

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50349850 (CHEMBL1738787) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Superacide et Chimie des syst£mes£ team-Universit£ de Poitiers Curated by ChEMBL | Assay Description Inhibition of human catalytic domain of carbonic anhydrase 9 preincubated for 15 mins by CO2 hydration stopped-flow assay | Bioorg Med Chem 21: 1555-63 (2013) Article DOI: 10.1016/j.bmc.2012.05.037 BindingDB Entry DOI: 10.7270/Q25H7HM8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Rattus norvegicus (Rat)) | BDBM50001885 ((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | US Patent | 0.880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Richter Gedeon Nyrt. US Patent | Assay Description Alpha-1 receptor binding studies were performed according to the methods described by Greengrass and Bremner (Eur. J. Pharmacol., 55:323-326, 1979) o... | US Patent US8802672 (2014) BindingDB Entry DOI: 10.7270/Q2FF3R1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Rattus norvegicus (Rat)) | BDBM129721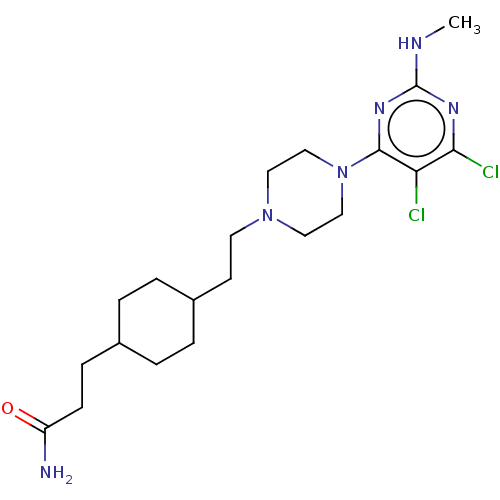 (US8802672, 11) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Richter Gedeon Nyrt. US Patent | Assay Description Binding assays were carried out on rat recombinant D3 receptors (Perkin-Elmer, Cat. No. 6110139) expressed in Sf9 cells using [3H]spiperone (0.44-1.4... | US Patent US8802672 (2014) BindingDB Entry DOI: 10.7270/Q2FF3R1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Rattus norvegicus (Rat)) | BDBM129720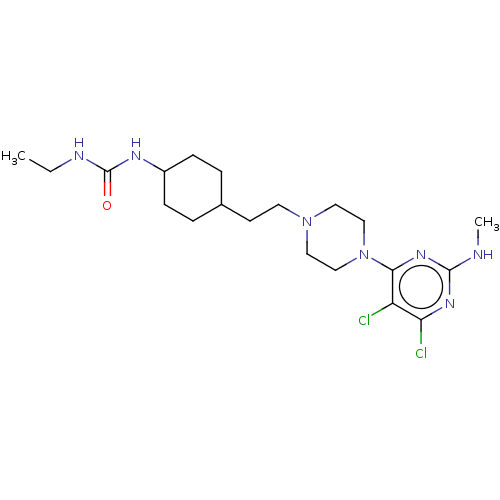 (US8802672, 10) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Richter Gedeon Nyrt. US Patent | Assay Description Binding assays were carried out on rat recombinant D3 receptors (Perkin-Elmer, Cat. No. 6110139) expressed in Sf9 cells using [3H]spiperone (0.44-1.4... | US Patent US8802672 (2014) BindingDB Entry DOI: 10.7270/Q2FF3R1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Rattus norvegicus (Rat)) | BDBM129716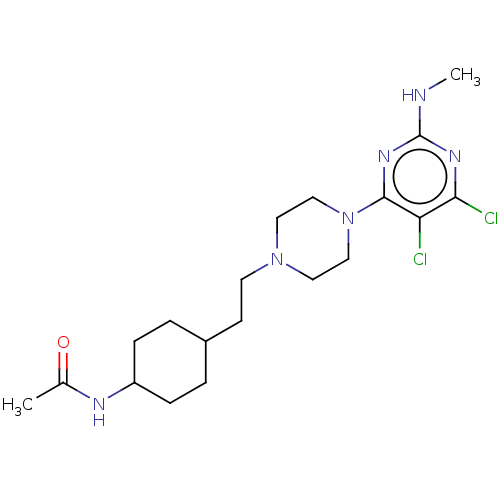 (US8802672, 1) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Richter Gedeon Nyrt. US Patent | Assay Description Binding assays were carried out on rat recombinant D3 receptors (Perkin-Elmer, Cat. No. 6110139) expressed in Sf9 cells using [3H]spiperone (0.44-1.4... | US Patent US8802672 (2014) BindingDB Entry DOI: 10.7270/Q2FF3R1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50349858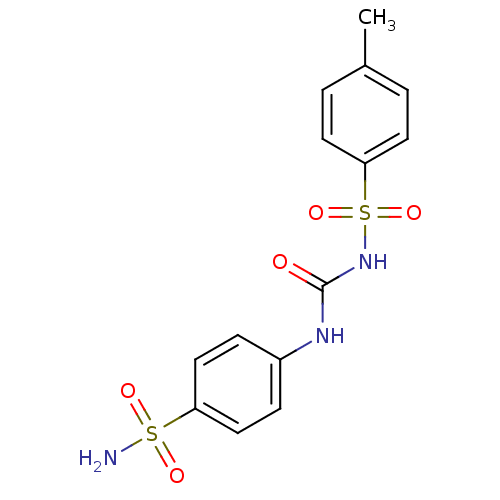 (CHEMBL78755) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Superacide et Chimie des syst£mes£ team-Universit£ de Poitiers Curated by ChEMBL | Assay Description Inhibition of human catalytic domain of carbonic anhydrase 9 preincubated for 15 mins by CO2 hydration stopped-flow assay | Bioorg Med Chem 21: 1555-63 (2013) Article DOI: 10.1016/j.bmc.2012.05.037 BindingDB Entry DOI: 10.7270/Q25H7HM8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50349858 (CHEMBL78755) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Superacide et Chimie des syst£mes£ team-Universit£ de Poitiers Curated by ChEMBL | Assay Description Inhibition of human catalytic domain of carbonic anhydrase 12 preincubated for 15 mins by CO2 hydration stopped-flow assay | Bioorg Med Chem 21: 1555-63 (2013) Article DOI: 10.1016/j.bmc.2012.05.037 BindingDB Entry DOI: 10.7270/Q25H7HM8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50349850 (CHEMBL1738787) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Superacide et Chimie des syst£mes£ team-Universit£ de Poitiers Curated by ChEMBL | Assay Description Inhibition of human catalytic domain of carbonic anhydrase 12 preincubated for 15 mins by CO2 hydration stopped-flow assay | Bioorg Med Chem 21: 1555-63 (2013) Article DOI: 10.1016/j.bmc.2012.05.037 BindingDB Entry DOI: 10.7270/Q25H7HM8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Cavia porcellus (Guinea pig)) | BDBM50036738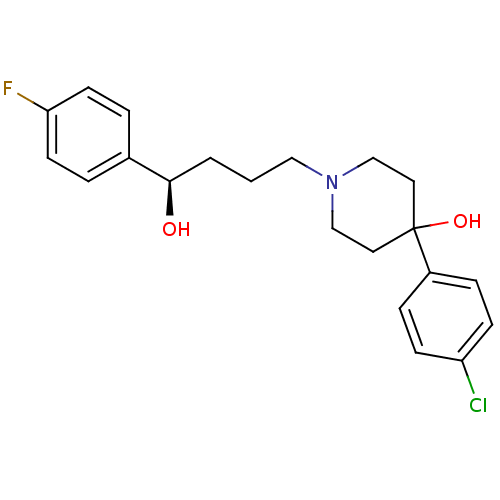 ((R)-4-(4-Chloro-phenyl)-1-[4-(4-fluoro-phenyl)-4-h...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H](+)-pentazocine from sigma 1 receptor in guinea pig brain membranes after 150 mins by microbeta scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00995 BindingDB Entry DOI: 10.7270/Q2DZ0D56 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Rattus norvegicus (Rat)) | BDBM129719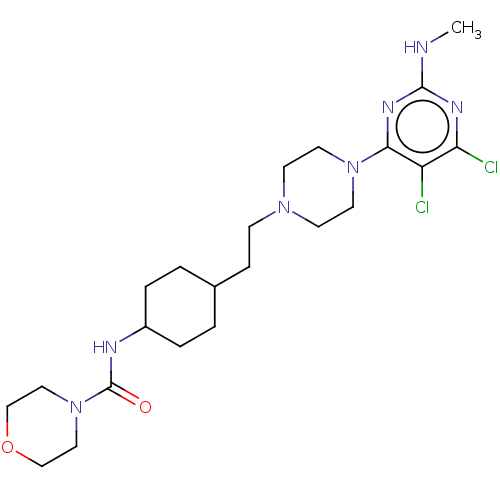 (US8802672, 7) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Richter Gedeon Nyrt. US Patent | Assay Description Binding assays were carried out on rat recombinant D3 receptors (Perkin-Elmer, Cat. No. 6110139) expressed in Sf9 cells using [3H]spiperone (0.44-1.4... | US Patent US8802672 (2014) BindingDB Entry DOI: 10.7270/Q2FF3R1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Rattus norvegicus (Rat)) | BDBM129717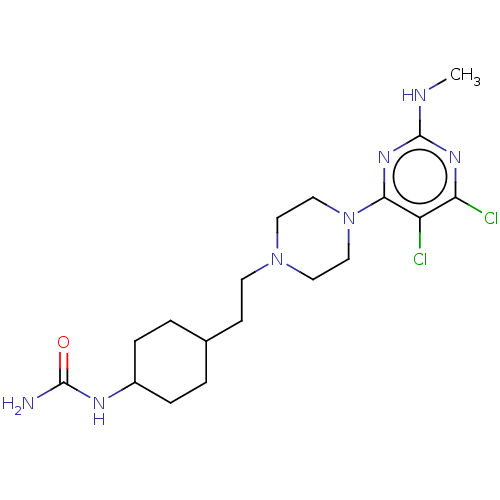 (US8802672, 2) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Richter Gedeon Nyrt. US Patent | Assay Description Binding assays were carried out on rat recombinant D3 receptors (Perkin-Elmer, Cat. No. 6110139) expressed in Sf9 cells using [3H]spiperone (0.44-1.4... | US Patent US8802672 (2014) BindingDB Entry DOI: 10.7270/Q2FF3R1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Rattus norvegicus (Rat)) | BDBM6775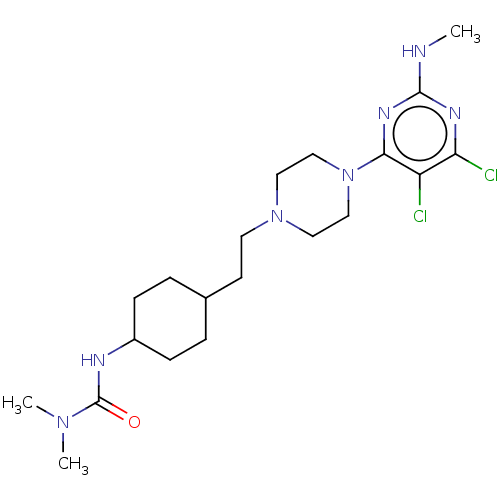 (US8802672, 8) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Richter Gedeon Nyrt. US Patent | Assay Description Binding assays were carried out on rat recombinant D3 receptors (Perkin-Elmer, Cat. No. 6110139) expressed in Sf9 cells using [3H]spiperone (0.44-1.4... | US Patent US8802672 (2014) BindingDB Entry DOI: 10.7270/Q2FF3R1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Rattus norvegicus (Rat)) | BDBM129718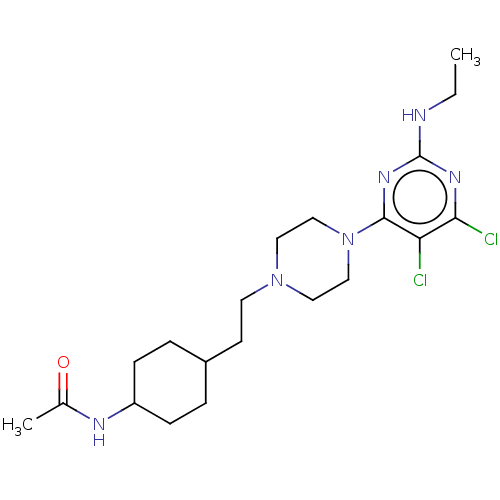 (US8802672, 6) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Richter Gedeon Nyrt. US Patent | Assay Description Binding assays were carried out on rat recombinant D3 receptors (Perkin-Elmer, Cat. No. 6110139) expressed in Sf9 cells using [3H]spiperone (0.44-1.4... | US Patent US8802672 (2014) BindingDB Entry DOI: 10.7270/Q2FF3R1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Cavia porcellus (Guinea pig)) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H](+)-pentazocine from sigma 1 receptor in guinea pig brain membranes after 150 mins by microbeta scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00995 BindingDB Entry DOI: 10.7270/Q2DZ0D56 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sigma non-opioid intracellular receptor 1 (Cavia porcellus (Guinea pig)) | BDBM50064946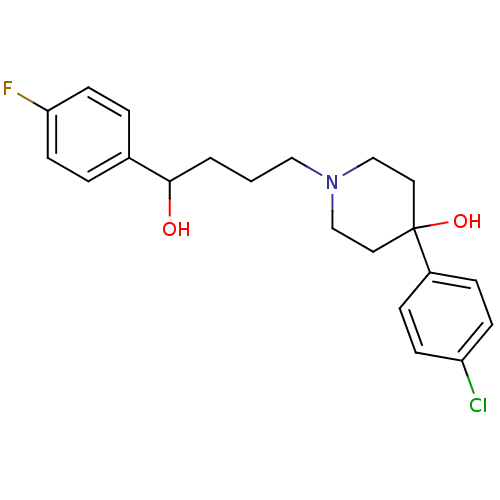 (4-(4-Chloro-phenyl)-1-[4-(4-fluoro-phenyl)-4-hydro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H](+)-pentazocine from sigma 1 receptor in guinea pig brain membranes after 150 mins by microbeta scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00995 BindingDB Entry DOI: 10.7270/Q2DZ0D56 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Cavia porcellus (Guinea pig)) | BDBM50036734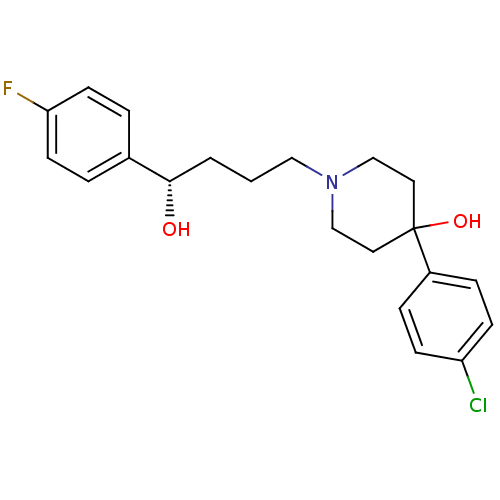 ((S)-4-(4-Chloro-phenyl)-1-[4-(4-fluoro-phenyl)-4-h...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H](+)-pentazocine from sigma 1 receptor in guinea pig brain membranes after 150 mins by microbeta scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00995 BindingDB Entry DOI: 10.7270/Q2DZ0D56 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50349858 (CHEMBL78755) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Superacide et Chimie des syst£mes£ team-Universit£ de Poitiers Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 1 preincubated for 15 mins by CO2 hydration stopped-flow assay | Bioorg Med Chem 21: 1555-63 (2013) Article DOI: 10.1016/j.bmc.2012.05.037 BindingDB Entry DOI: 10.7270/Q25H7HM8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50062680 (2-[[2-[[[3-(4-chlorophenyl)-8-methyl-8-azabicyclo[...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC sid UniChem Similars | Article PubMed | 8.42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description In vitro bindingaffinity towards dopamine transporter in rat striatal homogenatewith [125I]-IPT as the radioligand | J Med Chem 41: 428-36 (1998) Article DOI: 10.1021/jm970742b BindingDB Entry DOI: 10.7270/Q2DN4459 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM129721 (US8802672, 11) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Richter Gedeon Nyrt. US Patent | Assay Description D2 receptor binding was determined as described by Creese et al. (Eur. J. Pharmacol., 60:55-66, 1979) on rat brain striatal membrane preparation usin... | US Patent US8802672 (2014) BindingDB Entry DOI: 10.7270/Q2FF3R1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM129720 (US8802672, 10) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Richter Gedeon Nyrt. US Patent | Assay Description D2 receptor binding was determined as described by Creese et al. (Eur. J. Pharmacol., 60:55-66, 1979) on rat brain striatal membrane preparation usin... | US Patent US8802672 (2014) BindingDB Entry DOI: 10.7270/Q2FF3R1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM129719 (US8802672, 7) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Richter Gedeon Nyrt. US Patent | Assay Description D2 receptor binding was determined as described by Creese et al. (Eur. J. Pharmacol., 60:55-66, 1979) on rat brain striatal membrane preparation usin... | US Patent US8802672 (2014) BindingDB Entry DOI: 10.7270/Q2FF3R1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50349858 (CHEMBL78755) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Superacide et Chimie des syst£mes£ team-Universit£ de Poitiers Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 2 preincubated for 15 mins by CO2 hydration stopped-flow assay | Bioorg Med Chem 21: 1555-63 (2013) Article DOI: 10.1016/j.bmc.2012.05.037 BindingDB Entry DOI: 10.7270/Q25H7HM8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50001885 ((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | US Patent | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Richter Gedeon Nyrt. US Patent | Assay Description D2 receptor binding was determined as described by Creese et al. (Eur. J. Pharmacol., 60:55-66, 1979) on rat brain striatal membrane preparation usin... | US Patent US8802672 (2014) BindingDB Entry DOI: 10.7270/Q2FF3R1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Cavia porcellus (Guinea pig)) | BDBM50550237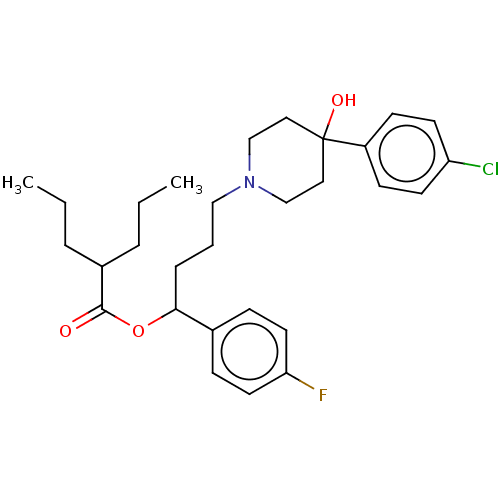 (CHEMBL4747257) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H](+)-pentazocine from sigma 1 receptor in guinea pig brain membranes after 150 mins by microbeta scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00995 BindingDB Entry DOI: 10.7270/Q2DZ0D56 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Rattus norvegicus (Rat)) | BDBM50001885 ((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | US Patent | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Richter Gedeon Nyrt. US Patent | Assay Description Binding assays were carried out on rat recombinant D3 receptors (Perkin-Elmer, Cat. No. 6110139) expressed in Sf9 cells using [3H]spiperone (0.44-1.4... | US Patent US8802672 (2014) BindingDB Entry DOI: 10.7270/Q2FF3R1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50062679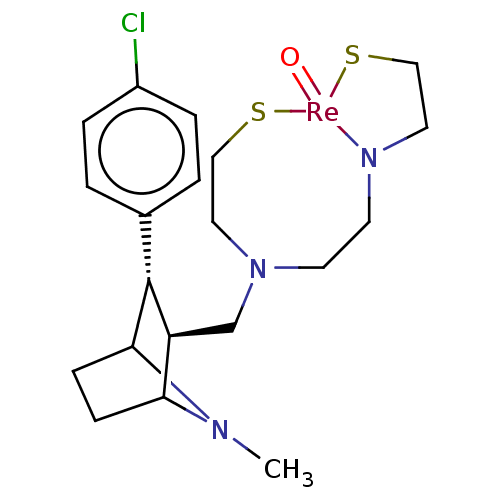 (2-[[2-[[[3-(4-chlorophenyl)-8-methyl-8-azabicyclo[...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC sid UniChem Similars | Article PubMed | 13.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description In vitro bindingaffinity towards dopamine transporter in rat striatal homogenatewith [125I]-IPT as the radioligand | J Med Chem 41: 428-36 (1998) Article DOI: 10.1021/jm970742b BindingDB Entry DOI: 10.7270/Q2DN4459 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Cavia porcellus (Guinea pig)) | BDBM50580184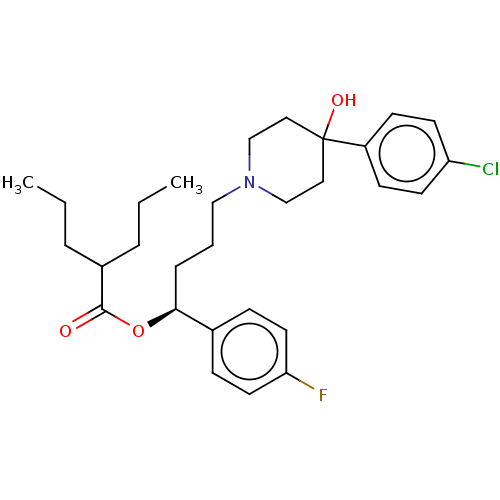 (CHEMBL5079556) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H](+)-pentazocine from sigma 1 receptor in guinea pig brain membranes after 150 mins by microbeta scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00995 BindingDB Entry DOI: 10.7270/Q2DZ0D56 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Rattus norvegicus (Rat)) | BDBM35254 (2-methyl-4-(4-methylpiperazin-1-yl)-10H-thieno[2,3...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | US Patent | 25.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Richter Gedeon Nyrt. US Patent | Assay Description Alpha-1 receptor binding studies were performed according to the methods described by Greengrass and Bremner (Eur. J. Pharmacol., 55:323-326, 1979) o... | US Patent US8802672 (2014) BindingDB Entry DOI: 10.7270/Q2FF3R1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50080733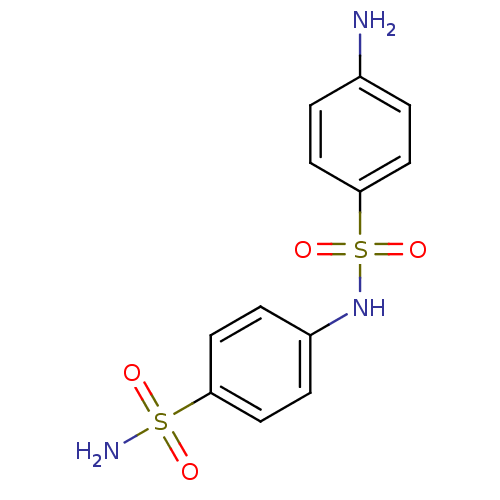 (4-(4-aminophenylsulfonamido)-1-benzenesulfonamide ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Superacide et Chimie des syst£mes£ team-Universit£ de Poitiers Curated by ChEMBL | Assay Description Inhibition of human catalytic domain of carbonic anhydrase 9 preincubated for 15 mins by CO2 hydration stopped-flow assay | Bioorg Med Chem 21: 1555-63 (2013) Article DOI: 10.1016/j.bmc.2012.05.037 BindingDB Entry DOI: 10.7270/Q25H7HM8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM129718 (US8802672, 6) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Richter Gedeon Nyrt. US Patent | Assay Description D2 receptor binding was determined as described by Creese et al. (Eur. J. Pharmacol., 60:55-66, 1979) on rat brain striatal membrane preparation usin... | US Patent US8802672 (2014) BindingDB Entry DOI: 10.7270/Q2FF3R1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM6775 (US8802672, 8) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Richter Gedeon Nyrt. US Patent | Assay Description D2 receptor binding was determined as described by Creese et al. (Eur. J. Pharmacol., 60:55-66, 1979) on rat brain striatal membrane preparation usin... | US Patent US8802672 (2014) BindingDB Entry DOI: 10.7270/Q2FF3R1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM129716 (US8802672, 1) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Richter Gedeon Nyrt. US Patent | Assay Description D2 receptor binding was determined as described by Creese et al. (Eur. J. Pharmacol., 60:55-66, 1979) on rat brain striatal membrane preparation usin... | US Patent US8802672 (2014) BindingDB Entry DOI: 10.7270/Q2FF3R1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM129717 (US8802672, 2) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Richter Gedeon Nyrt. US Patent | Assay Description D2 receptor binding was determined as described by Creese et al. (Eur. J. Pharmacol., 60:55-66, 1979) on rat brain striatal membrane preparation usin... | US Patent US8802672 (2014) BindingDB Entry DOI: 10.7270/Q2FF3R1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM10857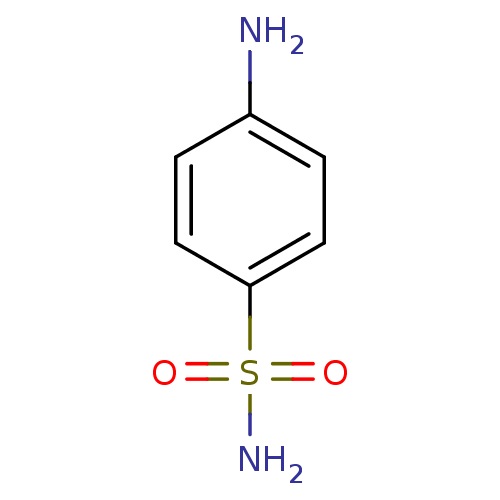 (4-aminobenzene-1-sulfonamide | CHEMBL21 | Sulfanil...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Superacide et Chimie des syst£mes£ team-Universit£ de Poitiers Curated by ChEMBL | Assay Description Inhibition of human catalytic domain of carbonic anhydrase 12 preincubated for 15 mins by CO2 hydration stopped-flow assay | Bioorg Med Chem 21: 1555-63 (2013) Article DOI: 10.1016/j.bmc.2012.05.037 BindingDB Entry DOI: 10.7270/Q25H7HM8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50349850 (CHEMBL1738787) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Superacide et Chimie des syst£mes£ team-Universit£ de Poitiers Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 2 preincubated for 15 mins by CO2 hydration stopped-flow assay | Bioorg Med Chem 21: 1555-63 (2013) Article DOI: 10.1016/j.bmc.2012.05.037 BindingDB Entry DOI: 10.7270/Q25H7HM8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50428408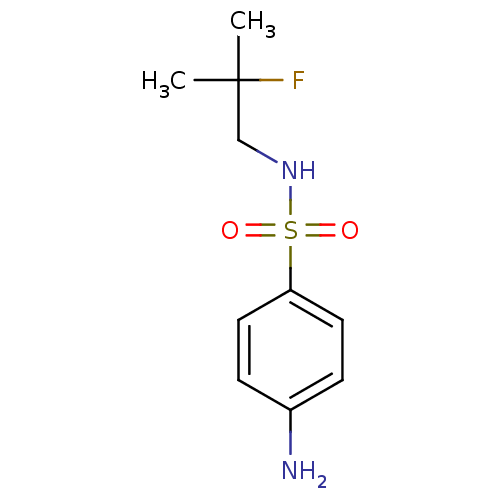 (CHEMBL2333970) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Superacide et Chimie des syst£mes£ team-Universit£ de Poitiers Curated by ChEMBL | Assay Description Inhibition of human catalytic domain of carbonic anhydrase 12 preincubated for 15 mins by CO2 hydration stopped-flow assay | Bioorg Med Chem 21: 1555-63 (2013) Article DOI: 10.1016/j.bmc.2012.05.037 BindingDB Entry DOI: 10.7270/Q25H7HM8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50428412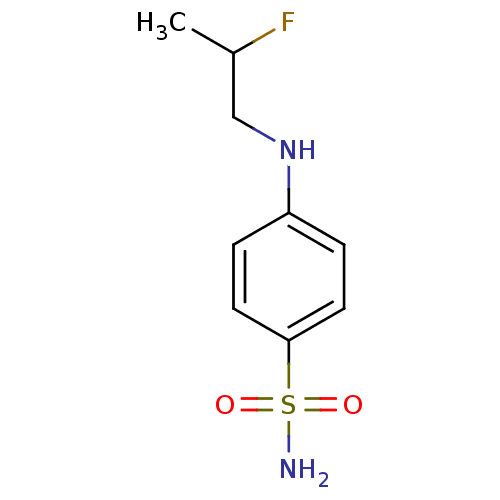 (4-(2-Fluoropropylamino)Benzenesulfonamide | CHEMBL...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Superacide et Chimie des syst£mes£ team-Universit£ de Poitiers Curated by ChEMBL | Assay Description Inhibition of human catalytic domain of carbonic anhydrase 9 preincubated for 15 mins by CO2 hydration stopped-flow assay | Bioorg Med Chem 21: 1555-63 (2013) Article DOI: 10.1016/j.bmc.2012.05.037 BindingDB Entry DOI: 10.7270/Q25H7HM8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50080733 (4-(4-aminophenylsulfonamido)-1-benzenesulfonamide ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Superacide et Chimie des syst£mes£ team-Universit£ de Poitiers Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 2 preincubated for 15 mins by CO2 hydration stopped-flow assay | Bioorg Med Chem 21: 1555-63 (2013) Article DOI: 10.1016/j.bmc.2012.05.037 BindingDB Entry DOI: 10.7270/Q25H7HM8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50428405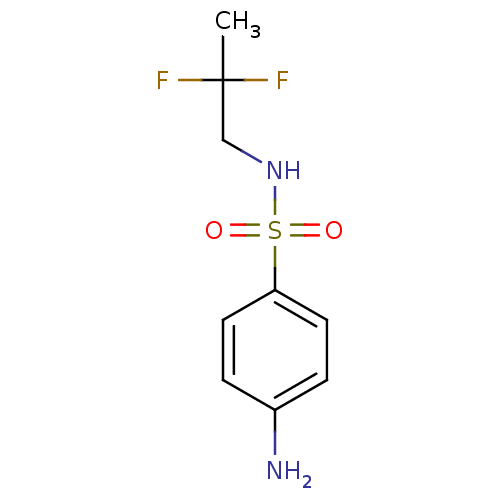 (CHEMBL2333973) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Superacide et Chimie des syst£mes£ team-Universit£ de Poitiers Curated by ChEMBL | Assay Description Inhibition of human catalytic domain of carbonic anhydrase 9 preincubated for 15 mins by CO2 hydration stopped-flow assay | Bioorg Med Chem 21: 1555-63 (2013) Article DOI: 10.1016/j.bmc.2012.05.037 BindingDB Entry DOI: 10.7270/Q25H7HM8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50138983 ((S)-2-Amino-5-(2-methyl-isothioureido)-pentanoic a...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Texas | Assay Description Inhibition assay using DDAH-1 and nNOS. | Biochemistry 48: 8624-35 (2009) Article DOI: 10.1021/bi9007098 BindingDB Entry DOI: 10.7270/Q29C6W1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50428398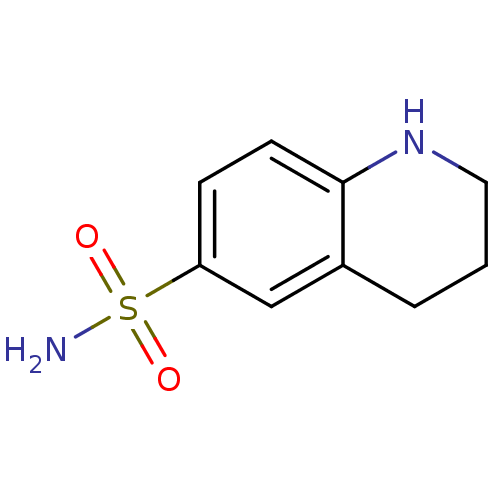 (CHEMBL2333965) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Superacide et Chimie des syst£mes£ team-Universit£ de Poitiers Curated by ChEMBL | Assay Description Inhibition of human catalytic domain of carbonic anhydrase 9 preincubated for 15 mins by CO2 hydration stopped-flow assay | Bioorg Med Chem 21: 1555-63 (2013) Article DOI: 10.1016/j.bmc.2012.05.037 BindingDB Entry DOI: 10.7270/Q25H7HM8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50428412 (4-(2-Fluoropropylamino)Benzenesulfonamide | CHEMBL...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Superacide et Chimie des syst£mes£ team-Universit£ de Poitiers Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 2 preincubated for 15 mins by CO2 hydration stopped-flow assay | Bioorg Med Chem 21: 1555-63 (2013) Article DOI: 10.1016/j.bmc.2012.05.037 BindingDB Entry DOI: 10.7270/Q25H7HM8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Cavia porcellus (Guinea pig)) | BDBM50580183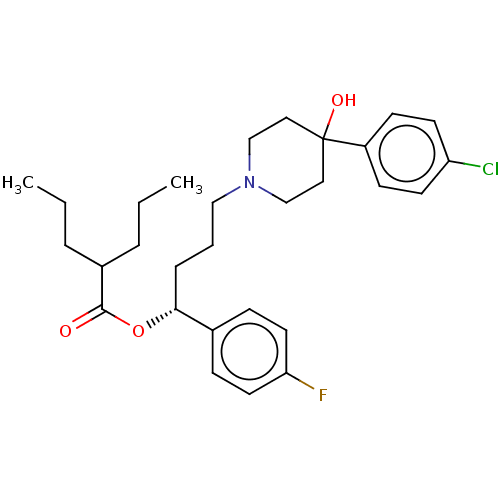 (CHEMBL5094340) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H](+)-pentazocine from sigma 1 receptor in guinea pig brain membranes after 150 mins by microbeta scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00995 BindingDB Entry DOI: 10.7270/Q2DZ0D56 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50428401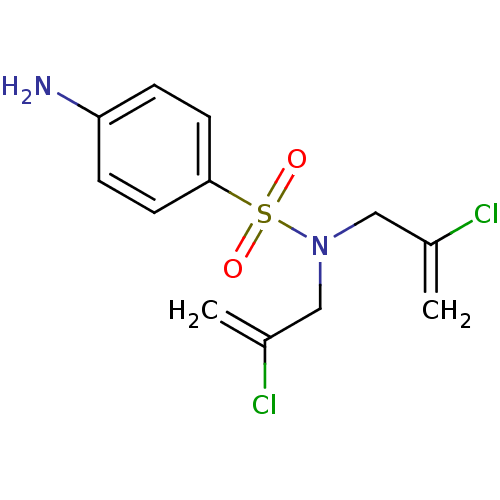 (CHEMBL2333977) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 73 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Superacide et Chimie des syst£mes£ team-Universit£ de Poitiers Curated by ChEMBL | Assay Description Inhibition of human catalytic domain of carbonic anhydrase 9 preincubated for 15 mins by CO2 hydration stopped-flow assay | Bioorg Med Chem 21: 1555-63 (2013) Article DOI: 10.1016/j.bmc.2012.05.037 BindingDB Entry DOI: 10.7270/Q25H7HM8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50428412 (4-(2-Fluoropropylamino)Benzenesulfonamide | CHEMBL...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 74 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Superacide et Chimie des syst£mes£ team-Universit£ de Poitiers Curated by ChEMBL | Assay Description Inhibition of human catalytic domain of carbonic anhydrase 12 preincubated for 15 mins by CO2 hydration stopped-flow assay | Bioorg Med Chem 21: 1555-63 (2013) Article DOI: 10.1016/j.bmc.2012.05.037 BindingDB Entry DOI: 10.7270/Q25H7HM8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50428408 (CHEMBL2333970) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Superacide et Chimie des syst£mes£ team-Universit£ de Poitiers Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 2 preincubated for 15 mins by CO2 hydration stopped-flow assay | Bioorg Med Chem 21: 1555-63 (2013) Article DOI: 10.1016/j.bmc.2012.05.037 BindingDB Entry DOI: 10.7270/Q25H7HM8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Rattus norvegicus (Rat)) | BDBM35254 (2-methyl-4-(4-methylpiperazin-1-yl)-10H-thieno[2,3...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | US Patent | 76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Richter Gedeon Nyrt. US Patent | Assay Description Binding assays were carried out on rat recombinant D3 receptors (Perkin-Elmer, Cat. No. 6110139) expressed in Sf9 cells using [3H]spiperone (0.44-1.4... | US Patent US8802672 (2014) BindingDB Entry DOI: 10.7270/Q2FF3R1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50428405 (CHEMBL2333973) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Superacide et Chimie des syst£mes£ team-Universit£ de Poitiers Curated by ChEMBL | Assay Description Inhibition of human catalytic domain of carbonic anhydrase 12 preincubated for 15 mins by CO2 hydration stopped-flow assay | Bioorg Med Chem 21: 1555-63 (2013) Article DOI: 10.1016/j.bmc.2012.05.037 BindingDB Entry DOI: 10.7270/Q25H7HM8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50428398 (CHEMBL2333965) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Superacide et Chimie des syst£mes£ team-Universit£ de Poitiers Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 2 preincubated for 15 mins by CO2 hydration stopped-flow assay | Bioorg Med Chem 21: 1555-63 (2013) Article DOI: 10.1016/j.bmc.2012.05.037 BindingDB Entry DOI: 10.7270/Q25H7HM8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50428401 (CHEMBL2333977) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Superacide et Chimie des syst£mes£ team-Universit£ de Poitiers Curated by ChEMBL | Assay Description Inhibition of human catalytic domain of carbonic anhydrase 12 preincubated for 15 mins by CO2 hydration stopped-flow assay | Bioorg Med Chem 21: 1555-63 (2013) Article DOI: 10.1016/j.bmc.2012.05.037 BindingDB Entry DOI: 10.7270/Q25H7HM8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 624 total ) | Next | Last >> |