 Found 69 hits with Last Name = 'nickel' and Initial = 'a'
Found 69 hits with Last Name = 'nickel' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Casein kinase II subunit alpha'/beta
(Homo sapiens (Human)) | BDBM50530315

(CHEMBL4520531)Show InChI InChI=1S/C20H14N2O3S/c23-18-10-15(7-8-16(18)19(24)25)21-20-22-17(11-26-20)14-6-5-12-3-1-2-4-13(12)9-14/h1-11,23H,(H,21,22)(H,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 86 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t zu K£ln
Curated by ChEMBL
| Assay Description
ATP competitive inhibition of recombinant human CK2alpha2 (1 to 335 residues)/CK2beta2 (1 to 193 residues) expressed in Escherichia coli BL21(DE3) us... |
J Med Chem 63: 7766-7772 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00587
BindingDB Entry DOI: 10.7270/Q24M9834 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Casein kinase II subunit alpha'/beta
(Homo sapiens (Human)) | BDBM50540033

(CHEMBL4635554)Show InChI InChI=1S/C16H11ClN2O3S/c17-10-3-1-9(2-4-10)13-8-23-16(19-13)18-11-5-6-12(15(21)22)14(20)7-11/h1-8,20H,(H,18,19)(H,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 206 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t zu K£ln
Curated by ChEMBL
| Assay Description
ATP competitive inhibition of recombinant human CK2alpha2 (1 to 335 residues)/CK2beta2 (1 to 193 residues) expressed in Escherichia coli BL21(DE3) us... |
J Med Chem 63: 7766-7772 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00587
BindingDB Entry DOI: 10.7270/Q24M9834 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Casein kinase II subunit alpha'/beta
(Homo sapiens (Human)) | BDBM50540034

(CHEMBL4647986)Show InChI InChI=1S/C15H11N3O2S/c19-14(20)10-4-3-5-11(8-10)17-15-18-13(9-21-15)12-6-1-2-7-16-12/h1-9H,(H,17,18)(H,19,20) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 1.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t zu K£ln
Curated by ChEMBL
| Assay Description
ATP competitive inhibition of recombinant human CK2alpha2 (1 to 335 residues)/CK2beta2 (1 to 193 residues) expressed in Escherichia coli BL21(DE3) us... |
J Med Chem 63: 7766-7772 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00587
BindingDB Entry DOI: 10.7270/Q24M9834 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50239250

(CHEMBL4086080)Show SMILES CNC(=O)c1c(oc2ccc(cc12)-c1cc(ccc1C)C(=O)NC1(CC1)c1ccncc1)-c1ccc(F)cc1 Show InChI InChI=1S/C32H26FN3O3/c1-19-3-4-22(30(37)36-32(13-14-32)23-11-15-35-16-12-23)18-25(19)21-7-10-27-26(17-21)28(31(38)34-2)29(39-27)20-5-8-24(33)9-6-20/h3-12,15-18H,13-14H2,1-2H3,(H,34,38)(H,36,37) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of microsomal CYP2C8 (unknown origin) |
J Med Chem 60: 4369-4385 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00328
BindingDB Entry DOI: 10.7270/Q20R9RKB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50239251

(CHEMBL4096241)Show SMILES CNC(=O)c1c(oc2ccc(cc12)-c1cc(ccc1C)C(=O)NC1(CC1)c1cccnc1)-c1ccc(F)cc1 Show InChI InChI=1S/C32H26FN3O3/c1-19-5-6-22(30(37)36-32(13-14-32)23-4-3-15-35-18-23)17-25(19)21-9-12-27-26(16-21)28(31(38)34-2)29(39-27)20-7-10-24(33)11-8-20/h3-12,15-18H,13-14H2,1-2H3,(H,34,38)(H,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of microsomal CYP3A4 (unknown origin) |
J Med Chem 60: 4369-4385 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00328
BindingDB Entry DOI: 10.7270/Q20R9RKB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50239250

(CHEMBL4086080)Show SMILES CNC(=O)c1c(oc2ccc(cc12)-c1cc(ccc1C)C(=O)NC1(CC1)c1ccncc1)-c1ccc(F)cc1 Show InChI InChI=1S/C32H26FN3O3/c1-19-3-4-22(30(37)36-32(13-14-32)23-11-15-35-16-12-23)18-25(19)21-7-10-27-26(17-21)28(31(38)34-2)29(39-27)20-5-8-24(33)9-6-20/h3-12,15-18H,13-14H2,1-2H3,(H,34,38)(H,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of microsomal CYP3A4 (unknown origin) |
J Med Chem 60: 4369-4385 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00328
BindingDB Entry DOI: 10.7270/Q20R9RKB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50239251

(CHEMBL4096241)Show SMILES CNC(=O)c1c(oc2ccc(cc12)-c1cc(ccc1C)C(=O)NC1(CC1)c1cccnc1)-c1ccc(F)cc1 Show InChI InChI=1S/C32H26FN3O3/c1-19-5-6-22(30(37)36-32(13-14-32)23-4-3-15-35-18-23)17-25(19)21-9-12-27-26(16-21)28(31(38)34-2)29(39-27)20-7-10-24(33)11-8-20/h3-12,15-18H,13-14H2,1-2H3,(H,34,38)(H,36,37) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of microsomal CYP2C8 (unknown origin) |
J Med Chem 60: 4369-4385 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00328
BindingDB Entry DOI: 10.7270/Q20R9RKB |
More data for this
Ligand-Target Pair | |
Casein kinase II subunit alpha
(Homo sapiens (Human)) | BDBM50252622
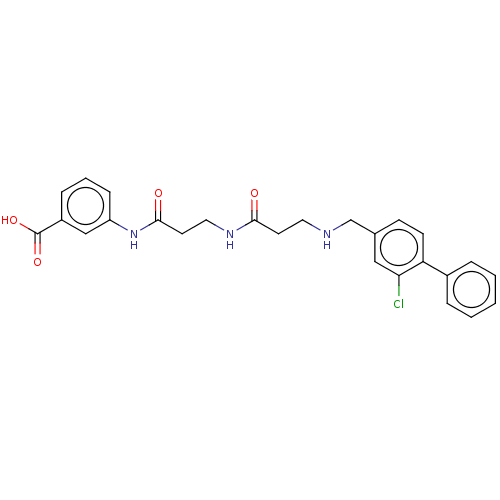
(CHEMBL4070389)Show SMILES OC(=O)c1cccc(NC(=O)CCNC(=O)CCNCc2ccc(c(Cl)c2)-c2ccccc2)c1 Show InChI InChI=1S/C26H26ClN3O4/c27-23-15-18(9-10-22(23)19-5-2-1-3-6-19)17-28-13-11-24(31)29-14-12-25(32)30-21-8-4-7-20(16-21)26(33)34/h1-10,15-16,28H,11-14,17H2,(H,29,31)(H,30,32)(H,33,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00063
BindingDB Entry DOI: 10.7270/Q2ZP4B53 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50239252

(CHEMBL4078188)Show SMILES CNC(=O)c1c(oc2ccc(cc12)-c1cc(ccc1C)C(=O)NC1(CC1)c1ccccn1)-c1ccc(F)cc1 Show InChI InChI=1S/C32H26FN3O3/c1-19-6-7-22(30(37)36-32(14-15-32)27-5-3-4-16-35-27)18-24(19)21-10-13-26-25(17-21)28(31(38)34-2)29(39-26)20-8-11-23(33)12-9-20/h3-13,16-18H,14-15H2,1-2H3,(H,34,38)(H,36,37) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of microsomal CYP2C8 (unknown origin) |
J Med Chem 60: 4369-4385 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00328
BindingDB Entry DOI: 10.7270/Q20R9RKB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50239253
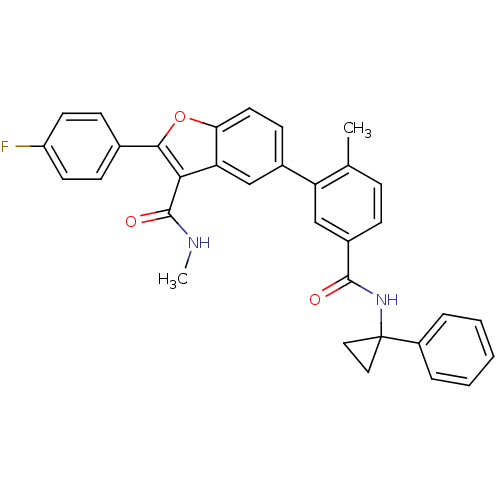
(CHEMBL4088517)Show SMILES CNC(=O)c1c(oc2ccc(cc12)-c1cc(ccc1C)C(=O)NC1(CC1)c1ccccc1)-c1ccc(F)cc1 Show InChI InChI=1S/C33H27FN2O3/c1-20-8-9-23(31(37)36-33(16-17-33)24-6-4-3-5-7-24)19-26(20)22-12-15-28-27(18-22)29(32(38)35-2)30(39-28)21-10-13-25(34)14-11-21/h3-15,18-19H,16-17H2,1-2H3,(H,35,38)(H,36,37) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of microsomal CYP2C8 (unknown origin) |
J Med Chem 60: 4369-4385 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00328
BindingDB Entry DOI: 10.7270/Q20R9RKB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50239253

(CHEMBL4088517)Show SMILES CNC(=O)c1c(oc2ccc(cc12)-c1cc(ccc1C)C(=O)NC1(CC1)c1ccccc1)-c1ccc(F)cc1 Show InChI InChI=1S/C33H27FN2O3/c1-20-8-9-23(31(37)36-33(16-17-33)24-6-4-3-5-7-24)19-26(20)22-12-15-28-27(18-22)29(32(38)35-2)30(39-28)21-10-13-25(34)14-11-21/h3-15,18-19H,16-17H2,1-2H3,(H,35,38)(H,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of microsomal CYP3A4 (unknown origin) |
J Med Chem 60: 4369-4385 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00328
BindingDB Entry DOI: 10.7270/Q20R9RKB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50239249

(CHEMBL4104000)Show SMILES CNC(=O)c1c(oc2ccc(cc12)-c1cc(ccc1C)C(=O)NC1(CC1)c1ncccn1)-c1ccc(F)cc1 Show InChI InChI=1S/C31H25FN4O3/c1-18-4-5-21(28(37)36-31(12-13-31)30-34-14-3-15-35-30)17-23(18)20-8-11-25-24(16-20)26(29(38)33-2)27(39-25)19-6-9-22(32)10-7-19/h3-11,14-17H,12-13H2,1-2H3,(H,33,38)(H,36,37) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of microsomal CYP2C8 (unknown origin) |
J Med Chem 60: 4369-4385 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00328
BindingDB Entry DOI: 10.7270/Q20R9RKB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50239252

(CHEMBL4078188)Show SMILES CNC(=O)c1c(oc2ccc(cc12)-c1cc(ccc1C)C(=O)NC1(CC1)c1ccccn1)-c1ccc(F)cc1 Show InChI InChI=1S/C32H26FN3O3/c1-19-6-7-22(30(37)36-32(14-15-32)27-5-3-4-16-35-27)18-24(19)21-10-13-26-25(17-21)28(31(38)34-2)29(39-26)20-8-11-23(33)12-9-20/h3-13,16-18H,14-15H2,1-2H3,(H,34,38)(H,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of microsomal CYP3A4 (unknown origin) |
J Med Chem 60: 4369-4385 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00328
BindingDB Entry DOI: 10.7270/Q20R9RKB |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50239249

(CHEMBL4104000)Show SMILES CNC(=O)c1c(oc2ccc(cc12)-c1cc(ccc1C)C(=O)NC1(CC1)c1ncccn1)-c1ccc(F)cc1 Show InChI InChI=1S/C31H25FN4O3/c1-18-4-5-21(28(37)36-31(12-13-31)30-34-14-3-15-35-30)17-23(18)20-8-11-25-24(16-20)26(29(38)33-2)27(39-25)19-6-9-22(32)10-7-19/h3-11,14-17H,12-13H2,1-2H3,(H,33,38)(H,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by thallium flux assay |
J Med Chem 60: 4369-4385 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00328
BindingDB Entry DOI: 10.7270/Q20R9RKB |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50239252

(CHEMBL4078188)Show SMILES CNC(=O)c1c(oc2ccc(cc12)-c1cc(ccc1C)C(=O)NC1(CC1)c1ccccn1)-c1ccc(F)cc1 Show InChI InChI=1S/C32H26FN3O3/c1-19-6-7-22(30(37)36-32(14-15-32)27-5-3-4-16-35-27)18-24(19)21-10-13-26-25(17-21)28(31(38)34-2)29(39-26)20-8-11-23(33)12-9-20/h3-13,16-18H,14-15H2,1-2H3,(H,34,38)(H,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by thallium flux assay |
J Med Chem 60: 4369-4385 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00328
BindingDB Entry DOI: 10.7270/Q20R9RKB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50239248
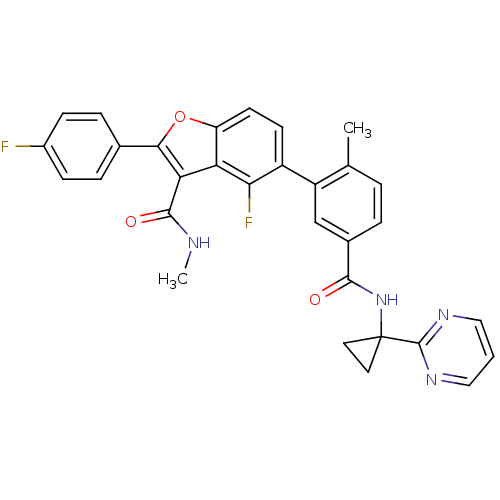
(CHEMBL4093031)Show SMILES CNC(=O)c1c(oc2ccc(c(F)c12)-c1cc(ccc1C)C(=O)NC1(CC1)c1ncccn1)-c1ccc(F)cc1 Show InChI InChI=1S/C31H24F2N4O3/c1-17-4-5-19(28(38)37-31(12-13-31)30-35-14-3-15-36-30)16-22(17)21-10-11-23-24(26(21)33)25(29(39)34-2)27(40-23)18-6-8-20(32)9-7-18/h3-11,14-16H,12-13H2,1-2H3,(H,34,39)(H,37,38) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of microsomal CYP2C8 (unknown origin) |
J Med Chem 60: 4369-4385 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00328
BindingDB Entry DOI: 10.7270/Q20R9RKB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50239249

(CHEMBL4104000)Show SMILES CNC(=O)c1c(oc2ccc(cc12)-c1cc(ccc1C)C(=O)NC1(CC1)c1ncccn1)-c1ccc(F)cc1 Show InChI InChI=1S/C31H25FN4O3/c1-18-4-5-21(28(37)36-31(12-13-31)30-34-14-3-15-35-30)17-23(18)20-8-11-25-24(16-20)26(29(38)33-2)27(39-25)19-6-9-22(32)10-7-19/h3-11,14-17H,12-13H2,1-2H3,(H,33,38)(H,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.14E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of microsomal CYP3A4 (unknown origin) |
J Med Chem 60: 4369-4385 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00328
BindingDB Entry DOI: 10.7270/Q20R9RKB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50239248

(CHEMBL4093031)Show SMILES CNC(=O)c1c(oc2ccc(c(F)c12)-c1cc(ccc1C)C(=O)NC1(CC1)c1ncccn1)-c1ccc(F)cc1 Show InChI InChI=1S/C31H24F2N4O3/c1-17-4-5-19(28(38)37-31(12-13-31)30-35-14-3-15-36-30)16-22(17)21-10-11-23-24(26(21)33)25(29(39)34-2)27(40-23)18-6-8-20(32)9-7-18/h3-11,14-16H,12-13H2,1-2H3,(H,34,39)(H,37,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of microsomal CYP3A4 (unknown origin) |
J Med Chem 60: 4369-4385 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00328
BindingDB Entry DOI: 10.7270/Q20R9RKB |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50239248

(CHEMBL4093031)Show SMILES CNC(=O)c1c(oc2ccc(c(F)c12)-c1cc(ccc1C)C(=O)NC1(CC1)c1ncccn1)-c1ccc(F)cc1 Show InChI InChI=1S/C31H24F2N4O3/c1-17-4-5-19(28(38)37-31(12-13-31)30-35-14-3-15-36-30)16-22(17)21-10-11-23-24(26(21)33)25(29(39)34-2)27(40-23)18-6-8-20(32)9-7-18/h3-11,14-16H,12-13H2,1-2H3,(H,34,39)(H,37,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch clamp assay |
J Med Chem 60: 4369-4385 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00328
BindingDB Entry DOI: 10.7270/Q20R9RKB |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50239248

(CHEMBL4093031)Show SMILES CNC(=O)c1c(oc2ccc(c(F)c12)-c1cc(ccc1C)C(=O)NC1(CC1)c1ncccn1)-c1ccc(F)cc1 Show InChI InChI=1S/C31H24F2N4O3/c1-17-4-5-19(28(38)37-31(12-13-31)30-35-14-3-15-36-30)16-22(17)21-10-11-23-24(26(21)33)25(29(39)34-2)27(40-23)18-6-8-20(32)9-7-18/h3-11,14-16H,12-13H2,1-2H3,(H,34,39)(H,37,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by thallium flux assay |
J Med Chem 60: 4369-4385 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00328
BindingDB Entry DOI: 10.7270/Q20R9RKB |
More data for this
Ligand-Target Pair | |
Casein kinase I isoform epsilon
(Homo sapiens (Human)) | BDBM50602484

(CHEMBL5195963)Show SMILES Clc1ccc(CCNC(=O)CCC(=O)NCCCCn2cnc3c(Br)c(Br)c(Br)c(Br)c23)cc1Cl | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00063
BindingDB Entry DOI: 10.7270/Q2ZP4B53 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50448498
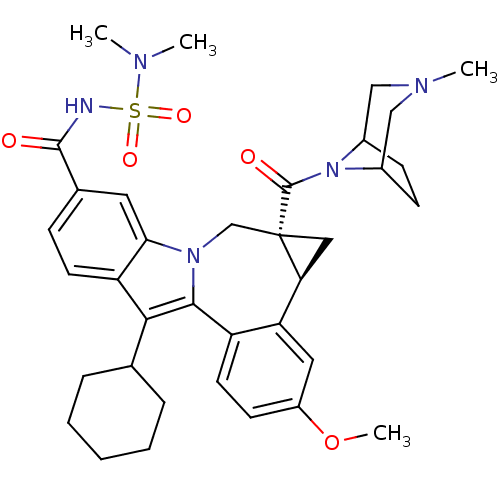
(BMS-791325 | Beclabuvir)Show SMILES COc1ccc2-c3c(C4CCCCC4)c4ccc(cc4n3C[C@]3(C[C@H]3c2c1)C(=O)N1C2CCC1CN(C)C2)C(=O)NS(=O)(=O)N(C)C |r,THB:36:35:29:31.32| Show InChI InChI=1S/C36H45N5O5S/c1-38(2)47(44,45)37-34(42)23-10-14-28-31(16-23)40-21-36(35(43)41-24-11-12-25(41)20-39(3)19-24)18-30(36)29-17-26(46-4)13-15-27(29)33(40)32(28)22-8-6-5-7-9-22/h10,13-17,22,24-25,30H,5-9,11-12,18-21H2,1-4H3,(H,37,42)/t24?,25?,30-,36-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
J Med Chem 57: 1855-79 (2014)
Article DOI: 10.1021/jm4016894
BindingDB Entry DOI: 10.7270/Q2V989JD |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50448498

(BMS-791325 | Beclabuvir)Show SMILES COc1ccc2-c3c(C4CCCCC4)c4ccc(cc4n3C[C@]3(C[C@H]3c2c1)C(=O)N1C2CCC1CN(C)C2)C(=O)NS(=O)(=O)N(C)C |r,THB:36:35:29:31.32| Show InChI InChI=1S/C36H45N5O5S/c1-38(2)47(44,45)37-34(42)23-10-14-28-31(16-23)40-21-36(35(43)41-24-11-12-25(41)20-39(3)19-24)18-30(36)29-17-26(46-4)13-15-27(29)33(40)32(28)22-8-6-5-7-9-22/h10,13-17,22,24-25,30H,5-9,11-12,18-21H2,1-4H3,(H,37,42)/t24?,25?,30-,36-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
J Med Chem 57: 1855-79 (2014)
Article DOI: 10.1021/jm4016894
BindingDB Entry DOI: 10.7270/Q2V989JD |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50448498

(BMS-791325 | Beclabuvir)Show SMILES COc1ccc2-c3c(C4CCCCC4)c4ccc(cc4n3C[C@]3(C[C@H]3c2c1)C(=O)N1C2CCC1CN(C)C2)C(=O)NS(=O)(=O)N(C)C |r,THB:36:35:29:31.32| Show InChI InChI=1S/C36H45N5O5S/c1-38(2)47(44,45)37-34(42)23-10-14-28-31(16-23)40-21-36(35(43)41-24-11-12-25(41)20-39(3)19-24)18-30(36)29-17-26(46-4)13-15-27(29)33(40)32(28)22-8-6-5-7-9-22/h10,13-17,22,24-25,30H,5-9,11-12,18-21H2,1-4H3,(H,37,42)/t24?,25?,30-,36-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 (unknown origin) |
J Med Chem 57: 1855-79 (2014)
Article DOI: 10.1021/jm4016894
BindingDB Entry DOI: 10.7270/Q2V989JD |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50448498

(BMS-791325 | Beclabuvir)Show SMILES COc1ccc2-c3c(C4CCCCC4)c4ccc(cc4n3C[C@]3(C[C@H]3c2c1)C(=O)N1C2CCC1CN(C)C2)C(=O)NS(=O)(=O)N(C)C |r,THB:36:35:29:31.32| Show InChI InChI=1S/C36H45N5O5S/c1-38(2)47(44,45)37-34(42)23-10-14-28-31(16-23)40-21-36(35(43)41-24-11-12-25(41)20-39(3)19-24)18-30(36)29-17-26(46-4)13-15-27(29)33(40)32(28)22-8-6-5-7-9-22/h10,13-17,22,24-25,30H,5-9,11-12,18-21H2,1-4H3,(H,37,42)/t24?,25?,30-,36-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 (unknown origin) |
J Med Chem 57: 1855-79 (2014)
Article DOI: 10.1021/jm4016894
BindingDB Entry DOI: 10.7270/Q2V989JD |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50448494
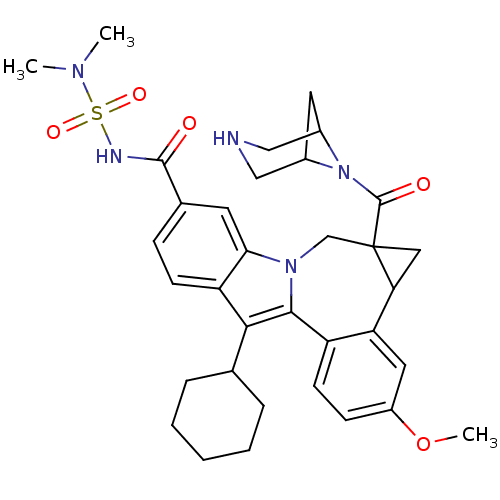
(CHEMBL3126657)Show SMILES COc1ccc2-c3c(C4CCCCC4)c4ccc(cc4n3CC3(CC3c2c1)C(=O)N1C2CC1CNC2)C(=O)NS(=O)(=O)N(C)C |TLB:27:29:35.34.33:31| Show InChI InChI=1S/C34H41N5O5S/c1-37(2)45(42,43)36-32(40)21-9-11-26-29(13-21)38-19-34(33(41)39-22-14-23(39)18-35-17-22)16-28(34)27-15-24(44-3)10-12-25(27)31(38)30(26)20-7-5-4-6-8-20/h9-13,15,20,22-23,28,35H,4-8,14,16-19H2,1-3H3,(H,36,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 in human liver microsomes after 30 mins in presence of NADPH |
J Med Chem 57: 1855-79 (2014)
Article DOI: 10.1021/jm4016894
BindingDB Entry DOI: 10.7270/Q2V989JD |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50448495
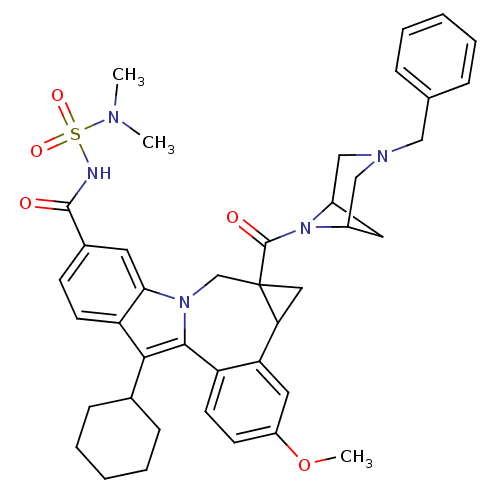
(CHEMBL3126836)Show SMILES COc1ccc2-c3c(C4CCCCC4)c4ccc(cc4n3CC3(CC3c2c1)C(=O)N1C2CC1CN(Cc1ccccc1)C2)C(=O)NS(=O)(=O)N(C)C |TLB:27:29:42.34.33:31,THB:35:34:29:31| Show InChI InChI=1S/C41H47N5O5S/c1-43(2)52(49,50)42-39(47)28-14-16-33-36(18-28)45-25-41(40(48)46-29-19-30(46)24-44(23-29)22-26-10-6-4-7-11-26)21-35(41)34-20-31(51-3)15-17-32(34)38(45)37(33)27-12-8-5-9-13-27/h4,6-7,10-11,14-18,20,27,29-30,35H,5,8-9,12-13,19,21-25H2,1-3H3,(H,42,47) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.30E+4 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 in human liver microsomes after 30 mins in presence of NADPH |
J Med Chem 57: 1855-79 (2014)
Article DOI: 10.1021/jm4016894
BindingDB Entry DOI: 10.7270/Q2V989JD |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50448496
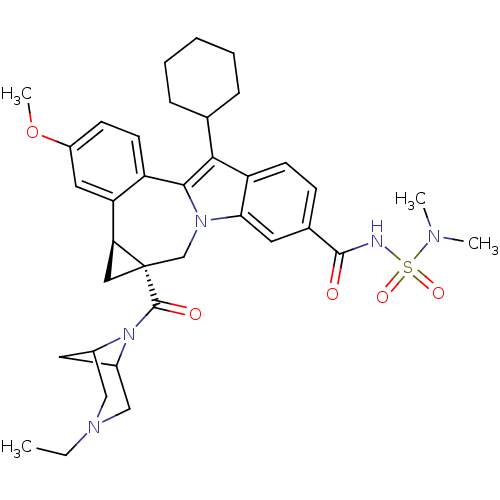
(CHEMBL3126835)Show SMILES CCN1CC2CC(C1)N2C(=O)[C@]12C[C@H]1c1cc(OC)ccc1-c1c(C3CCCCC3)c3ccc(cc3n1C2)C(=O)NS(=O)(=O)N(C)C |r,TLB:9:8:3.2.7:5,1:2:8:5| Show InChI InChI=1S/C36H45N5O5S/c1-5-39-19-24-16-25(20-39)41(24)35(43)36-18-30(36)29-17-26(46-4)12-14-27(29)33-32(22-9-7-6-8-10-22)28-13-11-23(15-31(28)40(33)21-36)34(42)37-47(44,45)38(2)3/h11-15,17,22,24-25,30H,5-10,16,18-21H2,1-4H3,(H,37,42)/t24?,25?,30-,36-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 in human liver microsomes after 30 mins in presence of NADPH |
J Med Chem 57: 1855-79 (2014)
Article DOI: 10.1021/jm4016894
BindingDB Entry DOI: 10.7270/Q2V989JD |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50448497
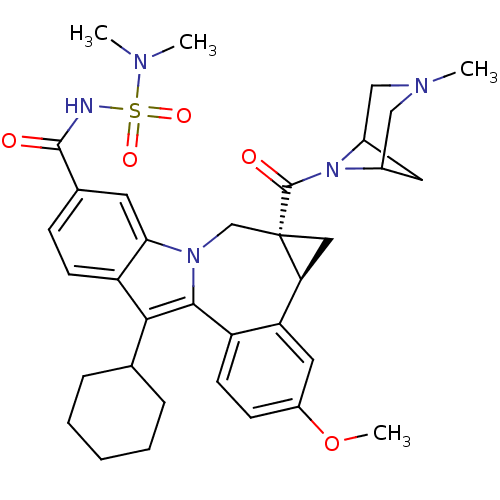
(CHEMBL3126834)Show SMILES COc1ccc2-c3c(C4CCCCC4)c4ccc(cc4n3C[C@]3(C[C@H]3c2c1)C(=O)N1C2CC1CN(C)C2)C(=O)NS(=O)(=O)N(C)C |r,TLB:27:29:36.34.33:31,THB:35:34:29:31| Show InChI InChI=1S/C35H43N5O5S/c1-37(2)46(43,44)36-33(41)22-10-12-27-30(14-22)39-20-35(34(42)40-23-15-24(40)19-38(3)18-23)17-29(35)28-16-25(45-4)11-13-26(28)32(39)31(27)21-8-6-5-7-9-21/h10-14,16,21,23-24,29H,5-9,15,17-20H2,1-4H3,(H,36,41)/t23?,24?,29-,35-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 in human liver microsomes after 30 mins in presence of NADPH |
J Med Chem 57: 1855-79 (2014)
Article DOI: 10.1021/jm4016894
BindingDB Entry DOI: 10.7270/Q2V989JD |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50448494

(CHEMBL3126657)Show SMILES COc1ccc2-c3c(C4CCCCC4)c4ccc(cc4n3CC3(CC3c2c1)C(=O)N1C2CC1CNC2)C(=O)NS(=O)(=O)N(C)C |TLB:27:29:35.34.33:31| Show InChI InChI=1S/C34H41N5O5S/c1-37(2)45(42,43)36-32(40)21-9-11-26-29(13-21)38-19-34(33(41)39-22-14-23(39)18-35-17-22)16-28(34)27-15-24(44-3)10-12-25(27)31(38)30(26)20-7-5-4-6-8-20/h9-13,15,20,22-23,28,35H,4-8,14,16-19H2,1-3H3,(H,36,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 in human liver microsomes after 30 mins in presence of NADPH |
J Med Chem 57: 1855-79 (2014)
Article DOI: 10.1021/jm4016894
BindingDB Entry DOI: 10.7270/Q2V989JD |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50448495

(CHEMBL3126836)Show SMILES COc1ccc2-c3c(C4CCCCC4)c4ccc(cc4n3CC3(CC3c2c1)C(=O)N1C2CC1CN(Cc1ccccc1)C2)C(=O)NS(=O)(=O)N(C)C |TLB:27:29:42.34.33:31,THB:35:34:29:31| Show InChI InChI=1S/C41H47N5O5S/c1-43(2)52(49,50)42-39(47)28-14-16-33-36(18-28)45-25-41(40(48)46-29-19-30(46)24-44(23-29)22-26-10-6-4-7-11-26)21-35(41)34-20-31(51-3)15-17-32(34)38(45)37(33)27-12-8-5-9-13-27/h4,6-7,10-11,14-18,20,27,29-30,35H,5,8-9,12-13,19,21-25H2,1-3H3,(H,42,47) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 in human liver microsomes after 30 mins in presence of NADPH |
J Med Chem 57: 1855-79 (2014)
Article DOI: 10.1021/jm4016894
BindingDB Entry DOI: 10.7270/Q2V989JD |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50448496

(CHEMBL3126835)Show SMILES CCN1CC2CC(C1)N2C(=O)[C@]12C[C@H]1c1cc(OC)ccc1-c1c(C3CCCCC3)c3ccc(cc3n1C2)C(=O)NS(=O)(=O)N(C)C |r,TLB:9:8:3.2.7:5,1:2:8:5| Show InChI InChI=1S/C36H45N5O5S/c1-5-39-19-24-16-25(20-39)41(24)35(43)36-18-30(36)29-17-26(46-4)12-14-27(29)33-32(22-9-7-6-8-10-22)28-13-11-23(15-31(28)40(33)21-36)34(42)37-47(44,45)38(2)3/h11-15,17,22,24-25,30H,5-10,16,18-21H2,1-4H3,(H,37,42)/t24?,25?,30-,36-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 in human liver microsomes after 30 mins in presence of NADPH |
J Med Chem 57: 1855-79 (2014)
Article DOI: 10.1021/jm4016894
BindingDB Entry DOI: 10.7270/Q2V989JD |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50448497

(CHEMBL3126834)Show SMILES COc1ccc2-c3c(C4CCCCC4)c4ccc(cc4n3C[C@]3(C[C@H]3c2c1)C(=O)N1C2CC1CN(C)C2)C(=O)NS(=O)(=O)N(C)C |r,TLB:27:29:36.34.33:31,THB:35:34:29:31| Show InChI InChI=1S/C35H43N5O5S/c1-37(2)46(43,44)36-33(41)22-10-12-27-30(14-22)39-20-35(34(42)40-23-15-24(40)19-38(3)18-23)17-29(35)28-16-25(45-4)11-13-26(28)32(39)31(27)21-8-6-5-7-9-21/h10-14,16,21,23-24,29H,5-9,15,17-20H2,1-4H3,(H,36,41)/t23?,24?,29-,35-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 in human liver microsomes after 30 mins in presence of NADPH |
J Med Chem 57: 1855-79 (2014)
Article DOI: 10.1021/jm4016894
BindingDB Entry DOI: 10.7270/Q2V989JD |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50448494

(CHEMBL3126657)Show SMILES COc1ccc2-c3c(C4CCCCC4)c4ccc(cc4n3CC3(CC3c2c1)C(=O)N1C2CC1CNC2)C(=O)NS(=O)(=O)N(C)C |TLB:27:29:35.34.33:31| Show InChI InChI=1S/C34H41N5O5S/c1-37(2)45(42,43)36-32(40)21-9-11-26-29(13-21)38-19-34(33(41)39-22-14-23(39)18-35-17-22)16-28(34)27-15-24(44-3)10-12-25(27)31(38)30(26)20-7-5-4-6-8-20/h9-13,15,20,22-23,28,35H,4-8,14,16-19H2,1-3H3,(H,36,40) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 in human liver microsomes after 30 mins in presence of NADPH |
J Med Chem 57: 1855-79 (2014)
Article DOI: 10.1021/jm4016894
BindingDB Entry DOI: 10.7270/Q2V989JD |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50448495

(CHEMBL3126836)Show SMILES COc1ccc2-c3c(C4CCCCC4)c4ccc(cc4n3CC3(CC3c2c1)C(=O)N1C2CC1CN(Cc1ccccc1)C2)C(=O)NS(=O)(=O)N(C)C |TLB:27:29:42.34.33:31,THB:35:34:29:31| Show InChI InChI=1S/C41H47N5O5S/c1-43(2)52(49,50)42-39(47)28-14-16-33-36(18-28)45-25-41(40(48)46-29-19-30(46)24-44(23-29)22-26-10-6-4-7-11-26)21-35(41)34-20-31(51-3)15-17-32(34)38(45)37(33)27-12-8-5-9-13-27/h4,6-7,10-11,14-18,20,27,29-30,35H,5,8-9,12-13,19,21-25H2,1-3H3,(H,42,47) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.10E+4 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 in human liver microsomes after 30 mins in presence of NADPH |
J Med Chem 57: 1855-79 (2014)
Article DOI: 10.1021/jm4016894
BindingDB Entry DOI: 10.7270/Q2V989JD |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50448496

(CHEMBL3126835)Show SMILES CCN1CC2CC(C1)N2C(=O)[C@]12C[C@H]1c1cc(OC)ccc1-c1c(C3CCCCC3)c3ccc(cc3n1C2)C(=O)NS(=O)(=O)N(C)C |r,TLB:9:8:3.2.7:5,1:2:8:5| Show InChI InChI=1S/C36H45N5O5S/c1-5-39-19-24-16-25(20-39)41(24)35(43)36-18-30(36)29-17-26(46-4)12-14-27(29)33-32(22-9-7-6-8-10-22)28-13-11-23(15-31(28)40(33)21-36)34(42)37-47(44,45)38(2)3/h11-15,17,22,24-25,30H,5-10,16,18-21H2,1-4H3,(H,37,42)/t24?,25?,30-,36-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 in human liver microsomes after 30 mins in presence of NADPH |
J Med Chem 57: 1855-79 (2014)
Article DOI: 10.1021/jm4016894
BindingDB Entry DOI: 10.7270/Q2V989JD |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50448497

(CHEMBL3126834)Show SMILES COc1ccc2-c3c(C4CCCCC4)c4ccc(cc4n3C[C@]3(C[C@H]3c2c1)C(=O)N1C2CC1CN(C)C2)C(=O)NS(=O)(=O)N(C)C |r,TLB:27:29:36.34.33:31,THB:35:34:29:31| Show InChI InChI=1S/C35H43N5O5S/c1-37(2)46(43,44)36-33(41)22-10-12-27-30(14-22)39-20-35(34(42)40-23-15-24(40)19-38(3)18-23)17-29(35)28-16-25(45-4)11-13-26(28)32(39)31(27)21-8-6-5-7-9-21/h10-14,16,21,23-24,29H,5-9,15,17-20H2,1-4H3,(H,36,41)/t23?,24?,29-,35-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 in human liver microsomes after 30 mins in presence of NADPH |
J Med Chem 57: 1855-79 (2014)
Article DOI: 10.1021/jm4016894
BindingDB Entry DOI: 10.7270/Q2V989JD |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50448494

(CHEMBL3126657)Show SMILES COc1ccc2-c3c(C4CCCCC4)c4ccc(cc4n3CC3(CC3c2c1)C(=O)N1C2CC1CNC2)C(=O)NS(=O)(=O)N(C)C |TLB:27:29:35.34.33:31| Show InChI InChI=1S/C34H41N5O5S/c1-37(2)45(42,43)36-32(40)21-9-11-26-29(13-21)38-19-34(33(41)39-22-14-23(39)18-35-17-22)16-28(34)27-15-24(44-3)10-12-25(27)31(38)30(26)20-7-5-4-6-8-20/h9-13,15,20,22-23,28,35H,4-8,14,16-19H2,1-3H3,(H,36,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 in human liver microsomes after 30 mins in presence of NADPH |
J Med Chem 57: 1855-79 (2014)
Article DOI: 10.1021/jm4016894
BindingDB Entry DOI: 10.7270/Q2V989JD |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50448497

(CHEMBL3126834)Show SMILES COc1ccc2-c3c(C4CCCCC4)c4ccc(cc4n3C[C@]3(C[C@H]3c2c1)C(=O)N1C2CC1CN(C)C2)C(=O)NS(=O)(=O)N(C)C |r,TLB:27:29:36.34.33:31,THB:35:34:29:31| Show InChI InChI=1S/C35H43N5O5S/c1-37(2)46(43,44)36-33(41)22-10-12-27-30(14-22)39-20-35(34(42)40-23-15-24(40)19-38(3)18-23)17-29(35)28-16-25(45-4)11-13-26(28)32(39)31(27)21-8-6-5-7-9-21/h10-14,16,21,23-24,29H,5-9,15,17-20H2,1-4H3,(H,36,41)/t23?,24?,29-,35-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 in human liver microsomes after 30 mins in presence of NADPH |
J Med Chem 57: 1855-79 (2014)
Article DOI: 10.1021/jm4016894
BindingDB Entry DOI: 10.7270/Q2V989JD |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 1 group I member 2
(Homo sapiens (Human)) | BDBM50448498

(BMS-791325 | Beclabuvir)Show SMILES COc1ccc2-c3c(C4CCCCC4)c4ccc(cc4n3C[C@]3(C[C@H]3c2c1)C(=O)N1C2CCC1CN(C)C2)C(=O)NS(=O)(=O)N(C)C |r,THB:36:35:29:31.32| Show InChI InChI=1S/C36H45N5O5S/c1-38(2)47(44,45)37-34(42)23-10-14-28-31(16-23)40-21-36(35(43)41-24-11-12-25(41)20-39(3)19-24)18-30(36)29-17-26(46-4)13-15-27(29)33(40)32(28)22-8-6-5-7-9-22/h10,13-17,22,24-25,30H,5-9,11-12,18-21H2,1-4H3,(H,37,42)/t24?,25?,30-,36-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Transactivation of human PXR expressed in human HepG2 cells by luciferase reporter gene assay |
J Med Chem 57: 1855-79 (2014)
Article DOI: 10.1021/jm4016894
BindingDB Entry DOI: 10.7270/Q2V989JD |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 1 group I member 2
(Homo sapiens (Human)) | BDBM50448499
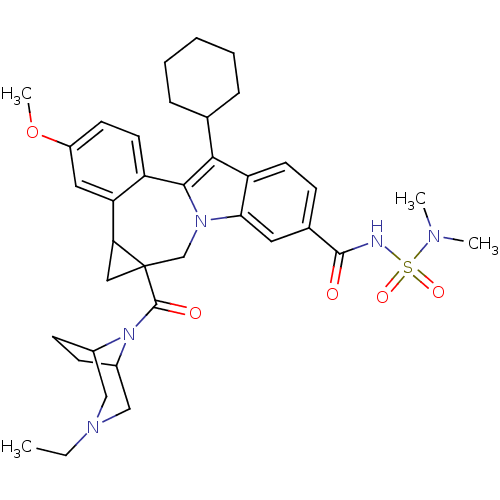
(CHEMBL3126841)Show SMILES CCN1CC2CCC(C1)N2C(=O)C12CC1c1cc(OC)ccc1-c1c(C3CCCCC3)c3ccc(cc3n1C2)C(=O)NS(=O)(=O)N(C)C |TLB:1:2:9:5.6,10:9:8.2.3:5.6| Show InChI InChI=1S/C37H47N5O5S/c1-5-40-20-25-12-13-26(21-40)42(25)36(44)37-19-31(37)30-18-27(47-4)14-16-28(30)34-33(23-9-7-6-8-10-23)29-15-11-24(17-32(29)41(34)22-37)35(43)38-48(45,46)39(2)3/h11,14-18,23,25-26,31H,5-10,12-13,19-22H2,1-4H3,(H,38,43) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Transactivation of human PXR expressed in human HepG2 cells by luciferase reporter gene assay |
J Med Chem 57: 1855-79 (2014)
Article DOI: 10.1021/jm4016894
BindingDB Entry DOI: 10.7270/Q2V989JD |
More data for this
Ligand-Target Pair | |
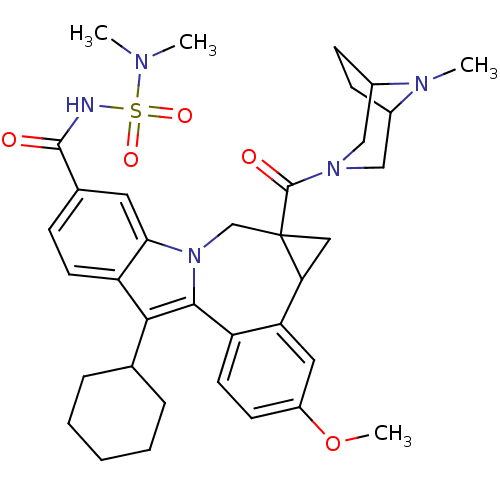
Nuclear receptor subfamily 1 group I member 2
(Homo sapiens (Human)) | BDBM50448500
(CHEMBL3126840)Show SMILES COc1ccc2-c3c(C4CCCCC4)c4ccc(cc4n3CC3(CC3c2c1)C(=O)N1CC2CCC(C1)N2C)C(=O)NS(=O)(=O)N(C)C |TLB:27:29:36:32.33| Show InChI InChI=1S/C36H45N5O5S/c1-38(2)47(44,45)37-34(42)23-10-14-28-31(16-23)41-21-36(35(43)40-19-24-11-12-25(20-40)39(24)3)18-30(36)29-17-26(46-4)13-15-27(29)33(41)32(28)22-8-6-5-7-9-22/h10,13-17,22,24-25,30H,5-9,11-12,18-21H2,1-4H3,(H,37,42) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Transactivation of human PXR expressed in human HepG2 cells by luciferase reporter gene assay |
J Med Chem 57: 1855-79 (2014)
Article DOI: 10.1021/jm4016894
BindingDB Entry DOI: 10.7270/Q2V989JD |
More data for this
Ligand-Target Pair | |
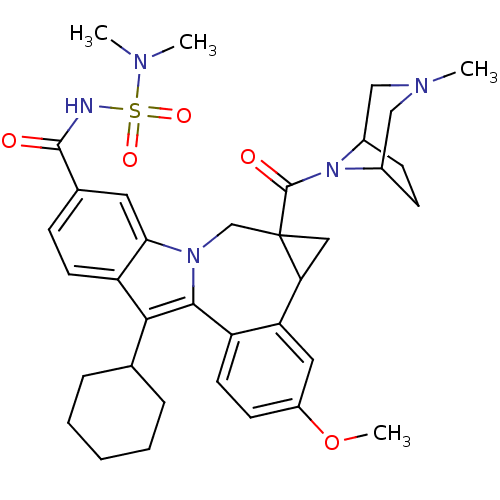
Nuclear receptor subfamily 1 group I member 2
(Homo sapiens (Human)) | BDBM50448501
(CHEMBL3126839)Show SMILES COc1ccc2-c3c(C4CCCCC4)c4ccc(cc4n3CC3(CC3c2c1)C(=O)N1C2CCC1CN(C)C2)C(=O)NS(=O)(=O)N(C)C |TLB:27:29:34.35.37:31.32,THB:36:35:29:31.32| Show InChI InChI=1S/C36H45N5O5S/c1-38(2)47(44,45)37-34(42)23-10-14-28-31(16-23)40-21-36(35(43)41-24-11-12-25(41)20-39(3)19-24)18-30(36)29-17-26(46-4)13-15-27(29)33(40)32(28)22-8-6-5-7-9-22/h10,13-17,22,24-25,30H,5-9,11-12,18-21H2,1-4H3,(H,37,42) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Transactivation of human PXR expressed in human HepG2 cells by luciferase reporter gene assay |
J Med Chem 57: 1855-79 (2014)
Article DOI: 10.1021/jm4016894
BindingDB Entry DOI: 10.7270/Q2V989JD |
More data for this
Ligand-Target Pair | |
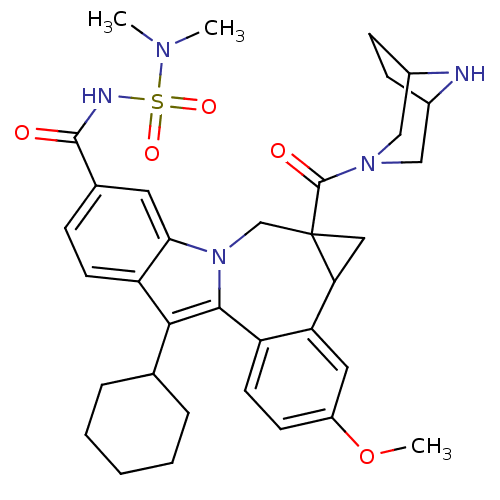
Nuclear receptor subfamily 1 group I member 2
(Homo sapiens (Human)) | BDBM50448502
(CHEMBL3126838)Show SMILES COc1ccc2-c3c(C4CCCCC4)c4ccc(cc4n3CC3(CC3c2c1)C(=O)N1CC2CCC(C1)N2)C(=O)NS(=O)(=O)N(C)C |TLB:27:29:36:32.33| Show InChI InChI=1S/C35H43N5O5S/c1-38(2)46(43,44)37-33(41)22-9-13-27-30(15-22)40-20-35(34(42)39-18-23-10-11-24(19-39)36-23)17-29(35)28-16-25(45-3)12-14-26(28)32(40)31(27)21-7-5-4-6-8-21/h9,12-16,21,23-24,29,36H,4-8,10-11,17-20H2,1-3H3,(H,37,41) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Transactivation of human PXR expressed in human HepG2 cells by luciferase reporter gene assay |
J Med Chem 57: 1855-79 (2014)
Article DOI: 10.1021/jm4016894
BindingDB Entry DOI: 10.7270/Q2V989JD |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 1 group I member 2
(Homo sapiens (Human)) | BDBM50448503
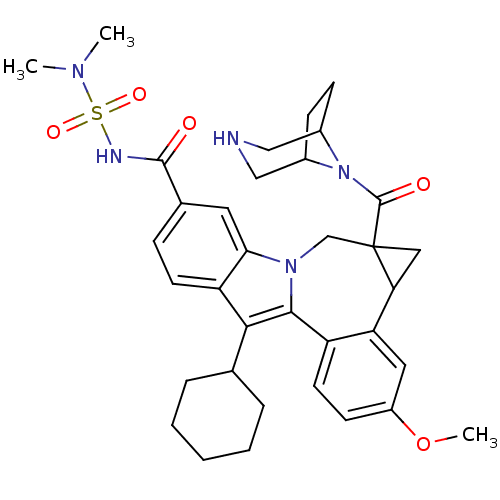
(CHEMBL3126837)Show SMILES COc1ccc2-c3c(C4CCCCC4)c4ccc(cc4n3CC3(CC3c2c1)C(=O)N1C2CCC1CNC2)C(=O)NS(=O)(=O)N(C)C |TLB:27:29:34.35.36:31.32| Show InChI InChI=1S/C35H43N5O5S/c1-38(2)46(43,44)37-33(41)22-9-13-27-30(15-22)39-20-35(34(42)40-23-10-11-24(40)19-36-18-23)17-29(35)28-16-25(45-3)12-14-26(28)32(39)31(27)21-7-5-4-6-8-21/h9,12-16,21,23-24,29,36H,4-8,10-11,17-20H2,1-3H3,(H,37,41) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Transactivation of human PXR expressed in human HepG2 cells by luciferase reporter gene assay |
J Med Chem 57: 1855-79 (2014)
Article DOI: 10.1021/jm4016894
BindingDB Entry DOI: 10.7270/Q2V989JD |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 1 group I member 2
(Homo sapiens (Human)) | BDBM50448495

(CHEMBL3126836)Show SMILES COc1ccc2-c3c(C4CCCCC4)c4ccc(cc4n3CC3(CC3c2c1)C(=O)N1C2CC1CN(Cc1ccccc1)C2)C(=O)NS(=O)(=O)N(C)C |TLB:27:29:42.34.33:31,THB:35:34:29:31| Show InChI InChI=1S/C41H47N5O5S/c1-43(2)52(49,50)42-39(47)28-14-16-33-36(18-28)45-25-41(40(48)46-29-19-30(46)24-44(23-29)22-26-10-6-4-7-11-26)21-35(41)34-20-31(51-3)15-17-32(34)38(45)37(33)27-12-8-5-9-13-27/h4,6-7,10-11,14-18,20,27,29-30,35H,5,8-9,12-13,19,21-25H2,1-3H3,(H,42,47) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Transactivation of human PXR expressed in human HepG2 cells by luciferase reporter gene assay |
J Med Chem 57: 1855-79 (2014)
Article DOI: 10.1021/jm4016894
BindingDB Entry DOI: 10.7270/Q2V989JD |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 1 group I member 2
(Homo sapiens (Human)) | BDBM50448496

(CHEMBL3126835)Show SMILES CCN1CC2CC(C1)N2C(=O)[C@]12C[C@H]1c1cc(OC)ccc1-c1c(C3CCCCC3)c3ccc(cc3n1C2)C(=O)NS(=O)(=O)N(C)C |r,TLB:9:8:3.2.7:5,1:2:8:5| Show InChI InChI=1S/C36H45N5O5S/c1-5-39-19-24-16-25(20-39)41(24)35(43)36-18-30(36)29-17-26(46-4)12-14-27(29)33-32(22-9-7-6-8-10-22)28-13-11-23(15-31(28)40(33)21-36)34(42)37-47(44,45)38(2)3/h11-15,17,22,24-25,30H,5-10,16,18-21H2,1-4H3,(H,37,42)/t24?,25?,30-,36-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Transactivation of human PXR expressed in human HepG2 cells by luciferase reporter gene assay |
J Med Chem 57: 1855-79 (2014)
Article DOI: 10.1021/jm4016894
BindingDB Entry DOI: 10.7270/Q2V989JD |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 1 group I member 2
(Homo sapiens (Human)) | BDBM50448497

(CHEMBL3126834)Show SMILES COc1ccc2-c3c(C4CCCCC4)c4ccc(cc4n3C[C@]3(C[C@H]3c2c1)C(=O)N1C2CC1CN(C)C2)C(=O)NS(=O)(=O)N(C)C |r,TLB:27:29:36.34.33:31,THB:35:34:29:31| Show InChI InChI=1S/C35H43N5O5S/c1-37(2)46(43,44)36-33(41)22-10-12-27-30(14-22)39-20-35(34(42)40-23-15-24(40)19-38(3)18-23)17-29(35)28-16-25(45-4)11-13-26(28)32(39)31(27)21-8-6-5-7-9-21/h10-14,16,21,23-24,29H,5-9,15,17-20H2,1-4H3,(H,36,41)/t23?,24?,29-,35-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Transactivation of human PXR expressed in human HepG2 cells by luciferase reporter gene assay |
J Med Chem 57: 1855-79 (2014)
Article DOI: 10.1021/jm4016894
BindingDB Entry DOI: 10.7270/Q2V989JD |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 1 group I member 2
(Homo sapiens (Human)) | BDBM50448504
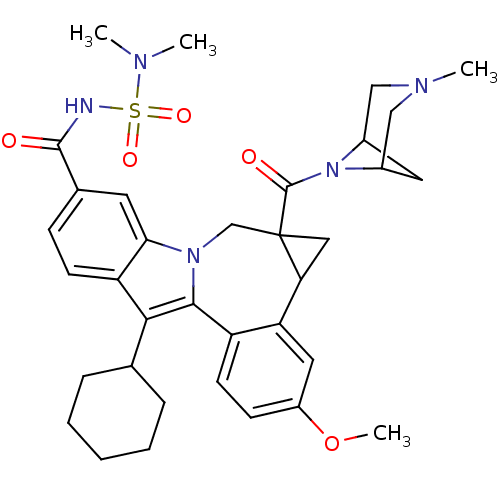
(CHEMBL3126658)Show SMILES COc1ccc2-c3c(C4CCCCC4)c4ccc(cc4n3CC3(CC3c2c1)C(=O)N1C2CC1CN(C)C2)C(=O)NS(=O)(=O)N(C)C |TLB:27:29:36.34.33:31,THB:35:34:29:31| Show InChI InChI=1S/C35H43N5O5S/c1-37(2)46(43,44)36-33(41)22-10-12-27-30(14-22)39-20-35(34(42)40-23-15-24(40)19-38(3)18-23)17-29(35)28-16-25(45-4)11-13-26(28)32(39)31(27)21-8-6-5-7-9-21/h10-14,16,21,23-24,29H,5-9,15,17-20H2,1-4H3,(H,36,41) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Transactivation of human PXR expressed in human HepG2 cells by luciferase reporter gene assay |
J Med Chem 57: 1855-79 (2014)
Article DOI: 10.1021/jm4016894
BindingDB Entry DOI: 10.7270/Q2V989JD |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 1 group I member 2
(Homo sapiens (Human)) | BDBM50448494

(CHEMBL3126657)Show SMILES COc1ccc2-c3c(C4CCCCC4)c4ccc(cc4n3CC3(CC3c2c1)C(=O)N1C2CC1CNC2)C(=O)NS(=O)(=O)N(C)C |TLB:27:29:35.34.33:31| Show InChI InChI=1S/C34H41N5O5S/c1-37(2)45(42,43)36-32(40)21-9-11-26-29(13-21)38-19-34(33(41)39-22-14-23(39)18-35-17-22)16-28(34)27-15-24(44-3)10-12-25(27)31(38)30(26)20-7-5-4-6-8-20/h9-13,15,20,22-23,28,35H,4-8,14,16-19H2,1-3H3,(H,36,40) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Transactivation of human PXR expressed in human HepG2 cells by luciferase reporter gene assay |
J Med Chem 57: 1855-79 (2014)
Article DOI: 10.1021/jm4016894
BindingDB Entry DOI: 10.7270/Q2V989JD |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data