

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50174419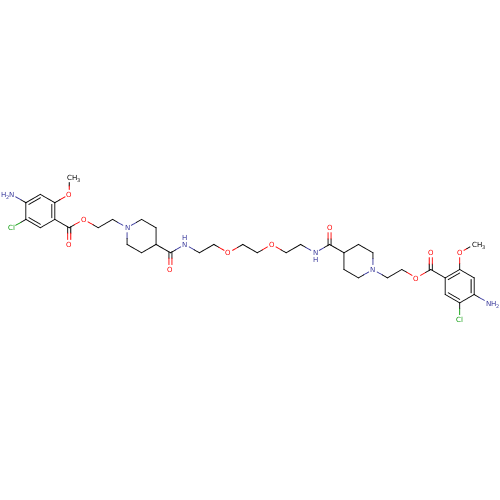 (1-[2-(4-amino-5-chloro-2-methoxyphenylcarbonyloxy)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris XI Curated by ChEMBL | Assay Description Binding affinity for 5-HT4 receptor using [3H]-GR-113,808 | J Med Chem 48: 6220-8 (2005) Article DOI: 10.1021/jm050234z BindingDB Entry DOI: 10.7270/Q22N51V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50000040 (((2S,3S)-2-Benzhydryl-1-aza-bicyclo[2.2.2]oct-3-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoire de Chimie Thérapeutique associé au CNRS et à l'Université René Descartes (UMR 8638) Curated by ChEMBL | Assay Description Binding affinity towards Tachykinin receptor 1 expressed in CHO cells using [3H]-[Pro9]-SP as radioligand | Bioorg Med Chem Lett 11: 659-61 (2001) BindingDB Entry DOI: 10.7270/Q2CJ8CR0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM29526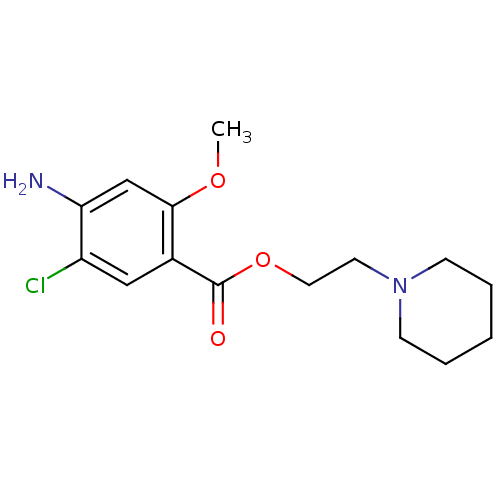 (2-Piperidinoethyl 4-amino-5-chloro-2-methoxybenzoa...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris XI Curated by ChEMBL | Assay Description Binding affinity for 5-HT4 receptor using [3H]-GR-113,808 | J Med Chem 48: 6220-8 (2005) Article DOI: 10.1021/jm050234z BindingDB Entry DOI: 10.7270/Q22N51V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50174440 (1-[2-(4-amino-5-chloro-2-hydroxyphenylcarbonyloxy)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris XI Curated by ChEMBL | Assay Description Binding affinity for 5-HT4 receptor using [3H]-GR-113,808 | J Med Chem 48: 6220-8 (2005) Article DOI: 10.1021/jm050234z BindingDB Entry DOI: 10.7270/Q22N51V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50174432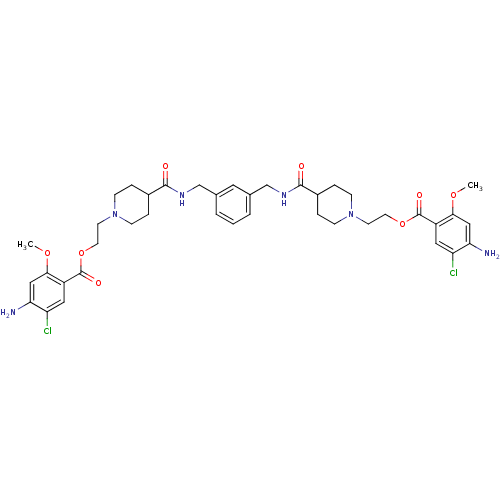 (1-[2-(3-amino-4-chloro-2-methoxyphenylcarbonyloxy)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris XI Curated by ChEMBL | Assay Description Binding affinity for 5-HT4 receptor using [3H]-GR-113,808 | J Med Chem 48: 6220-8 (2005) Article DOI: 10.1021/jm050234z BindingDB Entry DOI: 10.7270/Q22N51V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50174414 (4-(3-{1-[2-(4-amino-5-chloro-2-hydroxyphenylcarbon...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris XI Curated by ChEMBL | Assay Description Binding affinity for 5-HT4 receptor using [3H]-GR-113,808 | J Med Chem 48: 6220-8 (2005) Article DOI: 10.1021/jm050234z BindingDB Entry DOI: 10.7270/Q22N51V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50174416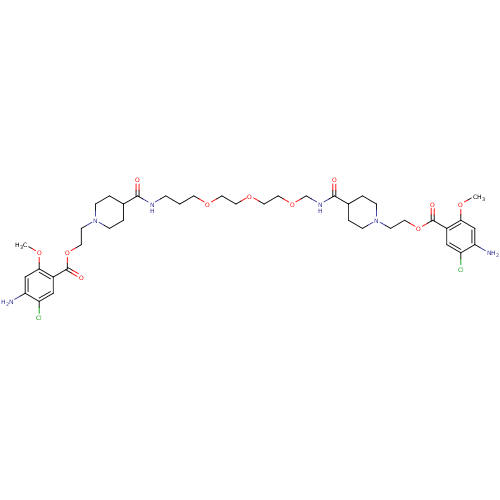 (1-[2-(4-amino-5-chloro-2-methoxyphenylcarbonyloxy)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris XI Curated by ChEMBL | Assay Description Binding affinity for 5-HT4 receptor using [3H]-GR-113,808 | J Med Chem 48: 6220-8 (2005) Article DOI: 10.1021/jm050234z BindingDB Entry DOI: 10.7270/Q22N51V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50174434 (1-[2-(4-amino-5-chloro-2-hydroxyphenylcarbonyloxy)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris XI Curated by ChEMBL | Assay Description Binding affinity for 5-HT4 receptor using [3H]-GR-113,808 | J Med Chem 48: 6220-8 (2005) Article DOI: 10.1021/jm050234z BindingDB Entry DOI: 10.7270/Q22N51V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50174430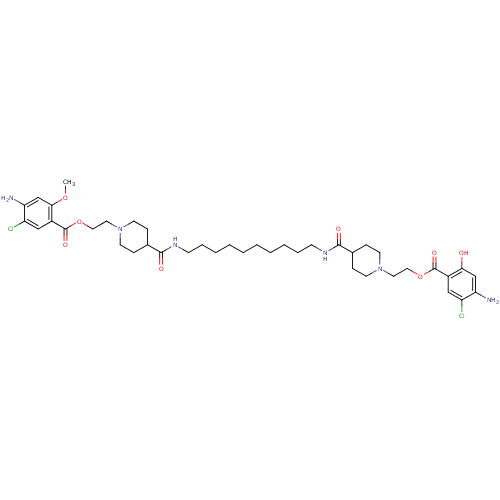 (1-[2-(4-amino-5-chloro-2-hydroxyphenylcarbonyloxy)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris XI Curated by ChEMBL | Assay Description Binding affinity for 5-HT4 receptor using [3H]-GR-113,808 | J Med Chem 48: 6220-8 (2005) Article DOI: 10.1021/jm050234z BindingDB Entry DOI: 10.7270/Q22N51V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50174421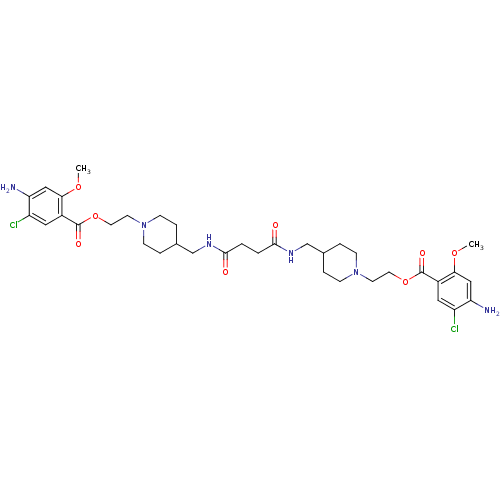 (1-[2-(4-amino-5-chloro-2-methoxyphenylcarbonyloxy)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris XI Curated by ChEMBL | Assay Description Binding affinity for 5-HT4 receptor using [3H]-GR-113,808 | J Med Chem 48: 6220-8 (2005) Article DOI: 10.1021/jm050234z BindingDB Entry DOI: 10.7270/Q22N51V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50174435 (1-[2-(4-amino-5-chloro-2-methoxyphenylcarbonyloxy)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris XI Curated by ChEMBL | Assay Description Binding affinity for 5-HT4 receptor using [3H]-GR-113,808 | J Med Chem 48: 6220-8 (2005) Article DOI: 10.1021/jm050234z BindingDB Entry DOI: 10.7270/Q22N51V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50174420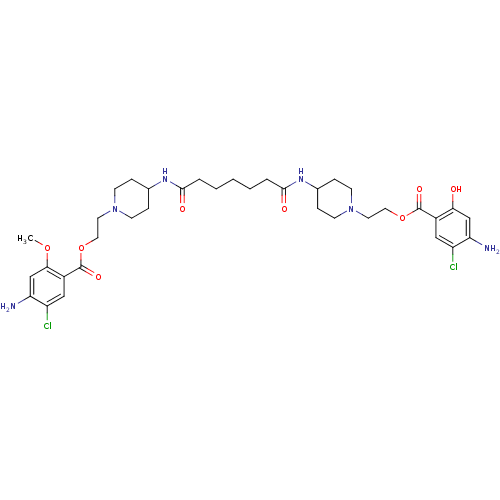 (1-[2-(4-amino-5-chloro-2-hydroxyphenylcarbonyloxy)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris XI Curated by ChEMBL | Assay Description Binding affinity for 5-HT4 receptor using [3H]-GR-113,808 | J Med Chem 48: 6220-8 (2005) Article DOI: 10.1021/jm050234z BindingDB Entry DOI: 10.7270/Q22N51V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50174443 (1-[2-(4-amino-5-chloro-2-methoxyphenylcarbonyloxy)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris XI Curated by ChEMBL | Assay Description Binding affinity for 5-HT4 receptor using [3H]-GR-113,808 | J Med Chem 48: 6220-8 (2005) Article DOI: 10.1021/jm050234z BindingDB Entry DOI: 10.7270/Q22N51V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50174444 (1-[2-(4-amino-5-chloro-2-methoxyphenylcarbonyloxy)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris XI Curated by ChEMBL | Assay Description Binding affinity for 5-HT4 receptor using [3H]-GR-113,808 | J Med Chem 48: 6220-8 (2005) Article DOI: 10.1021/jm050234z BindingDB Entry DOI: 10.7270/Q22N51V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50174422 (1-[2-(4-amino-5-chloro-2-methoxyphenylcarbonyloxy)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris XI Curated by ChEMBL | Assay Description Binding affinity for 5-HT4 receptor using [3H]-GR-113,808 | J Med Chem 48: 6220-8 (2005) Article DOI: 10.1021/jm050234z BindingDB Entry DOI: 10.7270/Q22N51V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50174417 (1-[2-(4-amino-5-chloro-2-methoxyphenylcarbonyloxy)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris XI Curated by ChEMBL | Assay Description Binding affinity for 5-HT4 receptor using [3H]-GR-113,808 | J Med Chem 48: 6220-8 (2005) Article DOI: 10.1021/jm050234z BindingDB Entry DOI: 10.7270/Q22N51V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50174439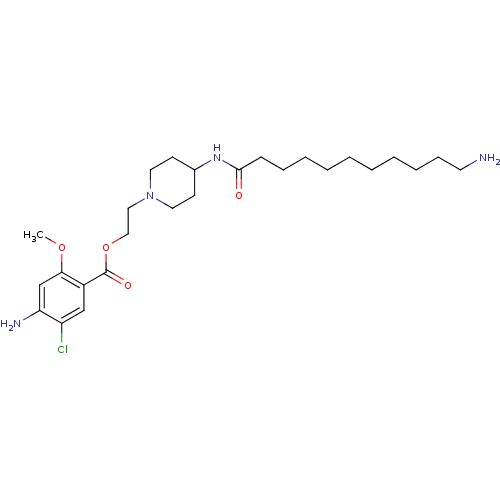 (4-Amino-5-chloro-2-methoxy-benzoic acid 2-[4-(11-a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris XI Curated by ChEMBL | Assay Description Binding affinity for 5-HT4 receptor using [3H]-GR-113,808 | J Med Chem 48: 6220-8 (2005) Article DOI: 10.1021/jm050234z BindingDB Entry DOI: 10.7270/Q22N51V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50174415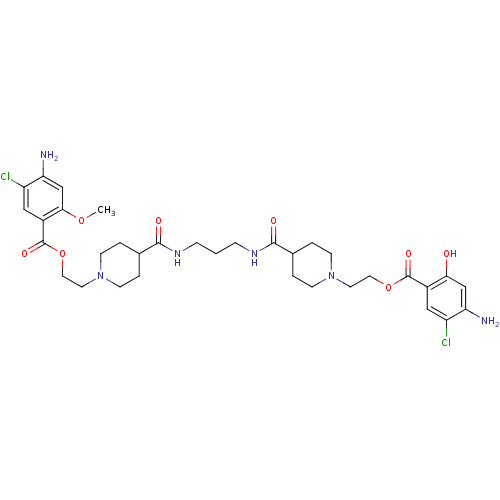 (1-[2-(4-amino-5-chloro-2-hydroxyphenylcarbonyloxy)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris XI Curated by ChEMBL | Assay Description Binding affinity for 5-HT4 receptor using [3H]-GR-113,808 | J Med Chem 48: 6220-8 (2005) Article DOI: 10.1021/jm050234z BindingDB Entry DOI: 10.7270/Q22N51V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50174433 (1-[2-(4-amino-5-chloro-2-methoxyphenylcarbonyloxy)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris XI Curated by ChEMBL | Assay Description Binding affinity for 5-HT4 receptor using [3H]-GR-113,808 | J Med Chem 48: 6220-8 (2005) Article DOI: 10.1021/jm050234z BindingDB Entry DOI: 10.7270/Q22N51V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50174425 (1-[2-(4-amino-5-chloro-2-methoxyphenylcarbonyloxy)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris XI Curated by ChEMBL | Assay Description Binding affinity for 5-HT4 receptor using [3H]-GR-113,808 | J Med Chem 48: 6220-8 (2005) Article DOI: 10.1021/jm050234z BindingDB Entry DOI: 10.7270/Q22N51V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50174442 (4-(10-{1-[2-(4-amino-5-chloro-2-hydroxyphenylcarbo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris XI Curated by ChEMBL | Assay Description Binding affinity for 5-HT4 receptor using [3H]-GR-113,808 | J Med Chem 48: 6220-8 (2005) Article DOI: 10.1021/jm050234z BindingDB Entry DOI: 10.7270/Q22N51V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50174431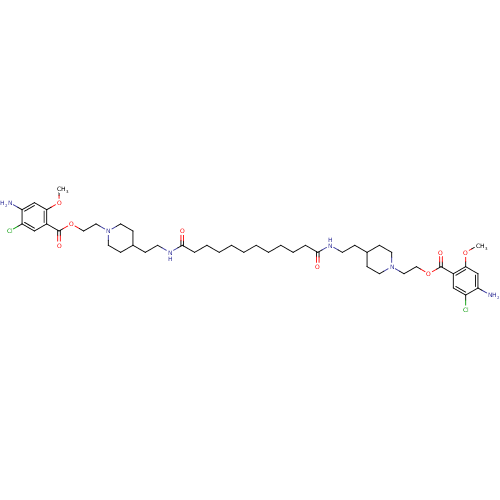 (1-[2-(4-amino-5-chloro-2-methoxyphenylcarbonyloxy)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris XI Curated by ChEMBL | Assay Description Binding affinity for 5-HT4 receptor using [3H]-GR-113,808 | J Med Chem 48: 6220-8 (2005) Article DOI: 10.1021/jm050234z BindingDB Entry DOI: 10.7270/Q22N51V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50174427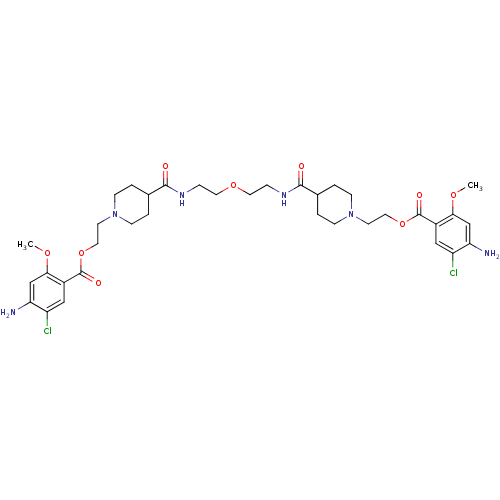 (1-[2-(4-amino-5-chloro-2-methoxyphenylcarbonyloxy)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris XI Curated by ChEMBL | Assay Description Binding affinity for 5-HT4 receptor using [3H]-GR-113,808 | J Med Chem 48: 6220-8 (2005) Article DOI: 10.1021/jm050234z BindingDB Entry DOI: 10.7270/Q22N51V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50174424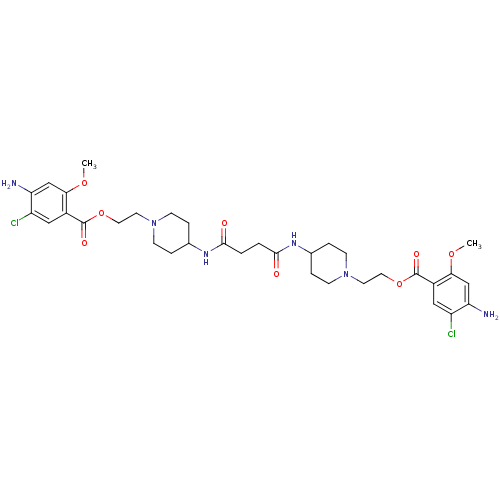 (4-(2-{1-[2-(4-amino-5-chloro-2-hydroxyphenylcarbon...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris XI Curated by ChEMBL | Assay Description Binding affinity for 5-HT4 receptor using [3H]-GR-113,808 | J Med Chem 48: 6220-8 (2005) Article DOI: 10.1021/jm050234z BindingDB Entry DOI: 10.7270/Q22N51V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50174428 (1-[2-(3-amino-4-chloro-2-methoxyphenylcarbonyloxy)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris XI Curated by ChEMBL | Assay Description Binding affinity for 5-HT4 receptor using [3H]-GR-113,808 | J Med Chem 48: 6220-8 (2005) Article DOI: 10.1021/jm050234z BindingDB Entry DOI: 10.7270/Q22N51V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50174437 (1-[2-(4-amino-5-chloro-2-methoxyphenylcarbonyloxy)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris XI Curated by ChEMBL | Assay Description Binding affinity for 5-HT4 receptor using [3H]-GR-113,808 | J Med Chem 48: 6220-8 (2005) Article DOI: 10.1021/jm050234z BindingDB Entry DOI: 10.7270/Q22N51V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50485783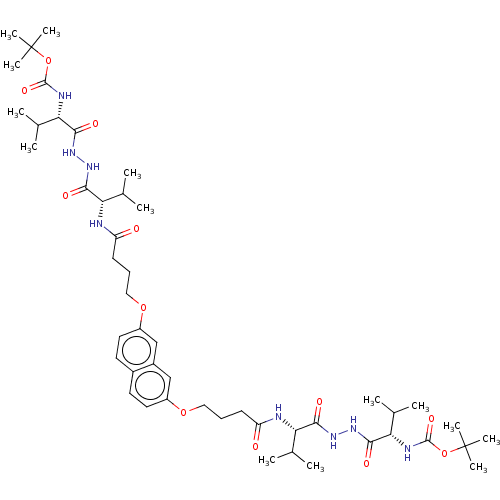 (CHEMBL2164408) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Paris-Sud 11 Curated by ChEMBL | Assay Description Inhibition of HIV1 protease dimerization expressed in Escherichia coli Rosetta(DE3) using DABCYL-gamma-abu-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-EDANS as s... | J Med Chem 55: 6762-75 (2012) Article DOI: 10.1021/jm300181j BindingDB Entry DOI: 10.7270/Q2125WJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50156909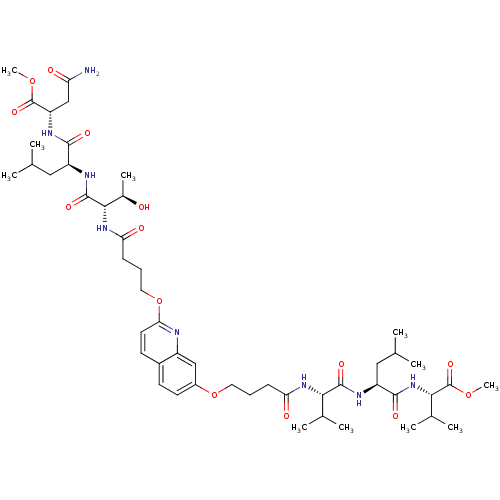 (CHEMBL376817 | methyl (2S)-3-carbamoyl-2-[(2S)-2-[...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris-Sud Curated by ChEMBL | Assay Description Inhibition of dimerization of HIV1 protease | J Med Chem 47: 6392-400 (2004) Article DOI: 10.1021/jm040833q BindingDB Entry DOI: 10.7270/Q2CV4H7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50485784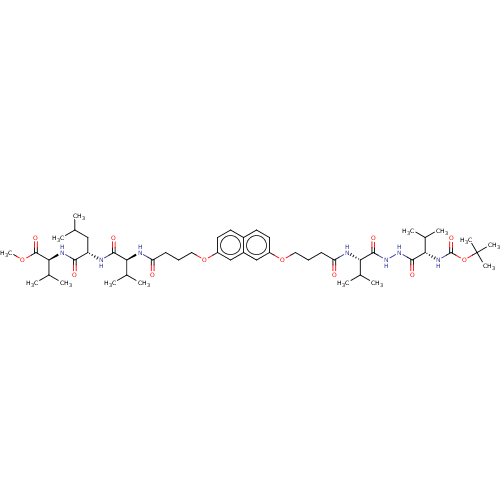 (CHEMBL2164407) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Paris-Sud 11 Curated by ChEMBL | Assay Description Inhibition of HIV1 protease dimerization expressed in Escherichia coli Rosetta(DE3) using DABCYL-gamma-abu-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-EDANS as s... | J Med Chem 55: 6762-75 (2012) Article DOI: 10.1021/jm300181j BindingDB Entry DOI: 10.7270/Q2125WJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50191463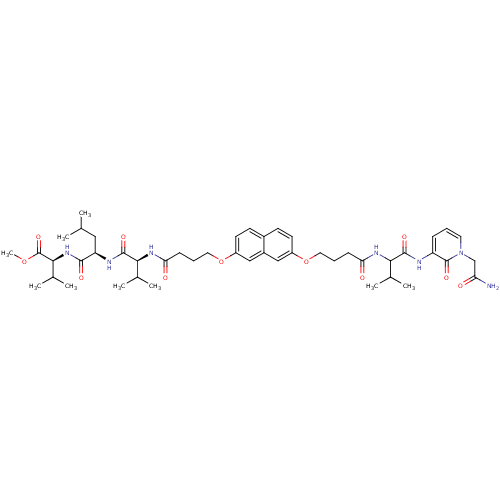 (CHEMBL378467 | methyl (2S)-2-[(2R)-2-[(2S)-2-{4-[(...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris-Sud XI Curated by ChEMBL | Assay Description Inhibition of dimerization of HIV1 protease 150V mutant | J Med Chem 49: 4657-64 (2006) Article DOI: 10.1021/jm060576k BindingDB Entry DOI: 10.7270/Q2KD1XJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50156906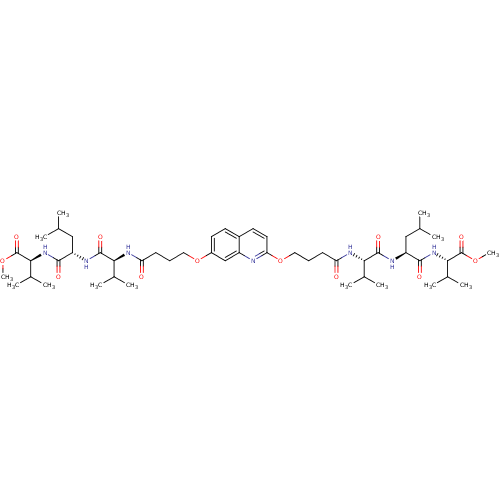 (CHEMBL375050 | methyl (2S)-2-[(2S)-2-[(2S)-2-(4-{[...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris-Sud Curated by ChEMBL | Assay Description Inhibition of dimerization of HIV1 protease | J Med Chem 47: 6392-400 (2004) Article DOI: 10.1021/jm040833q BindingDB Entry DOI: 10.7270/Q2CV4H7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50097646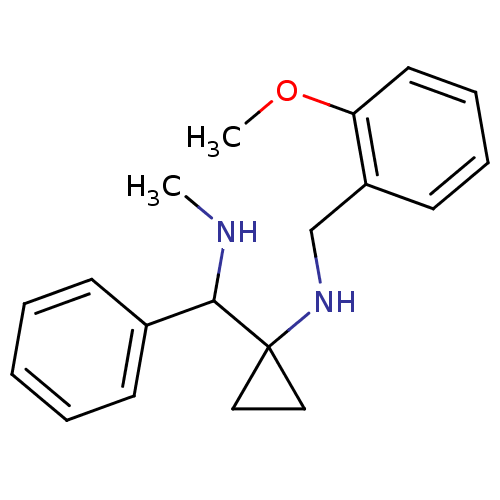 ((2-Methoxy-benzyl)-[1-(methylamino-phenyl-methyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoire de Chimie Thérapeutique associé au CNRS et à l'Université René Descartes (UMR 8638) Curated by ChEMBL | Assay Description Binding affinity towards Tachykinin receptor 1 expressed in CHO cells using [3H]-[Pro9]-SP as radioligand | Bioorg Med Chem Lett 11: 659-61 (2001) BindingDB Entry DOI: 10.7270/Q2CJ8CR0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50485785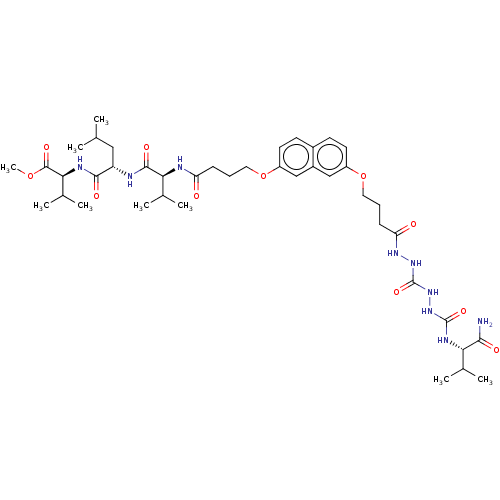 (CHEMBL2164409) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Paris-Sud 11 Curated by ChEMBL | Assay Description Inhibition of HIV1 protease dimerization expressed in Escherichia coli Rosetta(DE3) using DABCYL-gamma-abu-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-EDANS as s... | J Med Chem 55: 6762-75 (2012) Article DOI: 10.1021/jm300181j BindingDB Entry DOI: 10.7270/Q2125WJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50191463 (CHEMBL378467 | methyl (2S)-2-[(2R)-2-[(2S)-2-{4-[(...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris-Sud XI Curated by ChEMBL | Assay Description Inhibition of dimerization of HIV1 protease ANAM-11 mutant | J Med Chem 49: 4657-64 (2006) Article DOI: 10.1021/jm060576k BindingDB Entry DOI: 10.7270/Q2KD1XJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50174423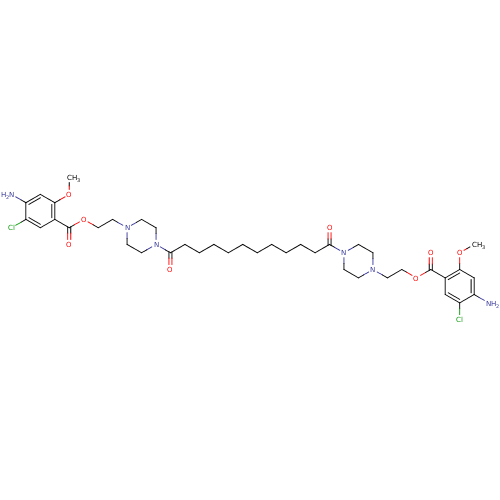 (1-[2-(3-amino-4-chloro-2-methoxyphenylcarbonyloxy)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris XI Curated by ChEMBL | Assay Description Binding affinity for 5-HT4 receptor using [3H]-GR-113,808 | J Med Chem 48: 6220-8 (2005) Article DOI: 10.1021/jm050234z BindingDB Entry DOI: 10.7270/Q22N51V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50191464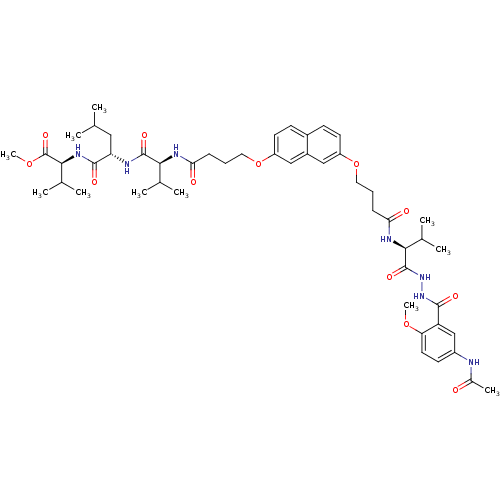 (CHEMBL212749 | methyl (2S)-2-[(2S)-2-[(2S)-2-(4-{[...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris-Sud XI Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 protease dimerization | J Med Chem 49: 4657-64 (2006) Article DOI: 10.1021/jm060576k BindingDB Entry DOI: 10.7270/Q2KD1XJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50156901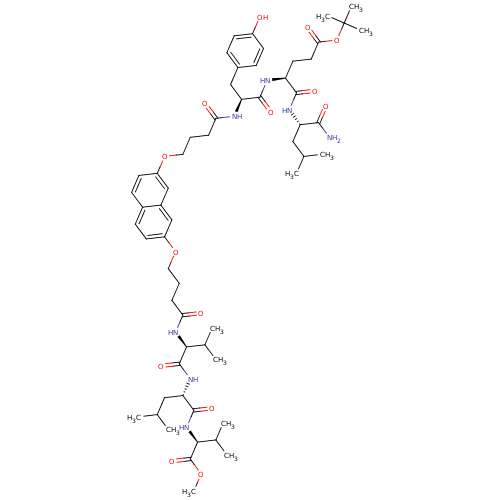 ((S)-tert-butyl 5-((S)-1-amino-4-methyl-1-oxopentan...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris-Sud Curated by ChEMBL | Assay Description Inhibition of dimerization of HIV1 protease | J Med Chem 47: 6392-400 (2004) Article DOI: 10.1021/jm040833q BindingDB Entry DOI: 10.7270/Q2CV4H7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50191463 (CHEMBL378467 | methyl (2S)-2-[(2R)-2-[(2S)-2-{4-[(...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris-Sud XI Curated by ChEMBL | Assay Description Inhibition of dimerization of HIV1 protease V82A mutant | J Med Chem 49: 4657-64 (2006) Article DOI: 10.1021/jm060576k BindingDB Entry DOI: 10.7270/Q2KD1XJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50156902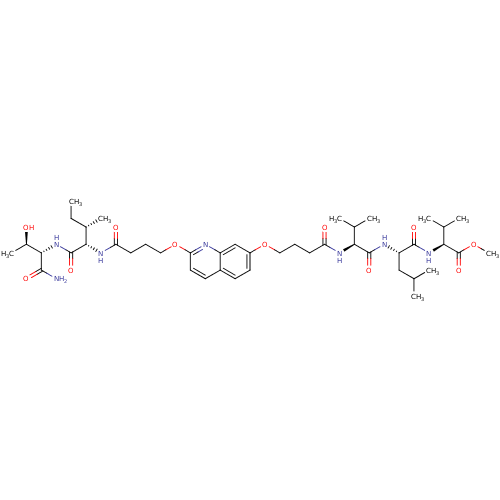 ((S)-methyl 2-((S)-2-((S)-2-(4-(2-(4-((2S,3S)-1-((2...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris-Sud Curated by ChEMBL | Assay Description Inhibition of dimerization of HIV1 protease | J Med Chem 47: 6392-400 (2004) Article DOI: 10.1021/jm040833q BindingDB Entry DOI: 10.7270/Q2CV4H7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50191463 (CHEMBL378467 | methyl (2S)-2-[(2R)-2-[(2S)-2-{4-[(...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris-Sud XI Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 protease dimerization | J Med Chem 49: 4657-64 (2006) Article DOI: 10.1021/jm060576k BindingDB Entry DOI: 10.7270/Q2KD1XJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50156903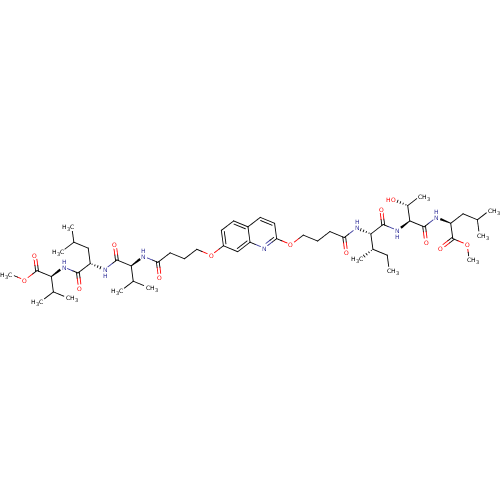 ((S)-methyl 2-((2S,3R)-3-hydroxy-2-((2S,3S)-2-(4-(7...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris-Sud Curated by ChEMBL | Assay Description Inhibition of dimerization of HIV1 protease | J Med Chem 47: 6392-400 (2004) Article DOI: 10.1021/jm040833q BindingDB Entry DOI: 10.7270/Q2CV4H7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50156910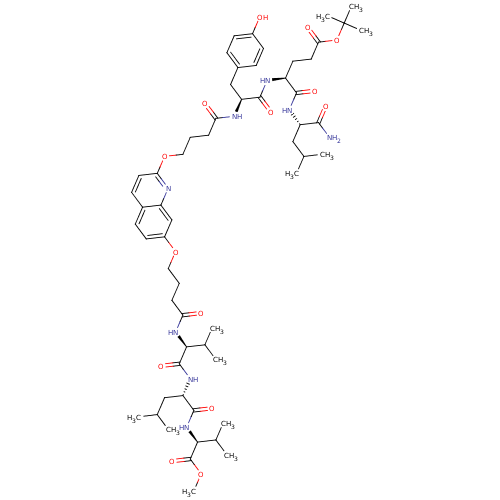 ((S)-tert-butyl 5-((S)-1-amino-4-methyl-1-oxopentan...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris-Sud Curated by ChEMBL | Assay Description Inhibition of dimerization of HIV1 protease | J Med Chem 47: 6392-400 (2004) Article DOI: 10.1021/jm040833q BindingDB Entry DOI: 10.7270/Q2CV4H7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50156908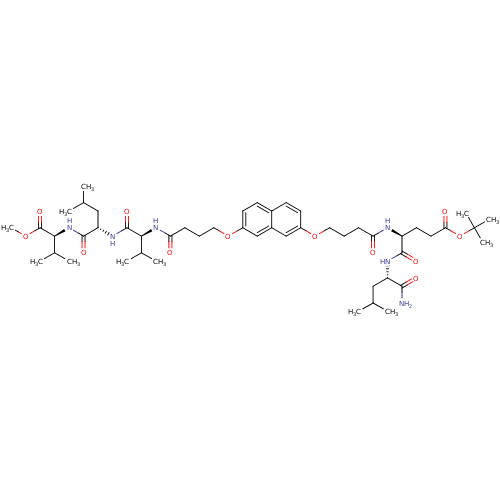 ((S)-tert-butyl 5-((S)-1-amino-4-methyl-1-oxopentan...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris-Sud Curated by ChEMBL | Assay Description Inhibition of dimerization of HIV1 protease | J Med Chem 47: 6392-400 (2004) Article DOI: 10.1021/jm040833q BindingDB Entry DOI: 10.7270/Q2CV4H7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50174438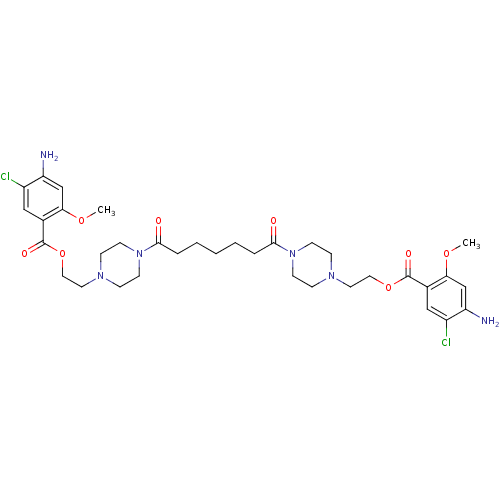 (1-[2-(3-amino-4-chloro-2-methoxyphenylcarbonyloxy)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 488 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris XI Curated by ChEMBL | Assay Description Binding affinity for 5-HT4 receptor using [3H]-GR-113,808 | J Med Chem 48: 6220-8 (2005) Article DOI: 10.1021/jm050234z BindingDB Entry DOI: 10.7270/Q22N51V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50191463 (CHEMBL378467 | methyl (2S)-2-[(2R)-2-[(2S)-2-{4-[(...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris-Sud XI Curated by ChEMBL | Assay Description Inhibition of dimerization of HIV1 protease G48V/L90M mutant | J Med Chem 49: 4657-64 (2006) Article DOI: 10.1021/jm060576k BindingDB Entry DOI: 10.7270/Q2KD1XJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50191463 (CHEMBL378467 | methyl (2S)-2-[(2R)-2-[(2S)-2-{4-[(...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris-Sud XI Curated by ChEMBL | Assay Description Inhibition of dimerization of HIV1 protease D30N mutant | J Med Chem 49: 4657-64 (2006) Article DOI: 10.1021/jm060576k BindingDB Entry DOI: 10.7270/Q2KD1XJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50156904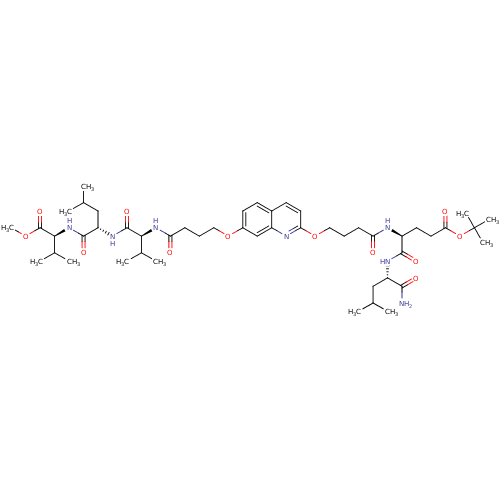 ((S)-tert-butyl 5-((S)-1-amino-4-methyl-1-oxopentan...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris-Sud Curated by ChEMBL | Assay Description Inhibition of dimerization of HIV1 protease | J Med Chem 47: 6392-400 (2004) Article DOI: 10.1021/jm040833q BindingDB Entry DOI: 10.7270/Q2CV4H7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50074687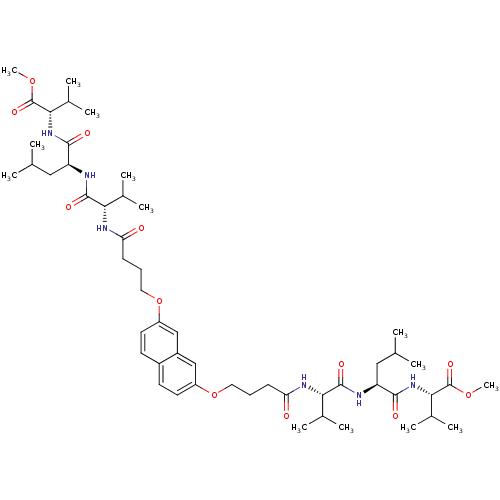 (2-[2-(2-{4-[7-(3-{1-[1-(1-Methoxycarbonyl-2-methyl...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Paris-Sud 11 Curated by ChEMBL | Assay Description Inhibition of HIV1 protease dimerization expressed in Escherichia coli Rosetta(DE3) using DABCYL-gamma-abu-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-EDANS as s... | J Med Chem 55: 6762-75 (2012) Article DOI: 10.1021/jm300181j BindingDB Entry DOI: 10.7270/Q2125WJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50074687 (2-[2-(2-{4-[7-(3-{1-[1-(1-Methoxycarbonyl-2-methyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris-Sud Curated by ChEMBL | Assay Description Inhibition of dimerization of HIV1 protease | J Med Chem 47: 6392-400 (2004) Article DOI: 10.1021/jm040833q BindingDB Entry DOI: 10.7270/Q2CV4H7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50097647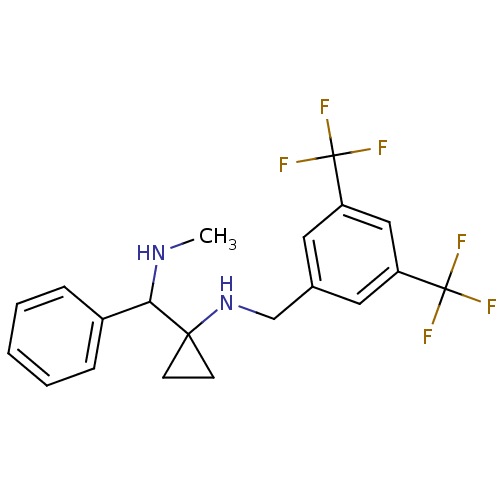 ((3,5-Bis-trifluoromethyl-benzyl)-[1-(methylamino-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoire de Chimie Thérapeutique associé au CNRS et à l'Université René Descartes (UMR 8638) Curated by ChEMBL | Assay Description Binding affinity towards Tachykinin receptor 1 expressed in CHO cells using [3H]-[Pro9]-SP as radioligand | Bioorg Med Chem Lett 11: 659-61 (2001) BindingDB Entry DOI: 10.7270/Q2CJ8CR0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 77 total ) | Next | Last >> |