 Found 126 hits with Last Name = 'ona' and Initial = 'sm'
Found 126 hits with Last Name = 'ona' and Initial = 'sm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50063544
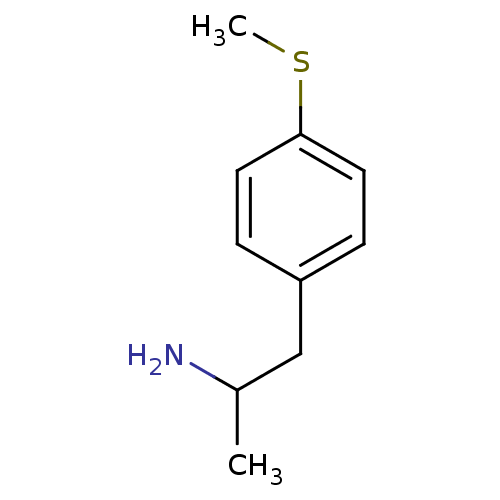
(1-(6-(methylthio)naphthalen-2-yl)propan-2-amine | ...)Show InChI InChI=1S/C10H15NS/c1-8(11)7-9-3-5-10(12-2)6-4-9/h3-6,8H,7,11H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College
Curated by ChEMBL
| Assay Description
Inhibition of [3H]5-HT uptake at human SERT expressed in HEK293 cells after 10 mins by scintillation counting |
Eur J Med Chem 44: 4862-88 (2009)
Article DOI: 10.1016/j.ejmech.2009.07.027
BindingDB Entry DOI: 10.7270/Q24J0F7W |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50316213
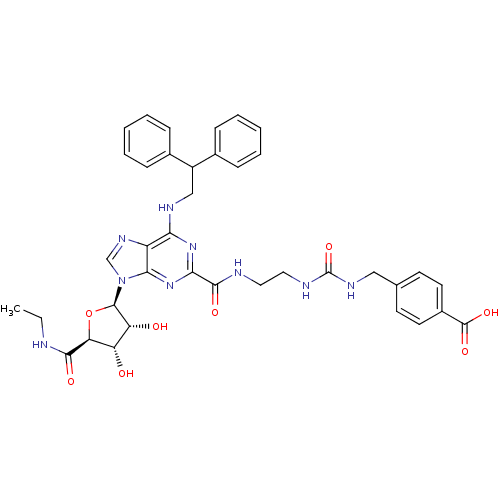
(4-((3-(2-(6-(2,2-diphenylethylamino)-9-((2R,3R,4S,...)Show SMILES CCNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCC(c3ccccc3)c3ccccc3)nc(nc12)C(=O)NCCNC(=O)NCc1ccc(cc1)C(O)=O |r| Show InChI InChI=1S/C38H41N9O8/c1-2-39-34(50)30-28(48)29(49)36(55-30)47-21-44-27-31(42-20-26(23-9-5-3-6-10-23)24-11-7-4-8-12-24)45-32(46-33(27)47)35(51)40-17-18-41-38(54)43-19-22-13-15-25(16-14-22)37(52)53/h3-16,21,26,28-30,36,48-49H,2,17-20H2,1H3,(H,39,50)(H,40,51)(H,52,53)(H2,41,43,54)(H,42,45,46)/t28-,29+,30-,36+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Agonist activity at adenosine A2A receptor in fMLP-stimulated human neutrophils assessed as inhibition of superoxide production by colorimetric analy... |
Bioorg Med Chem Lett 19: 4471-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.027
BindingDB Entry DOI: 10.7270/Q2ST7Q0C |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM25870
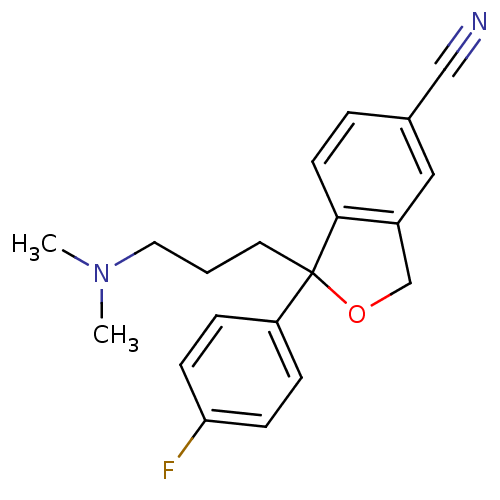
(1-[3-(dimethylamino)propyl]-1-(4-fluorophenyl)-1,3...)Show InChI InChI=1S/C20H21FN2O/c1-23(2)11-3-10-20(17-5-7-18(21)8-6-17)19-9-4-15(13-22)12-16(19)14-24-20/h4-9,12H,3,10-11,14H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 3.17 | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College
Curated by ChEMBL
| Assay Description
Inhibition of [3H]5-HT reuptake at rat SERT expressed in HEK293 cells after 2 mins by liquid scintillation counting |
Eur J Med Chem 44: 4862-88 (2009)
Article DOI: 10.1016/j.ejmech.2009.07.027
BindingDB Entry DOI: 10.7270/Q24J0F7W |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50316212
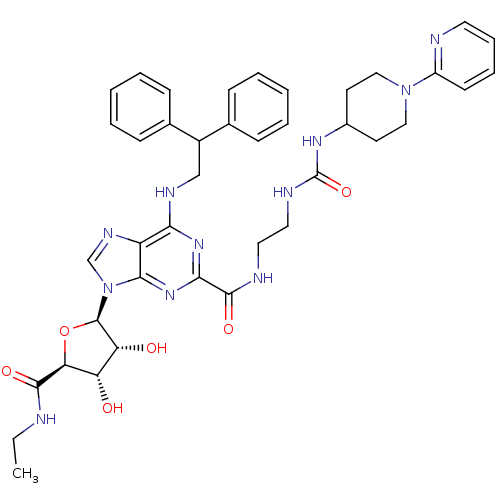
(6-(2,2-diphenylethylamino)-9-((2R,3R,4S,5S)-5-(eth...)Show SMILES CCNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCC(c3ccccc3)c3ccccc3)nc(nc12)C(=O)NCCNC(=O)NC1CCN(CC1)c1ccccn1 |r| Show InChI InChI=1S/C40H47N11O6/c1-2-41-37(54)33-31(52)32(53)39(57-33)51-24-46-30-34(45-23-28(25-11-5-3-6-12-25)26-13-7-4-8-14-26)48-35(49-36(30)51)38(55)43-19-20-44-40(56)47-27-16-21-50(22-17-27)29-15-9-10-18-42-29/h3-15,18,24,27-28,31-33,39,52-53H,2,16-17,19-23H2,1H3,(H,41,54)(H,43,55)(H2,44,47,56)(H,45,48,49)/t31-,32+,33-,39+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Agonist activity at adenosine A2A receptor in fMLP-stimulated human neutrophils assessed as inhibition of superoxide production by colorimetric analy... |
Bioorg Med Chem Lett 19: 4471-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.027
BindingDB Entry DOI: 10.7270/Q2ST7Q0C |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50316210
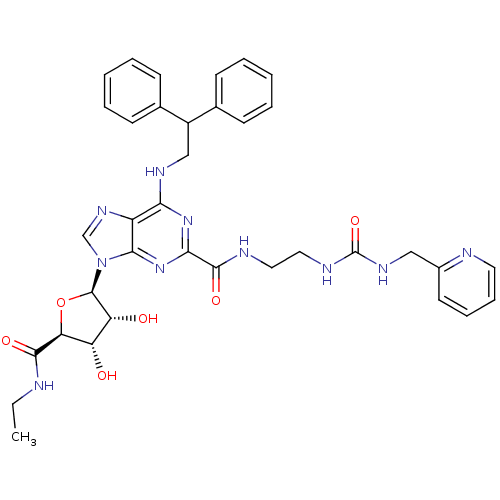
(6-(2,2-diphenylethylamino)-9-((2R,3R,4S,5S)-5-(eth...)Show SMILES CCNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCC(c3ccccc3)c3ccccc3)nc(nc12)C(=O)NCCNC(=O)NCc1ccccn1 |r| Show InChI InChI=1S/C36H40N10O6/c1-2-37-33(49)29-27(47)28(48)35(52-29)46-21-43-26-30(41-20-25(22-11-5-3-6-12-22)23-13-7-4-8-14-23)44-31(45-32(26)46)34(50)39-17-18-40-36(51)42-19-24-15-9-10-16-38-24/h3-16,21,25,27-29,35,47-48H,2,17-20H2,1H3,(H,37,49)(H,39,50)(H2,40,42,51)(H,41,44,45)/t27-,28+,29-,35+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Agonist activity at adenosine A2A receptor in fMLP-stimulated human neutrophils assessed as inhibition of superoxide production by colorimetric analy... |
Bioorg Med Chem Lett 19: 4471-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.027
BindingDB Entry DOI: 10.7270/Q2ST7Q0C |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50316211

(CHEMBL1096895 | N-(2-(3-(4-((diethylamino)methyl)b...)Show SMILES CCNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCC(c3ccccc3)c3ccccc3)nc(nc12)C(=O)NCCNC(=O)NCc1ccc(CN(CC)CC)cc1 |r| Show InChI InChI=1S/C42H52N10O6/c1-4-43-39(55)35-33(53)34(54)41(58-35)52-26-48-32-36(46-24-31(29-13-9-7-10-14-29)30-15-11-8-12-16-30)49-37(50-38(32)52)40(56)44-21-22-45-42(57)47-23-27-17-19-28(20-18-27)25-51(5-2)6-3/h7-20,26,31,33-35,41,53-54H,4-6,21-25H2,1-3H3,(H,43,55)(H,44,56)(H2,45,47,57)(H,46,49,50)/t33-,34+,35-,41+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Agonist activity at adenosine A2A receptor in fMLP-stimulated human neutrophils assessed as inhibition of superoxide production by colorimetric analy... |
Bioorg Med Chem Lett 19: 4471-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.027
BindingDB Entry DOI: 10.7270/Q2ST7Q0C |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50316202

(6-(2,2-diphenylethylamino)-9-((2R,3R,4S,5S)-5-(eth...)Show SMILES CCNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCC(c3ccccc3)c3ccccc3)nc(nc12)C(=O)NCCNC(=O)NCCN1CCCCC1 |r| Show InChI InChI=1S/C37H48N10O6/c1-2-38-34(50)30-28(48)29(49)36(53-30)47-23-43-27-31(42-22-26(24-12-6-3-7-13-24)25-14-8-4-9-15-25)44-32(45-33(27)47)35(51)39-16-17-40-37(52)41-18-21-46-19-10-5-11-20-46/h3-4,6-9,12-15,23,26,28-30,36,48-49H,2,5,10-11,16-22H2,1H3,(H,38,50)(H,39,51)(H2,40,41,52)(H,42,44,45)/t28-,29+,30-,36+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Agonist activity at adenosine A2A receptor in fMLP-stimulated human neutrophils assessed as inhibition of superoxide production by colorimetric analy... |
Bioorg Med Chem Lett 19: 4471-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.027
BindingDB Entry DOI: 10.7270/Q2ST7Q0C |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50316205
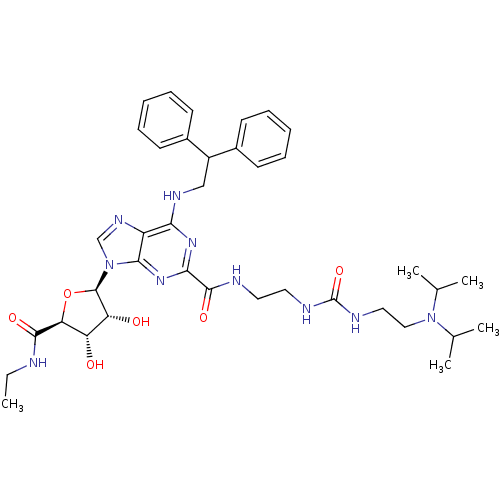
(CHEMBL1096889 | N-(2-(3-(2-(diisopropylamino)ethyl...)Show SMILES CCNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCC(c3ccccc3)c3ccccc3)nc(nc12)C(=O)NCCNC(=O)NCCN(C(C)C)C(C)C |r| Show InChI InChI=1S/C38H52N10O6/c1-6-39-35(51)31-29(49)30(50)37(54-31)48-22-44-28-32(43-21-27(25-13-9-7-10-14-25)26-15-11-8-12-16-26)45-33(46-34(28)48)36(52)40-17-18-41-38(53)42-19-20-47(23(2)3)24(4)5/h7-16,22-24,27,29-31,37,49-50H,6,17-21H2,1-5H3,(H,39,51)(H,40,52)(H2,41,42,53)(H,43,45,46)/t29-,30+,31-,37+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Agonist activity at adenosine A2A receptor in fMLP-stimulated human neutrophils assessed as inhibition of superoxide production by colorimetric analy... |
Bioorg Med Chem Lett 19: 4471-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.027
BindingDB Entry DOI: 10.7270/Q2ST7Q0C |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50233816

((2S,3S,4R,5R)-5-(2-((3-(2-(cyclopentyl(isopropyl)a...)Show SMILES CCNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCC(c3ccccc3)c3ccccc3)nc(CNC(=O)NCCN(C(C)C)C3CCCC3)nc12 Show InChI InChI=1S/C38H51N9O5/c1-4-39-36(50)33-31(48)32(49)37(52-33)47-23-43-30-34(41-21-28(25-13-7-5-8-14-25)26-15-9-6-10-16-26)44-29(45-35(30)47)22-42-38(51)40-19-20-46(24(2)3)27-17-11-12-18-27/h5-10,13-16,23-24,27-28,31-33,37,48-49H,4,11-12,17-22H2,1-3H3,(H,39,50)(H2,40,42,51)(H,41,44,45)/t31-,32+,33-,37+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at adenosine A2A receptor |
Bioorg Med Chem Lett 18: 1284-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.033
BindingDB Entry DOI: 10.7270/Q22808F4 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50233816

((2S,3S,4R,5R)-5-(2-((3-(2-(cyclopentyl(isopropyl)a...)Show SMILES CCNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCC(c3ccccc3)c3ccccc3)nc(CNC(=O)NCCN(C(C)C)C3CCCC3)nc12 Show InChI InChI=1S/C38H51N9O5/c1-4-39-36(50)33-31(48)32(49)37(52-33)47-23-43-30-34(41-21-28(25-13-7-5-8-14-25)26-15-9-6-10-16-26)44-29(45-35(30)47)22-42-38(51)40-19-20-46(24(2)3)27-17-11-12-18-27/h5-10,13-16,23-24,27-28,31-33,37,48-49H,4,11-12,17-22H2,1-3H3,(H,39,50)(H2,40,42,51)(H,41,44,45)/t31-,32+,33-,37+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Agonist activity at adenosine A2A receptor in fMLP-stimulated human neutrophils assessed as inhibition of superoxide production by colorimetric analy... |
Bioorg Med Chem Lett 19: 4471-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.027
BindingDB Entry DOI: 10.7270/Q2ST7Q0C |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50316209

(CHEMBL1096893 | N-(2-(3-benzylureido)ethyl)-6-(2,2...)Show SMILES CCNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCC(c3ccccc3)c3ccccc3)nc(nc12)C(=O)NCCNC(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C37H41N9O6/c1-2-38-34(49)30-28(47)29(48)36(52-30)46-22-43-27-31(41-21-26(24-14-8-4-9-15-24)25-16-10-5-11-17-25)44-32(45-33(27)46)35(50)39-18-19-40-37(51)42-20-23-12-6-3-7-13-23/h3-17,22,26,28-30,36,47-48H,2,18-21H2,1H3,(H,38,49)(H,39,50)(H2,40,42,51)(H,41,44,45)/t28-,29+,30-,36+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Agonist activity at adenosine A2A receptor in fMLP-stimulated human neutrophils assessed as inhibition of superoxide production by colorimetric analy... |
Bioorg Med Chem Lett 19: 4471-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.027
BindingDB Entry DOI: 10.7270/Q2ST7Q0C |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50316204
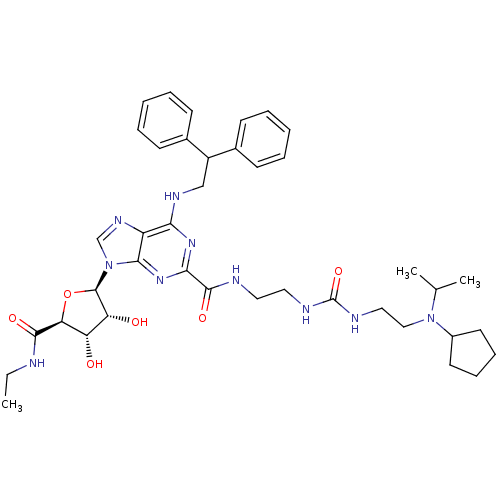
(CHEMBL1096888 | N-(2-(3-(2-(cyclopentyl(isopropyl)...)Show SMILES CCNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCC(c3ccccc3)c3ccccc3)nc(nc12)C(=O)NCCNC(=O)NCCN(C(C)C)C1CCCC1 |r| Show InChI InChI=1S/C40H54N10O6/c1-4-41-37(53)33-31(51)32(52)39(56-33)50-24-46-30-34(45-23-29(26-13-7-5-8-14-26)27-15-9-6-10-16-27)47-35(48-36(30)50)38(54)42-19-20-43-40(55)44-21-22-49(25(2)3)28-17-11-12-18-28/h5-10,13-16,24-25,28-29,31-33,39,51-52H,4,11-12,17-23H2,1-3H3,(H,41,53)(H,42,54)(H2,43,44,55)(H,45,47,48)/t31-,32+,33-,39+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Agonist activity at adenosine A2A receptor in fMLP-stimulated human neutrophils assessed as inhibition of superoxide production by colorimetric analy... |
Bioorg Med Chem Lett 19: 4471-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.027
BindingDB Entry DOI: 10.7270/Q2ST7Q0C |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50372023

(CHEMBL403478)Show SMILES CCNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCC(c3ccccc3)c3ccccc3)nc(nc12)C(=O)NCCN1CCCCC1 Show InChI InChI=1S/C34H42N8O5/c1-2-35-32(45)28-26(43)27(44)34(47-28)42-21-38-25-29(37-20-24(22-12-6-3-7-13-22)23-14-8-4-9-15-23)39-30(40-31(25)42)33(46)36-16-19-41-17-10-5-11-18-41/h3-4,6-9,12-15,21,24,26-28,34,43-44H,2,5,10-11,16-20H2,1H3,(H,35,45)(H,36,46)(H,37,39,40)/t26-,27+,28-,34+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at adenosine A2A receptor |
Bioorg Med Chem Lett 18: 1284-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.033
BindingDB Entry DOI: 10.7270/Q22808F4 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50316206

(CHEMBL1096890 | N-(2-(3-(2-(3,4-dihydroisoquinolin...)Show SMILES CCNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCC(c3ccccc3)c3ccccc3)nc(nc12)C(=O)NCCNC(=O)NCCN1CCc2ccccc2C1 |r| Show InChI InChI=1S/C41H48N10O6/c1-2-42-38(54)34-32(52)33(53)40(57-34)51-25-47-31-35(46-23-30(27-12-5-3-6-13-27)28-14-7-4-8-15-28)48-36(49-37(31)51)39(55)43-18-19-44-41(56)45-20-22-50-21-17-26-11-9-10-16-29(26)24-50/h3-16,25,30,32-34,40,52-53H,2,17-24H2,1H3,(H,42,54)(H,43,55)(H2,44,45,56)(H,46,48,49)/t32-,33+,34-,40+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Agonist activity at adenosine A2A receptor in fMLP-stimulated human neutrophils assessed as inhibition of superoxide production by colorimetric analy... |
Bioorg Med Chem Lett 19: 4471-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.027
BindingDB Entry DOI: 10.7270/Q2ST7Q0C |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50316208
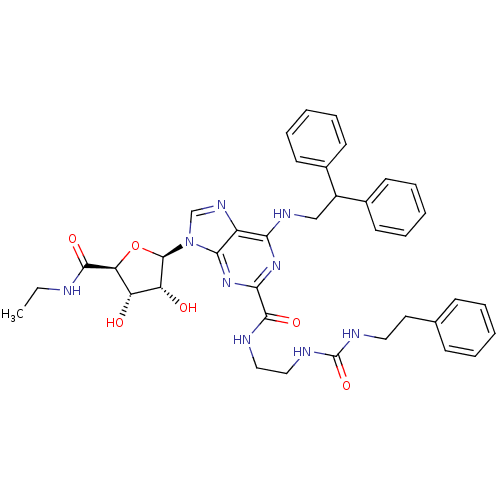
(6-(2,2-diphenylethylamino)-9-((2R,3R,4S,5S)-5-(eth...)Show SMILES CCNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCC(c3ccccc3)c3ccccc3)nc(nc12)C(=O)NCCNC(=O)NCCc1ccccc1 |r| Show InChI InChI=1S/C38H43N9O6/c1-2-39-35(50)31-29(48)30(49)37(53-31)47-23-44-28-32(43-22-27(25-14-8-4-9-15-25)26-16-10-5-11-17-26)45-33(46-34(28)47)36(51)40-20-21-42-38(52)41-19-18-24-12-6-3-7-13-24/h3-17,23,27,29-31,37,48-49H,2,18-22H2,1H3,(H,39,50)(H,40,51)(H2,41,42,52)(H,43,45,46)/t29-,30+,31-,37+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Agonist activity at adenosine A2A receptor in fMLP-stimulated human neutrophils assessed as inhibition of superoxide production by colorimetric analy... |
Bioorg Med Chem Lett 19: 4471-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.027
BindingDB Entry DOI: 10.7270/Q2ST7Q0C |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50372024

(CHEMBL257213)Show SMILES CCNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCC(c3ccccc3)c3ccccc3)nc(CNC(=O)NCCN(C(C)C)C(C)C)nc12 Show InChI InChI=1S/C36H49N9O5/c1-6-37-34(48)31-29(46)30(47)35(50-31)45-21-41-28-32(39-19-26(24-13-9-7-10-14-24)25-15-11-8-12-16-25)42-27(43-33(28)45)20-40-36(49)38-17-18-44(22(2)3)23(4)5/h7-16,21-23,26,29-31,35,46-47H,6,17-20H2,1-5H3,(H,37,48)(H2,38,40,49)(H,39,42,43)/t29-,30+,31-,35+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at adenosine A2A receptor |
Bioorg Med Chem Lett 18: 1284-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.033
BindingDB Entry DOI: 10.7270/Q22808F4 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50233809

((2S,3S,4R,5R)-5-(6-(2,2-diphenylethylamino)-2-((3-...)Show SMILES CCNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCC(c3ccccc3)c3ccccc3)nc(CNC(=O)NCCN3CCCCC3)nc12 Show InChI InChI=1S/C35H45N9O5/c1-2-36-33(47)30-28(45)29(46)34(49-30)44-22-40-27-31(38-20-25(23-12-6-3-7-13-23)24-14-8-4-9-15-24)41-26(42-32(27)44)21-39-35(48)37-16-19-43-17-10-5-11-18-43/h3-4,6-9,12-15,22,25,28-30,34,45-46H,2,5,10-11,16-21H2,1H3,(H,36,47)(H2,37,39,48)(H,38,41,42)/t28-,29+,30-,34+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at adenosine A2A receptor |
Bioorg Med Chem Lett 18: 1284-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.033
BindingDB Entry DOI: 10.7270/Q22808F4 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50233809

((2S,3S,4R,5R)-5-(6-(2,2-diphenylethylamino)-2-((3-...)Show SMILES CCNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCC(c3ccccc3)c3ccccc3)nc(CNC(=O)NCCN3CCCCC3)nc12 Show InChI InChI=1S/C35H45N9O5/c1-2-36-33(47)30-28(45)29(46)34(49-30)44-22-40-27-31(38-20-25(23-12-6-3-7-13-23)24-14-8-4-9-15-24)41-26(42-32(27)44)21-39-35(48)37-16-19-43-17-10-5-11-18-43/h3-4,6-9,12-15,22,25,28-30,34,45-46H,2,5,10-11,16-21H2,1H3,(H,36,47)(H2,37,39,48)(H,38,41,42)/t28-,29+,30-,34+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Agonist activity at adenosine A2A receptor in fMLP-stimulated human neutrophils assessed as inhibition of superoxide production by colorimetric analy... |
Bioorg Med Chem Lett 19: 4471-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.027
BindingDB Entry DOI: 10.7270/Q2ST7Q0C |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM25870

(1-[3-(dimethylamino)propyl]-1-(4-fluorophenyl)-1,3...)Show InChI InChI=1S/C20H21FN2O/c1-23(2)11-3-10-20(17-5-7-18(21)8-6-17)19-9-4-15(13-22)12-16(19)14-24-20/h4-9,12H,3,10-11,14H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-serotonin reuptake at human SERT expressed in HEK293 cells after 15 to 20 mins by fluorescence neurotransmitter transporter assay |
Bioorg Med Chem 19: 1328-48 (2011)
Article DOI: 10.1016/j.bmc.2010.11.054
BindingDB Entry DOI: 10.7270/Q28C9WJ7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50372026

(CHEMBL257429)Show SMILES COC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCC(c3ccccc3)c3ccccc3)nc(CCNC3CCCCC3)nc12 Show InChI InChI=1S/C33H42N6O4/c1-42-20-26-29(40)30(41)33(43-26)39-21-36-28-31(37-27(38-32(28)39)17-18-34-24-15-9-4-10-16-24)35-19-25(22-11-5-2-6-12-22)23-13-7-3-8-14-23/h2-3,5-8,11-14,21,24-26,29-30,33-34,40-41H,4,9-10,15-20H2,1H3,(H,35,37,38)/t26-,29-,30-,33-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at adenosine A2A receptor |
Bioorg Med Chem Lett 18: 1284-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.033
BindingDB Entry DOI: 10.7270/Q22808F4 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50372029

(CHEMBL256489)Show SMILES CCNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCC(c3ccccc3)c3ccccc3)nc(CNC(=O)NCCN(C3CCCCC3)C(C)(C)C)nc12 Show InChI InChI=1S/C40H55N9O5/c1-5-41-37(52)34-32(50)33(51)38(54-34)48-25-45-31-35(43-23-29(26-15-9-6-10-16-26)27-17-11-7-12-18-27)46-30(47-36(31)48)24-44-39(53)42-21-22-49(40(2,3)4)28-19-13-8-14-20-28/h6-7,9-12,15-18,25,28-29,32-34,38,50-51H,5,8,13-14,19-24H2,1-4H3,(H,41,52)(H2,42,44,53)(H,43,46,47)/t32-,33+,34-,38+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at adenosine A2A receptor |
Bioorg Med Chem Lett 18: 1284-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.033
BindingDB Entry DOI: 10.7270/Q22808F4 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50316203
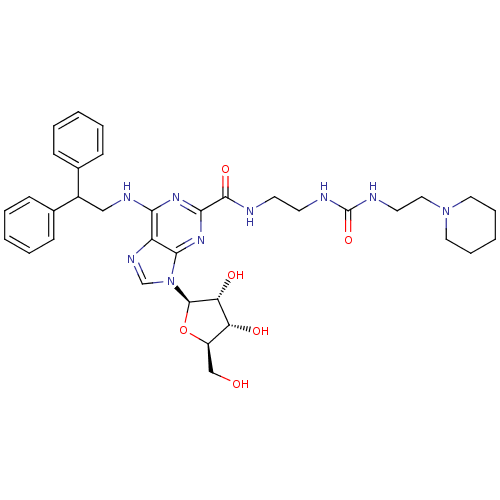
(9-((2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)te...)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCC(c3ccccc3)c3ccccc3)nc(nc12)C(=O)NCCNC(=O)NCCN1CCCCC1 |r| Show InChI InChI=1S/C35H45N9O6/c45-21-26-28(46)29(47)34(50-26)44-22-40-27-30(39-20-25(23-10-4-1-5-11-23)24-12-6-2-7-13-24)41-31(42-32(27)44)33(48)36-14-15-37-35(49)38-16-19-43-17-8-3-9-18-43/h1-2,4-7,10-13,22,25-26,28-29,34,45-47H,3,8-9,14-21H2,(H,36,48)(H2,37,38,49)(H,39,41,42)/t26-,28-,29-,34-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Agonist activity at adenosine A2A receptor in fMLP-stimulated human neutrophils assessed as inhibition of superoxide production by colorimetric analy... |
Bioorg Med Chem Lett 19: 4471-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.027
BindingDB Entry DOI: 10.7270/Q2ST7Q0C |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50372027
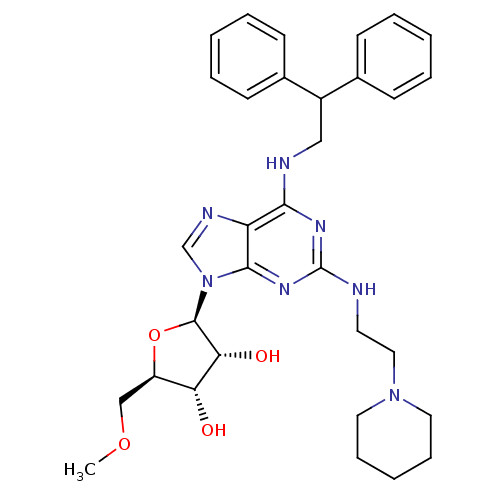
(CHEMBL270378)Show SMILES COC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCC(c3ccccc3)c3ccccc3)nc(NCCN3CCCCC3)nc12 Show InChI InChI=1S/C32H41N7O4/c1-42-20-25-27(40)28(41)31(43-25)39-21-35-26-29(36-32(37-30(26)39)33-15-18-38-16-9-4-10-17-38)34-19-24(22-11-5-2-6-12-22)23-13-7-3-8-14-23/h2-3,5-8,11-14,21,24-25,27-28,31,40-41H,4,9-10,15-20H2,1H3,(H2,33,34,36,37)/t25-,27-,28-,31-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at adenosine A2A receptor |
Bioorg Med Chem Lett 18: 1284-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.033
BindingDB Entry DOI: 10.7270/Q22808F4 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50233810

(9-((2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)-t...)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCC(c3ccccc3)c3ccccc3)nc(nc12)C(=O)NCCN1CCCCC1 Show InChI InChI=1S/C32H39N7O5/c40-19-24-26(41)27(42)32(44-24)39-20-35-25-28(34-18-23(21-10-4-1-5-11-21)22-12-6-2-7-13-22)36-29(37-30(25)39)31(43)33-14-17-38-15-8-3-9-16-38/h1-2,4-7,10-13,20,23-24,26-27,32,40-42H,3,8-9,14-19H2,(H,33,43)(H,34,36,37)/t24-,26-,27-,32-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at adenosine A2A receptor |
Bioorg Med Chem Lett 18: 1284-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.033
BindingDB Entry DOI: 10.7270/Q22808F4 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50233810

(9-((2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)-t...)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCC(c3ccccc3)c3ccccc3)nc(nc12)C(=O)NCCN1CCCCC1 Show InChI InChI=1S/C32H39N7O5/c40-19-24-26(41)27(42)32(44-24)39-20-35-25-28(34-18-23(21-10-4-1-5-11-21)22-12-6-2-7-13-22)36-29(37-30(25)39)31(43)33-14-17-38-15-8-3-9-16-38/h1-2,4-7,10-13,20,23-24,26-27,32,40-42H,3,8-9,14-19H2,(H,33,43)(H,34,36,37)/t24-,26-,27-,32-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Agonist activity at adenosine A2A receptor in fMLP-stimulated human neutrophils assessed as inhibition of superoxide production by colorimetric analy... |
Bioorg Med Chem Lett 19: 4471-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.027
BindingDB Entry DOI: 10.7270/Q2ST7Q0C |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50063544

(1-(6-(methylthio)naphthalen-2-yl)propan-2-amine | ...)Show InChI InChI=1S/C10H15NS/c1-8(11)7-9-3-5-10(12-2)6-4-9/h3-6,8H,7,11H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College
Curated by ChEMBL
| Assay Description
Inhibition of [3H]5-HT uptake at SERT |
Eur J Med Chem 44: 4862-88 (2009)
Article DOI: 10.1016/j.ejmech.2009.07.027
BindingDB Entry DOI: 10.7270/Q24J0F7W |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50063544

(1-(6-(methylthio)naphthalen-2-yl)propan-2-amine | ...)Show InChI InChI=1S/C10H15NS/c1-8(11)7-9-3-5-10(12-2)6-4-9/h3-6,8H,7,11H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College
Curated by ChEMBL
| Assay Description
Inhibition of [3H]5-HT uptake at SERT in rat brain synaptosome |
Eur J Med Chem 44: 4862-88 (2009)
Article DOI: 10.1016/j.ejmech.2009.07.027
BindingDB Entry DOI: 10.7270/Q24J0F7W |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50372025
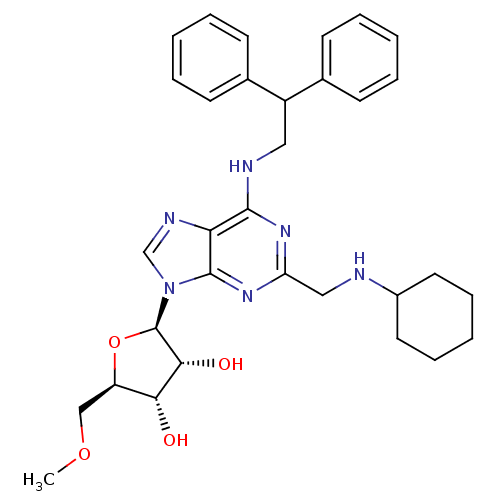
(CHEMBL401571)Show SMILES COC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCC(c3ccccc3)c3ccccc3)nc(CNC3CCCCC3)nc12 Show InChI InChI=1S/C32H40N6O4/c1-41-19-25-28(39)29(40)32(42-25)38-20-35-27-30(36-26(37-31(27)38)18-33-23-15-9-4-10-16-23)34-17-24(21-11-5-2-6-12-21)22-13-7-3-8-14-22/h2-3,5-8,11-14,20,23-25,28-29,32-33,39-40H,4,9-10,15-19H2,1H3,(H,34,36,37)/t25-,28-,29-,32-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at adenosine A2A receptor |
Bioorg Med Chem Lett 18: 1284-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.033
BindingDB Entry DOI: 10.7270/Q22808F4 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50024209
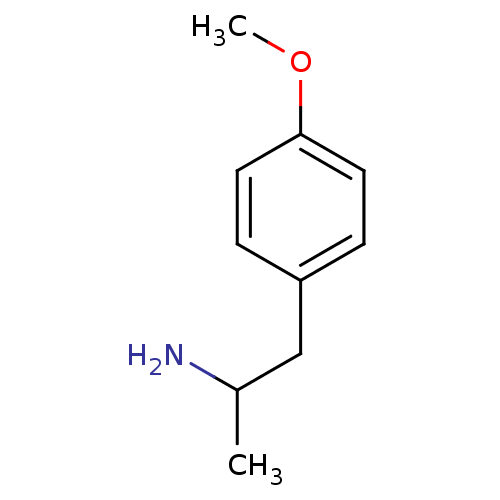
((+/-)2-(4-Methoxy-phenyl)-1-methyl-ethylamine | (-...)Show InChI InChI=1S/C10H15NO/c1-8(11)7-9-3-5-10(12-2)6-4-9/h3-6,8H,7,11H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 173 | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College
Curated by ChEMBL
| Assay Description
Inhibition of [3H]5-HT reuptake at rat SERT expressed in HEK293 cells after 2 mins by liquid scintillation counting |
Eur J Med Chem 44: 4862-88 (2009)
Article DOI: 10.1016/j.ejmech.2009.07.027
BindingDB Entry DOI: 10.7270/Q24J0F7W |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50029100
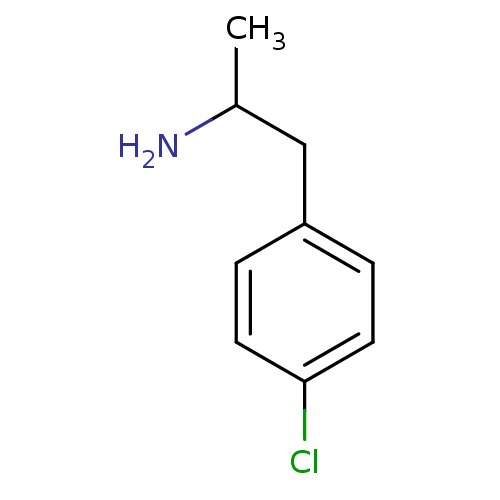
(2-(4-Chloro-phenyl)-1-methyl-ethylamine | CHEMBL35...)Show InChI InChI=1S/C9H12ClN/c1-7(11)6-8-2-4-9(10)5-3-8/h2-5,7H,6,11H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 182 | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College
Curated by ChEMBL
| Assay Description
Inhibition of [3H]5-HT uptake at SERT in rat brain synaptosome |
Eur J Med Chem 44: 4862-88 (2009)
Article DOI: 10.1016/j.ejmech.2009.07.027
BindingDB Entry DOI: 10.7270/Q24J0F7W |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50063544

(1-(6-(methylthio)naphthalen-2-yl)propan-2-amine | ...)Show InChI InChI=1S/C10H15NS/c1-8(11)7-9-3-5-10(12-2)6-4-9/h3-6,8H,7,11H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-serotonin reuptake at human SERT expressed in HEK293 cells after 15 to 20 mins by fluorescence neurotransmitter transporter assay |
Bioorg Med Chem 19: 1328-48 (2011)
Article DOI: 10.1016/j.bmc.2010.11.054
BindingDB Entry DOI: 10.7270/Q28C9WJ7 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50063544

(1-(6-(methylthio)naphthalen-2-yl)propan-2-amine | ...)Show InChI InChI=1S/C10H15NS/c1-8(11)7-9-3-5-10(12-2)6-4-9/h3-6,8H,7,11H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 207 | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College
Curated by ChEMBL
| Assay Description
Inhibition of [3H]5-HT reuptake at rat SERT expressed in HEK293 cells after 2 mins by liquid scintillation counting |
Eur J Med Chem 44: 4862-88 (2009)
Article DOI: 10.1016/j.ejmech.2009.07.027
BindingDB Entry DOI: 10.7270/Q24J0F7W |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50029100

(2-(4-Chloro-phenyl)-1-methyl-ethylamine | CHEMBL35...)Show InChI InChI=1S/C9H12ClN/c1-7(11)6-8-2-4-9(10)5-3-8/h2-5,7H,6,11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 207 | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College
Curated by ChEMBL
| Assay Description
Inhibition of human noradrenaline transporter |
Eur J Med Chem 44: 4862-88 (2009)
Article DOI: 10.1016/j.ejmech.2009.07.027
BindingDB Entry DOI: 10.7270/Q24J0F7W |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50005247

((+/-)2-Benzo[1,3]dioxol-5-yl-1-methyl-ethylamine |...)Show InChI InChI=1S/C10H13NO2/c1-7(11)4-8-2-3-9-10(5-8)13-6-12-9/h2-3,5,7H,4,6,11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 266 | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College
Curated by ChEMBL
| Assay Description
Binding affinity to human NET |
Bioorg Med Chem 18: 4009-31 (2010)
Article DOI: 10.1016/j.bmc.2010.04.022
BindingDB Entry DOI: 10.7270/Q2ST7Q1T |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50005247

((+/-)2-Benzo[1,3]dioxol-5-yl-1-methyl-ethylamine |...)Show InChI InChI=1S/C10H13NO2/c1-7(11)4-8-2-3-9-10(5-8)13-6-12-9/h2-3,5,7H,4,6,11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 266 | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College
Curated by ChEMBL
| Assay Description
Inhibition of human noradrenaline transporter |
Eur J Med Chem 44: 4862-88 (2009)
Article DOI: 10.1016/j.ejmech.2009.07.027
BindingDB Entry DOI: 10.7270/Q24J0F7W |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50316207
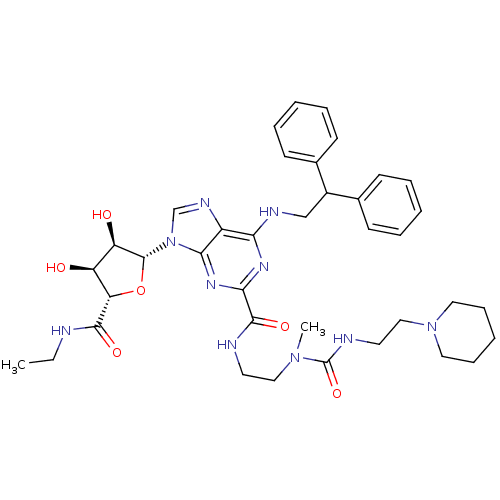
(6-(2,2-diphenylethylamino)-9-((2R,3R,4S,5S)-5-(eth...)Show SMILES CCNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCC(c3ccccc3)c3ccccc3)nc(nc12)C(=O)NCCN(C)C(=O)NCCN1CCCCC1 |r| Show InChI InChI=1S/C38H50N10O6/c1-3-39-35(51)31-29(49)30(50)37(54-31)48-24-43-28-32(42-23-27(25-13-7-4-8-14-25)26-15-9-5-10-16-26)44-33(45-34(28)48)36(52)40-17-21-46(2)38(53)41-18-22-47-19-11-6-12-20-47/h4-5,7-10,13-16,24,27,29-31,37,49-50H,3,6,11-12,17-23H2,1-2H3,(H,39,51)(H,40,52)(H,41,53)(H,42,44,45)/t29-,30+,31-,37+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 305 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Agonist activity at adenosine A2A receptor in fMLP-stimulated human neutrophils assessed as inhibition of superoxide production by colorimetric analy... |
Bioorg Med Chem Lett 19: 4471-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.027
BindingDB Entry DOI: 10.7270/Q2ST7Q0C |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50310850
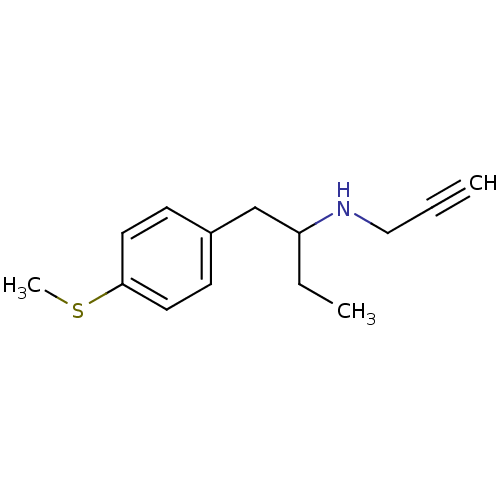
(2-N-Propargylamino-1-(4-methylthiophenyl)butane | ...)Show InChI InChI=1S/C14H19NS/c1-4-10-15-13(5-2)11-12-6-8-14(16-3)9-7-12/h1,6-9,13,15H,5,10-11H2,2-3H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 399 | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College
Curated by ChEMBL
| Assay Description
Inhibition of [3H]5-HT reuptake at rat SERT expressed in HEK293 cells after 2 mins by liquid scintillation counting |
Eur J Med Chem 44: 4862-88 (2009)
Article DOI: 10.1016/j.ejmech.2009.07.027
BindingDB Entry DOI: 10.7270/Q24J0F7W |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50010588

((RS)-3,4-(methylenedioxy)methamphetamine | 1-(1,3-...)Show InChI InChI=1S/C11H15NO2/c1-8(12-2)5-9-3-4-10-11(6-9)14-7-13-10/h3-4,6,8,12H,5,7H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 405 | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College
Curated by ChEMBL
| Assay Description
Binding affinity to human NET |
Bioorg Med Chem 18: 4009-31 (2010)
Article DOI: 10.1016/j.bmc.2010.04.022
BindingDB Entry DOI: 10.7270/Q2ST7Q1T |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50010588

((RS)-3,4-(methylenedioxy)methamphetamine | 1-(1,3-...)Show InChI InChI=1S/C11H15NO2/c1-8(12-2)5-9-3-4-10-11(6-9)14-7-13-10/h3-4,6,8,12H,5,7H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 405 | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College
Curated by ChEMBL
| Assay Description
Inhibition of human noradrenaline transporter |
Eur J Med Chem 44: 4862-88 (2009)
Article DOI: 10.1016/j.ejmech.2009.07.027
BindingDB Entry DOI: 10.7270/Q24J0F7W |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50310855
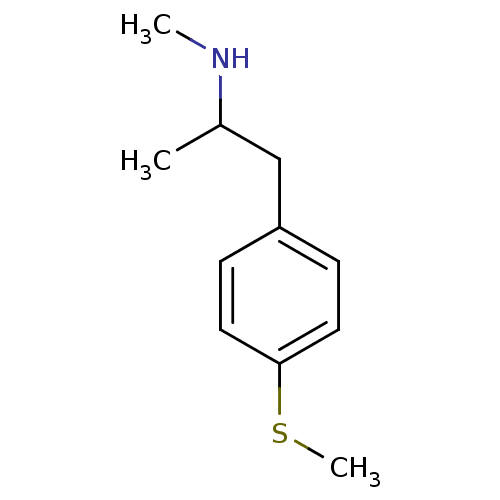
(2-Amino-1-(4-methylthiophenyl)butane | 2-N-Methyla...)Show InChI InChI=1S/C11H17NS/c1-9(12-2)8-10-4-6-11(13-3)7-5-10/h4-7,9,12H,8H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 417 | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College
Curated by ChEMBL
| Assay Description
Inhibition of [3H]5-HT reuptake at rat SERT expressed in HEK293 cells after 2 mins by liquid scintillation counting |
Eur J Med Chem 44: 4862-88 (2009)
Article DOI: 10.1016/j.ejmech.2009.07.027
BindingDB Entry DOI: 10.7270/Q24J0F7W |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM50029100

(2-(4-Chloro-phenyl)-1-methyl-ethylamine | CHEMBL35...)Show InChI InChI=1S/C9H12ClN/c1-7(11)6-8-2-4-9(10)5-3-8/h2-5,7H,6,11H2,1H3 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 424 | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College
Curated by ChEMBL
| Assay Description
Inhibition of human dopamine transporter |
Eur J Med Chem 44: 4862-88 (2009)
Article DOI: 10.1016/j.ejmech.2009.07.027
BindingDB Entry DOI: 10.7270/Q24J0F7W |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50010588

((RS)-3,4-(methylenedioxy)methamphetamine | 1-(1,3-...)Show InChI InChI=1S/C11H15NO2/c1-8(12-2)5-9-3-4-10-11(6-9)14-7-13-10/h3-4,6,8,12H,5,7H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 425 | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College
Curated by ChEMBL
| Assay Description
Binding affinity to human SERT |
Bioorg Med Chem 18: 4009-31 (2010)
Article DOI: 10.1016/j.bmc.2010.04.022
BindingDB Entry DOI: 10.7270/Q2ST7Q1T |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50010588

((RS)-3,4-(methylenedioxy)methamphetamine | 1-(1,3-...)Show InChI InChI=1S/C11H15NO2/c1-8(12-2)5-9-3-4-10-11(6-9)14-7-13-10/h3-4,6,8,12H,5,7H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 425 | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College
Curated by ChEMBL
| Assay Description
Inhibition of [3H]5-HT uptake at SERT |
Eur J Med Chem 44: 4862-88 (2009)
Article DOI: 10.1016/j.ejmech.2009.07.027
BindingDB Entry DOI: 10.7270/Q24J0F7W |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50310866
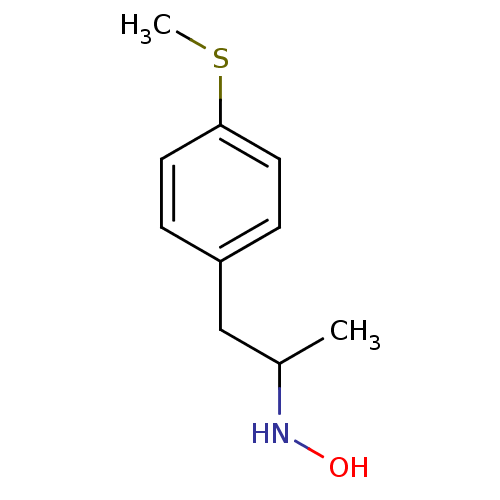
(2-N-Hydroxyamino-1-(4-methylthiophenyl)propane | C...)Show InChI InChI=1S/C10H15NOS/c1-8(11-12)7-9-3-5-10(13-2)6-4-9/h3-6,8,11-12H,7H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 436 | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College
Curated by ChEMBL
| Assay Description
Inhibition of [3H]5-HT reuptake at rat SERT expressed in HEK293 cells after 2 mins by liquid scintillation counting |
Eur J Med Chem 44: 4862-88 (2009)
Article DOI: 10.1016/j.ejmech.2009.07.027
BindingDB Entry DOI: 10.7270/Q24J0F7W |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50310845
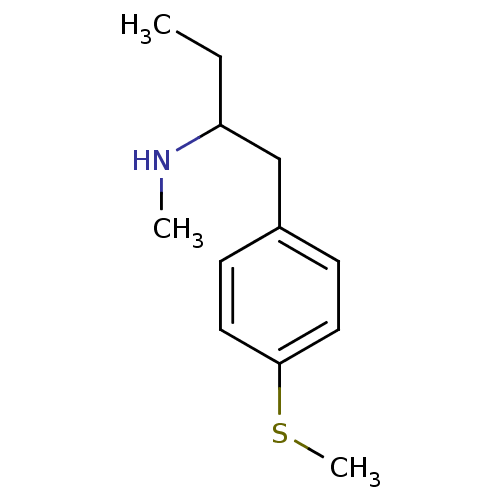
(2-N-Methylamino-1-(4-methylthiophenyl)butane | CHE...)Show InChI InChI=1S/C12H19NS/c1-4-11(13-2)9-10-5-7-12(14-3)8-6-10/h5-8,11,13H,4,9H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 437 | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College
Curated by ChEMBL
| Assay Description
Inhibition of [3H]5-HT reuptake at rat SERT expressed in HEK293 cells after 2 mins by liquid scintillation counting |
Eur J Med Chem 44: 4862-88 (2009)
Article DOI: 10.1016/j.ejmech.2009.07.027
BindingDB Entry DOI: 10.7270/Q24J0F7W |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50005247

((+/-)2-Benzo[1,3]dioxol-5-yl-1-methyl-ethylamine |...)Show InChI InChI=1S/C10H13NO2/c1-7(11)4-8-2-3-9-10(5-8)13-6-12-9/h2-3,5,7H,4,6,11H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 478 | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College
Curated by ChEMBL
| Assay Description
Inhibition of [3H]5-HT uptake at SERT in rat brain synaptosome |
Eur J Med Chem 44: 4862-88 (2009)
Article DOI: 10.1016/j.ejmech.2009.07.027
BindingDB Entry DOI: 10.7270/Q24J0F7W |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50005247

((+/-)2-Benzo[1,3]dioxol-5-yl-1-methyl-ethylamine |...)Show InChI InChI=1S/C10H13NO2/c1-7(11)4-8-2-3-9-10(5-8)13-6-12-9/h2-3,5,7H,4,6,11H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 478 | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College
Curated by ChEMBL
| Assay Description
Binding affinity to human SERT |
Bioorg Med Chem 18: 4009-31 (2010)
Article DOI: 10.1016/j.bmc.2010.04.022
BindingDB Entry DOI: 10.7270/Q2ST7Q1T |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50310837

(2-N-Ethylamino-1-(4-ethylthiophenyl)propane | CHEM...)Show InChI InChI=1S/C13H21NS/c1-4-14-11(3)10-12-6-8-13(9-7-12)15-5-2/h6-9,11,14H,4-5,10H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 478 | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College
Curated by ChEMBL
| Assay Description
Inhibition of [3H]5-HT reuptake at rat SERT expressed in HEK293 cells after 2 mins by liquid scintillation counting |
Eur J Med Chem 44: 4862-88 (2009)
Article DOI: 10.1016/j.ejmech.2009.07.027
BindingDB Entry DOI: 10.7270/Q24J0F7W |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50310863
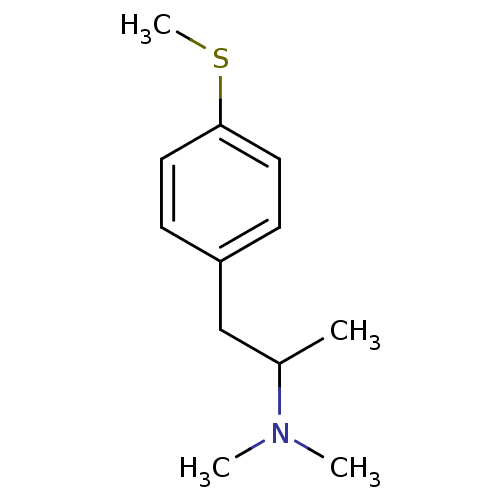
(2-N,N-Dimethylamino-1-(4-methylthiophenyl)propane ...)Show InChI InChI=1S/C12H19NS/c1-10(13(2)3)9-11-5-7-12(14-4)8-6-11/h5-8,10H,9H2,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College
Curated by ChEMBL
| Assay Description
Inhibition of [3H]5-HT reuptake at rat SERT expressed in HEK293 cells after 2 mins by liquid scintillation counting |
Eur J Med Chem 44: 4862-88 (2009)
Article DOI: 10.1016/j.ejmech.2009.07.027
BindingDB Entry DOI: 10.7270/Q24J0F7W |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50310859
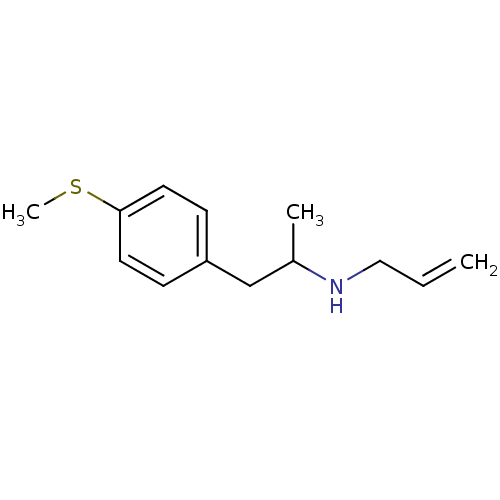
(2-N-Allylamino-1-(4-methylthiophenyl)propan | CHEM...)Show InChI InChI=1S/C13H19NS/c1-4-9-14-11(2)10-12-5-7-13(15-3)8-6-12/h4-8,11,14H,1,9-10H2,2-3H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 524 | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College
Curated by ChEMBL
| Assay Description
Inhibition of [3H]5-HT reuptake at rat SERT expressed in HEK293 cells after 2 mins by liquid scintillation counting |
Eur J Med Chem 44: 4862-88 (2009)
Article DOI: 10.1016/j.ejmech.2009.07.027
BindingDB Entry DOI: 10.7270/Q24J0F7W |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data