 Found 206 hits with Last Name = 'papini' and Initial = 'am'
Found 206 hits with Last Name = 'papini' and Initial = 'am' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50154334

((S)-2-[3-(4-Sulfamoyl-phenyl)-thioureido]-succinam...)Show SMILES CNC(=O)COC(=O)[C@H](CC(N)=O)NC(=S)Nc1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C14H19N5O6S2/c1-17-12(21)7-25-13(22)10(6-11(15)20)19-14(26)18-8-2-4-9(5-3-8)27(16,23)24/h2-5,10H,6-7H2,1H3,(H2,15,20)(H,17,21)(H2,16,23,24)(H2,18,19,26)/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Carbonic anhydrase II |
J Med Chem 47: 5224-9 (2004)
Article DOI: 10.1021/jm049692i
BindingDB Entry DOI: 10.7270/Q24F1RHJ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50154318

((2S,3S)-3-Methyl-2-[3-(4-sulfamoyl-phenyl)-thioure...)Show SMILES CC[C@H](C)[C@H](NC(=S)Nc1ccc(cc1)S(N)(=O)=O)C(O)=O Show InChI InChI=1S/C13H19N3O4S2/c1-3-8(2)11(12(17)18)16-13(21)15-9-4-6-10(7-5-9)22(14,19)20/h4-8,11H,3H2,1-2H3,(H,17,18)(H2,14,19,20)(H2,15,16,21)/t8-,11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Carbonic anhydrase II |
J Med Chem 47: 5224-9 (2004)
Article DOI: 10.1021/jm049692i
BindingDB Entry DOI: 10.7270/Q24F1RHJ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50154325

((S)-4-Methylsulfanyl-2-[3-(4-sulfamoyl-phenyl)-thi...)Show SMILES CSCC[C@H](NC(=S)Nc1ccc(cc1)S(N)(=O)=O)C(O)=O Show InChI InChI=1S/C12H17N3O4S3/c1-21-7-6-10(11(16)17)15-12(20)14-8-2-4-9(5-3-8)22(13,18)19/h2-5,10H,6-7H2,1H3,(H,16,17)(H2,13,18,19)(H2,14,15,20)/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Carbonic anhydrase II |
J Med Chem 47: 5224-9 (2004)
Article DOI: 10.1021/jm049692i
BindingDB Entry DOI: 10.7270/Q24F1RHJ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50154323

((S)-4-Methyl-2-[3-(4-sulfamoyl-phenyl)-thioureido]...)Show SMILES CC(C)C[C@H](NC(=S)Nc1ccc(cc1)S(N)(=O)=O)C(O)=O Show InChI InChI=1S/C13H19N3O4S2/c1-8(2)7-11(12(17)18)16-13(21)15-9-3-5-10(6-4-9)22(14,19)20/h3-6,8,11H,7H2,1-2H3,(H,17,18)(H2,14,19,20)(H2,15,16,21)/t11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Carbonic anhydrase II |
J Med Chem 47: 5224-9 (2004)
Article DOI: 10.1021/jm049692i
BindingDB Entry DOI: 10.7270/Q24F1RHJ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50154328
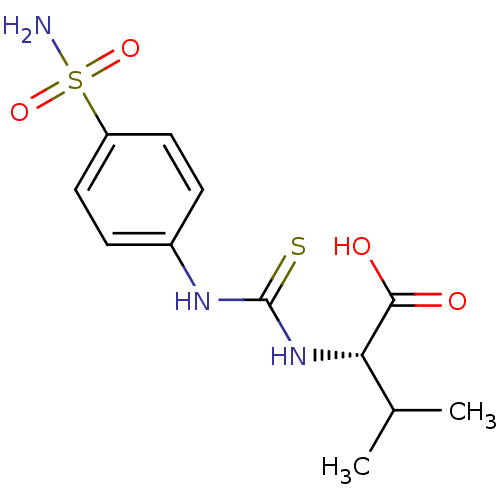
((S)-3-Methyl-2-[3-(4-sulfamoyl-phenyl)-thioureido]...)Show SMILES CC(C)[C@H](NC(=S)Nc1ccc(cc1)S(N)(=O)=O)C(O)=O Show InChI InChI=1S/C12H17N3O4S2/c1-7(2)10(11(16)17)15-12(20)14-8-3-5-9(6-4-8)21(13,18)19/h3-7,10H,1-2H3,(H,16,17)(H2,13,18,19)(H2,14,15,20)/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Carbonic anhydrase II |
J Med Chem 47: 5224-9 (2004)
Article DOI: 10.1021/jm049692i
BindingDB Entry DOI: 10.7270/Q24F1RHJ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50154335
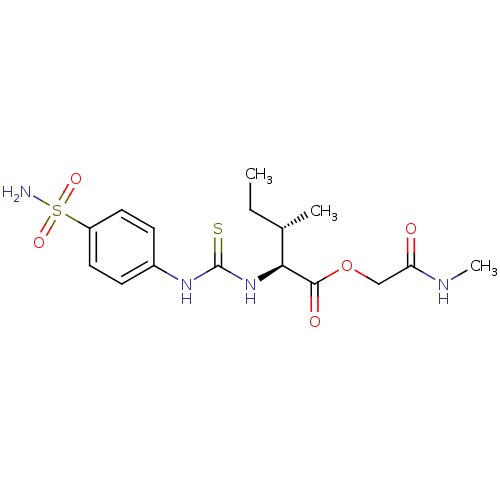
((1S,3S)-3-Methyl-2-[3-(4-sulfamoyl-phenyl)-thioure...)Show SMILES CC[C@H](C)[C@H](NC(=S)Nc1ccc(cc1)S(N)(=O)=O)C(=O)OCC(=O)NC Show InChI InChI=1S/C16H24N4O5S2/c1-4-10(2)14(15(22)25-9-13(21)18-3)20-16(26)19-11-5-7-12(8-6-11)27(17,23)24/h5-8,10,14H,4,9H2,1-3H3,(H,18,21)(H2,17,23,24)(H2,19,20,26)/t10-,14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Carbonic anhydrase II |
J Med Chem 47: 5224-9 (2004)
Article DOI: 10.1021/jm049692i
BindingDB Entry DOI: 10.7270/Q24F1RHJ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50154329

((S)-3-Methyl-2-[3-(4-sulfamoyl-phenyl)-thioureido]...)Show SMILES CNC(=O)COC(=O)[C@@H](NC(=S)Nc1ccc(cc1)S(N)(=O)=O)C(C)C Show InChI InChI=1S/C15H22N4O5S2/c1-9(2)13(14(21)24-8-12(20)17-3)19-15(25)18-10-4-6-11(7-5-10)26(16,22)23/h4-7,9,13H,8H2,1-3H3,(H,17,20)(H2,16,22,23)(H2,18,19,25)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Carbonic anhydrase II |
J Med Chem 47: 5224-9 (2004)
Article DOI: 10.1021/jm049692i
BindingDB Entry DOI: 10.7270/Q24F1RHJ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50154321
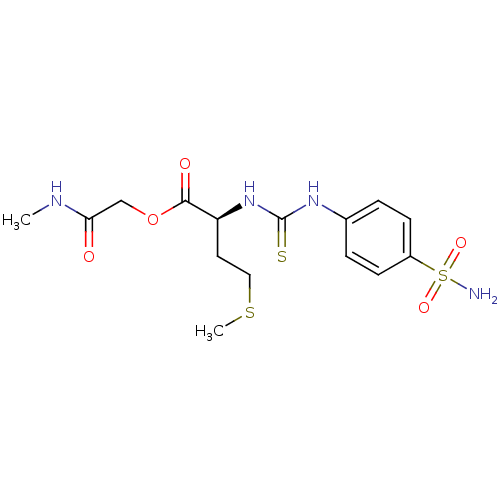
((S)-4-Methylsulfanyl-2-[3-(4-sulfamoyl-phenyl)-thi...)Show SMILES CNC(=O)COC(=O)[C@H](CCSC)NC(=S)Nc1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C15H22N4O5S3/c1-17-13(20)9-24-14(21)12(7-8-26-2)19-15(25)18-10-3-5-11(6-4-10)27(16,22)23/h3-6,12H,7-9H2,1-2H3,(H,17,20)(H2,16,22,23)(H2,18,19,25)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Carbonic anhydrase II |
J Med Chem 47: 5224-9 (2004)
Article DOI: 10.1021/jm049692i
BindingDB Entry DOI: 10.7270/Q24F1RHJ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50154327
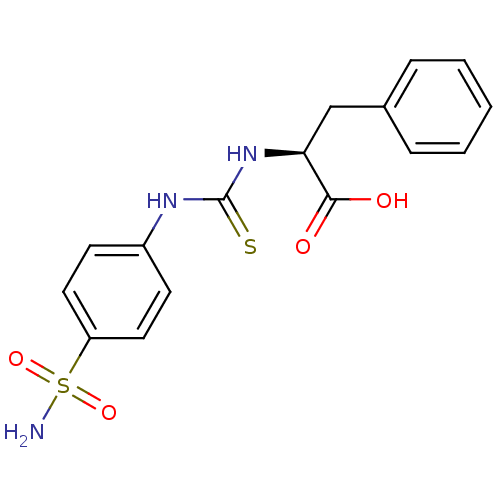
((S)-3-Phenyl-2-[3-(4-sulfamoyl-phenyl)-thioureido]...)Show SMILES NS(=O)(=O)c1ccc(NC(=S)N[C@@H](Cc2ccccc2)C(O)=O)cc1 Show InChI InChI=1S/C16H17N3O4S2/c17-25(22,23)13-8-6-12(7-9-13)18-16(24)19-14(15(20)21)10-11-4-2-1-3-5-11/h1-9,14H,10H2,(H,20,21)(H2,17,22,23)(H2,18,19,24)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Carbonic anhydrase II |
J Med Chem 47: 5224-9 (2004)
Article DOI: 10.1021/jm049692i
BindingDB Entry DOI: 10.7270/Q24F1RHJ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50154320

((S)-2-[3-(4-Sulfamoyl-phenyl)-thioureido]-succinam...)Show SMILES NC(=O)C[C@H](NC(=S)Nc1ccc(cc1)S(N)(=O)=O)C(O)=O Show InChI InChI=1S/C11H14N4O5S2/c12-9(16)5-8(10(17)18)15-11(21)14-6-1-3-7(4-2-6)22(13,19)20/h1-4,8H,5H2,(H2,12,16)(H,17,18)(H2,13,19,20)(H2,14,15,21)/t8-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Carbonic anhydrase II |
J Med Chem 47: 5224-9 (2004)
Article DOI: 10.1021/jm049692i
BindingDB Entry DOI: 10.7270/Q24F1RHJ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50154332

((S)-4-Methyl-2-[3-(4-sulfamoyl-phenyl)-thioureido]...)Show SMILES CNC(=O)COC(=O)[C@H](CC(C)C)NC(=S)Nc1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C16H24N4O5S2/c1-10(2)8-13(15(22)25-9-14(21)18-3)20-16(26)19-11-4-6-12(7-5-11)27(17,23)24/h4-7,10,13H,8-9H2,1-3H3,(H,18,21)(H2,17,23,24)(H2,19,20,26)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Carbonic anhydrase II |
J Med Chem 47: 5224-9 (2004)
Article DOI: 10.1021/jm049692i
BindingDB Entry DOI: 10.7270/Q24F1RHJ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50154336
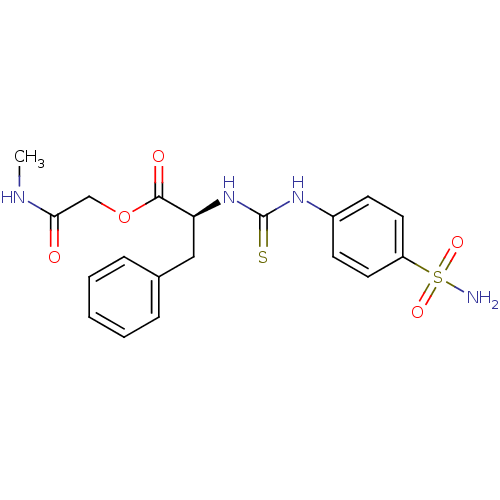
((S)-3-Phenyl-2-[3-(4-sulfamoyl-phenyl)-thioureido]...)Show SMILES CNC(=O)COC(=O)[C@H](Cc1ccccc1)NC(=S)Nc1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C19H22N4O5S2/c1-21-17(24)12-28-18(25)16(11-13-5-3-2-4-6-13)23-19(29)22-14-7-9-15(10-8-14)30(20,26)27/h2-10,16H,11-12H2,1H3,(H,21,24)(H2,20,26,27)(H2,22,23,29)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Carbonic anhydrase II |
J Med Chem 47: 5224-9 (2004)
Article DOI: 10.1021/jm049692i
BindingDB Entry DOI: 10.7270/Q24F1RHJ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10882

(6-ethoxy-1,3-benzothiazole-2-sulfonamide | CHEMBL1...)Show InChI InChI=1S/C9H10N2O3S2/c1-2-14-6-3-4-7-8(5-6)15-9(11-7)16(10,12)13/h3-5H,2H2,1H3,(H2,10,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Carbonic anhydrase II |
J Med Chem 47: 5224-9 (2004)
Article DOI: 10.1021/jm049692i
BindingDB Entry DOI: 10.7270/Q24F1RHJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50154326

((S)-4-Carbamoyl-2-[3-(4-sulfamoyl-phenyl)-thiourei...)Show SMILES NC(=O)CC[C@H](NC(=S)Nc1ccc(cc1)S(N)(=O)=O)C(O)=O Show InChI InChI=1S/C12H16N4O5S2/c13-10(17)6-5-9(11(18)19)16-12(22)15-7-1-3-8(4-2-7)23(14,20)21/h1-4,9H,5-6H2,(H2,13,17)(H,18,19)(H2,14,20,21)(H2,15,16,22)/t9-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Carbonic anhydrase II |
J Med Chem 47: 5224-9 (2004)
Article DOI: 10.1021/jm049692i
BindingDB Entry DOI: 10.7270/Q24F1RHJ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10884

((2S,4S)-4-(ethylamino)-2-methyl-1,1-dioxo-2H,3H,4H...)Show SMILES CCN[C@H]1C[C@H](C)S(=O)(=O)c2sc(cc12)S(N)(=O)=O |r| Show InChI InChI=1S/C10H16N2O4S3/c1-3-12-8-4-6(2)18(13,14)10-7(8)5-9(17-10)19(11,15)16/h5-6,8,12H,3-4H2,1-2H3,(H2,11,15,16)/t6-,8-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Carbonic anhydrase II |
J Med Chem 47: 5224-9 (2004)
Article DOI: 10.1021/jm049692i
BindingDB Entry DOI: 10.7270/Q24F1RHJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50154324
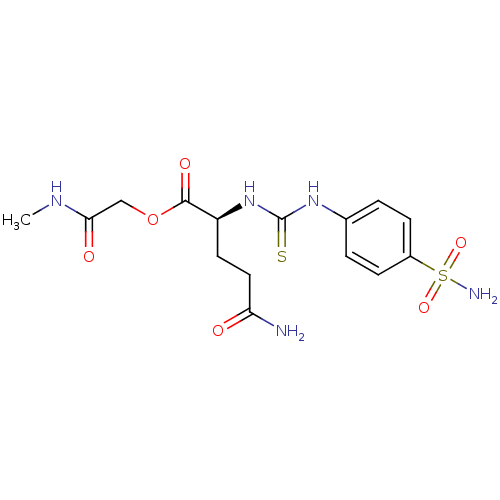
(4-Carbamoyl-2-[3-(4-sulfamoyl-phenyl)-thioureido]-...)Show SMILES CNC(=O)COC(=O)[C@H](CCC(N)=O)NC(=S)Nc1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C15H21N5O6S2/c1-18-13(22)8-26-14(23)11(6-7-12(16)21)20-15(27)19-9-2-4-10(5-3-9)28(17,24)25/h2-5,11H,6-8H2,1H3,(H2,16,21)(H,18,22)(H2,17,24,25)(H2,19,20,27)/t11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Carbonic anhydrase II |
J Med Chem 47: 5224-9 (2004)
Article DOI: 10.1021/jm049692i
BindingDB Entry DOI: 10.7270/Q24F1RHJ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50154330
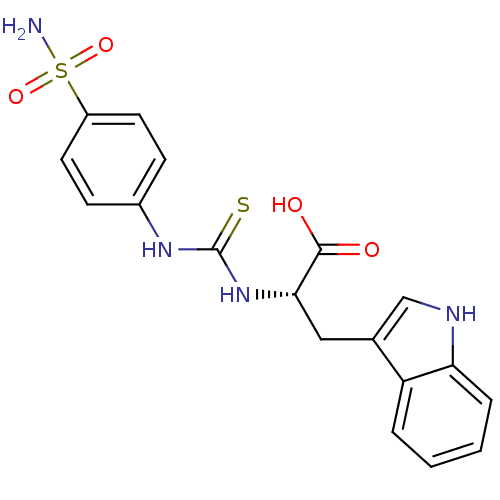
((S)-3-(1H-Indol-3-yl)-2-[3-(4-sulfamoyl-phenyl)-th...)Show SMILES NS(=O)(=O)c1ccc(NC(=S)N[C@@H](Cc2c[nH]c3ccccc23)C(O)=O)cc1 Show InChI InChI=1S/C18H18N4O4S2/c19-28(25,26)13-7-5-12(6-8-13)21-18(27)22-16(17(23)24)9-11-10-20-15-4-2-1-3-14(11)15/h1-8,10,16,20H,9H2,(H,23,24)(H2,19,25,26)(H2,21,22,27)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Carbonic anhydrase II |
J Med Chem 47: 5224-9 (2004)
Article DOI: 10.1021/jm049692i
BindingDB Entry DOI: 10.7270/Q24F1RHJ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Carbonic anhydrase II |
J Med Chem 47: 5224-9 (2004)
Article DOI: 10.1021/jm049692i
BindingDB Entry DOI: 10.7270/Q24F1RHJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50154319

((S)-2-[3-(4-Sulfamoyl-phenyl)-thioureido]-propioni...)Show InChI InChI=1S/C10H13N3O4S2/c1-6(9(14)15)12-10(18)13-7-2-4-8(5-3-7)19(11,16)17/h2-6H,1H3,(H,14,15)(H2,11,16,17)(H2,12,13,18)/t6-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Carbonic anhydrase II |
J Med Chem 47: 5224-9 (2004)
Article DOI: 10.1021/jm049692i
BindingDB Entry DOI: 10.7270/Q24F1RHJ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10881

(CHEMBL288100 | MZA3 | Methazolamide | Methazolamid...)Show InChI InChI=1S/C5H8N4O3S2/c1-3(10)7-4-9(2)8-5(13-4)14(6,11)12/h1-2H3,(H2,6,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Carbonic anhydrase II |
J Med Chem 47: 5224-9 (2004)
Article DOI: 10.1021/jm049692i
BindingDB Entry DOI: 10.7270/Q24F1RHJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50154317
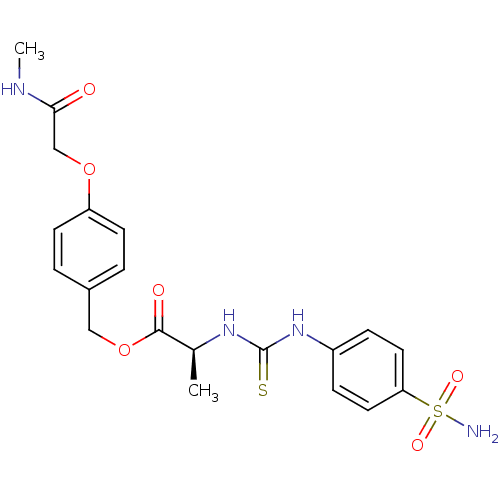
((S)-2-[3-(4-Sulfamoyl-phenyl)-thioureido]-propioni...)Show SMILES CNC(=O)COc1ccc(COC(=O)[C@H](C)NC(=S)Nc2ccc(cc2)S(N)(=O)=O)cc1 Show InChI InChI=1S/C20H24N4O6S2/c1-13(23-20(31)24-15-5-9-17(10-6-15)32(21,27)28)19(26)30-11-14-3-7-16(8-4-14)29-12-18(25)22-2/h3-10,13H,11-12H2,1-2H3,(H,22,25)(H2,21,27,28)(H2,23,24,31)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Carbonic anhydrase II |
J Med Chem 47: 5224-9 (2004)
Article DOI: 10.1021/jm049692i
BindingDB Entry DOI: 10.7270/Q24F1RHJ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50154331
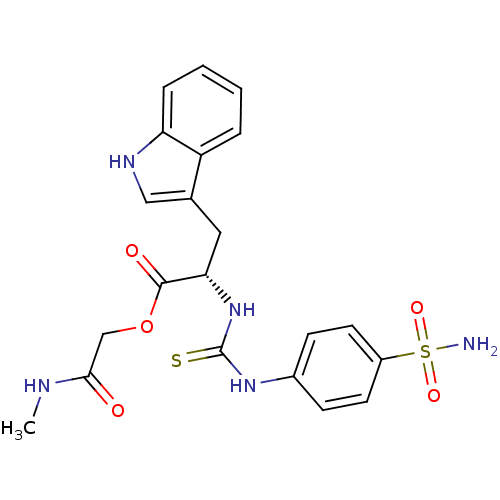
((S)-3-(1H-Indol-3-yl)-2-[3-(4-sulfamoyl-phenyl)-th...)Show SMILES CNC(=O)COC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=S)Nc1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C21H23N5O5S2/c1-23-19(27)12-31-20(28)18(10-13-11-24-17-5-3-2-4-16(13)17)26-21(32)25-14-6-8-15(9-7-14)33(22,29)30/h2-9,11,18,24H,10,12H2,1H3,(H,23,27)(H2,22,29,30)(H2,25,26,32)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Carbonic anhydrase II |
J Med Chem 47: 5224-9 (2004)
Article DOI: 10.1021/jm049692i
BindingDB Entry DOI: 10.7270/Q24F1RHJ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50154323

((S)-4-Methyl-2-[3-(4-sulfamoyl-phenyl)-thioureido]...)Show SMILES CC(C)C[C@H](NC(=S)Nc1ccc(cc1)S(N)(=O)=O)C(O)=O Show InChI InChI=1S/C13H19N3O4S2/c1-8(2)7-11(12(17)18)16-13(21)15-9-3-5-10(6-4-9)22(14,19)20/h3-6,8,11H,7H2,1-2H3,(H,17,18)(H2,14,19,20)(H2,15,16,21)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Carbonic anhydrase I |
J Med Chem 47: 5224-9 (2004)
Article DOI: 10.1021/jm049692i
BindingDB Entry DOI: 10.7270/Q24F1RHJ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50154318

((2S,3S)-3-Methyl-2-[3-(4-sulfamoyl-phenyl)-thioure...)Show SMILES CC[C@H](C)[C@H](NC(=S)Nc1ccc(cc1)S(N)(=O)=O)C(O)=O Show InChI InChI=1S/C13H19N3O4S2/c1-3-8(2)11(12(17)18)16-13(21)15-9-4-6-10(7-5-9)22(14,19)20/h4-8,11H,3H2,1-2H3,(H,17,18)(H2,14,19,20)(H2,15,16,21)/t8-,11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Carbonic anhydrase I |
J Med Chem 47: 5224-9 (2004)
Article DOI: 10.1021/jm049692i
BindingDB Entry DOI: 10.7270/Q24F1RHJ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50154328

((S)-3-Methyl-2-[3-(4-sulfamoyl-phenyl)-thioureido]...)Show SMILES CC(C)[C@H](NC(=S)Nc1ccc(cc1)S(N)(=O)=O)C(O)=O Show InChI InChI=1S/C12H17N3O4S2/c1-7(2)10(11(16)17)15-12(20)14-8-3-5-9(6-4-8)21(13,18)19/h3-7,10H,1-2H3,(H,16,17)(H2,13,18,19)(H2,14,15,20)/t10-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Carbonic anhydrase I |
J Med Chem 47: 5224-9 (2004)
Article DOI: 10.1021/jm049692i
BindingDB Entry DOI: 10.7270/Q24F1RHJ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50154332

((S)-4-Methyl-2-[3-(4-sulfamoyl-phenyl)-thioureido]...)Show SMILES CNC(=O)COC(=O)[C@H](CC(C)C)NC(=S)Nc1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C16H24N4O5S2/c1-10(2)8-13(15(22)25-9-14(21)18-3)20-16(26)19-11-4-6-12(7-5-11)27(17,23)24/h4-7,10,13H,8-9H2,1-3H3,(H,18,21)(H2,17,23,24)(H2,19,20,26)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Carbonic anhydrase I |
J Med Chem 47: 5224-9 (2004)
Article DOI: 10.1021/jm049692i
BindingDB Entry DOI: 10.7270/Q24F1RHJ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50154335

((1S,3S)-3-Methyl-2-[3-(4-sulfamoyl-phenyl)-thioure...)Show SMILES CC[C@H](C)[C@H](NC(=S)Nc1ccc(cc1)S(N)(=O)=O)C(=O)OCC(=O)NC Show InChI InChI=1S/C16H24N4O5S2/c1-4-10(2)14(15(22)25-9-13(21)18-3)20-16(26)19-11-5-7-12(8-6-11)27(17,23)24/h5-8,10,14H,4,9H2,1-3H3,(H,18,21)(H2,17,23,24)(H2,19,20,26)/t10-,14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Carbonic anhydrase I |
J Med Chem 47: 5224-9 (2004)
Article DOI: 10.1021/jm049692i
BindingDB Entry DOI: 10.7270/Q24F1RHJ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50154333

((S)-1-(4-Sulfamoyl-phenylthiocarbamoyl)-pyrrolidin...)Show SMILES NS(=O)(=O)c1ccc(NC(=S)N2CCC[C@H]2C(O)=O)cc1 Show InChI InChI=1S/C12H15N3O4S2/c13-21(18,19)9-5-3-8(4-6-9)14-12(20)15-7-1-2-10(15)11(16)17/h3-6,10H,1-2,7H2,(H,14,20)(H,16,17)(H2,13,18,19)/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Carbonic anhydrase II |
J Med Chem 47: 5224-9 (2004)
Article DOI: 10.1021/jm049692i
BindingDB Entry DOI: 10.7270/Q24F1RHJ |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM10882

(6-ethoxy-1,3-benzothiazole-2-sulfonamide | CHEMBL1...)Show InChI InChI=1S/C9H10N2O3S2/c1-2-14-6-3-4-7-8(5-6)15-9(11-7)16(10,12)13/h3-5H,2H2,1H3,(H2,10,12,13) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Carbonic anhydrase I |
J Med Chem 47: 5224-9 (2004)
Article DOI: 10.1021/jm049692i
BindingDB Entry DOI: 10.7270/Q24F1RHJ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50154325

((S)-4-Methylsulfanyl-2-[3-(4-sulfamoyl-phenyl)-thi...)Show SMILES CSCC[C@H](NC(=S)Nc1ccc(cc1)S(N)(=O)=O)C(O)=O Show InChI InChI=1S/C12H17N3O4S3/c1-21-7-6-10(11(16)17)15-12(20)14-8-2-4-9(5-3-8)22(13,18)19/h2-5,10H,6-7H2,1H3,(H,16,17)(H2,13,18,19)(H2,14,15,20)/t10-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Carbonic anhydrase I |
J Med Chem 47: 5224-9 (2004)
Article DOI: 10.1021/jm049692i
BindingDB Entry DOI: 10.7270/Q24F1RHJ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50154320

((S)-2-[3-(4-Sulfamoyl-phenyl)-thioureido]-succinam...)Show SMILES NC(=O)C[C@H](NC(=S)Nc1ccc(cc1)S(N)(=O)=O)C(O)=O Show InChI InChI=1S/C11H14N4O5S2/c12-9(16)5-8(10(17)18)15-11(21)14-6-1-3-7(4-2-6)22(13,19)20/h1-4,8H,5H2,(H2,12,16)(H,17,18)(H2,13,19,20)(H2,14,15,21)/t8-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Carbonic anhydrase I |
J Med Chem 47: 5224-9 (2004)
Article DOI: 10.1021/jm049692i
BindingDB Entry DOI: 10.7270/Q24F1RHJ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50154334

((S)-2-[3-(4-Sulfamoyl-phenyl)-thioureido]-succinam...)Show SMILES CNC(=O)COC(=O)[C@H](CC(N)=O)NC(=S)Nc1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C14H19N5O6S2/c1-17-12(21)7-25-13(22)10(6-11(15)20)19-14(26)18-8-2-4-9(5-3-8)27(16,23)24/h2-5,10H,6-7H2,1H3,(H2,15,20)(H,17,21)(H2,16,23,24)(H2,18,19,26)/t10-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Carbonic anhydrase I |
J Med Chem 47: 5224-9 (2004)
Article DOI: 10.1021/jm049692i
BindingDB Entry DOI: 10.7270/Q24F1RHJ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50154327

((S)-3-Phenyl-2-[3-(4-sulfamoyl-phenyl)-thioureido]...)Show SMILES NS(=O)(=O)c1ccc(NC(=S)N[C@@H](Cc2ccccc2)C(O)=O)cc1 Show InChI InChI=1S/C16H17N3O4S2/c17-25(22,23)13-8-6-12(7-9-13)18-16(24)19-14(15(20)21)10-11-4-2-1-3-5-11/h1-9,14H,10H2,(H,20,21)(H2,17,22,23)(H2,18,19,24)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Carbonic anhydrase I |
J Med Chem 47: 5224-9 (2004)
Article DOI: 10.1021/jm049692i
BindingDB Entry DOI: 10.7270/Q24F1RHJ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50154329

((S)-3-Methyl-2-[3-(4-sulfamoyl-phenyl)-thioureido]...)Show SMILES CNC(=O)COC(=O)[C@@H](NC(=S)Nc1ccc(cc1)S(N)(=O)=O)C(C)C Show InChI InChI=1S/C15H22N4O5S2/c1-9(2)13(14(21)24-8-12(20)17-3)19-15(25)18-10-4-6-11(7-5-10)26(16,22)23/h4-7,9,13H,8H2,1-3H3,(H,17,20)(H2,16,22,23)(H2,18,19,25)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Carbonic anhydrase I |
J Med Chem 47: 5224-9 (2004)
Article DOI: 10.1021/jm049692i
BindingDB Entry DOI: 10.7270/Q24F1RHJ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50154321

((S)-4-Methylsulfanyl-2-[3-(4-sulfamoyl-phenyl)-thi...)Show SMILES CNC(=O)COC(=O)[C@H](CCSC)NC(=S)Nc1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C15H22N4O5S3/c1-17-13(20)9-24-14(21)12(7-8-26-2)19-15(25)18-10-3-5-11(6-4-10)27(16,22)23/h3-6,12H,7-9H2,1-2H3,(H,17,20)(H2,16,22,23)(H2,18,19,25)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Carbonic anhydrase I |
J Med Chem 47: 5224-9 (2004)
Article DOI: 10.1021/jm049692i
BindingDB Entry DOI: 10.7270/Q24F1RHJ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10883

(4,5-dichlorobenzene-1,3-disulfonamide | CHEMBL17 |...)Show InChI InChI=1S/C6H6Cl2N2O4S2/c7-4-1-3(15(9,11)12)2-5(6(4)8)16(10,13)14/h1-2H,(H2,9,11,12)(H2,10,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| DrugBank
MMDB
PDB
Article
PubMed
| 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Carbonic anhydrase II |
J Med Chem 47: 5224-9 (2004)
Article DOI: 10.1021/jm049692i
BindingDB Entry DOI: 10.7270/Q24F1RHJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50154326

((S)-4-Carbamoyl-2-[3-(4-sulfamoyl-phenyl)-thiourei...)Show SMILES NC(=O)CC[C@H](NC(=S)Nc1ccc(cc1)S(N)(=O)=O)C(O)=O Show InChI InChI=1S/C12H16N4O5S2/c13-10(17)6-5-9(11(18)19)16-12(22)15-7-1-3-8(4-2-7)23(14,20)21/h1-4,9H,5-6H2,(H2,13,17)(H,18,19)(H2,14,20,21)(H2,15,16,22)/t9-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Carbonic anhydrase I |
J Med Chem 47: 5224-9 (2004)
Article DOI: 10.1021/jm049692i
BindingDB Entry DOI: 10.7270/Q24F1RHJ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50154336

((S)-3-Phenyl-2-[3-(4-sulfamoyl-phenyl)-thioureido]...)Show SMILES CNC(=O)COC(=O)[C@H](Cc1ccccc1)NC(=S)Nc1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C19H22N4O5S2/c1-21-17(24)12-28-18(25)16(11-13-5-3-2-4-6-13)23-19(29)22-14-7-9-15(10-8-14)30(20,26)27/h2-10,16H,11-12H2,1H3,(H,21,24)(H2,20,26,27)(H2,22,23,29)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Carbonic anhydrase I |
J Med Chem 47: 5224-9 (2004)
Article DOI: 10.1021/jm049692i
BindingDB Entry DOI: 10.7270/Q24F1RHJ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50154322
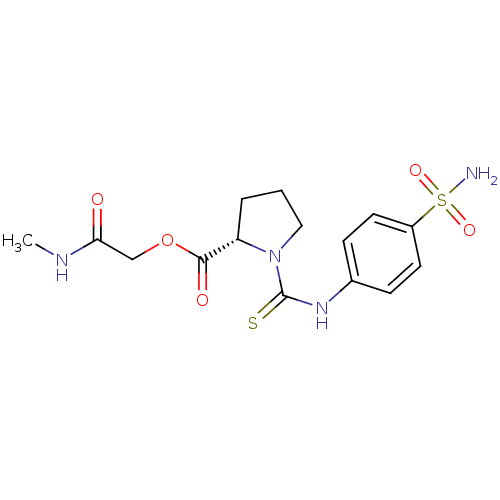
(1-(4-Sulfamoyl-phenylthiocarbamoyl)-pyrrolidine-2-...)Show SMILES CNC(=O)COC(=O)[C@@H]1CCCN1C(=S)Nc1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C15H20N4O5S2/c1-17-13(20)9-24-14(21)12-3-2-8-19(12)15(25)18-10-4-6-11(7-5-10)26(16,22)23/h4-7,12H,2-3,8-9H2,1H3,(H,17,20)(H,18,25)(H2,16,22,23)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Carbonic anhydrase II |
J Med Chem 47: 5224-9 (2004)
Article DOI: 10.1021/jm049692i
BindingDB Entry DOI: 10.7270/Q24F1RHJ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50154330

((S)-3-(1H-Indol-3-yl)-2-[3-(4-sulfamoyl-phenyl)-th...)Show SMILES NS(=O)(=O)c1ccc(NC(=S)N[C@@H](Cc2c[nH]c3ccccc23)C(O)=O)cc1 Show InChI InChI=1S/C18H18N4O4S2/c19-28(25,26)13-7-5-12(6-8-13)21-18(27)22-16(17(23)24)9-11-10-20-15-4-2-1-3-14(11)15/h1-8,10,16,20H,9H2,(H,23,24)(H2,19,25,26)(H2,21,22,27)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Carbonic anhydrase I |
J Med Chem 47: 5224-9 (2004)
Article DOI: 10.1021/jm049692i
BindingDB Entry DOI: 10.7270/Q24F1RHJ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50154324

(4-Carbamoyl-2-[3-(4-sulfamoyl-phenyl)-thioureido]-...)Show SMILES CNC(=O)COC(=O)[C@H](CCC(N)=O)NC(=S)Nc1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C15H21N5O6S2/c1-18-13(22)8-26-14(23)11(6-7-12(16)21)20-15(27)19-9-2-4-10(5-3-9)28(17,24)25/h2-5,11H,6-8H2,1H3,(H2,16,21)(H,18,22)(H2,17,24,25)(H2,19,20,27)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Carbonic anhydrase I |
J Med Chem 47: 5224-9 (2004)
Article DOI: 10.1021/jm049692i
BindingDB Entry DOI: 10.7270/Q24F1RHJ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50154319

((S)-2-[3-(4-Sulfamoyl-phenyl)-thioureido]-propioni...)Show InChI InChI=1S/C10H13N3O4S2/c1-6(9(14)15)12-10(18)13-7-2-4-8(5-3-7)19(11,16)17/h2-6H,1H3,(H,14,15)(H2,11,16,17)(H2,12,13,18)/t6-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Carbonic anhydrase I |
J Med Chem 47: 5224-9 (2004)
Article DOI: 10.1021/jm049692i
BindingDB Entry DOI: 10.7270/Q24F1RHJ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50154317

((S)-2-[3-(4-Sulfamoyl-phenyl)-thioureido]-propioni...)Show SMILES CNC(=O)COc1ccc(COC(=O)[C@H](C)NC(=S)Nc2ccc(cc2)S(N)(=O)=O)cc1 Show InChI InChI=1S/C20H24N4O6S2/c1-13(23-20(31)24-15-5-9-17(10-6-15)32(21,27)28)19(26)30-11-14-3-7-16(8-4-14)29-12-18(25)22-2/h3-10,13H,11-12H2,1-2H3,(H,22,25)(H2,21,27,28)(H2,23,24,31)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Carbonic anhydrase I |
J Med Chem 47: 5224-9 (2004)
Article DOI: 10.1021/jm049692i
BindingDB Entry DOI: 10.7270/Q24F1RHJ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50154331

((S)-3-(1H-Indol-3-yl)-2-[3-(4-sulfamoyl-phenyl)-th...)Show SMILES CNC(=O)COC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=S)Nc1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C21H23N5O5S2/c1-23-19(27)12-31-20(28)18(10-13-11-24-17-5-3-2-4-16(13)17)26-21(32)25-14-6-8-15(9-7-14)33(22,29)30/h2-9,11,18,24H,10,12H2,1H3,(H,23,27)(H2,22,29,30)(H2,25,26,32)/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Carbonic anhydrase I |
J Med Chem 47: 5224-9 (2004)
Article DOI: 10.1021/jm049692i
BindingDB Entry DOI: 10.7270/Q24F1RHJ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50154333

((S)-1-(4-Sulfamoyl-phenylthiocarbamoyl)-pyrrolidin...)Show SMILES NS(=O)(=O)c1ccc(NC(=S)N2CCC[C@H]2C(O)=O)cc1 Show InChI InChI=1S/C12H15N3O4S2/c13-21(18,19)9-5-3-8(4-6-9)14-12(20)15-7-1-2-10(15)11(16)17/h3-6,10H,1-2,7H2,(H,14,20)(H,16,17)(H2,13,18,19)/t10-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Carbonic anhydrase I |
J Med Chem 47: 5224-9 (2004)
Article DOI: 10.1021/jm049692i
BindingDB Entry DOI: 10.7270/Q24F1RHJ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50094812
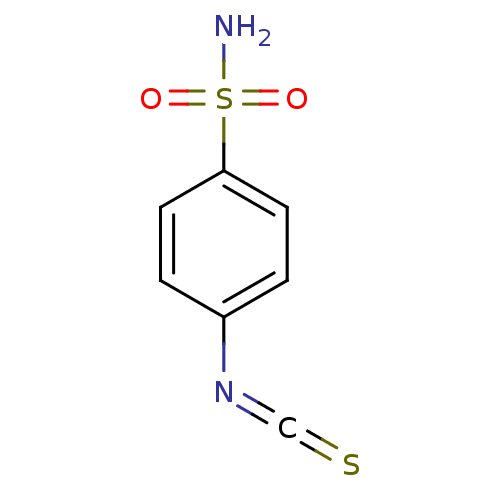
(4-Isothiocyanato-benzenesulfonamide | 4-isothiocya...)Show InChI InChI=1S/C7H6N2O2S2/c8-13(10,11)7-3-1-6(2-4-7)9-5-12/h1-4H,(H2,8,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 185 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Carbonic anhydrase II |
J Med Chem 47: 5224-9 (2004)
Article DOI: 10.1021/jm049692i
BindingDB Entry DOI: 10.7270/Q24F1RHJ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50154322

(1-(4-Sulfamoyl-phenylthiocarbamoyl)-pyrrolidine-2-...)Show SMILES CNC(=O)COC(=O)[C@@H]1CCCN1C(=S)Nc1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C15H20N4O5S2/c1-17-13(20)9-24-14(21)12-3-2-8-19(12)15(25)18-10-4-6-11(7-5-10)26(16,22)23/h4-7,12H,2-3,8-9H2,1H3,(H,17,20)(H,18,25)(H2,16,22,23)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 197 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Carbonic anhydrase I |
J Med Chem 47: 5224-9 (2004)
Article DOI: 10.1021/jm049692i
BindingDB Entry DOI: 10.7270/Q24F1RHJ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM10881

(CHEMBL288100 | MZA3 | Methazolamide | Methazolamid...)Show InChI InChI=1S/C5H8N4O3S2/c1-3(10)7-4-9(2)8-5(13-4)14(6,11)12/h1-2H3,(H2,6,11,12) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Carbonic anhydrase I |
J Med Chem 47: 5224-9 (2004)
Article DOI: 10.1021/jm049692i
BindingDB Entry DOI: 10.7270/Q24F1RHJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Carbonic anhydrase I |
J Med Chem 47: 5224-9 (2004)
Article DOI: 10.1021/jm049692i
BindingDB Entry DOI: 10.7270/Q24F1RHJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM10883

(4,5-dichlorobenzene-1,3-disulfonamide | CHEMBL17 |...)Show InChI InChI=1S/C6H6Cl2N2O4S2/c7-4-1-3(15(9,11)12)2-5(6(4)8)16(10,13)14/h1-2H,(H2,9,11,12)(H2,10,13,14) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| DrugBank
Article
PubMed
| 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Carbonic anhydrase I |
J Med Chem 47: 5224-9 (2004)
Article DOI: 10.1021/jm049692i
BindingDB Entry DOI: 10.7270/Q24F1RHJ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data