 Found 104 hits with Last Name = 'newman' and Initial = 'sd'
Found 104 hits with Last Name = 'newman' and Initial = 'sd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50356880
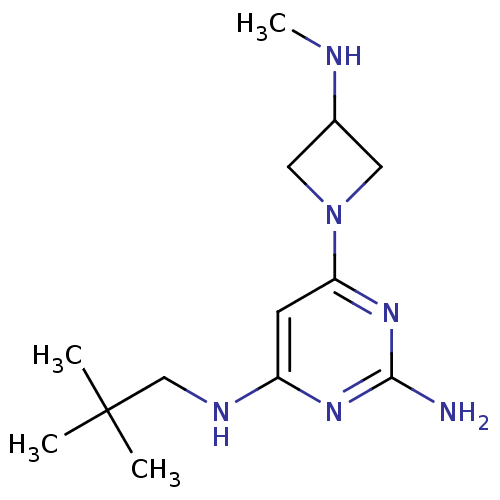
(CHEMBL1915536)Show InChI InChI=1S/C13H24N6/c1-13(2,3)8-16-10-5-11(18-12(14)17-10)19-6-9(7-19)15-4/h5,9,15H,6-8H2,1-4H3,(H3,14,16,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at full length human H4R expressed in HEK293 cells assessed as reversal of forskolin-induced cAMP production by CRE-beta-lactamas... |
Bioorg Med Chem Lett 21: 6596-602 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.125
BindingDB Entry DOI: 10.7270/Q20C4W6C |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50343152
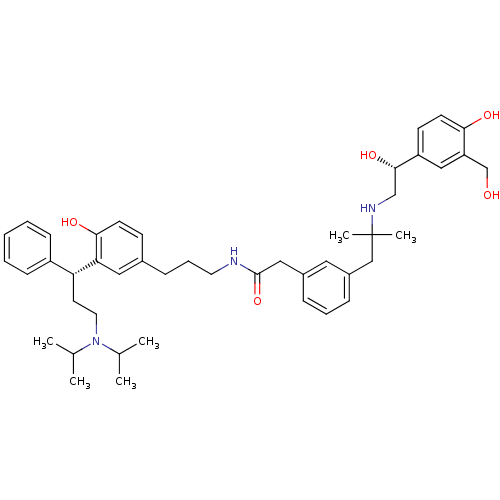
(CHEMBL1773196 | N-(3-(3-((R)-3-(diisopropylamino)-...)Show SMILES CC(C)N(CC[C@H](c1ccccc1)c1cc(CCCNC(=O)Cc2cccc(CC(C)(C)NC[C@H](O)c3ccc(O)c(CO)c3)c2)ccc1O)C(C)C |r| Show InChI InChI=1S/C45H61N3O5/c1-31(2)48(32(3)4)23-21-39(36-15-8-7-9-16-36)40-25-33(17-19-42(40)51)14-11-22-46-44(53)26-34-12-10-13-35(24-34)28-45(5,6)47-29-43(52)37-18-20-41(50)38(27-37)30-49/h7-10,12-13,15-20,24-25,27,31-32,39,43,47,49-52H,11,14,21-23,26,28-30H2,1-6H3,(H,46,53)/t39-,43+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl Scopolamine from human muscarinic M3 receptor expressed in CHO cells by scintillation proximity assay |
Bioorg Med Chem Lett 21: 2759-63 (2011)
Article DOI: 10.1016/j.bmcl.2010.10.132
BindingDB Entry DOI: 10.7270/Q2G73F1B |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50343153
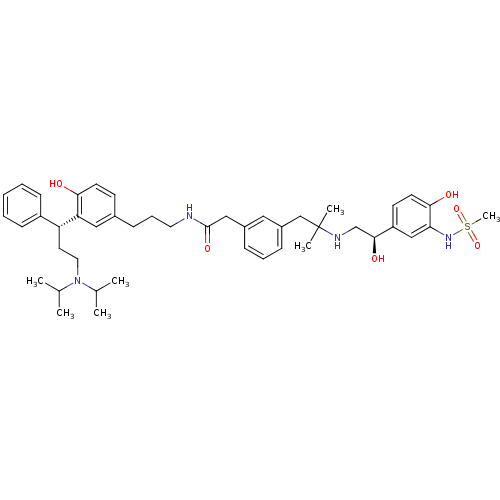
(CHEMBL1773197 | N-(3-(3-((R)-3-(diisopropylamino)-...)Show SMILES CC(C)N(CC[C@H](c1ccccc1)c1cc(CCCNC(=O)Cc2cccc(CC(C)(C)NC[C@H](O)c3ccc(O)c(NS(C)(=O)=O)c3)c2)ccc1O)C(C)C |r| Show InChI InChI=1S/C45H62N4O6S/c1-31(2)49(32(3)4)24-22-38(36-16-9-8-10-17-36)39-26-33(18-20-41(39)50)15-12-23-46-44(53)27-34-13-11-14-35(25-34)29-45(5,6)47-30-43(52)37-19-21-42(51)40(28-37)48-56(7,54)55/h8-11,13-14,16-21,25-26,28,31-32,38,43,47-48,50-52H,12,15,22-24,27,29-30H2,1-7H3,(H,46,53)/t38-,43+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.276 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl Scopolamine from human muscarinic M3 receptor expressed in CHO cells by scintillation proximity assay |
Bioorg Med Chem Lett 21: 2759-63 (2011)
Article DOI: 10.1016/j.bmcl.2010.10.132
BindingDB Entry DOI: 10.7270/Q2G73F1B |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50343161
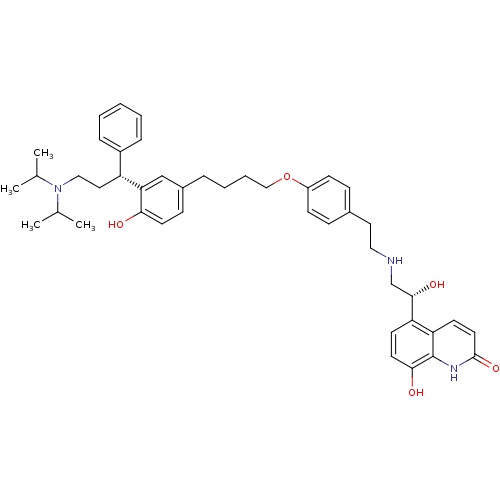
(5-((R)-2-(4-(4-(3-((R)-3-(diisopropylamino)-1-phen...)Show SMILES CC(C)N(CC[C@H](c1ccccc1)c1cc(CCCCOc2ccc(CCNC[C@H](O)c3ccc(O)c4[nH]c(=O)ccc34)cc2)ccc1O)C(C)C |r| Show InChI InChI=1S/C44H55N3O5/c1-30(2)47(31(3)4)26-24-36(34-11-6-5-7-12-34)39-28-33(15-20-40(39)48)10-8-9-27-52-35-16-13-32(14-17-35)23-25-45-29-42(50)37-18-21-41(49)44-38(37)19-22-43(51)46-44/h5-7,11-22,28,30-31,36,42,45,48-50H,8-10,23-27,29H2,1-4H3,(H,46,51)/t36-,42+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.305 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl Scopolamine from human muscarinic M3 receptor expressed in CHO cells by scintillation proximity assay |
Bioorg Med Chem Lett 21: 2759-63 (2011)
Article DOI: 10.1016/j.bmcl.2010.10.132
BindingDB Entry DOI: 10.7270/Q2G73F1B |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50343154
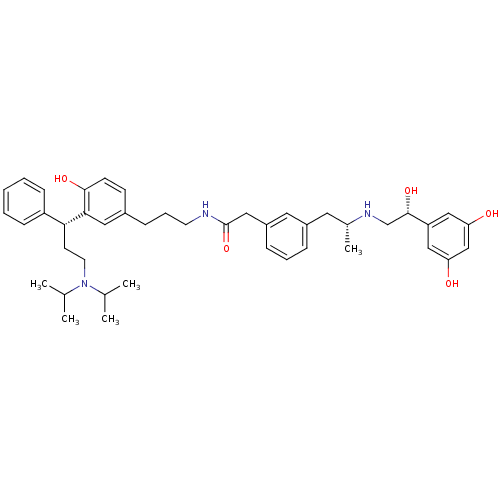
(2-(3-((R)-2-((R)-2-(3,5-dihydroxyphenyl)-2-hydroxy...)Show SMILES CC(C)N(CC[C@H](c1ccccc1)c1cc(CCCNC(=O)Cc2cccc(C[C@@H](C)NC[C@H](O)c3cc(O)cc(O)c3)c2)ccc1O)C(C)C |r| Show InChI InChI=1S/C43H57N3O5/c1-29(2)46(30(3)4)20-18-39(35-14-7-6-8-15-35)40-23-32(16-17-41(40)49)13-10-19-44-43(51)24-34-12-9-11-33(22-34)21-31(5)45-28-42(50)36-25-37(47)27-38(48)26-36/h6-9,11-12,14-17,22-23,25-27,29-31,39,42,45,47-50H,10,13,18-21,24,28H2,1-5H3,(H,44,51)/t31-,39-,42+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.397 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl Scopolamine from human muscarinic M3 receptor expressed in CHO cells by scintillation proximity assay |
Bioorg Med Chem Lett 21: 2759-63 (2011)
Article DOI: 10.1016/j.bmcl.2010.10.132
BindingDB Entry DOI: 10.7270/Q2G73F1B |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50343157
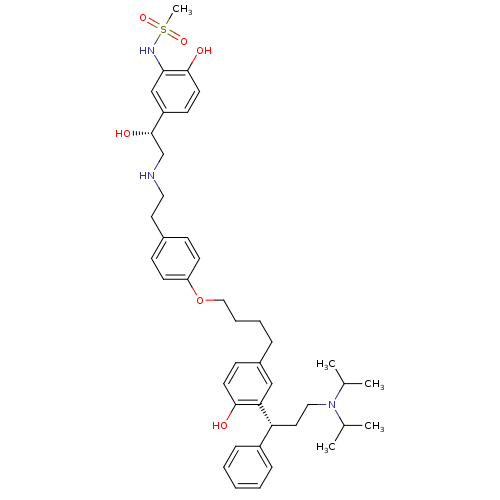
(CHEMBL1773264 | N-(5-((R)-2-(4-(4-(3-((R)-3-(diiso...)Show SMILES CC(C)N(CC[C@H](c1ccccc1)c1cc(CCCCOc2ccc(CCNC[C@H](O)c3ccc(O)c(NS(C)(=O)=O)c3)cc2)ccc1O)C(C)C |r| Show InChI InChI=1S/C42H57N3O6S/c1-30(2)45(31(3)4)25-23-37(34-12-7-6-8-13-34)38-27-33(16-20-40(38)46)11-9-10-26-51-36-18-14-32(15-19-36)22-24-43-29-42(48)35-17-21-41(47)39(28-35)44-52(5,49)50/h6-8,12-21,27-28,30-31,37,42-44,46-48H,9-11,22-26,29H2,1-5H3/t37-,42+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.634 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl Scopolamine from human muscarinic M3 receptor expressed in CHO cells by scintillation proximity assay |
Bioorg Med Chem Lett 21: 2759-63 (2011)
Article DOI: 10.1016/j.bmcl.2010.10.132
BindingDB Entry DOI: 10.7270/Q2G73F1B |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50343158
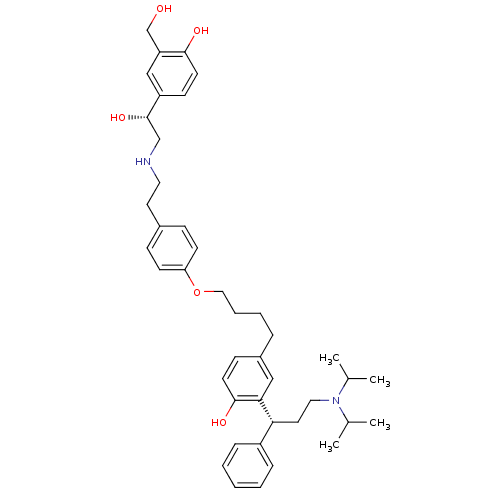
(2-((R)-3-(diisopropylamino)-1-phenylpropyl)-4-(4-(...)Show SMILES CC(C)N(CC[C@H](c1ccccc1)c1cc(CCCCOc2ccc(CCNC[C@H](O)c3ccc(O)c(CO)c3)cc2)ccc1O)C(C)C |r| Show InChI InChI=1S/C42H56N2O5/c1-30(2)44(31(3)4)24-22-38(34-11-6-5-7-12-34)39-26-33(15-19-41(39)47)10-8-9-25-49-37-17-13-32(14-18-37)21-23-43-28-42(48)35-16-20-40(46)36(27-35)29-45/h5-7,11-20,26-27,30-31,38,42-43,45-48H,8-10,21-25,28-29H2,1-4H3/t38-,42+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.765 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl Scopolamine from human muscarinic M3 receptor expressed in CHO cells by scintillation proximity assay |
Bioorg Med Chem Lett 21: 2759-63 (2011)
Article DOI: 10.1016/j.bmcl.2010.10.132
BindingDB Entry DOI: 10.7270/Q2G73F1B |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50343160
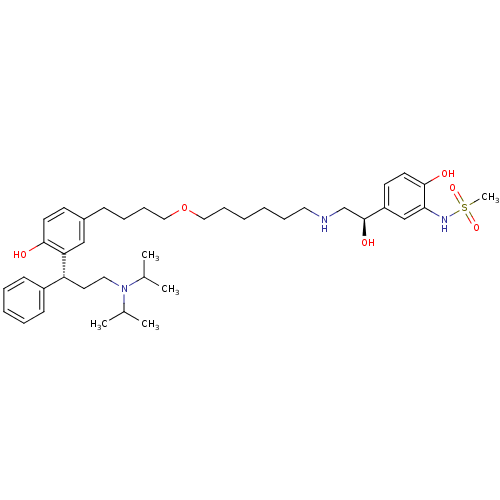
(CHEMBL1773266 | N-(5-((R)-2-(6-(4-(3-((R)-3-(diiso...)Show SMILES CC(C)N(CC[C@H](c1ccccc1)c1cc(CCCCOCCCCCCNC[C@H](O)c2ccc(O)c(NS(C)(=O)=O)c2)ccc1O)C(C)C |r| Show InChI InChI=1S/C40H61N3O6S/c1-30(2)43(31(3)4)24-22-35(33-16-9-8-10-17-33)36-27-32(18-20-38(36)44)15-11-14-26-49-25-13-7-6-12-23-41-29-40(46)34-19-21-39(45)37(28-34)42-50(5,47)48/h8-10,16-21,27-28,30-31,35,40-42,44-46H,6-7,11-15,22-26,29H2,1-5H3/t35-,40+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.990 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl Scopolamine from human muscarinic M3 receptor expressed in CHO cells by scintillation proximity assay |
Bioorg Med Chem Lett 21: 2759-63 (2011)
Article DOI: 10.1016/j.bmcl.2010.10.132
BindingDB Entry DOI: 10.7270/Q2G73F1B |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50356881

(CHEMBL1915537)Show InChI InChI=1S/C14H26N6/c1-14(2,3)5-6-17-11-7-12(19-13(15)18-11)20-8-10(9-20)16-4/h7,10,16H,5-6,8-9H2,1-4H3,(H3,15,17,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at full length human H4R expressed in HEK293 cells assessed as reversal of forskolin-induced cAMP production by CRE-beta-lactamas... |
Bioorg Med Chem Lett 21: 6596-602 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.125
BindingDB Entry DOI: 10.7270/Q20C4W6C |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50343156
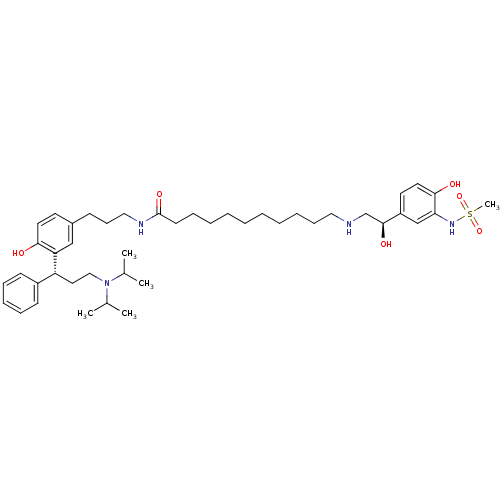
(CHEMBL1773263 | N-(3-(3-((R)-3-(diisopropylamino)-...)Show SMILES CC(C)N(CC[C@H](c1ccccc1)c1cc(CCCNC(=O)CCCCCCCCCCNC[C@H](O)c2ccc(O)c(NS(C)(=O)=O)c2)ccc1O)C(C)C |r| Show InChI InChI=1S/C44H68N4O6S/c1-33(2)48(34(3)4)29-26-38(36-19-13-12-14-20-36)39-30-35(22-24-41(39)49)18-17-28-46-44(52)21-15-10-8-6-7-9-11-16-27-45-32-43(51)37-23-25-42(50)40(31-37)47-55(5,53)54/h12-14,19-20,22-25,30-31,33-34,38,43,45,47,49-51H,6-11,15-18,21,26-29,32H2,1-5H3,(H,46,52)/t38-,43+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl Scopolamine from human muscarinic M3 receptor expressed in CHO cells by scintillation proximity assay |
Bioorg Med Chem Lett 21: 2759-63 (2011)
Article DOI: 10.1016/j.bmcl.2010.10.132
BindingDB Entry DOI: 10.7270/Q2G73F1B |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50356884

(CHEMBL1915540)Show InChI InChI=1S/C13H22N6/c1-15-10-4-5-19(8-10)12-6-11(17-13(14)18-12)16-7-9-2-3-9/h6,9-10,15H,2-5,7-8H2,1H3,(H3,14,16,17,18)/t10-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at full length human H4R expressed in HEK293 cells assessed as reversal of forskolin-induced cAMP production by CRE-beta-lactamas... |
Bioorg Med Chem Lett 21: 6596-602 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.125
BindingDB Entry DOI: 10.7270/Q20C4W6C |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50356880

(CHEMBL1915536)Show InChI InChI=1S/C13H24N6/c1-13(2,3)8-16-10-5-11(18-12(14)17-10)19-6-9(7-19)15-4/h5,9,15H,6-8H2,1-4H3,(H3,14,16,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-histamine from full length human H4R expressed in HEK293 cells |
Bioorg Med Chem Lett 21: 6596-602 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.125
BindingDB Entry DOI: 10.7270/Q20C4W6C |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50356884

(CHEMBL1915540)Show InChI InChI=1S/C13H22N6/c1-15-10-4-5-19(8-10)12-6-11(17-13(14)18-12)16-7-9-2-3-9/h6,9-10,15H,2-5,7-8H2,1H3,(H3,14,16,17,18)/t10-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-histamine from full length human H4R expressed in HEK293 cells |
Bioorg Med Chem Lett 21: 6596-602 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.125
BindingDB Entry DOI: 10.7270/Q20C4W6C |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50165008

((+)-(R)-2-(alpha-(2-(Diisopropylamino)ethyl)benzyl...)Show InChI InChI=1S/C22H31NO/c1-16(2)23(17(3)4)14-13-20(19-9-7-6-8-10-19)21-15-18(5)11-12-22(21)24/h6-12,15-17,20,24H,13-14H2,1-5H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl Scopolamine from human muscarinic M3 receptor expressed in CHO cells by scintillation proximity assay |
Bioorg Med Chem Lett 21: 2759-63 (2011)
Article DOI: 10.1016/j.bmcl.2010.10.132
BindingDB Entry DOI: 10.7270/Q2G73F1B |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Mus musculus (mouse)) | BDBM22566

(5-chloro-2-[(4-methylpiperazin-1-yl)carbonyl]-1H-i...)Show InChI InChI=1S/C14H16ClN3O/c1-17-4-6-18(7-5-17)14(19)13-9-10-8-11(15)2-3-12(10)16-13/h2-3,8-9,16H,4-7H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.89 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-histamine from mouse H4R expressed in HEK293T cells |
Bioorg Med Chem Lett 21: 6596-602 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.125
BindingDB Entry DOI: 10.7270/Q20C4W6C |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Rattus norvegicus (rat)) | BDBM22566

(5-chloro-2-[(4-methylpiperazin-1-yl)carbonyl]-1H-i...)Show InChI InChI=1S/C14H16ClN3O/c1-17-4-6-18(7-5-17)14(19)13-9-10-8-11(15)2-3-12(10)16-13/h2-3,8-9,16H,4-7H2,1H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-histamine from rat H4R expressed in HEK293T cells |
Bioorg Med Chem Lett 21: 6596-602 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.125
BindingDB Entry DOI: 10.7270/Q20C4W6C |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50356881

(CHEMBL1915537)Show InChI InChI=1S/C14H26N6/c1-14(2,3)5-6-17-11-7-12(19-13(15)18-11)20-8-10(9-20)16-4/h7,10,16H,5-6,8-9H2,1-4H3,(H3,15,17,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-histamine from full length human H4R expressed in HEK293 cells |
Bioorg Med Chem Lett 21: 6596-602 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.125
BindingDB Entry DOI: 10.7270/Q20C4W6C |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50343159

(4-((R)-2-(4-(4-(3-((R)-3-(diisopropylamino)-1-phen...)Show SMILES CC(C)N(CC[C@H](c1ccccc1)c1cc(CCCCOc2ccc(CCNC[C@H](O)c3ccc(O)c(O)c3)cc2)ccc1O)C(C)C |r| Show InChI InChI=1S/C41H54N2O5/c1-29(2)43(30(3)4)24-22-36(33-11-6-5-7-12-33)37-26-32(15-19-38(37)44)10-8-9-25-48-35-17-13-31(14-18-35)21-23-42-28-41(47)34-16-20-39(45)40(46)27-34/h5-7,11-20,26-27,29-30,36,41-42,44-47H,8-10,21-25,28H2,1-4H3/t36-,41+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl Scopolamine from human muscarinic M3 receptor expressed in CHO cells by scintillation proximity assay |
Bioorg Med Chem Lett 21: 2759-63 (2011)
Article DOI: 10.1016/j.bmcl.2010.10.132
BindingDB Entry DOI: 10.7270/Q2G73F1B |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50343155
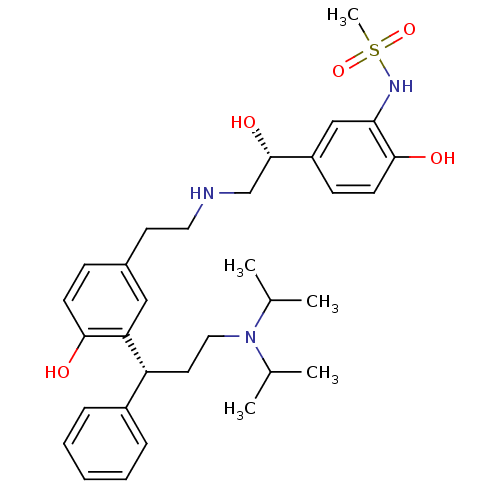
(CHEMBL1773262 | N-(5-((R)-2-(3-((R)-3-(diisopropyl...)Show SMILES CC(C)N(CC[C@H](c1ccccc1)c1cc(CCNC[C@H](O)c2ccc(O)c(NS(C)(=O)=O)c2)ccc1O)C(C)C |r| Show InChI InChI=1S/C32H45N3O5S/c1-22(2)35(23(3)4)18-16-27(25-9-7-6-8-10-25)28-19-24(11-13-30(28)36)15-17-33-21-32(38)26-12-14-31(37)29(20-26)34-41(5,39)40/h6-14,19-20,22-23,27,32-34,36-38H,15-18,21H2,1-5H3/t27-,32+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl Scopolamine from human muscarinic M3 receptor expressed in CHO cells by scintillation proximity assay |
Bioorg Med Chem Lett 21: 2759-63 (2011)
Article DOI: 10.1016/j.bmcl.2010.10.132
BindingDB Entry DOI: 10.7270/Q2G73F1B |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM22566

(5-chloro-2-[(4-methylpiperazin-1-yl)carbonyl]-1H-i...)Show InChI InChI=1S/C14H16ClN3O/c1-17-4-6-18(7-5-17)14(19)13-9-10-8-11(15)2-3-12(10)16-13/h2-3,8-9,16H,4-7H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at full length human H4R expressed in HEK293 cells assessed as reversal of forskolin-induced cAMP production by CRE-beta-lactamas... |
Bioorg Med Chem Lett 21: 6596-602 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.125
BindingDB Entry DOI: 10.7270/Q20C4W6C |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM22566

(5-chloro-2-[(4-methylpiperazin-1-yl)carbonyl]-1H-i...)Show InChI InChI=1S/C14H16ClN3O/c1-17-4-6-18(7-5-17)14(19)13-9-10-8-11(15)2-3-12(10)16-13/h2-3,8-9,16H,4-7H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-histamine from full length human H4R expressed in HEK293 cells |
Bioorg Med Chem Lett 21: 6596-602 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.125
BindingDB Entry DOI: 10.7270/Q20C4W6C |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50356888
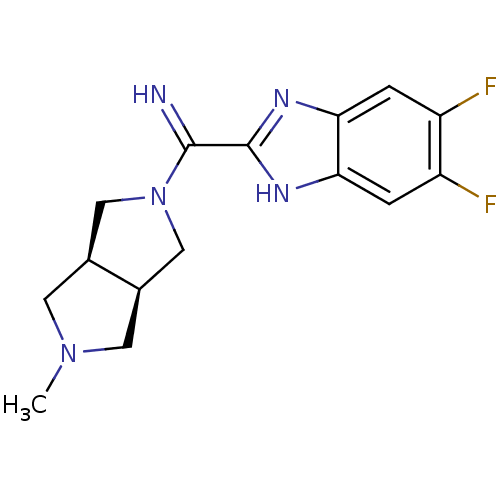
(CHEMBL1915348)Show SMILES CN1C[C@H]2CN(C[C@H]2C1)C(=N)c1nc2cc(F)c(F)cc2[nH]1 |r| Show InChI InChI=1S/C15H17F2N5/c1-21-4-8-6-22(7-9(8)5-21)14(18)15-19-12-2-10(16)11(17)3-13(12)20-15/h2-3,8-9,18H,4-7H2,1H3,(H,19,20)/t8-,9+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at full length human H4R expressed in HEK293 cells assessed as reversal of forskolin-induced cAMP production by CRE-beta-lactamas... |
Bioorg Med Chem Lett 21: 6596-602 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.125
BindingDB Entry DOI: 10.7270/Q20C4W6C |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50356888

(CHEMBL1915348)Show SMILES CN1C[C@H]2CN(C[C@H]2C1)C(=N)c1nc2cc(F)c(F)cc2[nH]1 |r| Show InChI InChI=1S/C15H17F2N5/c1-21-4-8-6-22(7-9(8)5-21)14(18)15-19-12-2-10(16)11(17)3-13(12)20-15/h2-3,8-9,18H,4-7H2,1H3,(H,19,20)/t8-,9+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-histamine from full length human H4R expressed in HEK293 cells |
Bioorg Med Chem Lett 21: 6596-602 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.125
BindingDB Entry DOI: 10.7270/Q20C4W6C |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Rattus norvegicus (rat)) | BDBM50356884

(CHEMBL1915540)Show InChI InChI=1S/C13H22N6/c1-15-10-4-5-19(8-10)12-6-11(17-13(14)18-12)16-7-9-2-3-9/h6,9-10,15H,2-5,7-8H2,1H3,(H3,14,16,17,18)/t10-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 12.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-histamine from rat H4R expressed in HEK293T cells |
Bioorg Med Chem Lett 21: 6596-602 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.125
BindingDB Entry DOI: 10.7270/Q20C4W6C |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50356882
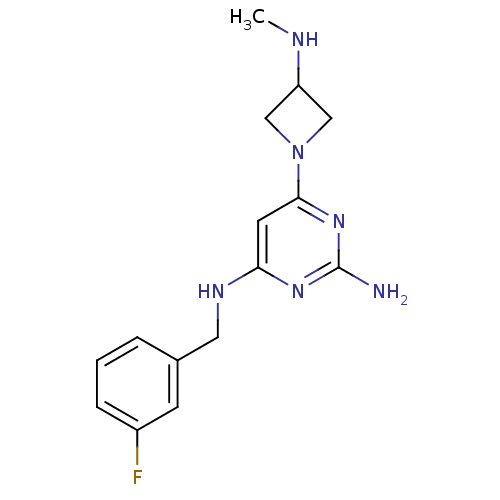
(CHEMBL1915538)Show InChI InChI=1S/C15H19FN6/c1-18-12-8-22(9-12)14-6-13(20-15(17)21-14)19-7-10-3-2-4-11(16)5-10/h2-6,12,18H,7-9H2,1H3,(H3,17,19,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at full length human H4R expressed in HEK293 cells assessed as reversal of forskolin-induced cAMP production by CRE-beta-lactamas... |
Bioorg Med Chem Lett 21: 6596-602 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.125
BindingDB Entry DOI: 10.7270/Q20C4W6C |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50356885
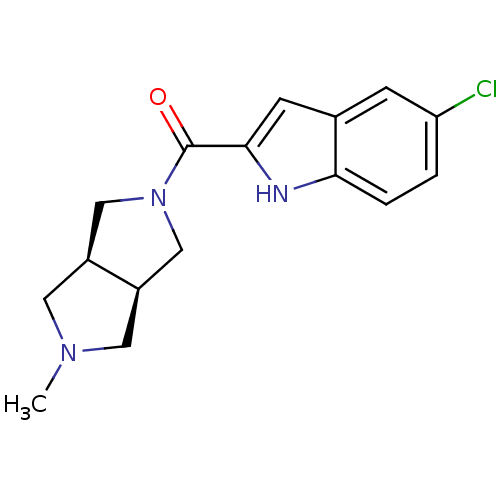
(CHEMBL1915345)Show SMILES CN1C[C@H]2CN(C[C@H]2C1)C(=O)c1cc2cc(Cl)ccc2[nH]1 |r| Show InChI InChI=1S/C16H18ClN3O/c1-19-6-11-8-20(9-12(11)7-19)16(21)15-5-10-4-13(17)2-3-14(10)18-15/h2-5,11-12,18H,6-9H2,1H3/t11-,12+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-histamine from full length human H4R expressed in HEK293 cells |
Bioorg Med Chem Lett 21: 6596-602 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.125
BindingDB Entry DOI: 10.7270/Q20C4W6C |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50356882

(CHEMBL1915538)Show InChI InChI=1S/C15H19FN6/c1-18-12-8-22(9-12)14-6-13(20-15(17)21-14)19-7-10-3-2-4-11(16)5-10/h2-6,12,18H,7-9H2,1H3,(H3,17,19,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-histamine from full length human H4R expressed in HEK293 cells |
Bioorg Med Chem Lett 21: 6596-602 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.125
BindingDB Entry DOI: 10.7270/Q20C4W6C |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Mus musculus (mouse)) | BDBM50356884

(CHEMBL1915540)Show InChI InChI=1S/C13H22N6/c1-15-10-4-5-19(8-10)12-6-11(17-13(14)18-12)16-7-9-2-3-9/h6,9-10,15H,2-5,7-8H2,1H3,(H3,14,16,17,18)/t10-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 20.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-histamine from mouse H4R expressed in HEK293T cells |
Bioorg Med Chem Lett 21: 6596-602 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.125
BindingDB Entry DOI: 10.7270/Q20C4W6C |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50356885

(CHEMBL1915345)Show SMILES CN1C[C@H]2CN(C[C@H]2C1)C(=O)c1cc2cc(Cl)ccc2[nH]1 |r| Show InChI InChI=1S/C16H18ClN3O/c1-19-6-11-8-20(9-12(11)7-19)16(21)15-5-10-4-13(17)2-3-14(10)18-15/h2-5,11-12,18H,6-9H2,1H3/t11-,12+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at full length human H4R expressed in HEK293 cells assessed as reversal of forskolin-induced cAMP production by CRE-beta-lactamas... |
Bioorg Med Chem Lett 21: 6596-602 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.125
BindingDB Entry DOI: 10.7270/Q20C4W6C |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50356886
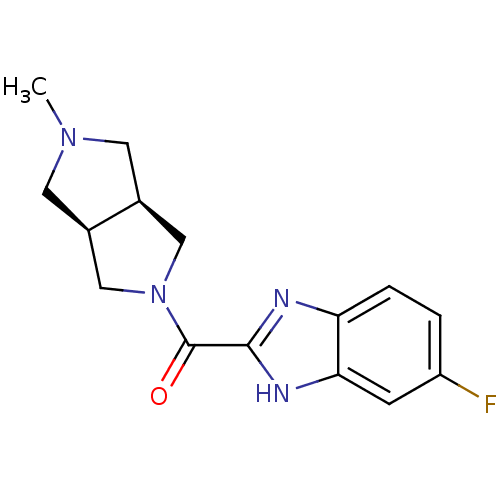
(CHEMBL1915346)Show SMILES CN1C[C@H]2CN(C[C@H]2C1)C(=O)c1nc2ccc(F)cc2[nH]1 |r| Show InChI InChI=1S/C15H17FN4O/c1-19-5-9-7-20(8-10(9)6-19)15(21)14-17-12-3-2-11(16)4-13(12)18-14/h2-4,9-10H,5-8H2,1H3,(H,17,18)/t9-,10+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at full length human H4R expressed in HEK293 cells assessed as reversal of forskolin-induced cAMP production by CRE-beta-lactamas... |
Bioorg Med Chem Lett 21: 6596-602 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.125
BindingDB Entry DOI: 10.7270/Q20C4W6C |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50356887
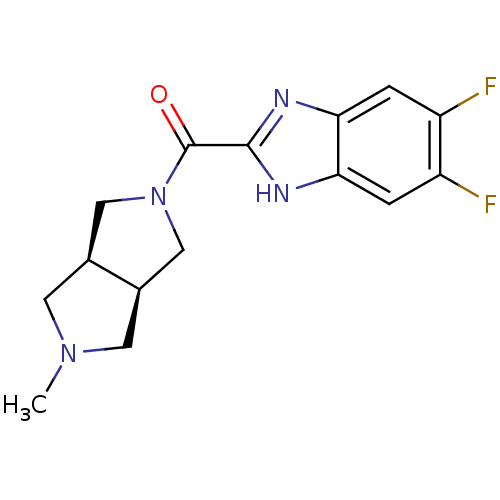
(CHEMBL1915347)Show SMILES CN1C[C@H]2CN(C[C@H]2C1)C(=O)c1nc2cc(F)c(F)cc2[nH]1 |r| Show InChI InChI=1S/C15H16F2N4O/c1-20-4-8-6-21(7-9(8)5-20)15(22)14-18-12-2-10(16)11(17)3-13(12)19-14/h2-3,8-9H,4-7H2,1H3,(H,18,19)/t8-,9+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at full length human H4R expressed in HEK293 cells assessed as reversal of forskolin-induced cAMP production by CRE-beta-lactamas... |
Bioorg Med Chem Lett 21: 6596-602 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.125
BindingDB Entry DOI: 10.7270/Q20C4W6C |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50356879

(CHEMBL1915535)Show InChI InChI=1S/C15H27N5/c1-15(2,3)5-6-16-13-11-14(18-12-17-13)20-9-7-19(4)8-10-20/h11-12H,5-10H2,1-4H3,(H,16,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 82 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-histamine from full length human H4R expressed in HEK293 cells |
Bioorg Med Chem Lett 21: 6596-602 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.125
BindingDB Entry DOI: 10.7270/Q20C4W6C |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50356886

(CHEMBL1915346)Show SMILES CN1C[C@H]2CN(C[C@H]2C1)C(=O)c1nc2ccc(F)cc2[nH]1 |r| Show InChI InChI=1S/C15H17FN4O/c1-19-5-9-7-20(8-10(9)6-19)15(21)14-17-12-3-2-11(16)4-13(12)18-14/h2-4,9-10H,5-8H2,1H3,(H,17,18)/t9-,10+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 83 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-histamine from full length human H4R expressed in HEK293 cells |
Bioorg Med Chem Lett 21: 6596-602 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.125
BindingDB Entry DOI: 10.7270/Q20C4W6C |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50356879

(CHEMBL1915535)Show InChI InChI=1S/C15H27N5/c1-15(2,3)5-6-16-13-11-14(18-12-17-13)20-9-7-19(4)8-10-20/h11-12H,5-10H2,1-4H3,(H,16,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 92 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at full length human H4R expressed in HEK293 cells assessed as reversal of forskolin-induced cAMP production by CRE-beta-lactamas... |
Bioorg Med Chem Lett 21: 6596-602 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.125
BindingDB Entry DOI: 10.7270/Q20C4W6C |
More data for this
Ligand-Target Pair | |
Histamine receptor H4
(GUINEA PIG) | BDBM50356884

(CHEMBL1915540)Show InChI InChI=1S/C13H22N6/c1-15-10-4-5-19(8-10)12-6-11(17-13(14)18-12)16-7-9-2-3-9/h6,9-10,15H,2-5,7-8H2,1H3,(H3,14,16,17,18)/t10-/m1/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 123 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-histamine from guinea pig H4R expressed in HEK293T cells |
Bioorg Med Chem Lett 21: 6596-602 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.125
BindingDB Entry DOI: 10.7270/Q20C4W6C |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50356887

(CHEMBL1915347)Show SMILES CN1C[C@H]2CN(C[C@H]2C1)C(=O)c1nc2cc(F)c(F)cc2[nH]1 |r| Show InChI InChI=1S/C15H16F2N4O/c1-20-4-8-6-21(7-9(8)5-20)15(22)14-18-12-2-10(16)11(17)3-13(12)19-14/h2-3,8-9H,4-7H2,1H3,(H,18,19)/t8-,9+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 128 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-histamine from full length human H4R expressed in HEK293 cells |
Bioorg Med Chem Lett 21: 6596-602 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.125
BindingDB Entry DOI: 10.7270/Q20C4W6C |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50356890
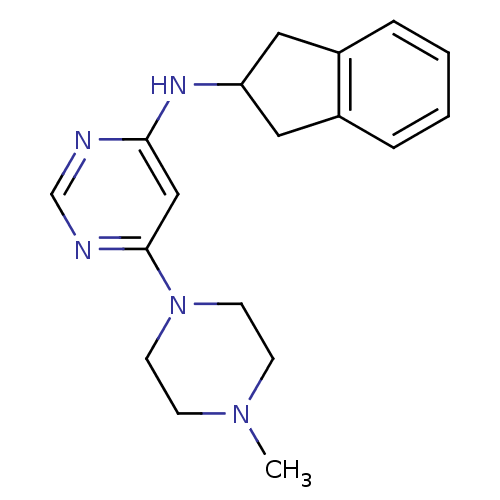
(CHEMBL1915533)Show InChI InChI=1S/C18H23N5/c1-22-6-8-23(9-7-22)18-12-17(19-13-20-18)21-16-10-14-4-2-3-5-15(14)11-16/h2-5,12-13,16H,6-11H2,1H3,(H,19,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 212 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-histamine from full length human H4R expressed in HEK293 cells |
Bioorg Med Chem Lett 21: 6596-602 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.125
BindingDB Entry DOI: 10.7270/Q20C4W6C |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50356878
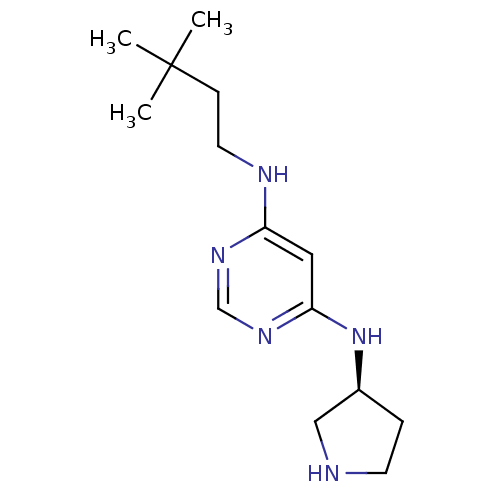
(CHEMBL1915534)Show InChI InChI=1S/C14H25N5/c1-14(2,3)5-7-16-12-8-13(18-10-17-12)19-11-4-6-15-9-11/h8,10-11,15H,4-7,9H2,1-3H3,(H2,16,17,18,19)/t11-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 502 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-histamine from full length human H4R expressed in HEK293 cells |
Bioorg Med Chem Lett 21: 6596-602 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.125
BindingDB Entry DOI: 10.7270/Q20C4W6C |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50356878

(CHEMBL1915534)Show InChI InChI=1S/C14H25N5/c1-14(2,3)5-7-16-12-8-13(18-10-17-12)19-11-4-6-15-9-11/h8,10-11,15H,4-7,9H2,1-3H3,(H2,16,17,18,19)/t11-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 572 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at full length human H4R expressed in HEK293 cells assessed as reversal of forskolin-induced cAMP production by CRE-beta-lactamas... |
Bioorg Med Chem Lett 21: 6596-602 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.125
BindingDB Entry DOI: 10.7270/Q20C4W6C |
More data for this
Ligand-Target Pair | |
Histamine receptor H4
(GUINEA PIG) | BDBM22566

(5-chloro-2-[(4-methylpiperazin-1-yl)carbonyl]-1H-i...)Show InChI InChI=1S/C14H16ClN3O/c1-17-4-6-18(7-5-17)14(19)13-9-10-8-11(15)2-3-12(10)16-13/h2-3,8-9,16H,4-7H2,1H3 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-histamine from guinea pig H4R expressed in HEK293T cells |
Bioorg Med Chem Lett 21: 6596-602 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.125
BindingDB Entry DOI: 10.7270/Q20C4W6C |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50356883
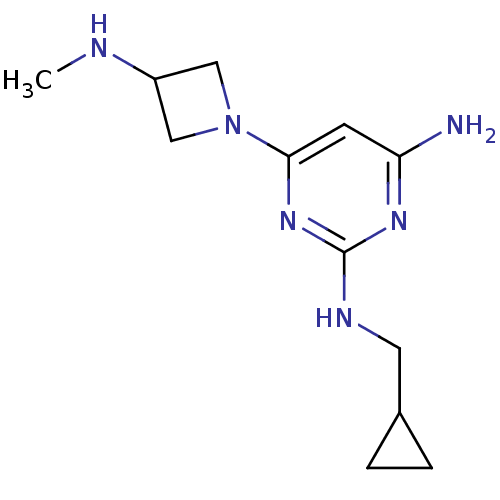
(CHEMBL1915539)Show InChI InChI=1S/C12H20N6/c1-14-9-6-18(7-9)11-4-10(13)16-12(17-11)15-5-8-2-3-8/h4,8-9,14H,2-3,5-7H2,1H3,(H3,13,15,16,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at full length human H4R expressed in HEK293 cells assessed as reversal of forskolin-induced cAMP production by CRE-beta-lactamas... |
Bioorg Med Chem Lett 21: 6596-602 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.125
BindingDB Entry DOI: 10.7270/Q20C4W6C |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50356889
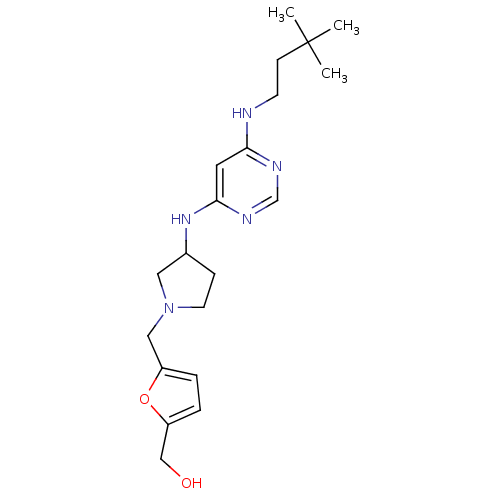
(CHEMBL1915532)Show SMILES CC(C)(C)CCNc1cc(NC2CCN(Cc3ccc(CO)o3)C2)ncn1 Show InChI InChI=1S/C20H31N5O2/c1-20(2,3)7-8-21-18-10-19(23-14-22-18)24-15-6-9-25(11-15)12-16-4-5-17(13-26)27-16/h4-5,10,14-15,26H,6-9,11-13H2,1-3H3,(H2,21,22,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-histamine from full length human H4R expressed in HEK293 cells |
Bioorg Med Chem Lett 21: 6596-602 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.125
BindingDB Entry DOI: 10.7270/Q20C4W6C |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50356883

(CHEMBL1915539)Show InChI InChI=1S/C12H20N6/c1-14-9-6-18(7-9)11-4-10(13)16-12(17-11)15-5-8-2-3-8/h4,8-9,14H,2-3,5-7H2,1H3,(H3,13,15,16,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-histamine from full length human H4R expressed in HEK293 cells |
Bioorg Med Chem Lett 21: 6596-602 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.125
BindingDB Entry DOI: 10.7270/Q20C4W6C |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Homo sapiens (Human)) | BDBM50170969

((S)-1-(1-Cyclopropyl-cyclopentyl)-5-(3,4-dichloro-...)Show SMILES Clc1ccc(cc1Cl)[C@@]1(CCN2CC(C2)N2CCOCC2)CCC(=O)N(C1)C1(CCCC1)C1CC1 Show InChI InChI=1S/C28H39Cl2N3O2/c29-24-6-5-22(17-25(24)30)27(11-12-31-18-23(19-31)32-13-15-35-16-14-32)10-7-26(34)33(20-27)28(21-3-4-21)8-1-2-9-28/h5-6,17,21,23H,1-4,7-16,18-20H2/t27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Ki for human Neurokinin 2 receptor |
Bioorg Med Chem Lett 15: 3957-61 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.134
BindingDB Entry DOI: 10.7270/Q20G3JQM |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Homo sapiens (Human)) | BDBM50170967
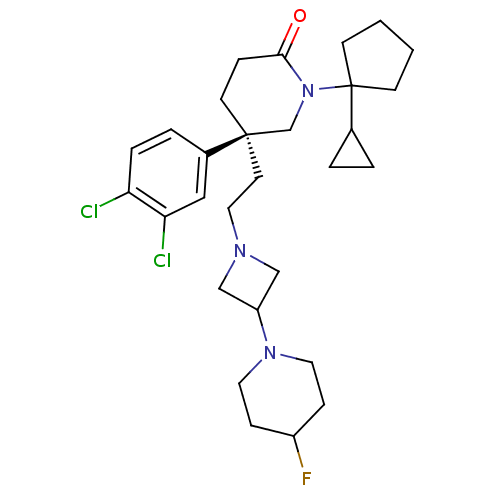
((S)-1-(1-Cyclopropyl-cyclopentyl)-5-(3,4-dichloro-...)Show SMILES FC1CCN(CC1)C1CN(CC[C@@]2(CCC(=O)N(C2)C2(CCCC2)C2CC2)c2ccc(Cl)c(Cl)c2)C1 Show InChI InChI=1S/C29H40Cl2FN3O/c30-25-6-5-22(17-26(25)31)28(13-16-33-18-24(19-33)34-14-8-23(32)9-15-34)12-7-27(36)35(20-28)29(21-3-4-21)10-1-2-11-29/h5-6,17,21,23-24H,1-4,7-16,18-20H2/t28-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Ki for human Neurokinin 2 receptor |
Bioorg Med Chem Lett 15: 3957-61 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.134
BindingDB Entry DOI: 10.7270/Q20G3JQM |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Homo sapiens (Human)) | BDBM50170968
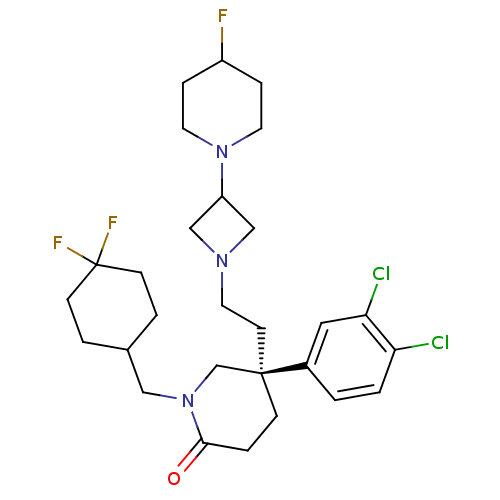
((S)-5-(3,4-Dichloro-phenyl)-1-(4,4-difluoro-cycloh...)Show SMILES FC1CCN(CC1)C1CN(CC[C@@]2(CCC(=O)N(CC3CCC(F)(F)CC3)C2)c2ccc(Cl)c(Cl)c2)C1 Show InChI InChI=1S/C28H38Cl2F3N3O/c29-24-2-1-21(15-25(24)30)27(11-14-34-17-23(18-34)35-12-6-22(31)7-13-35)8-5-26(37)36(19-27)16-20-3-9-28(32,33)10-4-20/h1-2,15,20,22-23H,3-14,16-19H2/t27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Neurokinin 2 receptor |
Bioorg Med Chem Lett 15: 3957-61 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.134
BindingDB Entry DOI: 10.7270/Q20G3JQM |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50356884

(CHEMBL1915540)Show InChI InChI=1S/C13H22N6/c1-15-10-4-5-19(8-10)12-6-11(17-13(14)18-12)16-7-9-2-3-9/h6,9-10,15H,2-5,7-8H2,1H3,(H3,14,16,17,18)/t10-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at H4R in human eosinophils assessed as inhibition of histamine-induced shape change by GAFS assay |
Bioorg Med Chem Lett 21: 6596-602 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.125
BindingDB Entry DOI: 10.7270/Q20C4W6C |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Homo sapiens (Human)) | BDBM50409627
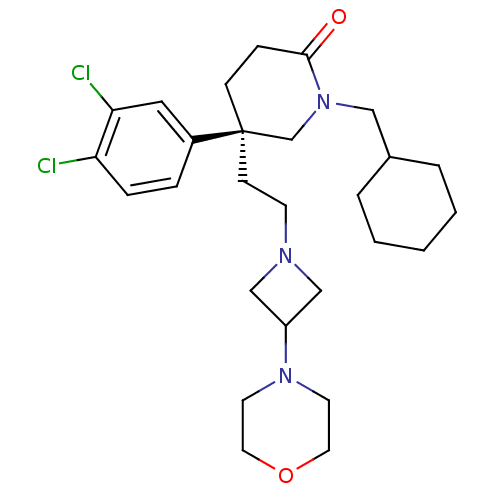
(CHEMBL185572)Show SMILES Clc1ccc(cc1Cl)[C@@]1(CCN2CC(C2)N2CCOCC2)CCC(=O)N(CC2CCCCC2)C1 Show InChI InChI=1S/C27H39Cl2N3O2/c28-24-7-6-22(16-25(24)29)27(10-11-30-18-23(19-30)31-12-14-34-15-13-31)9-8-26(33)32(20-27)17-21-4-2-1-3-5-21/h6-7,16,21,23H,1-5,8-15,17-20H2/t27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Neurokinin 2 receptor |
Bioorg Med Chem Lett 15: 3957-61 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.134
BindingDB Entry DOI: 10.7270/Q20G3JQM |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Homo sapiens (Human)) | BDBM50410049
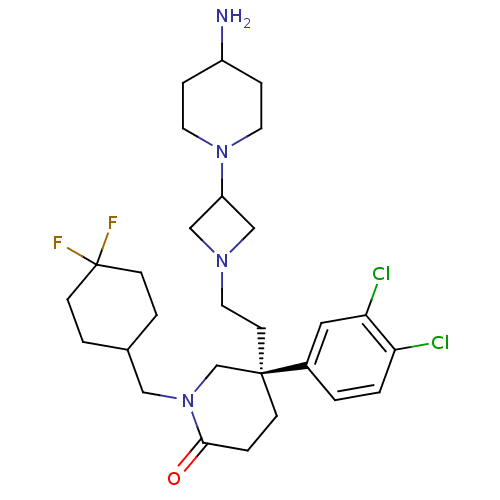
(CHEMBL434142)Show SMILES NC1CCN(CC1)C1CN(CC[C@@]2(CCC(=O)N(CC3CCC(F)(F)CC3)C2)c2ccc(Cl)c(Cl)c2)C1 Show InChI InChI=1S/C28H40Cl2F2N4O/c29-24-2-1-21(15-25(24)30)27(11-14-34-17-23(18-34)35-12-6-22(33)7-13-35)8-5-26(37)36(19-27)16-20-3-9-28(31,32)10-4-20/h1-2,15,20,22-23H,3-14,16-19,33H2/t27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Neurokinin 2 receptor |
Bioorg Med Chem Lett 15: 3957-61 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.134
BindingDB Entry DOI: 10.7270/Q20G3JQM |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50356884

(CHEMBL1915540)Show InChI InChI=1S/C13H22N6/c1-15-10-4-5-19(8-10)12-6-11(17-13(14)18-12)16-7-9-2-3-9/h6,9-10,15H,2-5,7-8H2,1H3,(H3,14,16,17,18)/t10-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at H4R in human eosinophils assessed as inhibition of histamine-induced actin polymerisation |
Bioorg Med Chem Lett 21: 6596-602 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.125
BindingDB Entry DOI: 10.7270/Q20C4W6C |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data