

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM1803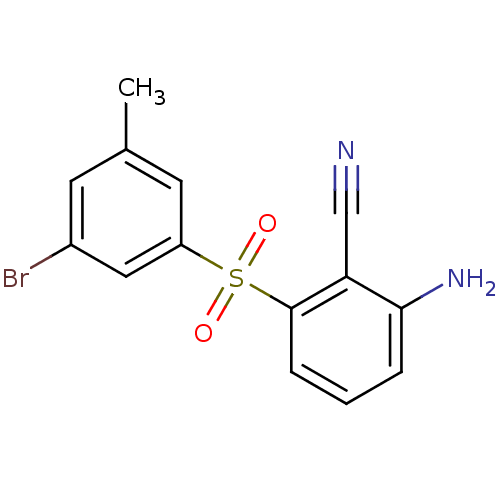 (2-Amino-6-arylthiobenzonitrile deriv. 3w | 2-amino...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 44: 1866-82 (2001) Article DOI: 10.1021/jm0004906 BindingDB Entry DOI: 10.7270/Q2FJ2F09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM1804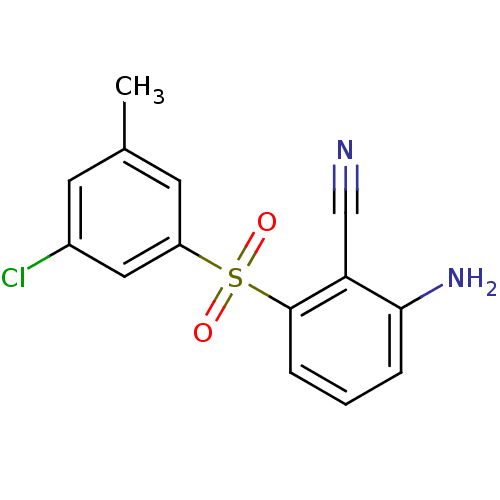 (2-Amino-6-arylthiobenzonitrile deriv. 3x | 2-amino...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 44: 1866-82 (2001) Article DOI: 10.1021/jm0004906 BindingDB Entry DOI: 10.7270/Q2FJ2F09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM1802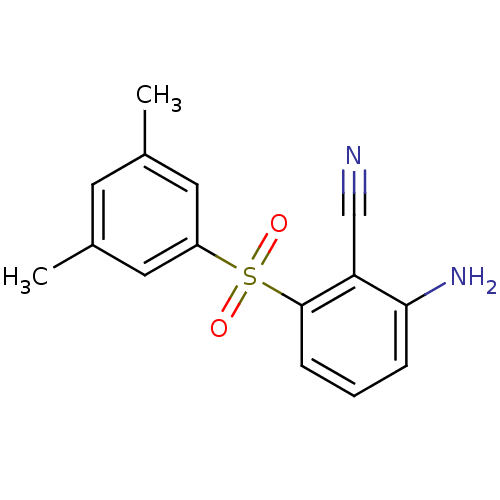 (2-Amino-6-arylthiobenzonitrile deriv. 3v, 739W94 |...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 44: 1866-82 (2001) Article DOI: 10.1021/jm0004906 BindingDB Entry DOI: 10.7270/Q2FJ2F09 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM1805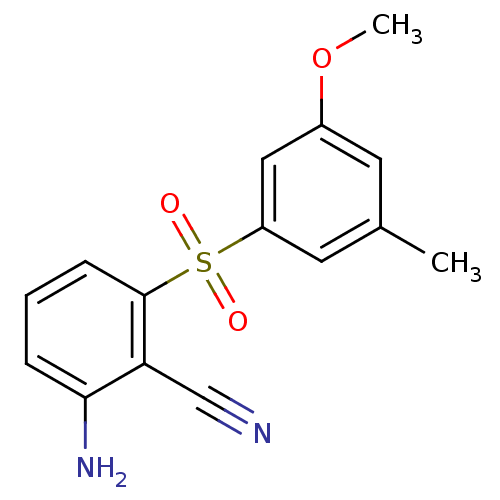 (2-Amino-6-arylthiobenzonitrile deriv. 3y | 2-amino...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 44: 1866-82 (2001) Article DOI: 10.1021/jm0004906 BindingDB Entry DOI: 10.7270/Q2FJ2F09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uridine phosphorylase 1 (Mus musculus) | BDBM50030972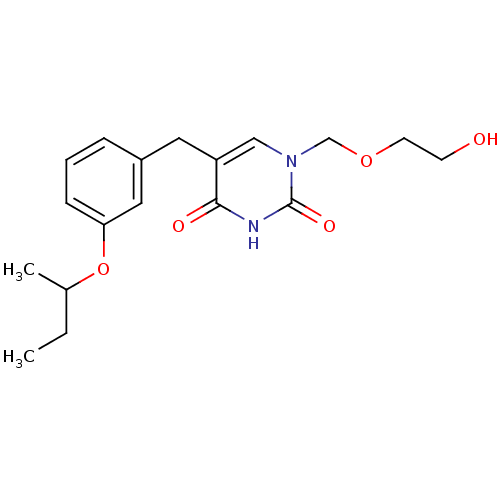 (5-(3-sec-Butoxy-benzyl)-1-(2-hydroxy-ethoxymethyl)...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description Inhibition of uridine phosphorylase (UrdPase) from murine liver. | J Med Chem 38: 3850-6 (1995) BindingDB Entry DOI: 10.7270/Q2B27T91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM1778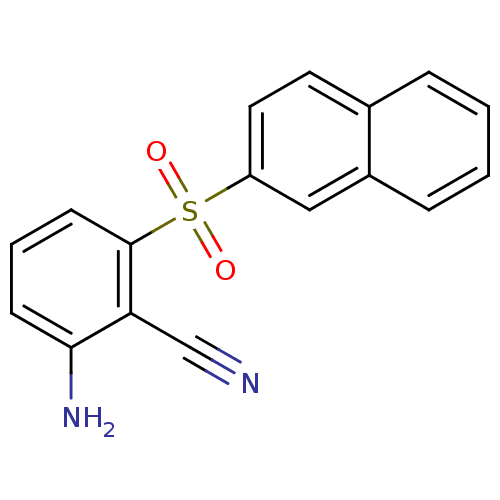 (2-Amino-6-arylthiobenzonitrile deriv. 3ff | 2-amin...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 44: 1866-82 (2001) Article DOI: 10.1021/jm0004906 BindingDB Entry DOI: 10.7270/Q2FJ2F09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM1801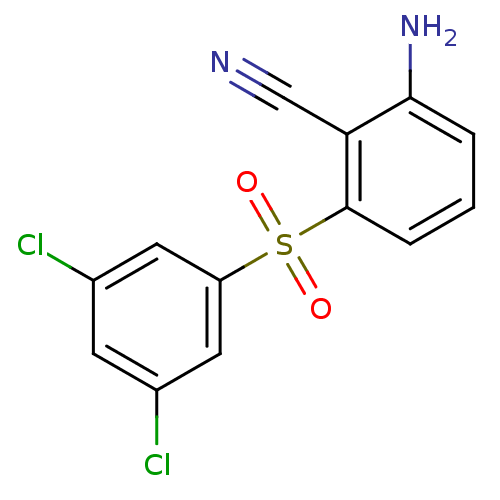 (2-Amino-6-arylthiobenzonitrile deriv. 3u | 2-amino...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 44: 1866-82 (2001) Article DOI: 10.1021/jm0004906 BindingDB Entry DOI: 10.7270/Q2FJ2F09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM1806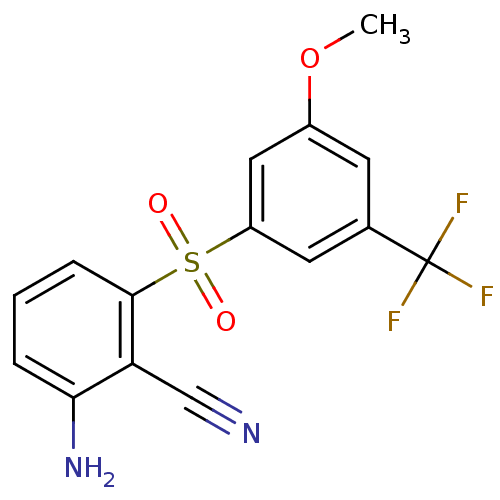 (2-Amino-6-arylthiobenzonitrile deriv. 3z | 2-amino...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 44: 1866-82 (2001) Article DOI: 10.1021/jm0004906 BindingDB Entry DOI: 10.7270/Q2FJ2F09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uridine phosphorylase 1 (Mus musculus) | BDBM50031005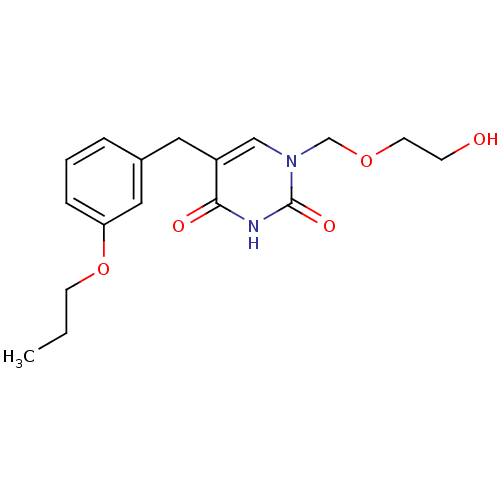 (1-(2-Hydroxy-ethoxymethyl)-5-(3-propoxy-benzyl)-1H...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description Inhibition of uridine phosphorylase (UrdPase) from murine liver. | J Med Chem 38: 3850-6 (1995) BindingDB Entry DOI: 10.7270/Q2B27T91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uridine phosphorylase 1 (Mus musculus) | BDBM50030974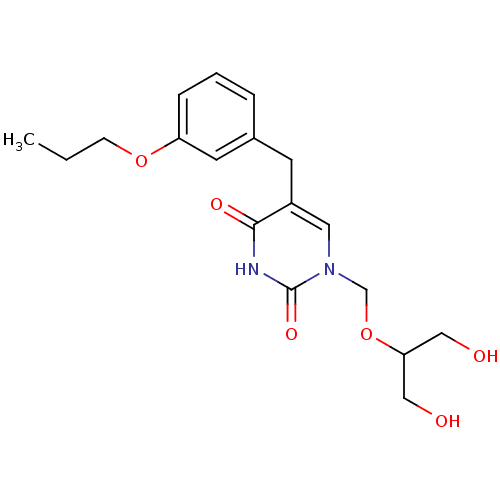 (1-(2-Hydroxy-1-hydroxymethyl-ethoxymethyl)-5-(3-pr...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description Inhibition of uridine phosphorylase (UrdPase) from murine liver. | J Med Chem 38: 3850-6 (1995) BindingDB Entry DOI: 10.7270/Q2B27T91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uridine phosphorylase 1 (Mus musculus) | BDBM50031009 (1-(2-Hydroxy-ethoxymethyl)-5-(3-isobutoxy-benzyl)-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description Inhibition of uridine phosphorylase (UrdPase) from murine liver. | J Med Chem 38: 3850-6 (1995) BindingDB Entry DOI: 10.7270/Q2B27T91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uridine phosphorylase 1 (Mus musculus) | BDBM50030990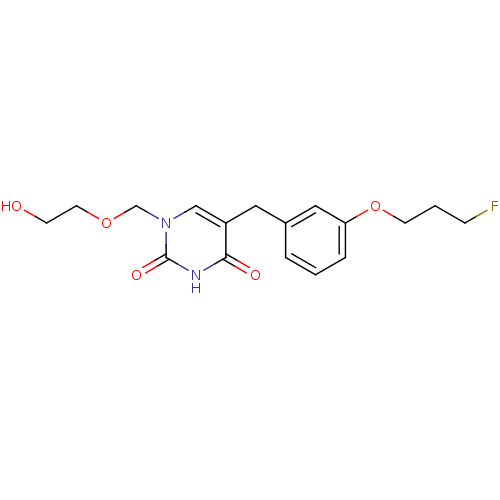 (5-[3-(3-Fluoro-propoxy)-benzyl]-1-(2-hydroxy-ethox...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description Inhibition of uridine phosphorylase (UrdPase) from murine liver. | J Med Chem 38: 3850-6 (1995) BindingDB Entry DOI: 10.7270/Q2B27T91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uridine phosphorylase 1 (Mus musculus) | BDBM50030980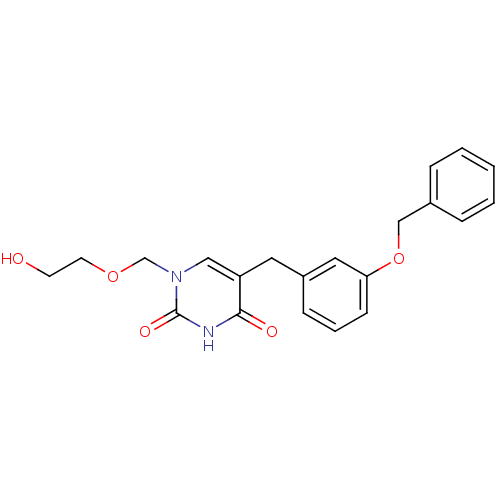 (1-((2-HYDROXYETHOXY)METHYL)-5-(3-(BENZYLOXY)BENZYL...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 84 | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description Inhibition of uridine phosphorylase (UrdPase) from murine liver. | J Med Chem 38: 3850-6 (1995) BindingDB Entry DOI: 10.7270/Q2B27T91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uridine phosphorylase 1 (Mus musculus) | BDBM50031006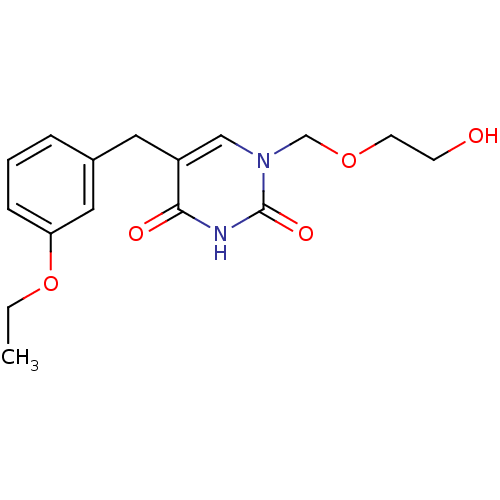 (5-(3-Ethoxy-benzyl)-1-(2-hydroxy-ethoxymethyl)-1H-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description Inhibition of uridine phosphorylase (UrdPase) from murine liver. | J Med Chem 38: 3850-6 (1995) BindingDB Entry DOI: 10.7270/Q2B27T91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uridine phosphorylase 1 (Mus musculus) | BDBM50030999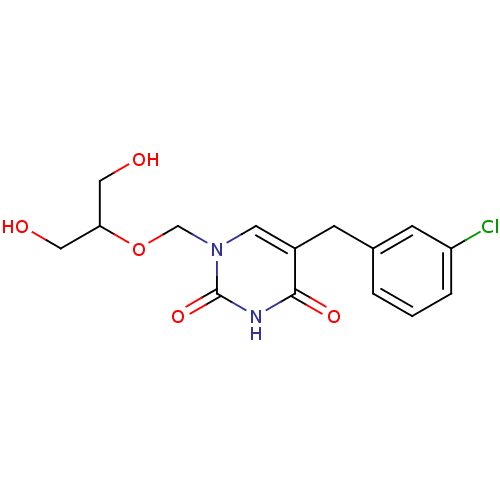 (5-(3-Chloro-benzyl)-1-(2-hydroxy-1-hydroxymethyl-e...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description Inhibition of uridine phosphorylase (UrdPase) from murine liver. | J Med Chem 38: 3850-6 (1995) BindingDB Entry DOI: 10.7270/Q2B27T91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uridine phosphorylase 1 (Mus musculus) | BDBM50031007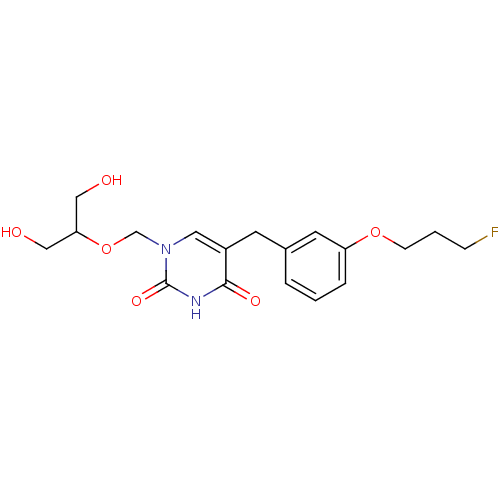 (5-[3-(3-Fluoro-propoxy)-benzyl]-1-(2-hydroxy-1-hyd...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description Inhibition of uridine phosphorylase (UrdPase) from murine liver. | J Med Chem 38: 3850-6 (1995) BindingDB Entry DOI: 10.7270/Q2B27T91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM1751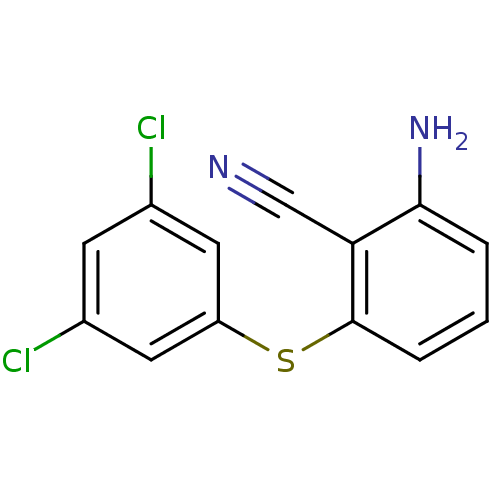 (2-Amino-6-arylthiobenzonitrile deriv. 1u | 2-amino...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | 8.0 | n/a |
GlaxoSmithKline | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 44: 1866-82 (2001) Article DOI: 10.1021/jm0004906 BindingDB Entry DOI: 10.7270/Q2FJ2F09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uridine phosphorylase 1 (Mus musculus) | BDBM50030983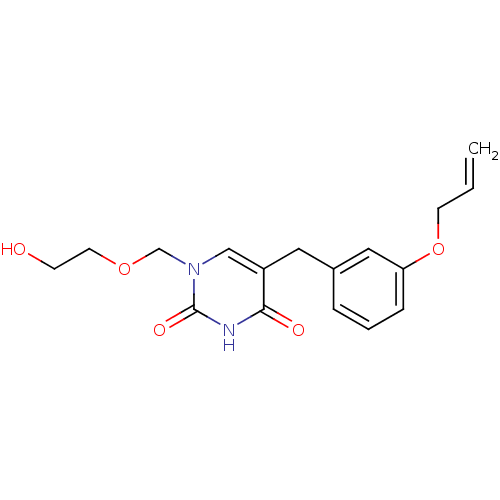 (5-(3-Allyloxy-benzyl)-1-(2-hydroxy-ethoxymethyl)-1...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description Inhibition of uridine phosphorylase (UrdPase) from murine liver. | J Med Chem 38: 3850-6 (1995) BindingDB Entry DOI: 10.7270/Q2B27T91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM1753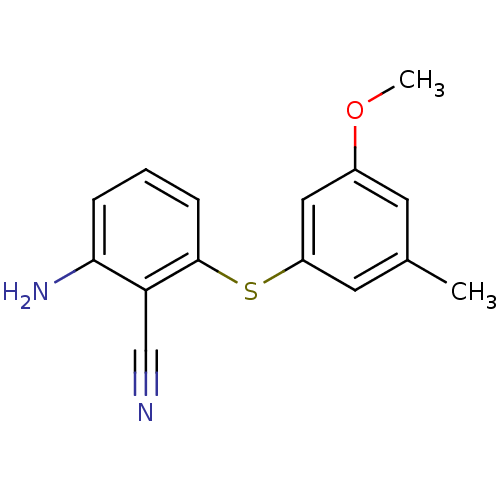 (2-Amino-6-arylthiobenzonitrile deriv. 1w | 2-amino...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | 8.0 | n/a |
GlaxoSmithKline | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 44: 1866-82 (2001) Article DOI: 10.1021/jm0004906 BindingDB Entry DOI: 10.7270/Q2FJ2F09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uridine phosphorylase 1 (Mus musculus) | BDBM50031004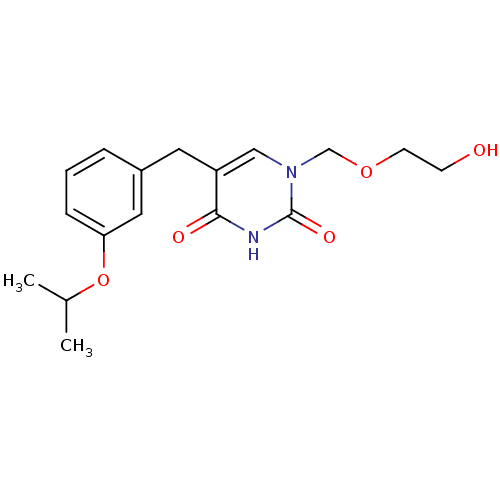 (1-(2-Hydroxy-ethoxymethyl)-5-(3-isopropoxy-benzyl)...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description Inhibition of uridine phosphorylase (UrdPase) from murine liver. | J Med Chem 38: 3850-6 (1995) BindingDB Entry DOI: 10.7270/Q2B27T91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uridine phosphorylase 1 (Mus musculus) | BDBM50030995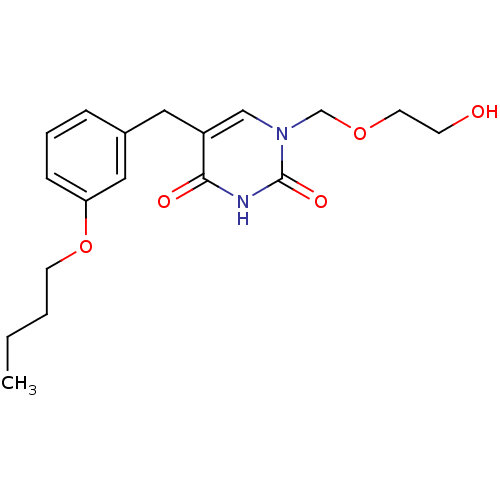 (5-(3-Butoxy-benzyl)-1-(2-hydroxy-ethoxymethyl)-1H-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description Inhibition of uridine phosphorylase (UrdPase) from murine liver. | J Med Chem 38: 3850-6 (1995) BindingDB Entry DOI: 10.7270/Q2B27T91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM1786 (2-Amino-6-arylthiobenzonitrile deriv. 3f | 2-amino...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 44: 1866-82 (2001) Article DOI: 10.1021/jm0004906 BindingDB Entry DOI: 10.7270/Q2FJ2F09 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50122955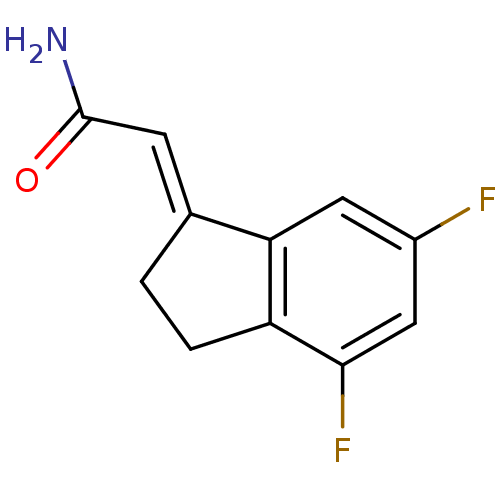 (2-(4,6-Difluoro-indan-1-ylidene)-acetamide | CHEMB...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibitory concentration of the compound against Monoamine oxidase B (MAO-B) | J Med Chem 46: 399-408 (2003) Article DOI: 10.1021/jm020067s BindingDB Entry DOI: 10.7270/Q2VH5N7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM1792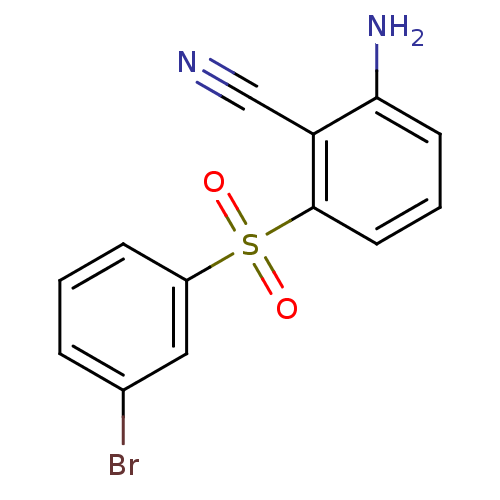 (2-Amino-6-arylthiobenzonitrile deriv. 3l | 2-amino...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 44: 1866-82 (2001) Article DOI: 10.1021/jm0004906 BindingDB Entry DOI: 10.7270/Q2FJ2F09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uridine phosphorylase 1 (Mus musculus) | BDBM50030984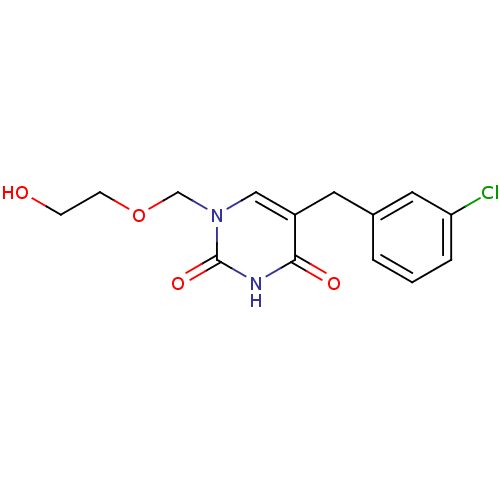 (5-(3-Chloro-benzyl)-1-(2-hydroxy-ethoxymethyl)-1H-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description Inhibition of uridine phosphorylase (UrdPase) from murine liver. | J Med Chem 38: 3850-6 (1995) BindingDB Entry DOI: 10.7270/Q2B27T91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM1800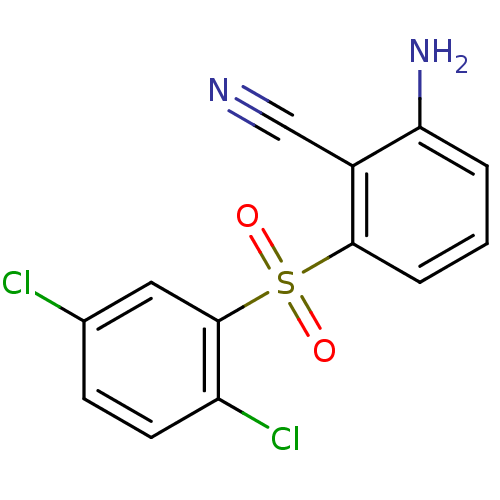 (2-Amino-6-arylthiobenzonitrile deriv. 3t | 2-amino...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 44: 1866-82 (2001) Article DOI: 10.1021/jm0004906 BindingDB Entry DOI: 10.7270/Q2FJ2F09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uridine phosphorylase 1 (Mus musculus) | BDBM50030981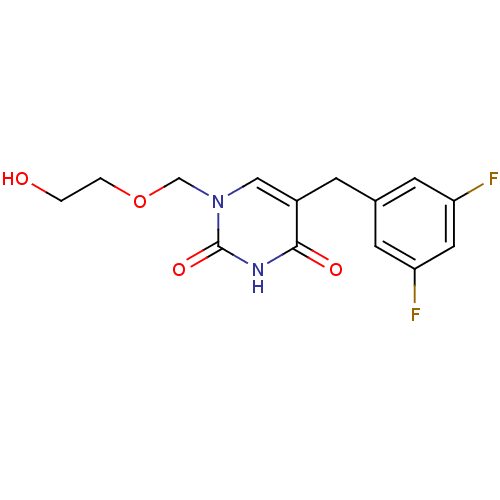 (5-(3,5-Difluoro-benzyl)-1-(2-hydroxy-ethoxymethyl)...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description Inhibition of uridine phosphorylase (UrdPase) from murine liver. | J Med Chem 38: 3850-6 (1995) BindingDB Entry DOI: 10.7270/Q2B27T91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM1789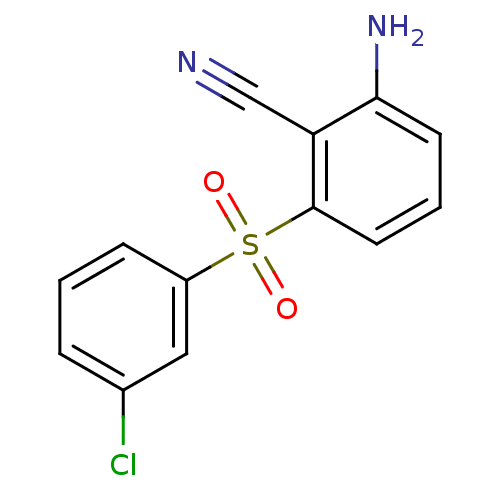 (2-Amino-6-arylthiobenzonitrile deriv. 3i | 2-amino...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 44: 1866-82 (2001) Article DOI: 10.1021/jm0004906 BindingDB Entry DOI: 10.7270/Q2FJ2F09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM1776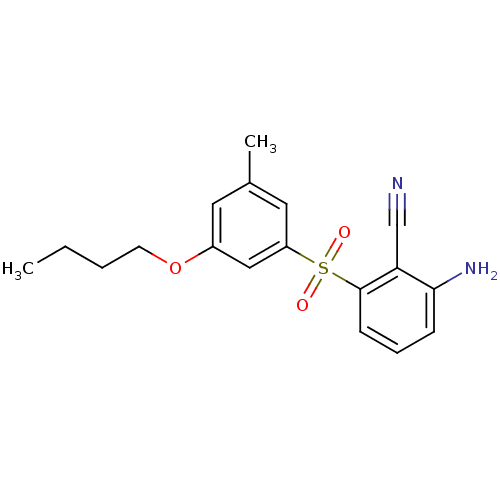 (2-Amino-6-arylthiobenzonitrile deriv. 3dd | 2-amin...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 44: 1866-82 (2001) Article DOI: 10.1021/jm0004906 BindingDB Entry DOI: 10.7270/Q2FJ2F09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uridine phosphorylase 1 (Mus musculus) | BDBM50026387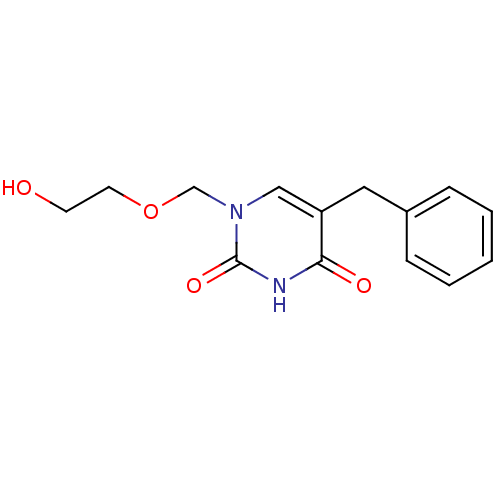 (1-((2-HYDROXYETHOXY)METHYL)-5-BENZYLPYRIMIDINE-2,4...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description Inhibition of uridine phosphorylase (UrdPase) from murine liver. | J Med Chem 38: 3850-6 (1995) BindingDB Entry DOI: 10.7270/Q2B27T91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM1769 (2-Amino-6-arylthiobenzonitrile deriv. 2n | 2-amino...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 44: 1866-82 (2001) Article DOI: 10.1021/jm0004906 BindingDB Entry DOI: 10.7270/Q2FJ2F09 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM1771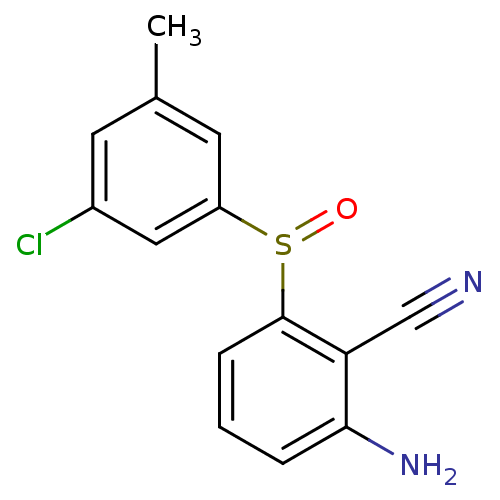 (2-Amino-6-arylthiobenzonitrile deriv. 2p | 2-amino...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 44: 1866-82 (2001) Article DOI: 10.1021/jm0004906 BindingDB Entry DOI: 10.7270/Q2FJ2F09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uridine phosphorylase 1 (Mus musculus) | BDBM50030976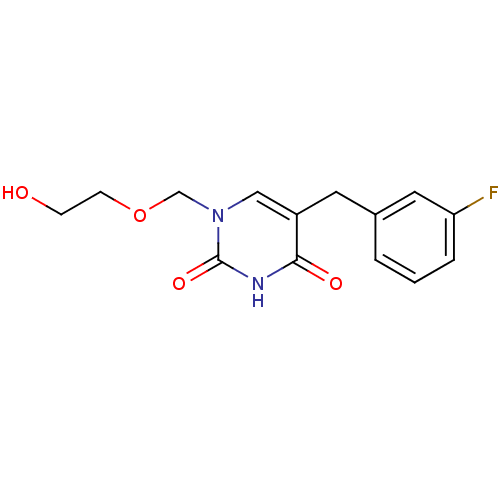 (5-(3-Fluoro-benzyl)-1-(2-hydroxy-ethoxymethyl)-1H-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description Inhibition of uridine phosphorylase (UrdPase) from murine liver. | J Med Chem 38: 3850-6 (1995) BindingDB Entry DOI: 10.7270/Q2B27T91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM1783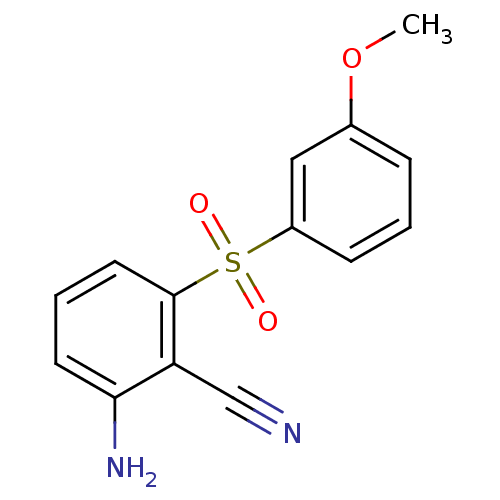 (2-Amino-6-arylthiobenzonitrile deriv. 3c | 2-amino...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 44: 1866-82 (2001) Article DOI: 10.1021/jm0004906 BindingDB Entry DOI: 10.7270/Q2FJ2F09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uridine phosphorylase 1 (Mus musculus) | BDBM50031001 (5-(3-Propoxy-benzyl)-1H-pyrimidine-2,4-dione | CHE...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 840 | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description Inhibition of uridine phosphorylase (UrdPase) from murine liver. | J Med Chem 38: 3850-6 (1995) BindingDB Entry DOI: 10.7270/Q2B27T91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uridine phosphorylase 1 (Mus musculus) | BDBM50030975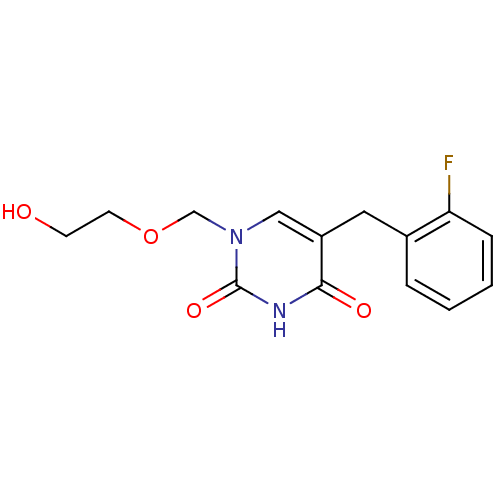 (5-(2-Fluoro-benzyl)-1-(2-hydroxy-ethoxymethyl)-1H-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 870 | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description Inhibition of uridine phosphorylase (UrdPase) from murine liver. | J Med Chem 38: 3850-6 (1995) BindingDB Entry DOI: 10.7270/Q2B27T91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM1772 (2-Amino-6-arylthiobenzonitrile deriv. 2q | 2-amino...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 44: 1866-82 (2001) Article DOI: 10.1021/jm0004906 BindingDB Entry DOI: 10.7270/Q2FJ2F09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM1736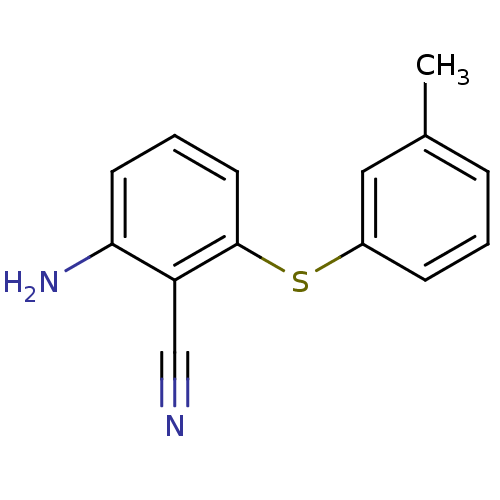 (2-Amino-6-arylthiobenzonitrile deriv. 1f | 2-amino...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 960 | n/a | n/a | n/a | n/a | 8.0 | n/a |
GlaxoSmithKline | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 44: 1866-82 (2001) Article DOI: 10.1021/jm0004906 BindingDB Entry DOI: 10.7270/Q2FJ2F09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM1777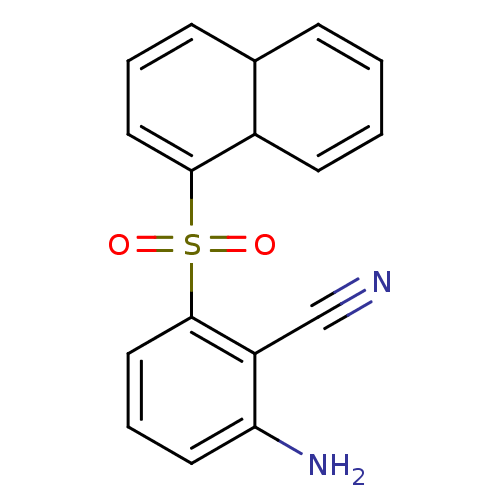 (2-(4a,8a-dihydronaphthalene-1-sulfonyl)-6-aminoben...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 44: 1866-82 (2001) Article DOI: 10.1021/jm0004906 BindingDB Entry DOI: 10.7270/Q2FJ2F09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM1745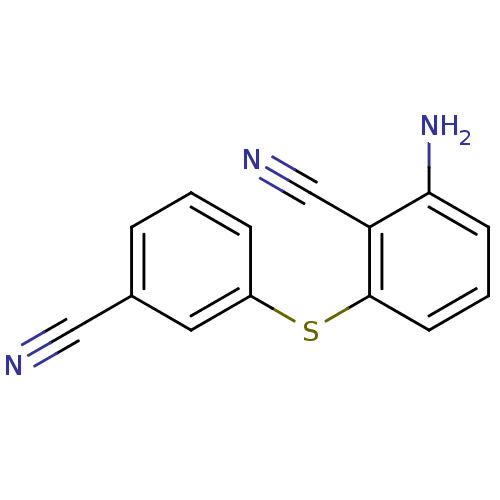 (2-Amino-6-arylthiobenzonitrile deriv. 1o | 2-amino...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | 8.0 | n/a |
GlaxoSmithKline | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 44: 1866-82 (2001) Article DOI: 10.1021/jm0004906 BindingDB Entry DOI: 10.7270/Q2FJ2F09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uridine phosphorylase 1 (Mus musculus) | BDBM50031000 (5-(3-sec-Butoxy-benzyl)-1H-pyrimidine-2,4-dione | ...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description Inhibition of uridine phosphorylase (UrdPase) from murine liver. | J Med Chem 38: 3850-6 (1995) BindingDB Entry DOI: 10.7270/Q2B27T91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM1750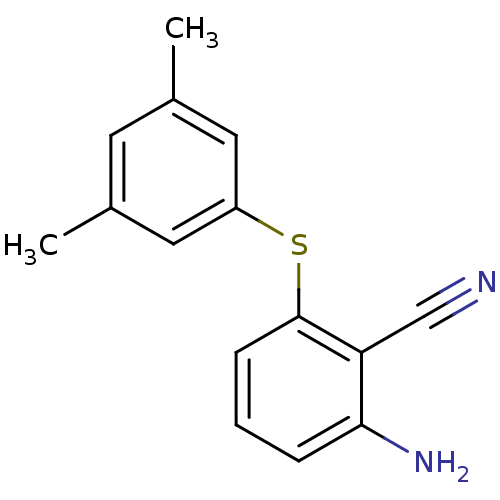 (2-Amino-6-arylthiobenzonitrile deriv. 1t | 2-amino...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | 8.0 | n/a |
GlaxoSmithKline | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 44: 1866-82 (2001) Article DOI: 10.1021/jm0004906 BindingDB Entry DOI: 10.7270/Q2FJ2F09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uridine phosphorylase 1 (Mus musculus) | BDBM50031002 (5-(3-Isobutoxy-benzyl)-1H-pyrimidine-2,4-dione | C...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description Inhibition of uridine phosphorylase (UrdPase) from murine liver. | J Med Chem 38: 3850-6 (1995) BindingDB Entry DOI: 10.7270/Q2B27T91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM1782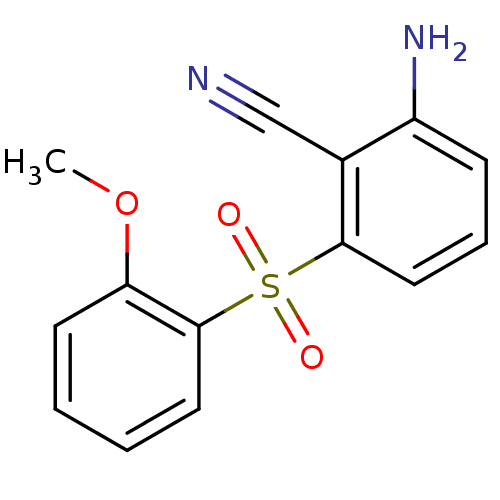 (2-Amino-6-arylthiobenzonitrile deriv. 3b | 2-amino...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 44: 1866-82 (2001) Article DOI: 10.1021/jm0004906 BindingDB Entry DOI: 10.7270/Q2FJ2F09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uridine phosphorylase 1 (Mus musculus) | BDBM50031012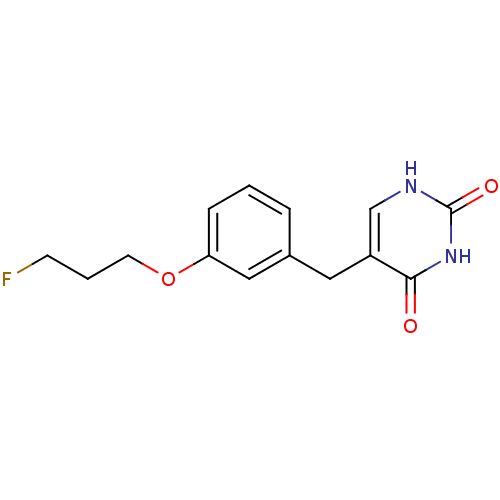 (5-[3-(3-Fluoro-propoxy)-benzyl]-1H-pyrimidine-2,4-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description Inhibition of uridine phosphorylase (UrdPase) from murine liver. | J Med Chem 38: 3850-6 (1995) BindingDB Entry DOI: 10.7270/Q2B27T91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM1733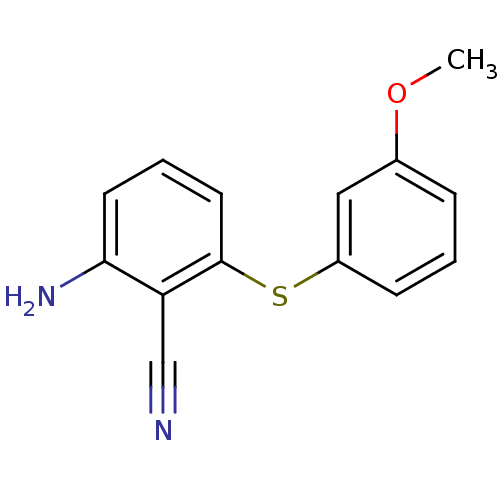 (2-Amino-6-arylthiobenzonitrile deriv. 1c | 2-amino...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | 8.0 | n/a |
GlaxoSmithKline | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 44: 1866-82 (2001) Article DOI: 10.1021/jm0004906 BindingDB Entry DOI: 10.7270/Q2FJ2F09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM1752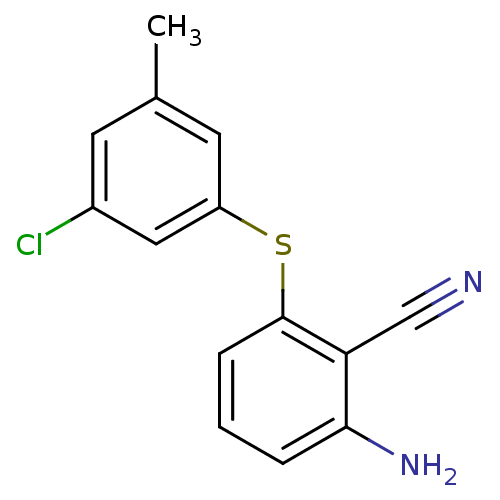 (2-Amino-6-arylthiobenzonitrile deriv. 1v | 2-amino...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | 8.0 | n/a |
GlaxoSmithKline | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 44: 1866-82 (2001) Article DOI: 10.1021/jm0004906 BindingDB Entry DOI: 10.7270/Q2FJ2F09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM1797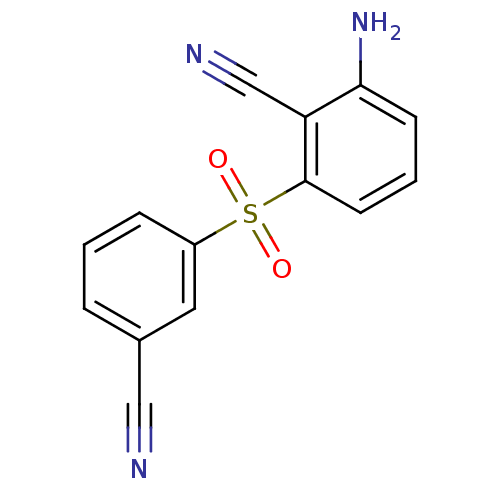 (2-Amino-6-arylthiobenzonitrile deriv. 3q | 2-amino...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 44: 1866-82 (2001) Article DOI: 10.1021/jm0004906 BindingDB Entry DOI: 10.7270/Q2FJ2F09 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Uridine phosphorylase 1 (Mus musculus) | BDBM50031010 (1-(2-Hydroxy-ethoxymethyl)-5-(3-methoxy-benzyl)-1H...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.86E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description The compound was tested for inhibition of uridine phosphorylase (UrdPase) from murine liver Value refers to activity for apparent Ki value | J Med Chem 38: 3850-6 (1995) BindingDB Entry DOI: 10.7270/Q2B27T91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uridine phosphorylase 1 (Mus musculus) | BDBM50030992 (5-(3-Isopropoxy-benzyl)-1H-pyrimidine-2,4-dione | ...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description Inhibition of uridine phosphorylase (UrdPase) from murine liver. | J Med Chem 38: 3850-6 (1995) BindingDB Entry DOI: 10.7270/Q2B27T91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 122 total ) | Next | Last >> |