

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sigma non-opioid intracellular receptor 1 (Cavia porcellus (Guinea pig)) | BDBM50036738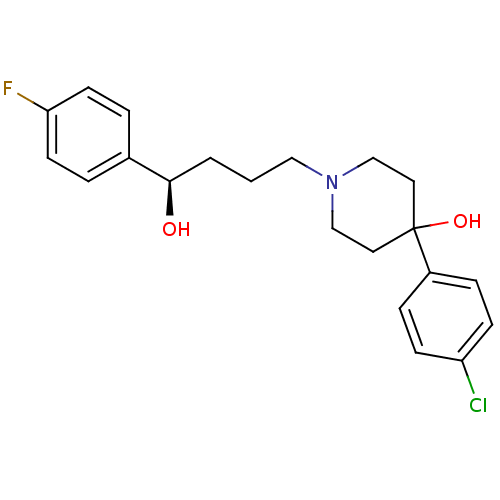 ((R)-4-(4-Chloro-phenyl)-1-[4-(4-fluoro-phenyl)-4-h...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Chieti Gabriele D'Annunzio Curated by ChEMBL | Assay Description Displacement of [3H]-(+)-pentazocine from sigma 1 receptor guinea pig brain membranes incubated for 150 mins by scintillation counting method | Eur J Med Chem 90: 1-9 (2015) Article DOI: 10.1016/j.ejmech.2014.11.012 BindingDB Entry DOI: 10.7270/Q2WS8VWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Chieti Gabriele D'Annunzio Curated by ChEMBL | Assay Description Displacement of [3H]-spiperone from dopamine D2 receptor in rat striatum by scintillation counting method | Eur J Med Chem 90: 1-9 (2015) Article DOI: 10.1016/j.ejmech.2014.11.012 BindingDB Entry DOI: 10.7270/Q2WS8VWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Cavia porcellus (Guinea pig)) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Chieti Gabriele D'Annunzio Curated by ChEMBL | Assay Description Displacement of [3H]-(+)-pentazocine from sigma 1 receptor guinea pig brain membranes incubated for 150 mins by scintillation counting method | Eur J Med Chem 90: 1-9 (2015) Article DOI: 10.1016/j.ejmech.2014.11.012 BindingDB Entry DOI: 10.7270/Q2WS8VWN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sigma non-opioid intracellular receptor 1 (Cavia porcellus (Guinea pig)) | BDBM50036734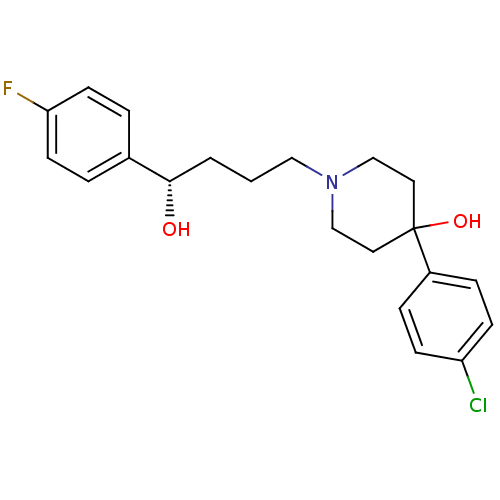 ((S)-4-(4-Chloro-phenyl)-1-[4-(4-fluoro-phenyl)-4-h...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Chieti Gabriele D'Annunzio Curated by ChEMBL | Assay Description Displacement of [3H]-(+)-pentazocine from sigma 1 receptor guinea pig brain membranes incubated for 150 mins by scintillation counting method | Eur J Med Chem 90: 1-9 (2015) Article DOI: 10.1016/j.ejmech.2014.11.012 BindingDB Entry DOI: 10.7270/Q2WS8VWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Rattus norvegicus (Rat)) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Chieti Gabriele D'Annunzio Curated by ChEMBL | Assay Description Displacement of [3H]-7-OH-DPAT from dopamine D3 receptor in rat olfactory tubercle by scintillation counting method | Eur J Med Chem 90: 1-9 (2015) Article DOI: 10.1016/j.ejmech.2014.11.012 BindingDB Entry DOI: 10.7270/Q2WS8VWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Cavia porcellus (Guinea pig)) | BDBM50009307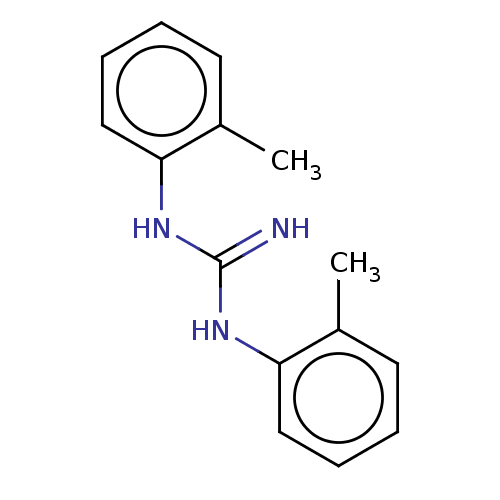 (DITOLYLGUANIDINE | Di-o-tolylguanidine) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Chieti Gabriele D'Annunzio Curated by ChEMBL | Assay Description Displacement of [3H]-(+)-pentazocine from sigma 1 receptor guinea pig brain membranes incubated for 150 mins by scintillation counting method | Eur J Med Chem 90: 1-9 (2015) Article DOI: 10.1016/j.ejmech.2014.11.012 BindingDB Entry DOI: 10.7270/Q2WS8VWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50036734 ((S)-4-(4-Chloro-phenyl)-1-[4-(4-fluoro-phenyl)-4-h...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Chieti Gabriele D'Annunzio Curated by ChEMBL | Assay Description Displacement of [3H]-spiperone from dopamine D2 receptor in rat striatum by scintillation counting method | Eur J Med Chem 90: 1-9 (2015) Article DOI: 10.1016/j.ejmech.2014.11.012 BindingDB Entry DOI: 10.7270/Q2WS8VWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Cavia porcellus (Guinea pig)) | BDBM50059789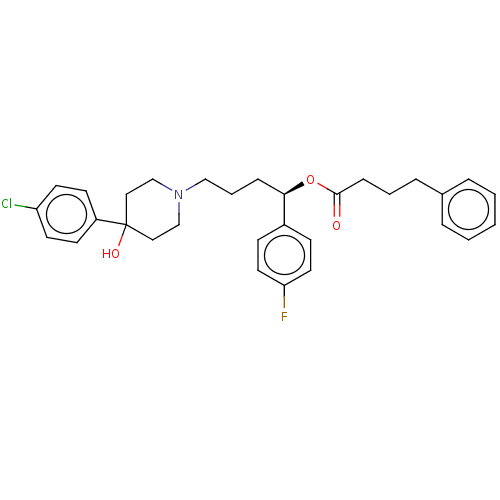 (CHEMBL3393789) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 88 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Chieti Gabriele D'Annunzio Curated by ChEMBL | Assay Description Displacement of [3H]-(+)-pentazocine from sigma 1 receptor guinea pig brain membranes incubated for 150 mins by scintillation counting method | Eur J Med Chem 90: 1-9 (2015) Article DOI: 10.1016/j.ejmech.2014.11.012 BindingDB Entry DOI: 10.7270/Q2WS8VWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Cavia porcellus (Guinea pig)) | BDBM50059787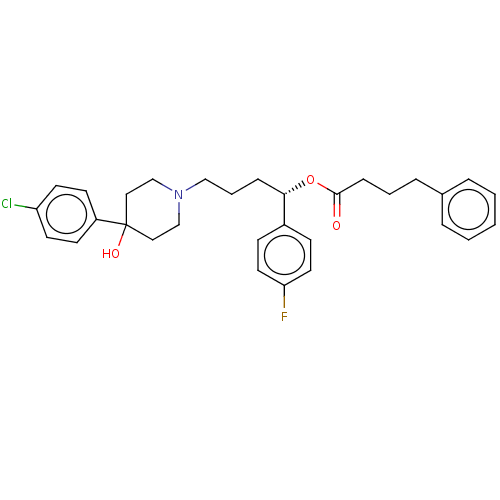 (CHEMBL3393790) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Chieti Gabriele D'Annunzio Curated by ChEMBL | Assay Description Displacement of [3H]-(+)-pentazocine from sigma 1 receptor guinea pig brain membranes incubated for 150 mins by scintillation counting method | Eur J Med Chem 90: 1-9 (2015) Article DOI: 10.1016/j.ejmech.2014.11.012 BindingDB Entry DOI: 10.7270/Q2WS8VWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50036738 ((R)-4-(4-Chloro-phenyl)-1-[4-(4-fluoro-phenyl)-4-h...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 256 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Chieti Gabriele D'Annunzio Curated by ChEMBL | Assay Description Displacement of [3H]-spiperone from dopamine D2 receptor in rat striatum by scintillation counting method | Eur J Med Chem 90: 1-9 (2015) Article DOI: 10.1016/j.ejmech.2014.11.012 BindingDB Entry DOI: 10.7270/Q2WS8VWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Rattus norvegicus (Rat)) | BDBM50036734 ((S)-4-(4-Chloro-phenyl)-1-[4-(4-fluoro-phenyl)-4-h...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 353 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Chieti Gabriele D'Annunzio Curated by ChEMBL | Assay Description Displacement of [3H]-7-OH-DPAT from dopamine D3 receptor in rat olfactory tubercle by scintillation counting method | Eur J Med Chem 90: 1-9 (2015) Article DOI: 10.1016/j.ejmech.2014.11.012 BindingDB Entry DOI: 10.7270/Q2WS8VWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Rattus norvegicus (Rat)) | BDBM50036738 ((R)-4-(4-Chloro-phenyl)-1-[4-(4-fluoro-phenyl)-4-h...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Chieti Gabriele D'Annunzio Curated by ChEMBL | Assay Description Displacement of [3H]-7-OH-DPAT from dopamine D3 receptor in rat olfactory tubercle by scintillation counting method | Eur J Med Chem 90: 1-9 (2015) Article DOI: 10.1016/j.ejmech.2014.11.012 BindingDB Entry DOI: 10.7270/Q2WS8VWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50059787 (CHEMBL3393790) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Chieti Gabriele D'Annunzio Curated by ChEMBL | Assay Description Displacement of [3H]-spiperone from dopamine D2 receptor in rat striatum by scintillation counting method | Eur J Med Chem 90: 1-9 (2015) Article DOI: 10.1016/j.ejmech.2014.11.012 BindingDB Entry DOI: 10.7270/Q2WS8VWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Rattus norvegicus (Rat)) | BDBM50059789 (CHEMBL3393789) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Chieti Gabriele D'Annunzio Curated by ChEMBL | Assay Description Displacement of [3H]-7-OH-DPAT from dopamine D3 receptor in rat olfactory tubercle by scintillation counting method | Eur J Med Chem 90: 1-9 (2015) Article DOI: 10.1016/j.ejmech.2014.11.012 BindingDB Entry DOI: 10.7270/Q2WS8VWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Rattus norvegicus (Rat)) | BDBM50059787 (CHEMBL3393790) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Chieti Gabriele D'Annunzio Curated by ChEMBL | Assay Description Displacement of [3H]-7-OH-DPAT from dopamine D3 receptor in rat olfactory tubercle by scintillation counting method | Eur J Med Chem 90: 1-9 (2015) Article DOI: 10.1016/j.ejmech.2014.11.012 BindingDB Entry DOI: 10.7270/Q2WS8VWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50059789 (CHEMBL3393789) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Chieti Gabriele D'Annunzio Curated by ChEMBL | Assay Description Displacement of [3H]-spiperone from dopamine D2 receptor in rat striatum by scintillation counting method | Eur J Med Chem 90: 1-9 (2015) Article DOI: 10.1016/j.ejmech.2014.11.012 BindingDB Entry DOI: 10.7270/Q2WS8VWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50492037 (CHEMBL2391055) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Sapienza Curated by ChEMBL | Assay Description Inhibition of COX2 in mouse J774 cells assessed as inhibition of LPS-induced PGE2 production by radioimmunoassay | Bioorg Med Chem 21: 3695-701 (2013) Article DOI: 10.1016/j.bmc.2013.04.031 BindingDB Entry DOI: 10.7270/Q2GT5R3K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50142932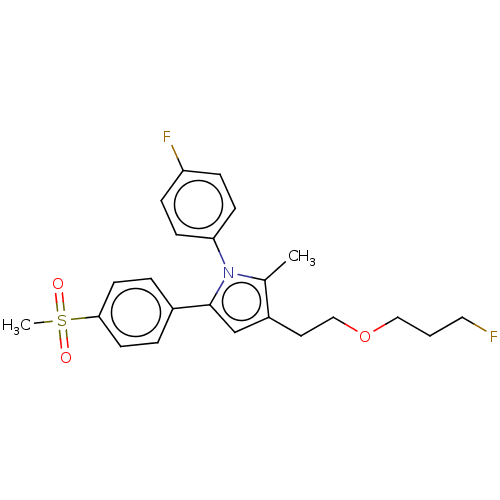 (CHEMBL3759842) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena Curated by ChEMBL | Assay Description Inhibition of COX-2 in mouse J774 cells assessed as reduction in LPS-induced PGE2 level incubated for 24 hrs by radio immunoassay | Eur J Med Chem 109: 99-106 (2016) Article DOI: 10.1016/j.ejmech.2015.12.044 BindingDB Entry DOI: 10.7270/Q2Q2423H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50492043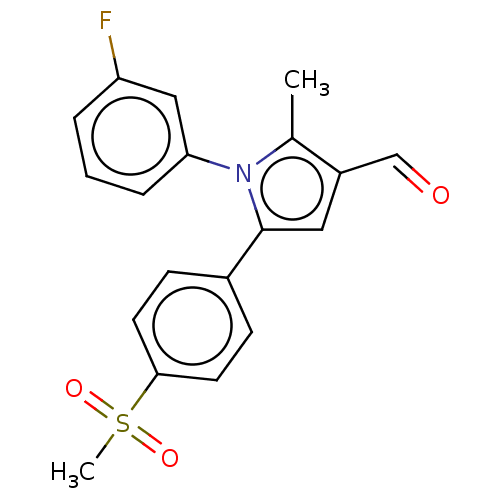 (CHEMBL2391047) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Sapienza Curated by ChEMBL | Assay Description Inhibition of COX2 in mouse J774 cells assessed as inhibition of LPS-induced PGE2 production by radioimmunoassay | Bioorg Med Chem 21: 3695-701 (2013) Article DOI: 10.1016/j.bmc.2013.04.031 BindingDB Entry DOI: 10.7270/Q2GT5R3K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50517099 (CHEMBL4563969) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena Curated by ChEMBL | Assay Description Inhibition of COX-2 in LPS-induced mouse J774 cells assessed as inhibition of PEG2 production after 24 hrs by radioimmuno assay | Bioorg Med Chem 27: (2019) Article DOI: 10.1016/j.bmc.2019.115045 BindingDB Entry DOI: 10.7270/Q21C2171 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid lipoxygenase ALOX15 (Homo sapiens (Human)) | BDBM50020589 (CHEMBL3290441) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University Jena Curated by ChEMBL | Assay Description Inhibition of 15-LO-mediated 15-HETE formation in LPS-stimulated human monocytes preincubated for 15 mins before arachidonic acid substrate addition ... | J Med Chem 57: 5638-48 (2014) Article DOI: 10.1021/jm500308c BindingDB Entry DOI: 10.7270/Q2HT2QWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50020589 (CHEMBL3290441) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University Jena Curated by ChEMBL | Assay Description Inhibition of COX2-mediated PGD2 formation in LPS-stimulated human monocytes preincubated for 15 mins before arachidonic acid substrate addition meas... | J Med Chem 57: 5638-48 (2014) Article DOI: 10.1021/jm500308c BindingDB Entry DOI: 10.7270/Q2HT2QWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50020589 (CHEMBL3290441) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University Jena Curated by ChEMBL | Assay Description Inhibition of COX2-mediated PGF2alpha formation in LPS-stimulated human monocytes preincubated for 15 mins before arachidonic acid substrate addition... | J Med Chem 57: 5638-48 (2014) Article DOI: 10.1021/jm500308c BindingDB Entry DOI: 10.7270/Q2HT2QWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50020589 (CHEMBL3290441) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University Jena Curated by ChEMBL | Assay Description Inhibition of COX1-mediated PGD2 formation in LPS-stimulated human monocytes preincubated for 15 mins before arachidonic acid substrate addition meas... | J Med Chem 57: 5638-48 (2014) Article DOI: 10.1021/jm500308c BindingDB Entry DOI: 10.7270/Q2HT2QWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50020589 (CHEMBL3290441) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University Jena Curated by ChEMBL | Assay Description Inhibition of COX1-mediated PGF2alpha formation in LPS-stimulated human monocytes preincubated for 15 mins before arachidonic acid substrate addition... | J Med Chem 57: 5638-48 (2014) Article DOI: 10.1021/jm500308c BindingDB Entry DOI: 10.7270/Q2HT2QWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50020589 (CHEMBL3290441) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University Jena Curated by ChEMBL | Assay Description Inhibition of mPGES1-mediated PGE2 formation in LPS-stimulated human monocytes preincubated for 15 mins before arachidonic acid substrate addition me... | J Med Chem 57: 5638-48 (2014) Article DOI: 10.1021/jm500308c BindingDB Entry DOI: 10.7270/Q2HT2QWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM22334 (BW A4C | BW4C | BWA4C | BWA4C, 10 | CHEMBL314360 |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University Jena Curated by ChEMBL | Assay Description Inhibition of 5-LO-mediated 5,12-DiHETE formation in LPS-stimulated human monocytes preincubated for 15 mins before arachidonic acid substrate additi... | J Med Chem 57: 5638-48 (2014) Article DOI: 10.1021/jm500308c BindingDB Entry DOI: 10.7270/Q2HT2QWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50020589 (CHEMBL3290441) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University Jena Curated by ChEMBL | Assay Description Inhibition of 5-LO-mediated 5,12-DiHETE formation in LPS-stimulated human monocytes preincubated for 15 mins before arachidonic acid substrate additi... | J Med Chem 57: 5638-48 (2014) Article DOI: 10.1021/jm500308c BindingDB Entry DOI: 10.7270/Q2HT2QWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50020584 (CHEMBL2413471) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University Jena Curated by ChEMBL | Assay Description Inhibition of 5-LO-mediated 5,12-DiHETE formation in LPS-stimulated human monocytes preincubated for 15 mins before arachidonic acid substrate additi... | J Med Chem 57: 5638-48 (2014) Article DOI: 10.1021/jm500308c BindingDB Entry DOI: 10.7270/Q2HT2QWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50163748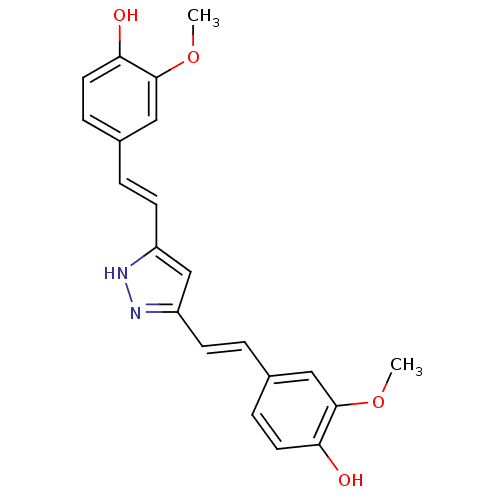 ((E)-3,5-Bis[beta-(4-Hydroxy-3-methoxyphenyl)-ethen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University Jena Curated by ChEMBL | Assay Description Inhibition of 5-LO-mediated 5,12-DiHETE formation in LPS-stimulated human monocytes preincubated for 15 mins before arachidonic acid substrate additi... | J Med Chem 57: 5638-48 (2014) Article DOI: 10.1021/jm500308c BindingDB Entry DOI: 10.7270/Q2HT2QWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM22334 (BW A4C | BW4C | BWA4C | BWA4C, 10 | CHEMBL314360 |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University Jena Curated by ChEMBL | Assay Description Inhibition of 5-LO-mediated 5-HETE formation in LPS-stimulated human monocytes preincubated for 15 mins before arachidonic acid substrate addition me... | J Med Chem 57: 5638-48 (2014) Article DOI: 10.1021/jm500308c BindingDB Entry DOI: 10.7270/Q2HT2QWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50020589 (CHEMBL3290441) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University Jena Curated by ChEMBL | Assay Description Inhibition of 5-LO-mediated 5-HETE formation in LPS-stimulated human monocytes preincubated for 15 mins before arachidonic acid substrate addition me... | J Med Chem 57: 5638-48 (2014) Article DOI: 10.1021/jm500308c BindingDB Entry DOI: 10.7270/Q2HT2QWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50020584 (CHEMBL2413471) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University Jena Curated by ChEMBL | Assay Description Inhibition of 5-LO-mediated 5-HETE formation in LPS-stimulated human monocytes preincubated for 15 mins before arachidonic acid substrate addition me... | J Med Chem 57: 5638-48 (2014) Article DOI: 10.1021/jm500308c BindingDB Entry DOI: 10.7270/Q2HT2QWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50163748 ((E)-3,5-Bis[beta-(4-Hydroxy-3-methoxyphenyl)-ethen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University Jena Curated by ChEMBL | Assay Description Inhibition of 5-LO-mediated 5-HETE formation in LPS-stimulated human monocytes preincubated for 15 mins before arachidonic acid substrate addition me... | J Med Chem 57: 5638-48 (2014) Article DOI: 10.1021/jm500308c BindingDB Entry DOI: 10.7270/Q2HT2QWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM22334 (BW A4C | BW4C | BWA4C | BWA4C, 10 | CHEMBL314360 |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University Jena Curated by ChEMBL | Assay Description Inhibition of 5-LO-mediated LTB4 formation in LPS-stimulated human monocytes preincubated for 15 mins before arachidonic acid substrate addition meas... | J Med Chem 57: 5638-48 (2014) Article DOI: 10.1021/jm500308c BindingDB Entry DOI: 10.7270/Q2HT2QWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50020589 (CHEMBL3290441) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University Jena Curated by ChEMBL | Assay Description Inhibition of 5-LO-mediated LTB4 formation in LPS-stimulated human monocytes preincubated for 15 mins before arachidonic acid substrate addition meas... | J Med Chem 57: 5638-48 (2014) Article DOI: 10.1021/jm500308c BindingDB Entry DOI: 10.7270/Q2HT2QWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50020584 (CHEMBL2413471) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University Jena Curated by ChEMBL | Assay Description Inhibition of 5-LO-mediated LTB4 formation in LPS-stimulated human monocytes preincubated for 15 mins before arachidonic acid substrate addition meas... | J Med Chem 57: 5638-48 (2014) Article DOI: 10.1021/jm500308c BindingDB Entry DOI: 10.7270/Q2HT2QWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50163748 ((E)-3,5-Bis[beta-(4-Hydroxy-3-methoxyphenyl)-ethen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University Jena Curated by ChEMBL | Assay Description Inhibition of 5-LO-mediated LTB4 formation in LPS-stimulated human monocytes preincubated for 15 mins before arachidonic acid substrate addition meas... | J Med Chem 57: 5638-48 (2014) Article DOI: 10.1021/jm500308c BindingDB Entry DOI: 10.7270/Q2HT2QWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50142937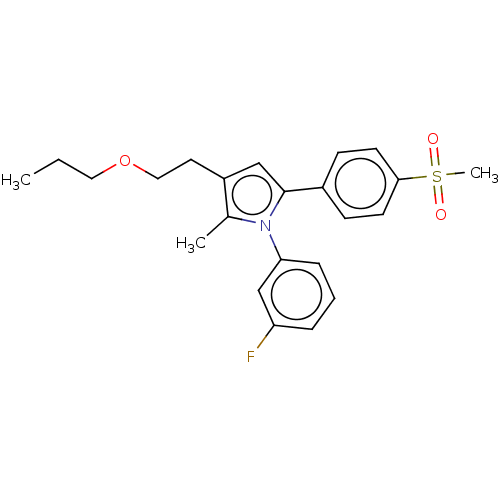 (CHEMBL3759726) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena Curated by ChEMBL | Assay Description Inhibition of COX-2 in mouse J774 cells assessed as reduction in LPS-induced PGE2 level incubated for 24 hrs by radio immunoassay | Eur J Med Chem 109: 99-106 (2016) Article DOI: 10.1016/j.ejmech.2015.12.044 BindingDB Entry DOI: 10.7270/Q2Q2423H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50142935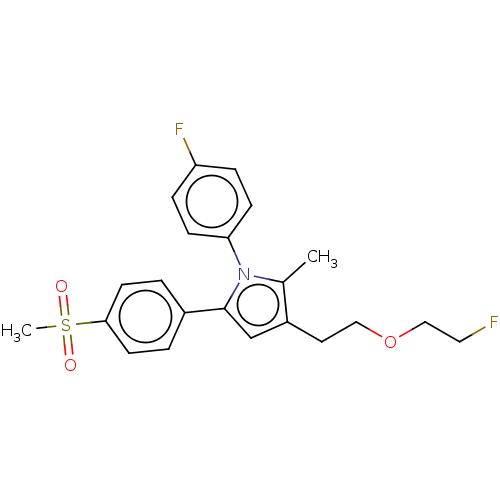 (CHEMBL3759195) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena Curated by ChEMBL | Assay Description Inhibition of COX-2 in mouse J774 cells assessed as reduction in LPS-induced PGE2 level incubated for 24 hrs by radio immunoassay | Eur J Med Chem 109: 99-106 (2016) Article DOI: 10.1016/j.ejmech.2015.12.044 BindingDB Entry DOI: 10.7270/Q2Q2423H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50492035 (CHEMBL2391054) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Sapienza Curated by ChEMBL | Assay Description Inhibition of COX2 in mouse J774 cells assessed as inhibition of LPS-induced PGE2 production by radioimmunoassay | Bioorg Med Chem 21: 3695-701 (2013) Article DOI: 10.1016/j.bmc.2013.04.031 BindingDB Entry DOI: 10.7270/Q2GT5R3K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50517095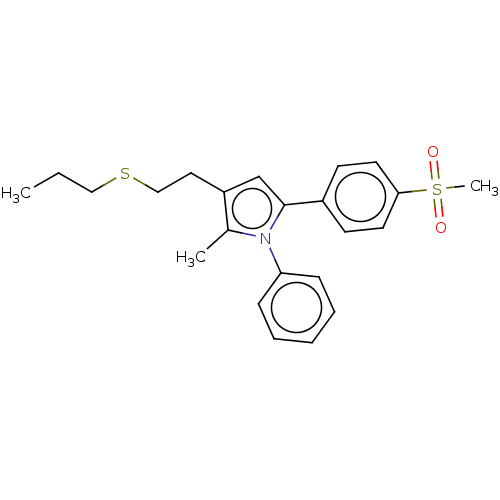 (CHEMBL4535137) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena Curated by ChEMBL | Assay Description Inhibition of COX-2 in LPS-induced mouse J774 cells assessed as inhibition of PEG2 production after 24 hrs by radioimmuno assay | Bioorg Med Chem 27: (2019) Article DOI: 10.1016/j.bmc.2019.115045 BindingDB Entry DOI: 10.7270/Q21C2171 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50430948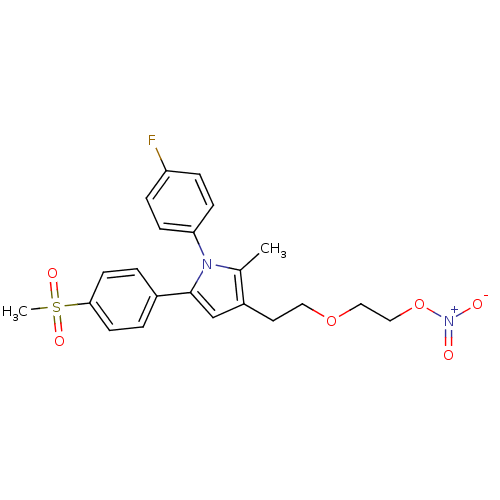 (CHEMBL2337402) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of Escherichia coli lipopolysaccharide-induced COX2 activity in mouse J774 cells assessed as decrease in PGE2 levels after 24 hrs by RIA | J Med Chem 56: 3191-206 (2013) Article DOI: 10.1021/jm301370e BindingDB Entry DOI: 10.7270/Q2JS9RSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50430957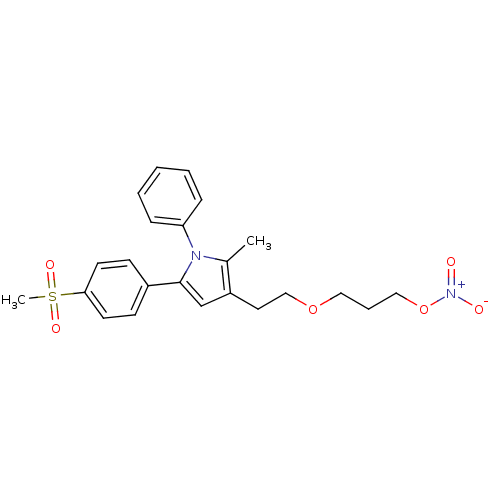 (CHEMBL2337404) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of Escherichia coli lipopolysaccharide-induced COX2 activity in mouse J774 cells assessed as decrease in PGE2 levels after 24 hrs by RIA | J Med Chem 56: 3191-206 (2013) Article DOI: 10.1021/jm301370e BindingDB Entry DOI: 10.7270/Q2JS9RSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50430960 (CHEMBL2337400) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of Escherichia coli lipopolysaccharide-induced COX2 activity in mouse J774 cells assessed as decrease in PGE2 levels after 24 hrs by RIA | J Med Chem 56: 3191-206 (2013) Article DOI: 10.1021/jm301370e BindingDB Entry DOI: 10.7270/Q2JS9RSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50142936 (CHEMBL3758384) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena Curated by ChEMBL | Assay Description Inhibition of COX-2 in mouse J774 cells assessed as reduction in LPS-induced PGE2 level incubated for 24 hrs by radio immunoassay | Eur J Med Chem 109: 99-106 (2016) Article DOI: 10.1016/j.ejmech.2015.12.044 BindingDB Entry DOI: 10.7270/Q2Q2423H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50142930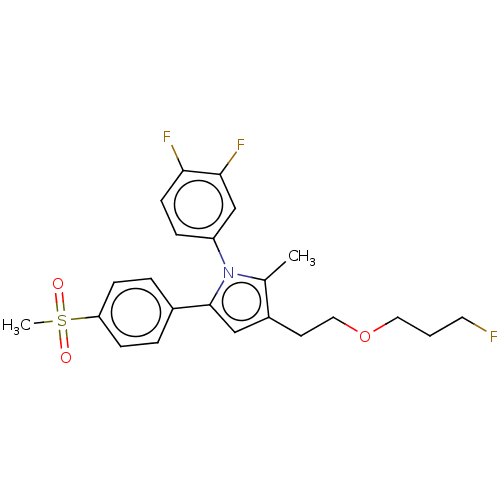 (CHEMBL3759435) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena Curated by ChEMBL | Assay Description Inhibition of COX-2 in mouse J774 cells assessed as reduction in LPS-induced PGE2 level incubated for 24 hrs by radio immunoassay | Eur J Med Chem 109: 99-106 (2016) Article DOI: 10.1016/j.ejmech.2015.12.044 BindingDB Entry DOI: 10.7270/Q2Q2423H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM22334 (BW A4C | BW4C | BWA4C | BWA4C, 10 | CHEMBL314360 |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
University Jena Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-LO expressed in Escherichia coli Bl21 (DE3) using arachidonic acid as substrate preincubated for 10 mins measured a... | J Med Chem 57: 5638-48 (2014) Article DOI: 10.1021/jm500308c BindingDB Entry DOI: 10.7270/Q2HT2QWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50020588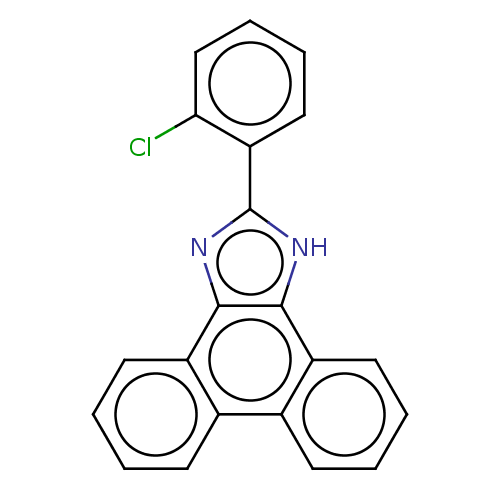 (CHEMBL270718) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
University Jena Curated by ChEMBL | Assay Description Inhibition of mPGES1-mediated PGE2 production in microsomes of IL-1beta stimulated human A549 cells preincubated for 15 mins by RP-HPLC analysis | J Med Chem 57: 5638-48 (2014) Article DOI: 10.1021/jm500308c BindingDB Entry DOI: 10.7270/Q2HT2QWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50430956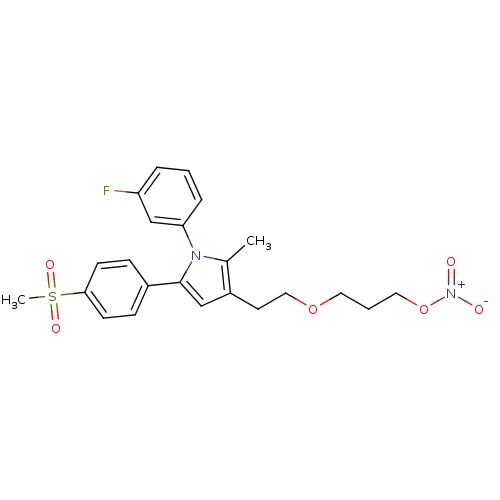 (CHEMBL2337405) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of Escherichia coli lipopolysaccharide-induced COX2 activity in mouse J774 cells assessed as decrease in PGE2 levels after 24 hrs by RIA | J Med Chem 56: 3191-206 (2013) Article DOI: 10.1021/jm301370e BindingDB Entry DOI: 10.7270/Q2JS9RSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 612 total ) | Next | Last >> |