 Found 1554 hits with Last Name = 'nelson' and Initial = 'dl'
Found 1554 hits with Last Name = 'nelson' and Initial = 'dl' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50068612
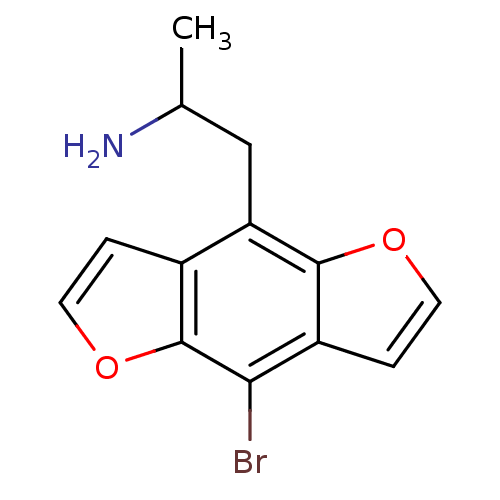
(2-(8-Bromo-benzo[1,2-b;4,5-b']difuran-4-yl)-1-meth...)Show InChI InChI=1S/C13H12BrNO2/c1-7(15)6-10-8-2-4-17-13(8)11(14)9-3-5-16-12(9)10/h2-5,7H,6,15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Binding activity against cloned human 5-hydroxytryptamine 2C receptor using [125I]-DOI as the radioligand. |
J Med Chem 41: 5148-9 (1999)
Article DOI: 10.1021/jm9803525
BindingDB Entry DOI: 10.7270/Q2862FKX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50068612

(2-(8-Bromo-benzo[1,2-b;4,5-b']difuran-4-yl)-1-meth...)Show InChI InChI=1S/C13H12BrNO2/c1-7(15)6-10-8-2-4-17-13(8)11(14)9-3-5-16-12(9)10/h2-5,7H,6,15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Binding activity against cloned human 5-hydroxytryptamine 2A receptor using [125I]-DOI as the radioligand. |
J Med Chem 41: 5148-9 (1999)
Article DOI: 10.1021/jm9803525
BindingDB Entry DOI: 10.7270/Q2862FKX |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50130172
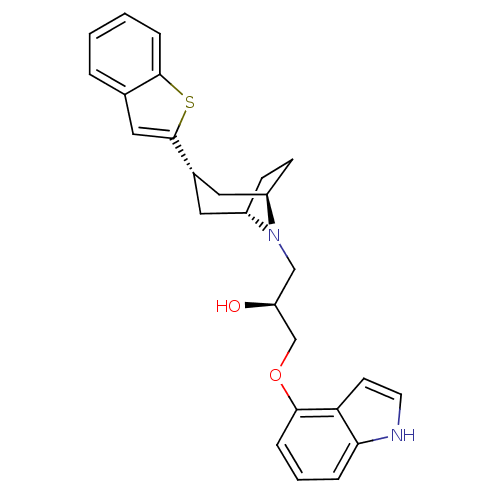
(1-(3-Benzo[b]thiophen-2-yl-8-aza-bicyclo[3.2.1]oct...)Show SMILES O[C@H](COc1cccc2[nH]ccc12)CN1[C@H]2CC[C@@H]1C[C@H](C2)c1cc2ccccc2s1 |THB:13:14:20.19.21:16.17| Show InChI InChI=1S/C26H28N2O2S/c29-21(16-30-24-6-3-5-23-22(24)10-11-27-23)15-28-19-8-9-20(28)13-18(12-19)26-14-17-4-1-2-7-25(17)31-26/h1-7,10-11,14,18-21,27,29H,8-9,12-13,15-16H2/t18-,19-,20+,21-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro affinity of the compound at the 5-HT reuptake site using [3H]-paroxetine as radioligand in rat frontal cortex membranes |
Bioorg Med Chem Lett 13: 2393-7 (2003)
BindingDB Entry DOI: 10.7270/Q2Z31Z1R |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM86231

(ATR | ATROPINE | Atropine,(-) | CAS_51-55-8 | CHEM...)Show SMILES CN1C2CCC1CC(C2)OC(=O)C(CO)c1ccccc1 |THB:9:7:1:3.4| Show InChI InChI=1S/C17H23NO3/c1-18-13-7-8-14(18)10-15(9-13)21-17(20)16(11-19)12-5-3-2-4-6-12/h2-6,13-16,19H,7-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by PDSP Ki Database
| |
Schizophr Res 37: 107-22 (1999)
Article DOI: 10.1016/s0920-9964(98)00146-7
BindingDB Entry DOI: 10.7270/Q23F4N5N |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50014997
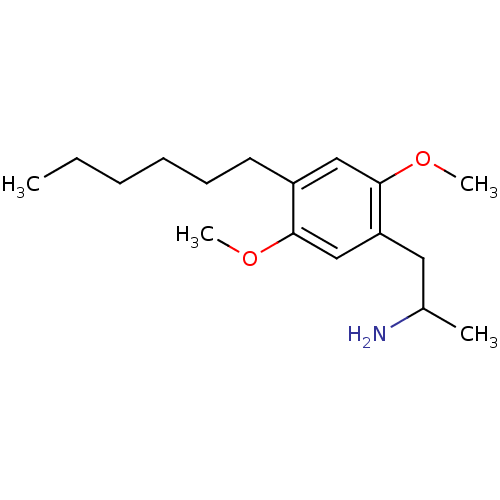
(1-(4-hexyl-2,5-dimethoxyphenyl)propan-2-amine | 2-...)Show InChI InChI=1S/C17H29NO2/c1-5-6-7-8-9-14-11-17(20-4)15(10-13(2)18)12-16(14)19-3/h11-13H,5-10,18H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by PDSP Ki Database
| |
Naunyn Schmiedebergs Arch Pharmacol 359: 1-6 (1999)
Article DOI: 10.1007/pl00005315
BindingDB Entry DOI: 10.7270/Q2862F09 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50130170

((S)-1-(1H-Indol-4-yloxy)-3-[(1R,3S,5S)-3-(4-methox...)Show SMILES COc1cccc2sc(cc12)[C@H]1C[C@@H]2CC[C@H](C1)N2C[C@H](O)COc1cccc2[nH]ccc12 |THB:19:18:11.17.12:14.15| Show InChI InChI=1S/C27H30N2O3S/c1-31-24-5-3-7-26-22(24)14-27(33-26)17-12-18-8-9-19(13-17)29(18)15-20(30)16-32-25-6-2-4-23-21(25)10-11-28-23/h2-7,10-11,14,17-20,28,30H,8-9,12-13,15-16H2,1H3/t17-,18-,19+,20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity at 5-hydroxytryptamine 1A receptor by [3H]-8-OH-DPAT displacement. |
Bioorg Med Chem Lett 13: 2393-7 (2003)
BindingDB Entry DOI: 10.7270/Q2Z31Z1R |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50130170

((S)-1-(1H-Indol-4-yloxy)-3-[(1R,3S,5S)-3-(4-methox...)Show SMILES COc1cccc2sc(cc12)[C@H]1C[C@@H]2CC[C@H](C1)N2C[C@H](O)COc1cccc2[nH]ccc12 |THB:19:18:11.17.12:14.15| Show InChI InChI=1S/C27H30N2O3S/c1-31-24-5-3-7-26-22(24)14-27(33-26)17-12-18-8-9-19(13-17)29(18)15-20(30)16-32-25-6-2-4-23-21(25)10-11-28-23/h2-7,10-11,14,17-20,28,30H,8-9,12-13,15-16H2,1H3/t17-,18-,19+,20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro affinity of the compound at the 5-HT reuptake site using [3H]-paroxetine as radioligand in rat frontal cortex membranes |
Bioorg Med Chem Lett 13: 2393-7 (2003)
BindingDB Entry DOI: 10.7270/Q2Z31Z1R |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50135254
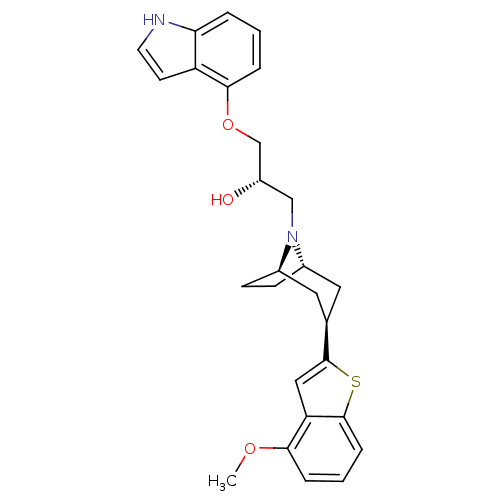
((S)-1-(1H-Indol-4-yloxy)-3-[(1S,3R,5R)-3-(4-methox...)Show SMILES COc1cccc2sc(cc12)[C@@H]1C[C@@H]2CC[C@H](C1)N2C[C@H](O)COc1cccc2[nH]ccc12 |THB:19:18:11.17.12:14.15| Show InChI InChI=1S/C27H30N2O3S/c1-31-24-5-3-7-26-22(24)14-27(33-26)17-12-18-8-9-19(13-17)29(18)15-20(30)16-32-25-6-2-4-23-21(25)10-11-28-23/h2-7,10-11,14,17-20,28,30H,8-9,12-13,15-16H2,1H3/t17-,18+,19-,20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Affinity at the 5-HT reuptake site labeled with [3H]-paroxetine using rat frontal cortex membranes |
Bioorg Med Chem Lett 13: 3939-42 (2003)
BindingDB Entry DOI: 10.7270/Q24T6HSX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM50068612

(2-(8-Bromo-benzo[1,2-b;4,5-b']difuran-4-yl)-1-meth...)Show InChI InChI=1S/C13H12BrNO2/c1-7(15)6-10-8-2-4-17-13(8)11(14)9-3-5-16-12(9)10/h2-5,7H,6,15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Binding activity against cloned human 5-hydroxytryptamine 2B receptor using [3H]-5-HT as the radioligand. |
J Med Chem 41: 5148-9 (1999)
Article DOI: 10.1021/jm9803525
BindingDB Entry DOI: 10.7270/Q2862FKX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50068612

(2-(8-Bromo-benzo[1,2-b;4,5-b']difuran-4-yl)-1-meth...)Show InChI InChI=1S/C13H12BrNO2/c1-7(15)6-10-8-2-4-17-13(8)11(14)9-3-5-16-12(9)10/h2-5,7H,6,15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Antagonistic activity measured for 5-hydroxytryptamine 2A receptor using [3H]-MDL- 100,907 as radioligand in rat cortical homogenates. |
J Med Chem 41: 5148-9 (1999)
Article DOI: 10.1021/jm9803525
BindingDB Entry DOI: 10.7270/Q2862FKX |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50135249

((S)-1-((2S,4R)-4-(5-fluorobenzo[b]thiophen-2-yl)-2...)Show SMILES C[C@H]1C[C@@H](CCN1C[C@H](O)COc1cccc2[nH]c(C)cc12)c1cc2cc(F)ccc2s1 Show InChI InChI=1S/C26H29FN2O2S/c1-16-10-22-23(28-16)4-3-5-24(22)31-15-21(30)14-29-9-8-18(11-17(29)2)26-13-19-12-20(27)6-7-25(19)32-26/h3-7,10,12-13,17-18,21,28,30H,8-9,11,14-15H2,1-2H3/t17-,18+,21-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Affinity at the 5-HT reuptake site labeled with [3H]-paroxetine using rat frontal cortex membranes |
Bioorg Med Chem Lett 13: 3939-42 (2003)
BindingDB Entry DOI: 10.7270/Q24T6HSX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50076428
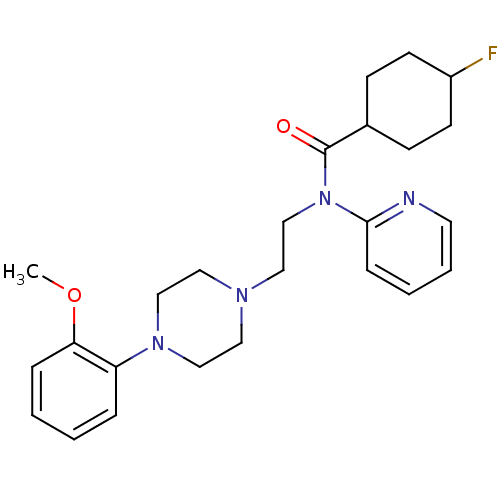
(4-Fluoro-cyclohexanecarboxylic acid {2-[4-(2-metho...)Show SMILES COc1ccccc1N1CCN(CCN(C(=O)C2CCC(F)CC2)c2ccccn2)CC1 |(2.32,-7.79,;3.65,-8.56,;3.63,-10.1,;2.31,-10.86,;2.31,-12.41,;3.63,-13.18,;4.99,-12.41,;4.99,-10.87,;6.3,-10.12,;7.65,-10.89,;8.99,-10.12,;8.96,-8.58,;10.31,-7.82,;11.65,-8.58,;12.96,-7.82,;14.31,-8.58,;14.31,-10.13,;15.63,-7.82,;16.96,-8.58,;18.94,-8.27,;19.49,-9.96,;21.03,-9.96,;18.27,-9.22,;16.18,-9.51,;12.96,-6.27,;11.65,-5.51,;11.65,-3.96,;12.96,-3.19,;14.31,-3.96,;14.31,-5.51,;7.65,-7.81,;6.32,-8.58,)| Show InChI InChI=1S/C25H33FN4O2/c1-32-23-7-3-2-6-22(23)29-17-14-28(15-18-29)16-19-30(24-8-4-5-13-27-24)25(31)20-9-11-21(26)12-10-20/h2-8,13,20-21H,9-12,14-19H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.247 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by ChEMBL
| Assay Description
In vitro inhibition of [3H]- 8-OH-DPAT binding to cloned cell line containing human 5-hydroxytryptamine 1A receptor by Panlabs assay |
J Med Chem 42: 1576-86 (1999)
Article DOI: 10.1021/jm980456f
BindingDB Entry DOI: 10.7270/Q23R0S2J |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50130163

((S)-1-(1H-Indol-4-yloxy)-3-[(4S,6R)-4-(4-methoxy-b...)Show SMILES COc1cccc2sc(cc12)[C@@H]1CCN(C[C@H](O)COc2cccc3[nH]ccc23)[C@@H](C)C1 Show InChI InChI=1S/C26H30N2O3S/c1-17-13-18(26-14-21-23(30-2)6-4-8-25(21)32-26)10-12-28(17)15-19(29)16-31-24-7-3-5-22-20(24)9-11-27-22/h3-9,11,14,17-19,27,29H,10,12-13,15-16H2,1-2H3/t17-,18+,19-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro affinity of the compound at the 5-HT reuptake site using [3H]-paroxetine as radioligand in rat frontal cortex membranes |
Bioorg Med Chem Lett 13: 2393-7 (2003)
BindingDB Entry DOI: 10.7270/Q2Z31Z1R |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50052337
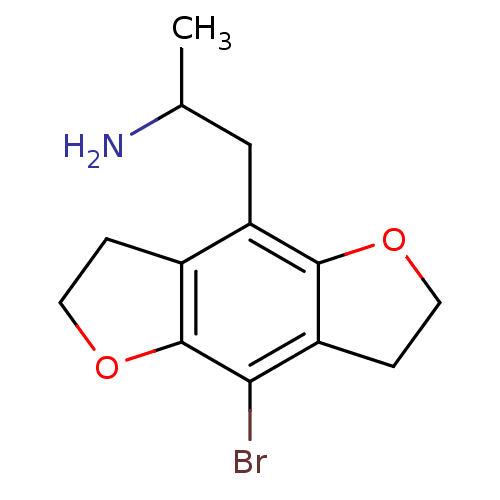
(2-(8-bromo-2,3,6,7-tetrahydro-benzo[1,2-b;4,5-b']d...)Show InChI InChI=1S/C13H16BrNO2/c1-7(15)6-10-8-2-4-17-13(8)11(14)9-3-5-16-12(9)10/h7H,2-6,15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Binding activity against cloned human 5-hydroxytryptamine 2C receptor using [125I]-DOI as the radioligand. |
J Med Chem 41: 5148-9 (1999)
Article DOI: 10.1021/jm9803525
BindingDB Entry DOI: 10.7270/Q2862FKX |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50145598
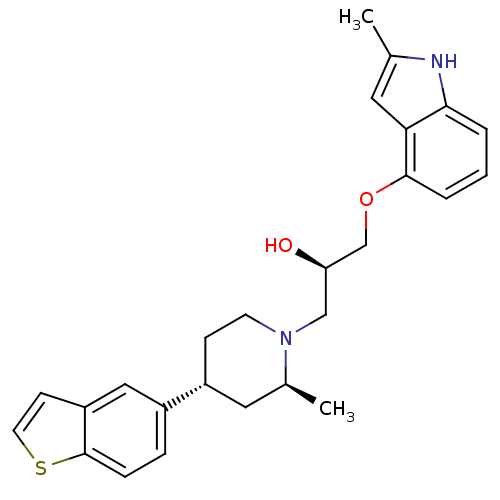
((R)-1-((3S,4R)-4-Benzo[b]thiophen-5-yl-2-methyl-pi...)Show SMILES C[C@H]1C[C@@H](CCN1C[C@@H](O)COc1cccc2[nH]c(C)cc12)c1ccc2sccc2c1 Show InChI InChI=1S/C26H30N2O2S/c1-17-12-23-24(27-17)4-3-5-25(23)30-16-22(29)15-28-10-8-20(13-18(28)2)19-6-7-26-21(14-19)9-11-31-26/h3-7,9,11-12,14,18,20,22,27,29H,8,10,13,15-16H2,1-2H3/t18-,20+,22+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity at 5-HT reuptake site labeled with [3H]-paroxetine |
Bioorg Med Chem Lett 14: 2653-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.088
BindingDB Entry DOI: 10.7270/Q2GF0SZH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50052337

(2-(8-bromo-2,3,6,7-tetrahydro-benzo[1,2-b;4,5-b']d...)Show InChI InChI=1S/C13H16BrNO2/c1-7(15)6-10-8-2-4-17-13(8)11(14)9-3-5-16-12(9)10/h7H,2-6,15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Agonistic activity at cloned human 5-hydroxytryptamine 2C receptor using [125I]-DOI as radioligand |
J Med Chem 39: 2953-61 (1996)
Article DOI: 10.1021/jm960199j
BindingDB Entry DOI: 10.7270/Q28914ZS |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50064703
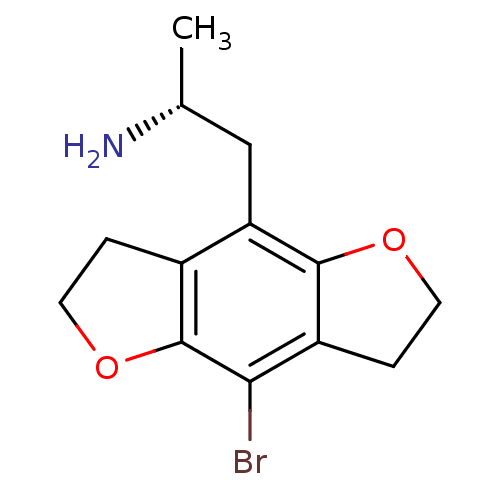
((R)-2-(8-Bromo-2,3,6,7-tetrahydro-benzo[1,2-b;4,5-...)Show InChI InChI=1S/C13H16BrNO2/c1-7(15)6-10-8-2-4-17-13(8)11(14)9-3-5-16-12(9)10/h7H,2-6,15H2,1H3/t7-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Binding ability of compound at cloned human 5-hydroxytryptamine 2C receptor using [125 I]DOI as radioligand |
J Med Chem 41: 2134-45 (1998)
Article DOI: 10.1021/jm980076u
BindingDB Entry DOI: 10.7270/Q2XD10T8 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50130152
((S)-1-(1H-indol-4-yloxy)-3-((2S,4R)-4-(6-fluoroben...)Show SMILES C[C@H]1C[C@@H](CCN1C[C@H](O)COc1cccc2[nH]ccc12)c1cc2ccc(F)cc2s1 Show InChI InChI=1S/C25H27FN2O2S/c1-16-11-18(24-12-17-5-6-19(26)13-25(17)31-24)8-10-28(16)14-20(29)15-30-23-4-2-3-22-21(23)7-9-27-22/h2-7,9,12-13,16,18,20,27,29H,8,10-11,14-15H2,1H3/t16-,18+,20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro affinity of the compound at the 5-HT reuptake site using [3H]-paroxetine as radioligand in rat frontal cortex membranes |
Bioorg Med Chem Lett 13: 2393-7 (2003)
BindingDB Entry DOI: 10.7270/Q2Z31Z1R |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50452111

(CHEMBL2112353)Show SMILES COc1cccc2ccc(cc12)[C@@H]1CCN(C[C@H](O)COc2cccc3[nH]c(C)cc23)[C@@H](C)C1 Show InChI InChI=1S/C29H34N2O3/c1-19-14-26-27(30-19)7-5-9-29(26)34-18-24(32)17-31-13-12-23(15-20(31)2)22-11-10-21-6-4-8-28(33-3)25(21)16-22/h4-11,14,16,20,23-24,30,32H,12-13,15,17-18H2,1-3H3/t20-,23+,24-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity at 5-HT reuptake site labeled with [3H]-paroxetine |
Bioorg Med Chem Lett 14: 2653-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.088
BindingDB Entry DOI: 10.7270/Q2GF0SZH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Mus musculus (Mouse)) | BDBM86708

(CAS_146714-97-8 | CHEMBL31354 | CHEMBL514874 | CHE...)Show SMILES COc1ccccc1N1CCN(CCN(C(=O)C2CCCCC2)c2ccccn2)CC1 Show InChI InChI=1S/C25H34N4O2/c1-31-23-12-6-5-11-22(23)28-18-15-27(16-19-28)17-20-29(24-13-7-8-14-26-24)25(30)21-9-3-2-4-10-21/h5-8,11-14,21H,2-4,9-10,15-20H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by ChEMBL
| Assay Description
Receptor-linked G protein activation at 5-hydroxytryptamine receptor was determined by measuring the stimulation of [35S]-GTP-gammaS, binding (Experi... |
J Med Chem 42: 1576-86 (1999)
Article DOI: 10.1021/jm980456f
BindingDB Entry DOI: 10.7270/Q23R0S2J |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50135248
(4-{(S)-2-Hydroxy-3-[(1R,3S,5S)-3-(4-methoxy-benzo[...)Show SMILES COc1cccc2sc(cc12)[C@H]1C[C@@H]2CC[C@H](C1)N2C[C@H](O)COc1cccc2[nH]c(cc12)C(N)=O |THB:19:18:11.17.12:14.15| Show InChI InChI=1S/C28H31N3O4S/c1-34-24-5-3-7-26-21(24)13-27(36-26)16-10-17-8-9-18(11-16)31(17)14-19(32)15-35-25-6-2-4-22-20(25)12-23(30-22)28(29)33/h2-7,12-13,16-19,30,32H,8-11,14-15H2,1H3,(H2,29,33)/t16-,17-,18+,19-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Affinity at the 5-HT reuptake site labeled with [3H]-paroxetine using rat frontal cortex membranes |
Bioorg Med Chem Lett 13: 3939-42 (2003)
BindingDB Entry DOI: 10.7270/Q24T6HSX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(PIG) | BDBM30704
((phenylmethyl) N-[[(6aR,9S,10aR)-4,7-dimethyl-6,6a...)Show SMILES CN1C[C@H](CNC(=O)OCc2ccccc2)C[C@H]2[C@H]1Cc1cn(C)c3cccc2c13 Show InChI InChI=1S/C25H29N3O2/c1-27-14-18(13-26-25(29)30-16-17-7-4-3-5-8-17)11-21-20-9-6-10-22-24(20)19(12-23(21)27)15-28(22)2/h3-10,15,18,21,23H,11-14,16H2,1-2H3,(H,26,29)/t18-,21+,23+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 265: 1272-9 (1993)
BindingDB Entry DOI: 10.7270/Q2833QJ4 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50135244

((S)-1-[(2S,4R)-4-(6-Fluoro-benzo[b]thiophen-2-yl)-...)Show SMILES C[C@H]1C[C@@H](CCN1C[C@H](O)COc1cccc2[nH]c(C)cc12)c1cc2ccc(F)cc2s1 Show InChI InChI=1S/C26H29FN2O2S/c1-16-10-22-23(28-16)4-3-5-24(22)31-15-21(30)14-29-9-8-19(11-17(29)2)25-12-18-6-7-20(27)13-26(18)32-25/h3-7,10,12-13,17,19,21,28,30H,8-9,11,14-15H2,1-2H3/t17-,19+,21-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Affinity at the 5-HT reuptake site labeled with [3H]-paroxetine using rat frontal cortex membranes |
Bioorg Med Chem Lett 13: 3939-42 (2003)
BindingDB Entry DOI: 10.7270/Q24T6HSX |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50130173

((S)-1-[(4S,6R)-4-(5-Fluoro-benzo[b]thiophen-2-yl)-...)Show SMILES C[C@H]1C[C@@H](CCN1C[C@H](O)COc1cccc2[nH]ccc12)c1cc2cc(F)ccc2s1 Show InChI InChI=1S/C25H27FN2O2S/c1-16-11-17(25-13-18-12-19(26)5-6-24(18)31-25)8-10-28(16)14-20(29)15-30-23-4-2-3-22-21(23)7-9-27-22/h2-7,9,12-13,16-17,20,27,29H,8,10-11,14-15H2,1H3/t16-,17+,20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro affinity of the compound at the 5-HT reuptake site using [3H]-paroxetine as radioligand in rat frontal cortex membranes |
Bioorg Med Chem Lett 13: 2393-7 (2003)
BindingDB Entry DOI: 10.7270/Q2Z31Z1R |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M5
(Homo sapiens (Human)) | BDBM86231

(ATR | ATROPINE | Atropine,(-) | CAS_51-55-8 | CHEM...)Show SMILES CN1C2CCC1CC(C2)OC(=O)C(CO)c1ccccc1 |THB:9:7:1:3.4| Show InChI InChI=1S/C17H23NO3/c1-18-13-7-8-14(18)10-15(9-13)21-17(20)16(11-19)12-5-3-2-4-6-12/h2-6,13-16,19H,7-11H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by PDSP Ki Database
| |
Schizophr Res 37: 107-22 (1999)
Article DOI: 10.1016/s0920-9964(98)00146-7
BindingDB Entry DOI: 10.7270/Q23F4N5N |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50014999
(CHEMBL274589 | DOBz)Show InChI InChI=1S/C18H23NO2/c1-13(19)9-15-11-18(21-3)16(12-17(15)20-2)10-14-7-5-4-6-8-14/h4-8,11-13H,9-10,19H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by PDSP Ki Database
| |
Naunyn Schmiedebergs Arch Pharmacol 359: 1-6 (1999)
Article DOI: 10.1007/pl00005315
BindingDB Entry DOI: 10.7270/Q2862F09 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50019443
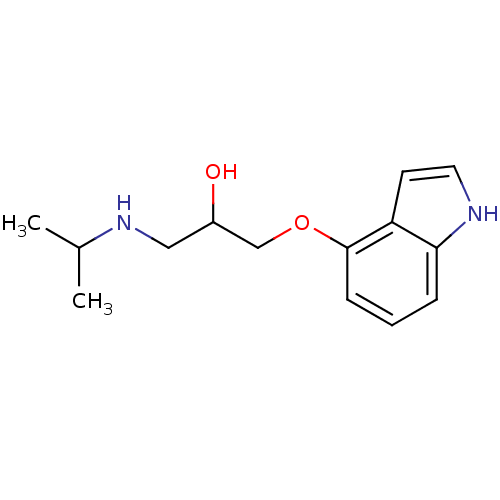
(1-(1H-Indol-4-yloxy)-3-isopropylamino-propan-2-ol ...)Show InChI InChI=1S/C14H20N2O2/c1-10(2)16-8-11(17)9-18-14-5-3-4-13-12(14)6-7-15-13/h3-7,10-11,15-17H,8-9H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity at human adrenergic beta2 receptor |
Bioorg Med Chem Lett 17: 5600-4 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.086
BindingDB Entry DOI: 10.7270/Q2FJ2GG0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM50001775

((ritanserin)6-(2-{4-[Bis-(4-fluoro-phenyl)-methyle...)Show SMILES [#6]-c1nc2sccn2c(=O)c1-[#6]-[#6]-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6](\c1ccc(F)cc1)-c1ccc(F)cc1 Show InChI InChI=1S/C27H25F2N3OS/c1-18-24(26(33)32-16-17-34-27(32)30-18)12-15-31-13-10-21(11-14-31)25(19-2-6-22(28)7-3-19)20-4-8-23(29)9-5-20/h2-9,16-17H,10-15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by PDSP Ki Database
| |
Naunyn Schmiedebergs Arch Pharmacol 357: 17-24 (1998)
Article DOI: 10.1007/pl00005133
BindingDB Entry DOI: 10.7270/Q2W66J9P |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50452114
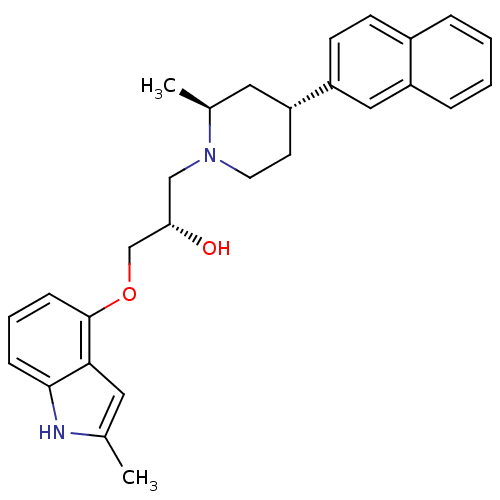
(CHEMBL2112350)Show SMILES C[C@H]1C[C@@H](CCN1C[C@H](O)COc1cccc2[nH]c(C)cc12)c1ccc2ccccc2c1 Show InChI InChI=1S/C28H32N2O2/c1-19-14-26-27(29-19)8-5-9-28(26)32-18-25(31)17-30-13-12-24(15-20(30)2)23-11-10-21-6-3-4-7-22(21)16-23/h3-11,14,16,20,24-25,29,31H,12-13,15,17-18H2,1-2H3/t20-,24+,25-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity at 5-HT reuptake site labeled with [3H]-paroxetine |
Bioorg Med Chem Lett 14: 2653-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.088
BindingDB Entry DOI: 10.7270/Q2GF0SZH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50001885

((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...)Show SMILES Cc1nc2CCCCn2c(=O)c1CCN1CCC(CC1)c1noc2cc(F)ccc12 Show InChI InChI=1S/C23H27FN4O2/c1-15-18(23(29)28-10-3-2-4-21(28)25-15)9-13-27-11-7-16(8-12-27)22-19-6-5-17(24)14-20(19)30-26-22/h5-6,14,16H,2-4,7-13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 276: 720-7 (1996)
BindingDB Entry DOI: 10.7270/Q29W0D1S |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50130168
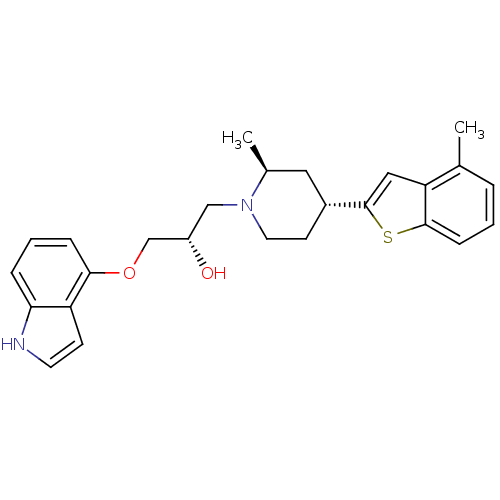
((S)-1-(1H-Indol-4-yloxy)-3-[(4S,6R)-2-methyl-4-(4-...)Show SMILES C[C@H]1C[C@@H](CCN1C[C@H](O)COc1cccc2[nH]ccc12)c1cc2c(C)cccc2s1 Show InChI InChI=1S/C26H30N2O2S/c1-17-5-3-8-25-22(17)14-26(31-25)19-10-12-28(18(2)13-19)15-20(29)16-30-24-7-4-6-23-21(24)9-11-27-23/h3-9,11,14,18-20,27,29H,10,12-13,15-16H2,1-2H3/t18-,19+,20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro affinity of the compound at the 5-HT reuptake site using [3H]-paroxetine as radioligand in rat frontal cortex membranes |
Bioorg Med Chem Lett 13: 2393-7 (2003)
BindingDB Entry DOI: 10.7270/Q2Z31Z1R |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50135253
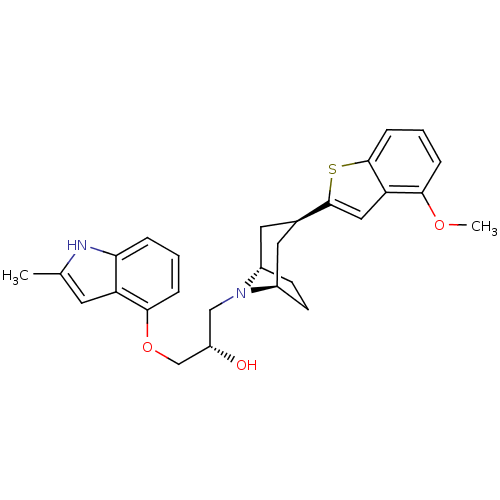
((S)-1-[(1S,3R,5R)-3-(4-Methoxy-benzo[b]thiophen-2-...)Show SMILES COc1cccc2sc(cc12)[C@@H]1C[C@@H]2CC[C@H](C1)N2C[C@H](O)COc1cccc2[nH]c(C)cc12 |THB:19:18:11.17.12:14.15| Show InChI InChI=1S/C28H32N2O3S/c1-17-11-22-24(29-17)5-3-7-26(22)33-16-21(31)15-30-19-9-10-20(30)13-18(12-19)28-14-23-25(32-2)6-4-8-27(23)34-28/h3-8,11,14,18-21,29,31H,9-10,12-13,15-16H2,1-2H3/t18-,19+,20-,21-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Affinity at the 5-HT reuptake site labeled with [3H]-paroxetine using rat frontal cortex membranes |
Bioorg Med Chem Lett 13: 3939-42 (2003)
BindingDB Entry DOI: 10.7270/Q24T6HSX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM28582
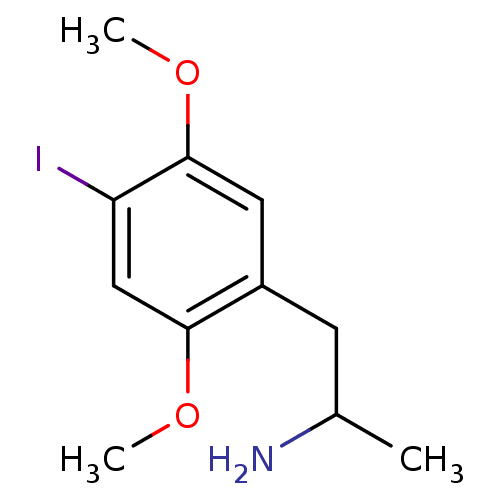
(1-(4-iodo-2,5-dimethoxyphenyl)propan-2-amine | CHE...)Show InChI InChI=1S/C11H16INO2/c1-7(13)4-8-5-11(15-3)9(12)6-10(8)14-2/h5-7H,4,13H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Binding activity against cloned human 5-hydroxytryptamine 2A receptor using [125I]-DOI as the radioligand. |
J Med Chem 41: 5148-9 (1999)
Article DOI: 10.1021/jm9803525
BindingDB Entry DOI: 10.7270/Q2862FKX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50052337

(2-(8-bromo-2,3,6,7-tetrahydro-benzo[1,2-b;4,5-b']d...)Show InChI InChI=1S/C13H16BrNO2/c1-7(15)6-10-8-2-4-17-13(8)11(14)9-3-5-16-12(9)10/h7H,2-6,15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Agonistic activity at cloned human 5-hydroxytryptamine 2A receptor using [125I]-DOI as radioligand |
J Med Chem 39: 2953-61 (1996)
Article DOI: 10.1021/jm960199j
BindingDB Entry DOI: 10.7270/Q28914ZS |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50064703

((R)-2-(8-Bromo-2,3,6,7-tetrahydro-benzo[1,2-b;4,5-...)Show InChI InChI=1S/C13H16BrNO2/c1-7(15)6-10-8-2-4-17-13(8)11(14)9-3-5-16-12(9)10/h7H,2-6,15H2,1H3/t7-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Binding ability of compound at cloned human 5-hydroxytryptamine 2A receptor using [125 I]DOI as radioligand |
J Med Chem 41: 2134-45 (1998)
Article DOI: 10.1021/jm980076u
BindingDB Entry DOI: 10.7270/Q2XD10T8 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50130269

((6aR,9R)-5-Bromo-7-methyl-4,6,6a,7,8,9-hexahydro-i...)Show SMILES CCN(CC)C(=O)[C@H]1CN(C)[C@@H]2Cc3c(Br)[nH]c4cccc(C2=C1)c34 |c:23| Show InChI InChI=1S/C20H24BrN3O/c1-4-24(5-2)20(25)12-9-14-13-7-6-8-16-18(13)15(19(21)22-16)10-17(14)23(3)11-12/h6-9,12,17,22H,4-5,10-11H2,1-3H3/t12-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 276: 720-7 (1996)
BindingDB Entry DOI: 10.7270/Q29W0D1S |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50052337

(2-(8-bromo-2,3,6,7-tetrahydro-benzo[1,2-b;4,5-b']d...)Show InChI InChI=1S/C13H16BrNO2/c1-7(15)6-10-8-2-4-17-13(8)11(14)9-3-5-16-12(9)10/h7H,2-6,15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Binding activity against cloned human 5-hydroxytryptamine 2A receptor using [125I]-DOI as the radioligand. |
J Med Chem 41: 5148-9 (1999)
Article DOI: 10.1021/jm9803525
BindingDB Entry DOI: 10.7270/Q2862FKX |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM84745

(CAS_136434-34-9 | DULOXETINE | LY-248686 | LY24868...)Show InChI InChI=1S/C18H19NOS/c1-19-12-11-17(18-10-5-13-21-18)20-16-9-4-7-14-6-2-3-8-15(14)16/h2-10,13,17,19H,11-12H2,1H3/t17-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by PDSP Ki Database
| |
Neuropsychopharmacology 25: 871-80 (2001)
Article DOI: 10.1016/S0893-133X(01)00298-6
BindingDB Entry DOI: 10.7270/Q25M648W |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50130157
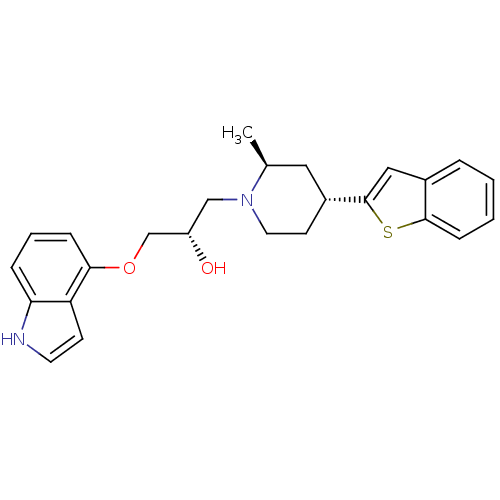
((S)-1-((4S,6R)-4-Benzo[b]thiophen-2-yl-2-methyl-pi...)Show SMILES C[C@H]1C[C@@H](CCN1C[C@H](O)COc1cccc2[nH]ccc12)c1cc2ccccc2s1 Show InChI InChI=1S/C25H28N2O2S/c1-17-13-19(25-14-18-5-2-3-8-24(18)30-25)10-12-27(17)15-20(28)16-29-23-7-4-6-22-21(23)9-11-26-22/h2-9,11,14,17,19-20,26,28H,10,12-13,15-16H2,1H3/t17-,19+,20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro affinity of the compound at the 5-HT reuptake site using [3H]-paroxetine as radioligand in rat frontal cortex membranes |
Bioorg Med Chem Lett 13: 2393-7 (2003)
BindingDB Entry DOI: 10.7270/Q2Z31Z1R |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50076428

(4-Fluoro-cyclohexanecarboxylic acid {2-[4-(2-metho...)Show SMILES COc1ccccc1N1CCN(CCN(C(=O)C2CCC(F)CC2)c2ccccn2)CC1 |(2.32,-7.79,;3.65,-8.56,;3.63,-10.1,;2.31,-10.86,;2.31,-12.41,;3.63,-13.18,;4.99,-12.41,;4.99,-10.87,;6.3,-10.12,;7.65,-10.89,;8.99,-10.12,;8.96,-8.58,;10.31,-7.82,;11.65,-8.58,;12.96,-7.82,;14.31,-8.58,;14.31,-10.13,;15.63,-7.82,;16.96,-8.58,;18.94,-8.27,;19.49,-9.96,;21.03,-9.96,;18.27,-9.22,;16.18,-9.51,;12.96,-6.27,;11.65,-5.51,;11.65,-3.96,;12.96,-3.19,;14.31,-3.96,;14.31,-5.51,;7.65,-7.81,;6.32,-8.58,)| Show InChI InChI=1S/C25H33FN4O2/c1-32-23-7-3-2-6-22(23)29-17-14-28(15-18-29)16-19-30(24-8-4-5-13-27-24)25(31)20-9-11-21(26)12-10-20/h2-8,13,20-21H,9-12,14-19H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.515 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by ChEMBL
| Assay Description
Compound was tested in vitro for the inhibition of [3H]- 8-OH-DPAT binding to cloned cell line containing human 5-hydroxytryptamine 1A receptor |
J Med Chem 42: 1576-86 (1999)
Article DOI: 10.1021/jm980456f
BindingDB Entry DOI: 10.7270/Q23R0S2J |
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(Homo sapiens (Human)) | BDBM50019443

(1-(1H-Indol-4-yloxy)-3-isopropylamino-propan-2-ol ...)Show InChI InChI=1S/C14H20N2O2/c1-10(2)16-8-11(17)9-18-14-5-3-4-13-12(14)6-7-15-13/h3-7,10-11,15-17H,8-9H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity at human adrenergic beta1 receptor |
Bioorg Med Chem Lett 17: 5600-4 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.086
BindingDB Entry DOI: 10.7270/Q2FJ2GG0 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50135246
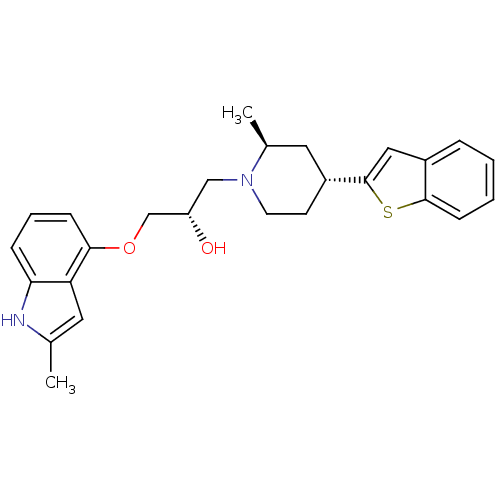
((S)-1-((2S,4R)-4-(benzo[b]thiophen-2-yl)-2-methylp...)Show SMILES C[C@H]1C[C@@H](CCN1C[C@H](O)COc1cccc2[nH]c(C)cc12)c1cc2ccccc2s1 Show InChI InChI=1S/C26H30N2O2S/c1-17-12-22-23(27-17)7-5-8-24(22)30-16-21(29)15-28-11-10-20(13-18(28)2)26-14-19-6-3-4-9-25(19)31-26/h3-9,12,14,18,20-21,27,29H,10-11,13,15-16H2,1-2H3/t18-,20+,21-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity at 5-HT reuptake site labeled with [3H]-paroxetine |
Bioorg Med Chem Lett 14: 2653-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.088
BindingDB Entry DOI: 10.7270/Q2GF0SZH |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50135246

((S)-1-((2S,4R)-4-(benzo[b]thiophen-2-yl)-2-methylp...)Show SMILES C[C@H]1C[C@@H](CCN1C[C@H](O)COc1cccc2[nH]c(C)cc12)c1cc2ccccc2s1 Show InChI InChI=1S/C26H30N2O2S/c1-17-12-22-23(27-17)7-5-8-24(22)30-16-21(29)15-28-11-10-20(13-18(28)2)26-14-19-6-3-4-9-25(19)31-26/h3-9,12,14,18,20-21,27,29H,10-11,13,15-16H2,1-2H3/t18-,20+,21-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Affinity at the 5-HT reuptake site labeled with [3H]-paroxetine using rat frontal cortex membranes |
Bioorg Med Chem Lett 13: 3939-42 (2003)
BindingDB Entry DOI: 10.7270/Q24T6HSX |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50452112
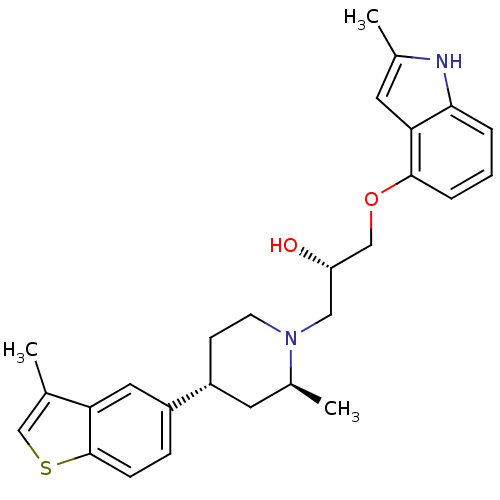
(CHEMBL2112356)Show SMILES C[C@H]1C[C@@H](CCN1C[C@H](O)COc1cccc2[nH]c(C)cc12)c1ccc2scc(C)c2c1 Show InChI InChI=1S/C27H32N2O2S/c1-17-16-32-27-8-7-20(13-23(17)27)21-9-10-29(19(3)12-21)14-22(30)15-31-26-6-4-5-25-24(26)11-18(2)28-25/h4-8,11,13,16,19,21-22,28,30H,9-10,12,14-15H2,1-3H3/t19-,21+,22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity at 5-HT reuptake site labeled with [3H]-paroxetine |
Bioorg Med Chem Lett 14: 2653-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.088
BindingDB Entry DOI: 10.7270/Q2GF0SZH |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50135263
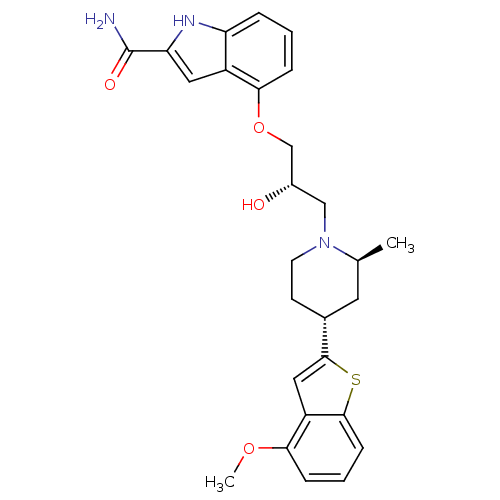
(4-{(S)-2-Hydroxy-3-[(2S,4R)-4-(4-methoxy-benzo[b]t...)Show SMILES COc1cccc2sc(cc12)[C@@H]1CCN(C[C@H](O)COc2cccc3[nH]c(cc23)C(N)=O)[C@@H](C)C1 Show InChI InChI=1S/C27H31N3O4S/c1-16-11-17(26-13-20-23(33-2)6-4-8-25(20)35-26)9-10-30(16)14-18(31)15-34-24-7-3-5-21-19(24)12-22(29-21)27(28)32/h3-8,12-13,16-18,29,31H,9-11,14-15H2,1-2H3,(H2,28,32)/t16-,17+,18-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Affinity at the 5-HT reuptake site labeled with [3H]-paroxetine using rat frontal cortex membranes |
Bioorg Med Chem Lett 13: 3939-42 (2003)
BindingDB Entry DOI: 10.7270/Q24T6HSX |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50452113
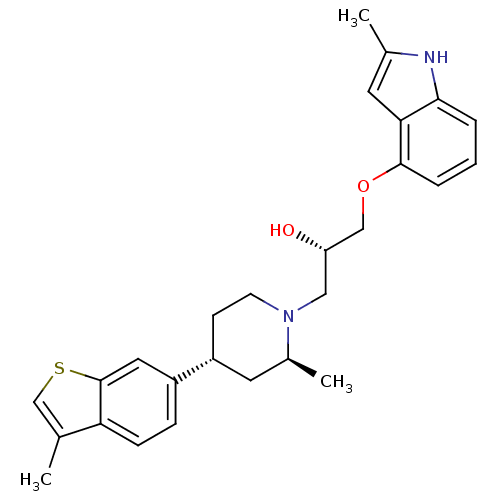
(CHEMBL2112355)Show SMILES C[C@H]1C[C@@H](CCN1C[C@H](O)COc1cccc2[nH]c(C)cc12)c1ccc2c(C)csc2c1 Show InChI InChI=1S/C27H32N2O2S/c1-17-16-32-27-13-20(7-8-23(17)27)21-9-10-29(19(3)12-21)14-22(30)15-31-26-6-4-5-25-24(26)11-18(2)28-25/h4-8,11,13,16,19,21-22,28,30H,9-10,12,14-15H2,1-3H3/t19-,21+,22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity at 5-HT reuptake site labeled with [3H]-paroxetine |
Bioorg Med Chem Lett 14: 2653-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.088
BindingDB Entry DOI: 10.7270/Q2GF0SZH |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50130169
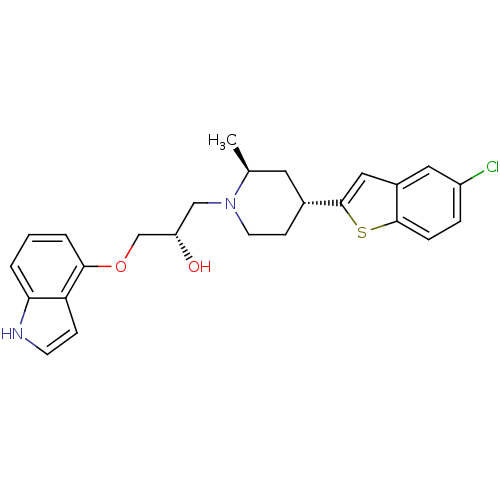
((S)-1-(1H-indol-4-yloxy)-3-((2S,4R)-4-(5-chloroben...)Show SMILES C[C@H]1C[C@@H](CCN1C[C@H](O)COc1cccc2[nH]ccc12)c1cc2cc(Cl)ccc2s1 Show InChI InChI=1S/C25H27ClN2O2S/c1-16-11-17(25-13-18-12-19(26)5-6-24(18)31-25)8-10-28(16)14-20(29)15-30-23-4-2-3-22-21(23)7-9-27-22/h2-7,9,12-13,16-17,20,27,29H,8,10-11,14-15H2,1H3/t16-,17+,20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro affinity of the compound at the 5-HT reuptake site using [3H]-paroxetine as radioligand in rat frontal cortex membranes |
Bioorg Med Chem Lett 13: 2393-7 (2003)
BindingDB Entry DOI: 10.7270/Q2Z31Z1R |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM78940

(METHIOTHEPIN | MLS000859918 | Methiothepin mesylat...)Show InChI InChI=1S/C20H24N2S2/c1-21-9-11-22(12-10-21)18-13-15-5-3-4-6-19(15)24-20-8-7-16(23-2)14-17(18)20/h3-8,14,18H,9-13H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by PDSP Ki Database
| |
Naunyn Schmiedebergs Arch Pharmacol 357: 17-24 (1998)
Article DOI: 10.1007/pl00005133
BindingDB Entry DOI: 10.7270/Q2W66J9P |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM86708

(CAS_146714-97-8 | CHEMBL31354 | CHEMBL514874 | CHE...)Show SMILES COc1ccccc1N1CCN(CCN(C(=O)C2CCCCC2)c2ccccn2)CC1 Show InChI InChI=1S/C25H34N4O2/c1-31-23-12-6-5-11-22(23)28-18-15-27(16-19-28)17-20-29(24-13-7-8-14-26-24)25(30)21-9-3-2-4-10-21/h5-8,11-14,21H,2-4,9-10,15-20H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by ChEMBL
| Assay Description
Compound was tested in vitro for the inhibition of [3H]- 8-OH-DPAT binding to cloned cell line containing human 5-hydroxytryptamine 1A receptor |
J Med Chem 42: 1576-86 (1999)
Article DOI: 10.1021/jm980456f
BindingDB Entry DOI: 10.7270/Q23R0S2J |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50005257
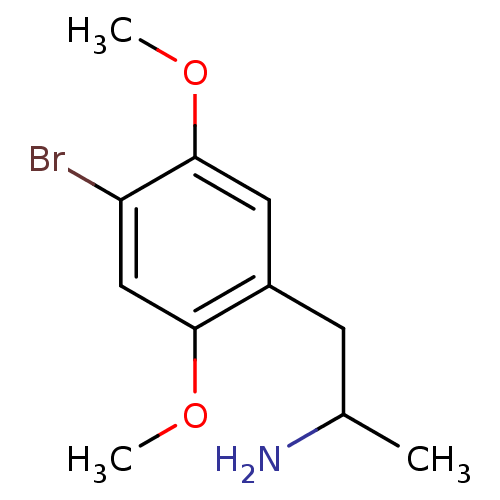
((+)-2-(4-Bromo-2,5-dimethoxy-phenyl)-1-methyl-ethy...)Show InChI InChI=1S/C11H16BrNO2/c1-7(13)4-8-5-11(15-3)9(12)6-10(8)14-2/h5-7H,4,13H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by PDSP Ki Database
| |
Naunyn Schmiedebergs Arch Pharmacol 359: 1-6 (1999)
Article DOI: 10.1007/pl00005315
BindingDB Entry DOI: 10.7270/Q2862F09 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data