

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM729 ((2S,4R)-N-tert-butyl-1-[(2R,3S)-2-hydroxy-3-[(2S)-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0310 | -62.4 | 4 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Cleavage products and substrate w... | J Med Chem 43: 1094-108 (2000) Article DOI: 10.1021/jm990336n BindingDB Entry DOI: 10.7270/Q2BZ647F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [514-612] (Human immunodeficiency virus type 2) | BDBM729 ((2S,4R)-N-tert-butyl-1-[(2R,3S)-2-hydroxy-3-[(2S)-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.130 | -58.7 | 10 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Cleavage products and substrate w... | J Med Chem 43: 1094-108 (2000) Article DOI: 10.1021/jm990336n BindingDB Entry DOI: 10.7270/Q2BZ647F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50069984 ((R)-1-((S)-2-((S)-2-(benzyloxycarbonyl)-4-methylpe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ProScript, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Cathepsin B | Bioorg Med Chem Lett 8: 333-8 (1999) BindingDB Entry DOI: 10.7270/Q2RV0MVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen B (Homo sapiens (Human)) | BDBM50069989 ((R)-3-methyl-1-((S)-3-phenyl-2-(pyrazine-2-carboxa...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ProScript, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against human Chymotrypsinogen | Bioorg Med Chem Lett 8: 333-8 (1999) BindingDB Entry DOI: 10.7270/Q2RV0MVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin G (Homo sapiens (Human)) | BDBM50069989 ((R)-3-methyl-1-((S)-3-phenyl-2-(pyrazine-2-carboxa...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ProScript, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against human cathepsin G | Bioorg Med Chem Lett 8: 333-8 (1999) BindingDB Entry DOI: 10.7270/Q2RV0MVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50069989 ((R)-3-methyl-1-((S)-3-phenyl-2-(pyrazine-2-carboxa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ProScript, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against human leukocyte elastase | Bioorg Med Chem Lett 8: 333-8 (1999) BindingDB Entry DOI: 10.7270/Q2RV0MVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50069989 ((R)-3-methyl-1-((S)-3-phenyl-2-(pyrazine-2-carboxa...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ProScript, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against thrombin | Bioorg Med Chem Lett 8: 333-8 (1999) BindingDB Entry DOI: 10.7270/Q2RV0MVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM748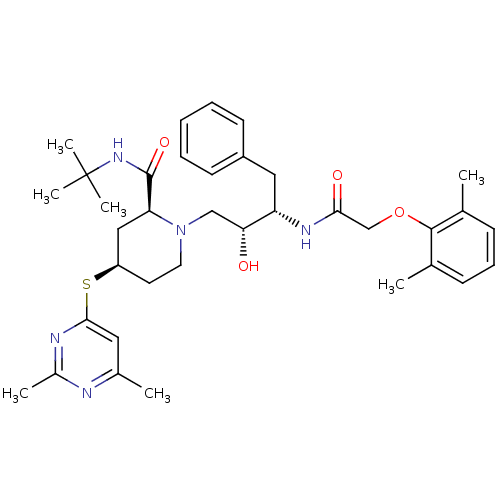 ((2S,4R)-N-tert-butyl-1-[(2R,3S)-3-[2-(2,6-dimethyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Cleavage products and substrate w... | J Med Chem 43: 1094-108 (2000) Article DOI: 10.1021/jm990336n BindingDB Entry DOI: 10.7270/Q2BZ647F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM747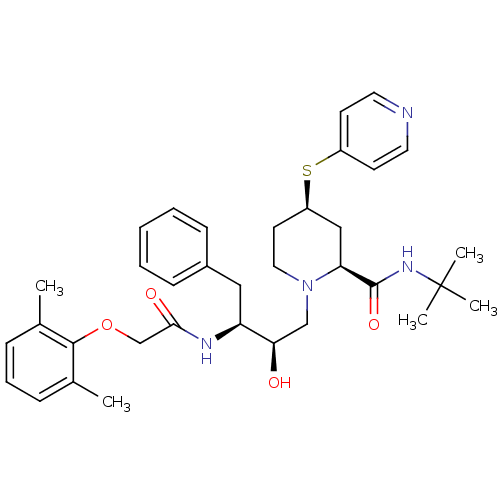 ((2S,4R)-N-tert-butyl-1-[(2R,3S)-3-[2-(2,6-dimethyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Cleavage products and substrate w... | J Med Chem 43: 1094-108 (2000) Article DOI: 10.1021/jm990336n BindingDB Entry DOI: 10.7270/Q2BZ647F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM746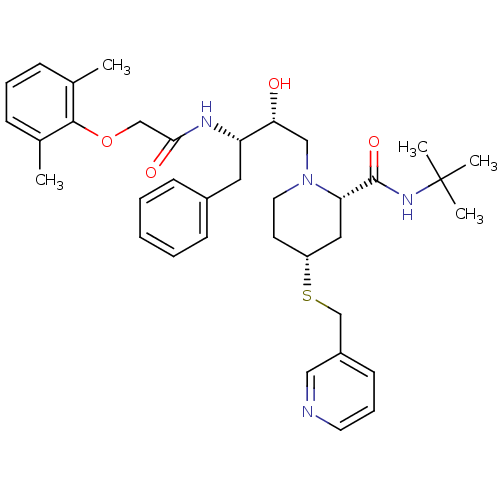 ((2S,4R)-N-tert-butyl-1-[(2R,3S)-3-[2-(2,6-dimethyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Cleavage products and substrate w... | J Med Chem 43: 1094-108 (2000) Article DOI: 10.1021/jm990336n BindingDB Entry DOI: 10.7270/Q2BZ647F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM745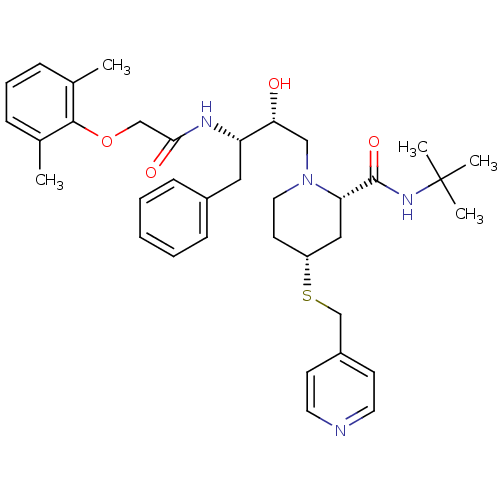 ((2S,4R)-N-tert-butyl-1-[(2R,3S)-3-[2-(2,6-dimethyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Cleavage products and substrate w... | J Med Chem 43: 1094-108 (2000) Article DOI: 10.1021/jm990336n BindingDB Entry DOI: 10.7270/Q2BZ647F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM577 ((3S)-oxolan-3-yl N-[(2S,3R)-4-[(4-aminobenzene)(2-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Cleavage products and substrate w... | J Med Chem 43: 1094-108 (2000) Article DOI: 10.1021/jm990336n BindingDB Entry DOI: 10.7270/Q2BZ647F | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Ribonucleoside-diphosphate reductase large subunit (Human herpesvirus 1 (strain 17) (HHV-1) (Human her...) | BDBM50050831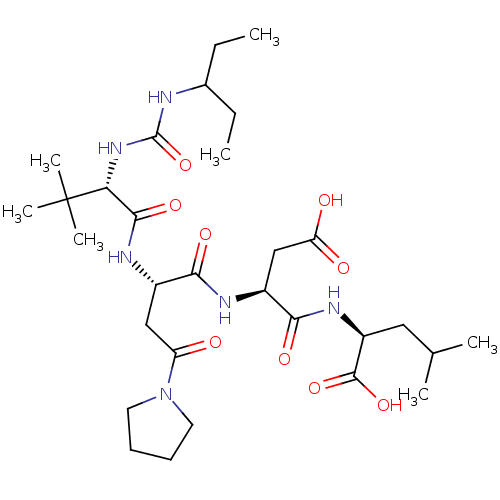 ((S)-2-[(S)-3-Carboxy-2-((S)-2-{(S)-2-[3-(1-ethyl-p...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-Méga/Boehringer Ingelheim Research Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Herpes simplex virus (HSV) ribonucleotide reductase (RR) | J Med Chem 39: 2178-87 (1996) Article DOI: 10.1021/jm950825x BindingDB Entry DOI: 10.7270/Q2BG2N22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribonucleoside-diphosphate reductase large subunit (Human herpesvirus 1 (strain 17) (HHV-1) (Human her...) | BDBM50050824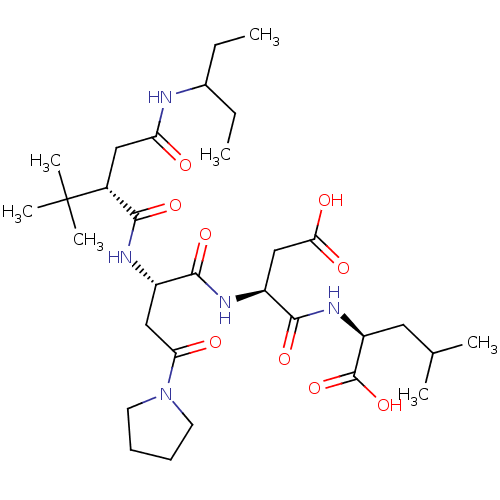 ((S)-2-[(S)-3-Carboxy-2-((S)-2-{(S)-2-[(1-ethyl-pro...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-Méga/Boehringer Ingelheim Research Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Herpes simplex virus (HSV) ribonucleotide reductase (RR) | J Med Chem 39: 2178-87 (1996) Article DOI: 10.1021/jm950825x BindingDB Entry DOI: 10.7270/Q2BG2N22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribonucleoside-diphosphate reductase large subunit (Human herpesvirus 1 (strain 17) (HHV-1) (Human her...) | BDBM50050828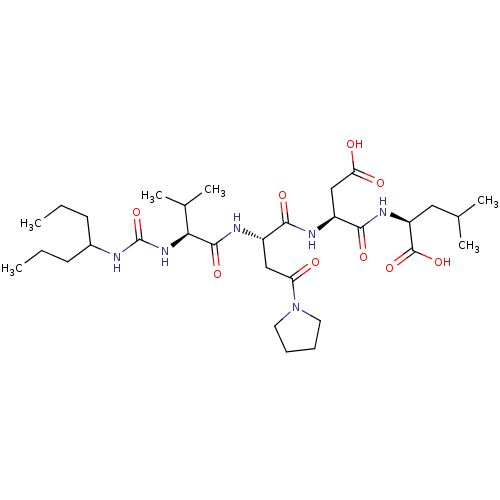 ((S)-2-[(S)-3-Carboxy-2-((S)-2-{(S)-3-methyl-2-[3-(...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-Méga/Boehringer Ingelheim Research Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Herpes simplex virus (HSV) ribonucleotide reductase (RR) | J Med Chem 39: 2178-87 (1996) Article DOI: 10.1021/jm950825x BindingDB Entry DOI: 10.7270/Q2BG2N22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM741 ((2S,4R)-N-tert-butyl-1-[(2R,3S)-3-[2-(2,6-dimethyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Cleavage products and substrate w... | J Med Chem 43: 1094-108 (2000) Article DOI: 10.1021/jm990336n BindingDB Entry DOI: 10.7270/Q2BZ647F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM519 ((2S)-N-[(2S,3R)-4-[(3S,4aS,8aS)-3-(tert-butylcarba...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Cleavage products and substrate w... | J Med Chem 43: 1094-108 (2000) Article DOI: 10.1021/jm990336n BindingDB Entry DOI: 10.7270/Q2BZ647F | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Ribonucleoside-diphosphate reductase large subunit (Human herpesvirus 1 (strain 17) (HHV-1) (Human her...) | BDBM50050822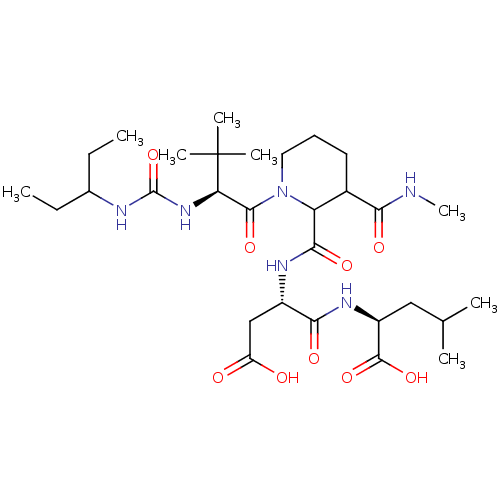 ((S)-2-{(S)-3-Carboxy-2-[(1-{(S)-2-[3-(1-ethyl-prop...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-Méga/Boehringer Ingelheim Research Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Herpes simplex virus (HSV) ribonucleotide reductase (RR) | J Med Chem 39: 2178-87 (1996) Article DOI: 10.1021/jm950825x BindingDB Entry DOI: 10.7270/Q2BG2N22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM743 ((2S,4R)-N-tert-butyl-1-[(2R,3S)-2-hydroxy-3-[(3-hy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Cleavage products and substrate w... | J Med Chem 43: 1094-108 (2000) Article DOI: 10.1021/jm990336n BindingDB Entry DOI: 10.7270/Q2BZ647F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribonucleoside-diphosphate reductase large subunit (Human herpesvirus 1 (strain 17) (HHV-1) (Human her...) | BDBM50050826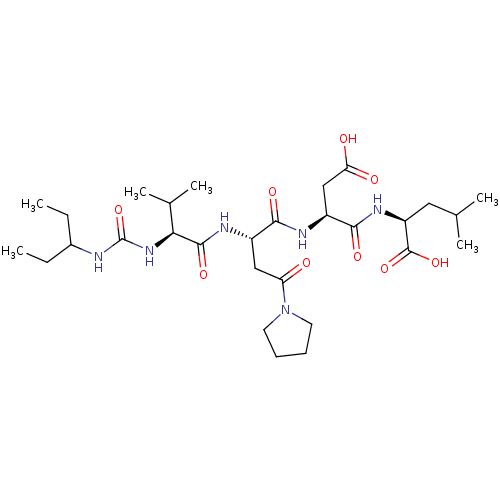 ((S)-2-[(S)-3-Carboxy-2-((S)-2-{(S)-2-[3-(1-ethyl-p...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-Méga/Boehringer Ingelheim Research Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Herpes simplex virus (HSV) ribonucleotide reductase (RR) | J Med Chem 39: 2178-87 (1996) Article DOI: 10.1021/jm950825x BindingDB Entry DOI: 10.7270/Q2BG2N22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM742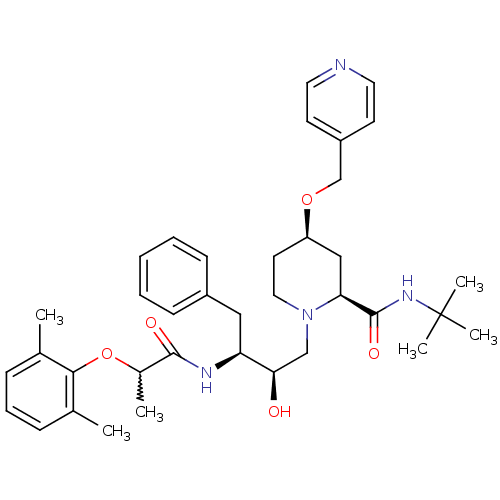 ((2S,4R)-N-tert-butyl-1-[(2R,3S)-3-[2-(2,6-dimethyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Cleavage products and substrate w... | J Med Chem 43: 1094-108 (2000) Article DOI: 10.1021/jm990336n BindingDB Entry DOI: 10.7270/Q2BZ647F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribonucleoside-diphosphate reductase large subunit (Human herpesvirus 1 (strain 17) (HHV-1) (Human her...) | BDBM50033462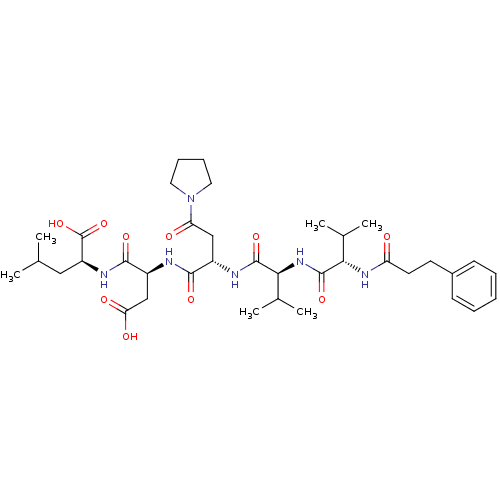 ((S)-2-[(S)-3-Carboxy-2-((S)-2-{(S)-3-methyl-2-[(S)...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-Méga/Boehringer Ingelheim Research Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Herpes simplex virus (HSV) ribonucleotide reductase (RR) | J Med Chem 39: 2178-87 (1996) Article DOI: 10.1021/jm950825x BindingDB Entry DOI: 10.7270/Q2BG2N22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribonucleoside-diphosphate reductase large subunit (Human herpesvirus 1 (strain 17) (HHV-1) (Human her...) | BDBM50050821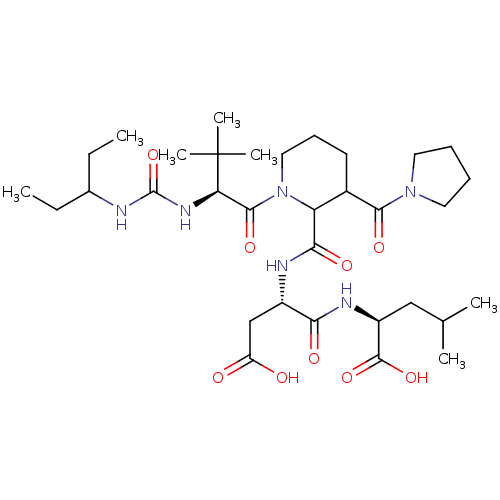 ((S)-2-((S)-3-Carboxy-2-{[1-{(S)-2-[3-(1-ethyl-prop...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-Méga/Boehringer Ingelheim Research Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Herpes simplex virus (HSV) ribonucleotide reductase (RR) | J Med Chem 39: 2178-87 (1996) Article DOI: 10.1021/jm950825x BindingDB Entry DOI: 10.7270/Q2BG2N22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribonucleoside-diphosphate reductase large subunit (Human herpesvirus 1 (strain 17) (HHV-1) (Human her...) | BDBM50050821 ((S)-2-((S)-3-Carboxy-2-{[1-{(S)-2-[3-(1-ethyl-prop...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-Méga/Boehringer Ingelheim Research Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Herpes simplex virus (HSV) ribonucleotide reductase (RR) | J Med Chem 39: 2178-87 (1996) Article DOI: 10.1021/jm950825x BindingDB Entry DOI: 10.7270/Q2BG2N22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribonucleoside-diphosphate reductase large subunit (Human herpesvirus 1 (strain 17) (HHV-1) (Human her...) | BDBM50050833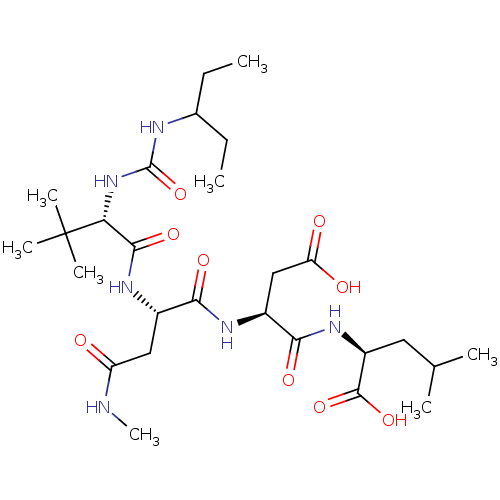 ((S)-2-[(S)-3-Carboxy-2-(2-{(S)-2-[3-(1-ethyl-propy...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-Méga/Boehringer Ingelheim Research Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Herpes simplex virus (HSV) ribonucleotide reductase (RR) | J Med Chem 39: 2178-87 (1996) Article DOI: 10.1021/jm950825x BindingDB Entry DOI: 10.7270/Q2BG2N22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribonucleoside-diphosphate reductase large subunit (Human herpesvirus 1 (strain 17) (HHV-1) (Human her...) | BDBM50050825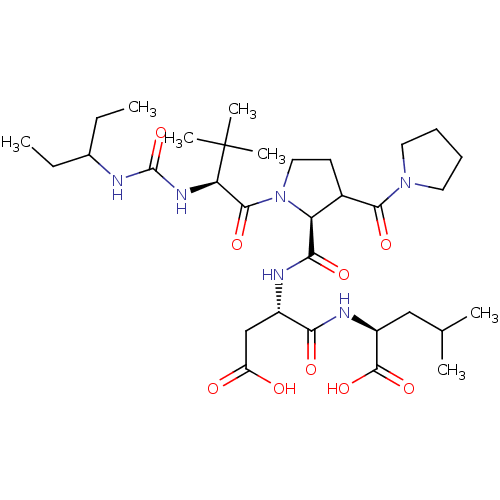 ((S)-2-((S)-3-Carboxy-2-{[1-{(S)-2-[3-(1-ethyl-prop...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 108 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-Méga/Boehringer Ingelheim Research Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Herpes simplex virus (HSV) ribonucleotide reductase (RR) | J Med Chem 39: 2178-87 (1996) Article DOI: 10.1021/jm950825x BindingDB Entry DOI: 10.7270/Q2BG2N22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM738 ((2S,4R)-N-tert-butyl-1-[(2R,3S)-2-hydroxy-3-[2-(2-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Cleavage products and substrate w... | J Med Chem 43: 1094-108 (2000) Article DOI: 10.1021/jm990336n BindingDB Entry DOI: 10.7270/Q2BZ647F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribonucleoside-diphosphate reductase large subunit (Human herpesvirus 1 (strain 17) (HHV-1) (Human her...) | BDBM50050834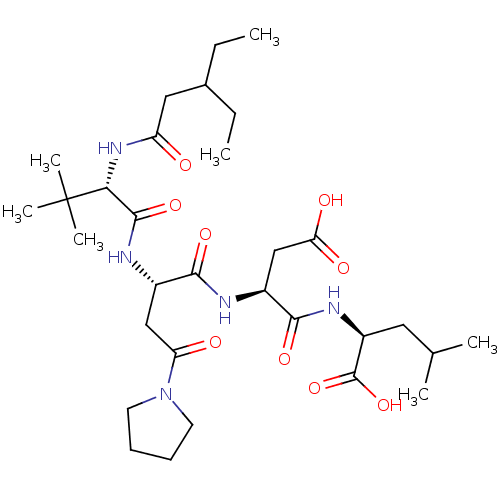 ((S)-2-((S)-3-Carboxy-2-{(S)-2-[(S)-2-(3-ethyl-pent...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-Méga/Boehringer Ingelheim Research Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Herpes simplex virus (HSV) ribonucleotide reductase (RR) | J Med Chem 39: 2178-87 (1996) Article DOI: 10.1021/jm950825x BindingDB Entry DOI: 10.7270/Q2BG2N22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM736 ((2-methylphenyl)methyl N-[(2S,3R)-4-[(2S,4R)-2-(te...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Cleavage products and substrate w... | J Med Chem 43: 1094-108 (2000) Article DOI: 10.1021/jm990336n BindingDB Entry DOI: 10.7270/Q2BZ647F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribonucleoside-diphosphate reductase large subunit (Human herpesvirus 1 (strain 17) (HHV-1) (Human her...) | BDBM50050835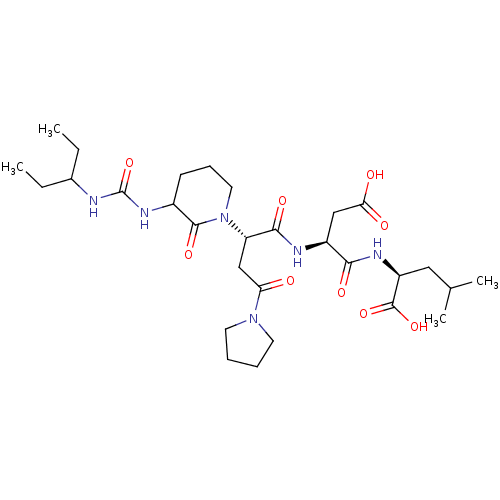 ((S)-2-[(S)-3-Carboxy-2-((S)-2-{3-[3-(1-ethyl-propy...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-Méga/Boehringer Ingelheim Research Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Herpes simplex virus (HSV) ribonucleotide reductase (RR) | J Med Chem 39: 2178-87 (1996) Article DOI: 10.1021/jm950825x BindingDB Entry DOI: 10.7270/Q2BG2N22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM744 ((3S)-oxolan-3-yl N-[(2S,3R)-4-[(2S,4R)-2-(tert-but...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Cleavage products and substrate w... | J Med Chem 43: 1094-108 (2000) Article DOI: 10.1021/jm990336n BindingDB Entry DOI: 10.7270/Q2BZ647F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM730 (Palinavir deriv. 2 | benzyl N-[(2S,3R)-4-[(2S,4R)-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Cleavage products and substrate w... | J Med Chem 43: 1094-108 (2000) Article DOI: 10.1021/jm990336n BindingDB Entry DOI: 10.7270/Q2BZ647F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM739 ((2,6-dimethylphenyl)methyl N-[(2S,3R)-4-[(2S,4R)-2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 420 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Cleavage products and substrate w... | J Med Chem 43: 1094-108 (2000) Article DOI: 10.1021/jm990336n BindingDB Entry DOI: 10.7270/Q2BZ647F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribonucleoside-diphosphate reductase large subunit (Human herpesvirus 1 (strain 17) (HHV-1) (Human her...) | BDBM50050829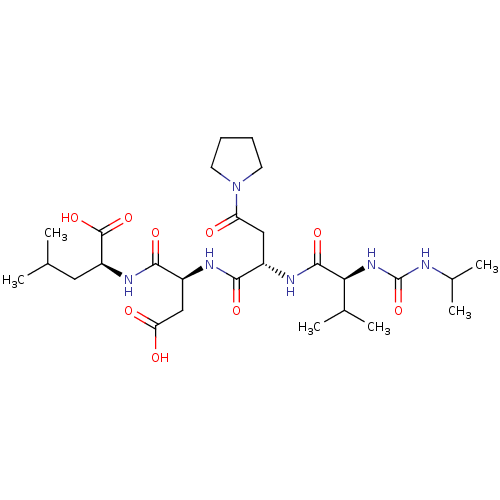 ((S)-2-((S)-3-Carboxy-2-{(S)-2-[(S)-2-(3-isopropyl-...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 435 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-Méga/Boehringer Ingelheim Research Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Herpes simplex virus (HSV) ribonucleotide reductase (RR) | J Med Chem 39: 2178-87 (1996) Article DOI: 10.1021/jm950825x BindingDB Entry DOI: 10.7270/Q2BG2N22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribonucleoside-diphosphate reductase large subunit (Human herpesvirus 1 (strain 17) (HHV-1) (Human her...) | BDBM50050830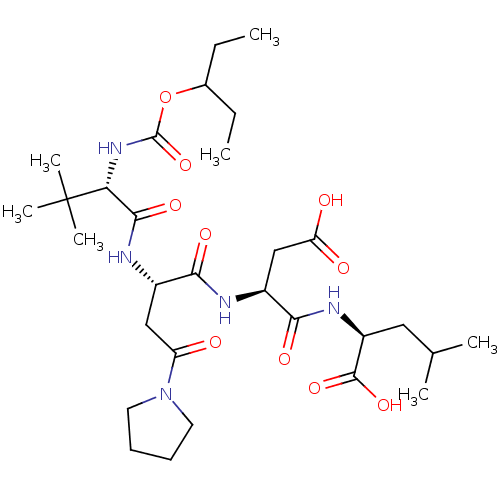 ((S)-2-((S)-3-Carboxy-2-{(S)-2-[(S)-2-(1-ethyl-prop...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 635 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-Méga/Boehringer Ingelheim Research Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Herpes simplex virus (HSV) ribonucleotide reductase (RR) | J Med Chem 39: 2178-87 (1996) Article DOI: 10.1021/jm950825x BindingDB Entry DOI: 10.7270/Q2BG2N22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribonucleoside-diphosphate reductase large subunit (Human herpesvirus 1 (strain 17) (HHV-1) (Human her...) | BDBM50050819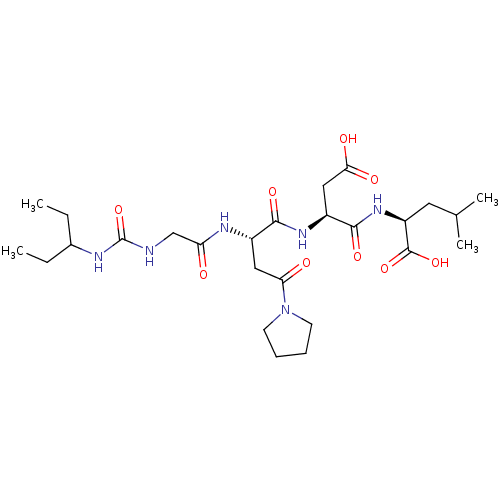 ((S)-2-[(S)-3-Carboxy-2-((S)-2-{2-[3-(1-ethyl-propy...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 670 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-Méga/Boehringer Ingelheim Research Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Herpes simplex virus (HSV) ribonucleotide reductase (RR) | J Med Chem 39: 2178-87 (1996) Article DOI: 10.1021/jm950825x BindingDB Entry DOI: 10.7270/Q2BG2N22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribonucleoside-diphosphate reductase large subunit (Human herpesvirus 1 (strain 17) (HHV-1) (Human her...) | BDBM50050837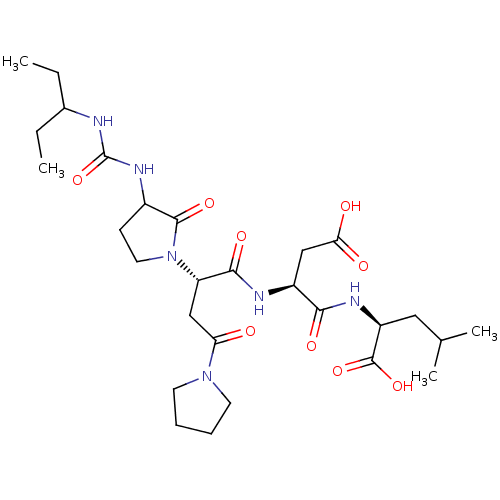 ((S)-2-[(S)-3-Carboxy-2-((S)-2-{3-[3-(1-ethyl-propy...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-Méga/Boehringer Ingelheim Research Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Herpes simplex virus (HSV) ribonucleotide reductase (RR) | J Med Chem 39: 2178-87 (1996) Article DOI: 10.1021/jm950825x BindingDB Entry DOI: 10.7270/Q2BG2N22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribonucleoside-diphosphate reductase large subunit (Human herpesvirus 1 (strain 17) (HHV-1) (Human her...) | BDBM50033460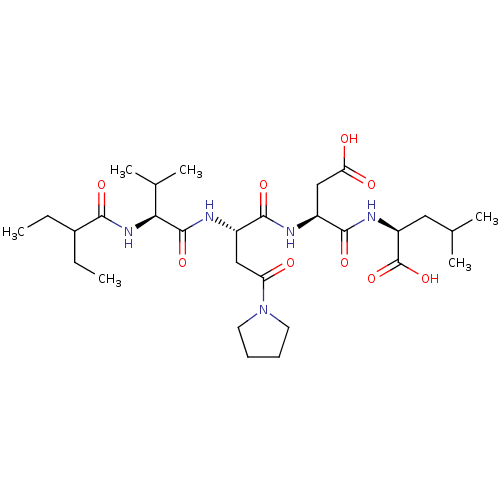 ((S)-2-((S)-3-Carboxy-2-{(S)-2-[(S)-2-(2-ethyl-buty...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 860 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-Méga/Boehringer Ingelheim Research Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Herpes simplex virus (HSV) ribonucleotide reductase (RR) | J Med Chem 39: 2178-87 (1996) Article DOI: 10.1021/jm950825x BindingDB Entry DOI: 10.7270/Q2BG2N22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribonucleoside-diphosphate reductase large subunit (Human herpesvirus 1 (strain 17) (HHV-1) (Human her...) | BDBM50033459 ((S)-2-((S)-2-{(S)-2-[(S)-2-((S)-2-Acetylamino-3-me...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 880 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-Méga/Boehringer Ingelheim Research Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Herpes simplex virus (HSV) ribonucleotide reductase (RR) | J Med Chem 39: 2178-87 (1996) Article DOI: 10.1021/jm950825x BindingDB Entry DOI: 10.7270/Q2BG2N22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM731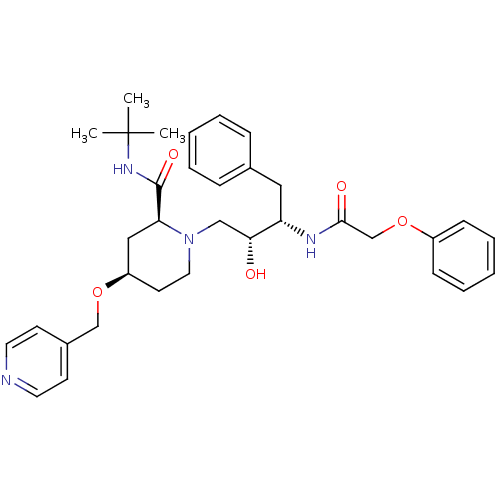 ((2S,4R)-N-tert-butyl-1-[(2R,3S)-2-hydroxy-3-(2-phe...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Cleavage products and substrate w... | J Med Chem 43: 1094-108 (2000) Article DOI: 10.1021/jm990336n BindingDB Entry DOI: 10.7270/Q2BZ647F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM732 ((2S,4R)-N-tert-butyl-1-[(2R,3S)-2-hydroxy-4-phenyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Cleavage products and substrate w... | J Med Chem 43: 1094-108 (2000) Article DOI: 10.1021/jm990336n BindingDB Entry DOI: 10.7270/Q2BZ647F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM740 ((2S,4R)-N-tert-butyl-1-[(2R,3S)-3-[3-(2,6-dimethyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Cleavage products and substrate w... | J Med Chem 43: 1094-108 (2000) Article DOI: 10.1021/jm990336n BindingDB Entry DOI: 10.7270/Q2BZ647F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM737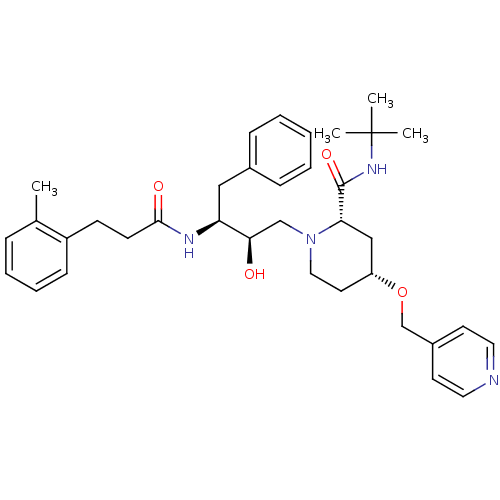 ((2S,4R)-N-tert-butyl-1-[(2R,3S)-2-hydroxy-3-[3-(2-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Cleavage products and substrate w... | J Med Chem 43: 1094-108 (2000) Article DOI: 10.1021/jm990336n BindingDB Entry DOI: 10.7270/Q2BZ647F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM733 ((2S,4R)-N-tert-butyl-1-[(2R,3S)-2-hydroxy-4-phenyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Cleavage products and substrate w... | J Med Chem 43: 1094-108 (2000) Article DOI: 10.1021/jm990336n BindingDB Entry DOI: 10.7270/Q2BZ647F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM735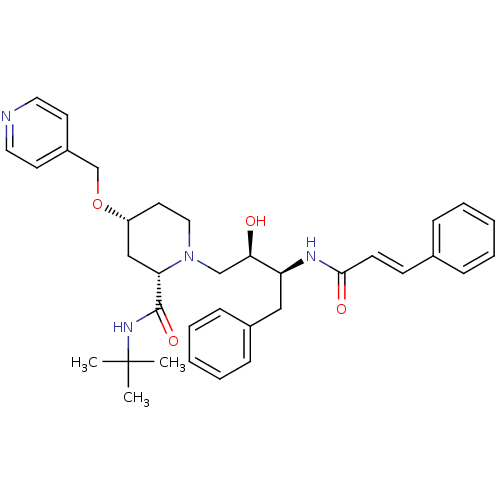 ((2S,4R)-N-tert-butyl-1-[(2R,3S)-2-hydroxy-4-phenyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Cleavage products and substrate w... | J Med Chem 43: 1094-108 (2000) Article DOI: 10.1021/jm990336n BindingDB Entry DOI: 10.7270/Q2BZ647F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM734 ((2S,4R)-N-tert-butyl-1-[(2R,3S)-2-hydroxy-4-phenyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Cleavage products and substrate w... | J Med Chem 43: 1094-108 (2000) Article DOI: 10.1021/jm990336n BindingDB Entry DOI: 10.7270/Q2BZ647F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribonucleoside-diphosphate reductase large subunit (Human herpesvirus 1 (strain 17) (HHV-1) (Human her...) | BDBM50050820 ((S)-2-[(S)-3-Carboxy-2-((S)-2-{2-[3-(1-ethyl-propy...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-Méga/Boehringer Ingelheim Research Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Herpes simplex virus (HSV) ribonucleotide reductase (RR) | J Med Chem 39: 2178-87 (1996) Article DOI: 10.1021/jm950825x BindingDB Entry DOI: 10.7270/Q2BG2N22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribonucleoside-diphosphate reductase large subunit (Human herpesvirus 1 (strain 17) (HHV-1) (Human her...) | BDBM50050827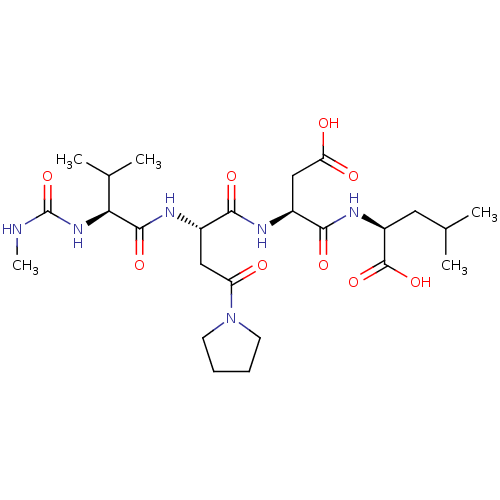 ((S)-2-((S)-3-Carboxy-2-{(S)-2-[(S)-3-methyl-2-(3-m...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-Méga/Boehringer Ingelheim Research Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Herpes simplex virus (HSV) ribonucleotide reductase (RR) | J Med Chem 39: 2178-87 (1996) Article DOI: 10.1021/jm950825x BindingDB Entry DOI: 10.7270/Q2BG2N22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribonucleoside-diphosphate reductase large subunit (Human herpesvirus 1 (strain 17) (HHV-1) (Human her...) | BDBM50050836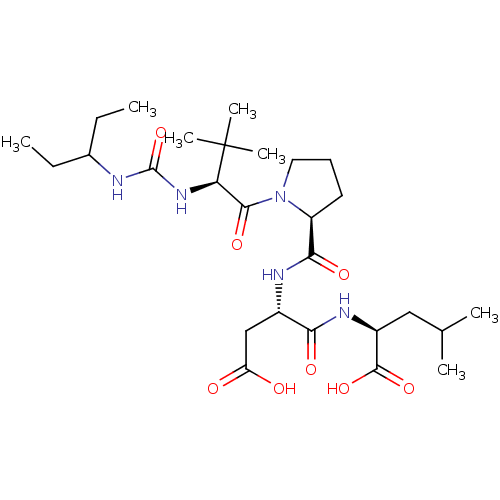 ((S)-2-{(S)-3-Carboxy-2-[(1-{(S)-2-[3-(1-ethyl-prop...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-Méga/Boehringer Ingelheim Research Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Herpes simplex virus (HSV) ribonucleotide reductase (RR) | J Med Chem 39: 2178-87 (1996) Article DOI: 10.1021/jm950825x BindingDB Entry DOI: 10.7270/Q2BG2N22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribonucleoside-diphosphate reductase large subunit (Human herpesvirus 1 (strain 17) (HHV-1) (Human her...) | BDBM50050832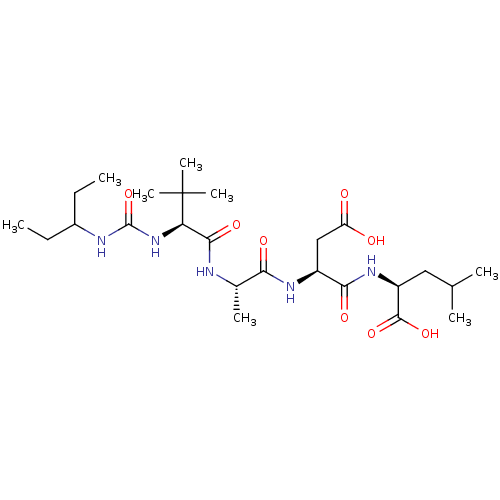 ((S)-2-[(S)-3-Carboxy-2-((S)-2-{(S)-2-[3-(1-ethyl-p...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-Méga/Boehringer Ingelheim Research Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Herpes simplex virus (HSV) ribonucleotide reductase (RR) | J Med Chem 39: 2178-87 (1996) Article DOI: 10.1021/jm950825x BindingDB Entry DOI: 10.7270/Q2BG2N22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 51 total ) | Next | Last >> |