

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| cAMP-dependent protein kinase (Oryctolagus cuniculus (Rabbit)) | BDBM36603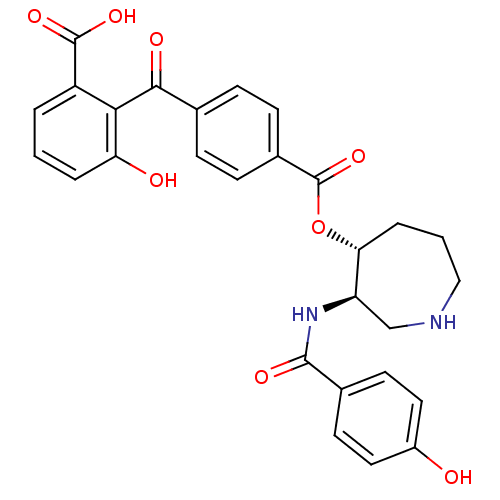 (4'',6''-Dideoxybalanol, 6) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.40 | -49.1 | 5.5 | n/a | n/a | n/a | n/a | 7.5 | 30 |
University of California, San Diego | Assay Description Cyclic AMP-dependent protein kinase (PKA) enzyme inhibition assay using purified recombinant C subunit of PKA (catalytic (C) subunit (cPKA)) with hol... | Chem Biol 2: 601-8 (1995) Article DOI: 10.1016/1074-5521(95)90124-8 BindingDB Entry DOI: 10.7270/Q2HQ3X8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-dependent protein kinase (Oryctolagus cuniculus (Rabbit)) | BDBM36604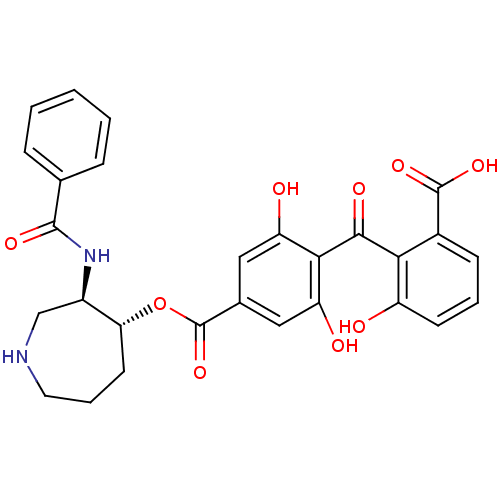 (5'-Deoxybalanol, 7) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 3.5 | -49.1 | 5.70 | n/a | n/a | n/a | n/a | 7.5 | 30 |
University of California, San Diego | Assay Description Cyclic AMP-dependent protein kinase (PKA) enzyme inhibition assay using purified recombinant C subunit of PKA (catalytic (C) subunit (cPKA)) with hol... | Chem Biol 2: 601-8 (1995) Article DOI: 10.1016/1074-5521(95)90124-8 BindingDB Entry DOI: 10.7270/Q2HQ3X8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-dependent protein kinase (Oryctolagus cuniculus (Rabbit)) | BDBM3207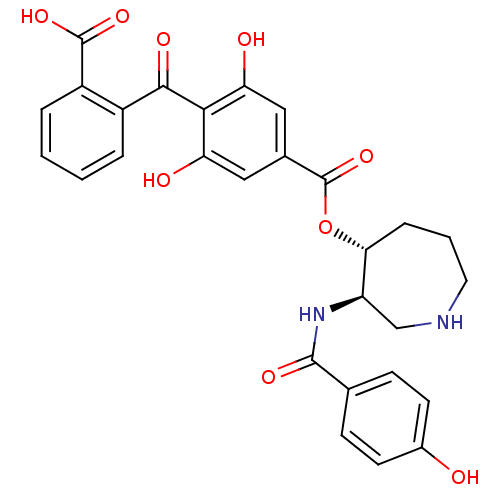 (10''-Deoxybalanol, 4 | 2-{[2,6-dihydroxy-4-({[(3R,...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.90 | -48.8 | 6.30 | n/a | n/a | n/a | n/a | 7.5 | 30 |
University of California, San Diego | Assay Description Cyclic AMP-dependent protein kinase (PKA) enzyme inhibition assay using purified recombinant C subunit of PKA (catalytic (C) subunit (cPKA)) with hol... | Chem Biol 2: 601-8 (1995) Article DOI: 10.1016/1074-5521(95)90124-8 BindingDB Entry DOI: 10.7270/Q2HQ3X8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-dependent protein kinase (Oryctolagus cuniculus (Rabbit)) | BDBM3149 (2-{[2,6-dihydroxy-4-({[(3R,4R)-3-[(4-hydroxybenzen...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 4.70 | -48.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
University of California, San Diego | Assay Description Cyclic AMP-dependent protein kinase (PKA) enzyme inhibition assay using purified recombinant C subunit of PKA (catalytic (C) subunit (cPKA)) with hol... | Chem Biol 2: 601-8 (1995) Article DOI: 10.1016/1074-5521(95)90124-8 BindingDB Entry DOI: 10.7270/Q2HQ3X8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Rattus norvegicus (rat)) | BDBM3149 (2-{[2,6-dihydroxy-4-({[(3R,4R)-3-[(4-hydroxybenzen...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 5.30 | -48.0 | n/a | n/a | n/a | n/a | n/a | n/a | 30 |
University of California, San Diego | Assay Description Protein kinase C (PKC) enzyme inhibition assay using purified rat brain protein kinase C from Calbiochem #539494. | Chem Biol 2: 601-8 (1995) Article DOI: 10.1016/1074-5521(95)90124-8 BindingDB Entry DOI: 10.7270/Q2HQ3X8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-dependent protein kinase (Oryctolagus cuniculus (Rabbit)) | BDBM36602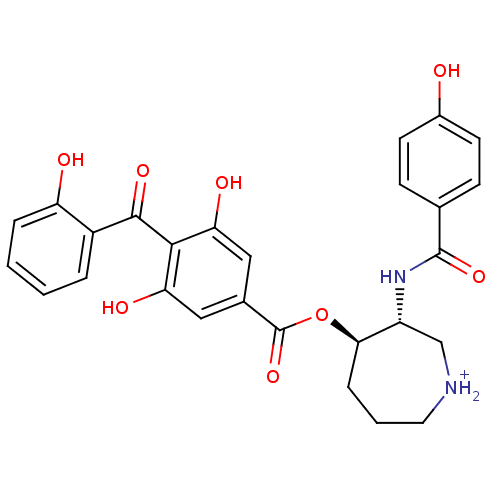 (14''-Decarboxybalanol hydrochloride, 5) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 11 | -46.2 | 18 | n/a | n/a | n/a | n/a | 7.5 | 30 |
University of California, San Diego | Assay Description Cyclic AMP-dependent protein kinase (PKA) enzyme inhibition assay using purified recombinant C subunit of PKA (catalytic (C) subunit (cPKA)) with hol... | Chem Biol 2: 601-8 (1995) Article DOI: 10.1016/1074-5521(95)90124-8 BindingDB Entry DOI: 10.7270/Q2HQ3X8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Rattus norvegicus (rat)) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 19 | -44.8 | n/a | n/a | n/a | n/a | n/a | n/a | 30 |
University of California, San Diego | Assay Description Protein kinase C (PKC) enzyme inhibition assay using purified rat brain protein kinase C from Calbiochem #539494. | Chem Biol 2: 601-8 (1995) Article DOI: 10.1016/1074-5521(95)90124-8 BindingDB Entry DOI: 10.7270/Q2HQ3X8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-dependent protein kinase (Oryctolagus cuniculus (Rabbit)) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
University of California, San Diego | Assay Description Cyclic AMP-dependent protein kinase (PKA) enzyme inhibition assay using purified recombinant C subunit of PKA (catalytic (C) subunit (cPKA)) with hol... | Chem Biol 2: 601-8 (1995) Article DOI: 10.1016/1074-5521(95)90124-8 BindingDB Entry DOI: 10.7270/Q2HQ3X8D | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cAMP-dependent protein kinase (Oryctolagus cuniculus (Rabbit)) | BDBM36606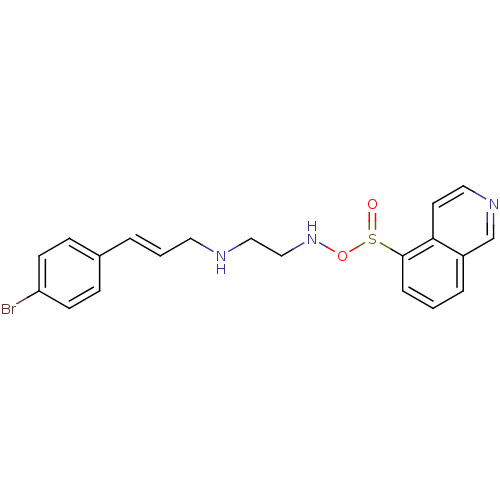 (H-89, 10) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 48 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
University of California, San Diego | Assay Description Cyclic AMP-dependent protein kinase (PKA) enzyme inhibition assay using purified recombinant C subunit of PKA (catalytic (C) subunit (cPKA)) with hol... | Chem Biol 2: 601-8 (1995) Article DOI: 10.1016/1074-5521(95)90124-8 BindingDB Entry DOI: 10.7270/Q2HQ3X8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-dependent protein kinase (Oryctolagus cuniculus (Rabbit)) | BDBM36605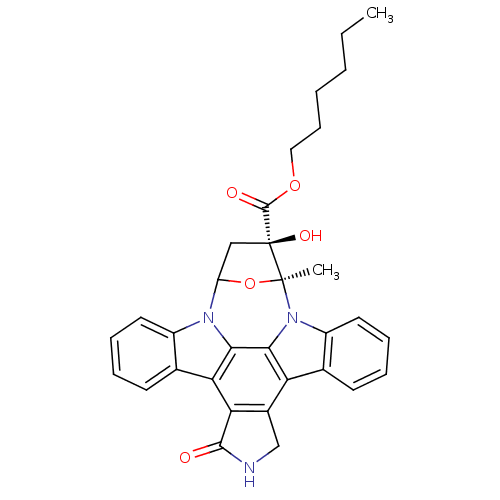 (KT5720, 9) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 56 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
University of California, San Diego | Assay Description Cyclic AMP-dependent protein kinase (PKA) enzyme inhibition assay using purified recombinant C subunit of PKA (catalytic (C) subunit (cPKA)) with hol... | Chem Biol 2: 601-8 (1995) Article DOI: 10.1016/1074-5521(95)90124-8 BindingDB Entry DOI: 10.7270/Q2HQ3X8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Rattus norvegicus (rat)) | BDBM36604 (5'-Deoxybalanol, 7) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 69 | -41.6 | 95 | n/a | n/a | n/a | n/a | 7.5 | 30 |
University of California, San Diego | Assay Description Protein kinase C (PKC) enzyme inhibition assay using purified rat brain protein kinase C from Calbiochem #539494. | Chem Biol 2: 601-8 (1995) Article DOI: 10.1016/1074-5521(95)90124-8 BindingDB Entry DOI: 10.7270/Q2HQ3X8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Rattus norvegicus (rat)) | BDBM36603 (4'',6''-Dideoxybalanol, 6) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 80 | -41.2 | 111 | n/a | n/a | n/a | n/a | 7.5 | 30 |
University of California, San Diego | Assay Description Protein kinase C (PKC) enzyme inhibition assay using purified rat brain protein kinase C from Calbiochem #539494. | Chem Biol 2: 601-8 (1995) Article DOI: 10.1016/1074-5521(95)90124-8 BindingDB Entry DOI: 10.7270/Q2HQ3X8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Rattus norvegicus (rat)) | BDBM3207 (10''-Deoxybalanol, 4 | 2-{[2,6-dihydroxy-4-({[(3R,...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 640 | -35.9 | 834 | n/a | n/a | n/a | n/a | 7.5 | 30 |
University of California, San Diego | Assay Description Protein kinase C (PKC) enzyme inhibition assay using purified rat brain protein kinase C from Calbiochem #539494. | Chem Biol 2: 601-8 (1995) Article DOI: 10.1016/1074-5521(95)90124-8 BindingDB Entry DOI: 10.7270/Q2HQ3X8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Rattus norvegicus (rat)) | BDBM36605 (KT5720, 9) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >2.00E+3 | >-33.1 | n/a | n/a | n/a | n/a | n/a | n/a | 30 |
University of California, San Diego | Assay Description Protein kinase C (PKC) enzyme inhibition assay using purified rat brain protein kinase C from Calbiochem #539494. | Chem Biol 2: 601-8 (1995) Article DOI: 10.1016/1074-5521(95)90124-8 BindingDB Entry DOI: 10.7270/Q2HQ3X8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Rattus norvegicus (rat)) | BDBM36602 (14''-Decarboxybalanol hydrochloride, 5) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5.00E+3 | -30.8 | 6.90E+3 | n/a | n/a | n/a | n/a | n/a | 30 |
University of California, San Diego | Assay Description Protein kinase C (PKC) enzyme inhibition assay using purified rat brain protein kinase C from Calbiochem #539494. | Chem Biol 2: 601-8 (1995) Article DOI: 10.1016/1074-5521(95)90124-8 BindingDB Entry DOI: 10.7270/Q2HQ3X8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-dependent protein kinase (Oryctolagus cuniculus (Rabbit)) | BDBM36600 (Benzophenone fragment, 2) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | >-29.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
University of California, San Diego | Assay Description Cyclic AMP-dependent protein kinase (PKA) enzyme inhibition assay using purified recombinant C subunit of PKA (catalytic (C) subunit (cPKA)) with hol... | Chem Biol 2: 601-8 (1995) Article DOI: 10.1016/1074-5521(95)90124-8 BindingDB Entry DOI: 10.7270/Q2HQ3X8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-dependent protein kinase (Oryctolagus cuniculus (Rabbit)) | BDBM36601 (Hexahydroazepine, 3) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
University of California, San Diego | Assay Description Cyclic AMP-dependent protein kinase (PKA) enzyme inhibition assay using purified recombinant C subunit of PKA (catalytic (C) subunit (cPKA)) with hol... | Chem Biol 2: 601-8 (1995) Article DOI: 10.1016/1074-5521(95)90124-8 BindingDB Entry DOI: 10.7270/Q2HQ3X8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Rattus norvegicus (rat)) | BDBM36606 (H-89, 10) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.17E+4 | -26.1 | n/a | n/a | n/a | n/a | n/a | n/a | 30 |
University of California, San Diego | Assay Description Protein kinase C (PKC) enzyme inhibition assay using purified rat brain protein kinase C from Calbiochem #539494. | Chem Biol 2: 601-8 (1995) Article DOI: 10.1016/1074-5521(95)90124-8 BindingDB Entry DOI: 10.7270/Q2HQ3X8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Rattus norvegicus (rat)) | BDBM36600 (Benzophenone fragment, 2) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | >1.00E+6 | >-17.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
University of California, San Diego | Assay Description Protein kinase C (PKC) enzyme inhibition assay using purified rat brain protein kinase C from Calbiochem #539494. | Chem Biol 2: 601-8 (1995) Article DOI: 10.1016/1074-5521(95)90124-8 BindingDB Entry DOI: 10.7270/Q2HQ3X8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Rattus norvegicus (rat)) | BDBM36601 (Hexahydroazepine, 3) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+6 | >-17.4 | n/a | n/a | n/a | n/a | n/a | n/a | 30 |
University of California, San Diego | Assay Description Protein kinase C (PKC) enzyme inhibition assay using purified rat brain protein kinase C from Calbiochem #539494. | Chem Biol 2: 601-8 (1995) Article DOI: 10.1016/1074-5521(95)90124-8 BindingDB Entry DOI: 10.7270/Q2HQ3X8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM36609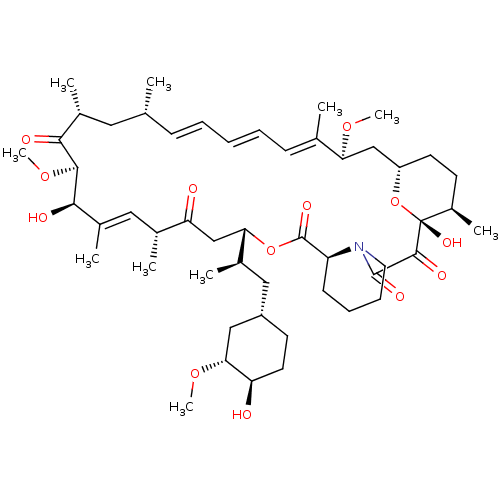 (Rapamycin C-7, analog 4 | SIROLIMUS | US11603377, ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against binding to FKBP12 | J Med Chem 48: 5613-38 (2005) Article DOI: 10.1021/jm050524f BindingDB Entry DOI: 10.7270/Q2V988V1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM36609 (Rapamycin C-7, analog 4 | SIROLIMUS | US11603377, ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against FKBP12 receptor | J Med Chem 48: 5613-38 (2005) Article DOI: 10.1021/jm050524f BindingDB Entry DOI: 10.7270/Q2V988V1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50172395 ((1R,9S,12R,21S,22S,24S,27R)-1-Hydroxy-22-methoxy-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against binding to FKBP12 | J Med Chem 48: 5613-38 (2005) Article DOI: 10.1021/jm050524f BindingDB Entry DOI: 10.7270/Q2V988V1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM50172395 ((1R,9S,12R,21S,22S,24S,27R)-1-Hydroxy-22-methoxy-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against FKBP12 receptor | J Med Chem 48: 5613-38 (2005) Article DOI: 10.1021/jm050524f BindingDB Entry DOI: 10.7270/Q2V988V1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50172384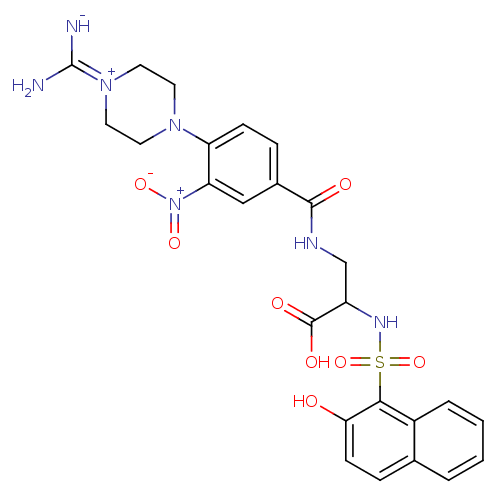 (3-[4-(4-Carbamimidoyl-piperazin-1-yl)-3-nitro-benz...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against integrin alpha-2b beta3 | J Med Chem 48: 5613-38 (2005) Article DOI: 10.1021/jm050524f BindingDB Entry DOI: 10.7270/Q2V988V1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50172384 (3-[4-(4-Carbamimidoyl-piperazin-1-yl)-3-nitro-benz...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against alpha IIb beta-3 receptor | J Med Chem 48: 5613-38 (2005) Article DOI: 10.1021/jm050524f BindingDB Entry DOI: 10.7270/Q2V988V1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 1/2/3 subunit alpha (Homo sapiens (Human)) | BDBM50172364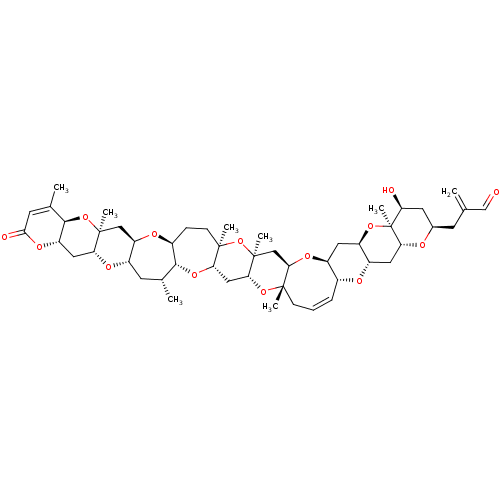 (2-{[(1R,3S,5R,7S,9R,11S,12S,14R,16R,18S,20R,21Z,24...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 15.0 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against synaptosomes using [3H]PbTx-3 | J Med Chem 48: 5613-38 (2005) Article DOI: 10.1021/jm050524f BindingDB Entry DOI: 10.7270/Q2V988V1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 1/2/3 subunit alpha (Homo sapiens (Human)) | BDBM50172364 (2-{[(1R,3S,5R,7S,9R,11S,12S,14R,16R,18S,20R,21Z,24...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 15.0 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against synaptosome using [3H]PbTx-3 | J Med Chem 48: 5613-38 (2005) Article DOI: 10.1021/jm050524f BindingDB Entry DOI: 10.7270/Q2V988V1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome c oxidase subunit NDUFA4 (Homo sapiens (Human)) | BDBM50172374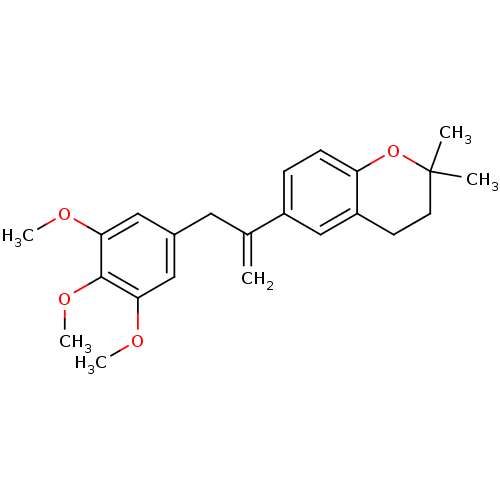 (2,2-Dimethyl-6-[1-(3,4,5-trimethoxy-benzyl)-vinyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against NADH-ubiquinone oxidoreductase | J Med Chem 48: 5613-38 (2005) Article DOI: 10.1021/jm050524f BindingDB Entry DOI: 10.7270/Q2V988V1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome c oxidase subunit NDUFA4 (Homo sapiens (Human)) | BDBM50172374 (2,2-Dimethyl-6-[1-(3,4,5-trimethoxy-benzyl)-vinyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against NADH ubiquinone oxidoreductase | J Med Chem 48: 5613-38 (2005) Article DOI: 10.1021/jm050524f BindingDB Entry DOI: 10.7270/Q2V988V1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome c oxidase subunit NDUFA4 (Homo sapiens (Human)) | BDBM50172381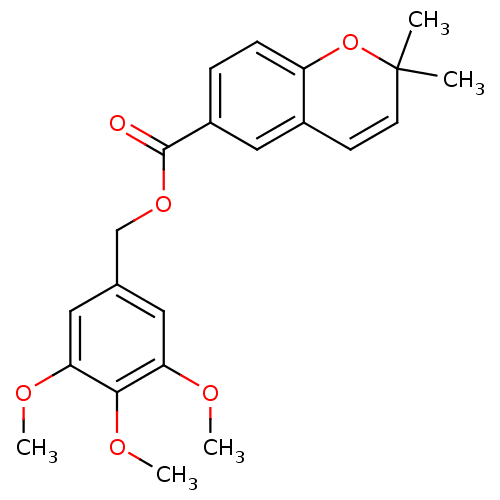 (2,2-Dimethyl-2H-chromene-6-carboxylic acid 3,4,5-t...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against NADH ubiquinone oxidoreductase | J Med Chem 48: 5613-38 (2005) Article DOI: 10.1021/jm050524f BindingDB Entry DOI: 10.7270/Q2V988V1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome c oxidase subunit NDUFA4 (Homo sapiens (Human)) | BDBM50172381 (2,2-Dimethyl-2H-chromene-6-carboxylic acid 3,4,5-t...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against NADH-ubiquinone oxidoreductase | J Med Chem 48: 5613-38 (2005) Article DOI: 10.1021/jm050524f BindingDB Entry DOI: 10.7270/Q2V988V1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial PAS domain-containing protein 1/Hypoxia-inducible factor 1-alpha (Homo sapiens (Human)) | BDBM50484273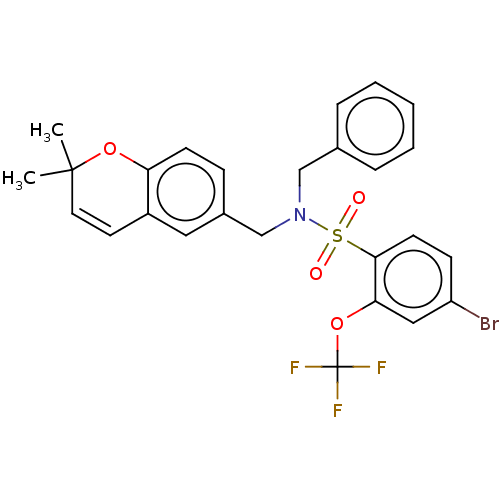 (CHEMBL1824104) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 508 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University School of Medicine Curated by ChEMBL | Assay Description Inhibition of hypoxia-induced HIF1 activation in human LN229 cells expressing HRE-AP reporter gene preincubated for 1 hr under normoxia condition fol... | Bioorg Med Chem Lett 21: 5528-32 (2011) Article DOI: 10.1016/j.bmcl.2011.06.099 BindingDB Entry DOI: 10.7270/Q2125WH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial PAS domain-containing protein 1/Hypoxia-inducible factor 1-alpha (Homo sapiens (Human)) | BDBM50484271 (CHEMBL1824130) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 532 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University School of Medicine Curated by ChEMBL | Assay Description Inhibition of hypoxia-induced HIF1 activation in human LN229 cells expressing HRE-AP reporter gene preincubated for 1 hr under normoxia condition fol... | Bioorg Med Chem Lett 21: 5528-32 (2011) Article DOI: 10.1016/j.bmcl.2011.06.099 BindingDB Entry DOI: 10.7270/Q2125WH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial PAS domain-containing protein 1/Hypoxia-inducible factor 1-alpha (Homo sapiens (Human)) | BDBM50396040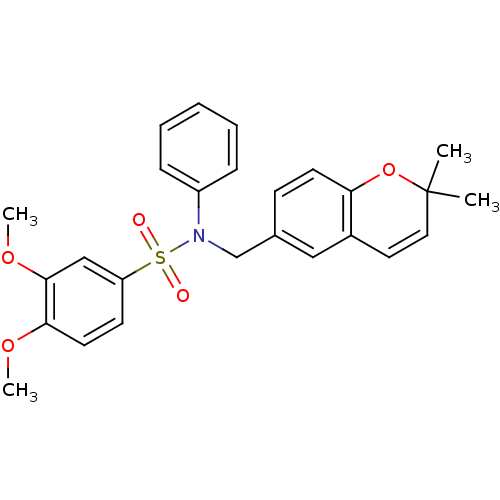 (CHEMBL1823895) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 593 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University School of Medicine Curated by ChEMBL | Assay Description Inhibition of hypoxia-induced HIF1 activation in human LN229 cells expressing HRE-AP reporter gene preincubated for 1 hr under normoxia condition fol... | Bioorg Med Chem Lett 21: 5528-32 (2011) Article DOI: 10.1016/j.bmcl.2011.06.099 BindingDB Entry DOI: 10.7270/Q2125WH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial PAS domain-containing protein 1/Hypoxia-inducible factor 1-alpha (Homo sapiens (Human)) | BDBM50484270 (CHEMBL1824127) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 656 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University School of Medicine Curated by ChEMBL | Assay Description Inhibition of hypoxia-induced HIF1 activation in human LN229 cells expressing HRE-AP reporter gene preincubated for 1 hr under normoxia condition fol... | Bioorg Med Chem Lett 21: 5528-32 (2011) Article DOI: 10.1016/j.bmcl.2011.06.099 BindingDB Entry DOI: 10.7270/Q2125WH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial PAS domain-containing protein 1/Hypoxia-inducible factor 1-alpha (Homo sapiens (Human)) | BDBM50484272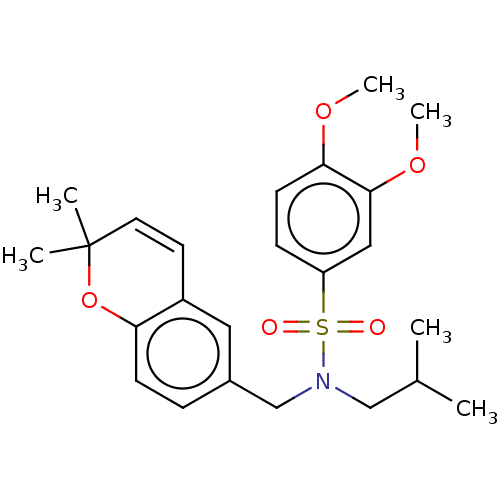 (CHEMBL1824131) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 672 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University School of Medicine Curated by ChEMBL | Assay Description Inhibition of hypoxia-induced HIF1 activation in human LN229 cells expressing HRE-AP reporter gene preincubated for 1 hr under normoxia condition fol... | Bioorg Med Chem Lett 21: 5528-32 (2011) Article DOI: 10.1016/j.bmcl.2011.06.099 BindingDB Entry DOI: 10.7270/Q2125WH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial PAS domain-containing protein 1/Hypoxia-inducible factor 1-alpha (Homo sapiens (Human)) | BDBM50484276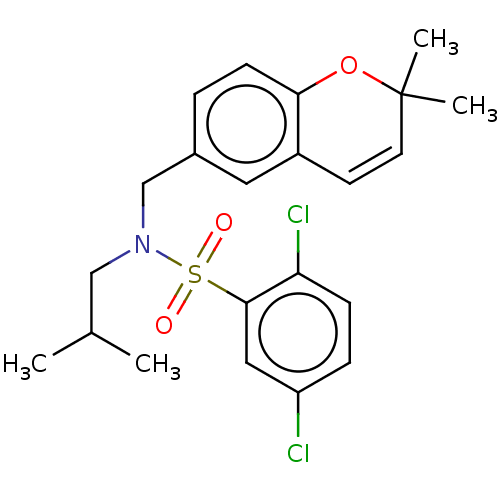 (CHEMBL1824129) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 731 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University School of Medicine Curated by ChEMBL | Assay Description Inhibition of hypoxia-induced HIF1 activation in human LN229 cells expressing HRE-AP reporter gene preincubated for 1 hr under normoxia condition fol... | Bioorg Med Chem Lett 21: 5528-32 (2011) Article DOI: 10.1016/j.bmcl.2011.06.099 BindingDB Entry DOI: 10.7270/Q2125WH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial PAS domain-containing protein 1/Hypoxia-inducible factor 1-alpha (Homo sapiens (Human)) | BDBM50484275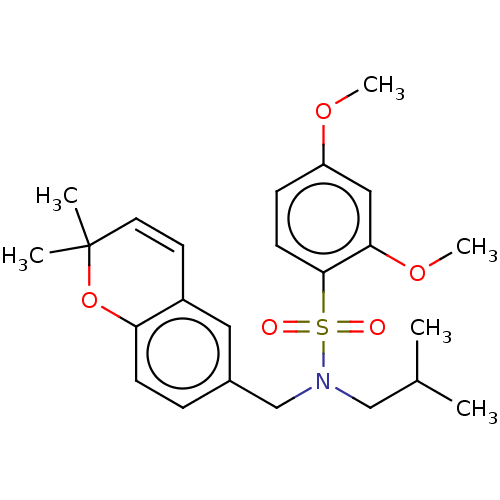 (CHEMBL1824132) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University School of Medicine Curated by ChEMBL | Assay Description Inhibition of hypoxia-induced HIF1 activation in human LN229 cells expressing HRE-AP reporter gene preincubated for 1 hr under normoxia condition fol... | Bioorg Med Chem Lett 21: 5528-32 (2011) Article DOI: 10.1016/j.bmcl.2011.06.099 BindingDB Entry DOI: 10.7270/Q2125WH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial PAS domain-containing protein 1/Hypoxia-inducible factor 1-alpha (Homo sapiens (Human)) | BDBM50484274 (CHEMBL1824124) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University School of Medicine Curated by ChEMBL | Assay Description Inhibition of hypoxia-induced HIF1 activation in human LN229 cells expressing HRE-AP reporter gene preincubated for 1 hr under normoxia condition fol... | Bioorg Med Chem Lett 21: 5528-32 (2011) Article DOI: 10.1016/j.bmcl.2011.06.099 BindingDB Entry DOI: 10.7270/Q2125WH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mycothiol S-conjugate amidase (Mycobacterium tuberculosis) | BDBM50117084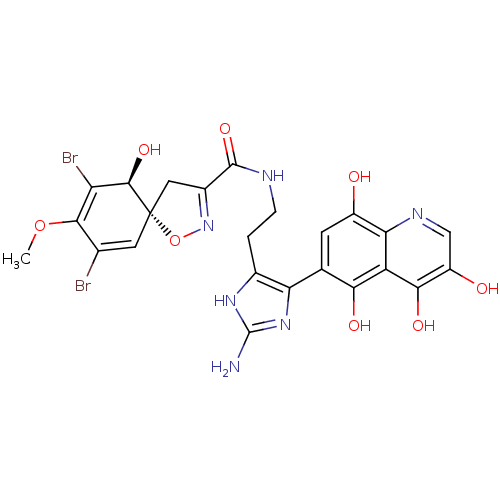 ((5R,10S)-7,9-Dibromo-10-hydroxy-8-methoxy-1-oxa-2-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of Mycothiol -S-conjugate amidase (MCA) from mycobacterium tuberculosis | Bioorg Med Chem Lett 12: 2487-90 (2002) BindingDB Entry DOI: 10.7270/Q2XD111C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mycothiol S-conjugate amidase (Mycobacterium tuberculosis) | BDBM50117077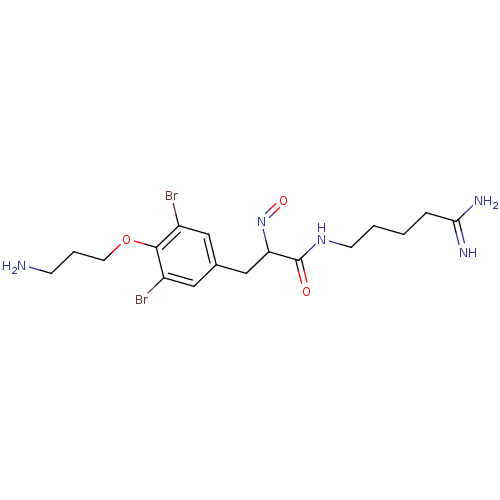 (3-[4-(3-Amino-propoxy)-3,5-dibromo-phenyl]-N-(4-ca...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of Mycothiol -S-conjugate amidase (MCA) from mycobacterium tuberculosis | Bioorg Med Chem Lett 12: 2487-90 (2002) BindingDB Entry DOI: 10.7270/Q2XD111C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mycothiol S-conjugate amidase (Mycobacterium tuberculosis) | BDBM50117090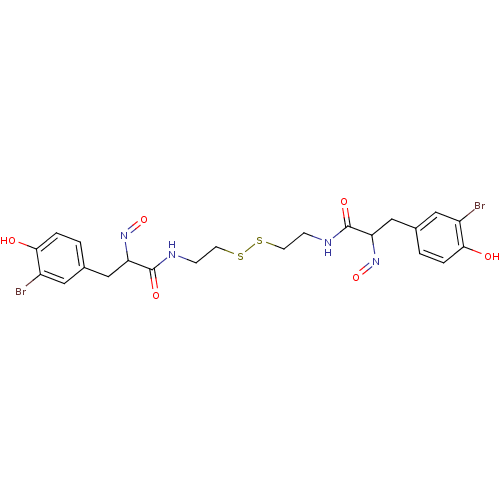 ((E,E)-Psammaplin A | 3-(3-Bromo-4-hydroxy-phenyl)-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis detoxification enzyme mycothiol-S-conjugate amidase (MCA) | J Med Chem 48: 5613-38 (2005) Article DOI: 10.1021/jm050524f BindingDB Entry DOI: 10.7270/Q2V988V1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mycothiol S-conjugate amidase (Mycobacterium tuberculosis) | BDBM50117090 ((E,E)-Psammaplin A | 3-(3-Bromo-4-hydroxy-phenyl)-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of Mycothiol -S-conjugate amidase (MCA) from mycobacterium tuberculosis | Bioorg Med Chem Lett 12: 2487-90 (2002) BindingDB Entry DOI: 10.7270/Q2XD111C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mycothiol S-conjugate amidase (Mycobacterium tuberculosis) | BDBM50117090 ((E,E)-Psammaplin A | 3-(3-Bromo-4-hydroxy-phenyl)-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis detoxification enzyme mycothiol-S-conjugate amidase (MCA) | J Med Chem 48: 5613-38 (2005) Article DOI: 10.1021/jm050524f BindingDB Entry DOI: 10.7270/Q2V988V1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 1/2/3 subunit alpha (Homo sapiens (Human)) | BDBM50172380 (CHEMBL370148 | Truncated brevetoxin B) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against synaptosomes using [3H]PbTx-3 | J Med Chem 48: 5613-38 (2005) Article DOI: 10.1021/jm050524f BindingDB Entry DOI: 10.7270/Q2V988V1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 1/2/3 subunit alpha (Homo sapiens (Human)) | BDBM50172380 (CHEMBL370148 | Truncated brevetoxin B) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against synaptosome using [3H]PbTx-3 | J Med Chem 48: 5613-38 (2005) Article DOI: 10.1021/jm050524f BindingDB Entry DOI: 10.7270/Q2V988V1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mycothiol S-conjugate amidase (Mycobacterium tuberculosis) | BDBM50117088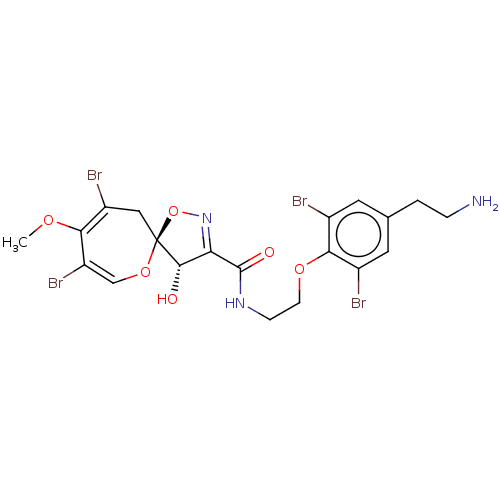 (8,10-Dibromo-4-hydroxy-9-methoxy-1,6-dioxa-2-aza-s...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of Mycothiol -S-conjugate amidase (MCA) from mycobacterium tuberculosis | Bioorg Med Chem Lett 12: 2487-90 (2002) BindingDB Entry DOI: 10.7270/Q2XD111C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mycothiol S-conjugate amidase (Mycobacterium tuberculosis) | BDBM50117086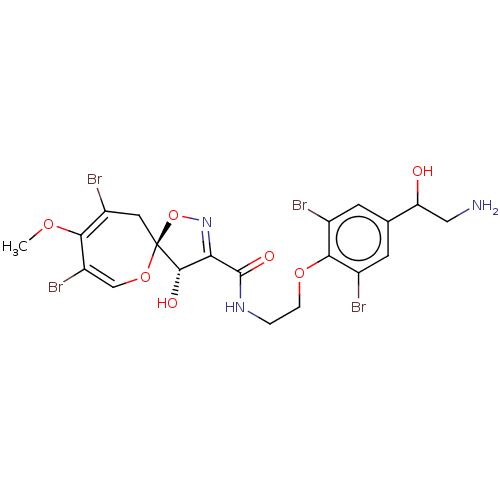 (8,10-Dibromo-4-hydroxy-9-methoxy-1,6-dioxa-2-aza-s...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of Mycothiol -S-conjugate amidase (MCA) from mycobacterium tuberculosis | Bioorg Med Chem Lett 12: 2487-90 (2002) BindingDB Entry DOI: 10.7270/Q2XD111C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mycothiol S-conjugate amidase (Mycobacterium tuberculosis) | BDBM50117078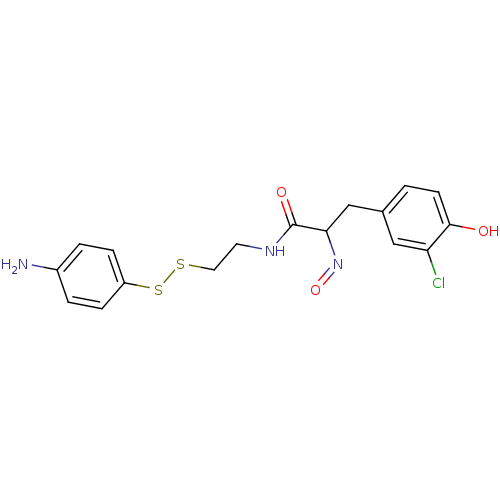 (CHEMBL197081 | CHEMBL312123 | N-[2-(4-Amino-phenyl...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of Mycothiol -S-conjugate amidase (MCA) from mycobacterium tuberculosis | Bioorg Med Chem Lett 12: 2487-90 (2002) BindingDB Entry DOI: 10.7270/Q2XD111C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 76 total ) | Next | Last >> |