

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Renin (Homo sapiens (Human)) | BDBM50405190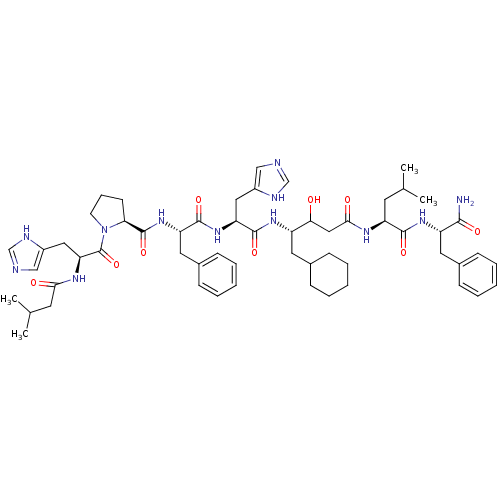 (CHEMBL2028991) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Renin | J Med Chem 28: 1779-90 (1986) BindingDB Entry DOI: 10.7270/Q2FJ2HC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50405190 (CHEMBL2028991) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description The compound was tested for inhibition of rhesus monkey plasma renin. | J Med Chem 28: 1779-90 (1986) BindingDB Entry DOI: 10.7270/Q2FJ2HC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50025937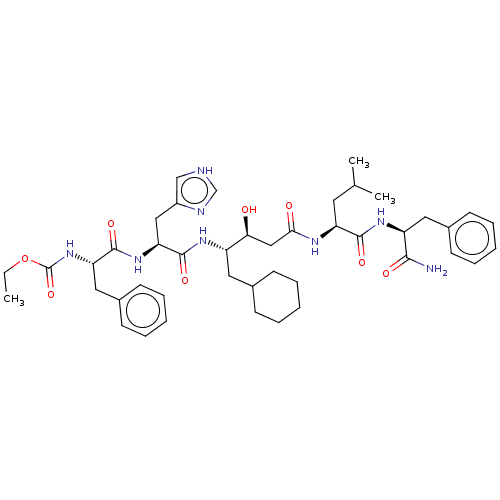 (CHEMBL3144417 | {1-[1-{3-[1-(1-Carbamoyl-2-phenyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Renin | J Med Chem 28: 1779-90 (1986) BindingDB Entry DOI: 10.7270/Q2FJ2HC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50405190 (CHEMBL2028991) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human plasma renin | J Med Chem 28: 1779-90 (1986) BindingDB Entry DOI: 10.7270/Q2FJ2HC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50077035 (1-(1-{2-[4-Morpholin-4-yl-2-(2,2,2-trifluoro-ethox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity for rat uterine oxytocin receptor (rOTr) | Bioorg Med Chem Lett 9: 1311-6 (1999) BindingDB Entry DOI: 10.7270/Q23N22K9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50406692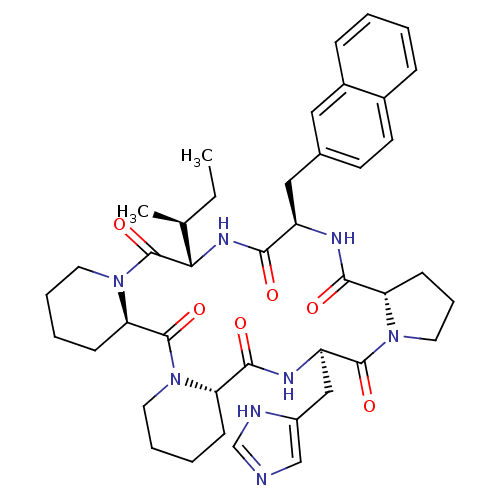 (CHEMBL2112249) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for OT receptor affinity by displacement of [3H]OT from binding sites in uterine tissue taken from pregnant rats | J Med Chem 35: 3905-18 (1992) BindingDB Entry DOI: 10.7270/Q2K64JP2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50077032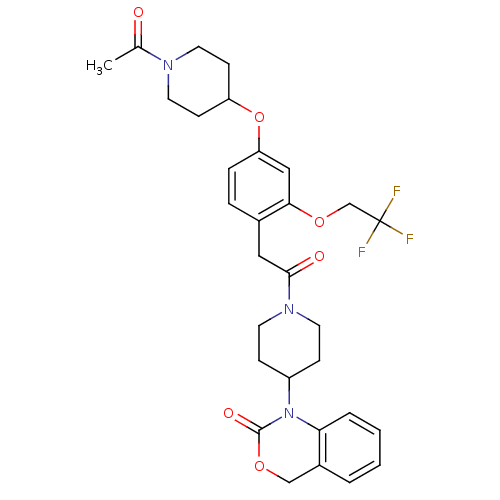 (1-(1-{2-[4-(1-Acetyl-piperidin-4-yloxy)-2-(2,2,2-t...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity for rat uterine oxytocin receptor (rOTr) | Bioorg Med Chem Lett 9: 1311-6 (1999) BindingDB Entry DOI: 10.7270/Q23N22K9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50077035 (1-(1-{2-[4-Morpholin-4-yl-2-(2,2,2-trifluoro-ethox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity for human oxytocin receptor | Bioorg Med Chem Lett 9: 1311-6 (1999) BindingDB Entry DOI: 10.7270/Q23N22K9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50025945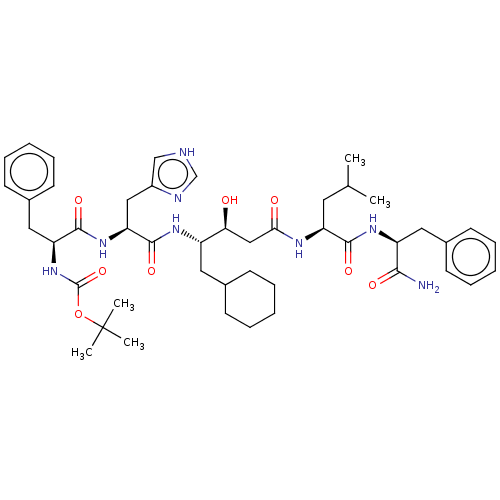 (CHEMBL3144416 | {1-[1-{3-[1-(1-Carbamoyl-2-phenyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0.880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human kidney renin | J Med Chem 28: 1779-90 (1986) BindingDB Entry DOI: 10.7270/Q2FJ2HC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50077041 (1-(1-{2-[4-(1-Cyclopropylmethyl-piperidin-4-yloxy)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity for human oxytocin receptor | Bioorg Med Chem Lett 9: 1311-6 (1999) BindingDB Entry DOI: 10.7270/Q23N22K9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50025941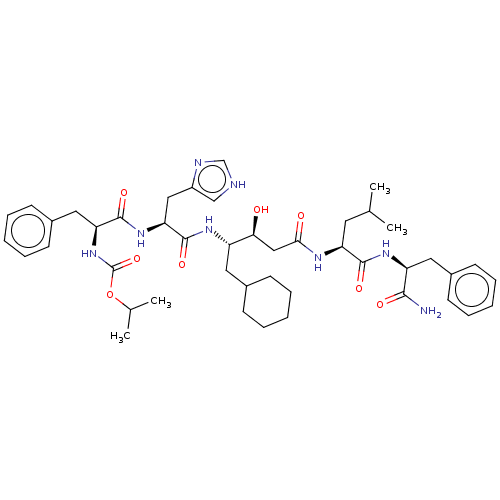 (CHEMBL3144415 | {1-[1-{3-[1-(1-Carbamoyl-2-phenyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human plasma renin | J Med Chem 28: 1779-90 (1986) BindingDB Entry DOI: 10.7270/Q2FJ2HC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50077041 (1-(1-{2-[4-(1-Cyclopropylmethyl-piperidin-4-yloxy)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity for rat uterine oxytocin receptor (rOTr) | Bioorg Med Chem Lett 9: 1311-6 (1999) BindingDB Entry DOI: 10.7270/Q23N22K9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM81894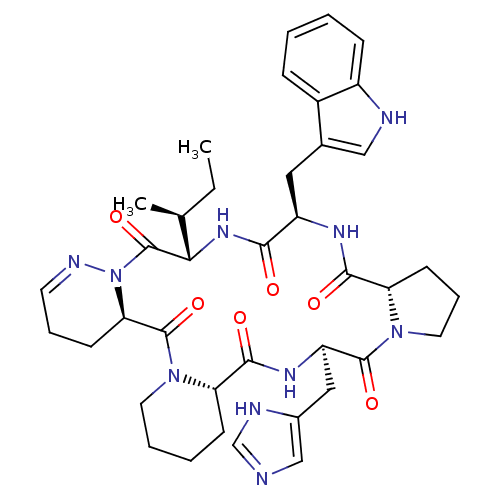 (Cyclo[L-Pro-D-Trp-L-Ile-1,6-didehydro-D-Pyz-L-Pip-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for OT receptor affinity by displacement of [3H]OT from binding sites in uterine tissue taken from near-term pregnant rhesus m... | J Med Chem 35: 3905-18 (1992) BindingDB Entry DOI: 10.7270/Q2K64JP2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM81894 (Cyclo[L-Pro-D-Trp-L-Ile-1,6-didehydro-D-Pyz-L-Pip-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Ability to inhibit AVP stimulation of adenylate cyclase activity in the rat kidney medulla (AVP-V2) receptor | J Med Chem 35: 3905-18 (1992) BindingDB Entry DOI: 10.7270/Q2K64JP2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM81894 (Cyclo[L-Pro-D-Trp-L-Ile-1,6-didehydro-D-Pyz-L-Pip-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for OT receptor affinity by displacement of [3H]OT from binding sites in uterine tissue taken from nonlabor pregnant women | J Med Chem 35: 3905-18 (1992) BindingDB Entry DOI: 10.7270/Q2K64JP2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50077037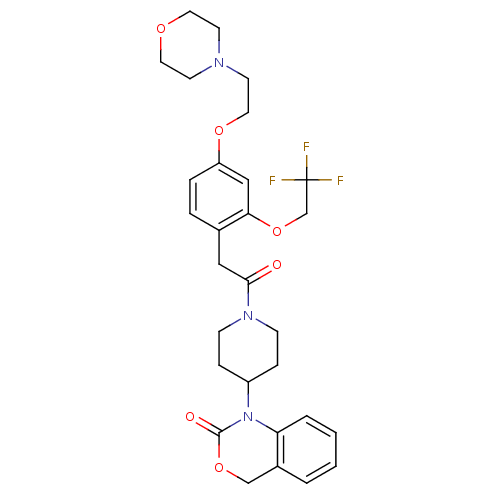 (1-(1-{2-[4-(2-Morpholin-4-yl-ethoxy)-2-(2,2,2-trif...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity for human oxytocin receptor | Bioorg Med Chem Lett 9: 1311-6 (1999) BindingDB Entry DOI: 10.7270/Q23N22K9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50077032 (1-(1-{2-[4-(1-Acetyl-piperidin-4-yloxy)-2-(2,2,2-t...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity for human oxytocin receptor | Bioorg Med Chem Lett 9: 1311-6 (1999) BindingDB Entry DOI: 10.7270/Q23N22K9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (RAT) | BDBM50077032 (1-(1-{2-[4-(1-Acetyl-piperidin-4-yloxy)-2-(2,2,2-t...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards rat liver Vasopressin V1a receptor by using functional assay | Bioorg Med Chem Lett 9: 1311-6 (1999) BindingDB Entry DOI: 10.7270/Q23N22K9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50072369 (1-{1-[5-Fluoro-2-methoxy-4-((R)-1-pyridin-4-yl-eth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against cloned human oxytocin receptor from human embryonic kidney cells | Bioorg Med Chem Lett 8: 3081-6 (1999) Article DOI: 10.1016/S0960-894X(98)00568-X BindingDB Entry DOI: 10.7270/Q298865X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50077037 (1-(1-{2-[4-(2-Morpholin-4-yl-ethoxy)-2-(2,2,2-trif...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity for rat uterine oxytocin receptor (rOTr) | Bioorg Med Chem Lett 9: 1311-6 (1999) BindingDB Entry DOI: 10.7270/Q23N22K9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50077039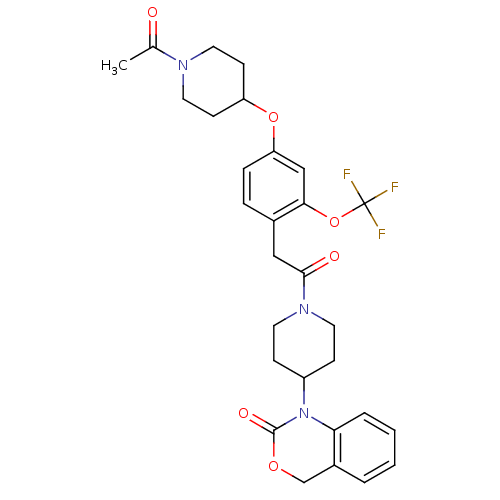 (1-(1-{2-[4-(1-Acetyl-piperidin-4-yloxy)-2-trifluor...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity for human oxytocin receptor | Bioorg Med Chem Lett 9: 1311-6 (1999) BindingDB Entry DOI: 10.7270/Q23N22K9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50077039 (1-(1-{2-[4-(1-Acetyl-piperidin-4-yloxy)-2-trifluor...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity for rat uterine oxytocin receptor (rOTr) | Bioorg Med Chem Lett 9: 1311-6 (1999) BindingDB Entry DOI: 10.7270/Q23N22K9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50001311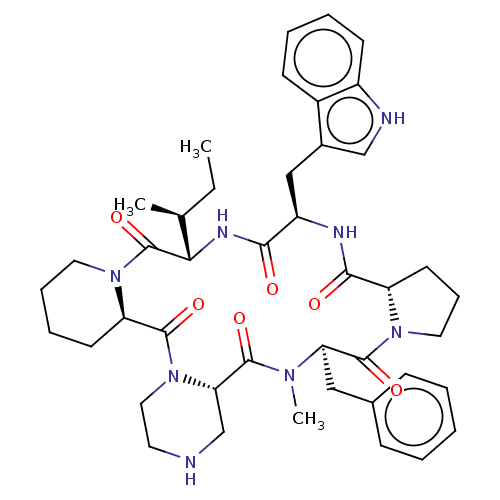 (24-benzyl-16-(1H-3-indolylmethyl)-25-methyl-13-[1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for OT receptor affinity by displacement of [3H]OT from binding sites in uterine tissue taken from pregnant rats | J Med Chem 35: 3905-18 (1992) BindingDB Entry DOI: 10.7270/Q2K64JP2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50405190 (CHEMBL2028991) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity of the compound was determined against Nicotinic acetylcholine receptor using [3H]-(-)-nicotine radioligand | J Med Chem 28: 1779-90 (1986) BindingDB Entry DOI: 10.7270/Q2FJ2HC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50072364 (1-{1-[2-Methoxy-4-((R)-1-pyridin-4-yl-ethoxy)-benz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against cloned human oxytocin receptor from human embryonic kidney cells | Bioorg Med Chem Lett 8: 3081-6 (1999) Article DOI: 10.1016/S0960-894X(98)00568-X BindingDB Entry DOI: 10.7270/Q298865X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50077034 (1-(1-{2-[2-(2,2,2-Trifluoro-ethoxy)-phenyl]-acetyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity for human oxytocin receptor | Bioorg Med Chem Lett 9: 1311-6 (1999) BindingDB Entry DOI: 10.7270/Q23N22K9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50001311 (24-benzyl-16-(1H-3-indolylmethyl)-25-methyl-13-[1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for OT receptor affinity by displacement of [3H]OT from binding sites in uterine tissue taken from nonlabor pregnant women | J Med Chem 35: 3905-18 (1992) BindingDB Entry DOI: 10.7270/Q2K64JP2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50001311 (24-benzyl-16-(1H-3-indolylmethyl)-25-methyl-13-[1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for OT receptor affinity by displacement of [3H]OT from binding sites in uterine tissue taken from near-term pregnant rhesus m... | J Med Chem 35: 3905-18 (1992) BindingDB Entry DOI: 10.7270/Q2K64JP2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50406692 (CHEMBL2112249) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for OT receptor affinity by displacement of [3H]OT from binding sites in uterine tissue taken from pregnant rats | J Med Chem 35: 3905-18 (1992) BindingDB Entry DOI: 10.7270/Q2K64JP2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50406692 (CHEMBL2112249) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for OT receptor affinity by displacement of [3H]OT from binding sites in uterine tissue taken from nonlabor pregnant women | J Med Chem 35: 3905-18 (1992) BindingDB Entry DOI: 10.7270/Q2K64JP2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50072367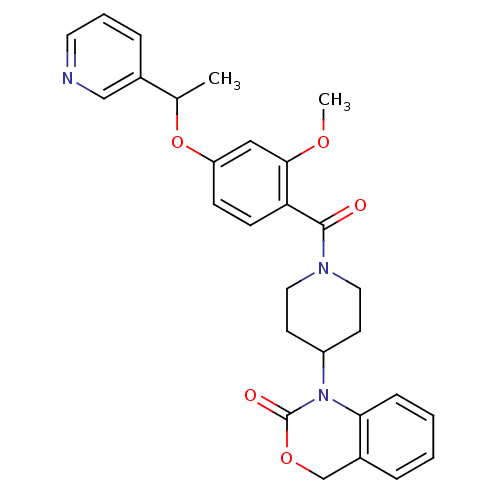 (1-{1-[2-Methoxy-4-(1-pyridin-3-yl-ethoxy)-benzoyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against cloned human oxytocin receptor from human embryonic kidney cells | Bioorg Med Chem Lett 8: 3081-6 (1999) Article DOI: 10.1016/S0960-894X(98)00568-X BindingDB Entry DOI: 10.7270/Q298865X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50368435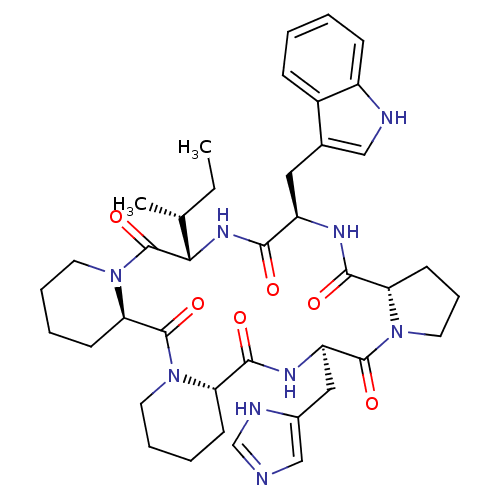 (CHEMBL1790937) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for OT receptor affinity by displacement of [3H]OT from binding sites in uterine tissue taken from pregnant rats | J Med Chem 35: 3905-18 (1992) BindingDB Entry DOI: 10.7270/Q2K64JP2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50077034 (1-(1-{2-[2-(2,2,2-Trifluoro-ethoxy)-phenyl]-acetyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity for rat uterine oxytocin receptor (rOTr) | Bioorg Med Chem Lett 9: 1311-6 (1999) BindingDB Entry DOI: 10.7270/Q23N22K9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50001307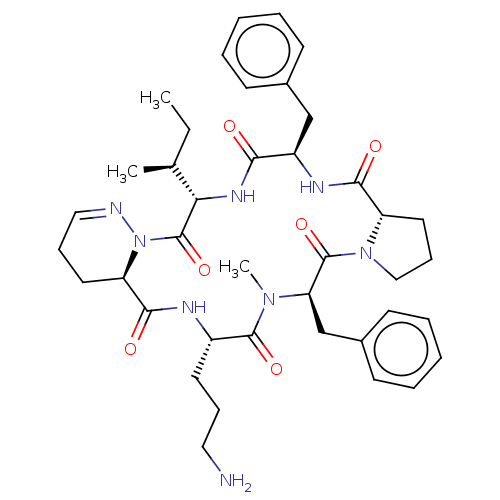 (18-(3-Amino-propyl)-6,15-dibenzyl-3-sec-butyl-16-m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [3H]arginine vasopressin binding to AVP-V2 site in rat kidney medulla. | J Med Chem 35: 3905-18 (1992) BindingDB Entry DOI: 10.7270/Q2K64JP2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50405191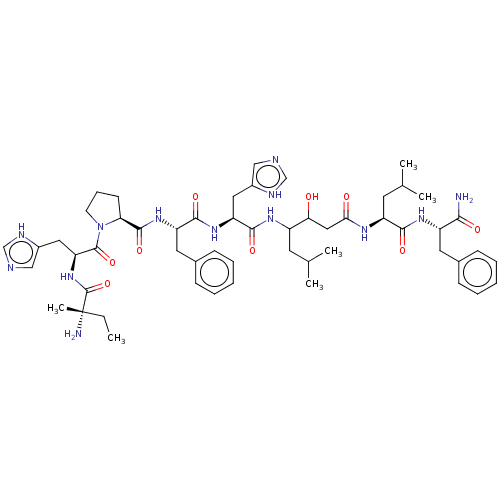 (CHEMBL269752) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description The compound was tested for inhibition of rabbit plasma renin. | J Med Chem 28: 1779-90 (1986) BindingDB Entry DOI: 10.7270/Q2FJ2HC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50072365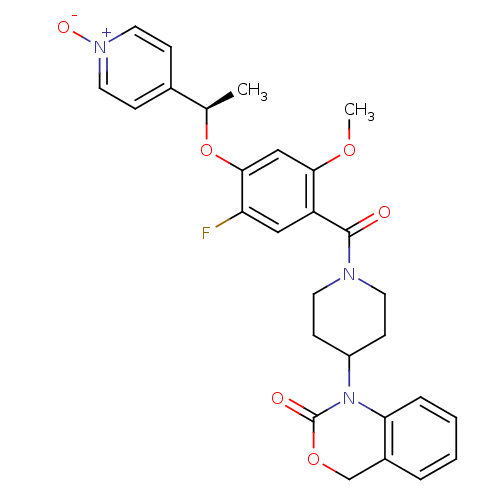 (1-(1-{5-Fluoro-2-methoxy-4-[(R)-1-(1-oxy-pyridin-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against cloned human oxytocin receptor from human embryonic kidney cells | Bioorg Med Chem Lett 8: 3081-6 (1999) Article DOI: 10.1016/S0960-894X(98)00568-X BindingDB Entry DOI: 10.7270/Q298865X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50001309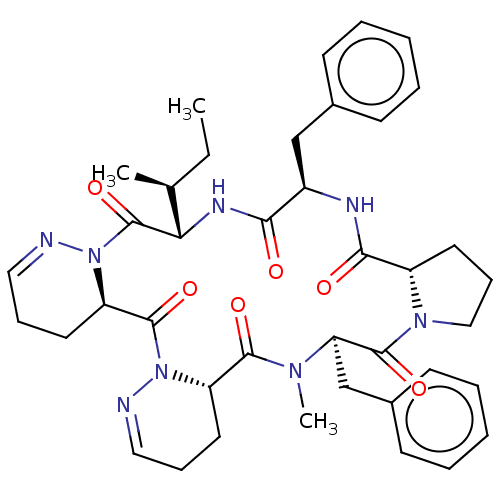 ((cyclo-[L-propyl-D-phenylalanyl-L-isoleucyl-D-dehy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards rat uterine receptor was determined using [3H]oxytocin as radioligand | J Med Chem 35: 3905-18 (1992) BindingDB Entry DOI: 10.7270/Q2K64JP2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50025946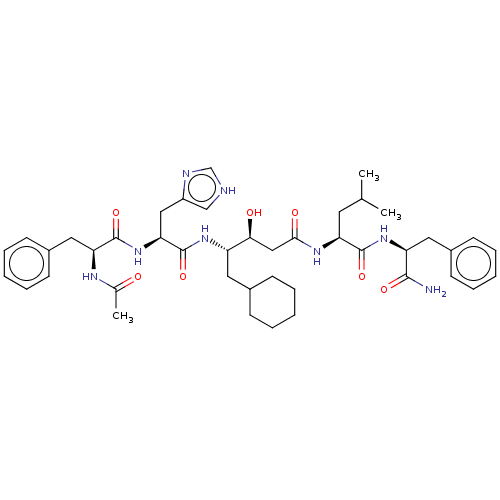 (4-[2-(2-Acetylamino-3-phenyl-propionylamino)-3-(1H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human kidney renin | J Med Chem 28: 1779-90 (1986) BindingDB Entry DOI: 10.7270/Q2FJ2HC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50072360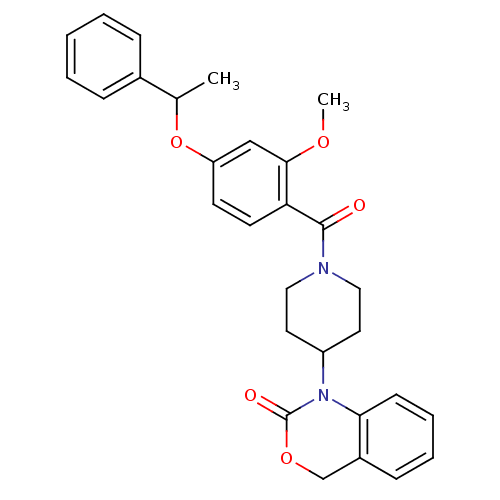 (1-{1-[2-Methoxy-4-(1-phenyl-ethoxy)-benzoyl]-piper...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against cloned human oxytocin receptor from human embryonic kidney cells | Bioorg Med Chem Lett 8: 3081-6 (1999) Article DOI: 10.1016/S0960-894X(98)00568-X BindingDB Entry DOI: 10.7270/Q298865X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50405191 (CHEMBL269752) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human kidney renin | J Med Chem 28: 1779-90 (1986) BindingDB Entry DOI: 10.7270/Q2FJ2HC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50029649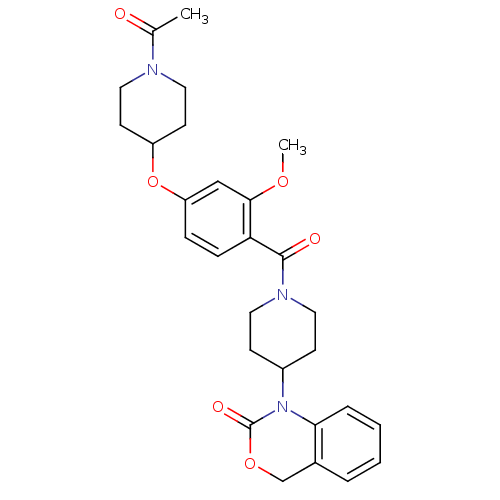 (1-(1-(4-(1-acetylpiperidin-4-yloxy)-2-methoxybenzo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against cloned human oxytocin receptor from human embryonic kidney cells | Bioorg Med Chem Lett 8: 3081-6 (1999) Article DOI: 10.1016/S0960-894X(98)00568-X BindingDB Entry DOI: 10.7270/Q298865X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50029649 (1-(1-(4-(1-acetylpiperidin-4-yloxy)-2-methoxybenzo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity for human oxytocin receptor | Bioorg Med Chem Lett 9: 1311-6 (1999) BindingDB Entry DOI: 10.7270/Q23N22K9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50368435 (CHEMBL1790937) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for OT receptor affinity by displacement of [3H]OT from binding sites in uterine tissue taken from nonlabor pregnant women | J Med Chem 35: 3905-18 (1992) BindingDB Entry DOI: 10.7270/Q2K64JP2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50077040 (1-(1-{2-[4-(1-Acetyl-piperidin-4-yloxy)-2-methoxy-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity for human oxytocin receptor | Bioorg Med Chem Lett 9: 1311-6 (1999) BindingDB Entry DOI: 10.7270/Q23N22K9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50072365 (1-(1-{5-Fluoro-2-methoxy-4-[(R)-1-(1-oxy-pyridin-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against oxytocin receptor (rOTr) in DES pretreated rat uterine | Bioorg Med Chem Lett 8: 3081-6 (1999) Article DOI: 10.1016/S0960-894X(98)00568-X BindingDB Entry DOI: 10.7270/Q298865X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50405191 (CHEMBL269752) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Renin | J Med Chem 28: 1779-90 (1986) BindingDB Entry DOI: 10.7270/Q2FJ2HC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50072367 (1-{1-[2-Methoxy-4-(1-pyridin-3-yl-ethoxy)-benzoyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against oxytocin receptor (rOTr) in DES pretreated rat uterine | Bioorg Med Chem Lett 8: 3081-6 (1999) Article DOI: 10.1016/S0960-894X(98)00568-X BindingDB Entry DOI: 10.7270/Q298865X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50025948 (5-Cyclohexyl-3-hydroxy-4-[3-(1H-imidazol-4-yl)-2-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Renin | J Med Chem 28: 1779-90 (1986) BindingDB Entry DOI: 10.7270/Q2FJ2HC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50072371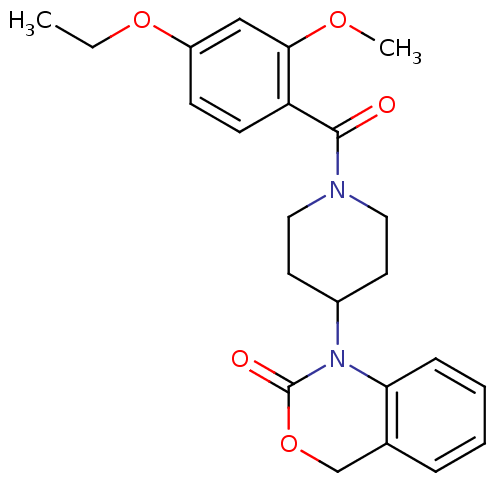 (1-[1-(4-Ethoxy-2-methoxy-benzoyl)-piperidin-4-yl]-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against cloned human oxytocin receptor from human embryonic kidney cells | Bioorg Med Chem Lett 8: 3081-6 (1999) Article DOI: 10.1016/S0960-894X(98)00568-X BindingDB Entry DOI: 10.7270/Q298865X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50072372 (1-[1-(4-Isopropoxy-2-methoxy-benzoyl)-piperidin-4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against cloned human oxytocin receptor from human embryonic kidney cells | Bioorg Med Chem Lett 8: 3081-6 (1999) Article DOI: 10.1016/S0960-894X(98)00568-X BindingDB Entry DOI: 10.7270/Q298865X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 309 total ) | Next | Last >> |