 Found 80 hits with Last Name = 'peters' and Initial = 'jw'
Found 80 hits with Last Name = 'peters' and Initial = 'jw' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Alkaline phosphatase, germ cell type
(Homo sapiens (Human)) | BDBM50131871
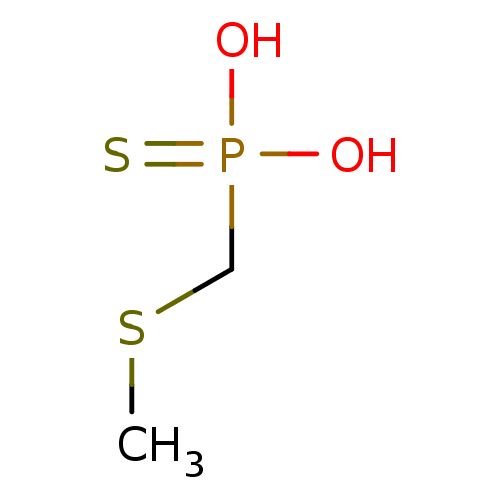
(CHEMBL122097 | Methylsulfanylmethyl-phosphonothioi...)Show InChI InChI=1S/C2H7O2PS2/c1-7-2-5(3,4)6/h2H2,1H3,(H2,3,4,6) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Utah State University
Curated by ChEMBL
| Assay Description
Inhibition of Human Placental alkaline Phosphatase (PLAP) |
J Med Chem 46: 3703-8 (2003)
Article DOI: 10.1021/jm030106f
BindingDB Entry DOI: 10.7270/Q2MG7NXN |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase
(Enterobacteria phage lambda) | BDBM50131868
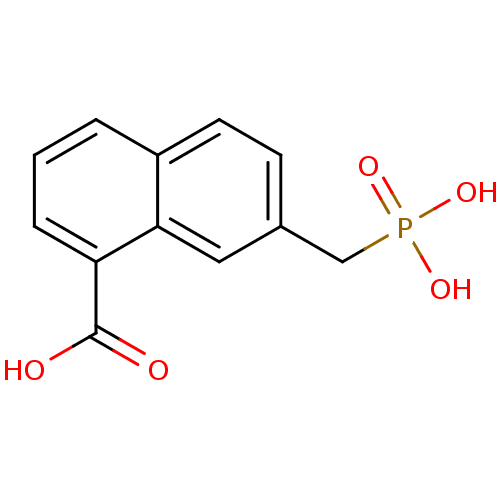
(7-Phosphonomethyl-naphthalene-1-carboxylic acid | ...)Show InChI InChI=1S/C12H11O5P/c13-12(14)10-3-1-2-9-5-4-8(6-11(9)10)7-18(15,16)17/h1-6H,7H2,(H,13,14)(H2,15,16,17) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Utah State University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Serine/Threonine Protein Phosphatase (Lamda protein phosphatase) |
J Med Chem 46: 3703-8 (2003)
Article DOI: 10.1021/jm030106f
BindingDB Entry DOI: 10.7270/Q2MG7NXN |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase
(Enterobacteria phage lambda) | BDBM50131866
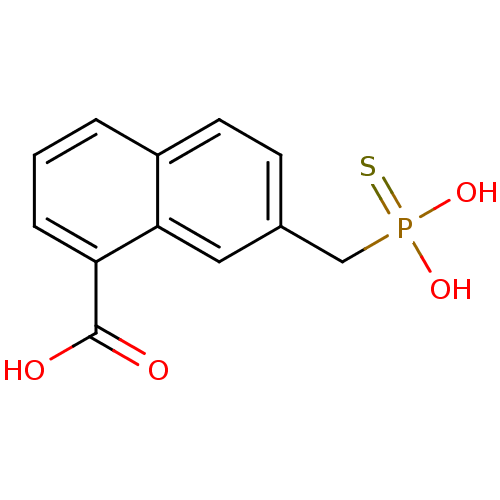
(7-Thiophosphonomethyl-naphthalene-1-carboxylic aci...)Show InChI InChI=1S/C12H11O4PS/c13-12(14)10-3-1-2-9-5-4-8(6-11(9)10)7-17(15,16)18/h1-6H,7H2,(H,13,14)(H2,15,16,18) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Utah State University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Serine/Threonine Protein Phosphatase (Lamda protein phosphatase) |
J Med Chem 46: 3703-8 (2003)
Article DOI: 10.1021/jm030106f
BindingDB Entry DOI: 10.7270/Q2MG7NXN |
More data for this
Ligand-Target Pair | |
Alkaline phosphatase, germ cell type
(Homo sapiens (Human)) | BDBM50131861
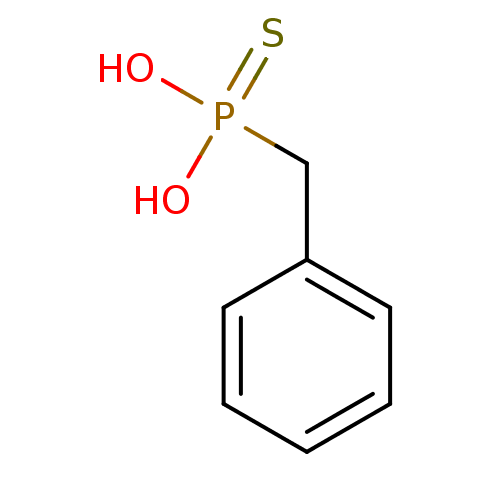
(Benzyl-phosphonothioic acid | CHEMBL123163)Show InChI InChI=1S/C7H9O2PS/c8-10(9,11)6-7-4-2-1-3-5-7/h1-5H,6H2,(H2,8,9,11) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Utah State University
Curated by ChEMBL
| Assay Description
Inhibition of Human Placental alkaline Phosphatase (PLAP) |
J Med Chem 46: 3703-8 (2003)
Article DOI: 10.1021/jm030106f
BindingDB Entry DOI: 10.7270/Q2MG7NXN |
More data for this
Ligand-Target Pair | |
Protein tyrosine phosphatase receptor type C-associated protein
(Homo sapiens (Human)) | BDBM50131866

(7-Thiophosphonomethyl-naphthalene-1-carboxylic aci...)Show InChI InChI=1S/C12H11O4PS/c13-12(14)10-3-1-2-9-5-4-8(6-11(9)10)7-17(15,16)18/h1-6H,7H2,(H,13,14)(H2,15,16,18) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Utah State University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Tyrosine phosphatase from Yersinia (Yop protein) |
J Med Chem 46: 3703-8 (2003)
Article DOI: 10.1021/jm030106f
BindingDB Entry DOI: 10.7270/Q2MG7NXN |
More data for this
Ligand-Target Pair | |
Alkaline phosphatase, tissue-nonspecific isozyme
(Rattus norvegicus) | BDBM50131868

(7-Phosphonomethyl-naphthalene-1-carboxylic acid | ...)Show InChI InChI=1S/C12H11O5P/c13-12(14)10-3-1-2-9-5-4-8(6-11(9)10)7-18(15,16)17/h1-6H,7H2,(H,13,14)(H2,15,16,17) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Utah State University
Curated by ChEMBL
| Assay Description
Inhibitory activity against E. coli Alkaline Phosphatase |
J Med Chem 46: 3703-8 (2003)
Article DOI: 10.1021/jm030106f
BindingDB Entry DOI: 10.7270/Q2MG7NXN |
More data for this
Ligand-Target Pair | |
Alkaline phosphatase, germ cell type
(Homo sapiens (Human)) | BDBM50131865
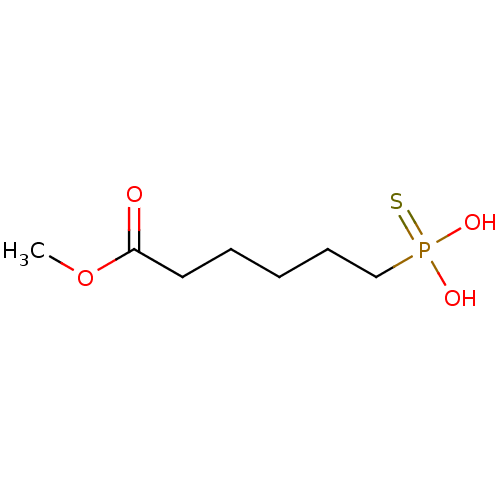
(6-Thiophosphono-hexanoic acid methyl ester | CHEMB...)Show InChI InChI=1S/C7H15O4PS/c1-11-7(8)5-3-2-4-6-12(9,10)13/h2-6H2,1H3,(H2,9,10,13) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Utah State University
Curated by ChEMBL
| Assay Description
Inhibition of Human Placental alkaline Phosphatase (PLAP) |
J Med Chem 46: 3703-8 (2003)
Article DOI: 10.1021/jm030106f
BindingDB Entry DOI: 10.7270/Q2MG7NXN |
More data for this
Ligand-Target Pair | |
Alkaline phosphatase, germ cell type
(Homo sapiens (Human)) | BDBM50131868

(7-Phosphonomethyl-naphthalene-1-carboxylic acid | ...)Show InChI InChI=1S/C12H11O5P/c13-12(14)10-3-1-2-9-5-4-8(6-11(9)10)7-18(15,16)17/h1-6H,7H2,(H,13,14)(H2,15,16,17) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 3.90E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Utah State University
Curated by ChEMBL
| Assay Description
Inhibition of Human Placental alkaline Phosphatase (PLAP) |
J Med Chem 46: 3703-8 (2003)
Article DOI: 10.1021/jm030106f
BindingDB Entry DOI: 10.7270/Q2MG7NXN |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase
(Enterobacteria phage lambda) | BDBM50131860
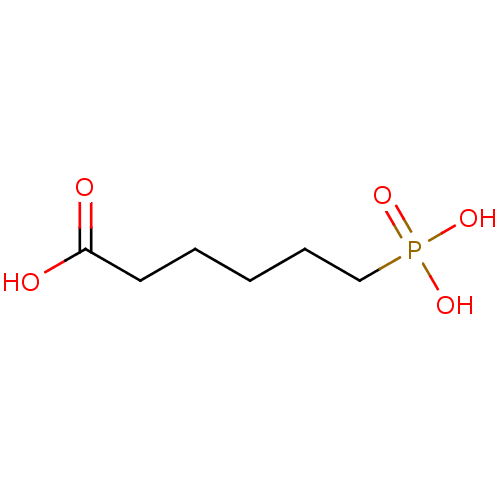
(6-Phosphono-hexanoic acid | CHEMBL122539)Show InChI InChI=1S/C6H13O5P/c7-6(8)4-2-1-3-5-12(9,10)11/h1-5H2,(H,7,8)(H2,9,10,11) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 4.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Utah State University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Serine/Threonine Protein Phosphatase (Lamda protein phosphatase) |
J Med Chem 46: 3703-8 (2003)
Article DOI: 10.1021/jm030106f
BindingDB Entry DOI: 10.7270/Q2MG7NXN |
More data for this
Ligand-Target Pair | |
Alkaline phosphatase, germ cell type
(Homo sapiens (Human)) | BDBM50131863
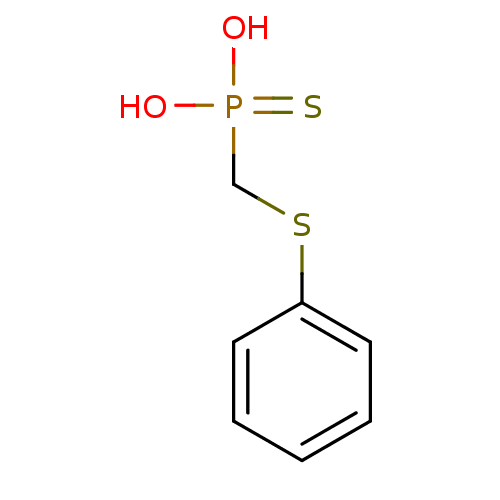
(CHEMBL331460 | Phenylsulfanylmethyl-phosphonothioi...)Show InChI InChI=1S/C7H9O2PS2/c8-10(9,11)6-12-7-4-2-1-3-5-7/h1-5H,6H2,(H2,8,9,11) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 4.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Utah State University
Curated by ChEMBL
| Assay Description
Inhibition of Human Placental alkaline Phosphatase (PLAP) |
J Med Chem 46: 3703-8 (2003)
Article DOI: 10.1021/jm030106f
BindingDB Entry DOI: 10.7270/Q2MG7NXN |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase
(Enterobacteria phage lambda) | BDBM50131863

(CHEMBL331460 | Phenylsulfanylmethyl-phosphonothioi...)Show InChI InChI=1S/C7H9O2PS2/c8-10(9,11)6-12-7-4-2-1-3-5-7/h1-5H,6H2,(H2,8,9,11) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 4.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Utah State University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Serine/Threonine Protein Phosphatase (Lamda protein phosphatase) |
J Med Chem 46: 3703-8 (2003)
Article DOI: 10.1021/jm030106f
BindingDB Entry DOI: 10.7270/Q2MG7NXN |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase
(Enterobacteria phage lambda) | BDBM50131861

(Benzyl-phosphonothioic acid | CHEMBL123163)Show InChI InChI=1S/C7H9O2PS/c8-10(9,11)6-7-4-2-1-3-5-7/h1-5H,6H2,(H2,8,9,11) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 5.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Utah State University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Serine/Threonine Protein Phosphatase (Lamda protein phosphatase) |
J Med Chem 46: 3703-8 (2003)
Article DOI: 10.1021/jm030106f
BindingDB Entry DOI: 10.7270/Q2MG7NXN |
More data for this
Ligand-Target Pair | |
Alkaline phosphatase, germ cell type
(Homo sapiens (Human)) | BDBM50080274
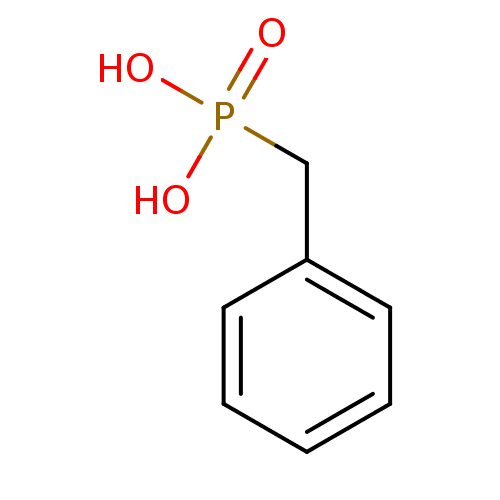
(Benzyl-phosphonic acid | CHEMBL299737 | Phenyl-met...)Show InChI InChI=1S/C7H9O3P/c8-11(9,10)6-7-4-2-1-3-5-7/h1-5H,6H2,(H2,8,9,10) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 5.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Utah State University
Curated by ChEMBL
| Assay Description
Inhibition of Human Placental alkaline Phosphatase (PLAP) |
J Med Chem 46: 3703-8 (2003)
Article DOI: 10.1021/jm030106f
BindingDB Entry DOI: 10.7270/Q2MG7NXN |
More data for this
Ligand-Target Pair | |
Alkaline phosphatase, germ cell type
(Homo sapiens (Human)) | BDBM50131870
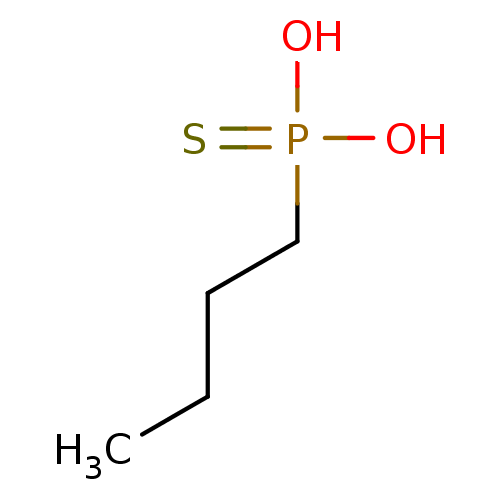
(Butyl-phosphonothioic acid | CHEMBL331046)Show InChI InChI=1S/C4H11O2PS/c1-2-3-4-7(5,6)8/h2-4H2,1H3,(H2,5,6,8) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 6.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Utah State University
Curated by ChEMBL
| Assay Description
Inhibition of Human Placental alkaline Phosphatase (PLAP) |
J Med Chem 46: 3703-8 (2003)
Article DOI: 10.1021/jm030106f
BindingDB Entry DOI: 10.7270/Q2MG7NXN |
More data for this
Ligand-Target Pair | |
Alkaline phosphatase, tissue-nonspecific isozyme
(Rattus norvegicus) | BDBM50131864
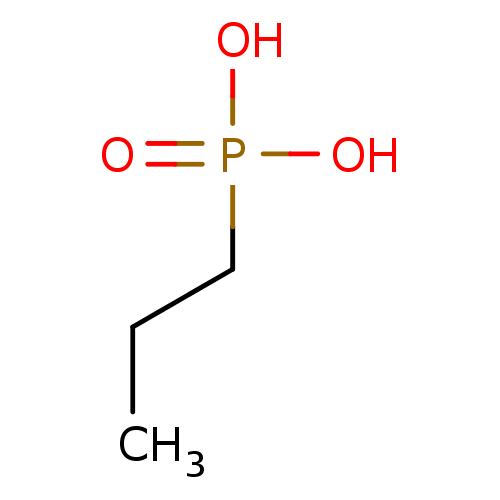
(CHEMBL125296 | Propyl-phosphonic acid)Show InChI InChI=1S/C3H9O3P/c1-2-3-7(4,5)6/h2-3H2,1H3,(H2,4,5,6) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 6.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Utah State University
Curated by ChEMBL
| Assay Description
Inhibitory activity against E. coli Alkaline Phosphatase |
J Med Chem 46: 3703-8 (2003)
Article DOI: 10.1021/jm030106f
BindingDB Entry DOI: 10.7270/Q2MG7NXN |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase
(Enterobacteria phage lambda) | BDBM50131862

(CHEMBL122938 | methylphosphonic acid)Show InChI InChI=1S/CH5O3P/c1-5(2,3)4/h1H3,(H2,2,3,4) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| 1.01E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Utah State University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Serine/Threonine Protein Phosphatase (Lamda protein phosphatase) |
J Med Chem 46: 3703-8 (2003)
Article DOI: 10.1021/jm030106f
BindingDB Entry DOI: 10.7270/Q2MG7NXN |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase
(Enterobacteria phage lambda) | BDBM50131865

(6-Thiophosphono-hexanoic acid methyl ester | CHEMB...)Show InChI InChI=1S/C7H15O4PS/c1-11-7(8)5-3-2-4-6-12(9,10)13/h2-6H2,1H3,(H2,9,10,13) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Utah State University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Serine/Threonine Protein Phosphatase (Lamda protein phosphatase) |
J Med Chem 46: 3703-8 (2003)
Article DOI: 10.1021/jm030106f
BindingDB Entry DOI: 10.7270/Q2MG7NXN |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase
(Enterobacteria phage lambda) | BDBM50131871

(CHEMBL122097 | Methylsulfanylmethyl-phosphonothioi...)Show InChI InChI=1S/C2H7O2PS2/c1-7-2-5(3,4)6/h2H2,1H3,(H2,3,4,6) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.20E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Utah State University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Serine/Threonine Protein Phosphatase (Lamda protein phosphatase) |
J Med Chem 46: 3703-8 (2003)
Article DOI: 10.1021/jm030106f
BindingDB Entry DOI: 10.7270/Q2MG7NXN |
More data for this
Ligand-Target Pair | |
Alkaline phosphatase, germ cell type
(Homo sapiens (Human)) | BDBM50131866

(7-Thiophosphonomethyl-naphthalene-1-carboxylic aci...)Show InChI InChI=1S/C12H11O4PS/c13-12(14)10-3-1-2-9-5-4-8(6-11(9)10)7-17(15,16)18/h1-6H,7H2,(H,13,14)(H2,15,16,18) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Utah State University
Curated by ChEMBL
| Assay Description
Inhibition of Human Placental alkaline Phosphatase (PLAP) |
J Med Chem 46: 3703-8 (2003)
Article DOI: 10.1021/jm030106f
BindingDB Entry DOI: 10.7270/Q2MG7NXN |
More data for this
Ligand-Target Pair | |
Alkaline phosphatase, germ cell type
(Homo sapiens (Human)) | BDBM50131867
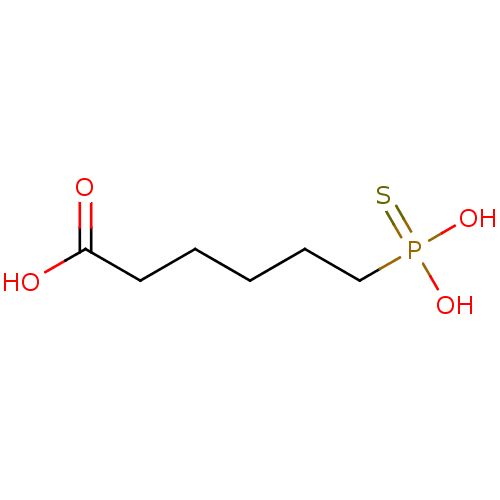
(6-Thiophosphono-hexanoic acid | CHEMBL123990)Show InChI InChI=1S/C6H13O4PS/c7-6(8)4-2-1-3-5-11(9,10)12/h1-5H2,(H,7,8)(H2,9,10,12) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Utah State University
Curated by ChEMBL
| Assay Description
Inhibition of Human Placental alkaline Phosphatase (PLAP) |
J Med Chem 46: 3703-8 (2003)
Article DOI: 10.1021/jm030106f
BindingDB Entry DOI: 10.7270/Q2MG7NXN |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase
(Enterobacteria phage lambda) | BDBM50131867

(6-Thiophosphono-hexanoic acid | CHEMBL123990)Show InChI InChI=1S/C6H13O4PS/c7-6(8)4-2-1-3-5-11(9,10)12/h1-5H2,(H,7,8)(H2,9,10,12) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Utah State University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Serine/Threonine Protein Phosphatase (Lamda protein phosphatase) |
J Med Chem 46: 3703-8 (2003)
Article DOI: 10.1021/jm030106f
BindingDB Entry DOI: 10.7270/Q2MG7NXN |
More data for this
Ligand-Target Pair | |
Alkaline phosphatase, germ cell type
(Homo sapiens (Human)) | BDBM50131864

(CHEMBL125296 | Propyl-phosphonic acid)Show InChI InChI=1S/C3H9O3P/c1-2-3-7(4,5)6/h2-3H2,1H3,(H2,4,5,6) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 1.55E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Utah State University
Curated by ChEMBL
| Assay Description
Inhibition of Human Placental alkaline Phosphatase (PLAP) |
J Med Chem 46: 3703-8 (2003)
Article DOI: 10.1021/jm030106f
BindingDB Entry DOI: 10.7270/Q2MG7NXN |
More data for this
Ligand-Target Pair | |
Alkaline phosphatase, germ cell type
(Homo sapiens (Human)) | BDBM50131860

(6-Phosphono-hexanoic acid | CHEMBL122539)Show InChI InChI=1S/C6H13O5P/c7-6(8)4-2-1-3-5-12(9,10)11/h1-5H2,(H,7,8)(H2,9,10,11) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 7.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Utah State University
Curated by ChEMBL
| Assay Description
Inhibition of Human Placental alkaline Phosphatase (PLAP) |
J Med Chem 46: 3703-8 (2003)
Article DOI: 10.1021/jm030106f
BindingDB Entry DOI: 10.7270/Q2MG7NXN |
More data for this
Ligand-Target Pair | |
Protein tyrosine phosphatase receptor type C-associated protein
(Homo sapiens (Human)) | BDBM50131869
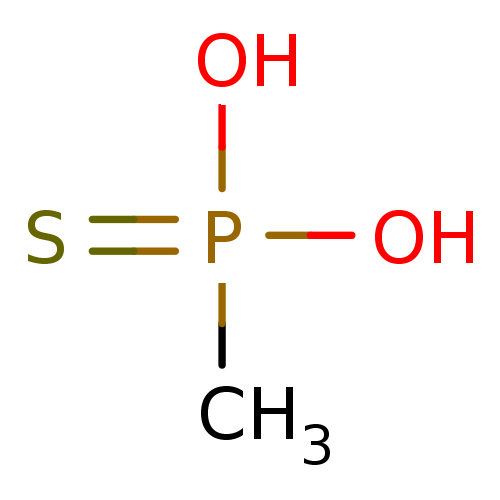
(CHEMBL122577 | Methyl-phosphonothioic acid)Show InChI InChI=1S/CH5O2PS/c1-4(2,3)5/h1H3,(H2,2,3,5) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 7.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Utah State University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Tyrosine phosphatase from Yersinia (Yop protein) |
J Med Chem 46: 3703-8 (2003)
Article DOI: 10.1021/jm030106f
BindingDB Entry DOI: 10.7270/Q2MG7NXN |
More data for this
Ligand-Target Pair | |
Alkaline phosphatase, germ cell type
(Homo sapiens (Human)) | BDBM50131862

(CHEMBL122938 | methylphosphonic acid)Show InChI InChI=1S/CH5O3P/c1-5(2,3)4/h1H3,(H2,2,3,4) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| 1.10E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Utah State University
Curated by ChEMBL
| Assay Description
Inhibition of Human Placental alkaline Phosphatase (PLAP) |
J Med Chem 46: 3703-8 (2003)
Article DOI: 10.1021/jm030106f
BindingDB Entry DOI: 10.7270/Q2MG7NXN |
More data for this
Ligand-Target Pair | |
Protein tyrosine phosphatase receptor type C-associated protein
(Homo sapiens (Human)) | BDBM50131863

(CHEMBL331460 | Phenylsulfanylmethyl-phosphonothioi...)Show InChI InChI=1S/C7H9O2PS2/c8-10(9,11)6-12-7-4-2-1-3-5-7/h1-5H,6H2,(H2,8,9,11) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.58E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Utah State University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Tyrosine phosphatase from Yersinia (Yop protein) |
J Med Chem 46: 3703-8 (2003)
Article DOI: 10.1021/jm030106f
BindingDB Entry DOI: 10.7270/Q2MG7NXN |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase
(Enterobacteria phage lambda) | BDBM50131869

(CHEMBL122577 | Methyl-phosphonothioic acid)Show InChI InChI=1S/CH5O2PS/c1-4(2,3)5/h1H3,(H2,2,3,5) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 1.70E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Utah State University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Serine/Threonine Protein Phosphatase (Lamda protein phosphatase) |
J Med Chem 46: 3703-8 (2003)
Article DOI: 10.1021/jm030106f
BindingDB Entry DOI: 10.7270/Q2MG7NXN |
More data for this
Ligand-Target Pair | |
Alkaline phosphatase, germ cell type
(Homo sapiens (Human)) | BDBM50131869

(CHEMBL122577 | Methyl-phosphonothioic acid)Show InChI InChI=1S/CH5O2PS/c1-4(2,3)5/h1H3,(H2,2,3,5) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 1.90E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Utah State University
Curated by ChEMBL
| Assay Description
Inhibition of Human Placental alkaline Phosphatase (PLAP) |
J Med Chem 46: 3703-8 (2003)
Article DOI: 10.1021/jm030106f
BindingDB Entry DOI: 10.7270/Q2MG7NXN |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase
(Enterobacteria phage lambda) | BDBM50131870

(Butyl-phosphonothioic acid | CHEMBL331046)Show InChI InChI=1S/C4H11O2PS/c1-2-3-4-7(5,6)8/h2-4H2,1H3,(H2,5,6,8) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 2.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Utah State University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Serine/Threonine Protein Phosphatase (Lamda protein phosphatase) |
J Med Chem 46: 3703-8 (2003)
Article DOI: 10.1021/jm030106f
BindingDB Entry DOI: 10.7270/Q2MG7NXN |
More data for this
Ligand-Target Pair | |
Protein tyrosine phosphatase receptor type C-associated protein
(Homo sapiens (Human)) | BDBM50131867

(6-Thiophosphono-hexanoic acid | CHEMBL123990)Show InChI InChI=1S/C6H13O4PS/c7-6(8)4-2-1-3-5-11(9,10)12/h1-5H2,(H,7,8)(H2,9,10,12) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.90E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Utah State University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Tyrosine phosphatase from Yersinia (Yop protein) |
J Med Chem 46: 3703-8 (2003)
Article DOI: 10.1021/jm030106f
BindingDB Entry DOI: 10.7270/Q2MG7NXN |
More data for this
Ligand-Target Pair | |
Protein tyrosine phosphatase receptor type C-associated protein
(Homo sapiens (Human)) | BDBM50131862

(CHEMBL122938 | methylphosphonic acid)Show InChI InChI=1S/CH5O3P/c1-5(2,3)4/h1H3,(H2,2,3,4) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| 3.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Utah State University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Tyrosine phosphatase from Yersinia (Yop protein) |
J Med Chem 46: 3703-8 (2003)
Article DOI: 10.1021/jm030106f
BindingDB Entry DOI: 10.7270/Q2MG7NXN |
More data for this
Ligand-Target Pair | |
Protein tyrosine phosphatase receptor type C-associated protein
(Homo sapiens (Human)) | BDBM50131860

(6-Phosphono-hexanoic acid | CHEMBL122539)Show InChI InChI=1S/C6H13O5P/c7-6(8)4-2-1-3-5-12(9,10)11/h1-5H2,(H,7,8)(H2,9,10,11) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 4.20E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Utah State University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Tyrosine phosphatase from Yersinia (Yop protein) |
J Med Chem 46: 3703-8 (2003)
Article DOI: 10.1021/jm030106f
BindingDB Entry DOI: 10.7270/Q2MG7NXN |
More data for this
Ligand-Target Pair | |
Calmodulin-sensitive adenylate cyclase
(Bacillus anthracis) | BDBM50486025
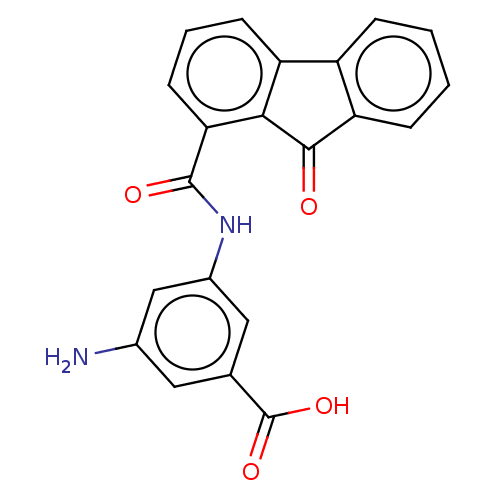
(CHEMBL2203672)Show SMILES Nc1cc(NC(=O)c2cccc3-c4ccccc4C(=O)c23)cc(c1)C(O)=O Show InChI InChI=1S/C21H14N2O4/c22-12-8-11(21(26)27)9-13(10-12)23-20(25)17-7-3-6-15-14-4-1-2-5-16(14)19(24)18(15)17/h1-10H,22H2,(H,23,25)(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 990 | n/a | n/a | n/a | n/a | n/a | n/a |
UTMB
Curated by ChEMBL
| Assay Description
Inhibition of Bacillus anthracis edema toxin-mediated cAMP production in mouse RAW264.7 cells after 4 hrs by ELISA |
Bioorg Med Chem 20: 368-76 (2012)
Article DOI: 10.1016/j.bmc.2011.10.091
BindingDB Entry DOI: 10.7270/Q2PK0K15 |
More data for this
Ligand-Target Pair | |
Calmodulin-sensitive adenylate cyclase
(Bacillus anthracis) | BDBM50486023
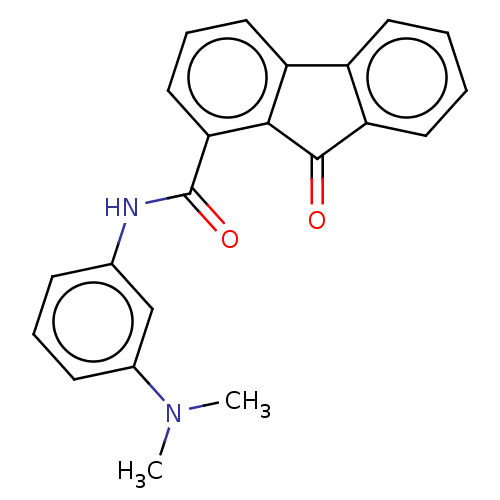
(CHEMBL2203681)Show SMILES CN(C)c1cccc(NC(=O)c2cccc3-c4ccccc4C(=O)c23)c1 Show InChI InChI=1S/C22H18N2O2/c1-24(2)15-8-5-7-14(13-15)23-22(26)19-12-6-11-17-16-9-3-4-10-18(16)21(25)20(17)19/h3-13H,1-2H3,(H,23,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
UTMB
Curated by ChEMBL
| Assay Description
Inhibition of Bacillus anthracis edema toxin-mediated cAMP production in mouse RAW264.7 cells after 4 hrs by ELISA |
Bioorg Med Chem 20: 368-76 (2012)
Article DOI: 10.1016/j.bmc.2011.10.091
BindingDB Entry DOI: 10.7270/Q2PK0K15 |
More data for this
Ligand-Target Pair | |
Calmodulin-sensitive adenylate cyclase
(Bacillus anthracis) | BDBM50262712
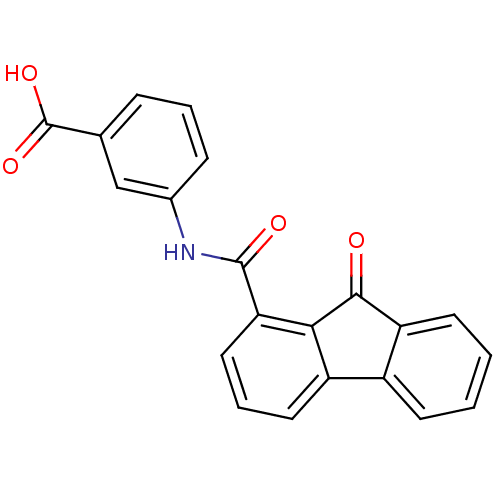
(3-[(9-oxo-9Hfluorene-1-carbonyl)-amino]-benzoic ac...)Show SMILES OC(=O)c1cccc(NC(=O)c2cccc3-c4ccccc4C(=O)c23)c1 Show InChI InChI=1S/C21H13NO4/c23-19-16-8-2-1-7-14(16)15-9-4-10-17(18(15)19)20(24)22-13-6-3-5-12(11-13)21(25)26/h1-11H,(H,22,24)(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
UTMB
Curated by ChEMBL
| Assay Description
Inhibition of Bacillus anthracis edema toxin-mediated cAMP production in mouse RAW264.7 cells after 4 hrs by ELISA |
Bioorg Med Chem 20: 368-76 (2012)
Article DOI: 10.1016/j.bmc.2011.10.091
BindingDB Entry DOI: 10.7270/Q2PK0K15 |
More data for this
Ligand-Target Pair | |
Calmodulin-sensitive adenylate cyclase
(Bacillus anthracis) | BDBM50262712

(3-[(9-oxo-9Hfluorene-1-carbonyl)-amino]-benzoic ac...)Show SMILES OC(=O)c1cccc(NC(=O)c2cccc3-c4ccccc4C(=O)c23)c1 Show InChI InChI=1S/C21H13NO4/c23-19-16-8-2-1-7-14(16)15-9-4-10-17(18(15)19)20(24)22-13-6-3-5-12(11-13)21(25)26/h1-11H,(H,22,24)(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas Medical Branch
Curated by ChEMBL
| Assay Description
Inhibition of Bacillus anthracis edema factor-induced cytotoxicity in mouse RAW264.7 cells assessed as LDH release after 4 hrs |
Bioorg Med Chem 16: 7225-33 (2008)
Article DOI: 10.1016/j.bmc.2008.06.036
BindingDB Entry DOI: 10.7270/Q2R2116M |
More data for this
Ligand-Target Pair | |
Calmodulin-sensitive adenylate cyclase
(Bacillus anthracis) | BDBM50262713

(4-(3-methoxy-phenyl)-3a,4,5,9b-tetrahydro-3H-cyclo...)Show SMILES COc1cccc(c1)[C@H]1Nc2ccc(cc2[C@@H]2C=CC[C@H]12)C(O)=O |r,c:19| Show InChI InChI=1S/C20H19NO3/c1-24-14-5-2-4-12(10-14)19-16-7-3-6-15(16)17-11-13(20(22)23)8-9-18(17)21-19/h2-6,8-11,15-16,19,21H,7H2,1H3,(H,22,23)/t15-,16+,19-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.73E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas Medical Branch
Curated by ChEMBL
| Assay Description
Inhibition of Bacillus anthracis edema factor-induced cAMP secretion in mouse RAW264.7 cells after 4 hrs by ELISA |
Bioorg Med Chem 16: 7225-33 (2008)
Article DOI: 10.1016/j.bmc.2008.06.036
BindingDB Entry DOI: 10.7270/Q2R2116M |
More data for this
Ligand-Target Pair | |
Calmodulin-sensitive adenylate cyclase
(Bacillus anthracis) | BDBM50262713

(4-(3-methoxy-phenyl)-3a,4,5,9b-tetrahydro-3H-cyclo...)Show SMILES COc1cccc(c1)[C@H]1Nc2ccc(cc2[C@@H]2C=CC[C@H]12)C(O)=O |r,c:19| Show InChI InChI=1S/C20H19NO3/c1-24-14-5-2-4-12(10-14)19-16-7-3-6-15(16)17-11-13(20(22)23)8-9-18(17)21-19/h2-6,8-11,15-16,19,21H,7H2,1H3,(H,22,23)/t15-,16+,19-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas Medical Branch
Curated by ChEMBL
| Assay Description
Inhibition of Bacillus anthracis edema factor-induced cytotoxicity in mouse RAW264.7 cells assessed as LDH release after 4 hrs |
Bioorg Med Chem 16: 7225-33 (2008)
Article DOI: 10.1016/j.bmc.2008.06.036
BindingDB Entry DOI: 10.7270/Q2R2116M |
More data for this
Ligand-Target Pair | |
Calmodulin-sensitive adenylate cyclase
(Bacillus anthracis) | BDBM50262712

(3-[(9-oxo-9Hfluorene-1-carbonyl)-amino]-benzoic ac...)Show SMILES OC(=O)c1cccc(NC(=O)c2cccc3-c4ccccc4C(=O)c23)c1 Show InChI InChI=1S/C21H13NO4/c23-19-16-8-2-1-7-14(16)15-9-4-10-17(18(15)19)20(24)22-13-6-3-5-12(11-13)21(25)26/h1-11H,(H,22,24)(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.83E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas Medical Branch
Curated by ChEMBL
| Assay Description
Inhibition of Bacillus anthracis edema factor-induced cAMP secretion in mouse RAW264.7 cells after 4 hrs by ELISA |
Bioorg Med Chem 16: 7225-33 (2008)
Article DOI: 10.1016/j.bmc.2008.06.036
BindingDB Entry DOI: 10.7270/Q2R2116M |
More data for this
Ligand-Target Pair | |
Calmodulin-sensitive adenylate cyclase
(Bacillus anthracis) | BDBM50486008
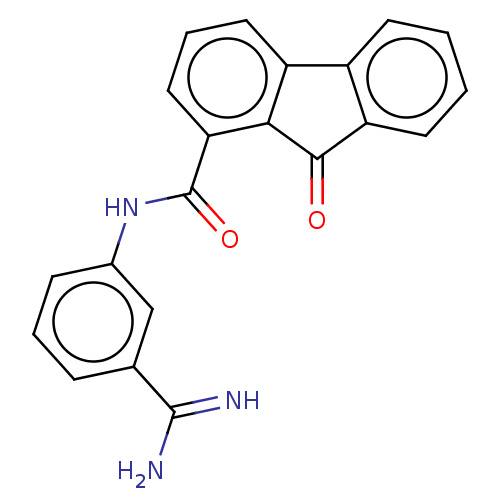
(CHEMBL2203684)Show SMILES NC(=N)c1cccc(NC(=O)c2cccc3-c4ccccc4C(=O)c23)c1 Show InChI InChI=1S/C21H15N3O2/c22-20(23)12-5-3-6-13(11-12)24-21(26)17-10-4-9-15-14-7-1-2-8-16(14)19(25)18(15)17/h1-11H,(H3,22,23)(H,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.71E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
UTMB
Curated by ChEMBL
| Assay Description
Inhibition of Bacillus anthracis edema toxin-mediated cAMP production in mouse RAW264.7 cells after 4 hrs by ELISA |
Bioorg Med Chem 20: 368-76 (2012)
Article DOI: 10.1016/j.bmc.2011.10.091
BindingDB Entry DOI: 10.7270/Q2PK0K15 |
More data for this
Ligand-Target Pair | |
Calmodulin-sensitive adenylate cyclase
(Bacillus anthracis) | BDBM50486009
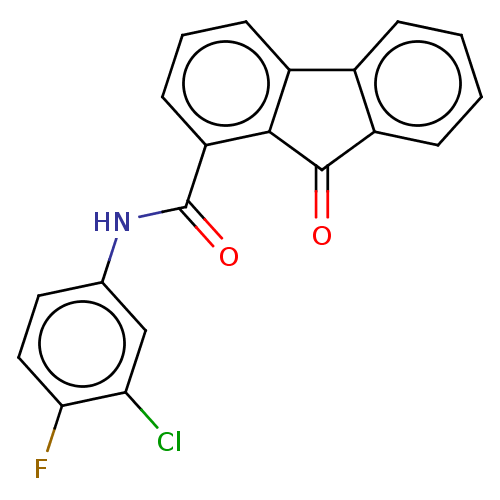
(CHEMBL2203682)Show SMILES Fc1ccc(NC(=O)c2cccc3-c4ccccc4C(=O)c23)cc1Cl Show InChI InChI=1S/C20H11ClFNO2/c21-16-10-11(8-9-17(16)22)23-20(25)15-7-3-6-13-12-4-1-2-5-14(12)19(24)18(13)15/h1-10H,(H,23,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
UTMB
Curated by ChEMBL
| Assay Description
Inhibition of Bacillus anthracis edema toxin-mediated cAMP production in mouse RAW264.7 cells after 4 hrs by ELISA |
Bioorg Med Chem 20: 368-76 (2012)
Article DOI: 10.1016/j.bmc.2011.10.091
BindingDB Entry DOI: 10.7270/Q2PK0K15 |
More data for this
Ligand-Target Pair | |
Calmodulin-sensitive adenylate cyclase
(Bacillus anthracis) | BDBM50486021
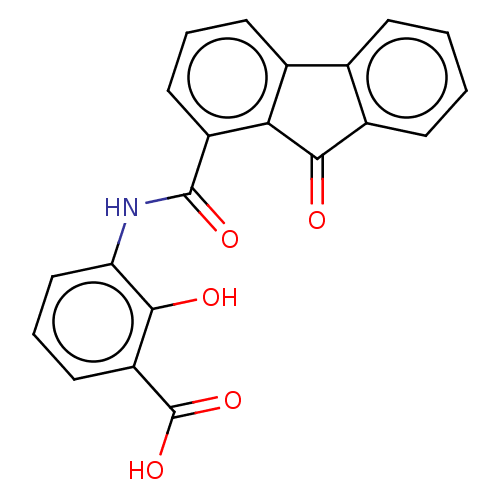
(CHEMBL2203670)Show SMILES OC(=O)c1cccc(NC(=O)c2cccc3-c4ccccc4C(=O)c23)c1O Show InChI InChI=1S/C21H13NO5/c23-18-15(21(26)27)9-4-10-16(18)22-20(25)14-8-3-7-12-11-5-1-2-6-13(11)19(24)17(12)14/h1-10,23H,(H,22,25)(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
UTMB
Curated by ChEMBL
| Assay Description
Inhibition of Bacillus anthracis edema toxin-mediated cAMP production in mouse RAW264.7 cells after 4 hrs by ELISA |
Bioorg Med Chem 20: 368-76 (2012)
Article DOI: 10.1016/j.bmc.2011.10.091
BindingDB Entry DOI: 10.7270/Q2PK0K15 |
More data for this
Ligand-Target Pair | |
Calmodulin-sensitive adenylate cyclase
(Bacillus anthracis) | BDBM50486014
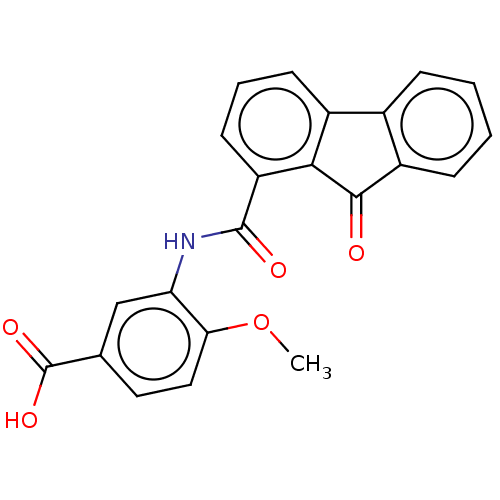
(CHEMBL2203673)Show SMILES COc1ccc(cc1NC(=O)c1cccc2-c3ccccc3C(=O)c12)C(O)=O Show InChI InChI=1S/C22H15NO5/c1-28-18-10-9-12(22(26)27)11-17(18)23-21(25)16-8-4-7-14-13-5-2-3-6-15(13)20(24)19(14)16/h2-11H,1H3,(H,23,25)(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.81E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
UTMB
Curated by ChEMBL
| Assay Description
Inhibition of Bacillus anthracis edema toxin-mediated cAMP production in mouse RAW264.7 cells after 4 hrs by ELISA |
Bioorg Med Chem 20: 368-76 (2012)
Article DOI: 10.1016/j.bmc.2011.10.091
BindingDB Entry DOI: 10.7270/Q2PK0K15 |
More data for this
Ligand-Target Pair | |
Calmodulin-sensitive adenylate cyclase
(Bacillus anthracis) | BDBM50486016
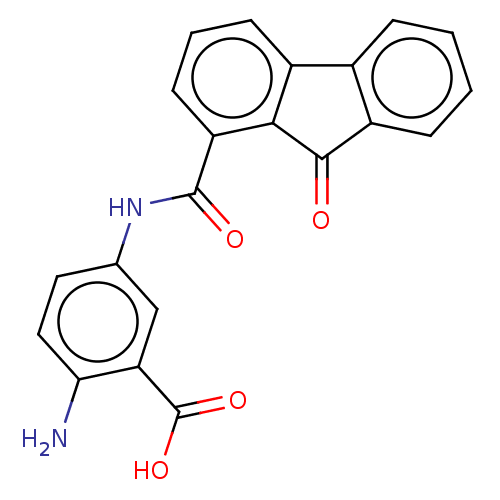
(CHEMBL2203669)Show SMILES Nc1ccc(NC(=O)c2cccc3-c4ccccc4C(=O)c23)cc1C(O)=O Show InChI InChI=1S/C21H14N2O4/c22-17-9-8-11(10-16(17)21(26)27)23-20(25)15-7-3-6-13-12-4-1-2-5-14(12)19(24)18(13)15/h1-10H,22H2,(H,23,25)(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
UTMB
Curated by ChEMBL
| Assay Description
Inhibition of Bacillus anthracis edema toxin-mediated cAMP production in mouse RAW264.7 cells after 4 hrs by ELISA |
Bioorg Med Chem 20: 368-76 (2012)
Article DOI: 10.1016/j.bmc.2011.10.091
BindingDB Entry DOI: 10.7270/Q2PK0K15 |
More data for this
Ligand-Target Pair | |
Calmodulin-sensitive adenylate cyclase
(Bacillus anthracis) | BDBM50486015
(CHEMBL2203671)Show SMILES OC(=O)c1cc(NC(=O)c2cccc3-c4ccccc4C(=O)c23)ccc1O Show InChI InChI=1S/C21H13NO5/c23-17-9-8-11(10-16(17)21(26)27)22-20(25)15-7-3-6-13-12-4-1-2-5-14(12)19(24)18(13)15/h1-10,23H,(H,22,25)(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
UTMB
Curated by ChEMBL
| Assay Description
Inhibition of Bacillus anthracis edema toxin-mediated cAMP production in mouse RAW264.7 cells after 4 hrs by ELISA |
Bioorg Med Chem 20: 368-76 (2012)
Article DOI: 10.1016/j.bmc.2011.10.091
BindingDB Entry DOI: 10.7270/Q2PK0K15 |
More data for this
Ligand-Target Pair | |
Calmodulin-sensitive adenylate cyclase
(Bacillus anthracis) | BDBM50486020
(CHEMBL2203680)Show SMILES FC(F)(F)Oc1cccc(NC(=O)c2cccc3-c4ccccc4C(=O)c23)c1 Show InChI InChI=1S/C21H12F3NO3/c22-21(23,24)28-13-6-3-5-12(11-13)25-20(27)17-10-4-9-15-14-7-1-2-8-16(14)19(26)18(15)17/h1-11H,(H,25,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
UTMB
Curated by ChEMBL
| Assay Description
Inhibition of Bacillus anthracis edema toxin-mediated cAMP production in mouse RAW264.7 cells after 4 hrs by ELISA |
Bioorg Med Chem 20: 368-76 (2012)
Article DOI: 10.1016/j.bmc.2011.10.091
BindingDB Entry DOI: 10.7270/Q2PK0K15 |
More data for this
Ligand-Target Pair | |
Calmodulin-sensitive adenylate cyclase
(Bacillus anthracis) | BDBM50486011
(CHEMBL2203678)Show SMILES COC(=O)c1ccc(OC)c(NC(=O)c2cccc3-c4ccccc4C(=O)c23)c1 Show InChI InChI=1S/C23H17NO5/c1-28-19-11-10-13(23(27)29-2)12-18(19)24-22(26)17-9-5-8-15-14-6-3-4-7-16(14)21(25)20(15)17/h3-12H,1-2H3,(H,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
UTMB
Curated by ChEMBL
| Assay Description
Inhibition of Bacillus anthracis edema toxin-mediated cAMP production in mouse RAW264.7 cells after 4 hrs by ELISA |
Bioorg Med Chem 20: 368-76 (2012)
Article DOI: 10.1016/j.bmc.2011.10.091
BindingDB Entry DOI: 10.7270/Q2PK0K15 |
More data for this
Ligand-Target Pair | |
Calmodulin-sensitive adenylate cyclase
(Bacillus anthracis) | BDBM50262711
(4-[(anthracen-9-ylmethylene)-amino]-2-hydroxy-benz...)Show InChI InChI=1S/C22H15NO3/c24-21-12-16(9-10-19(21)22(25)26)23-13-20-17-7-3-1-5-14(17)11-15-6-2-4-8-18(15)20/h1-13,24H,(H,25,26)/b23-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas Medical Branch
Curated by ChEMBL
| Assay Description
Inhibition of Bacillus anthracis edema factor-induced cAMP secretion in mouse RAW264.7 cells after 4 hrs by ELISA |
Bioorg Med Chem 16: 7225-33 (2008)
Article DOI: 10.1016/j.bmc.2008.06.036
BindingDB Entry DOI: 10.7270/Q2R2116M |
More data for this
Ligand-Target Pair | |
Calmodulin-sensitive adenylate cyclase
(Bacillus anthracis) | BDBM50262711

(4-[(anthracen-9-ylmethylene)-amino]-2-hydroxy-benz...)Show InChI InChI=1S/C22H15NO3/c24-21-12-16(9-10-19(21)22(25)26)23-13-20-17-7-3-1-5-14(17)11-15-6-2-4-8-18(15)20/h1-13,24H,(H,25,26)/b23-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas Medical Branch
Curated by ChEMBL
| Assay Description
Inhibition of Bacillus anthracis edema factor-induced cytotoxicity in mouse RAW264.7 cells assessed as LDH release after 4 hrs |
Bioorg Med Chem 16: 7225-33 (2008)
Article DOI: 10.1016/j.bmc.2008.06.036
BindingDB Entry DOI: 10.7270/Q2R2116M |
More data for this
Ligand-Target Pair | |
Calmodulin-sensitive adenylate cyclase
(Bacillus anthracis) | BDBM50486012
(CHEMBL2203675)Show SMILES OC(=O)c1cccc(NC(=O)c2cccc3-c4ccccc4C(=O)c23)c1Cl Show InChI InChI=1S/C21H12ClNO4/c22-18-15(21(26)27)9-4-10-16(18)23-20(25)14-8-3-7-12-11-5-1-2-6-13(11)19(24)17(12)14/h1-10H,(H,23,25)(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
UTMB
Curated by ChEMBL
| Assay Description
Inhibition of Bacillus anthracis edema toxin-mediated cAMP production in mouse RAW264.7 cells after 4 hrs by ELISA |
Bioorg Med Chem 20: 368-76 (2012)
Article DOI: 10.1016/j.bmc.2011.10.091
BindingDB Entry DOI: 10.7270/Q2PK0K15 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data