 Found 1573 hits with Last Name = 'palmer' and Initial = 'ws'
Found 1573 hits with Last Name = 'palmer' and Initial = 'ws' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Alpha-1A adrenergic receptor
(CALF) | BDBM50408198
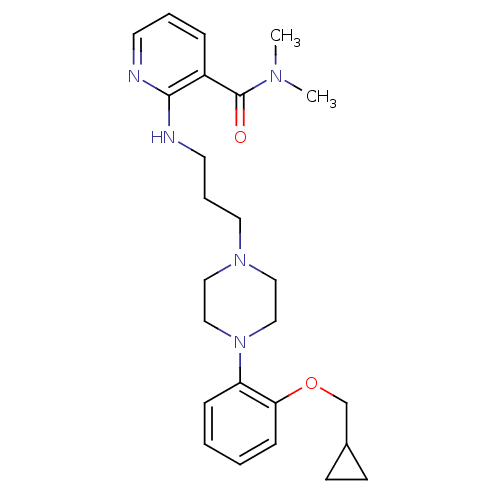
(CHEMBL91278)Show SMILES CN(C)C(=O)c1cccnc1NCCCN1CCN(CC1)c1ccccc1OCC1CC1 Show InChI InChI=1S/C25H35N5O2/c1-28(2)25(31)21-7-5-12-26-24(21)27-13-6-14-29-15-17-30(18-16-29)22-8-3-4-9-23(22)32-19-20-10-11-20/h3-5,7-9,12,20H,6,10-11,13-19H2,1-2H3,(H,26,27) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by ChEMBL
| Assay Description
In vitro for the displacement of [3H]prazosin binding to bovine Alpha-1A adrenergic receptor |
J Med Chem 40: 2674-87 (1997)
Checked by Author
Article DOI: 10.1021/jm970166j
BindingDB Entry DOI: 10.7270/Q2R78GD2 |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(CALF) | BDBM50408201
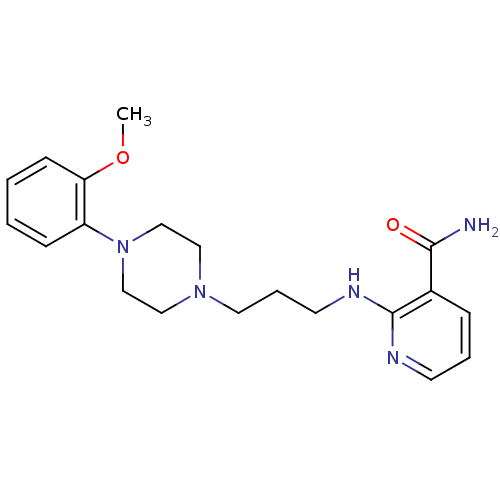
(CHEMBL88512)Show InChI InChI=1S/C20H27N5O2/c1-27-18-8-3-2-7-17(18)25-14-12-24(13-15-25)11-5-10-23-20-16(19(21)26)6-4-9-22-20/h2-4,6-9H,5,10-15H2,1H3,(H2,21,26)(H,22,23) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by ChEMBL
| Assay Description
In vitro for the displacement of [3H]prazosin binding to bovine Alpha-1A adrenergic receptor |
J Med Chem 40: 2674-87 (1997)
Checked by Author
Article DOI: 10.1021/jm970166j
BindingDB Entry DOI: 10.7270/Q2R78GD2 |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(CALF) | BDBM50060964
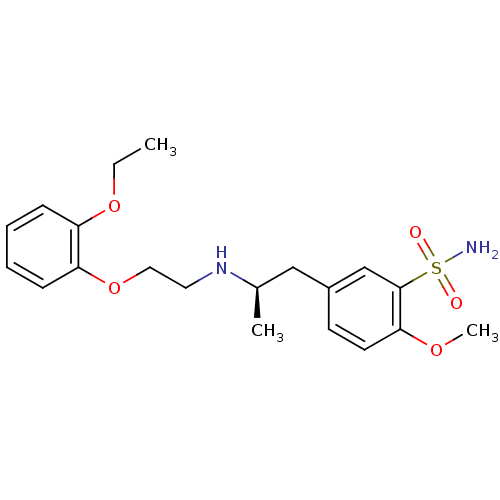
((R)-5-(2-((2-(2-ethoxyphenoxy)ethyl)amino)propyl)-...)Show SMILES CCOc1ccccc1OCCN[C@H](C)Cc1ccc(OC)c(c1)S(N)(=O)=O Show InChI InChI=1S/C20H28N2O5S/c1-4-26-17-7-5-6-8-18(17)27-12-11-22-15(2)13-16-9-10-19(25-3)20(14-16)28(21,23)24/h5-10,14-15,22H,4,11-13H2,1-3H3,(H2,21,23,24)/t15-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by ChEMBL
| Assay Description
In vitro for the displacement of [3H]prazosin binding to bovine Alpha-1A adrenergic receptor |
J Med Chem 40: 2674-87 (1997)
Checked by Author
Article DOI: 10.1021/jm970166j
BindingDB Entry DOI: 10.7270/Q2R78GD2 |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(CALF) | BDBM29568

(CHEMBL2 | PRAZOSIN | PRAZOSIN HYDROCHLORIDE | [3H]...)Show SMILES COc1cc2nc(nc(N)c2cc1OC)N1CCN(CC1)C(=O)c1ccco1 Show InChI InChI=1S/C19H21N5O4/c1-26-15-10-12-13(11-16(15)27-2)21-19(22-17(12)20)24-7-5-23(6-8-24)18(25)14-4-3-9-28-14/h3-4,9-11H,5-8H2,1-2H3,(H2,20,21,22) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by ChEMBL
| Assay Description
In vitro for the displacement of [3H]prazosin binding to bovine Alpha-1A adrenergic receptor |
J Med Chem 40: 2674-87 (1997)
Checked by Author
Article DOI: 10.1021/jm970166j
BindingDB Entry DOI: 10.7270/Q2R78GD2 |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(CALF) | BDBM50408246
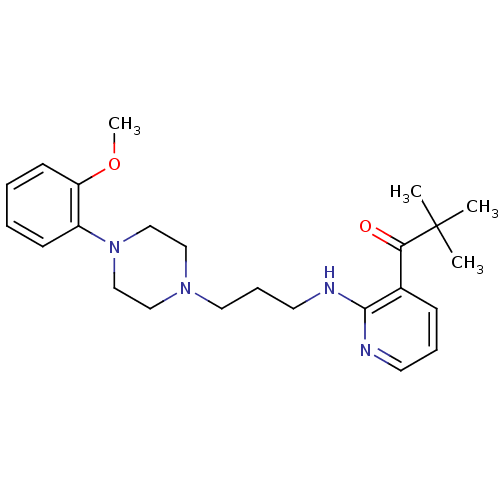
(CHEMBL92261)Show SMILES COc1ccccc1N1CCN(CCCNc2ncccc2C(=O)C(C)(C)C)CC1 Show InChI InChI=1S/C24H34N4O2/c1-24(2,3)22(29)19-9-7-12-25-23(19)26-13-8-14-27-15-17-28(18-16-27)20-10-5-6-11-21(20)30-4/h5-7,9-12H,8,13-18H2,1-4H3,(H,25,26) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by ChEMBL
| Assay Description
In vitro for the displacement of [3H]prazosin binding to bovine Alpha-1A adrenergic receptor |
J Med Chem 40: 2674-87 (1997)
Checked by Author
Article DOI: 10.1021/jm970166j
BindingDB Entry DOI: 10.7270/Q2R78GD2 |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(CALF) | BDBM50408248
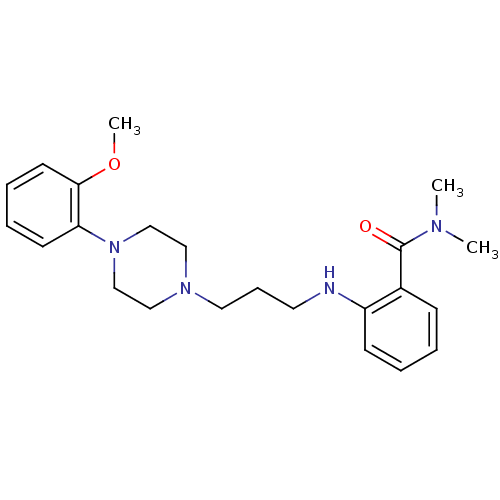
(CHEMBL330060)Show InChI InChI=1S/C23H32N4O2/c1-25(2)23(28)19-9-4-5-10-20(19)24-13-8-14-26-15-17-27(18-16-26)21-11-6-7-12-22(21)29-3/h4-7,9-12,24H,8,13-18H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by ChEMBL
| Assay Description
In vitro for the displacement of [3H]prazosin binding to bovine Alpha-1A adrenergic receptor |
J Med Chem 40: 2674-87 (1997)
Checked by Author
Article DOI: 10.1021/jm970166j
BindingDB Entry DOI: 10.7270/Q2R78GD2 |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(CALF) | BDBM50033112
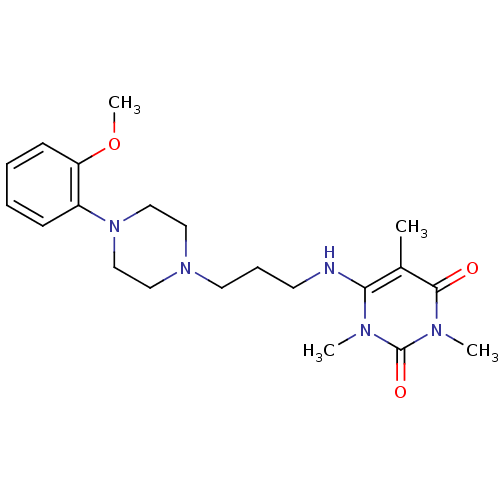
(6-{3-[4-(2-Methoxy-phenyl)-piperazin-1-yl]-propyla...)Show SMILES COc1ccccc1N1CCN(CCCNc2c(C)c(=O)n(C)c(=O)n2C)CC1 Show InChI InChI=1S/C21H31N5O3/c1-16-19(23(2)21(28)24(3)20(16)27)22-10-7-11-25-12-14-26(15-13-25)17-8-5-6-9-18(17)29-4/h5-6,8-9,22H,7,10-15H2,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by ChEMBL
| Assay Description
In vitro for the displacement of [3H]prazosin binding to bovine Alpha-1A adrenergic receptor |
J Med Chem 40: 2674-87 (1997)
Checked by Author
Article DOI: 10.1021/jm970166j
BindingDB Entry DOI: 10.7270/Q2R78GD2 |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(CALF) | BDBM50160165
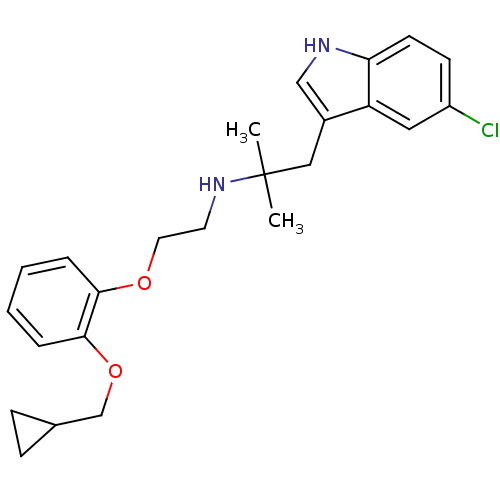
(CHEMBL88272 | RS-17053 | [2-(2-Cyclopropylmethoxy-...)Show SMILES CC(C)(Cc1c[nH]c2ccc(Cl)cc12)NCCOc1ccccc1OCC1CC1 Show InChI InChI=1S/C24H29ClN2O2/c1-24(2,14-18-15-26-21-10-9-19(25)13-20(18)21)27-11-12-28-22-5-3-4-6-23(22)29-16-17-7-8-17/h3-6,9-10,13,15,17,26-27H,7-8,11-12,14,16H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by ChEMBL
| Assay Description
In vitro for the displacement of [3H]prazosin binding to bovine Alpha-1A adrenergic receptor |
J Med Chem 40: 2674-87 (1997)
Checked by Author
Article DOI: 10.1021/jm970166j
BindingDB Entry DOI: 10.7270/Q2R78GD2 |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(CALF) | BDBM50408244
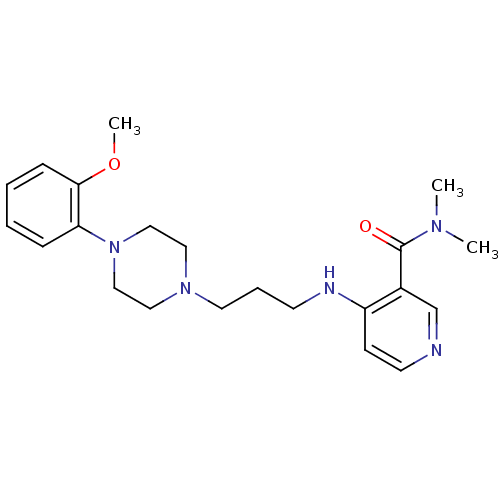
(CHEMBL93736)Show InChI InChI=1S/C22H31N5O2/c1-25(2)22(28)18-17-23-11-9-19(18)24-10-6-12-26-13-15-27(16-14-26)20-7-4-5-8-21(20)29-3/h4-5,7-9,11,17H,6,10,12-16H2,1-3H3,(H,23,24) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by ChEMBL
| Assay Description
In vitro for the displacement of [3H]prazosin binding to bovine Alpha-1A adrenergic receptor |
J Med Chem 40: 2674-87 (1997)
Checked by Author
Article DOI: 10.1021/jm970166j
BindingDB Entry DOI: 10.7270/Q2R78GD2 |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(CALF) | BDBM50408242
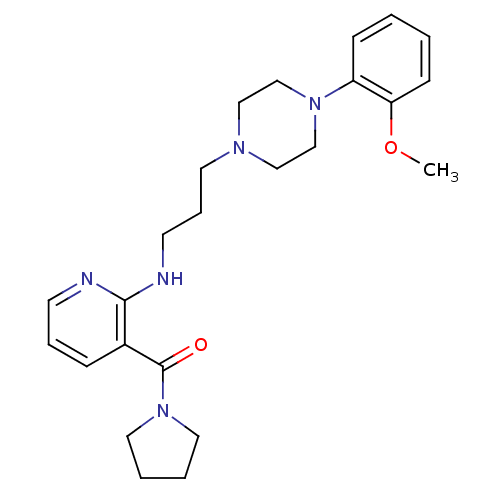
(CHEMBL91876)Show SMILES COc1ccccc1N1CCN(CCCNc2ncccc2C(=O)N2CCCC2)CC1 Show InChI InChI=1S/C24H33N5O2/c1-31-22-10-3-2-9-21(22)28-18-16-27(17-19-28)13-7-12-26-23-20(8-6-11-25-23)24(30)29-14-4-5-15-29/h2-3,6,8-11H,4-5,7,12-19H2,1H3,(H,25,26) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by ChEMBL
| Assay Description
In vitro for the displacement of [3H]prazosin binding to bovine Alpha-1A adrenergic receptor |
J Med Chem 40: 2674-87 (1997)
Checked by Author
Article DOI: 10.1021/jm970166j
BindingDB Entry DOI: 10.7270/Q2R78GD2 |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(CALF) | BDBM50408232
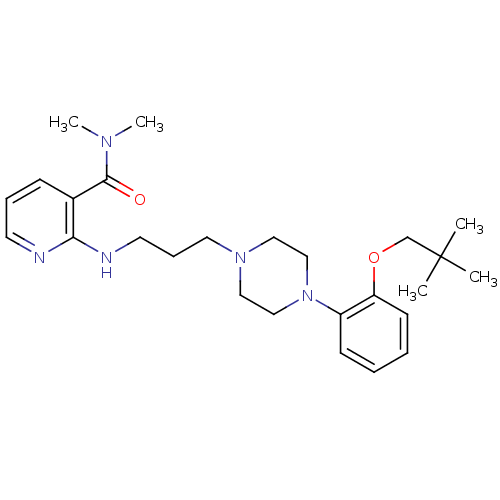
(CHEMBL91093)Show SMILES CN(C)C(=O)c1cccnc1NCCCN1CCN(CC1)c1ccccc1OCC(C)(C)C Show InChI InChI=1S/C26H39N5O2/c1-26(2,3)20-33-23-12-7-6-11-22(23)31-18-16-30(17-19-31)15-9-14-28-24-21(10-8-13-27-24)25(32)29(4)5/h6-8,10-13H,9,14-20H2,1-5H3,(H,27,28) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by ChEMBL
| Assay Description
In vitro for the displacement of [3H]prazosin binding to bovine Alpha-1A adrenergic receptor |
J Med Chem 40: 2674-87 (1997)
Checked by Author
Article DOI: 10.1021/jm970166j
BindingDB Entry DOI: 10.7270/Q2R78GD2 |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(CALF) | BDBM31046
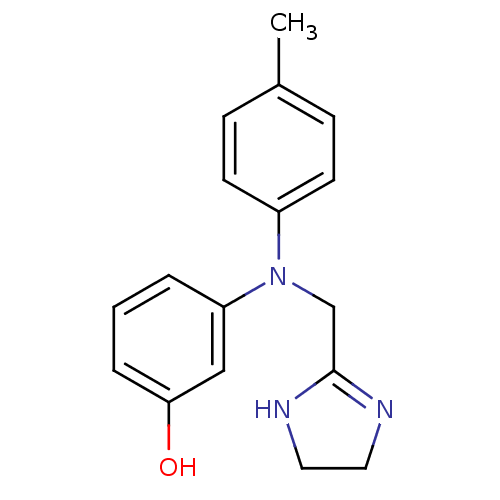
(3-[4,5-dihydro-1H-imidazol-2-ylmethyl-(4-methylphe...)Show InChI InChI=1S/C17H19N3O/c1-13-5-7-14(8-6-13)20(12-17-18-9-10-19-17)15-3-2-4-16(21)11-15/h2-8,11,21H,9-10,12H2,1H3,(H,18,19) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by ChEMBL
| Assay Description
In vitro for the displacement of [3H]prazosin binding to bovine Alpha-1A adrenergic receptor |
J Med Chem 40: 2674-87 (1997)
Checked by Author
Article DOI: 10.1021/jm970166j
BindingDB Entry DOI: 10.7270/Q2R78GD2 |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(CALF) | BDBM50408229
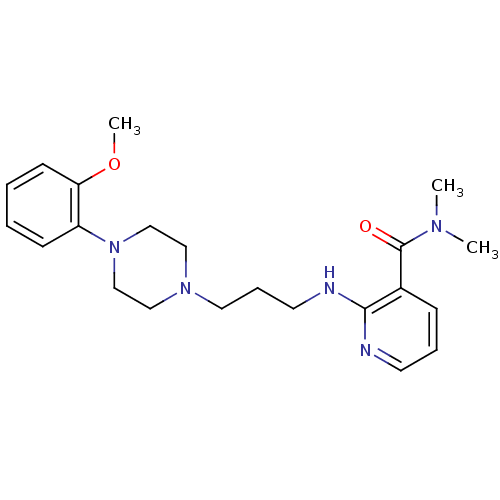
(CHEMBL329160)Show InChI InChI=1S/C22H31N5O2/c1-25(2)22(28)18-8-6-11-23-21(18)24-12-7-13-26-14-16-27(17-15-26)19-9-4-5-10-20(19)29-3/h4-6,8-11H,7,12-17H2,1-3H3,(H,23,24) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by ChEMBL
| Assay Description
The compound was screened in vitro for the displacement of [3H]prazosin binding to bovine Alpha-1A adrenergic receptor |
J Med Chem 40: 2674-87 (1997)
Checked by Author
Article DOI: 10.1021/jm970166j
BindingDB Entry DOI: 10.7270/Q2R78GD2 |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(CALF) | BDBM50408205
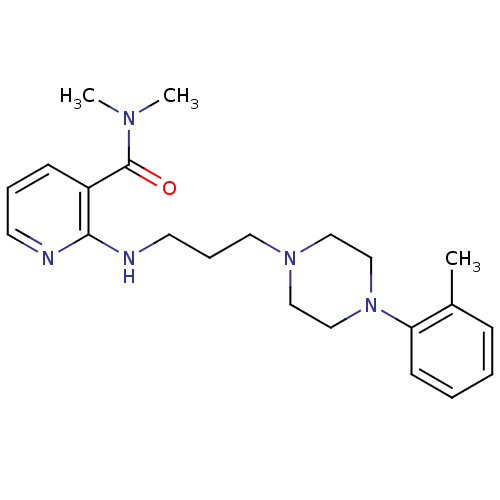
(CHEMBL88435)Show InChI InChI=1S/C22H31N5O/c1-18-8-4-5-10-20(18)27-16-14-26(15-17-27)13-7-12-24-21-19(9-6-11-23-21)22(28)25(2)3/h4-6,8-11H,7,12-17H2,1-3H3,(H,23,24) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by ChEMBL
| Assay Description
In vitro for the displacement of [3H]prazosin binding to bovine Alpha-1A adrenergic receptor |
J Med Chem 40: 2674-87 (1997)
Checked by Author
Article DOI: 10.1021/jm970166j
BindingDB Entry DOI: 10.7270/Q2R78GD2 |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(CALF) | BDBM50408239
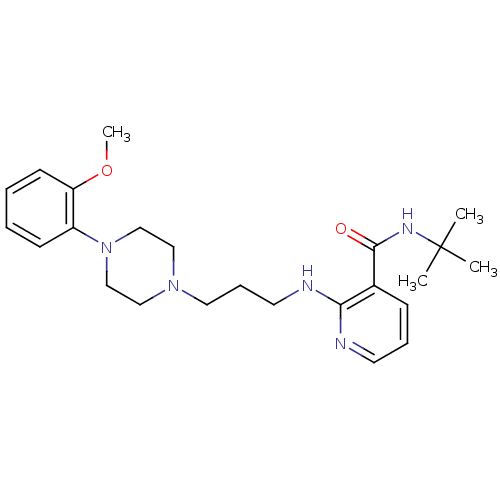
(CHEMBL90874)Show SMILES COc1ccccc1N1CCN(CCCNc2ncccc2C(=O)NC(C)(C)C)CC1 Show InChI InChI=1S/C24H35N5O2/c1-24(2,3)27-23(30)19-9-7-12-25-22(19)26-13-8-14-28-15-17-29(18-16-28)20-10-5-6-11-21(20)31-4/h5-7,9-12H,8,13-18H2,1-4H3,(H,25,26)(H,27,30) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by ChEMBL
| Assay Description
In vitro for the displacement of [3H]prazosin binding to bovine Alpha-1A adrenergic receptor |
J Med Chem 40: 2674-87 (1997)
Checked by Author
Article DOI: 10.1021/jm970166j
BindingDB Entry DOI: 10.7270/Q2R78GD2 |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(CALF) | BDBM50408202

(CHEMBL92901)Show SMILES COc1ccccc1N1CCN(CCCNc2nc(C)cc(C)c2C(N)=O)CC1 Show InChI InChI=1S/C22H31N5O2/c1-16-15-17(2)25-22(20(16)21(23)28)24-9-6-10-26-11-13-27(14-12-26)18-7-4-5-8-19(18)29-3/h4-5,7-8,15H,6,9-14H2,1-3H3,(H2,23,28)(H,24,25) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by ChEMBL
| Assay Description
In vitro for the displacement of [3H]prazosin binding to bovine Alpha-1A adrenergic receptor |
J Med Chem 40: 2674-87 (1997)
Checked by Author
Article DOI: 10.1021/jm970166j
BindingDB Entry DOI: 10.7270/Q2R78GD2 |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(CALF) | BDBM50408208
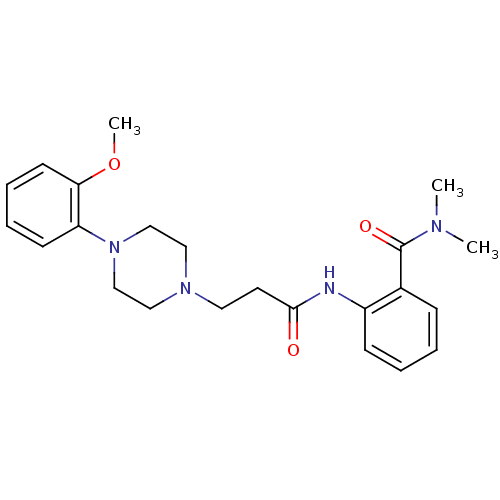
(CHEMBL88820)Show SMILES COc1ccccc1N1CCN(CCC(=O)Nc2ccccc2C(=O)N(C)C)CC1 Show InChI InChI=1S/C23H30N4O3/c1-25(2)23(29)18-8-4-5-9-19(18)24-22(28)12-13-26-14-16-27(17-15-26)20-10-6-7-11-21(20)30-3/h4-11H,12-17H2,1-3H3,(H,24,28) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by ChEMBL
| Assay Description
In vitro for the displacement of [3H]prazosin binding to bovine Alpha-1A adrenergic receptor |
J Med Chem 40: 2674-87 (1997)
Checked by Author
Article DOI: 10.1021/jm970166j
BindingDB Entry DOI: 10.7270/Q2R78GD2 |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(CALF) | BDBM50408238
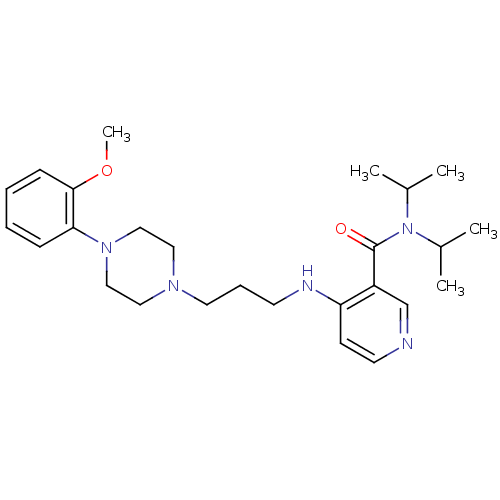
(CHEMBL89916)Show SMILES COc1ccccc1N1CCN(CCCNc2ccncc2C(=O)N(C(C)C)C(C)C)CC1 Show InChI InChI=1S/C26H39N5O2/c1-20(2)31(21(3)4)26(32)22-19-27-13-11-23(22)28-12-8-14-29-15-17-30(18-16-29)24-9-6-7-10-25(24)33-5/h6-7,9-11,13,19-21H,8,12,14-18H2,1-5H3,(H,27,28) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by ChEMBL
| Assay Description
In vitro for the displacement of [3H]prazosin binding to bovine Alpha-1A adrenergic receptor |
J Med Chem 40: 2674-87 (1997)
Checked by Author
Article DOI: 10.1021/jm970166j
BindingDB Entry DOI: 10.7270/Q2R78GD2 |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(CALF) | BDBM50408192
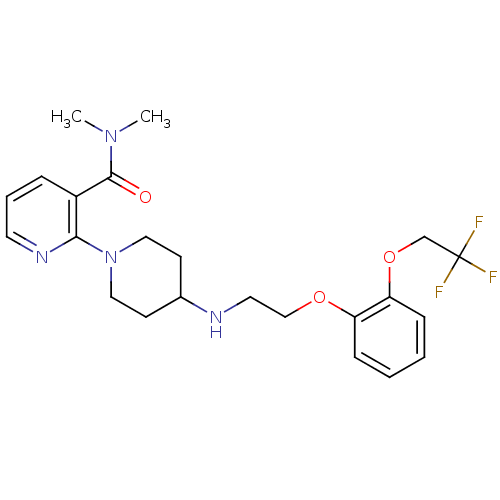
(CHEMBL90869)Show SMILES CN(C)C(=O)c1cccnc1N1CCC(CC1)NCCOc1ccccc1OCC(F)(F)F Show InChI InChI=1S/C23H29F3N4O3/c1-29(2)22(31)18-6-5-11-28-21(18)30-13-9-17(10-14-30)27-12-15-32-19-7-3-4-8-20(19)33-16-23(24,25)26/h3-8,11,17,27H,9-10,12-16H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by ChEMBL
| Assay Description
In vitro for the displacement of [3H]prazosin binding to bovine Alpha-1A adrenergic receptor |
J Med Chem 40: 2674-87 (1997)
Checked by Author
Article DOI: 10.1021/jm970166j
BindingDB Entry DOI: 10.7270/Q2R78GD2 |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(CALF) | BDBM50408194
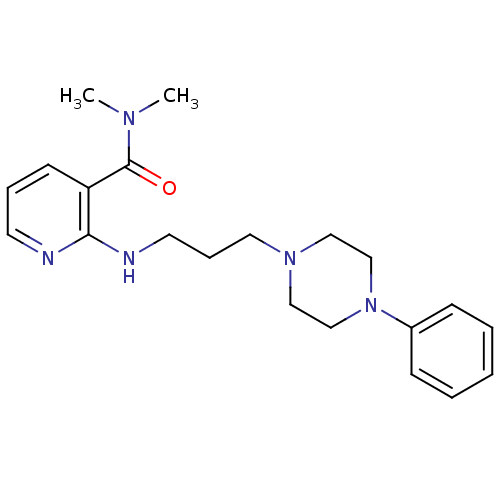
(CHEMBL90287)Show InChI InChI=1S/C21H29N5O/c1-24(2)21(27)19-10-6-11-22-20(19)23-12-7-13-25-14-16-26(17-15-25)18-8-4-3-5-9-18/h3-6,8-11H,7,12-17H2,1-2H3,(H,22,23) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by ChEMBL
| Assay Description
In vitro for the displacement of [3H]prazosin binding to bovine Alpha-1A adrenergic receptor |
J Med Chem 40: 2674-87 (1997)
Checked by Author
Article DOI: 10.1021/jm970166j
BindingDB Entry DOI: 10.7270/Q2R78GD2 |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(CALF) | BDBM50033111
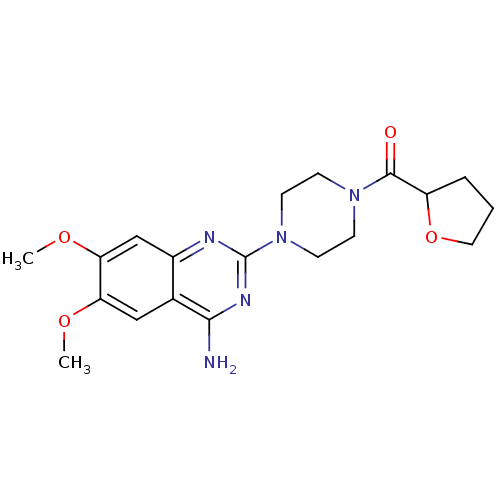
(1-(4-Amino-6,7-dimethoxy-2-quinazolinyl)-4-((tetra...)Show SMILES COc1cc2nc(nc(N)c2cc1OC)N1CCN(CC1)C(=O)C1CCCO1 Show InChI InChI=1S/C19H25N5O4/c1-26-15-10-12-13(11-16(15)27-2)21-19(22-17(12)20)24-7-5-23(6-8-24)18(25)14-4-3-9-28-14/h10-11,14H,3-9H2,1-2H3,(H2,20,21,22) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by ChEMBL
| Assay Description
In vitro for the displacement of [3H]prazosin binding to bovine Alpha-1A adrenergic receptor |
J Med Chem 40: 2674-87 (1997)
Checked by Author
Article DOI: 10.1021/jm970166j
BindingDB Entry DOI: 10.7270/Q2R78GD2 |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(CALF) | BDBM50408225
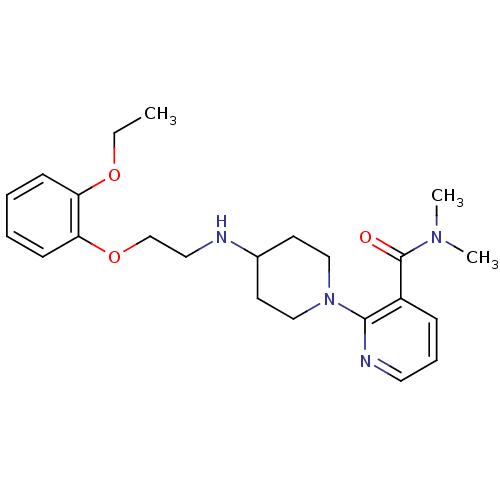
(CHEMBL312935)Show SMILES CCOc1ccccc1OCCNC1CCN(CC1)c1ncccc1C(=O)N(C)C Show InChI InChI=1S/C23H32N4O3/c1-4-29-20-9-5-6-10-21(20)30-17-14-24-18-11-15-27(16-12-18)22-19(8-7-13-25-22)23(28)26(2)3/h5-10,13,18,24H,4,11-12,14-17H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by ChEMBL
| Assay Description
In vitro for the displacement of [3H]prazosin binding to bovine Alpha-1A adrenergic receptor |
J Med Chem 40: 2674-87 (1997)
Checked by Author
Article DOI: 10.1021/jm970166j
BindingDB Entry DOI: 10.7270/Q2R78GD2 |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(CALF) | BDBM50408193
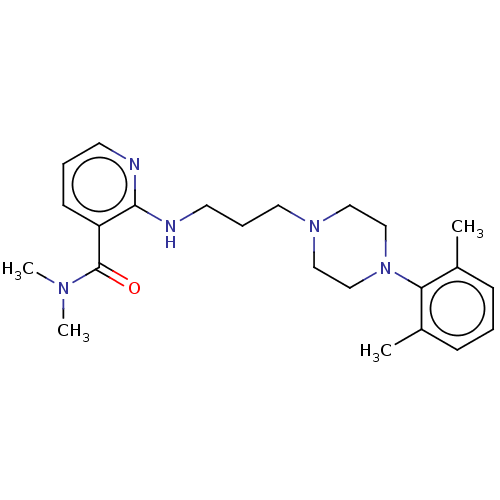
(CHEMBL92109)Show SMILES CN(C)C(=O)c1cccnc1NCCCN1CCN(CC1)c1c(C)cccc1C Show InChI InChI=1S/C23H33N5O/c1-18-8-5-9-19(2)21(18)28-16-14-27(15-17-28)13-7-12-25-22-20(10-6-11-24-22)23(29)26(3)4/h5-6,8-11H,7,12-17H2,1-4H3,(H,24,25) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by ChEMBL
| Assay Description
In vitro for the displacement of [3H]prazosin binding to bovine Alpha-1A adrenergic receptor |
J Med Chem 40: 2674-87 (1997)
Checked by Author
Article DOI: 10.1021/jm970166j
BindingDB Entry DOI: 10.7270/Q2R78GD2 |
More data for this
Ligand-Target Pair | |
Alpha-2B adrenergic receptor
(NEONATAL RAT) | BDBM50408216
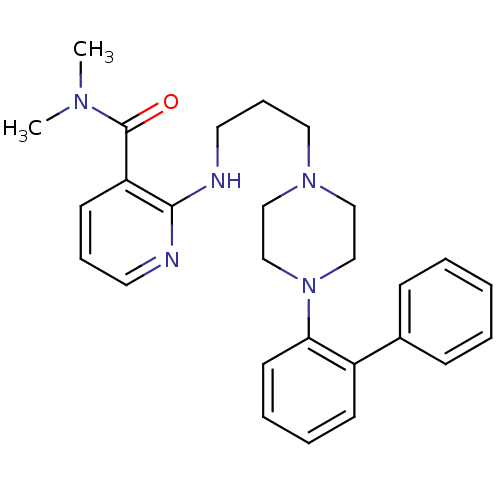
(CHEMBL90746)Show SMILES CN(C)C(=O)c1cccnc1NCCCN1CCN(CC1)c1ccccc1-c1ccccc1 Show InChI InChI=1S/C27H33N5O/c1-30(2)27(33)24-13-8-15-28-26(24)29-16-9-17-31-18-20-32(21-19-31)25-14-7-6-12-23(25)22-10-4-3-5-11-22/h3-8,10-15H,9,16-21H2,1-2H3,(H,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by ChEMBL
| Assay Description
The compound's binding affinity against Alpha-2B adrenergic receptor from rat kidney homogenate in the presence of phentolamine |
J Med Chem 40: 2674-87 (1997)
Checked by Author
Article DOI: 10.1021/jm970166j
BindingDB Entry DOI: 10.7270/Q2R78GD2 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50408216

(CHEMBL90746)Show SMILES CN(C)C(=O)c1cccnc1NCCCN1CCN(CC1)c1ccccc1-c1ccccc1 Show InChI InChI=1S/C27H33N5O/c1-30(2)27(33)24-13-8-15-28-26(24)29-16-9-17-31-18-20-32(21-19-31)25-14-7-6-12-23(25)22-10-4-3-5-11-22/h3-8,10-15H,9,16-21H2,1-2H3,(H,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by ChEMBL
| Assay Description
The compound's binding affinity against Dopamine receptor D2 |
J Med Chem 40: 2674-87 (1997)
Checked by Author
Article DOI: 10.1021/jm970166j
BindingDB Entry DOI: 10.7270/Q2R78GD2 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50408197

(CHEMBL91605)Show SMILES COc1cc(F)ccc1N1CCN(CCCNc2c(cnc3n(C)nc(C)c23)C(=O)N(C)C)CC1 Show InChI InChI=1S/C25H34FN7O2/c1-17-22-23(19(25(34)30(2)3)16-28-24(22)31(4)29-17)27-9-6-10-32-11-13-33(14-12-32)20-8-7-18(26)15-21(20)35-5/h7-8,15-16H,6,9-14H2,1-5H3,(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by ChEMBL
| Assay Description
The compound's binding affinity against Dopamine receptor D2 |
J Med Chem 40: 2674-87 (1997)
Checked by Author
Article DOI: 10.1021/jm970166j
BindingDB Entry DOI: 10.7270/Q2R78GD2 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Mus musculus (Mouse)) | BDBM50408197

(CHEMBL91605)Show SMILES COc1cc(F)ccc1N1CCN(CCCNc2c(cnc3n(C)nc(C)c23)C(=O)N(C)C)CC1 Show InChI InChI=1S/C25H34FN7O2/c1-17-22-23(19(25(34)30(2)3)16-28-24(22)31(4)29-17)27-9-6-10-32-11-13-33(14-12-32)20-8-7-18(26)15-21(20)35-5/h7-8,15-16H,6,9-14H2,1-5H3,(H,27,28) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by ChEMBL
| Assay Description
The compound's binding affinity against 5-hydroxytryptamine 1A receptor |
J Med Chem 40: 2674-87 (1997)
Checked by Author
Article DOI: 10.1021/jm970166j
BindingDB Entry DOI: 10.7270/Q2R78GD2 |
More data for this
Ligand-Target Pair | |
Alpha-2B adrenergic receptor
(NEONATAL RAT) | BDBM50408236
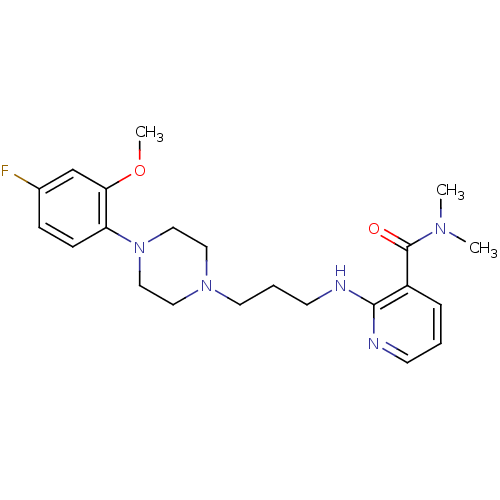
(CHEMBL420620)Show SMILES COc1cc(F)ccc1N1CCN(CCCNc2ncccc2C(=O)N(C)C)CC1 Show InChI InChI=1S/C22H30FN5O2/c1-26(2)22(29)18-6-4-9-24-21(18)25-10-5-11-27-12-14-28(15-13-27)19-8-7-17(23)16-20(19)30-3/h4,6-9,16H,5,10-15H2,1-3H3,(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by ChEMBL
| Assay Description
The compound's binding affinity against Alpha-2B adrenergic receptor from rat kidney homogenate in the presence of phentolamine |
J Med Chem 40: 2674-87 (1997)
Checked by Author
Article DOI: 10.1021/jm970166j
BindingDB Entry DOI: 10.7270/Q2R78GD2 |
More data for this
Ligand-Target Pair | |
Alpha-2B adrenergic receptor
(NEONATAL RAT) | BDBM50408241
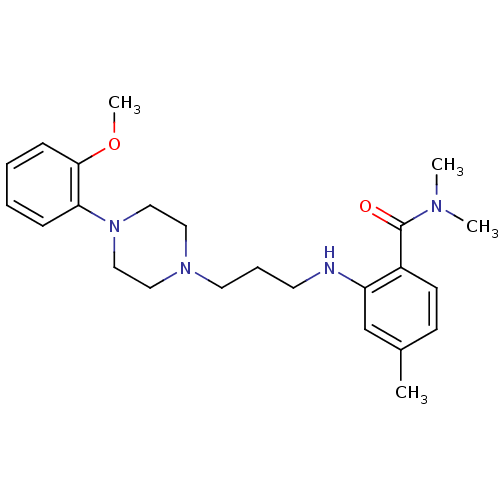
(CHEMBL89030)Show SMILES COc1ccccc1N1CCN(CCCNc2cc(C)ccc2C(=O)N(C)C)CC1 Show InChI InChI=1S/C24H34N4O2/c1-19-10-11-20(24(29)26(2)3)21(18-19)25-12-7-13-27-14-16-28(17-15-27)22-8-5-6-9-23(22)30-4/h5-6,8-11,18,25H,7,12-17H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by ChEMBL
| Assay Description
The compound's binding affinity against Alpha-2B adrenergic receptor from rat kidney homogenate in the presence of phentolamine |
J Med Chem 40: 2674-87 (1997)
Checked by Author
Article DOI: 10.1021/jm970166j
BindingDB Entry DOI: 10.7270/Q2R78GD2 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50394918
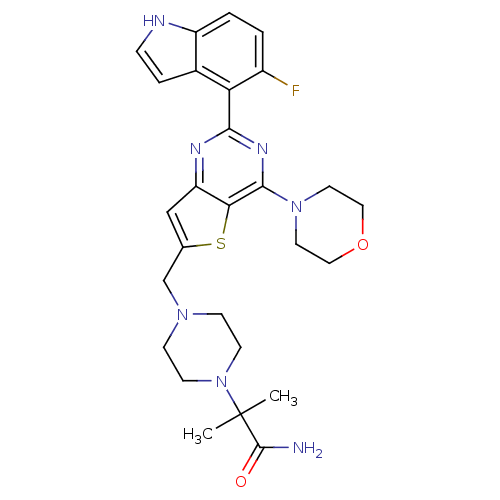
(CHEMBL2165504)Show SMILES CC(C)(N1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2c(F)ccc3[nH]ccc23)CC1)C(N)=O Show InChI InChI=1S/C27H32FN7O2S/c1-27(2,26(29)36)35-9-7-33(8-10-35)16-17-15-21-23(38-17)25(34-11-13-37-14-12-34)32-24(31-21)22-18-5-6-30-20(18)4-3-19(22)28/h3-6,15,30H,7-14,16H2,1-2H3,(H2,29,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Time dependent inhibition of CYP3A4 in human liver microsomes preincubated for 30 mins |
J Med Chem 55: 5887-900 (2012)
Article DOI: 10.1021/jm3003747
BindingDB Entry DOI: 10.7270/Q2V125XN |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50408236

(CHEMBL420620)Show SMILES COc1cc(F)ccc1N1CCN(CCCNc2ncccc2C(=O)N(C)C)CC1 Show InChI InChI=1S/C22H30FN5O2/c1-26(2)22(29)18-6-4-9-24-21(18)25-10-5-11-27-12-14-28(15-13-27)19-8-7-17(23)16-20(19)30-3/h4,6-9,16H,5,10-15H2,1-3H3,(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by ChEMBL
| Assay Description
The compound's binding affinity against Dopamine receptor D2 |
J Med Chem 40: 2674-87 (1997)
Checked by Author
Article DOI: 10.1021/jm970166j
BindingDB Entry DOI: 10.7270/Q2R78GD2 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Mus musculus (Mouse)) | BDBM50408236

(CHEMBL420620)Show SMILES COc1cc(F)ccc1N1CCN(CCCNc2ncccc2C(=O)N(C)C)CC1 Show InChI InChI=1S/C22H30FN5O2/c1-26(2)22(29)18-6-4-9-24-21(18)25-10-5-11-27-12-14-28(15-13-27)19-8-7-17(23)16-20(19)30-3/h4,6-9,16H,5,10-15H2,1-3H3,(H,24,25) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by ChEMBL
| Assay Description
The compound's binding affinity against 5-hydroxytryptamine 1A receptor |
J Med Chem 40: 2674-87 (1997)
Checked by Author
Article DOI: 10.1021/jm970166j
BindingDB Entry DOI: 10.7270/Q2R78GD2 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50408241

(CHEMBL89030)Show SMILES COc1ccccc1N1CCN(CCCNc2cc(C)ccc2C(=O)N(C)C)CC1 Show InChI InChI=1S/C24H34N4O2/c1-19-10-11-20(24(29)26(2)3)21(18-19)25-12-7-13-27-14-16-28(17-15-27)22-8-5-6-9-23(22)30-4/h5-6,8-11,18,25H,7,12-17H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by ChEMBL
| Assay Description
The compound's binding affinity against Dopamine receptor D2 |
J Med Chem 40: 2674-87 (1997)
Checked by Author
Article DOI: 10.1021/jm970166j
BindingDB Entry DOI: 10.7270/Q2R78GD2 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50408229

(CHEMBL329160)Show InChI InChI=1S/C22H31N5O2/c1-25(2)22(28)18-8-6-11-23-21(18)24-12-7-13-26-14-16-27(17-15-26)19-9-4-5-10-20(19)29-3/h4-6,8-11H,7,12-17H2,1-3H3,(H,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by ChEMBL
| Assay Description
The compound's binding affinity against Dopamine receptor D2 |
J Med Chem 40: 2674-87 (1997)
Checked by Author
Article DOI: 10.1021/jm970166j
BindingDB Entry DOI: 10.7270/Q2R78GD2 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Mus musculus (Mouse)) | BDBM50408199

(CHEMBL88160)Show SMILES CN(C)C(=O)c1cnc2n(C)nc(C)c2c1NCCCN1CCN(CC1)c1ccccc1OCC(F)(F)F Show InChI InChI=1S/C26H34F3N7O2/c1-18-22-23(19(25(37)33(2)3)16-31-24(22)34(4)32-18)30-10-7-11-35-12-14-36(15-13-35)20-8-5-6-9-21(20)38-17-26(27,28)29/h5-6,8-9,16H,7,10-15,17H2,1-4H3,(H,30,31) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by ChEMBL
| Assay Description
The compound's binding affinity against 5-hydroxytryptamine 1A receptor |
J Med Chem 40: 2674-87 (1997)
Checked by Author
Article DOI: 10.1021/jm970166j
BindingDB Entry DOI: 10.7270/Q2R78GD2 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Mus musculus (Mouse)) | BDBM50408229

(CHEMBL329160)Show InChI InChI=1S/C22H31N5O2/c1-25(2)22(28)18-8-6-11-23-21(18)24-12-7-13-26-14-16-27(17-15-26)19-9-4-5-10-20(19)29-3/h4-6,8-11H,7,12-17H2,1-3H3,(H,23,24) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by ChEMBL
| Assay Description
The compound's binding affinity against 5-hydroxytryptamine 1A receptor |
J Med Chem 40: 2674-87 (1997)
Checked by Author
Article DOI: 10.1021/jm970166j
BindingDB Entry DOI: 10.7270/Q2R78GD2 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Mus musculus (Mouse)) | BDBM50408241

(CHEMBL89030)Show SMILES COc1ccccc1N1CCN(CCCNc2cc(C)ccc2C(=O)N(C)C)CC1 Show InChI InChI=1S/C24H34N4O2/c1-19-10-11-20(24(29)26(2)3)21(18-19)25-12-7-13-27-14-16-28(17-15-27)22-8-5-6-9-23(22)30-4/h5-6,8-11,18,25H,7,12-17H2,1-4H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by ChEMBL
| Assay Description
The compound's binding affinity against 5-hydroxytryptamine 1A receptor |
J Med Chem 40: 2674-87 (1997)
Checked by Author
Article DOI: 10.1021/jm970166j
BindingDB Entry DOI: 10.7270/Q2R78GD2 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50408199

(CHEMBL88160)Show SMILES CN(C)C(=O)c1cnc2n(C)nc(C)c2c1NCCCN1CCN(CC1)c1ccccc1OCC(F)(F)F Show InChI InChI=1S/C26H34F3N7O2/c1-18-22-23(19(25(37)33(2)3)16-31-24(22)34(4)32-18)30-10-7-11-35-12-14-36(15-13-35)20-8-5-6-9-21(20)38-17-26(27,28)29/h5-6,8-9,16H,7,10-15,17H2,1-4H3,(H,30,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by ChEMBL
| Assay Description
The compound's binding affinity against Dopamine receptor D2 |
J Med Chem 40: 2674-87 (1997)
Checked by Author
Article DOI: 10.1021/jm970166j
BindingDB Entry DOI: 10.7270/Q2R78GD2 |
More data for this
Ligand-Target Pair | |
Alpha-2B adrenergic receptor
(NEONATAL RAT) | BDBM50408199

(CHEMBL88160)Show SMILES CN(C)C(=O)c1cnc2n(C)nc(C)c2c1NCCCN1CCN(CC1)c1ccccc1OCC(F)(F)F Show InChI InChI=1S/C26H34F3N7O2/c1-18-22-23(19(25(37)33(2)3)16-31-24(22)34(4)32-18)30-10-7-11-35-12-14-36(15-13-35)20-8-5-6-9-21(20)38-17-26(27,28)29/h5-6,8-9,16H,7,10-15,17H2,1-4H3,(H,30,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by ChEMBL
| Assay Description
The compound's binding affinity against Alpha-2B adrenergic receptor from rat kidney homogenate in the presence of phentolamine |
J Med Chem 40: 2674-87 (1997)
Checked by Author
Article DOI: 10.1021/jm970166j
BindingDB Entry DOI: 10.7270/Q2R78GD2 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50394917
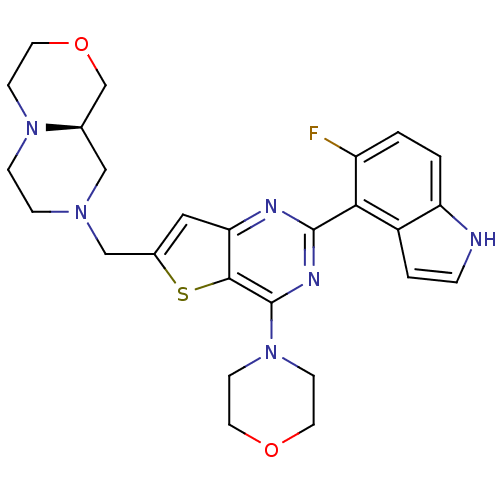
(CHEMBL2165505)Show SMILES Fc1ccc2[nH]ccc2c1-c1nc(N2CCOCC2)c2sc(CN3CCN4CCOC[C@H]4C3)cc2n1 |r| Show InChI InChI=1S/C26H29FN6O2S/c27-20-1-2-21-19(3-4-28-21)23(20)25-29-22-13-18(15-31-5-6-32-7-12-35-16-17(32)14-31)36-24(22)26(30-25)33-8-10-34-11-9-33/h1-4,13,17,28H,5-12,14-16H2/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Time dependent inhibition of CYP3A4 in human liver microsomes preincubated for 30 mins |
J Med Chem 55: 5887-900 (2012)
Article DOI: 10.1021/jm3003747
BindingDB Entry DOI: 10.7270/Q2V125XN |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50394916
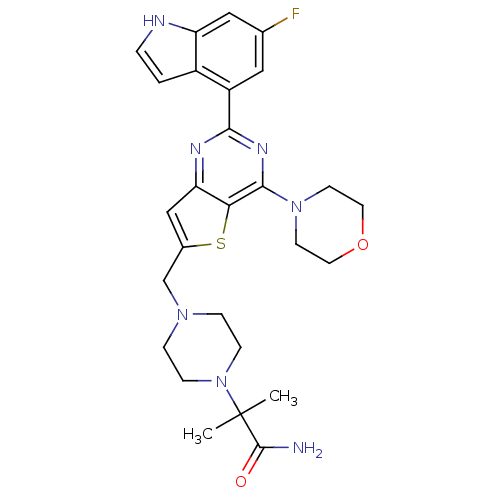
(CHEMBL2165506)Show SMILES CC(C)(N1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cc(F)cc3[nH]ccc23)CC1)C(N)=O Show InChI InChI=1S/C27H32FN7O2S/c1-27(2,26(29)36)35-7-5-33(6-8-35)16-18-15-22-23(38-18)25(34-9-11-37-12-10-34)32-24(31-22)20-13-17(28)14-21-19(20)3-4-30-21/h3-4,13-15,30H,5-12,16H2,1-2H3,(H2,29,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Binding affinity to PI3Kdelta after 30 mins by competitive fluorescence polarization assay |
J Med Chem 55: 5887-900 (2012)
Article DOI: 10.1021/jm3003747
BindingDB Entry DOI: 10.7270/Q2V125XN |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50394910
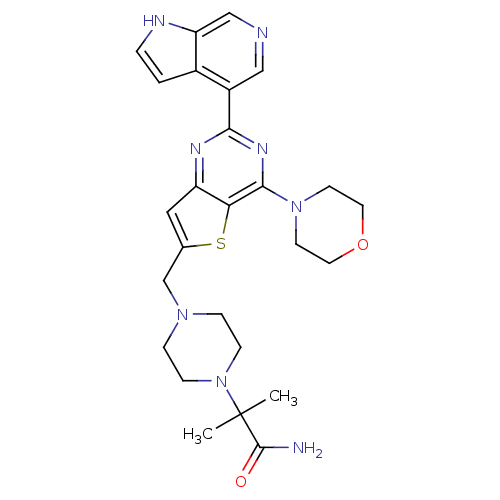
(CHEMBL2165512)Show SMILES CC(C)(N1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cncc3[nH]ccc23)CC1)C(N)=O Show InChI InChI=1S/C26H32N8O2S/c1-26(2,25(27)35)34-7-5-32(6-8-34)16-17-13-20-22(37-17)24(33-9-11-36-12-10-33)31-23(30-20)19-14-28-15-21-18(19)3-4-29-21/h3-4,13-15,29H,5-12,16H2,1-2H3,(H2,27,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Binding affinity to PI3Kdelta after 30 mins by competitive fluorescence polarization assay |
J Med Chem 55: 5887-900 (2012)
Article DOI: 10.1021/jm3003747
BindingDB Entry DOI: 10.7270/Q2V125XN |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50394913

(CHEMBL2165509)Show SMILES Cc1cc2c(cccc2[nH]1)-c1nc(N2CCOCC2)c2sc(CN3CCN(CC3)C(C)(C)C(N)=O)cc2n1 Show InChI InChI=1S/C28H35N7O2S/c1-18-15-21-20(5-4-6-22(21)30-18)25-31-23-16-19(38-24(23)26(32-25)34-11-13-37-14-12-34)17-33-7-9-35(10-8-33)28(2,3)27(29)36/h4-6,15-16,30H,7-14,17H2,1-3H3,(H2,29,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Binding affinity to PI3Kdelta after 30 mins by competitive fluorescence polarization assay |
J Med Chem 55: 5887-900 (2012)
Article DOI: 10.1021/jm3003747
BindingDB Entry DOI: 10.7270/Q2V125XN |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50394920
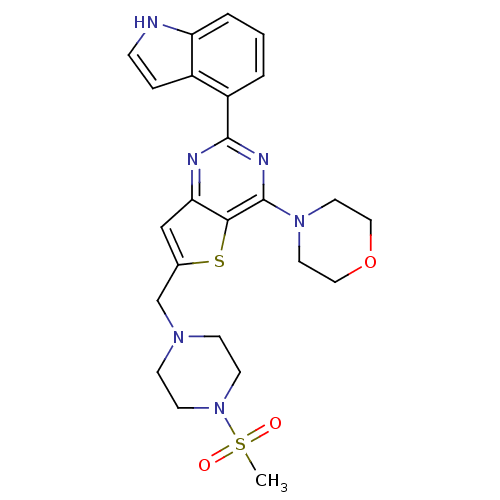
(CHEMBL2165672)Show SMILES CS(=O)(=O)N1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cccc3[nH]ccc23)CC1 Show InChI InChI=1S/C24H28N6O3S2/c1-35(31,32)30-9-7-28(8-10-30)16-17-15-21-22(34-17)24(29-11-13-33-14-12-29)27-23(26-21)19-3-2-4-20-18(19)5-6-25-20/h2-6,15,25H,7-14,16H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Binding affinity to PI3Kdelta after 30 mins by competitive fluorescence polarization assay |
J Med Chem 55: 5887-900 (2012)
Article DOI: 10.1021/jm3003747
BindingDB Entry DOI: 10.7270/Q2V125XN |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50394921
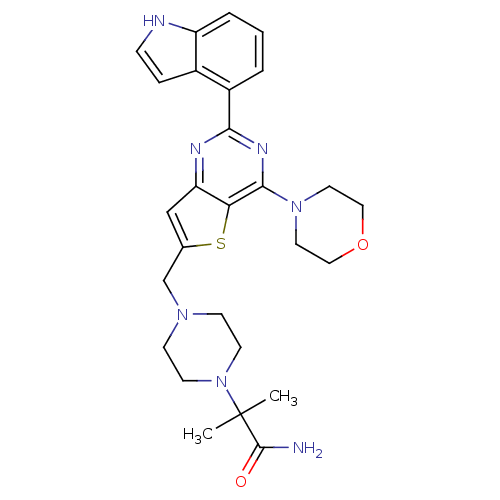
(CHEMBL2165671)Show SMILES CC(C)(N1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cccc3[nH]ccc23)CC1)C(N)=O Show InChI InChI=1S/C27H33N7O2S/c1-27(2,26(28)35)34-10-8-32(9-11-34)17-18-16-22-23(37-18)25(33-12-14-36-15-13-33)31-24(30-22)20-4-3-5-21-19(20)6-7-29-21/h3-7,16,29H,8-15,17H2,1-2H3,(H2,28,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Binding affinity to PI3Kdelta after 30 mins by competitive fluorescence polarization assay |
J Med Chem 55: 5887-900 (2012)
Article DOI: 10.1021/jm3003747
BindingDB Entry DOI: 10.7270/Q2V125XN |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50394908
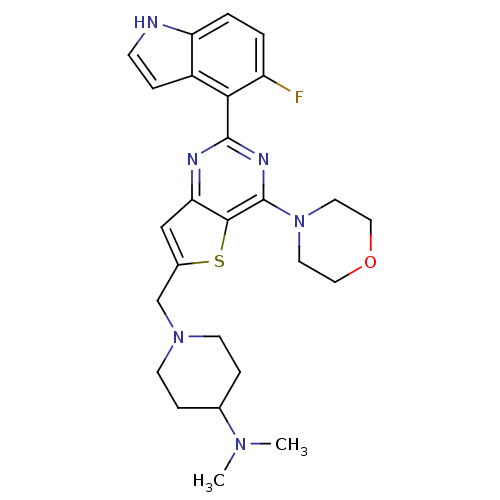
(CHEMBL2165666)Show SMILES CN(C)C1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2c(F)ccc3[nH]ccc23)CC1 Show InChI InChI=1S/C26H31FN6OS/c1-31(2)17-6-9-32(10-7-17)16-18-15-22-24(35-18)26(33-11-13-34-14-12-33)30-25(29-22)23-19-5-8-28-21(19)4-3-20(23)27/h3-5,8,15,17,28H,6-7,9-14,16H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Binding affinity to PI3Kdelta after 30 mins by competitive fluorescence polarization assay |
J Med Chem 55: 5887-900 (2012)
Article DOI: 10.1021/jm3003747
BindingDB Entry DOI: 10.7270/Q2V125XN |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM102578
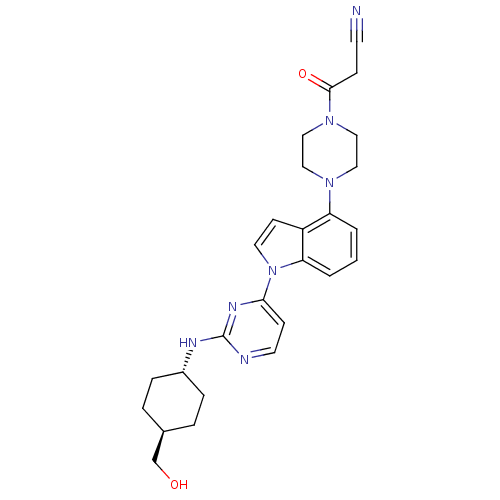
(US8536172, I-27)Show SMILES OC[C@H]1CC[C@@H](CC1)Nc1nccc(n1)-n1ccc2c(cccc12)N1CCN(CC1)C(=O)CC#N |r,wU:5.8,wD:2.1,(-5.68,-4.22,;-7.01,-3.45,;-7.01,-1.91,;-5.68,-1.14,;-5.68,.4,;-7.01,1.17,;-8.34,.4,;-8.34,-1.14,;-7.01,2.71,;-5.68,3.48,;-5.68,5.02,;-4.34,5.79,;-3.01,5.02,;-3.01,3.48,;-4.34,2.71,;-1.68,2.71,;-.21,3.18,;.69,1.94,;-.21,.69,;.11,-.82,;-1.04,-1.85,;-2.5,-1.37,;-2.82,.13,;-1.68,1.17,;1.6,-1.22,;2,-2.7,;3.48,-3.1,;4.57,-2.01,;4.17,-.53,;2.69,-.13,;6.06,-2.41,;7.15,-1.32,;6.46,-3.9,;7.95,-4.3,;8.34,-5.79,)| Show InChI InChI=1S/C26H31N7O2/c27-11-8-25(35)32-16-14-31(15-17-32)22-2-1-3-23-21(22)10-13-33(23)24-9-12-28-26(30-24)29-20-6-4-19(18-34)5-7-20/h1-3,9-10,12-13,19-20,34H,4-8,14-18H2,(H,28,29,30)/t19-,20- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Roche Palo Alto LLC
US Patent
| Assay Description
JNK activity was measured by phosphorylation of GST-ATF2 (19-96) with [gamma-33P] ATP. |
US Patent US8536172 (2013)
BindingDB Entry DOI: 10.7270/Q23N222K |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50394893
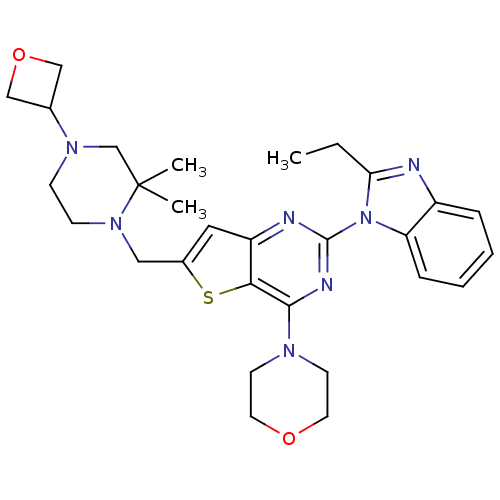
(CHEMBL2165502)Show SMILES CCc1nc2ccccc2n1-c1nc(N2CCOCC2)c2sc(CN3CCN(CC3(C)C)C3COC3)cc2n1 Show InChI InChI=1S/C29H37N7O2S/c1-4-25-30-22-7-5-6-8-24(22)36(25)28-31-23-15-21(39-26(23)27(32-28)33-11-13-37-14-12-33)16-35-10-9-34(19-29(35,2)3)20-17-38-18-20/h5-8,15,20H,4,9-14,16-19H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Binding affinity to PI3Kdelta after 30 mins by competitive fluorescence polarization assay |
J Med Chem 55: 5887-900 (2012)
Article DOI: 10.1021/jm3003747
BindingDB Entry DOI: 10.7270/Q2V125XN |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50394909
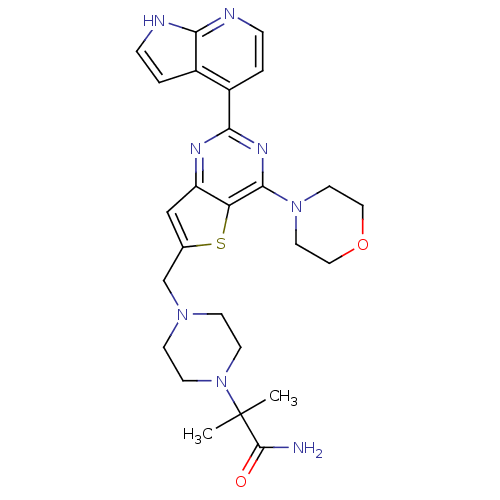
(CHEMBL2165513)Show SMILES CC(C)(N1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2ccnc3[nH]ccc23)CC1)C(N)=O Show InChI InChI=1S/C26H32N8O2S/c1-26(2,25(27)35)34-9-7-32(8-10-34)16-17-15-20-21(37-17)24(33-11-13-36-14-12-33)31-23(30-20)19-4-6-29-22-18(19)3-5-28-22/h3-6,15H,7-14,16H2,1-2H3,(H2,27,35)(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Binding affinity to PI3Kdelta after 30 mins by competitive fluorescence polarization assay |
J Med Chem 55: 5887-900 (2012)
Article DOI: 10.1021/jm3003747
BindingDB Entry DOI: 10.7270/Q2V125XN |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50394903
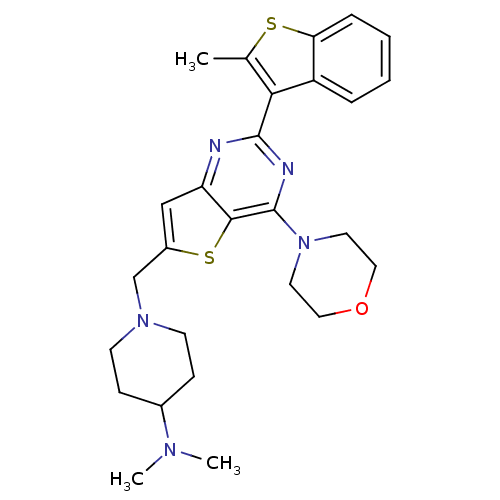
(CHEMBL2165492)Show SMILES CN(C)C1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2c(C)sc3ccccc23)CC1 Show InChI InChI=1S/C27H33N5OS2/c1-18-24(21-6-4-5-7-23(21)34-18)26-28-22-16-20(17-31-10-8-19(9-11-31)30(2)3)35-25(22)27(29-26)32-12-14-33-15-13-32/h4-7,16,19H,8-15,17H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Binding affinity to PI3Kdelta after 30 mins by competitive fluorescence polarization assay |
J Med Chem 55: 5887-900 (2012)
Article DOI: 10.1021/jm3003747
BindingDB Entry DOI: 10.7270/Q2V125XN |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data