

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DNA-directed RNA polymerase subunit beta (Escherichia coli) | BDBM50391000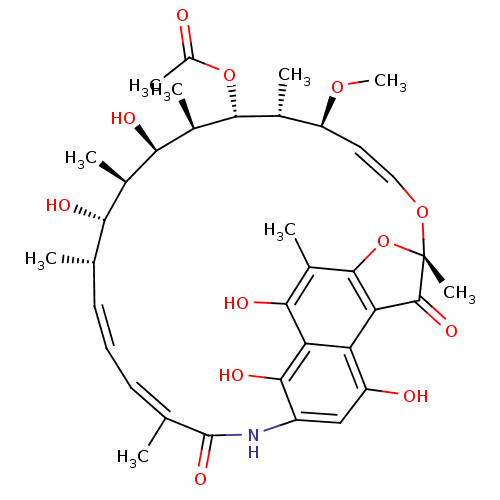 (CB-01-11 | RIFAMYCIN) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | 37 |
Merck & Co., Inc. | Assay Description Fluorescence-detected RNAP inhibition assays in Tables 1 and 4 were performed using a DNA fragment containing the lacUV5 promoter as a template and t... | ACS Chem Biol 12: 1346-1352 (2017) Article DOI: 10.1021/acschembio.6b01133 BindingDB Entry DOI: 10.7270/Q2VM4B41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA-directed RNA polymerase subunit beta (Escherichia coli) | BDBM50391000 (CB-01-11 | RIFAMYCIN) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | 37 |
Merck & Co., Inc. | Assay Description Fluorescence-detected RNAP inhibition assays in Tables 1 and 4 were performed using a DNA fragment containing the lacUV5 promoter as a template and t... | ACS Chem Biol 12: 1346-1352 (2017) Article DOI: 10.1021/acschembio.6b01133 BindingDB Entry DOI: 10.7270/Q2VM4B41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxa40 (Acinetobacter baumannii) | BDBM98796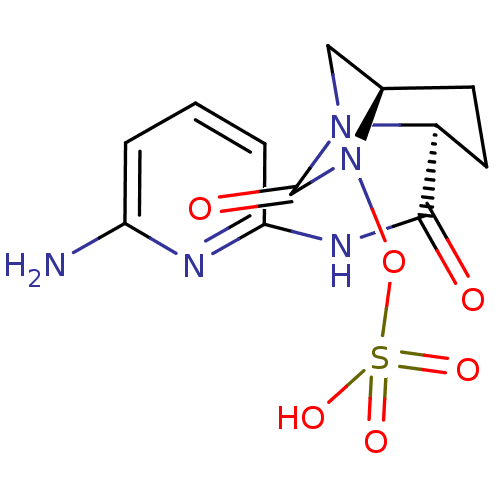 (US8487093, 184) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labs Curated by ChEMBL | Assay Description Inhibition of beta-lactamase Oxa40 in Acinetobacter baumannii assessed as inhibition of hydrolysis of nitrocefin | Bioorg Med Chem Lett 24: 780-5 (2014) Article DOI: 10.1016/j.bmcl.2013.12.101 BindingDB Entry DOI: 10.7270/Q2HD7X41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50309906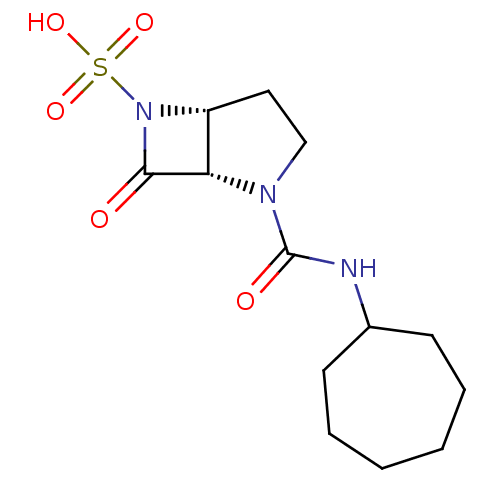 ((1S,5R)-2-(cycloheptylcarbamoyl)-7-oxo-2,6-diazabi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labs Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa beta lactamase AmpC by spectrophotometry | Bioorg Med Chem Lett 20: 918-21 (2010) Article DOI: 10.1016/j.bmcl.2009.12.069 BindingDB Entry DOI: 10.7270/Q2KW5G5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxa40 (Acinetobacter baumannii) | BDBM98792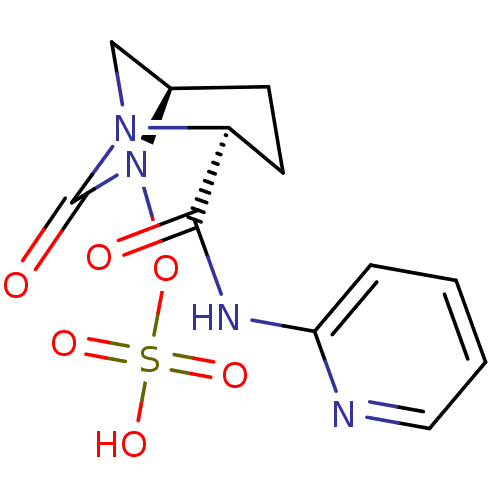 (US8487093, 180) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labs Curated by ChEMBL | Assay Description Inhibition of beta-lactamase Oxa40 in Acinetobacter baumannii assessed as inhibition of hydrolysis of nitrocefin | Bioorg Med Chem Lett 24: 780-5 (2014) Article DOI: 10.1016/j.bmcl.2013.12.101 BindingDB Entry DOI: 10.7270/Q2HD7X41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase SHV-5 (Klebsiella pneumoniae) | BDBM50315411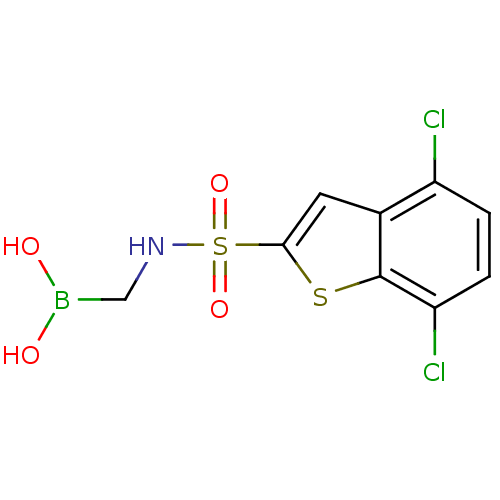 (4,7-Dichloro-1-benzothien-2-yl sulfonylaminomethyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Curated by ChEMBL | Assay Description Inhibition of Klebsiella pneumoniae beta-lactamase SHV5 | Bioorg Med Chem Lett 20: 2622-4 (2010) Article DOI: 10.1016/j.bmcl.2010.02.065 BindingDB Entry DOI: 10.7270/Q2ZS2WN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50348181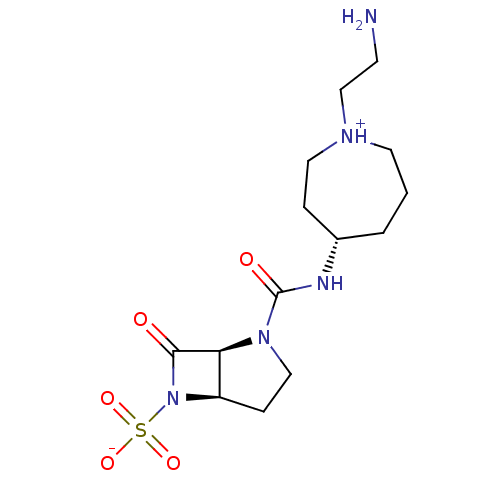 (CHEMBL1800870) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labs Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa CL5701 AmpC by spectrophotometric assay | Bioorg Med Chem Lett 21: 4267-70 (2011) Article DOI: 10.1016/j.bmcl.2011.05.065 BindingDB Entry DOI: 10.7270/Q2WW7J1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50309903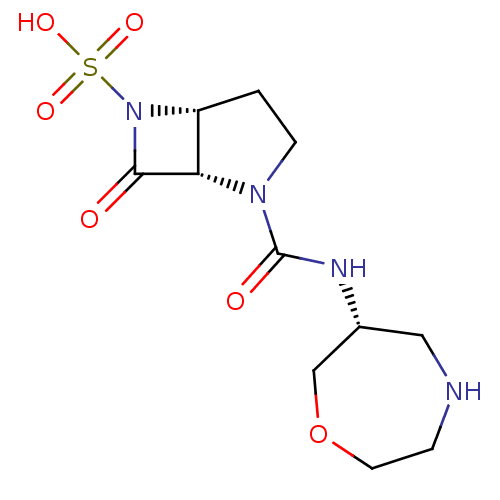 ((1S,5R)-2-((R)-1,4-oxazepan-6-ylcarbamoyl)-7-oxo-2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labs Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa beta lactamase AmpC by spectrophotometry | Bioorg Med Chem Lett 20: 918-21 (2010) Article DOI: 10.1016/j.bmcl.2009.12.069 BindingDB Entry DOI: 10.7270/Q2KW5G5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50315411 (4,7-Dichloro-1-benzothien-2-yl sulfonylaminomethyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 620 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Curated by ChEMBL | Assay Description Inhibition of Enterobacter colacae P99 Beta-lactamase | Bioorg Med Chem Lett 20: 2622-4 (2010) Article DOI: 10.1016/j.bmcl.2010.02.065 BindingDB Entry DOI: 10.7270/Q2ZS2WN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50309905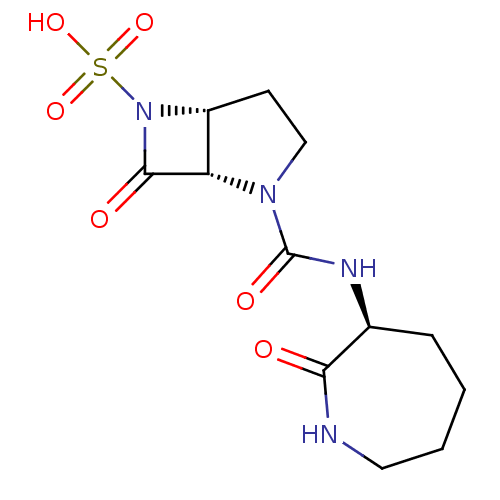 ((1S,5R)-7-oxo-2-((S)-2-oxoazepan-3-ylcarbamoyl)-2,...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labs Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa beta lactamase AmpC by spectrophotometry | Bioorg Med Chem Lett 20: 918-21 (2010) Article DOI: 10.1016/j.bmcl.2009.12.069 BindingDB Entry DOI: 10.7270/Q2KW5G5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50348182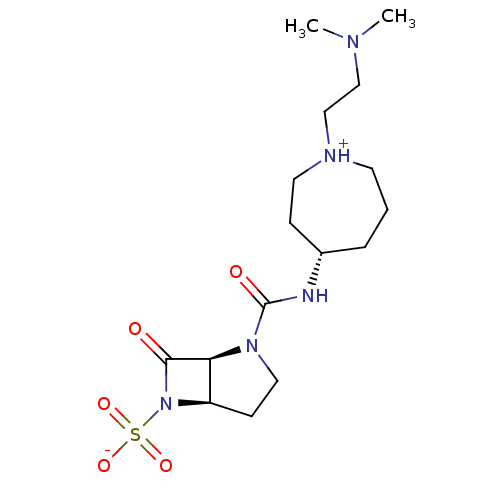 (CHEMBL1800871) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labs Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa CL5701 AmpC by spectrophotometric assay | Bioorg Med Chem Lett 21: 4267-70 (2011) Article DOI: 10.1016/j.bmcl.2011.05.065 BindingDB Entry DOI: 10.7270/Q2WW7J1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50348179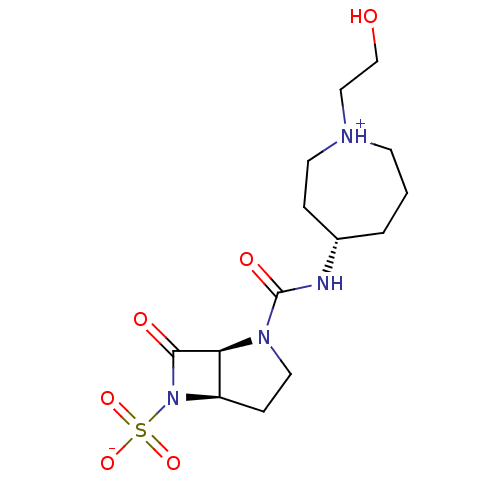 (CHEMBL1800868) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labs Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa CL5701 AmpC by spectrophotometric assay | Bioorg Med Chem Lett 21: 4267-70 (2011) Article DOI: 10.1016/j.bmcl.2011.05.065 BindingDB Entry DOI: 10.7270/Q2WW7J1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50309899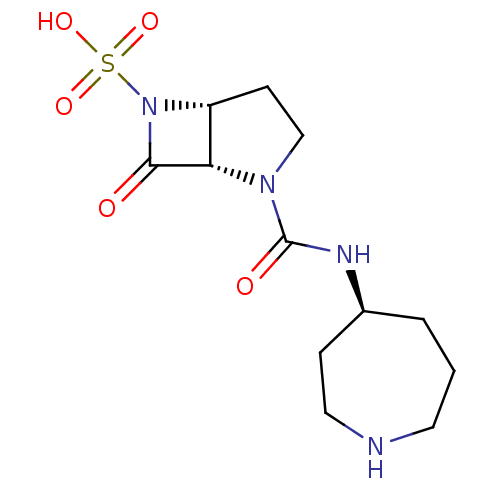 ((1S,5R)-2-((S)-azepan-4-ylcarbamoyl)-7-oxo-2,6-dia...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labs Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa beta lactamase AmpC by spectrophotometry | Bioorg Med Chem Lett 20: 918-21 (2010) Article DOI: 10.1016/j.bmcl.2009.12.069 BindingDB Entry DOI: 10.7270/Q2KW5G5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50309899 ((1S,5R)-2-((S)-azepan-4-ylcarbamoyl)-7-oxo-2,6-dia...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labs Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa CL5701 AmpC by spectrophotometric assay | Bioorg Med Chem Lett 21: 4267-70 (2011) Article DOI: 10.1016/j.bmcl.2011.05.065 BindingDB Entry DOI: 10.7270/Q2WW7J1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50309904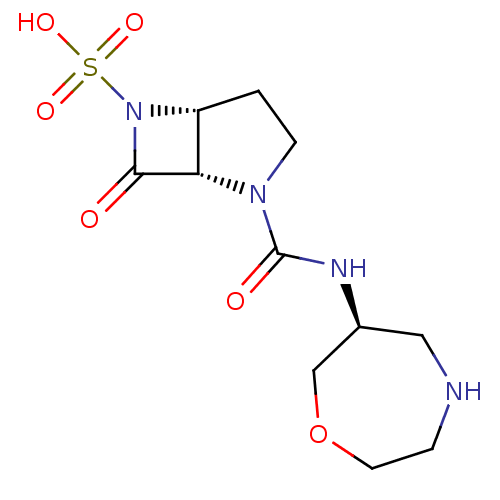 ((1S,5R)-2-((S)-1,4-oxazepan-6-ylcarbamoyl)-7-oxo-2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labs Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa beta lactamase AmpC by spectrophotometry | Bioorg Med Chem Lett 20: 918-21 (2010) Article DOI: 10.1016/j.bmcl.2009.12.069 BindingDB Entry DOI: 10.7270/Q2KW5G5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50348180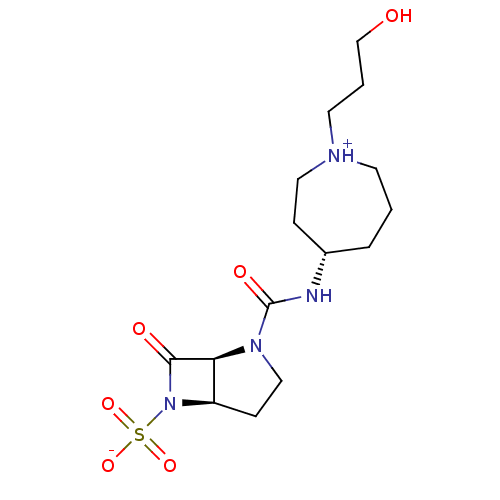 (CHEMBL1800869) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labs Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa CL5701 AmpC by spectrophotometric assay | Bioorg Med Chem Lett 21: 4267-70 (2011) Article DOI: 10.1016/j.bmcl.2011.05.065 BindingDB Entry DOI: 10.7270/Q2WW7J1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50315411 (4,7-Dichloro-1-benzothien-2-yl sulfonylaminomethyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa beta-lactamase AmpC | Bioorg Med Chem Lett 20: 2622-4 (2010) Article DOI: 10.1016/j.bmcl.2010.02.065 BindingDB Entry DOI: 10.7270/Q2ZS2WN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50309908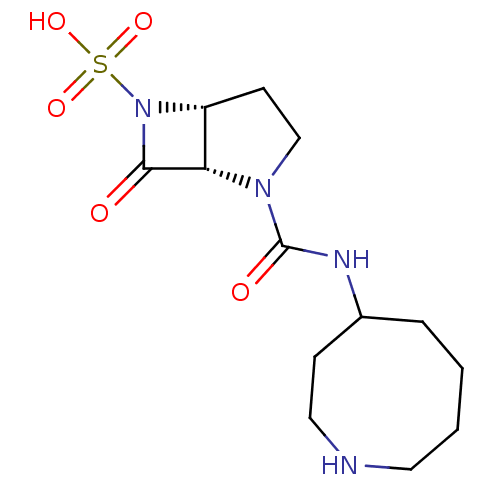 ((1S,5R)-2-(azocan-4-ylcarbamoyl)-7-oxo-2,6-diazabi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labs Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa beta lactamase AmpC by spectrophotometry | Bioorg Med Chem Lett 20: 918-21 (2010) Article DOI: 10.1016/j.bmcl.2009.12.069 BindingDB Entry DOI: 10.7270/Q2KW5G5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50309908 ((1S,5R)-2-(azocan-4-ylcarbamoyl)-7-oxo-2,6-diazabi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labs Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa beta lactamase AmpC by spectrophotometry | Bioorg Med Chem Lett 20: 918-21 (2010) Article DOI: 10.1016/j.bmcl.2009.12.069 BindingDB Entry DOI: 10.7270/Q2KW5G5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50348178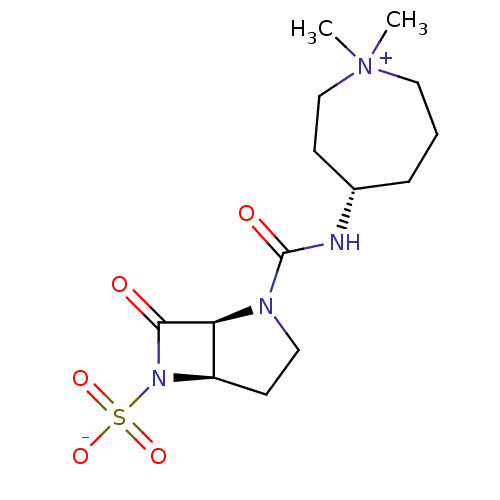 (CHEMBL1800867) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labs Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa CL5701 AmpC by spectrophotometric assay | Bioorg Med Chem Lett 21: 4267-70 (2011) Article DOI: 10.1016/j.bmcl.2011.05.065 BindingDB Entry DOI: 10.7270/Q2WW7J1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50348187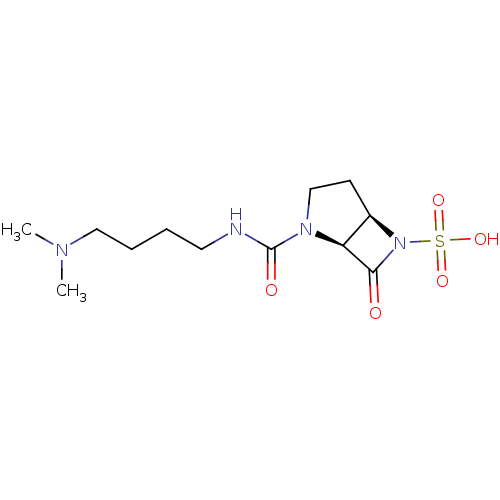 (CHEMBL1800876) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labs Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa CL5701 AmpC by spectrophotometric assay | Bioorg Med Chem Lett 21: 4267-70 (2011) Article DOI: 10.1016/j.bmcl.2011.05.065 BindingDB Entry DOI: 10.7270/Q2WW7J1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50348185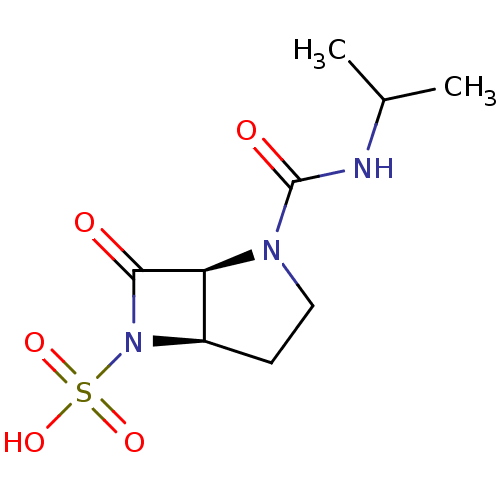 (CHEMBL1800874) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labs Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa CL5701 AmpC by spectrophotometric assay | Bioorg Med Chem Lett 21: 4267-70 (2011) Article DOI: 10.1016/j.bmcl.2011.05.065 BindingDB Entry DOI: 10.7270/Q2WW7J1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50309901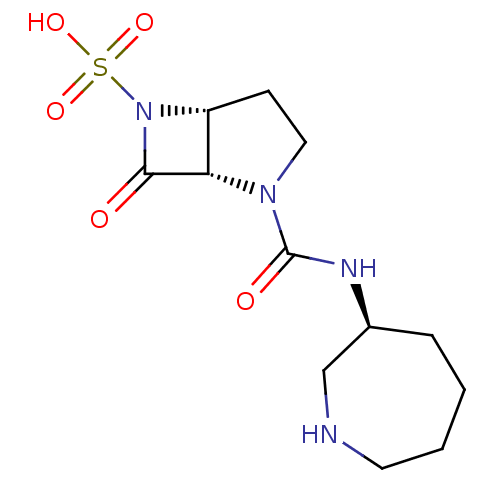 ((1S,5R)-2-((S)-azepan-3-ylcarbamoyl)-7-oxo-2,6-dia...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labs Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa beta lactamase AmpC by spectrophotometry | Bioorg Med Chem Lett 20: 918-21 (2010) Article DOI: 10.1016/j.bmcl.2009.12.069 BindingDB Entry DOI: 10.7270/Q2KW5G5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50348188 (CHEMBL1800877) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labs Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa CL5701 AmpC by spectrophotometric assay | Bioorg Med Chem Lett 21: 4267-70 (2011) Article DOI: 10.1016/j.bmcl.2011.05.065 BindingDB Entry DOI: 10.7270/Q2WW7J1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA-directed RNA polymerase subunit beta (Escherichia coli) | BDBM228835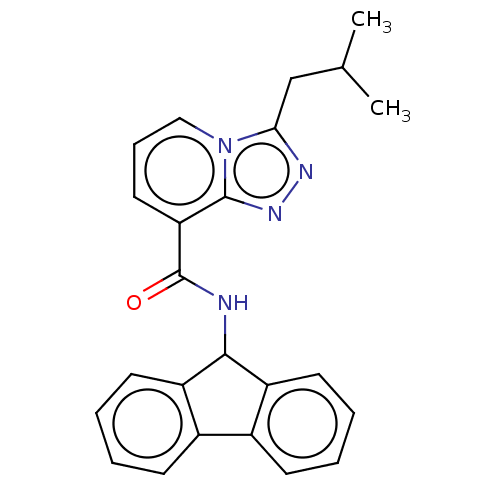 (MRL-436) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | 37 |
Merck & Co., Inc. | Assay Description Fluorescence-detected RNAP inhibition assays in Tables 1 and 4 were performed using a DNA fragment containing the lacUV5 promoter as a template and t... | ACS Chem Biol 12: 1346-1352 (2017) Article DOI: 10.1021/acschembio.6b01133 BindingDB Entry DOI: 10.7270/Q2VM4B41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50348186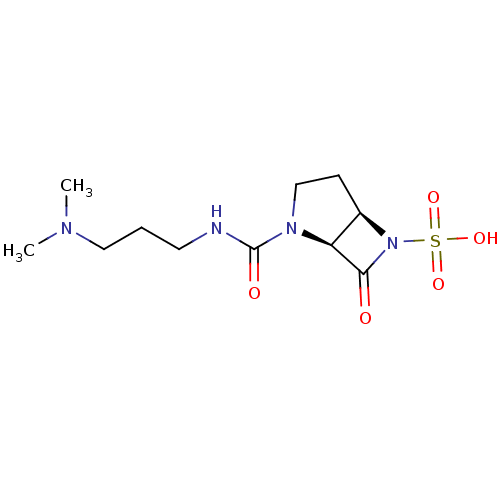 (CHEMBL1800875) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labs Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa CL5701 AmpC by spectrophotometric assay | Bioorg Med Chem Lett 21: 4267-70 (2011) Article DOI: 10.1016/j.bmcl.2011.05.065 BindingDB Entry DOI: 10.7270/Q2WW7J1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50348177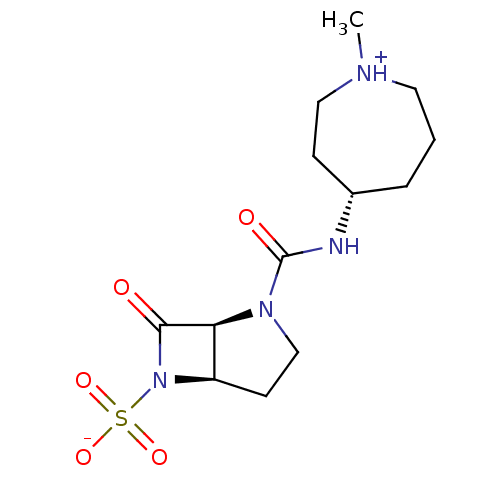 (CHEMBL1800866) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labs Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa CL5701 AmpC by spectrophotometric assay | Bioorg Med Chem Lett 21: 4267-70 (2011) Article DOI: 10.1016/j.bmcl.2011.05.065 BindingDB Entry DOI: 10.7270/Q2WW7J1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA-directed RNA polymerase subunit beta [H526D] (Escherichia coli) | BDBM228835 (MRL-436) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | 37 |
Merck & Co., Inc. | Assay Description Fluorescence-detected RNAP inhibition assays in Tables 1 and 4 were performed using a DNA fragment containing the lacUV5 promoter as a template and t... | ACS Chem Biol 12: 1346-1352 (2017) Article DOI: 10.1021/acschembio.6b01133 BindingDB Entry DOI: 10.7270/Q2VM4B41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA-directed RNA polymerase subunit beta (Escherichia coli) | BDBM228835 (MRL-436) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | 37 |
Merck & Co., Inc. | Assay Description Fluorescence-detected RNAP inhibition assays in Tables 1 and 4 were performed using a DNA fragment containing the lacUV5 promoter as a template and t... | ACS Chem Biol 12: 1346-1352 (2017) Article DOI: 10.1021/acschembio.6b01133 BindingDB Entry DOI: 10.7270/Q2VM4B41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50309907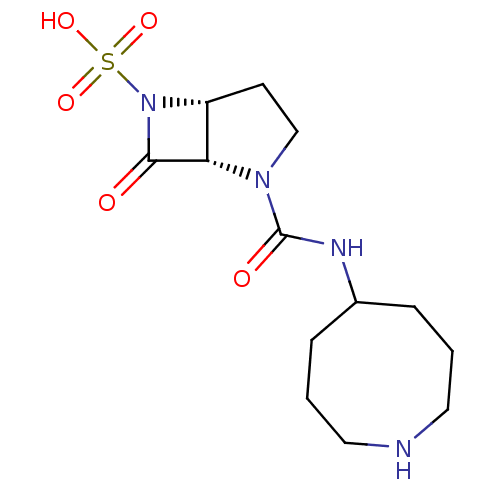 ((1S,5R)-2-(azocan-5-ylcarbamoyl)-7-oxo-2,6-diazabi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labs Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa beta lactamase AmpC by spectrophotometry | Bioorg Med Chem Lett 20: 918-21 (2010) Article DOI: 10.1016/j.bmcl.2009.12.069 BindingDB Entry DOI: 10.7270/Q2KW5G5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50348190 (CHEMBL1800183) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labs Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa CL5701 AmpC by spectrophotometric assay | Bioorg Med Chem Lett 21: 4267-70 (2011) Article DOI: 10.1016/j.bmcl.2011.05.065 BindingDB Entry DOI: 10.7270/Q2WW7J1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50309909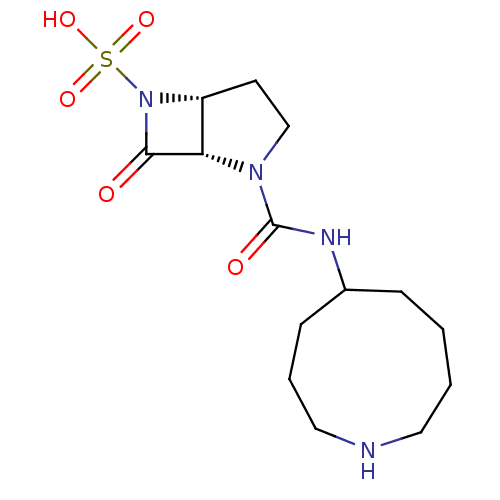 ((1S,5R)-2-(azonan-5-ylcarbamoyl)-7-oxo-2,6-diazabi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labs Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa beta lactamase AmpC by spectrophotometry | Bioorg Med Chem Lett 20: 918-21 (2010) Article DOI: 10.1016/j.bmcl.2009.12.069 BindingDB Entry DOI: 10.7270/Q2KW5G5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50309909 ((1S,5R)-2-(azonan-5-ylcarbamoyl)-7-oxo-2,6-diazabi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labs Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa beta lactamase AmpC by spectrophotometry | Bioorg Med Chem Lett 20: 918-21 (2010) Article DOI: 10.1016/j.bmcl.2009.12.069 BindingDB Entry DOI: 10.7270/Q2KW5G5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50309900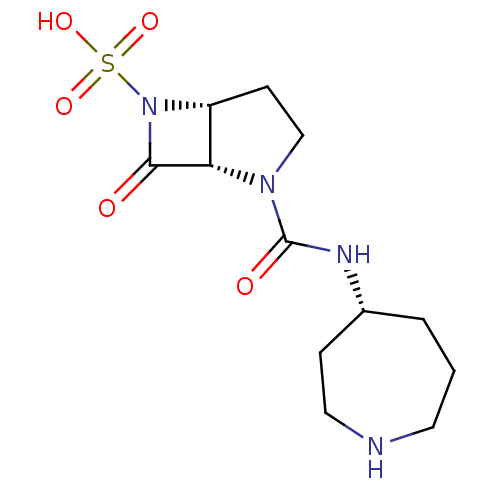 ((1S,5R)-2-((R)-azepan-4-ylcarbamoyl)-7-oxo-2,6-dia...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labs Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa beta lactamase AmpC by spectrophotometry | Bioorg Med Chem Lett 20: 918-21 (2010) Article DOI: 10.1016/j.bmcl.2009.12.069 BindingDB Entry DOI: 10.7270/Q2KW5G5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA-directed RNA polymerase subunit beta [S531L] (Escherichia coli) | BDBM228835 (MRL-436) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | 37 |
Merck & Co., Inc. | Assay Description Fluorescence-detected RNAP inhibition assays in Tables 1 and 4 were performed using a DNA fragment containing the lacUV5 promoter as a template and t... | ACS Chem Biol 12: 1346-1352 (2017) Article DOI: 10.1021/acschembio.6b01133 BindingDB Entry DOI: 10.7270/Q2VM4B41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA-directed RNA polymerase subunit beta [D516V] (Escherichia coli) | BDBM228835 (MRL-436) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | 37 |
Merck & Co., Inc. | Assay Description Fluorescence-detected RNAP inhibition assays in Tables 1 and 4 were performed using a DNA fragment containing the lacUV5 promoter as a template and t... | ACS Chem Biol 12: 1346-1352 (2017) Article DOI: 10.1021/acschembio.6b01133 BindingDB Entry DOI: 10.7270/Q2VM4B41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxa40 (Acinetobacter baumannii) | BDBM98800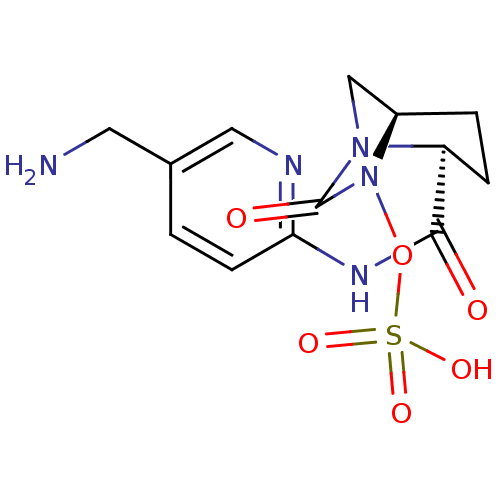 (US8487093, 190) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labs Curated by ChEMBL | Assay Description Inhibition of beta-lactamase Oxa40 in Acinetobacter baumannii assessed as inhibition of hydrolysis of nitrocefin | Bioorg Med Chem Lett 24: 780-5 (2014) Article DOI: 10.1016/j.bmcl.2013.12.101 BindingDB Entry DOI: 10.7270/Q2HD7X41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxa40 (Acinetobacter baumannii) | BDBM98787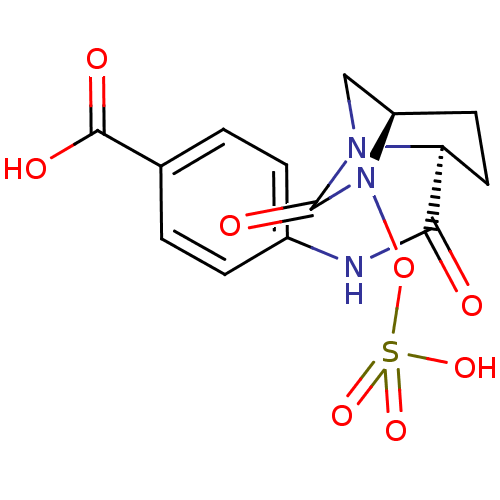 (US8487093, 170) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labs Curated by ChEMBL | Assay Description Inhibition of beta-lactamase Oxa40 in Acinetobacter baumannii assessed as inhibition of hydrolysis of nitrocefin | Bioorg Med Chem Lett 24: 780-5 (2014) Article DOI: 10.1016/j.bmcl.2013.12.101 BindingDB Entry DOI: 10.7270/Q2HD7X41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50348189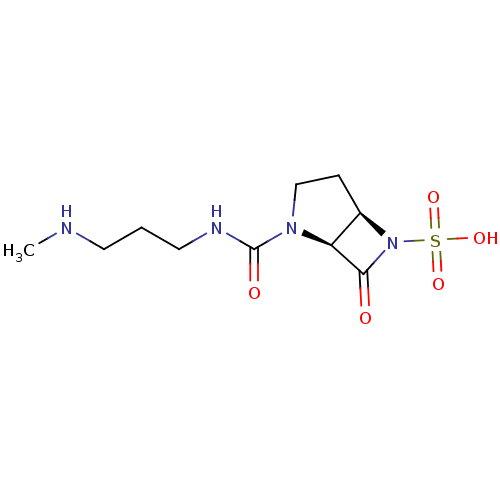 (CHEMBL1800878) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labs Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa CL5701 AmpC by spectrophotometric assay | Bioorg Med Chem Lett 21: 4267-70 (2011) Article DOI: 10.1016/j.bmcl.2011.05.065 BindingDB Entry DOI: 10.7270/Q2WW7J1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxa40 (Acinetobacter baumannii) | BDBM50315411 (4,7-Dichloro-1-benzothien-2-yl sulfonylaminomethyl...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Curated by ChEMBL | Assay Description Inhibition of Acinetobacter baumannii beta-lactamase OXA40 | Bioorg Med Chem Lett 20: 2622-4 (2010) Article DOI: 10.1016/j.bmcl.2010.02.065 BindingDB Entry DOI: 10.7270/Q2ZS2WN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50309898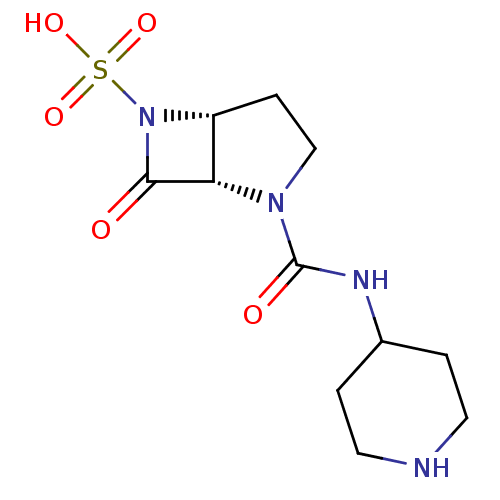 ((1S,5R)-7-oxo-2-(piperidin-4-ylcarbamoyl)-2,6-diaz...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labs Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa beta lactamase AmpC by spectrophotometry | Bioorg Med Chem Lett 20: 918-21 (2010) Article DOI: 10.1016/j.bmcl.2009.12.069 BindingDB Entry DOI: 10.7270/Q2KW5G5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxa40 (Acinetobacter baumannii) | BDBM98824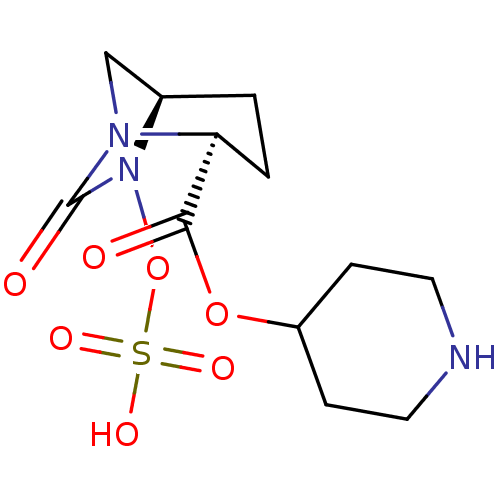 (US8487093, 222) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labs Curated by ChEMBL | Assay Description Inhibition of beta-lactamase Oxa40 in Acinetobacter baumannii assessed as inhibition of hydrolysis of nitrocefin | Bioorg Med Chem Lett 24: 780-5 (2014) Article DOI: 10.1016/j.bmcl.2013.12.101 BindingDB Entry DOI: 10.7270/Q2HD7X41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50348184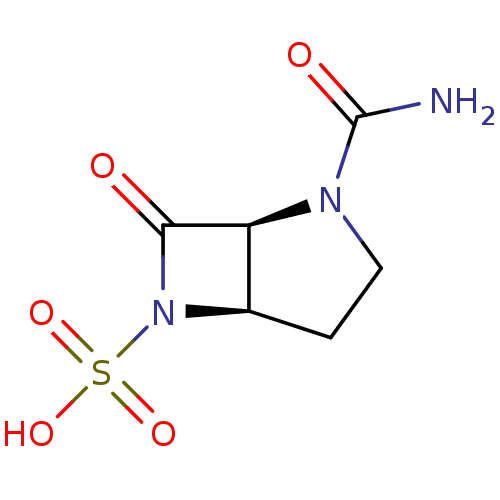 (CHEMBL1800873) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labs Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa CL5701 AmpC by spectrophotometric assay | Bioorg Med Chem Lett 21: 4267-70 (2011) Article DOI: 10.1016/j.bmcl.2011.05.065 BindingDB Entry DOI: 10.7270/Q2WW7J1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50309902 ((1S,5R)-2-(1,4-diazepan-6-ylcarbamoyl)-7-oxo-2,6-d...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labs Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa beta lactamase AmpC by spectrophotometry | Bioorg Med Chem Lett 20: 918-21 (2010) Article DOI: 10.1016/j.bmcl.2009.12.069 BindingDB Entry DOI: 10.7270/Q2KW5G5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxa40 (Acinetobacter baumannii) | BDBM50447649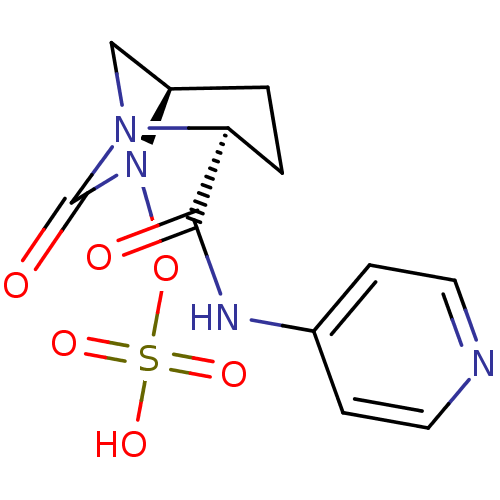 (CHEMBL3112746) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labs Curated by ChEMBL | Assay Description Inhibition of beta-lactamase Oxa40 in Acinetobacter baumannii assessed as inhibition of hydrolysis of nitrocefin | Bioorg Med Chem Lett 24: 780-5 (2014) Article DOI: 10.1016/j.bmcl.2013.12.101 BindingDB Entry DOI: 10.7270/Q2HD7X41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxa40 (Acinetobacter baumannii) | BDBM98791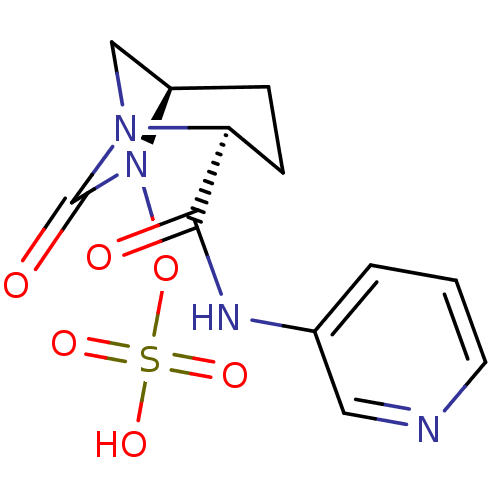 (US8487093, 178) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labs Curated by ChEMBL | Assay Description Inhibition of beta-lactamase Oxa40 in Acinetobacter baumannii assessed as inhibition of hydrolysis of nitrocefin | Bioorg Med Chem Lett 24: 780-5 (2014) Article DOI: 10.1016/j.bmcl.2013.12.101 BindingDB Entry DOI: 10.7270/Q2HD7X41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxa40 (Acinetobacter baumannii) | BDBM50447647 (CHEMBL3112752) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labs Curated by ChEMBL | Assay Description Inhibition of beta-lactamase Oxa40 in Acinetobacter baumannii assessed as inhibition of hydrolysis of nitrocefin | Bioorg Med Chem Lett 24: 780-5 (2014) Article DOI: 10.1016/j.bmcl.2013.12.101 BindingDB Entry DOI: 10.7270/Q2HD7X41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxa40 (Acinetobacter baumannii) | BDBM98755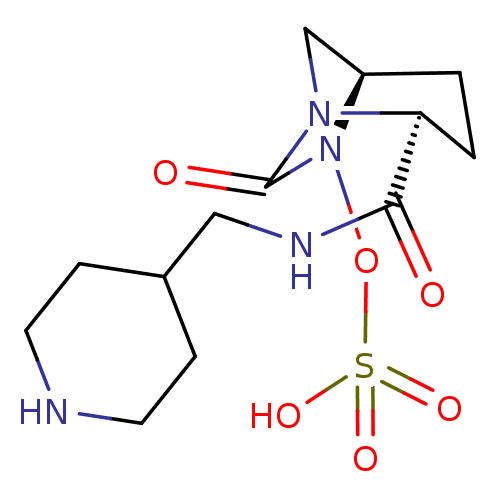 (US8487093, 116) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labs Curated by ChEMBL | Assay Description Inhibition of beta-lactamase Oxa40 in Acinetobacter baumannii assessed as inhibition of hydrolysis of nitrocefin | Bioorg Med Chem Lett 24: 780-5 (2014) Article DOI: 10.1016/j.bmcl.2013.12.101 BindingDB Entry DOI: 10.7270/Q2HD7X41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxa40 (Acinetobacter baumannii) | BDBM98764 (US8487093, 132) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labs Curated by ChEMBL | Assay Description Inhibition of beta-lactamase Oxa40 in Acinetobacter baumannii assessed as inhibition of hydrolysis of nitrocefin | Bioorg Med Chem Lett 24: 780-5 (2014) Article DOI: 10.1016/j.bmcl.2013.12.101 BindingDB Entry DOI: 10.7270/Q2HD7X41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxa40 (Acinetobacter baumannii) | BDBM50447648 (CHEMBL3112750) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labs Curated by ChEMBL | Assay Description Inhibition of beta-lactamase Oxa40 in Acinetobacter baumannii assessed as inhibition of hydrolysis of nitrocefin | Bioorg Med Chem Lett 24: 780-5 (2014) Article DOI: 10.1016/j.bmcl.2013.12.101 BindingDB Entry DOI: 10.7270/Q2HD7X41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 61 total ) | Next | Last >> |