

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50032675 (4-[1-(3,5,5,8,8-pentamethyl-5,6,7,8-tetrahydronaph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Graduate School of Medicine Curated by ChEMBL | Assay Description Displacement of 9-cis-[11,12-3H]-retinoic acid from human RXRalpha LBD incubated for overnight by scintillation counting method | J Med Chem 62: 8809-8818 (2019) Article DOI: 10.1021/acs.jmedchem.9b00995 BindingDB Entry DOI: 10.7270/Q2G44TRN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50032675 (4-[1-(3,5,5,8,8-pentamethyl-5,6,7,8-tetrahydronaph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Graduate School of Medicine Curated by ChEMBL | Assay Description Displacement of 9-cis-[11,12-3H]-retinoic acid from human RXRalpha LBD incubated for overnight by scintillation counting method | J Med Chem 62: 8809-8818 (2019) Article DOI: 10.1021/acs.jmedchem.9b00995 BindingDB Entry DOI: 10.7270/Q2G44TRN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50339081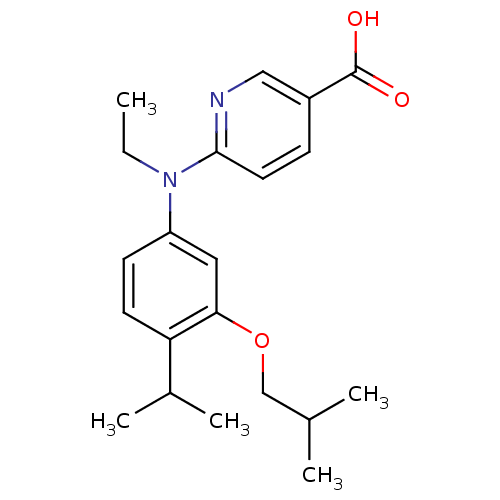 (6-[Ethyl-(3-isobutoxy-4-isopropylphenyl)amino]nico...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 182 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Graduate School of Medicine Curated by ChEMBL | Assay Description Displacement of CU-6PMN from human RXRalpha LBD incubated for 2 hrs by fluorescence based assay | J Med Chem 62: 8809-8818 (2019) Article DOI: 10.1021/acs.jmedchem.9b00995 BindingDB Entry DOI: 10.7270/Q2G44TRN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50339081 (6-[Ethyl-(3-isobutoxy-4-isopropylphenyl)amino]nico...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 182 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Graduate School of Medicine Curated by ChEMBL | Assay Description Displacement of CU-6PMN from human RXRalpha LBD incubated for 2 hrs by fluorescence based assay | J Med Chem 62: 8809-8818 (2019) Article DOI: 10.1021/acs.jmedchem.9b00995 BindingDB Entry DOI: 10.7270/Q2G44TRN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50530499 (CHEMBL4449685) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Graduate School of Medicine Curated by ChEMBL | Assay Description Displacement of 9-cis-[11,12-3H]-retinoic acid from human RXRalpha LBD incubated for overnight by scintillation counting method | J Med Chem 62: 8809-8818 (2019) Article DOI: 10.1021/acs.jmedchem.9b00995 BindingDB Entry DOI: 10.7270/Q2G44TRN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50530499 (CHEMBL4449685) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Graduate School of Medicine Curated by ChEMBL | Assay Description Displacement of 9-cis-[11,12-3H]-retinoic acid from human RXRalpha LBD incubated for overnight by scintillation counting method | J Med Chem 62: 8809-8818 (2019) Article DOI: 10.1021/acs.jmedchem.9b00995 BindingDB Entry DOI: 10.7270/Q2G44TRN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50032675 (4-[1-(3,5,5,8,8-pentamethyl-5,6,7,8-tetrahydronaph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | 379 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Graduate School of Medicine Curated by ChEMBL | Assay Description Displacement of CU-6PMN from human RXRalpha LBD incubated for 2 hrs by fluorescence based assay | J Med Chem 62: 8809-8818 (2019) Article DOI: 10.1021/acs.jmedchem.9b00995 BindingDB Entry DOI: 10.7270/Q2G44TRN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50032675 (4-[1-(3,5,5,8,8-pentamethyl-5,6,7,8-tetrahydronaph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | 379 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Graduate School of Medicine Curated by ChEMBL | Assay Description Displacement of CU-6PMN from human RXRalpha LBD incubated for 2 hrs by fluorescence based assay | J Med Chem 62: 8809-8818 (2019) Article DOI: 10.1021/acs.jmedchem.9b00995 BindingDB Entry DOI: 10.7270/Q2G44TRN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM31892 (9-cis retinoic acid | 9C-RA | CHEMBL705 | Panretin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | 583 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Graduate School of Medicine Curated by ChEMBL | Assay Description Displacement of CU-6PMN from human RXRalpha LBD incubated for 2 hrs by fluorescence based assay | J Med Chem 62: 8809-8818 (2019) Article DOI: 10.1021/acs.jmedchem.9b00995 BindingDB Entry DOI: 10.7270/Q2G44TRN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM31892 (9-cis retinoic acid | 9C-RA | CHEMBL705 | Panretin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | 583 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Graduate School of Medicine Curated by ChEMBL | Assay Description Displacement of CU-6PMN from human RXRalpha LBD incubated for 2 hrs by fluorescence based assay | J Med Chem 62: 8809-8818 (2019) Article DOI: 10.1021/acs.jmedchem.9b00995 BindingDB Entry DOI: 10.7270/Q2G44TRN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50210259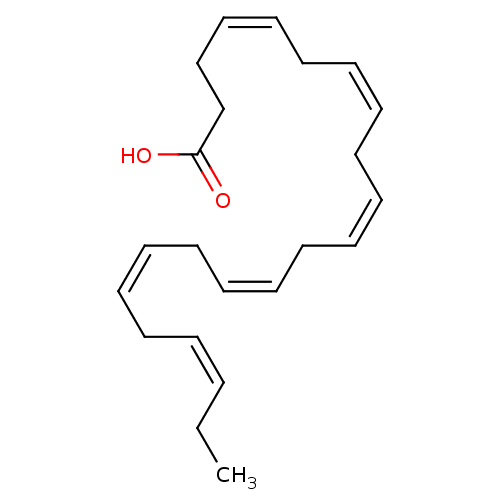 ((4Z,7Z,10Z,13Z,16Z,19Z)-docosa-4,7,10,13,16,19-hex...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid UniChem Patents | Article PubMed | 6.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Graduate School of Medicine Curated by ChEMBL | Assay Description Displacement of CU-6PMN from human RXRalpha LBD incubated for 2 hrs by fluorescence based assay | J Med Chem 62: 8809-8818 (2019) Article DOI: 10.1021/acs.jmedchem.9b00995 BindingDB Entry DOI: 10.7270/Q2G44TRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50210259 ((4Z,7Z,10Z,13Z,16Z,19Z)-docosa-4,7,10,13,16,19-hex...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid UniChem Patents | Article PubMed | 6.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Graduate School of Medicine Curated by ChEMBL | Assay Description Displacement of CU-6PMN from human RXRalpha LBD incubated for 2 hrs by fluorescence based assay | J Med Chem 62: 8809-8818 (2019) Article DOI: 10.1021/acs.jmedchem.9b00995 BindingDB Entry DOI: 10.7270/Q2G44TRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50242349 ((5Z,8Z,11Z,14Z,17Z)-5,8,11,14,17-eicosapentaenoic ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 7.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Graduate School of Medicine Curated by ChEMBL | Assay Description Displacement of CU-6PMN from human RXRalpha LBD incubated for 2 hrs by fluorescence based assay | J Med Chem 62: 8809-8818 (2019) Article DOI: 10.1021/acs.jmedchem.9b00995 BindingDB Entry DOI: 10.7270/Q2G44TRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50242349 ((5Z,8Z,11Z,14Z,17Z)-5,8,11,14,17-eicosapentaenoic ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 7.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Graduate School of Medicine Curated by ChEMBL | Assay Description Displacement of CU-6PMN from human RXRalpha LBD incubated for 2 hrs by fluorescence based assay | J Med Chem 62: 8809-8818 (2019) Article DOI: 10.1021/acs.jmedchem.9b00995 BindingDB Entry DOI: 10.7270/Q2G44TRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50038898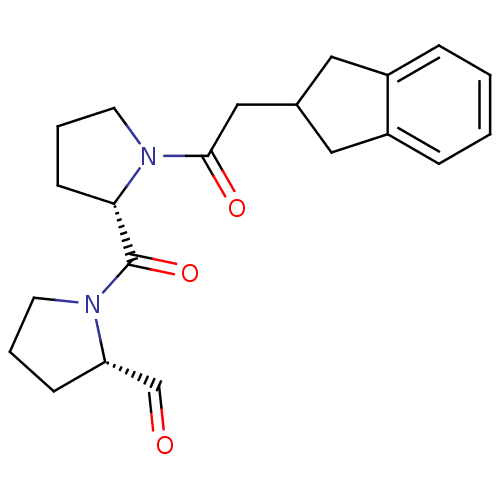 ((S)-1-((S)-1-(2-(2,3-dihydro-1H-inden-2-yl)acetyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeria Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory activity against purified prolyl endopeptidase (PEP) from canine brain. | J Med Chem 37: 2071-8 (1994) BindingDB Entry DOI: 10.7270/Q270822J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50038897 ((S)-1-[(S)-1-((S)-2-1,2,3,4-Tetrahydro-naphthalen-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeria Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory activity against purified prolyl endopeptidase (PEP) from canine brain. | J Med Chem 37: 2071-8 (1994) BindingDB Entry DOI: 10.7270/Q270822J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50038899 ((S)-1-[(R)-3-((S)-2-1,2,3,4-Tetrahydro-naphthalen-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeria Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory activity against purified prolyl endopeptidase (PEP) from canine brain. | J Med Chem 37: 2071-8 (1994) BindingDB Entry DOI: 10.7270/Q270822J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50038891 ((S)-1-[(S)-1-((S)-2-1,2,3,4-Tetrahydro-naphthalen-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeria Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory activity against purified prolyl endopeptidase (PEP) from canine brain. | J Med Chem 37: 2071-8 (1994) BindingDB Entry DOI: 10.7270/Q270822J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50038893 ((S)-1-[(R)-3-((S)-2-1,2,3,4-Tetrahydro-naphthalen-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeria Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory activity against purified prolyl endopeptidase (PEP) from canine brain. | J Med Chem 37: 2071-8 (1994) BindingDB Entry DOI: 10.7270/Q270822J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50038868 ((R)-3-[(R)-3-((S)-2-1,2,3,4-Tetrahydro-naphthalen-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeria Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory activity against purified prolyl endopeptidase (PEP) from canine brain. | J Med Chem 37: 2071-8 (1994) BindingDB Entry DOI: 10.7270/Q270822J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50038876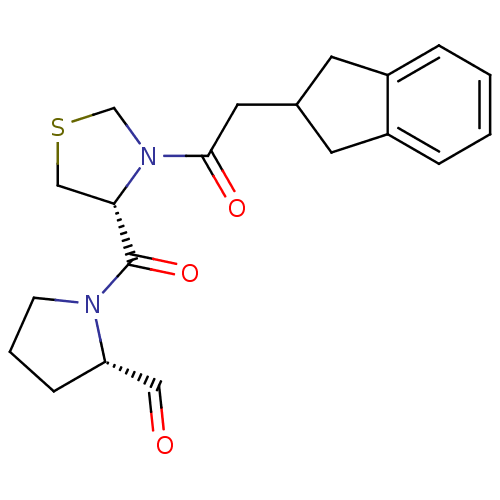 ((S)-1-[(R)-3-(2-Indan-2-yl-acetyl)-thiazolidine-4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeria Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory activity against purified prolyl endopeptidase (PEP) from canine brain. | J Med Chem 37: 2071-8 (1994) BindingDB Entry DOI: 10.7270/Q270822J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50038879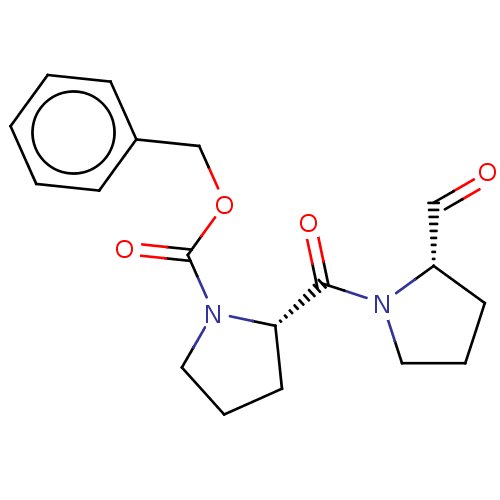 ((S)-2-(2-Formyl-pyrrolidine-1-carbonyl)-pyrrolidin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Similars | PDB PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeria Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory activity against purified prolyl endopeptidase (PEP) from canine brain. | J Med Chem 37: 2071-8 (1994) BindingDB Entry DOI: 10.7270/Q270822J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50038885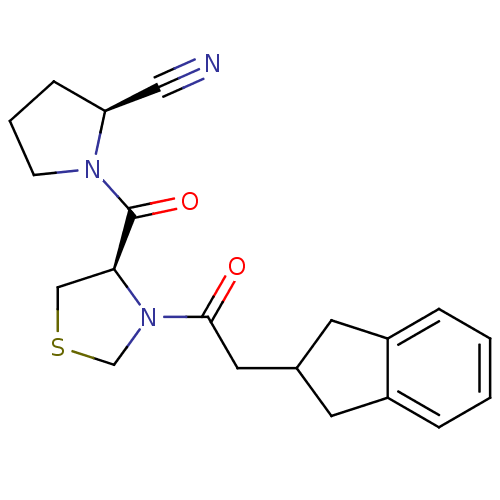 ((S)-1-[(R)-3-(2-Indan-2-yl-acetyl)-thiazolidine-4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeria Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory activity against purified prolyl endopeptidase (PEP) from canine brain. | J Med Chem 37: 2071-8 (1994) BindingDB Entry DOI: 10.7270/Q270822J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50038889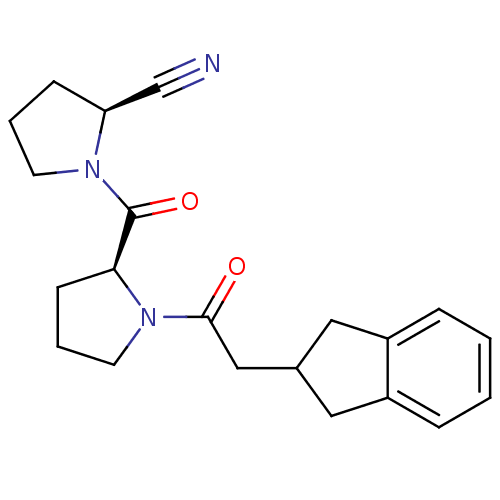 ((S)-1-((S)-1-(2-(2,3-dihydro-1H-inden-2-yl)acetyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeria Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory activity against purified prolyl endopeptidase (PEP) from canine brain. | J Med Chem 37: 2071-8 (1994) BindingDB Entry DOI: 10.7270/Q270822J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50038864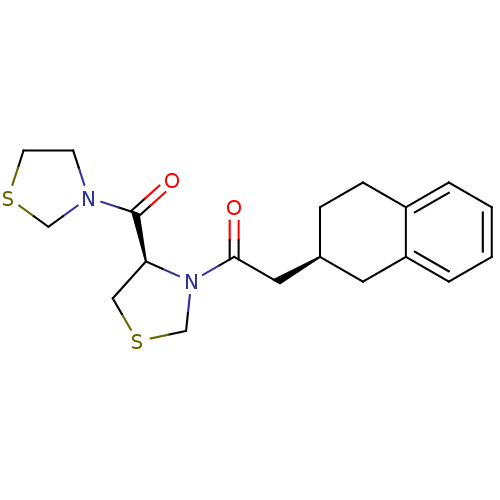 (2-(S)-1,2,3,4-Tetrahydro-naphthalen-2-yl-1-[(R)-4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeria Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory activity against purified prolyl endopeptidase (PEP) from canine brain. | J Med Chem 37: 2071-8 (1994) BindingDB Entry DOI: 10.7270/Q270822J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50038887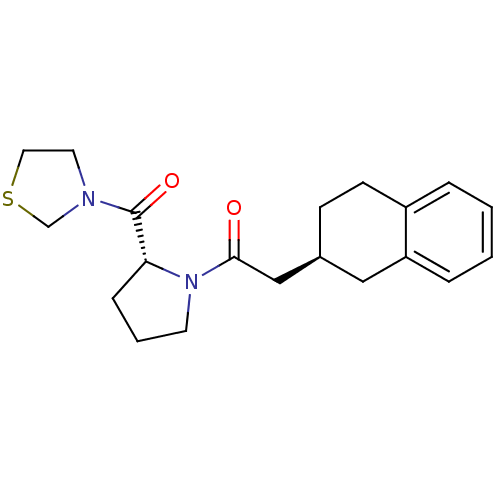 (2-(S)-1,2,3,4-Tetrahydro-naphthalen-2-yl-1-[(R)-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeria Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory activity against purified prolyl endopeptidase (PEP) from canine brain. | J Med Chem 37: 2071-8 (1994) BindingDB Entry DOI: 10.7270/Q270822J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50038895 (1-[(R)-4-(Pyrrolidine-1-carbonyl)-thiazolidin-3-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeria Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory activity against purified prolyl endopeptidase (PEP) from canine brain. | J Med Chem 37: 2071-8 (1994) BindingDB Entry DOI: 10.7270/Q270822J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50038883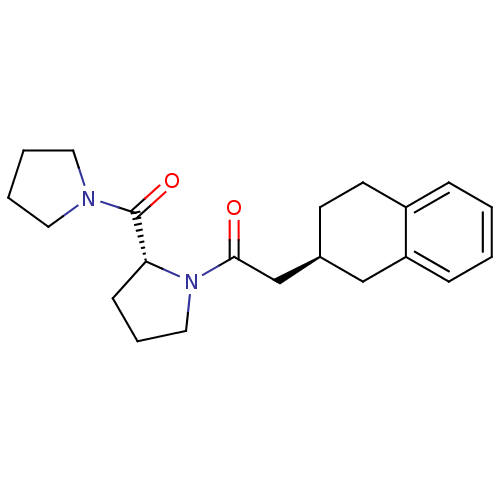 (1-[(R)-2-(Pyrrolidine-1-carbonyl)-pyrrolidin-1-yl]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeria Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory activity against purified prolyl endopeptidase (PEP) from canine brain. | J Med Chem 37: 2071-8 (1994) BindingDB Entry DOI: 10.7270/Q270822J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50038888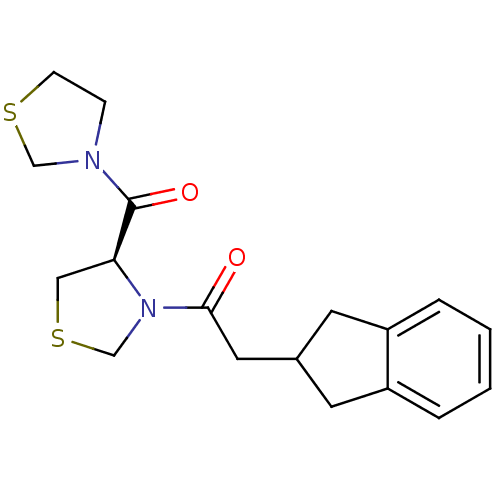 (2-Indan-2-yl-1-[(R)-4-(thiazolidine-3-carbonyl)-th...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeria Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory activity against purified prolyl endopeptidase (PEP) from canine brain. | J Med Chem 37: 2071-8 (1994) BindingDB Entry DOI: 10.7270/Q270822J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50038866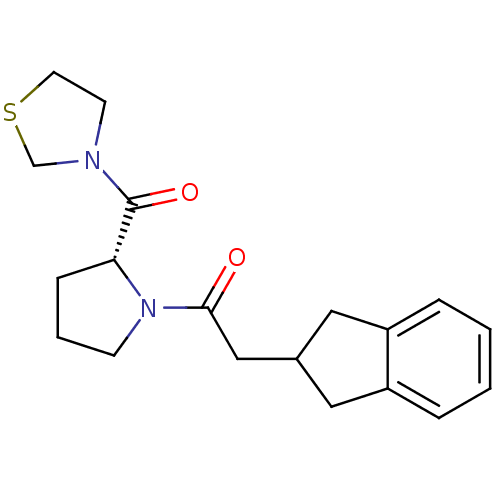 (2-Indan-2-yl-1-[(R)-2-(thiazolidine-3-carbonyl)-py...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeria Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory activity against purified prolyl endopeptidase (PEP) from canine brain. | J Med Chem 37: 2071-8 (1994) BindingDB Entry DOI: 10.7270/Q270822J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50038884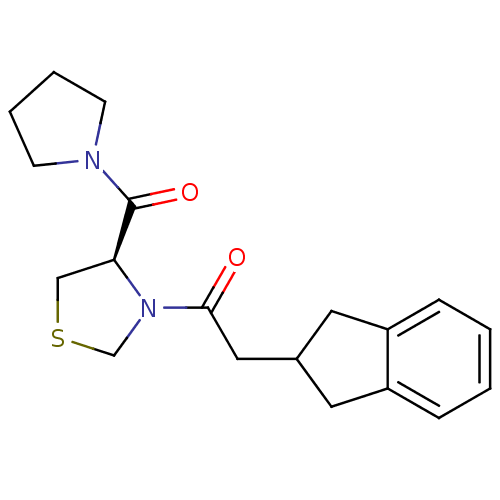 (2-Indan-2-yl-1-[(R)-4-(pyrrolidine-1-carbonyl)-thi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeria Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory activity against purified prolyl endopeptidase (PEP) from canine brain. | J Med Chem 37: 2071-8 (1994) BindingDB Entry DOI: 10.7270/Q270822J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50038894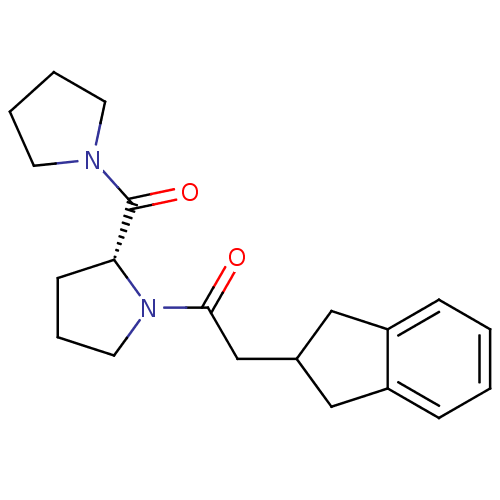 (2-Indan-2-yl-1-[(R)-2-(pyrrolidine-1-carbonyl)-pyr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeria Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory activity against purified prolyl endopeptidase (PEP) from canine brain. | J Med Chem 37: 2071-8 (1994) BindingDB Entry DOI: 10.7270/Q270822J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50038896 (1-[(R)-4-(Pyrrolidine-1-carbonyl)-thiazolidin-3-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeria Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory activity against purified prolyl endopeptidase (PEP) from canine brain. | J Med Chem 37: 2071-8 (1994) BindingDB Entry DOI: 10.7270/Q270822J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50038886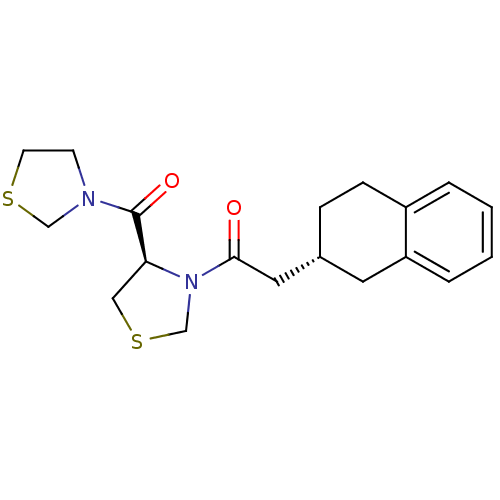 (2-(R)-1,2,3,4-Tetrahydro-naphthalen-2-yl-1-[(R)-4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeria Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory activity against purified prolyl endopeptidase (PEP) from canine brain. | J Med Chem 37: 2071-8 (1994) BindingDB Entry DOI: 10.7270/Q270822J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50038880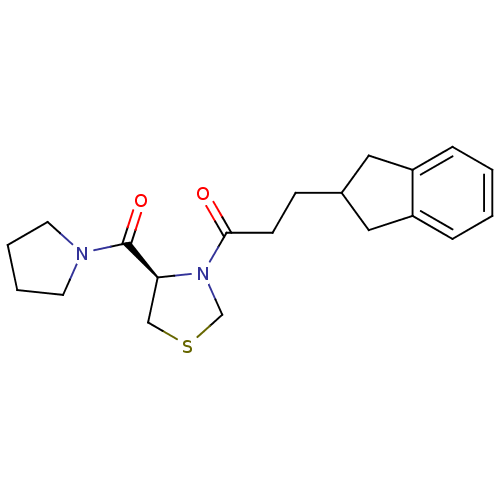 (3-Indan-2-yl-1-[(R)-4-(pyrrolidine-1-carbonyl)-thi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeria Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory activity against purified prolyl endopeptidase (PEP) from canine brain. | J Med Chem 37: 2071-8 (1994) BindingDB Entry DOI: 10.7270/Q270822J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50038890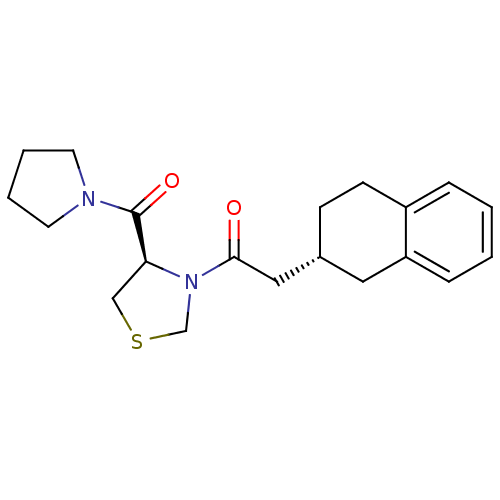 (1-[(R)-4-(Pyrrolidine-1-carbonyl)-thiazolidin-3-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeria Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory activity against purified prolyl endopeptidase (PEP) from canine brain. | J Med Chem 37: 2071-8 (1994) BindingDB Entry DOI: 10.7270/Q270822J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50038873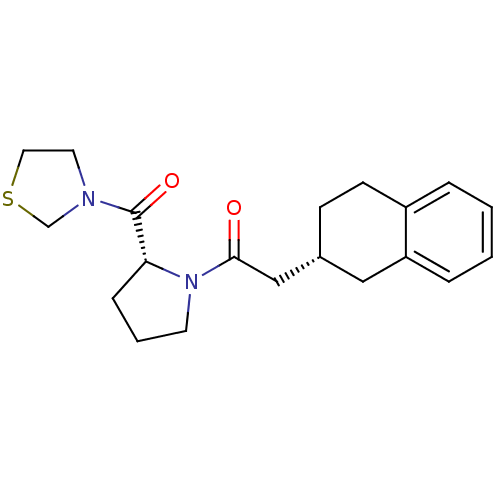 (2-(R)-1,2,3,4-Tetrahydro-naphthalen-2-yl-1-[(R)-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeria Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory activity against purified prolyl endopeptidase (PEP) from canine brain. | J Med Chem 37: 2071-8 (1994) BindingDB Entry DOI: 10.7270/Q270822J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50038871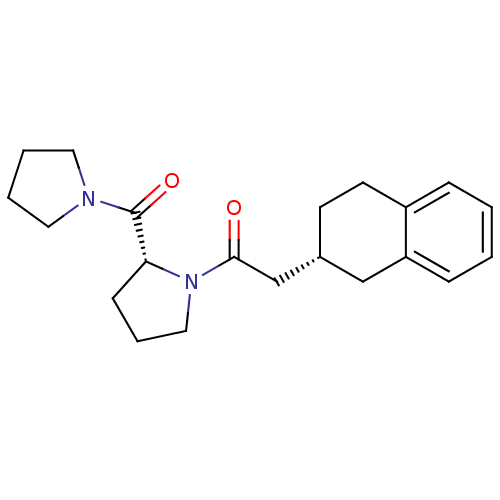 (1-[(R)-2-(Pyrrolidine-1-carbonyl)-pyrrolidin-1-yl]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeria Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory activity against purified prolyl endopeptidase (PEP) from canine brain. | J Med Chem 37: 2071-8 (1994) BindingDB Entry DOI: 10.7270/Q270822J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50051539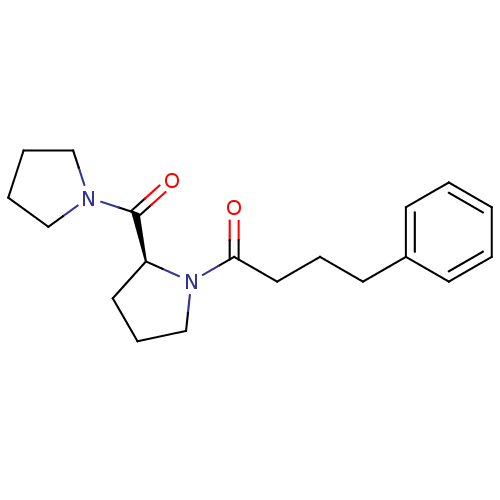 ((S)-4-phenyl-1-(2-(pyrrolidine-1-carbonyl)pyrrolid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeria Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory activity against purified prolyl endopeptidase (PEP) from canine brain. | J Med Chem 37: 2071-8 (1994) BindingDB Entry DOI: 10.7270/Q270822J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50038870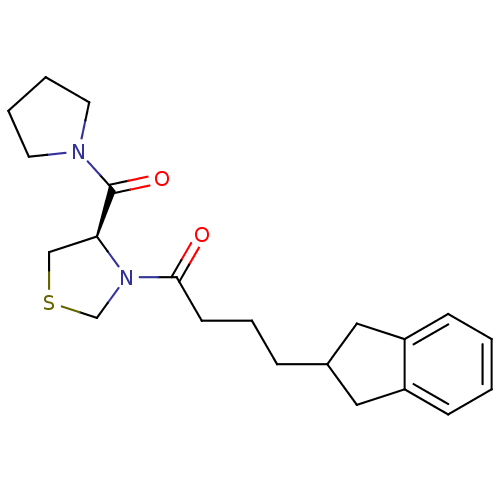 (4-Indan-2-yl-1-[(R)-4-(pyrrolidine-1-carbonyl)-thi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeria Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory activity against purified prolyl endopeptidase (PEP) from canine brain. | J Med Chem 37: 2071-8 (1994) BindingDB Entry DOI: 10.7270/Q270822J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50038892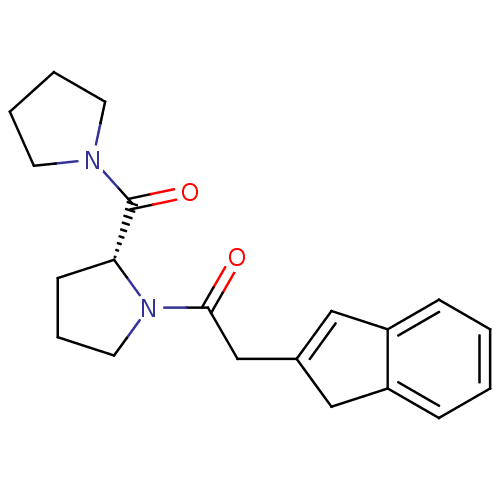 (2-(1H-Inden-2-yl)-1-[(R)-2-(pyrrolidine-1-carbonyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeria Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory activity against purified prolyl endopeptidase (PEP) from canine brain. | J Med Chem 37: 2071-8 (1994) BindingDB Entry DOI: 10.7270/Q270822J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50038869 (2-(Pyrrolidine-1-carbonyl)-pyrrolidine-1-carboxyli...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeria Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory activity against purified prolyl endopeptidase (PEP) from canine brain. | J Med Chem 37: 2071-8 (1994) BindingDB Entry DOI: 10.7270/Q270822J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50038867 (2-Benzo[b]thiophen-2-yl-1-[(R)-4-(pyrrolidine-1-ca...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeria Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory activity against purified prolyl endopeptidase (PEP) from canine brain. | J Med Chem 37: 2071-8 (1994) BindingDB Entry DOI: 10.7270/Q270822J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50038878 (1-[(R)-4-(Pyrrolidine-1-carbonyl)-thiazolidin-3-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeria Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory activity against purified prolyl endopeptidase (PEP) from canine brain. | J Med Chem 37: 2071-8 (1994) BindingDB Entry DOI: 10.7270/Q270822J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50038878 (1-[(R)-4-(Pyrrolidine-1-carbonyl)-thiazolidin-3-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeria Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory activity against purified prolyl endopeptidase (PEP) from canine brain. | J Med Chem 37: 2071-8 (1994) BindingDB Entry DOI: 10.7270/Q270822J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50038865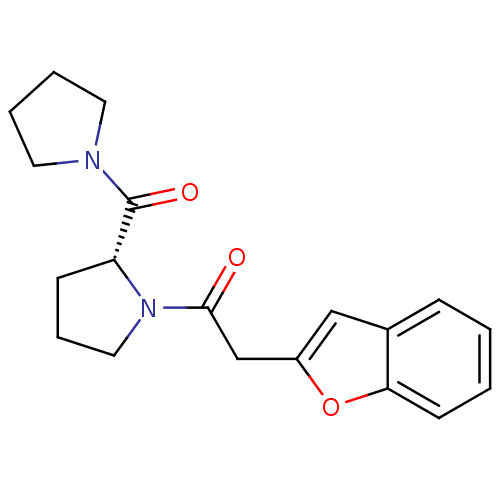 (2-Benzofuran-2-yl-1-[(R)-2-(pyrrolidine-1-carbonyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeria Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory activity against purified prolyl endopeptidase (PEP) from canine brain. | J Med Chem 37: 2071-8 (1994) BindingDB Entry DOI: 10.7270/Q270822J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50038874 (CHEMBL62629 | Indan-2-yl-[(R)-4-(pyrrolidine-1-car...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeria Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory activity against purified prolyl endopeptidase (PEP) from canine brain. | J Med Chem 37: 2071-8 (1994) BindingDB Entry DOI: 10.7270/Q270822J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50038881 (CHEMBL294803 | Y-29794 | [2-(8-Dimethylamino-octyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeria Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory activity against purified prolyl endopeptidase (PEP) from canine brain. | J Med Chem 37: 2071-8 (1994) BindingDB Entry DOI: 10.7270/Q270822J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50038872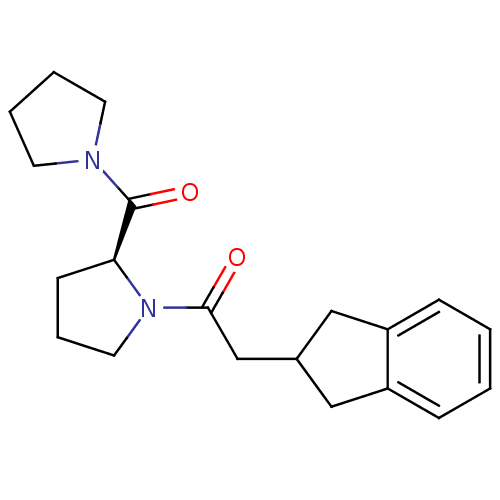 ((S)-2-(2,3-dihydro-1H-inden-2-yl)-1-(2-(pyrrolidin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeria Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory activity against purified prolyl endopeptidase (PEP) from canine brain. | J Med Chem 37: 2071-8 (1994) BindingDB Entry DOI: 10.7270/Q270822J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50038875 (1-((S)-2-(pyrrolidine-1-carbonyl)pyrrolidin-1-yl)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeria Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory activity against purified prolyl endopeptidase (PEP) from canine brain. | J Med Chem 37: 2071-8 (1994) BindingDB Entry DOI: 10.7270/Q270822J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 57 total ) | Next | Last >> |