

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM29061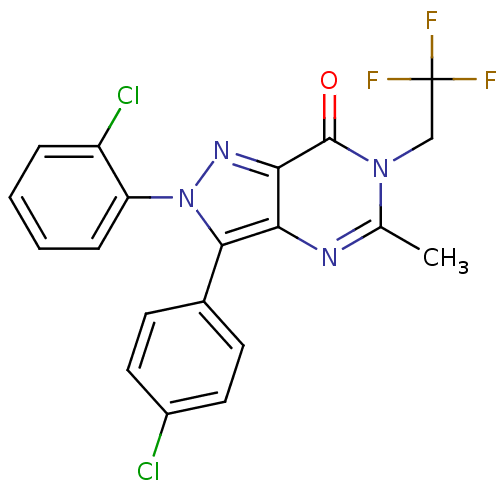 (CHEMBL201602 | pyrazolopyrimidinone-based antagoni...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.600 | -53.5 | n/a | n/a | 1 | n/a | n/a | 7.4 | 30 |
Pfizer | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 52: 2652-5 (2009) Article DOI: 10.1021/jm900255t BindingDB Entry DOI: 10.7270/Q2F47MGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM21278 (5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.650 | -53.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 52: 234-7 (2009) Article DOI: 10.1021/jm8012932 BindingDB Entry DOI: 10.7270/Q2CN7276 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM27337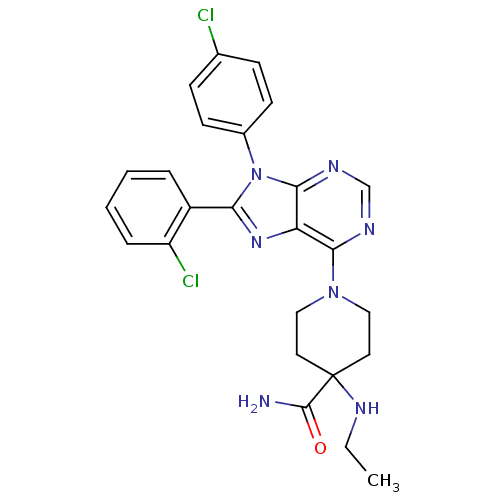 (1-[8-(2-chlorophenyl)-9-(4-chlorophenyl)-9H-purin-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.700 | -53.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 52: 234-7 (2009) Article DOI: 10.1021/jm8012932 BindingDB Entry DOI: 10.7270/Q2CN7276 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM29070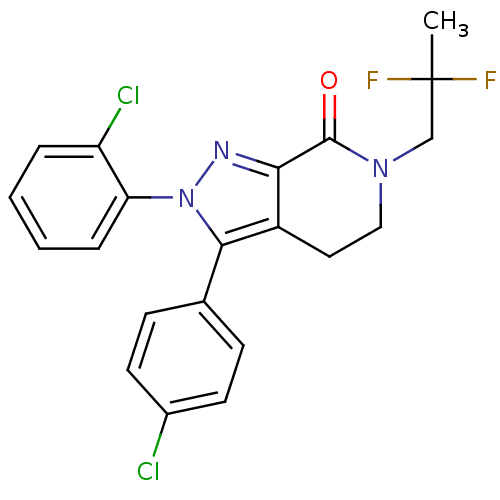 (lactam-based compound, 12i) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.700 | -53.1 | n/a | n/a | 0.800 | n/a | n/a | 7.4 | 30 |
Pfizer | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 52: 2652-5 (2009) Article DOI: 10.1021/jm900255t BindingDB Entry DOI: 10.7270/Q2F47MGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21278 (5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 0.900 | -52.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 52: 234-7 (2009) Article DOI: 10.1021/jm8012932 BindingDB Entry DOI: 10.7270/Q2CN7276 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM29075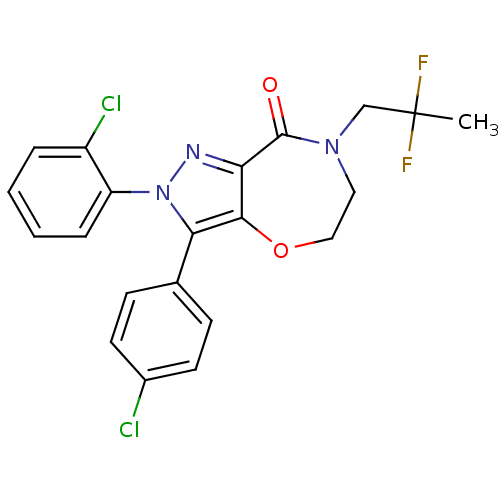 (PF-514273) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | -52.2 | n/a | n/a | 0.820 | n/a | n/a | 7.4 | 30 |
Pfizer | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 52: 2652-5 (2009) Article DOI: 10.1021/jm900255t BindingDB Entry DOI: 10.7270/Q2F47MGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM29073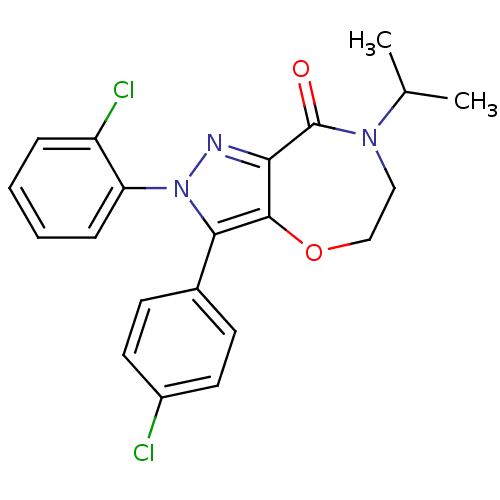 (ether-based lactam, 19b) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.30 | -51.6 | n/a | n/a | 4.80 | n/a | n/a | 7.4 | 30 |
Pfizer | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 52: 2652-5 (2009) Article DOI: 10.1021/jm900255t BindingDB Entry DOI: 10.7270/Q2F47MGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM29076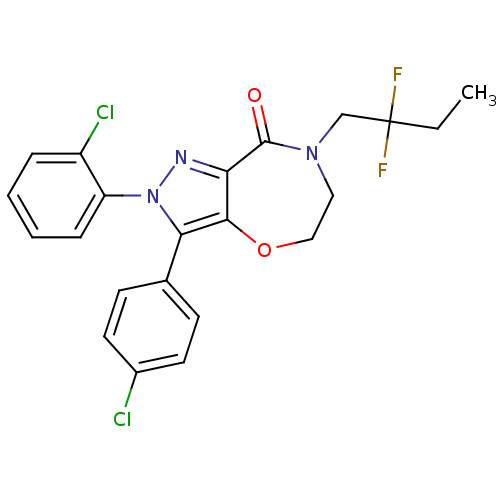 (ether-based lactam, 19e) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.40 | -51.4 | n/a | n/a | 0.700 | n/a | n/a | 7.4 | 30 |
Pfizer | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 52: 2652-5 (2009) Article DOI: 10.1021/jm900255t BindingDB Entry DOI: 10.7270/Q2F47MGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM29069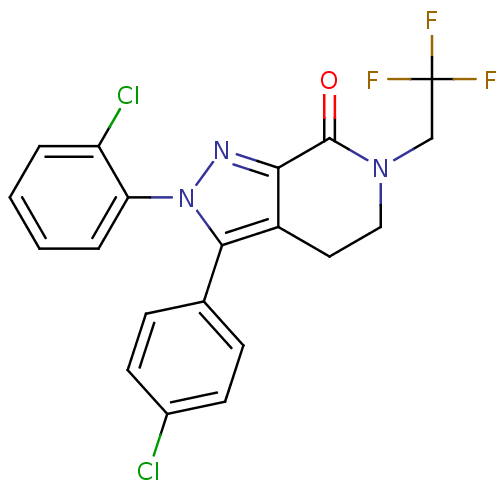 (lactam-based compound, 12h) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.70 | -50.9 | n/a | n/a | 3.10 | n/a | n/a | 7.4 | 30 |
Pfizer | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 52: 2652-5 (2009) Article DOI: 10.1021/jm900255t BindingDB Entry DOI: 10.7270/Q2F47MGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21278 (5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 1.80 | -50.7 | n/a | n/a | 1.60 | n/a | n/a | 7.4 | 30 |
Pfizer | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 52: 2652-5 (2009) Article DOI: 10.1021/jm900255t BindingDB Entry DOI: 10.7270/Q2F47MGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM29074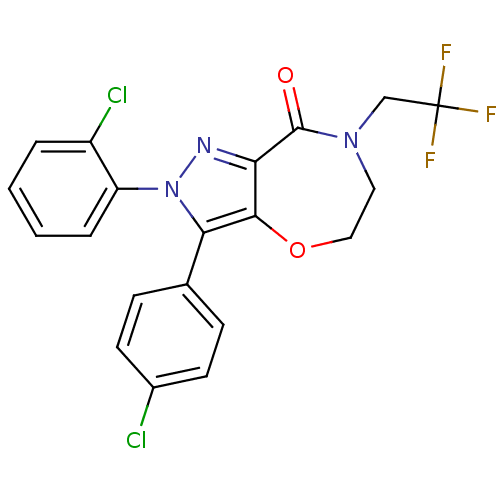 (ether-based lactam, 19c) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.90 | -50.6 | n/a | n/a | 1.5 | n/a | n/a | 7.4 | 30 |
Pfizer | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 52: 2652-5 (2009) Article DOI: 10.1021/jm900255t BindingDB Entry DOI: 10.7270/Q2F47MGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM27341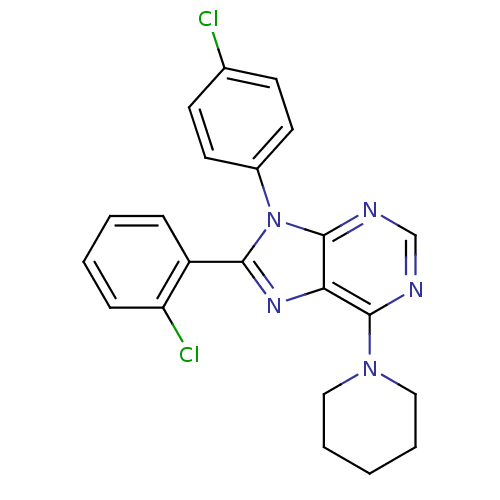 (8-(2-chlorophenyl)-9-(4-chlorophenyl)-6-(piperidin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.10 | -50.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 52: 234-7 (2009) Article DOI: 10.1021/jm8012932 BindingDB Entry DOI: 10.7270/Q2CN7276 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM27341 (8-(2-chlorophenyl)-9-(4-chlorophenyl)-6-(piperidin...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.30 | -50.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 52: 234-7 (2009) Article DOI: 10.1021/jm8012932 BindingDB Entry DOI: 10.7270/Q2CN7276 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM29064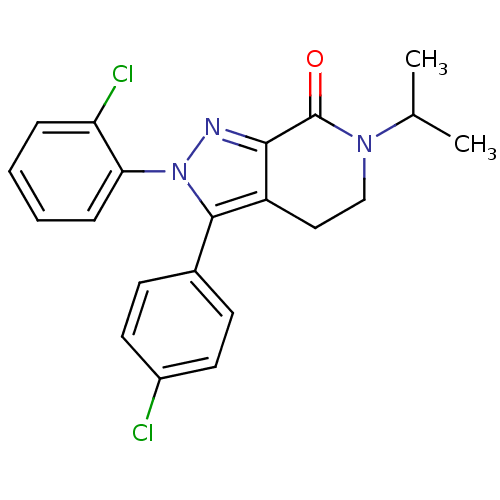 (lactam-based compound, 12c) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2.5 | -49.9 | n/a | n/a | 5.5 | n/a | n/a | 7.4 | 30 |
Pfizer | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 52: 2652-5 (2009) Article DOI: 10.1021/jm900255t BindingDB Entry DOI: 10.7270/Q2F47MGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM29065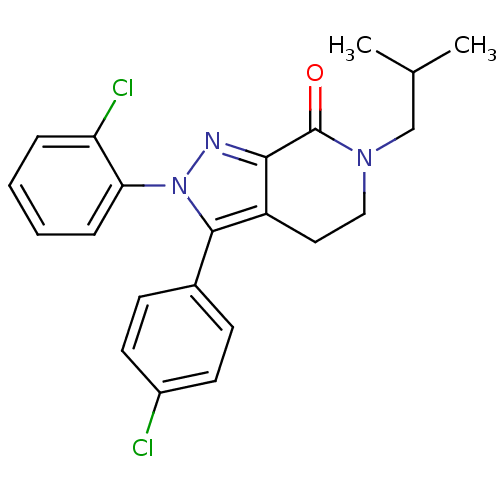 (lactam-based compound, 12d) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.5 | -49.9 | n/a | n/a | 9.80 | n/a | n/a | 7.4 | 30 |
Pfizer | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 52: 2652-5 (2009) Article DOI: 10.1021/jm900255t BindingDB Entry DOI: 10.7270/Q2F47MGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM27343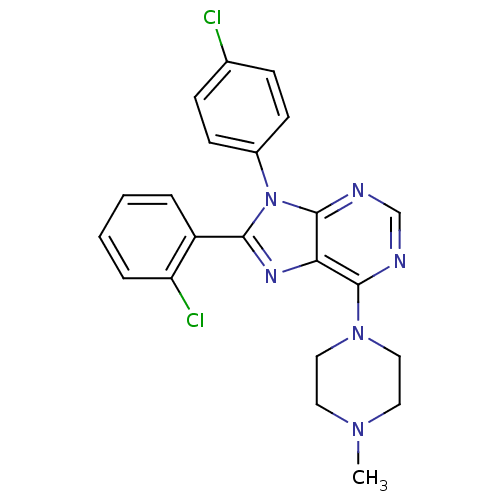 (8-(2-chlorophenyl)-9-(4-chlorophenyl)-6-(4-methylp...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.80 | -49.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 52: 234-7 (2009) Article DOI: 10.1021/jm8012932 BindingDB Entry DOI: 10.7270/Q2CN7276 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM27337 (1-[8-(2-chlorophenyl)-9-(4-chlorophenyl)-9H-purin-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 2.80 | -49.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 52: 234-7 (2009) Article DOI: 10.1021/jm8012932 BindingDB Entry DOI: 10.7270/Q2CN7276 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM27338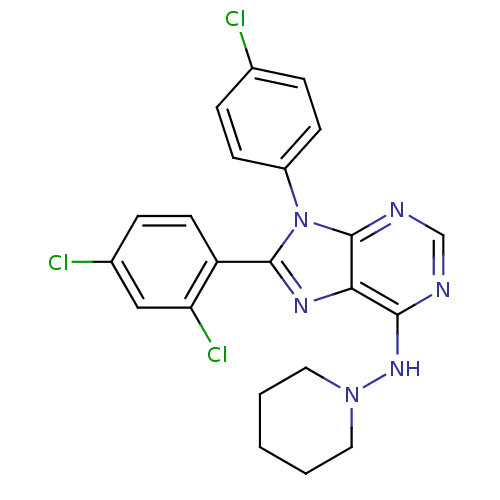 (9-(4-chlorophenyl)-8-(2,4-dichlorophenyl)-N-(piper...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.60 | -49.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 52: 234-7 (2009) Article DOI: 10.1021/jm8012932 BindingDB Entry DOI: 10.7270/Q2CN7276 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM27340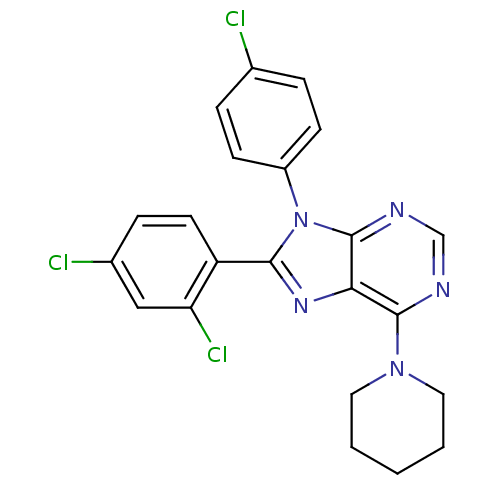 (9-(4-chlorophenyl)-8-(2,4-dichlorophenyl)-6-(piper...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.20 | -48.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 52: 234-7 (2009) Article DOI: 10.1021/jm8012932 BindingDB Entry DOI: 10.7270/Q2CN7276 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM29063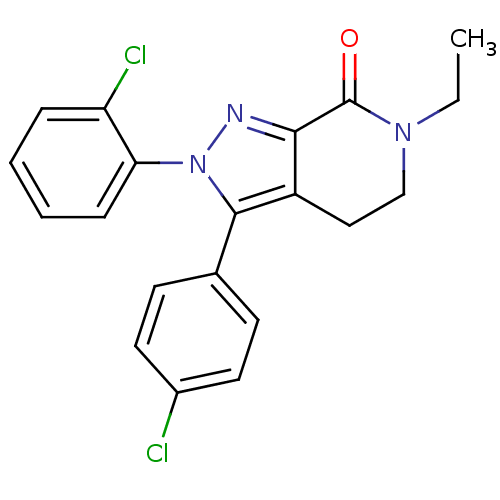 (lactam-based compound, 12b) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.70 | -48.3 | n/a | n/a | 25 | n/a | n/a | 7.4 | 30 |
Pfizer | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 52: 2652-5 (2009) Article DOI: 10.1021/jm900255t BindingDB Entry DOI: 10.7270/Q2F47MGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM29066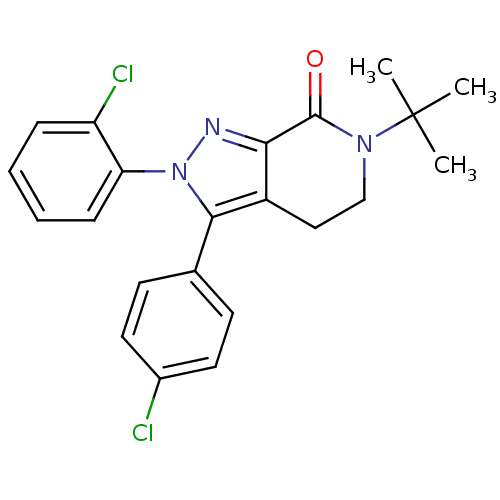 (lactam-based compound, 12e) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.70 | -48.3 | n/a | n/a | 11 | n/a | n/a | 7.4 | 30 |
Pfizer | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 52: 2652-5 (2009) Article DOI: 10.1021/jm900255t BindingDB Entry DOI: 10.7270/Q2F47MGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM27342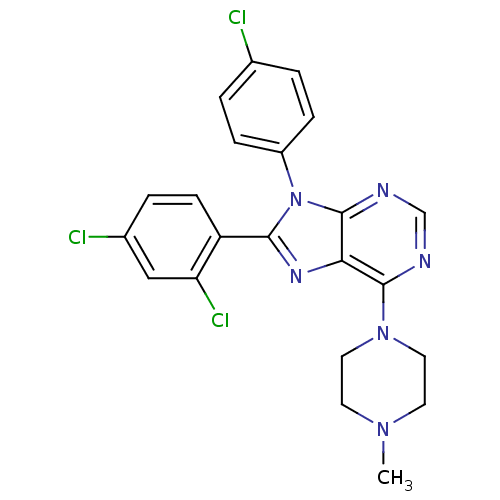 (9-(4-chlorophenyl)-8-(2,4-dichlorophenyl)-6-(4-met...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5.20 | -48.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 52: 234-7 (2009) Article DOI: 10.1021/jm8012932 BindingDB Entry DOI: 10.7270/Q2CN7276 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM27342 (9-(4-chlorophenyl)-8-(2,4-dichlorophenyl)-6-(4-met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5.90 | -47.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 52: 234-7 (2009) Article DOI: 10.1021/jm8012932 BindingDB Entry DOI: 10.7270/Q2CN7276 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM27340 (9-(4-chlorophenyl)-8-(2,4-dichlorophenyl)-6-(piper...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6.10 | -47.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 52: 234-7 (2009) Article DOI: 10.1021/jm8012932 BindingDB Entry DOI: 10.7270/Q2CN7276 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM27339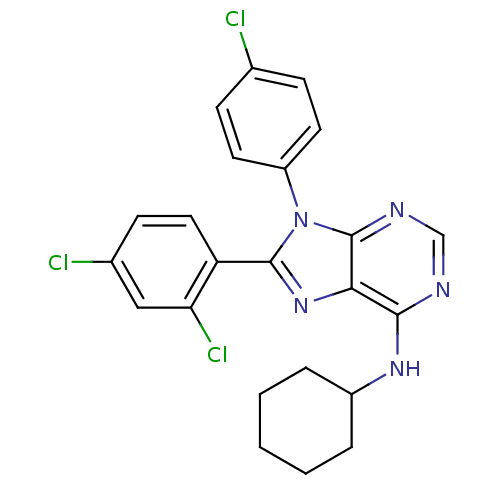 (9-(4-chlorophenyl)-N-cyclohexyl-8-(2,4-dichlorophe...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 7.10 | -47.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 52: 234-7 (2009) Article DOI: 10.1021/jm8012932 BindingDB Entry DOI: 10.7270/Q2CN7276 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM27338 (9-(4-chlorophenyl)-8-(2,4-dichlorophenyl)-N-(piper...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.40 | -47.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 52: 234-7 (2009) Article DOI: 10.1021/jm8012932 BindingDB Entry DOI: 10.7270/Q2CN7276 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM29068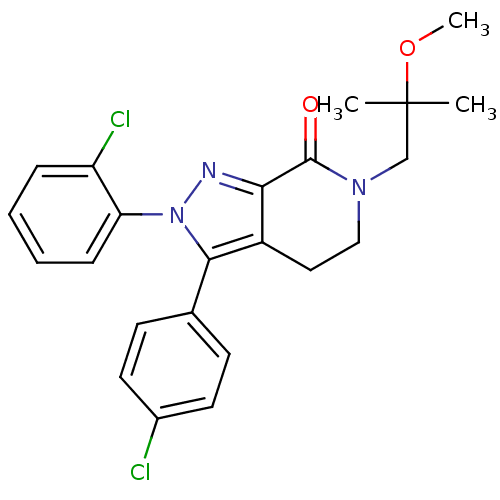 (lactam-based compound, 12g) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 11 | -46.2 | n/a | n/a | 13 | n/a | n/a | 7.4 | 30 |
Pfizer | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 52: 2652-5 (2009) Article DOI: 10.1021/jm900255t BindingDB Entry DOI: 10.7270/Q2F47MGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM27339 (9-(4-chlorophenyl)-N-cyclohexyl-8-(2,4-dichlorophe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 12 | -46.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 52: 234-7 (2009) Article DOI: 10.1021/jm8012932 BindingDB Entry DOI: 10.7270/Q2CN7276 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM29067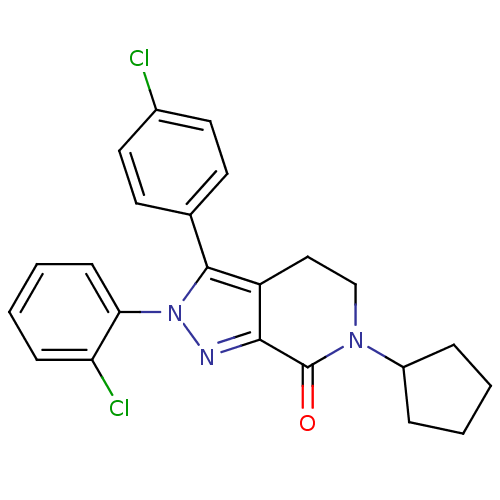 (lactam-based compound, 12f) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 24 | -44.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 52: 2652-5 (2009) Article DOI: 10.1021/jm900255t BindingDB Entry DOI: 10.7270/Q2F47MGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM27343 (8-(2-chlorophenyl)-9-(4-chlorophenyl)-6-(4-methylp...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 24 | -44.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 52: 234-7 (2009) Article DOI: 10.1021/jm8012932 BindingDB Entry DOI: 10.7270/Q2CN7276 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM29071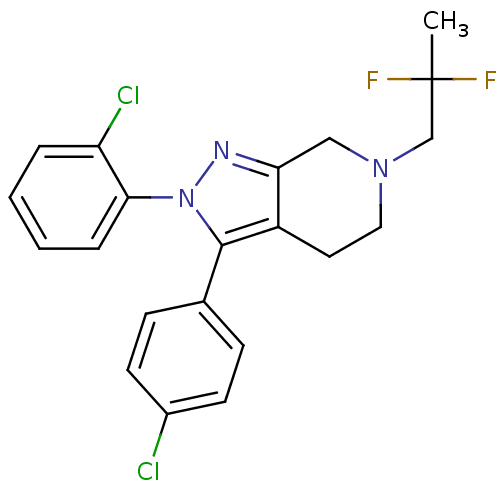 (amine-based bicyclic analogue, 13) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 30 | -43.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 52: 2652-5 (2009) Article DOI: 10.1021/jm900255t BindingDB Entry DOI: 10.7270/Q2F47MGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM29062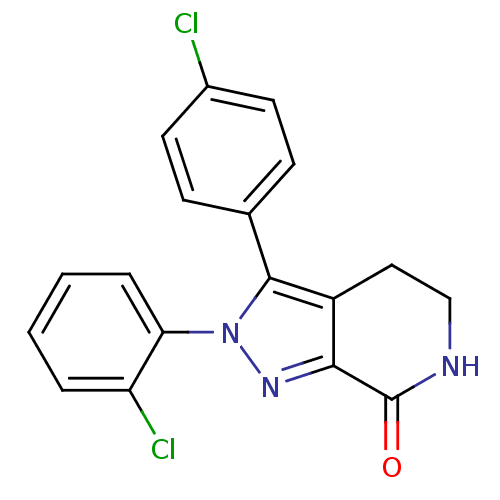 (lactam-based compound, 12a) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 61 | -41.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 52: 2652-5 (2009) Article DOI: 10.1021/jm900255t BindingDB Entry DOI: 10.7270/Q2F47MGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM29072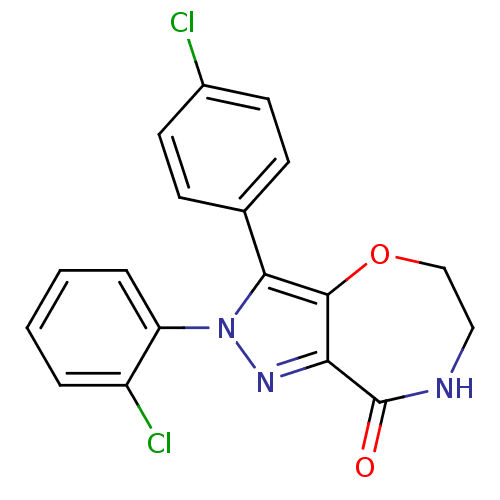 (ether-based lactam, 19a) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 83 | -41.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 52: 2652-5 (2009) Article DOI: 10.1021/jm900255t BindingDB Entry DOI: 10.7270/Q2F47MGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM29068 (lactam-based compound, 12g) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 162 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 52: 2652-5 (2009) Article DOI: 10.1021/jm900255t BindingDB Entry DOI: 10.7270/Q2F47MGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM21278 (5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.57E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 52: 2652-5 (2009) Article DOI: 10.1021/jm900255t BindingDB Entry DOI: 10.7270/Q2F47MGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM29065 (lactam-based compound, 12d) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 52: 2652-5 (2009) Article DOI: 10.1021/jm900255t BindingDB Entry DOI: 10.7270/Q2F47MGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM29066 (lactam-based compound, 12e) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6.81E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 52: 2652-5 (2009) Article DOI: 10.1021/jm900255t BindingDB Entry DOI: 10.7270/Q2F47MGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM29061 (CHEMBL201602 | pyrazolopyrimidinone-based antagoni...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 52: 2652-5 (2009) Article DOI: 10.1021/jm900255t BindingDB Entry DOI: 10.7270/Q2F47MGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM29076 (ether-based lactam, 19e) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 52: 2652-5 (2009) Article DOI: 10.1021/jm900255t BindingDB Entry DOI: 10.7270/Q2F47MGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM29075 (PF-514273) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 52: 2652-5 (2009) Article DOI: 10.1021/jm900255t BindingDB Entry DOI: 10.7270/Q2F47MGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM29074 (ether-based lactam, 19c) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 52: 2652-5 (2009) Article DOI: 10.1021/jm900255t BindingDB Entry DOI: 10.7270/Q2F47MGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM29073 (ether-based lactam, 19b) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 52: 2652-5 (2009) Article DOI: 10.1021/jm900255t BindingDB Entry DOI: 10.7270/Q2F47MGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM29070 (lactam-based compound, 12i) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 52: 2652-5 (2009) Article DOI: 10.1021/jm900255t BindingDB Entry DOI: 10.7270/Q2F47MGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM29069 (lactam-based compound, 12h) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 52: 2652-5 (2009) Article DOI: 10.1021/jm900255t BindingDB Entry DOI: 10.7270/Q2F47MGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM29064 (lactam-based compound, 12c) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 52: 2652-5 (2009) Article DOI: 10.1021/jm900255t BindingDB Entry DOI: 10.7270/Q2F47MGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM29063 (lactam-based compound, 12b) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.34E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 52: 2652-5 (2009) Article DOI: 10.1021/jm900255t BindingDB Entry DOI: 10.7270/Q2F47MGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Rattus norvegicus (rat)) | BDBM31592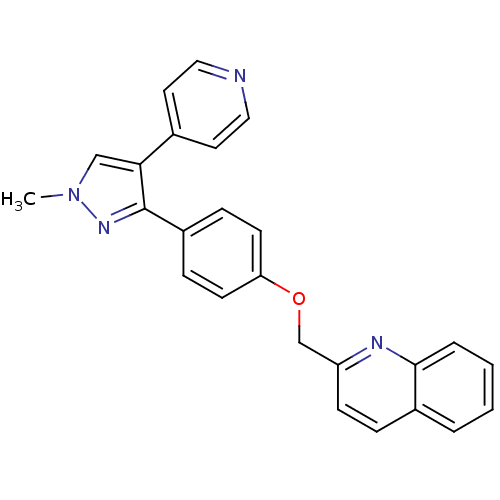 (PF-2545920 | US9138494, MP-10 | substituted pyraz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Pfizer | Assay Description PDE activity was measured using a plate-based Scintillation Proximity Assay (SPA). For competitive enzyme inhibition assays, the substrate [3H]cAMP c... | J Med Chem 52: 5188-96 (2009) Article DOI: 10.1021/jm900521k BindingDB Entry DOI: 10.7270/Q290223B | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Rattus norvegicus (rat)) | BDBM31591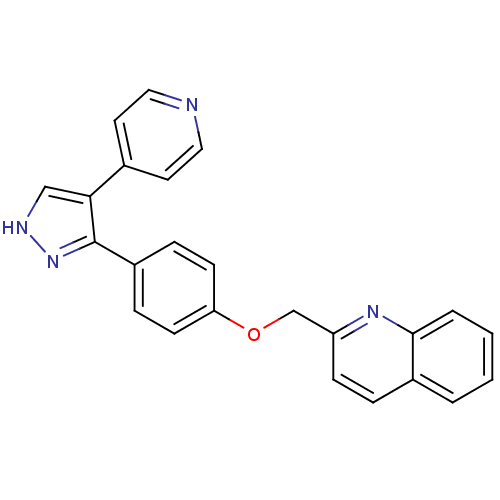 (pyrazole, 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Pfizer | Assay Description PDE activity was measured using a plate-based Scintillation Proximity Assay (SPA). For competitive enzyme inhibition assays, the substrate [3H]cAMP c... | J Med Chem 52: 5188-96 (2009) Article DOI: 10.1021/jm900521k BindingDB Entry DOI: 10.7270/Q290223B | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Rattus norvegicus (rat)) | BDBM31606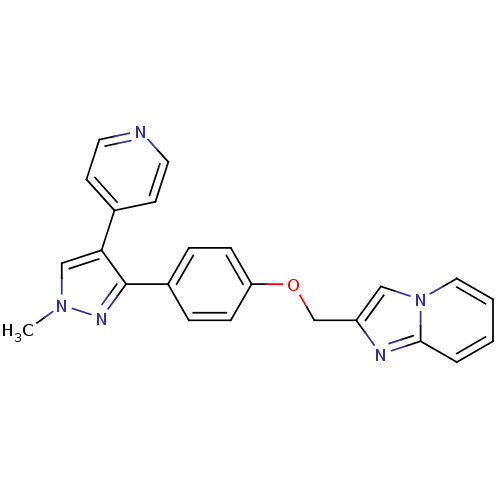 (methyl substituted pyrazole, 27) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.790 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Pfizer | Assay Description PDE activity was measured using a plate-based Scintillation Proximity Assay (SPA). For competitive enzyme inhibition assays, the substrate [3H]cAMP c... | J Med Chem 52: 5188-96 (2009) Article DOI: 10.1021/jm900521k BindingDB Entry DOI: 10.7270/Q290223B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Rattus norvegicus (rat)) | BDBM31594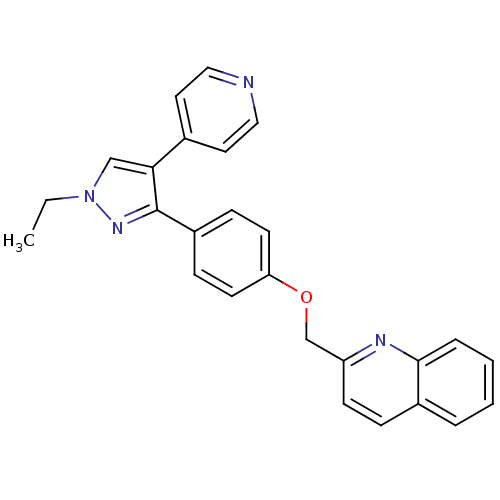 (substituted pyrazole, 11) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.920 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Pfizer | Assay Description PDE activity was measured using a plate-based Scintillation Proximity Assay (SPA). For competitive enzyme inhibition assays, the substrate [3H]cAMP c... | J Med Chem 52: 5188-96 (2009) Article DOI: 10.1021/jm900521k BindingDB Entry DOI: 10.7270/Q290223B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 65 total ) | Next | Last >> |