

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thymidylate synthase (Mus musculus) | BDBM18771 ((2S)-2-[(4-{[(2-amino-4-oxo-1,4-dihydroquinazolin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Mereside Curated by ChEMBL | Assay Description Binding affinity against Thymidylate synthase was measured in vitro | J Med Chem 33: 3060-7 (1990) BindingDB Entry DOI: 10.7270/Q2ZW1JWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50006687 ((S)-2-(4-(((2-methyl-4-oxo-3,4-dihydroquinazolin-6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Mereside Curated by ChEMBL | Assay Description Binding affinity against Thymidylate synthase was measured in vitro | J Med Chem 33: 3060-7 (1990) BindingDB Entry DOI: 10.7270/Q2ZW1JWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50014480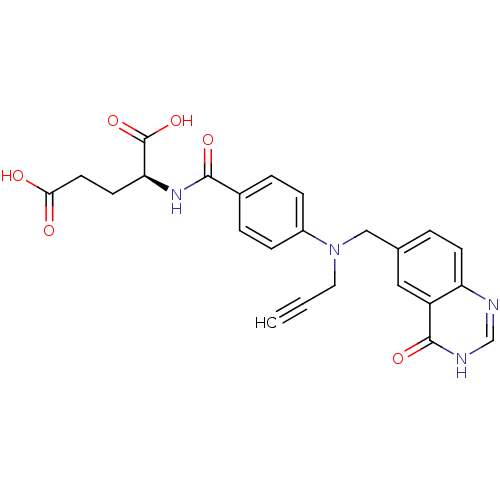 ((S)-2-(4-(((4-oxo-3,4-dihydroquinazolin-6-yl)methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Mereside Curated by ChEMBL | Assay Description Concentration required for in vitro inhibition of thymidylate synthase | J Med Chem 33: 3060-7 (1990) BindingDB Entry DOI: 10.7270/Q2ZW1JWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Rattus norvegicus (rat)) | BDBM18771 ((2S)-2-[(4-{[(2-amino-4-oxo-1,4-dihydroquinazolin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Mereside Curated by ChEMBL | Assay Description Inhibitory activity against Dihydrofolate reductase in rat liver | J Med Chem 33: 3060-7 (1990) BindingDB Entry DOI: 10.7270/Q2ZW1JWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Rattus norvegicus (rat)) | BDBM50014480 ((S)-2-(4-(((4-oxo-3,4-dihydroquinazolin-6-yl)methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Mereside Curated by ChEMBL | Assay Description Inhibitory activity against Dihydrofolate reductase in rat liver | J Med Chem 33: 3060-7 (1990) BindingDB Entry DOI: 10.7270/Q2ZW1JWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Rattus norvegicus (rat)) | BDBM50006687 ((S)-2-(4-(((2-methyl-4-oxo-3,4-dihydroquinazolin-6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >2.66E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Mereside Curated by ChEMBL | Assay Description Inhibitory activity against Dihydrofolate reductase in rat liver | J Med Chem 33: 3060-7 (1990) BindingDB Entry DOI: 10.7270/Q2ZW1JWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50033933 ((R)-{4-[(2,7-Dimethyl-4-oxo-3,4-dihydro-quinazolin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of thymidylate synthase purified from mouse L1210 leukemia cells that overproduce thymidylate synthase (TS) | J Med Chem 38: 994-1004 (1995) BindingDB Entry DOI: 10.7270/Q2J67FZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50033920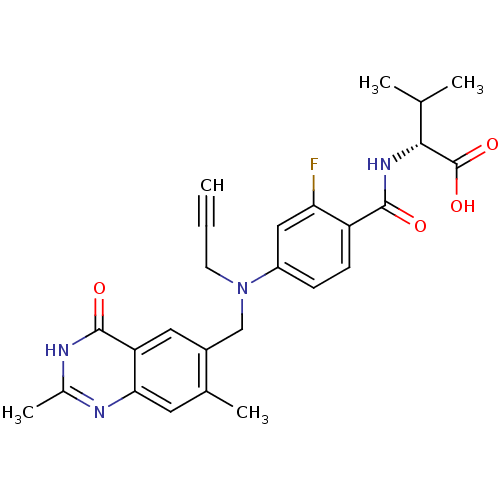 ((R)-2-{4-[(2,7-Dimethyl-4-oxo-3,4-dihydro-quinazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description Ability to inhibit Thymidylate synthase purified from mouse L1210 leukemia cells that overproduce thymidylate synthase (TS) | J Med Chem 38: 994-1004 (1995) BindingDB Entry DOI: 10.7270/Q2J67FZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50033901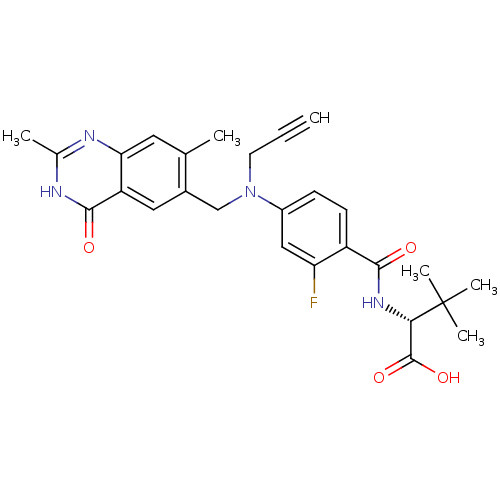 ((R)-2-{4-[(2,7-Dimethyl-4-oxo-3,4-dihydro-quinazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description Ability to inhibit Thymidylate synthase purified from mouse L1210 leukemia cells that overproduce thymidylate synthase (TS) | J Med Chem 38: 994-1004 (1995) BindingDB Entry DOI: 10.7270/Q2J67FZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50033913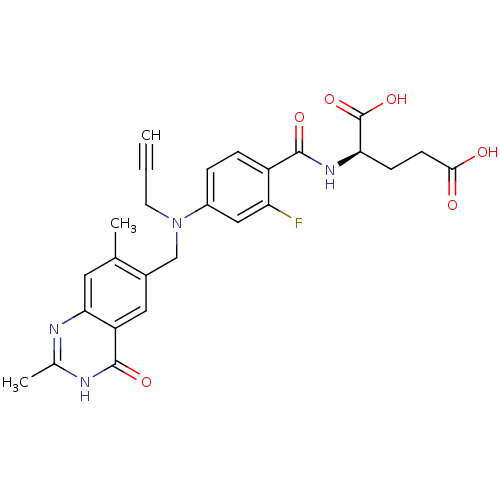 ((R)-2-{4-[(2,7-Dimethyl-4-oxo-3,4-dihydro-quinazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of thymidylate synthase purified from mouse L1210 leukemia cells that overproduce thymidylate synthase (TS) | J Med Chem 38: 994-1004 (1995) BindingDB Entry DOI: 10.7270/Q2J67FZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50014498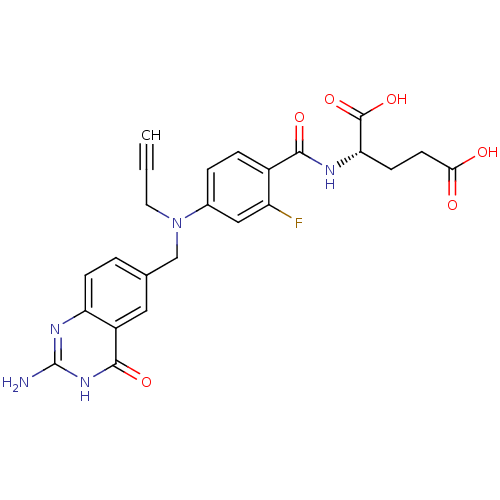 ((S)-2-(4-(((2-amino-4-oxo-3,4-dihydroquinazolin-6-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Concentration of the compound required to inhibit 50% activity of Thymidylate synthase was determined | J Med Chem 33: 3067-71 (1990) BindingDB Entry DOI: 10.7270/Q2V40T5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50011751 (2-{4-[(2-Amino-4-thioxo-3,4-dihydro-quinazolin-6-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Growth inhibition of thymidylate synthase(TS) in L1210 cells | J Med Chem 34: 978-84 (1991) BindingDB Entry DOI: 10.7270/Q2VD6XDB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM18771 ((2S)-2-[(4-{[(2-amino-4-oxo-1,4-dihydroquinazolin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Mereside Curated by ChEMBL | Assay Description Concentration required for in vitro inhibition of thymidylate synthase | J Med Chem 33: 3060-7 (1990) BindingDB Entry DOI: 10.7270/Q2ZW1JWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50006689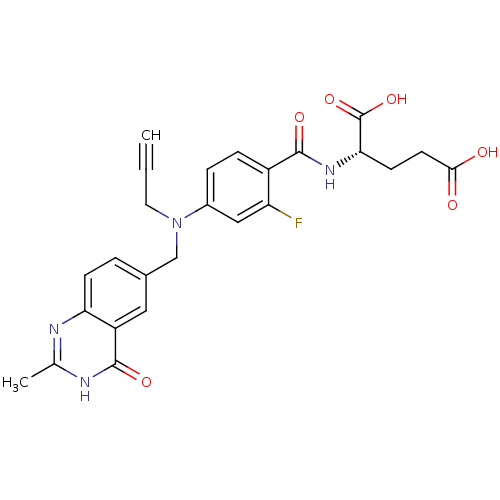 ((S)-2-(2-fluoro-4-(((2-methyl-4-oxo-3,4-dihydroqui...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Concentration of the compound required to inhibit 50% activity of Thymidylate synthase was determined | J Med Chem 33: 3067-71 (1990) BindingDB Entry DOI: 10.7270/Q2V40T5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50006689 ((S)-2-(2-fluoro-4-(((2-methyl-4-oxo-3,4-dihydroqui...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of thymidylate synthase from L1210 cells | J Med Chem 35: 2321-7 (1992) BindingDB Entry DOI: 10.7270/Q2XW4HRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50006689 ((S)-2-(2-fluoro-4-(((2-methyl-4-oxo-3,4-dihydroqui...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of partially purified thymidylate synthase (TS) | J Med Chem 33: 3072-8 (1990) BindingDB Entry DOI: 10.7270/Q2QC02G1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM18771 ((2S)-2-[(4-{[(2-amino-4-oxo-1,4-dihydroquinazolin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against thymidylate synthase (TS) from L1210 mouse leukemia cells | J Med Chem 35: 2761-8 (1992) BindingDB Entry DOI: 10.7270/Q2K9385P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM18771 ((2S)-2-[(4-{[(2-amino-4-oxo-1,4-dihydroquinazolin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Concentration of the compound required to inhibit 50% activity of Thymidylate synthase was determined | J Med Chem 33: 3067-71 (1990) BindingDB Entry DOI: 10.7270/Q2V40T5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50004387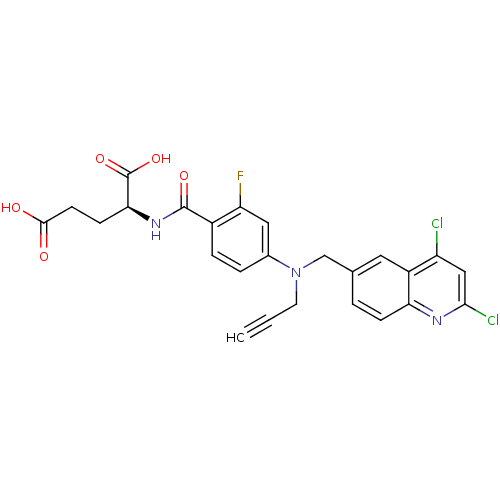 (2-{4-[(2,4-Dichloro-quinolin-6-ylmethyl)-prop-2-yn...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against thymidylate synthase from L1210 mouse leukemia cells | J Med Chem 35: 2761-8 (1992) BindingDB Entry DOI: 10.7270/Q2K9385P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50033921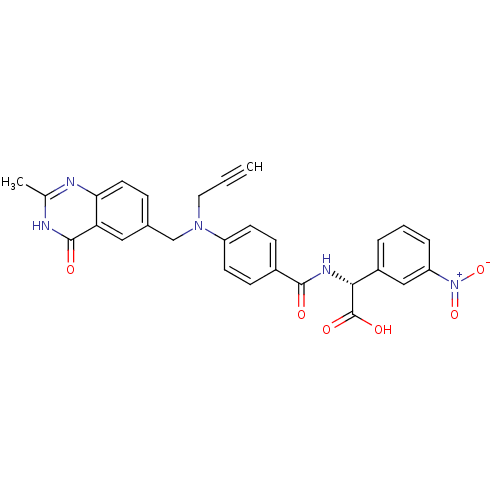 ((R)-{4-[(2-Methyl-4-oxo-3,4-dihydro-quinazolin-6-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of thymidylate synthase purified from mouse L1210 leukemia cells that overproduce thymidylate synthase (TS) | J Med Chem 38: 994-1004 (1995) BindingDB Entry DOI: 10.7270/Q2J67FZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50033902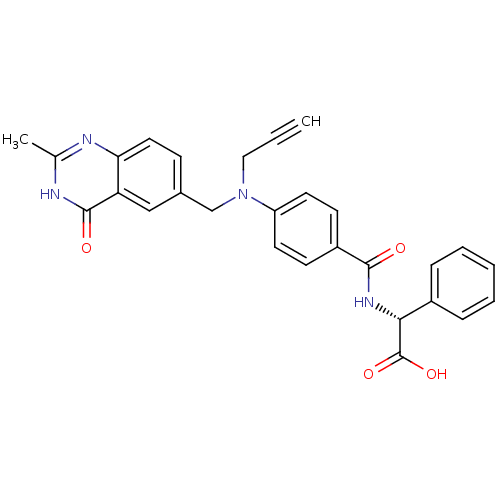 ((R)-{4-[(2-Methyl-4-oxo-3,4-dihydro-quinazolin-6-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of thymidylate synthase purified from mouse L1210 leukemia cells that overproduce thymidylate synthase (TS) | J Med Chem 38: 994-1004 (1995) BindingDB Entry DOI: 10.7270/Q2J67FZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50014496 ((S)-2-(2-fluoro-4-(((2-methoxy-4-oxo-3,4-dihydroqu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Concentration of the compound required to inhibit 50% activity of Thymidylate synthase was determined | J Med Chem 33: 3067-71 (1990) BindingDB Entry DOI: 10.7270/Q2V40T5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50006687 ((S)-2-(4-(((2-methyl-4-oxo-3,4-dihydroquinazolin-6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Mereside Curated by ChEMBL | Assay Description Binding affinity against Thymidylate synthase was measured in vitro | J Med Chem 33: 3060-7 (1990) BindingDB Entry DOI: 10.7270/Q2ZW1JWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50006687 ((S)-2-(4-(((2-methyl-4-oxo-3,4-dihydroquinazolin-6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of thymidylate synthase from L1210 cells | J Med Chem 35: 2321-7 (1992) BindingDB Entry DOI: 10.7270/Q2XW4HRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50012036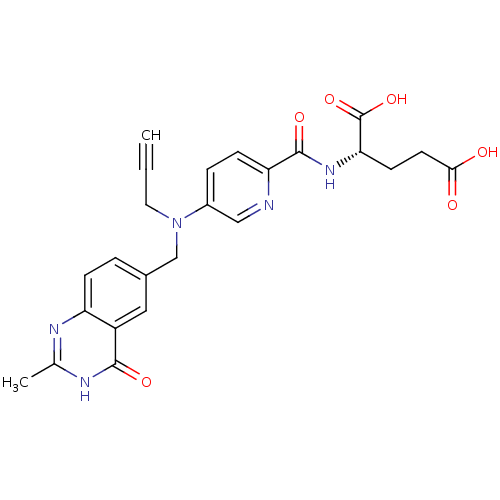 ((S)-2-(5-(((2-methyl-4-oxo-3,4-dihydroquinazolin-6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition of partially purified L1210 thymidylate synthase (TS). | J Med Chem 34: 1594-605 (1991) BindingDB Entry DOI: 10.7270/Q22N52VQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50006687 ((S)-2-(4-(((2-methyl-4-oxo-3,4-dihydroquinazolin-6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition of partially purified L1210 thymidylate synthase (TS). | J Med Chem 34: 1594-605 (1991) BindingDB Entry DOI: 10.7270/Q22N52VQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50033900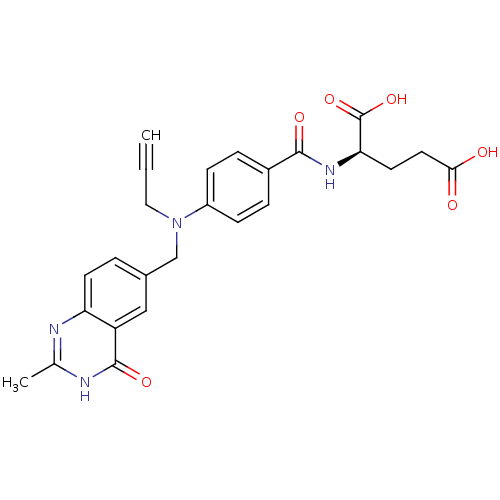 ((R)-2-{4-[(2-Methyl-4-oxo-3,4-dihydro-quinazolin-6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of thymidylate synthase purified from mouse L1210 leukemia cells that overproduce thymidylate synthase (TS) | J Med Chem 38: 994-1004 (1995) BindingDB Entry DOI: 10.7270/Q2J67FZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50006687 ((S)-2-(4-(((2-methyl-4-oxo-3,4-dihydroquinazolin-6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition of the compound against L1210 thymidylate synthase (TS) | J Med Chem 34: 2209-18 (1991) BindingDB Entry DOI: 10.7270/Q2D799D8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50006687 ((S)-2-(4-(((2-methyl-4-oxo-3,4-dihydroquinazolin-6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of partially purified thymidylate synthase (TS) | J Med Chem 33: 3072-8 (1990) BindingDB Entry DOI: 10.7270/Q2QC02G1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50033927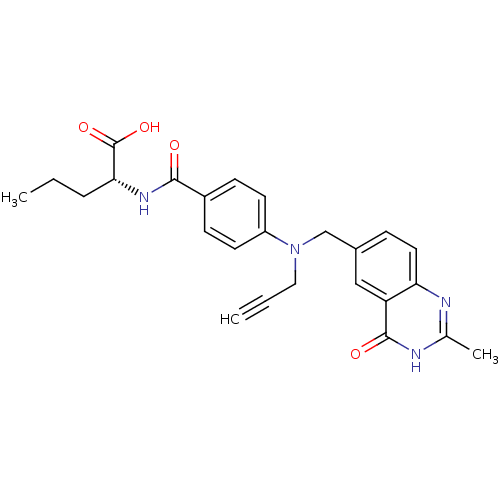 ((R)-2-{4-[(2-Methyl-4-oxo-3,4-dihydro-quinazolin-6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of thymidylate synthase purified from mouse L1210 leukemia cells that overproduce thymidylate synthase (TS) | J Med Chem 38: 994-1004 (1995) BindingDB Entry DOI: 10.7270/Q2J67FZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50033930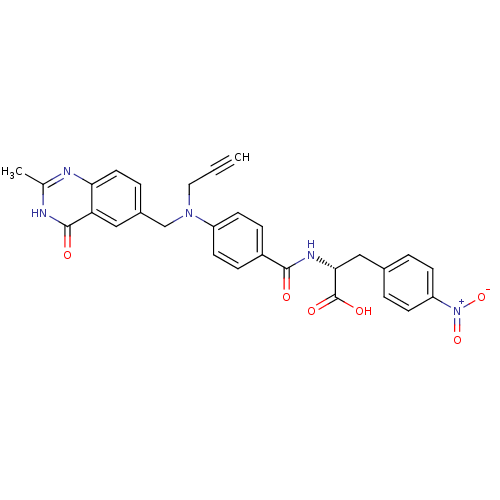 ((R)-2-{4-[(2-Methyl-4-oxo-3,4-dihydro-quinazolin-6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of thymidylate synthase purified from mouse L1210 leukemia cells that overproduce thymidylate synthase (TS) | J Med Chem 38: 994-1004 (1995) BindingDB Entry DOI: 10.7270/Q2J67FZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50014501 ((S)-2-(2-fluoro-4-((2-fluoroethyl)((2-methyl-4-oxo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of partially purified thymidylate synthase (TS) | J Med Chem 33: 3072-8 (1990) BindingDB Entry DOI: 10.7270/Q2QC02G1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50033910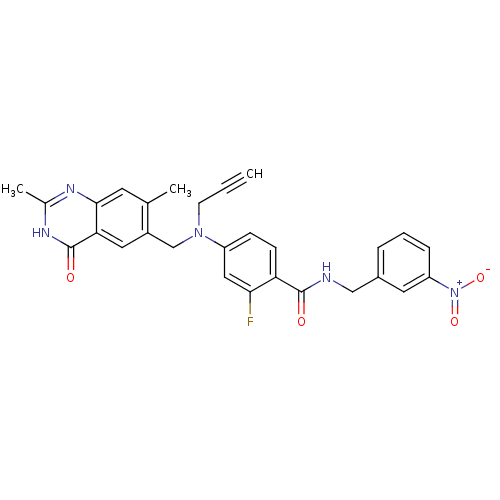 (4-[(2,7-Dimethyl-4-oxo-3,4-dihydro-quinazolin-6-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of thymidylate synthase purified from mouse L1210 leukemia cells that overproduce thymidylate synthase (TS) | J Med Chem 38: 994-1004 (1995) BindingDB Entry DOI: 10.7270/Q2J67FZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50014506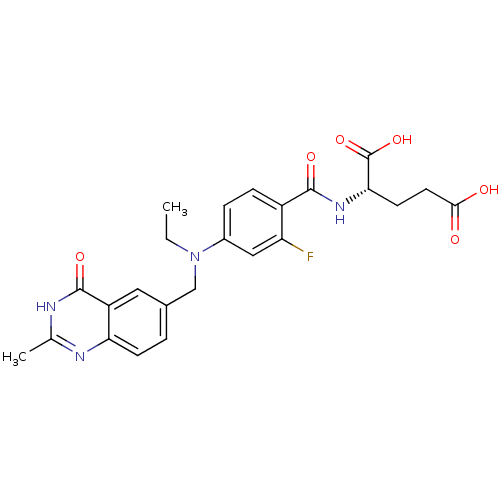 ((S)-2-(4-(ethyl((2-methyl-4-oxo-3,4-dihydroquinazo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of partially purified thymidylate synthase (TS) | J Med Chem 33: 3072-8 (1990) BindingDB Entry DOI: 10.7270/Q2QC02G1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50033899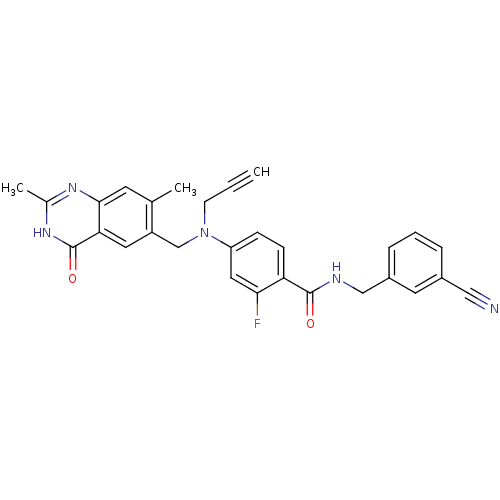 (CHEMBL264095 | N-(3-Cyano-benzyl)-4-[(2,7-dimethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description Ability to inhibit Thymidylate synthase purified from mouse L1210 leukemia cells that overproduce thymidylate synthase (TS) | J Med Chem 38: 994-1004 (1995) BindingDB Entry DOI: 10.7270/Q2J67FZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50004381 (2-{4-[(2-Amino-4-chloro-quinolin-6-ylmethyl)-prop-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against thymidylate synthase from L1210 mouse leukemia cells | J Med Chem 35: 2761-8 (1992) BindingDB Entry DOI: 10.7270/Q2K9385P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50006687 ((S)-2-(4-(((2-methyl-4-oxo-3,4-dihydroquinazolin-6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Concentration of the compound required to inhibit 50% activity of Thymidylate synthase was determined | J Med Chem 33: 3067-71 (1990) BindingDB Entry DOI: 10.7270/Q2V40T5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50011752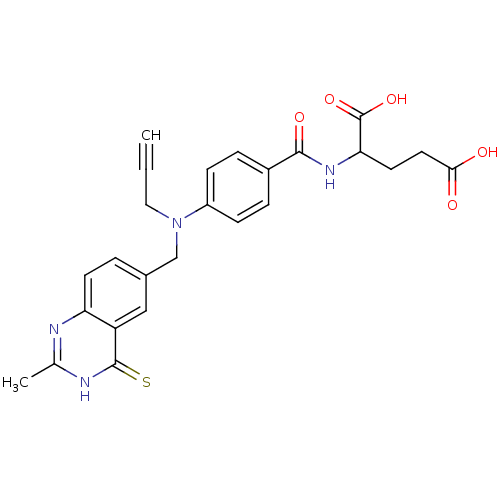 (2-{4-[(2-Methyl-4-thioxo-3,4-dihydro-quinazolin-6-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Growth inhibition of thymidylate synthase(TS) in L1210 cells | J Med Chem 34: 978-84 (1991) BindingDB Entry DOI: 10.7270/Q2VD6XDB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50033937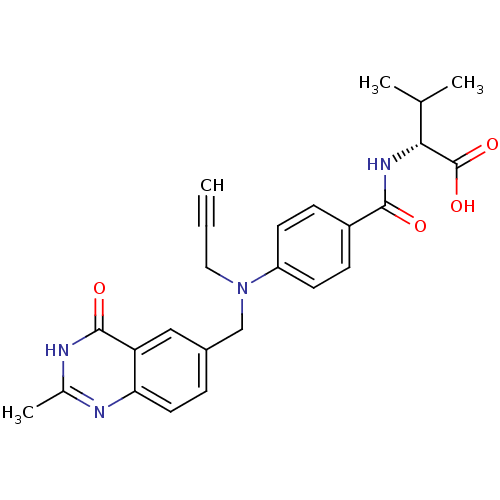 ((R)-3-Methyl-2-{4-[(2-methyl-4-oxo-3,4-dihydro-qui...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of thymidylate synthase purified from mouse L1210 leukemia cells that overproduce thymidylate synthase (TS) | J Med Chem 38: 994-1004 (1995) BindingDB Entry DOI: 10.7270/Q2J67FZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50033898 ((R)-3,3-Dimethyl-2-{4-[(2-methyl-4-oxo-3,4-dihydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of thymidylate synthase purified from mouse L1210 leukemia cells that overproduce thymidylate synthase (TS) | J Med Chem 38: 994-1004 (1995) BindingDB Entry DOI: 10.7270/Q2J67FZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50014497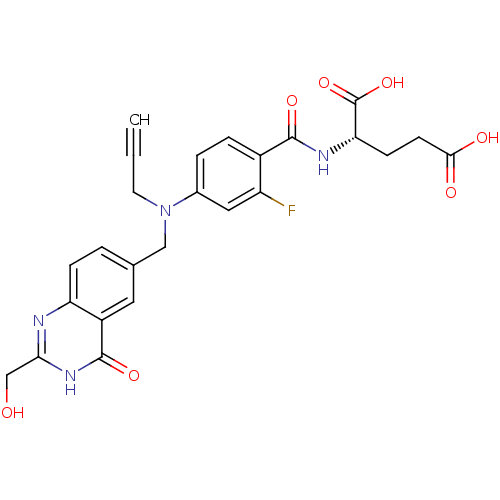 ((S)-2-(2-fluoro-4-(((2-(hydroxymethyl)-4-oxo-3,4-d...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Concentration of the compound required to inhibit 50% activity of Thymidylate synthase was determined | J Med Chem 33: 3067-71 (1990) BindingDB Entry DOI: 10.7270/Q2V40T5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50014494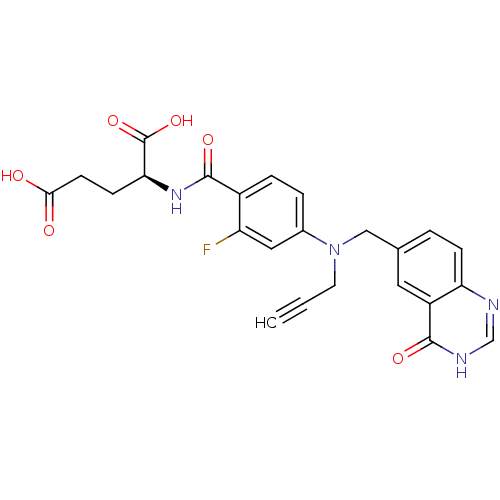 ((S)-2-(2-fluoro-4-(((4-oxo-3,4-dihydroquinazolin-6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Concentration of the compound required to inhibit 50% activity of Thymidylate synthase was determined | J Med Chem 33: 3067-71 (1990) BindingDB Entry DOI: 10.7270/Q2V40T5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50004395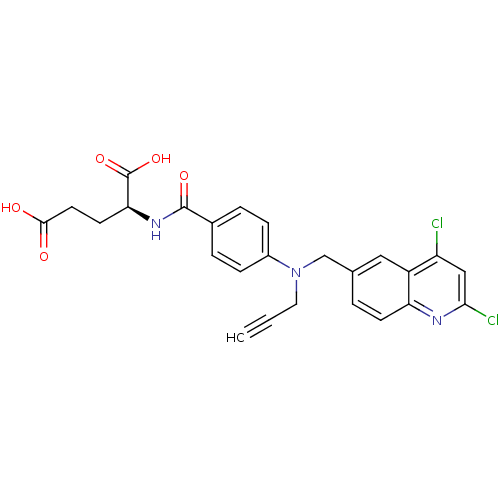 (2-{4-[(2,4-Dichloro-quinolin-6-ylmethyl)-prop-2-yn...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against thymidylate synthase from L1210 mouse leukemia cells | J Med Chem 35: 2761-8 (1992) BindingDB Entry DOI: 10.7270/Q2K9385P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50004372 (2-{4-[(4-Chloro-2-methyl-quinolin-6-ylmethyl)-prop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against thymidylate synthase from L1210 mouse leukemia cells | J Med Chem 35: 2761-8 (1992) BindingDB Entry DOI: 10.7270/Q2K9385P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50033928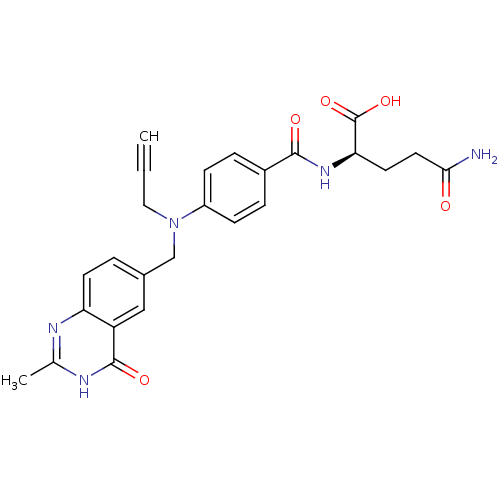 ((R)-4-Carbamoyl-2-{4-[(2-methyl-4-oxo-3,4-dihydro-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of thymidylate synthase purified from mouse L1210 leukemia cells that overproduce thymidylate synthase (TS) | J Med Chem 38: 994-1004 (1995) BindingDB Entry DOI: 10.7270/Q2J67FZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50014518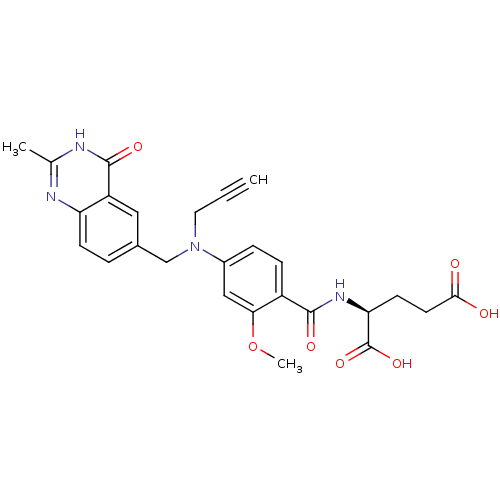 ((S)-2-(2-methoxy-4-(((2-methyl-4-oxo-3,4-dihydroqu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of partially purified thymidylate synthase (TS) | J Med Chem 33: 3072-8 (1990) BindingDB Entry DOI: 10.7270/Q2QC02G1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50014495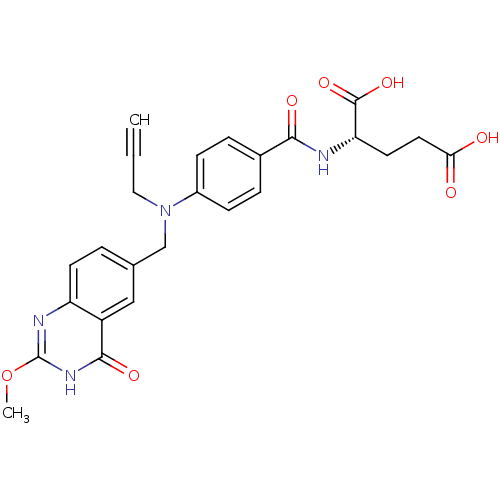 ((S)-2-(4-(((2-methoxy-4-oxo-3,4-dihydroquinazolin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Concentration of the compound required to inhibit 50% activity of Thymidylate synthase was determined | J Med Chem 33: 3067-71 (1990) BindingDB Entry DOI: 10.7270/Q2V40T5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50014514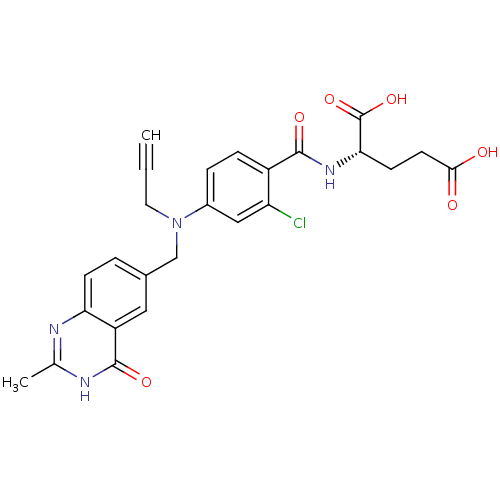 ((S)-2-(2-chloro-4-(((2-methyl-4-oxo-3,4-dihydroqui...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of partially purified thymidylate synthase (TS) | J Med Chem 33: 3072-8 (1990) BindingDB Entry DOI: 10.7270/Q2QC02G1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50014516 ((S)-2-(4-(allyl((2-methyl-4-oxo-3,4-dihydroquinazo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of partially purified thymidylate synthase (TS) | J Med Chem 33: 3072-8 (1990) BindingDB Entry DOI: 10.7270/Q2QC02G1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50014505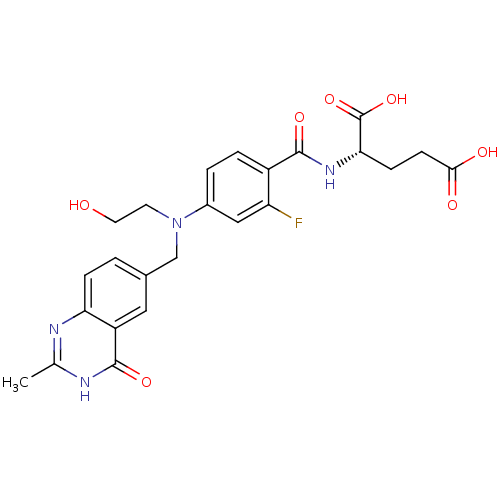 ((S)-2-(2-fluoro-4-((2-hydroxyethyl)((2-methyl-4-ox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of partially purified thymidylate synthase (TS) | J Med Chem 33: 3072-8 (1990) BindingDB Entry DOI: 10.7270/Q2QC02G1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 242 total ) | Next | Last >> |