H3/t18-,19+,20-,21+,24+,25-,28+,30-,31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Displacement of [3H]PDBu from PKCeta C1B domain (unknown origin) |
Bioorg Med Chem Lett 23: 4319-23 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.096
BindingDB Entry DOI: 10.7270/Q2VD72CX |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50295690
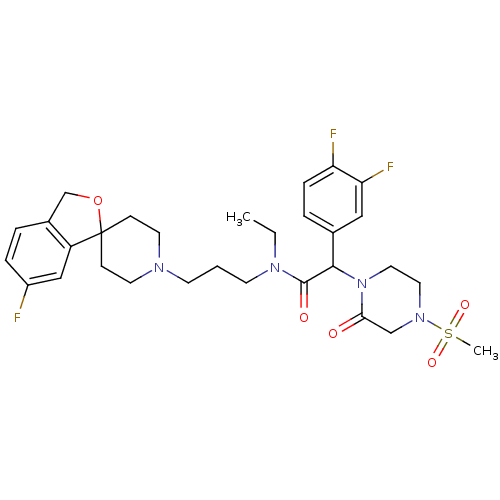
((+/-)-2-(3,4-difluorophenyl)-N-ethyl-N-(3-(6-fluor...)Show SMILES CCN(CCCN1CCC2(CC1)OCc1ccc(F)cc21)C(=O)C(N1CCN(CC1=O)S(C)(=O)=O)c1ccc(F)c(F)c1 Show InChI InChI=1S/C30H37F3N4O5S/c1-3-35(12-4-11-34-13-9-30(10-14-34)24-18-23(31)7-5-22(24)20-42-30)29(39)28(21-6-8-25(32)26(33)17-21)37-16-15-36(19-27(37)38)43(2,40)41/h5-8,17-18,28H,3-4,9-16,19-20H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human MCH1R expressed in CHO cells by scintillation counting per mg of protein |
Bioorg Med Chem Lett 19: 2835-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.102
BindingDB Entry DOI: 10.7270/Q2R49QS7 |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM50391386

(CHEMBL2148106)Show SMILES CO[C@@H](CC[C@H](C)[C@H]1O[C@@]23C[C@H](OC(=O)C[C@@H](OC(=O)C[C@](O)(O2)[C@H](C)CC3(C)C)[C@@H](C)O)[C@@H]1C)c1cccc(O)c1 |r| Show InChI InChI=1S/C32H48O10/c1-18(11-12-24(38-7)22-9-8-10-23(34)13-22)29-20(3)26-16-32(41-29)30(5,6)15-19(2)31(37,42-32)17-28(36)39-25(21(4)33)14-27(35)40-26/h8-10,13,18-21,24-26,29,33-34,37H,11-12,14-17H2,1-7H3/t18-,19+,20-,21+,24-,25+,26?,29+,31-,32-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]PDBu binding to PKCdelta C1B domain |
J Med Chem 55: 5614-26 (2012)
Article DOI: 10.1021/jm300566h
BindingDB Entry DOI: 10.7270/Q200036J |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50493217

(DEMETHOXYDEBROMOAPLYSIATOXIN)Show SMILES [H][C@]12C[C@]3(O[C@]([H])([C@@H](C)CCCc4cccc(O)c4)[C@H]1C)O[C@@](O)(CC(=O)O[C@]([H])(CC(=O)O2)[C@@H](C)O)[C@H](C)CC3(C)C |r| Show InChI InChI=1S/C31H46O9/c1-18(9-7-10-22-11-8-12-23(33)13-22)28-20(3)25-16-31(39-28)29(5,6)15-19(2)30(36,40-31)17-27(35)37-24(21(4)32)14-26(34)38-25/h8,11-13,18-21,24-25,28,32-33,36H,7,9-10,14-17H2,1-6H3/t18-,19+,20-,21+,24+,25-,28+,30-,31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Displacement of [3H]PDBu from PKCtheta C1B domain (unknown origin) |
Bioorg Med Chem Lett 23: 4319-23 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.096
BindingDB Entry DOI: 10.7270/Q2VD72CX |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Rattus norvegicus (Rat)) | BDBM29568

(CHEMBL2 | PRAZOSIN | PRAZOSIN HYDROCHLORIDE | [3H]...)Show SMILES COc1cc2nc(nc(N)c2cc1OC)N1CCN(CC1)C(=O)c1ccco1 Show InChI InChI=1S/C19H21N5O4/c1-26-15-10-12-13(11-16(15)27-2)21-19(22-17(12)20)24-7-5-23(6-8-24)18(25)14-4-3-9-28-14/h3-4,9-11H,5-8H2,1-2H3,(H2,20,21,22) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]prazosin from adrenergic alpha-1A receptor in rat submaxillary gland membranes |
Bioorg Med Chem Lett 16: 4045-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.002
BindingDB Entry DOI: 10.7270/Q2514001 |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50391386

(CHEMBL2148106)Show SMILES CO[C@@H](CC[C@H](C)[C@H]1O[C@@]23C[C@H](OC(=O)C[C@@H](OC(=O)C[C@](O)(O2)[C@H](C)CC3(C)C)[C@@H](C)O)[C@@H]1C)c1cccc(O)c1 |r| Show InChI InChI=1S/C32H48O10/c1-18(11-12-24(38-7)22-9-8-10-23(34)13-22)29-20(3)26-16-32(41-29)30(5,6)15-19(2)31(37,42-32)17-28(36)39-25(21(4)33)14-27(35)40-26/h8-10,13,18-21,24-26,29,33-34,37H,11-12,14-17H2,1-7H3/t18-,19+,20-,21+,24-,25+,26?,29+,31-,32-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]PDBu binding to PKCalpha C1A domain |
J Med Chem 55: 5614-26 (2012)
Article DOI: 10.1021/jm300566h
BindingDB Entry DOI: 10.7270/Q200036J |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM50493217

(DEMETHOXYDEBROMOAPLYSIATOXIN)Show SMILES [H][C@]12C[C@]3(O[C@]([H])([C@@H](C)CCCc4cccc(O)c4)[C@H]1C)O[C@@](O)(CC(=O)O[C@]([H])(CC(=O)O2)[C@@H](C)O)[C@H](C)CC3(C)C |r| Show InChI InChI=1S/C31H46O9/c1-18(9-7-10-22-11-8-12-23(33)13-22)28-20(3)25-16-31(39-28)29(5,6)15-19(2)30(36,40-31)17-27(35)37-24(21(4)32)14-26(34)38-25/h8,11-13,18-21,24-25,28,32-33,36H,7,9-10,14-17H2,1-6H3/t18-,19+,20-,21+,24+,25-,28+,30-,31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Displacement of [3H]PDBu from PKCdelta C1B domain (unknown origin) |
Bioorg Med Chem Lett 23: 4319-23 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.096
BindingDB Entry DOI: 10.7270/Q2VD72CX |
More data for this
Ligand-Target Pair | |
Protein kinase C gamma type
(Homo sapiens (Human)) | BDBM50391386

(CHEMBL2148106)Show SMILES CO[C@@H](CC[C@H](C)[C@H]1O[C@@]23C[C@H](OC(=O)C[C@@H](OC(=O)C[C@](O)(O2)[C@H](C)CC3(C)C)[C@@H](C)O)[C@@H]1C)c1cccc(O)c1 |r| Show InChI InChI=1S/C32H48O10/c1-18(11-12-24(38-7)22-9-8-10-23(34)13-22)29-20(3)26-16-32(41-29)30(5,6)15-19(2)31(37,42-32)17-28(36)39-25(21(4)33)14-27(35)40-26/h8-10,13,18-21,24-26,29,33-34,37H,11-12,14-17H2,1-7H3/t18-,19+,20-,21+,24-,25+,26?,29+,31-,32-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]PDBu binding to PKCgamma C1A domain |
J Med Chem 55: 5614-26 (2012)
Article DOI: 10.1021/jm300566h
BindingDB Entry DOI: 10.7270/Q200036J |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50295690

((+/-)-2-(3,4-difluorophenyl)-N-ethyl-N-(3-(6-fluor...)Show SMILES CCN(CCCN1CCC2(CC1)OCc1ccc(F)cc21)C(=O)C(N1CCN(CC1=O)S(C)(=O)=O)c1ccc(F)c(F)c1 Show InChI InChI=1S/C30H37F3N4O5S/c1-3-35(12-4-11-34-13-9-30(10-14-34)24-18-23(31)7-5-22(24)20-42-30)29(39)28(21-6-8-25(32)26(33)17-21)37-16-15-36(19-27(37)38)43(2,40)41/h5-8,17-18,28H,3-4,9-16,19-20H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [35S] 4-(1-(3,4-difluorophenyl)-2-(ethyl(3-(6-fluoro-3H-spiro[isobenzofuran-1,4'-piperidine]-1'-yl)propyl)amino)-2-oxoethyl)-3-oxopip... |
Bioorg Med Chem Lett 19: 2835-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.102
BindingDB Entry DOI: 10.7270/Q2R49QS7 |
More data for this
Ligand-Target Pair | |
Protein kinase C eta type
(Homo sapiens (Human)) | BDBM50391388
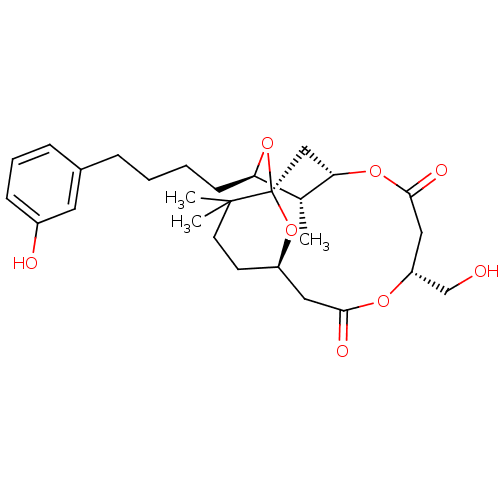
(CHEMBL2148108)Show SMILES C[C@@H]1[C@@H](CCCCc2cccc(O)c2)O[C@]23C[C@@H]1OC(=O)C[C@H](CO)OC(=O)C[C@@H](CCC2(C)C)O3 |r| Show InChI InChI=1S/C28H40O8/c1-18-23(10-5-4-7-19-8-6-9-20(30)13-19)36-28-16-24(18)34-26(32)15-22(17-29)33-25(31)14-21(35-28)11-12-27(28,2)3/h6,8-9,13,18,21-24,29-30H,4-5,7,10-12,14-17H2,1-3H3/t18-,21-,22-,23-,24?,28-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]PDBu binding to PKCeta C1B domain |
J Med Chem 55: 5614-26 (2012)
Article DOI: 10.1021/jm300566h
BindingDB Entry DOI: 10.7270/Q200036J |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM50391388

(CHEMBL2148108)Show SMILES C[C@@H]1[C@@H](CCCCc2cccc(O)c2)O[C@]23C[C@@H]1OC(=O)C[C@H](CO)OC(=O)C[C@@H](CCC2(C)C)O3 |r| Show InChI InChI=1S/C28H40O8/c1-18-23(10-5-4-7-19-8-6-9-20(30)13-19)36-28-16-24(18)34-26(32)15-22(17-29)33-25(31)14-21(35-28)11-12-27(28,2)3/h6,8-9,13,18,21-24,29-30H,4-5,7,10-12,14-17H2,1-3H3/t18-,21-,22-,23-,24?,28-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]PDBu binding to PKCdelta C1B domain |
J Med Chem 55: 5614-26 (2012)
Article DOI: 10.1021/jm300566h
BindingDB Entry DOI: 10.7270/Q200036J |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Mus musculus) | BDBM50295690

((+/-)-2-(3,4-difluorophenyl)-N-ethyl-N-(3-(6-fluor...)Show SMILES CCN(CCCN1CCC2(CC1)OCc1ccc(F)cc21)C(=O)C(N1CCN(CC1=O)S(C)(=O)=O)c1ccc(F)c(F)c1 Show InChI InChI=1S/C30H37F3N4O5S/c1-3-35(12-4-11-34-13-9-30(10-14-34)24-18-23(31)7-5-22(24)20-42-30)29(39)28(21-6-8-25(32)26(33)17-21)37-16-15-36(19-27(37)38)43(2,40)41/h5-8,17-18,28H,3-4,9-16,19-20H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [35S] 4-(1-(3,4-difluorophenyl)-2-(ethyl(3-(6-fluoro-3H-spiro[isobenzofuran-1,4'-piperidine]-1'-yl)propyl)amino)-2-oxoethyl)-3-oxopip... |
Bioorg Med Chem Lett 19: 2835-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.102
BindingDB Entry DOI: 10.7270/Q2R49QS7 |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50391388

(CHEMBL2148108)Show SMILES C[C@@H]1[C@@H](CCCCc2cccc(O)c2)O[C@]23C[C@@H]1OC(=O)C[C@H](CO)OC(=O)C[C@@H](CCC2(C)C)O3 |r| Show InChI InChI=1S/C28H40O8/c1-18-23(10-5-4-7-19-8-6-9-20(30)13-19)36-28-16-24(18)34-26(32)15-22(17-29)33-25(31)14-21(35-28)11-12-27(28,2)3/h6,8-9,13,18,21-24,29-30H,4-5,7,10-12,14-17H2,1-3H3/t18-,21-,22-,23-,24?,28-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]PDBu binding to PKCtheta C1B domain |
J Med Chem 55: 5614-26 (2012)
Article DOI: 10.1021/jm300566h
BindingDB Entry DOI: 10.7270/Q200036J |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50343939
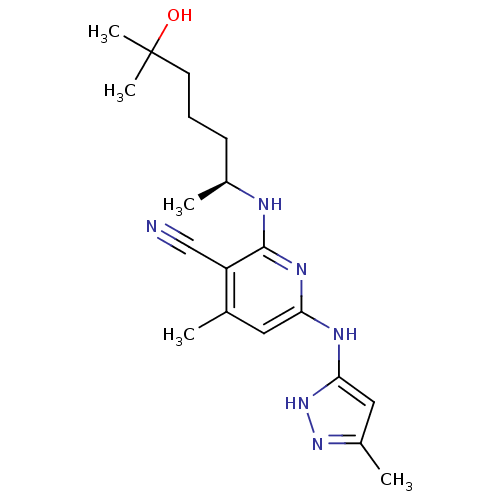
((S)-2-(6-hydroxy-6-methylheptan-2-ylamino)-4-methy...)Show SMILES C[C@@H](CCCC(C)(C)O)Nc1nc(Nc2cc(C)n[nH]2)cc(C)c1C#N |r| Show InChI InChI=1S/C19H28N6O/c1-12-9-16(22-17-10-14(3)24-25-17)23-18(15(12)11-20)21-13(2)7-6-8-19(4,5)26/h9-10,13,26H,6-8H2,1-5H3,(H3,21,22,23,24,25)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of aurora A |
Bioorg Med Chem Lett 20: 4709-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.119
BindingDB Entry DOI: 10.7270/Q2RF5VC3 |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM13534

(CHEMBL572878 | N-[4-({4-[(3-methyl-1H-pyrazol-5-yl...)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(C)n[nH]2)nc(Sc2ccc(NC(=O)C3CC3)cc2)n1 Show InChI InChI=1S/C23H28N8OS/c1-15-13-20(29-28-15)25-19-14-21(31-11-9-30(2)10-12-31)27-23(26-19)33-18-7-5-17(6-8-18)24-22(32)16-3-4-16/h5-8,13-14,16H,3-4,9-12H2,1-2H3,(H,24,32)(H2,25,26,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of aurora A |
Bioorg Med Chem Lett 20: 4709-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.119
BindingDB Entry DOI: 10.7270/Q2RF5VC3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Alpha-1B adrenergic receptor
(Rattus norvegicus (rat)) | BDBM50115122
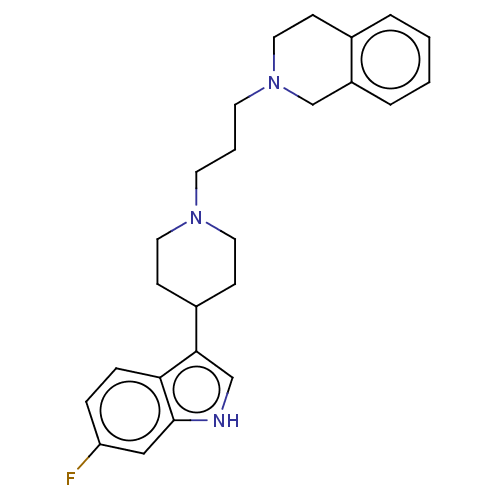
(CHEMBL3609360)Show SMILES Fc1ccc2c(c[nH]c2c1)C1CCN(CCCN2CCc3ccccc3C2)CC1 Show InChI InChI=1S/C25H30FN3/c26-22-6-7-23-24(17-27-25(23)16-22)20-9-13-28(14-10-20)11-3-12-29-15-8-19-4-1-2-5-21(19)18-29/h1-2,4-7,16-17,20,27H,3,8-15,18H2 | Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]prazosin from alpha1B adrenoceptor in rat liver membranes |
Bioorg Med Chem Lett 25: 3921-3 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.046
BindingDB Entry DOI: 10.7270/Q27M09Q4 |
More data for this
Ligand-Target Pair | |
Alpha-1B adrenergic receptor
(Rattus norvegicus (rat)) | BDBM50187753
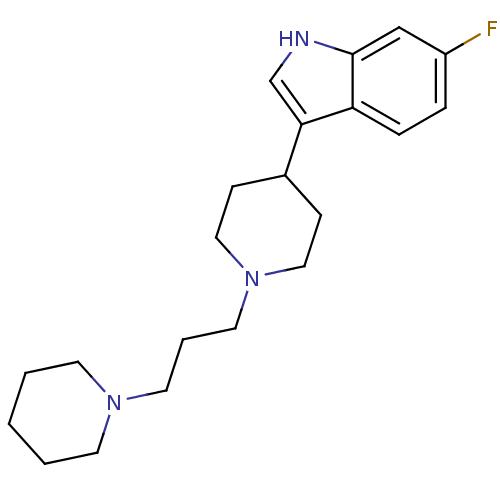
(6-fluoro-3-(1-(3-(piperidin-1-yl)propyl)piperidin-...)Show InChI InChI=1S/C21H30FN3/c22-18-5-6-19-20(16-23-21(19)15-18)17-7-13-25(14-8-17)12-4-11-24-9-2-1-3-10-24/h5-6,15-17,23H,1-4,7-14H2 | Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]prazosin from adrenergic alpha-1B receptor in rat liver membranes |
Bioorg Med Chem Lett 16: 4045-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.002
BindingDB Entry DOI: 10.7270/Q2514001 |
More data for this
Ligand-Target Pair | |
Protein kinase C epsilon type
(Homo sapiens (Human)) | BDBM50391386

(CHEMBL2148106)Show SMILES CO[C@@H](CC[C@H](C)[C@H]1O[C@@]23C[C@H](OC(=O)C[C@@H](OC(=O)C[C@](O)(O2)[C@H](C)CC3(C)C)[C@@H](C)O)[C@@H]1C)c1cccc(O)c1 |r| Show InChI InChI=1S/C32H48O10/c1-18(11-12-24(38-7)22-9-8-10-23(34)13-22)29-20(3)26-16-32(41-29)30(5,6)15-19(2)31(37,42-32)17-28(36)39-25(21(4)33)14-27(35)40-26/h8-10,13,18-21,24-26,29,33-34,37H,11-12,14-17H2,1-7H3/t18-,19+,20-,21+,24-,25+,26?,29+,31-,32-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]PDBu binding to PKCepsilon C1B domain |
J Med Chem 55: 5614-26 (2012)
Article DOI: 10.1021/jm300566h
BindingDB Entry DOI: 10.7270/Q200036J |
More data for this
Ligand-Target Pair | |
Protein kinase C gamma type
(Homo sapiens (Human)) | BDBM50493217

(DEMETHOXYDEBROMOAPLYSIATOXIN)Show SMILES [H][C@]12C[C@]3(O[C@]([H])([C@@H](C)CCCc4cccc(O)c4)[C@H]1C)O[C@@](O)(CC(=O)O[C@]([H])(CC(=O)O2)[C@@H](C)O)[C@H](C)CC3(C)C |r| Show InChI InChI=1S/C31H46O9/c1-18(9-7-10-22-11-8-12-23(33)13-22)28-20(3)25-16-31(39-28)29(5,6)15-19(2)30(36,40-31)17-27(35)37-24(21(4)32)14-26(34)38-25/h8,11-13,18-21,24-25,28,32-33,36H,7,9-10,14-17H2,1-6H3/t18-,19+,20-,21+,24+,25-,28+,30-,31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Displacement of [3H]PDBu from PKCgamma C1A domain (unknown origin) |
Bioorg Med Chem Lett 23: 4319-23 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.096
BindingDB Entry DOI: 10.7270/Q2VD72CX |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50343939

((S)-2-(6-hydroxy-6-methylheptan-2-ylamino)-4-methy...)Show SMILES C[C@@H](CCCC(C)(C)O)Nc1nc(Nc2cc(C)n[nH]2)cc(C)c1C#N |r| Show InChI InChI=1S/C19H28N6O/c1-12-9-16(22-17-10-14(3)24-25-17)23-18(15(12)11-20)21-13(2)7-6-8-19(4,5)26/h9-10,13,26H,6-8H2,1-5H3,(H3,21,22,23,24,25)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CXS Corporation
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Aurora A kinase derived from human Hela cells using kemptide as substrate in presence of [c-32P]ATP |
Bioorg Med Chem Lett 26: 5860-5862 (2016)
Article DOI: 10.1016/j.bmcl.2016.11.020
BindingDB Entry DOI: 10.7270/Q2WS8W80 |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50206860

(CHEMBL3974799)Show SMILES Cc1cc(Nc2cc(C)c(C#N)c(NCCOc3ccccc3Cl)n2)n[nH]1 Show InChI InChI=1S/C19H19ClN6O/c1-12-9-17(23-18-10-13(2)25-26-18)24-19(14(12)11-21)22-7-8-27-16-6-4-3-5-15(16)20/h3-6,9-10H,7-8H2,1-2H3,(H3,22,23,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CXS Corporation
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Aurora A kinase derived from human Hela cells using kemptide as substrate in presence of [c-32P]ATP |
Bioorg Med Chem Lett 26: 5860-5862 (2016)
Article DOI: 10.1016/j.bmcl.2016.11.020
BindingDB Entry DOI: 10.7270/Q2WS8W80 |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50206864

(CHEMBL3903916)Show SMILES Cc1cc(Nc2cc(C)c(C#N)c(NCCOc3ccccc3F)n2)n[nH]1 Show InChI InChI=1S/C19H19FN6O/c1-12-9-17(23-18-10-13(2)25-26-18)24-19(14(12)11-21)22-7-8-27-16-6-4-3-5-15(16)20/h3-6,9-10H,7-8H2,1-2H3,(H3,22,23,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CXS Corporation
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Aurora A kinase derived from human Hela cells using kemptide as substrate in presence of [c-32P]ATP |
Bioorg Med Chem Lett 26: 5860-5862 (2016)
Article DOI: 10.1016/j.bmcl.2016.11.020
BindingDB Entry DOI: 10.7270/Q2WS8W80 |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50434226
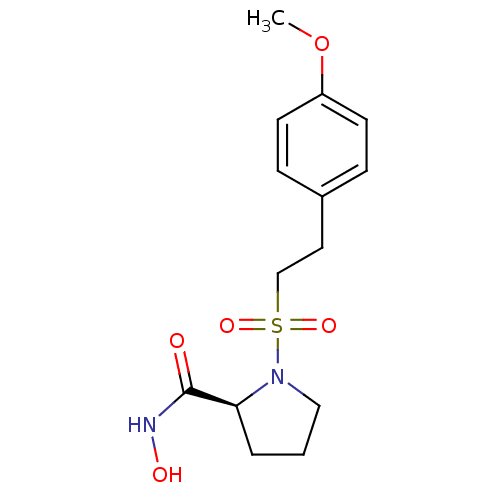
(CHEMBL2385483)Show SMILES COc1ccc(CCS(=O)(=O)N2CCC[C@H]2C(=O)NO)cc1 |r| Show InChI InChI=1S/C14H20N2O5S/c1-21-12-6-4-11(5-7-12)8-10-22(19,20)16-9-2-3-13(16)14(17)15-18/h4-7,13,18H,2-3,8-10H2,1H3,(H,15,17)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Inhibition of MMP12 (unknown origin)-mediated Mca-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 by fluorimetric assay |
ACS Med Chem Lett 4: 565-9 (2013)
Article DOI: 10.1021/ml300446a
BindingDB Entry DOI: 10.7270/Q2B859HT |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50493217

(DEMETHOXYDEBROMOAPLYSIATOXIN)Show SMILES [H][C@]12C[C@]3(O[C@]([H])([C@@H](C)CCCc4cccc(O)c4)[C@H]1C)O[C@@](O)(CC(=O)O[C@]([H])(CC(=O)O2)[C@@H](C)O)[C@H](C)CC3(C)C |r| Show InChI InChI=1S/C31H46O9/c1-18(9-7-10-22-11-8-12-23(33)13-22)28-20(3)25-16-31(39-28)29(5,6)15-19(2)30(36,40-31)17-27(35)37-24(21(4)32)14-26(34)38-25/h8,11-13,18-21,24-25,28,32-33,36H,7,9-10,14-17H2,1-6H3/t18-,19+,20-,21+,24+,25-,28+,30-,31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Displacement of [3H]PDBu from PKCalpha C1A domain (unknown origin) |
Bioorg Med Chem Lett 23: 4319-23 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.096
BindingDB Entry DOI: 10.7270/Q2VD72CX |
More data for this
Ligand-Target Pair | |
Alpha-1B adrenergic receptor
(Rattus norvegicus (rat)) | BDBM50187752
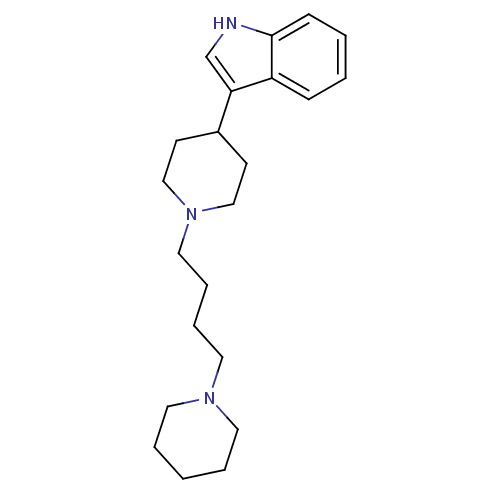
(3-(1-(4-(piperidin-1-yl)butyl)piperidin-4-yl)-1H-i...)Show InChI InChI=1S/C22H33N3/c1-4-12-24(13-5-1)14-6-7-15-25-16-10-19(11-17-25)21-18-23-22-9-3-2-8-20(21)22/h2-3,8-9,18-19,23H,1,4-7,10-17H2 | Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]prazosin from adrenergic alpha-1B receptor in rat liver membranes |
Bioorg Med Chem Lett 16: 4045-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.002
BindingDB Entry DOI: 10.7270/Q2514001 |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50343938
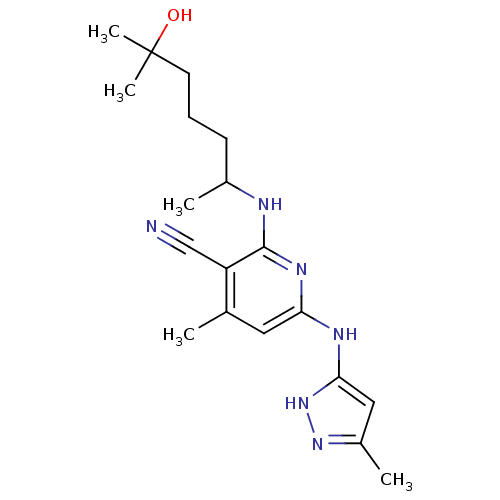
(CHEMBL1778662 | rac-2-(6-hydroxy-6-methylheptan-2-...)Show SMILES CC(CCCC(C)(C)O)Nc1nc(Nc2cc(C)n[nH]2)cc(C)c1C#N Show InChI InChI=1S/C19H28N6O/c1-12-9-16(22-17-10-14(3)24-25-17)23-18(15(12)11-20)21-13(2)7-6-8-19(4,5)26/h9-10,13,26H,6-8H2,1-5H3,(H3,21,22,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of aurora A |
Bioorg Med Chem Lett 20: 4709-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.119
BindingDB Entry DOI: 10.7270/Q2RF5VC3 |
More data for this
Ligand-Target Pair | |
Protein kinase C epsilon type
(Homo sapiens (Human)) | BDBM50493217

(DEMETHOXYDEBROMOAPLYSIATOXIN)Show SMILES [H][C@]12C[C@]3(O[C@]([H])([C@@H](C)CCCc4cccc(O)c4)[C@H]1C)O[C@@](O)(CC(=O)O[C@]([H])(CC(=O)O2)[C@@H](C)O)[C@H](C)CC3(C)C |r| Show InChI InChI=1S/C31H46O9/c1-18(9-7-10-22-11-8-12-23(33)13-22)28-20(3)25-16-31(39-28)29(5,6)15-19(2)30(36,40-31)17-27(35)37-24(21(4)32)14-26(34)38-25/h8,11-13,18-21,24-25,28,32-33,36H,7,9-10,14-17H2,1-6H3/t18-,19+,20-,21+,24+,25-,28+,30-,31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Displacement of [3H]PDBu from PKCepsilon C1B domain (unknown origin) |
Bioorg Med Chem Lett 23: 4319-23 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.096
BindingDB Entry DOI: 10.7270/Q2VD72CX |
More data for this
Ligand-Target Pair | |
Alpha-1B adrenergic receptor
(Rattus norvegicus (rat)) | BDBM50187747
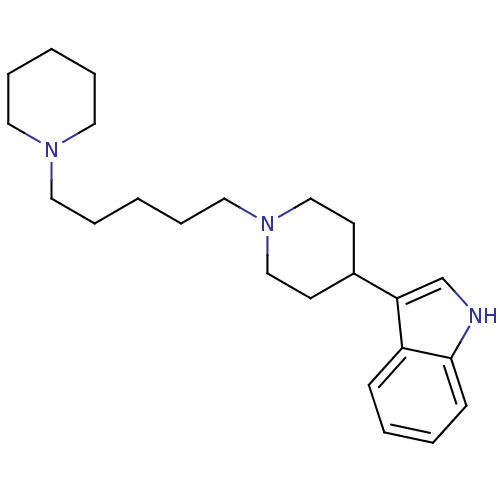
(3-(1-(5-(piperidin-1-yl)pentyl)piperidin-4-yl)-1H-...)Show InChI InChI=1S/C23H35N3/c1-5-13-25(14-6-1)15-7-2-8-16-26-17-11-20(12-18-26)22-19-24-23-10-4-3-9-21(22)23/h3-4,9-10,19-20,24H,1-2,5-8,11-18H2 | Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]prazosin from adrenergic alpha-1B receptor in rat liver membranes |
Bioorg Med Chem Lett 16: 4045-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.002
BindingDB Entry DOI: 10.7270/Q2514001 |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50493217

(DEMETHOXYDEBROMOAPLYSIATOXIN)Show SMILES [H][C@]12C[C@]3(O[C@]([H])([C@@H](C)CCCc4cccc(O)c4)[C@H]1C)O[C@@](O)(CC(=O)O[C@]([H])(CC(=O)O2)[C@@H](C)O)[C@H](C)CC3(C)C |r| Show InChI InChI=1S/C31H46O9/c1-18(9-7-10-22-11-8-12-23(33)13-22)28-20(3)25-16-31(39-28)29(5,6)15-19(2)30(36,40-31)17-27(35)37-24(21(4)32)14-26(34)38-25/h8,11-13,18-21,24-25,28,32-33,36H,7,9-10,14-17H2,1-6H3/t18-,19+,20-,21+,24+,25-,28+,30-,31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Displacement of [3H]PDBu from PKCbeta C1A domain (unknown origin) |
Bioorg Med Chem Lett 23: 4319-23 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.096
BindingDB Entry DOI: 10.7270/Q2VD72CX |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 2
(Homo sapiens (Human)) | BDBM50295693

(CHEMBL557629)Show SMILES CSCC[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](N)CC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@@H]2CCCN2C(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)CNC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCSC)NC1=O)C(C)C)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCC(N)=O)CN[C@@H](Cc1ccc(O)cc1)C(O)=O |r,wU:27.28,36.36,71.73,148.153,55.55,60.134,8.12,wD:112.115,4.4,97.100,93.131,82.84,16.24,134.137,44.44,64.69,156.161,120.123,(5.27,-20.83,;6.6,-20.06,;6.6,-18.52,;5.27,-17.75,;5.27,-16.21,;3.93,-15.44,;2.6,-16.21,;2.6,-17.75,;1.27,-15.44,;1.27,-13.9,;2.6,-13.13,;3.93,-13.9,;2.6,-11.59,;-.07,-16.21,;-1.4,-15.44,;-1.4,-13.9,;-2.73,-16.21,;-2.73,-17.75,;-1.4,-18.52,;-.07,-17.74,;1.27,-18.51,;1.27,-20.05,;-.08,-20.82,;-1.4,-20.05,;-4.07,-15.44,;-5.4,-16.21,;-5.4,-17.75,;-6.74,-15.44,;-8.07,-16.21,;-6.74,-13.9,;-8.07,-13.13,;-8.07,-11.59,;-9.4,-13.9,;6.6,-15.44,;6.6,-13.9,;7.93,-16.21,;9.27,-15.44,;9.27,-13.9,;10.6,-13.13,;10.6,-11.59,;11.94,-13.9,;10.6,-16.21,;10.6,-17.75,;11.94,-15.44,;13.27,-16.21,;13.27,-17.75,;11.94,-18.52,;11.94,-20.06,;10.6,-20.83,;10.6,-22.37,;9.27,-23.14,;11.94,-23.14,;14.6,-15.44,;14.6,-13.9,;15.94,-16.21,;17.27,-15.44,;17.27,-13.9,;15.94,-13.13,;15.71,-11.29,;14.63,-10.2,;15.04,-8.71,;16.59,-8.74,;17.38,-7.41,;18.92,-7.43,;16.62,-6.07,;15.22,-6.04,;14.79,-4.38,;16.14,-3.62,;17.27,-4.66,;18.6,-3.89,;18.6,-2.35,;19.94,-4.66,;19.94,-6.2,;21.27,-6.97,;21.27,-8.51,;19.94,-9.28,;19.94,-10.82,;18.6,-11.59,;21.27,-11.59,;21.27,-3.89,;22.61,-4.66,;22.61,-6.2,;23.94,-3.89,;23.94,-2.35,;22.61,-1.58,;22.61,-.04,;21.28,.73,;19.95,-.04,;19.95,-1.59,;21.27,-2.35,;25.27,-4.66,;26.61,-3.89,;26.61,-2.35,;27.94,-4.66,;27.94,-6.2,;26.61,-6.97,;25.27,-6.2,;26.61,-8.51,;27.94,-9.28,;27.94,-10.82,;29.27,-11.59,;29.27,-13.13,;30.61,-13.9,;31.94,-13.13,;30.61,-15.44,;25.27,-9.28,;25.27,-10.82,;23.94,-11.59,;26.61,-11.59,;26.61,-13.13,;25.27,-13.9,;23.94,-13.13,;25.27,-15.44,;26.61,-16.21,;26.61,-17.75,;27.94,-18.52,;25.27,-18.52,;23.94,-16.21,;22.61,-15.44,;22.61,-13.9,;21.27,-16.21,;21.27,-17.75,;19.94,-18.52,;19.94,-20.06,;18.6,-20.83,;19.94,-15.44,;18.6,-16.21,;18.6,-17.75,;29.27,-3.89,;30.61,-4.66,;29.27,-2.35,;13.71,-7.94,;13.71,-6.4,;12.38,-8.71,;11.04,-7.94,;11.05,-6.4,;9.71,-5.63,;8.29,-6.29,;7.28,-5.11,;8.05,-3.78,;7.58,-2.33,;8.6,-1.2,;10.11,-1.52,;10.57,-2.98,;9.54,-4.11,;9.71,-8.71,;9.71,-10.25,;8.38,-7.93,;7.04,-8.7,;7.04,-10.24,;5.71,-11.01,;5.7,-12.55,;7.19,-12.94,;5.3,-14.03,;5.71,-7.93,;4.38,-8.7,;3.04,-7.93,;3.04,-6.39,;1.71,-5.62,;1.72,-4.07,;.39,-3.3,;-.95,-4.07,;-2.28,-3.3,;-.94,-5.62,;.39,-6.38,;1.71,-8.7,;.38,-7.92,;1.71,-10.24,)| Show InChI InChI=1S/C109H162N30O25S4/c1-58(2)45-75-91(148)123-55-86(142)125-70(27-17-39-118-107(112)113)95(152)138-89(60(5)6)104(161)135-78(48-62-23-13-10-14-24-62)98(155)129-74(29-19-41-120-109(116)117)105(162)139-42-20-30-84(139)103(160)137-83(102(159)133-79(50-64-53-121-69-26-16-15-25-67(64)69)96(153)124-65(33-36-85(111)141)54-122-81(106(163)164)49-63-31-34-66(140)35-32-63)57-168-167-56-82(101(158)128-73(38-44-166-8)93(150)131-75)136-92(149)71(28-18-40-119-108(114)115)126-97(154)76(46-59(3)4)132-94(151)72(37-43-165-7)127-100(157)80(52-88(145)146)134-99(156)77(47-61-21-11-9-12-22-61)130-90(147)68(110)51-87(143)144/h9-16,21-26,31-32,34-35,53,58-60,65,68,70-84,89,121-122,140H,17-20,27-30,33,36-52,54-57,110H2,1-8H3,(H2,111,141)(H,123,148)(H,124,153)(H,125,142)(H,126,154)(H,127,157)(H,128,158)(H,129,155)(H,130,147)(H,131,150)(H,132,151)(H,133,159)(H,134,156)(H,135,161)(H,136,149)(H,137,160)(H,138,152)(H,143,144)(H,145,146)(H,163,164)(H4,112,113,118)(H4,114,115,119)(H4,116,117,120)/t65-,68-,70-,71-,72-,73-,74-,75-,76-,77-,78-,79-,80-,81-,82-,83-,84-,89-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [35S] 4-(1-(3,4-difluorophenyl)-2-(ethyl(3-(6-fluoro-3H-spiro[isobenzofuran-1,4'-piperidine]-1'-yl)propyl)amino)-2-oxoethyl)-3-oxopip... |
Bioorg Med Chem Lett 19: 2835-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.102
BindingDB Entry DOI: 10.7270/Q2R49QS7 |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM13534

(CHEMBL572878 | N-[4-({4-[(3-methyl-1H-pyrazol-5-yl...)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(C)n[nH]2)nc(Sc2ccc(NC(=O)C3CC3)cc2)n1 Show InChI InChI=1S/C23H28N8OS/c1-15-13-20(29-28-15)25-19-14-21(31-11-9-30(2)10-12-31)27-23(26-19)33-18-7-5-17(6-8-18)24-22(32)16-3-4-16/h5-8,13-14,16H,3-4,9-12H2,1-2H3,(H,24,32)(H2,25,26,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of aurora A |
Bioorg Med Chem Lett 20: 4709-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.119
BindingDB Entry DOI: 10.7270/Q2RF5VC3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50206865
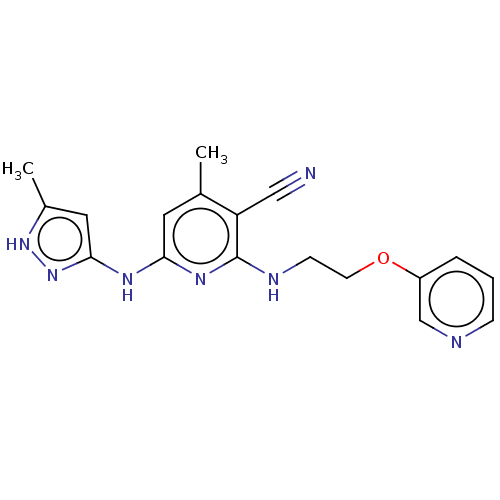
(CHEMBL3912923)Show SMILES Cc1cc(Nc2cc(C)c(C#N)c(NCCOc3cccnc3)n2)n[nH]1 Show InChI InChI=1S/C18H19N7O/c1-12-8-16(22-17-9-13(2)24-25-17)23-18(15(12)10-19)21-6-7-26-14-4-3-5-20-11-14/h3-5,8-9,11H,6-7H2,1-2H3,(H3,21,22,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CXS Corporation
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Aurora A kinase derived from human Hela cells using kemptide as substrate in presence of [c-32P]ATP |
Bioorg Med Chem Lett 26: 5860-5862 (2016)
Article DOI: 10.1016/j.bmcl.2016.11.020
BindingDB Entry DOI: 10.7270/Q2WS8W80 |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50434222

(CHEMBL2385487)Show SMILES ONC(=O)[C@H]1CCCN1S(=O)(=O)CCc1ccc(cc1)-c1ccccc1 |r| Show InChI InChI=1S/C19H22N2O4S/c22-19(20-23)18-7-4-13-21(18)26(24,25)14-12-15-8-10-17(11-9-15)16-5-2-1-3-6-16/h1-3,5-6,8-11,18,23H,4,7,12-14H2,(H,20,22)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Inhibition of MMP12 (unknown origin)-mediated Mca-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 by fluorimetric assay |
ACS Med Chem Lett 4: 565-9 (2013)
Article DOI: 10.1021/ml300446a
BindingDB Entry DOI: 10.7270/Q2B859HT |
More data for this
Ligand-Target Pair | |
Protein kinase C epsilon type
(Homo sapiens (Human)) | BDBM50391388

(CHEMBL2148108)Show SMILES C[C@@H]1[C@@H](CCCCc2cccc(O)c2)O[C@]23C[C@@H]1OC(=O)C[C@H](CO)OC(=O)C[C@@H](CCC2(C)C)O3 |r| Show InChI InChI=1S/C28H40O8/c1-18-23(10-5-4-7-19-8-6-9-20(30)13-19)36-28-16-24(18)34-26(32)15-22(17-29)33-25(31)14-21(35-28)11-12-27(28,2)3/h6,8-9,13,18,21-24,29-30H,4-5,7,10-12,14-17H2,1-3H3/t18-,21-,22-,23-,24?,28-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]PDBu binding to PKCepsilon C1B domain |
J Med Chem 55: 5614-26 (2012)
Article DOI: 10.1021/jm300566h
BindingDB Entry DOI: 10.7270/Q200036J |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50004205

(MK-045 | MK-0457 | TOZASERTIB | US9249124, VX680 |...)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(C)[nH]n2)nc(Sc2ccc(NC(=O)C3CC3)cc2)n1 Show InChI InChI=1S/C23H28N8OS/c1-15-13-20(29-28-15)25-19-14-21(31-11-9-30(2)10-12-31)27-23(26-19)33-18-7-5-17(6-8-18)24-22(32)16-3-4-16/h5-8,13-14,16H,3-4,9-12H2,1-2H3,(H,24,32)(H2,25,26,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CXS Corporation
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Aurora A kinase derived from human Hela cells using kemptide as substrate in presence of [c-32P]ATP |
Bioorg Med Chem Lett 26: 5860-5862 (2016)
Article DOI: 10.1016/j.bmcl.2016.11.020
BindingDB Entry DOI: 10.7270/Q2WS8W80 |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50294120

(2-(biphenyl-4-ylsulfonamido)-N-hydroxyacetamide | ...)Show InChI InChI=1S/C14H14N2O4S/c17-14(16-18)10-15-21(19,20)13-8-6-12(7-9-13)11-4-2-1-3-5-11/h1-9,15,18H,10H2,(H,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Inhibition of MMP12 (unknown origin)-mediated Mca-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 by fluorimetric assay |
ACS Med Chem Lett 4: 565-9 (2013)
Article DOI: 10.1021/ml300446a
BindingDB Entry DOI: 10.7270/Q2B859HT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50206863
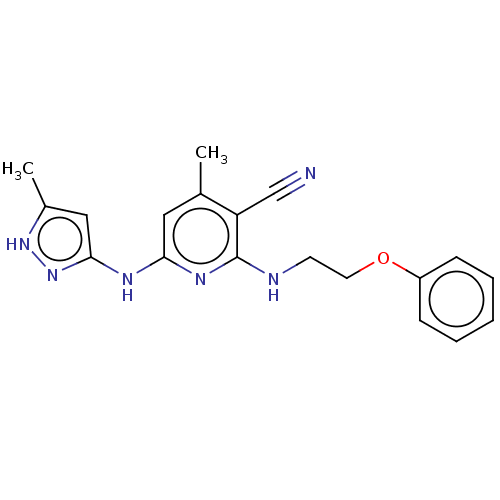
(CHEMBL3966303)Show SMILES Cc1cc(Nc2cc(C)c(C#N)c(NCCOc3ccccc3)n2)n[nH]1 Show InChI InChI=1S/C19H20N6O/c1-13-10-17(22-18-11-14(2)24-25-18)23-19(16(13)12-20)21-8-9-26-15-6-4-3-5-7-15/h3-7,10-11H,8-9H2,1-2H3,(H3,21,22,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CXS Corporation
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Aurora A kinase derived from human Hela cells using kemptide as substrate in presence of [c-32P]ATP |
Bioorg Med Chem Lett 26: 5860-5862 (2016)
Article DOI: 10.1016/j.bmcl.2016.11.020
BindingDB Entry DOI: 10.7270/Q2WS8W80 |
More data for this
Ligand-Target Pair | |
Alpha-1B adrenergic receptor
(Rattus norvegicus (rat)) | BDBM50115121
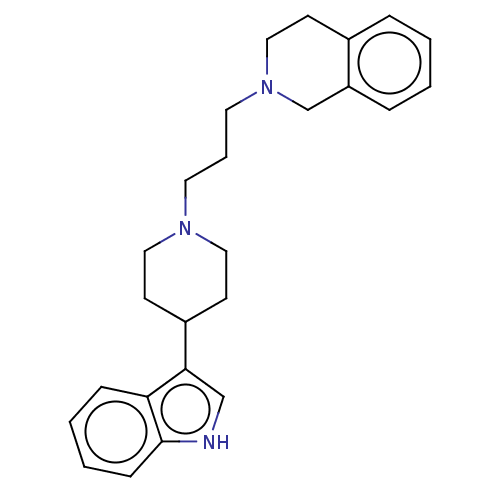
(CHEMBL3609359)Show SMILES C(CN1CCC(CC1)c1c[nH]c2ccccc12)CN1CCc2ccccc2C1 Show InChI InChI=1S/C25H31N3/c1-2-7-22-19-28(17-10-20(22)6-1)14-5-13-27-15-11-21(12-16-27)24-18-26-25-9-4-3-8-23(24)25/h1-4,6-9,18,21,26H,5,10-17,19H2 | Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]prazosin from alpha1B adrenoceptor in rat liver membranes |
Bioorg Med Chem Lett 25: 3921-3 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.046
BindingDB Entry DOI: 10.7270/Q27M09Q4 |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50343947

(2-(6-hydroxy-6-methylheptylamino)-4-methyl-6-(5-me...)Show SMILES Cc1cc(Nc2cc(C)c(C#N)c(NCCCCCC(C)(C)O)n2)[nH]n1 Show InChI InChI=1S/C19H28N6O/c1-13-10-16(22-17-11-14(2)24-25-17)23-18(15(13)12-20)21-9-7-5-6-8-19(3,4)26/h10-11,26H,5-9H2,1-4H3,(H3,21,22,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of aurora A |
Bioorg Med Chem Lett 20: 4709-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.119
BindingDB Entry DOI: 10.7270/Q2RF5VC3 |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50391387
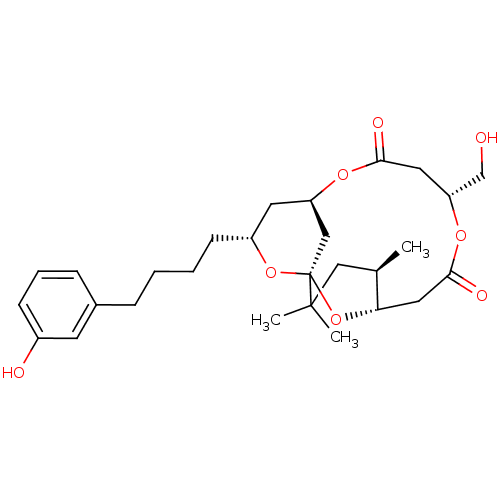
(CHEMBL2148107)Show SMILES C[C@@H]1CC(C)(C)[C@@]23C[C@@H](C[C@@H](CCCCc4cccc(O)c4)O2)OC(=O)C[C@H](CO)OC(=O)C[C@@H]1O3 |r| Show InChI InChI=1S/C28H40O8/c1-18-15-27(2,3)28-16-22(33-25(31)13-23(17-29)34-26(32)14-24(18)36-28)12-21(35-28)10-5-4-7-19-8-6-9-20(30)11-19/h6,8-9,11,18,21-24,29-30H,4-5,7,10,12-17H2,1-3H3/t18-,21-,22?,23-,24+,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]PDBu binding to PKCtheta C1B domain |
J Med Chem 55: 5614-26 (2012)
Article DOI: 10.1021/jm300566h
BindingDB Entry DOI: 10.7270/Q200036J |
More data for this
Ligand-Target Pair | |
Alpha-1B adrenergic receptor
(Rattus norvegicus (rat)) | BDBM50187749
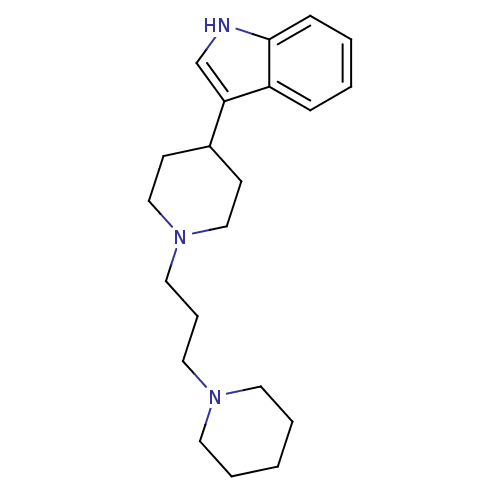
(3-(1-(3-(piperidin-1-yl)propyl)piperidin-4-yl)-1H-...)Show InChI InChI=1S/C21H31N3/c1-4-11-23(12-5-1)13-6-14-24-15-9-18(10-16-24)20-17-22-21-8-3-2-7-19(20)21/h2-3,7-8,17-18,22H,1,4-6,9-16H2 | Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]prazosin from adrenergic alpha-1B receptor in rat liver membranes |
Bioorg Med Chem Lett 16: 4045-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.002
BindingDB Entry DOI: 10.7270/Q2514001 |
More data for this
Ligand-Target Pair | |
Alpha-1B adrenergic receptor
(Rattus norvegicus (rat)) | BDBM50187749

(3-(1-(3-(piperidin-1-yl)propyl)piperidin-4-yl)-1H-...)Show InChI InChI=1S/C21H31N3/c1-4-11-23(12-5-1)13-6-14-24-15-9-18(10-16-24)20-17-22-21-8-3-2-7-19(20)21/h2-3,7-8,17-18,22H,1,4-6,9-16H2 | Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]prazosin from alpha1B adrenoceptor in rat liver membranes |
Bioorg Med Chem Lett 25: 3921-3 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.046
BindingDB Entry DOI: 10.7270/Q27M09Q4 |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM50391387

(CHEMBL2148107)Show SMILES C[C@@H]1CC(C)(C)[C@@]23C[C@@H](C[C@@H](CCCCc4cccc(O)c4)O2)OC(=O)C[C@H](CO)OC(=O)C[C@@H]1O3 |r| Show InChI InChI=1S/C28H40O8/c1-18-15-27(2,3)28-16-22(33-25(31)13-23(17-29)34-26(32)14-24(18)36-28)12-21(35-28)10-5-4-7-19-8-6-9-20(30)11-19/h6,8-9,11,18,21-24,29-30H,4-5,7,10,12-17H2,1-3H3/t18-,21-,22?,23-,24+,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]PDBu binding to PKCdelta C1B domain |
J Med Chem 55: 5614-26 (2012)
Article DOI: 10.1021/jm300566h
BindingDB Entry DOI: 10.7270/Q200036J |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data