

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8136 (N-(2,3-dihydro-1,4-benzodioxin-6-yl)-4-{pyrazolo[1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of human GSK3beta using biotinylated-aminohexyl-Ala-Ala-Ala-Lys-Arg-Arg-Glu-Ile-Leu-Ser-Arg-Arg-Pro-Ser(PO3)-Tyr-Arg-amide as substrate af... | J Med Chem 63: 756-783 (2020) Article DOI: 10.1021/acs.jmedchem.9b01741 BindingDB Entry DOI: 10.7270/Q2PR809G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8146 (N-(3,5-dimethylphenyl)-4-{pyrazolo[1,5-a]pyridazin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of human GSK3beta using biotinylated-aminohexyl-Ala-Ala-Ala-Lys-Arg-Arg-Glu-Ile-Leu-Ser-Arg-Arg-Pro-Ser(PO3)-Tyr-Arg-amide as substrate af... | J Med Chem 63: 756-783 (2020) Article DOI: 10.1021/acs.jmedchem.9b01741 BindingDB Entry DOI: 10.7270/Q2PR809G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM8128 (N-(3-methoxyphenyl)-4-{pyrazolo[1,5-a]pyridazin-3-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of human CDK2 using biotinylated-aminohexyl-Ala-Ala-Ala-Lys-Arg-Arg-Glu-Ile-Leu-Ser-Arg-Arg-Pro-Ser(PO3)-Tyr-Arg-amide as substrate after ... | J Med Chem 63: 756-783 (2020) Article DOI: 10.1021/acs.jmedchem.9b01741 BindingDB Entry DOI: 10.7270/Q2PR809G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM8137 (N-(3,4-difluorophenyl)-4-{pyrazolo[1,5-a]pyridazin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of human CDK2 using biotinylated-aminohexyl-Ala-Ala-Ala-Lys-Arg-Arg-Glu-Ile-Leu-Ser-Arg-Arg-Pro-Ser(PO3)-Tyr-Arg-amide as substrate after ... | J Med Chem 63: 756-783 (2020) Article DOI: 10.1021/acs.jmedchem.9b01741 BindingDB Entry DOI: 10.7270/Q2PR809G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM8146 (N-(3,5-dimethylphenyl)-4-{pyrazolo[1,5-a]pyridazin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of human CDK2 using biotinylated-aminohexyl-Ala-Ala-Ala-Lys-Arg-Arg-Glu-Ile-Leu-Ser-Arg-Arg-Pro-Ser(PO3)-Tyr-Arg-amide as substrate after ... | J Med Chem 63: 756-783 (2020) Article DOI: 10.1021/acs.jmedchem.9b01741 BindingDB Entry DOI: 10.7270/Q2PR809G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphodiesterase (Trypanosoma brucei) | BDBM50441529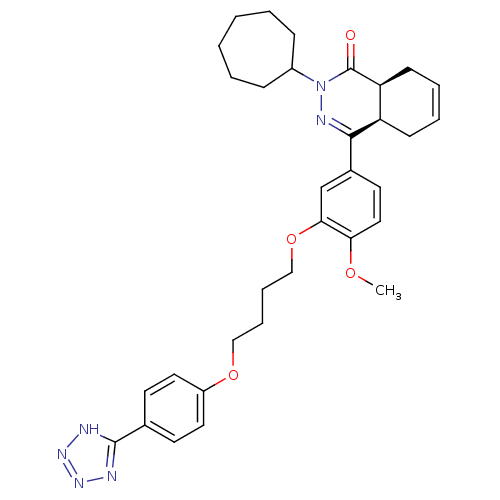 (CHEMBL2436771) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of Trypanosoma brucei PDEB1 | Bioorg Med Chem Lett 23: 5971-4 (2013) Article DOI: 10.1016/j.bmcl.2013.08.057 BindingDB Entry DOI: 10.7270/Q22B90GC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM92862 (US9284315, BEZ-235 | mTOR Inhibitor, BEZ235) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of human recombinant mTOR assessed as inhibition of 4EBP1 phosphorylation after 30 mins by TR-FRET analysis | J Med Chem 57: 4834-48 (2014) Article DOI: 10.1021/jm500361r BindingDB Entry DOI: 10.7270/Q2CJ8G1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM8126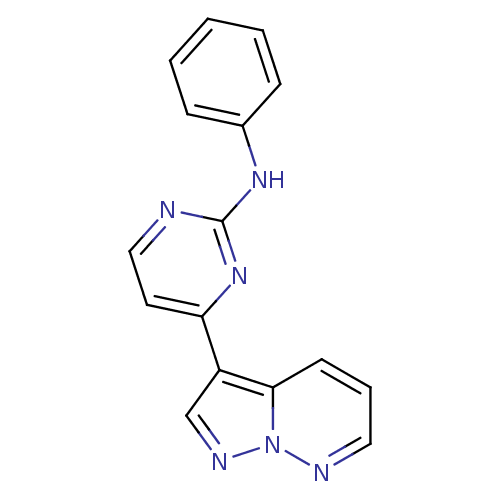 (N-phenyl-4-{pyrazolo[1,5-a]pyridazin-3-yl}pyrimidi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of human CDK2 using biotinylated-aminohexyl-Ala-Ala-Ala-Lys-Arg-Arg-Glu-Ile-Leu-Ser-Arg-Arg-Pro-Ser(PO3)-Tyr-Arg-amide as substrate after ... | J Med Chem 63: 756-783 (2020) Article DOI: 10.1021/acs.jmedchem.9b01741 BindingDB Entry DOI: 10.7270/Q2PR809G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM8136 (N-(2,3-dihydro-1,4-benzodioxin-6-yl)-4-{pyrazolo[1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of human CDK2 using biotinylated-aminohexyl-Ala-Ala-Ala-Lys-Arg-Arg-Glu-Ile-Leu-Ser-Arg-Arg-Pro-Ser(PO3)-Tyr-Arg-amide as substrate after ... | J Med Chem 63: 756-783 (2020) Article DOI: 10.1021/acs.jmedchem.9b01741 BindingDB Entry DOI: 10.7270/Q2PR809G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8137 (N-(3,4-difluorophenyl)-4-{pyrazolo[1,5-a]pyridazin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of human GSK3beta using biotinylated-aminohexyl-Ala-Ala-Ala-Lys-Arg-Arg-Glu-Ile-Leu-Ser-Arg-Arg-Pro-Ser(PO3)-Tyr-Arg-amide as substrate af... | J Med Chem 63: 756-783 (2020) Article DOI: 10.1021/acs.jmedchem.9b01741 BindingDB Entry DOI: 10.7270/Q2PR809G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8128 (N-(3-methoxyphenyl)-4-{pyrazolo[1,5-a]pyridazin-3-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of human GSK3beta using biotinylated-aminohexyl-Ala-Ala-Ala-Lys-Arg-Arg-Glu-Ile-Leu-Ser-Arg-Arg-Pro-Ser(PO3)-Tyr-Arg-amide as substrate af... | J Med Chem 63: 756-783 (2020) Article DOI: 10.1021/acs.jmedchem.9b01741 BindingDB Entry DOI: 10.7270/Q2PR809G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50506295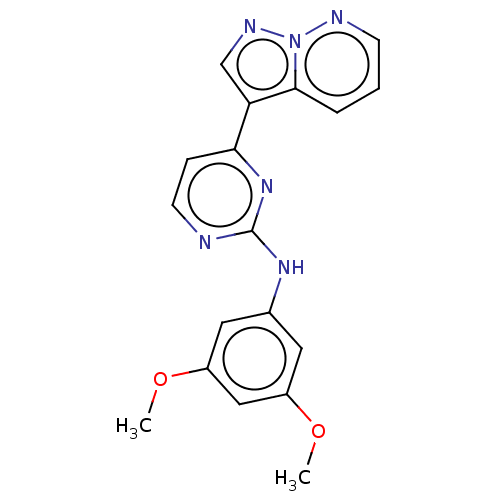 (GW801372X) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of human GSK3beta using biotinylated-aminohexyl-Ala-Ala-Ala-Lys-Arg-Arg-Glu-Ile-Leu-Ser-Arg-Arg-Pro-Ser(PO3)-Tyr-Arg-amide as substrate af... | J Med Chem 63: 756-783 (2020) Article DOI: 10.1021/acs.jmedchem.9b01741 BindingDB Entry DOI: 10.7270/Q2PR809G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM92862 (US9284315, BEZ-235 | mTOR Inhibitor, BEZ235) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of human PI3K-delta assessed as inhibition of Ptdlns(3,4,5)P3 phosphorylation after 1 hr by TR-FRET analysis | J Med Chem 57: 4834-48 (2014) Article DOI: 10.1021/jm500361r BindingDB Entry DOI: 10.7270/Q2CJ8G1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50506295 (GW801372X) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of human CDK2 using biotinylated-aminohexyl-Ala-Ala-Ala-Lys-Arg-Arg-Glu-Ile-Leu-Ser-Arg-Arg-Pro-Ser(PO3)-Tyr-Arg-amide as substrate after ... | J Med Chem 63: 756-783 (2020) Article DOI: 10.1021/acs.jmedchem.9b01741 BindingDB Entry DOI: 10.7270/Q2PR809G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM92862 (US9284315, BEZ-235 | mTOR Inhibitor, BEZ235) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of human PI3K-alpha assessed as inhibition of Ptdlns(3,4,5)P3 phosphorylation after 1 hr by TR-FRET analysis | J Med Chem 57: 4834-48 (2014) Article DOI: 10.1021/jm500361r BindingDB Entry DOI: 10.7270/Q2CJ8G1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4 (Homo sapiens (Human)) | BDBM50506295 (GW801372X) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of human CDK4 using biotinylated-aminohexyl-Ala-Ala-Ala-Lys-Arg-Arg-Glu-Ile-Leu-Ser-Arg-Arg-Pro-Ser(PO3)-Tyr-Arg-amide as substrate after ... | J Med Chem 63: 756-783 (2020) Article DOI: 10.1021/acs.jmedchem.9b01741 BindingDB Entry DOI: 10.7270/Q2PR809G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8126 (N-phenyl-4-{pyrazolo[1,5-a]pyridazin-3-yl}pyrimidi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of human GSK3beta using biotinylated-aminohexyl-Ala-Ala-Ala-Lys-Arg-Arg-Glu-Ile-Leu-Ser-Arg-Arg-Pro-Ser(PO3)-Tyr-Arg-amide as substrate af... | J Med Chem 63: 756-783 (2020) Article DOI: 10.1021/acs.jmedchem.9b01741 BindingDB Entry DOI: 10.7270/Q2PR809G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM50058062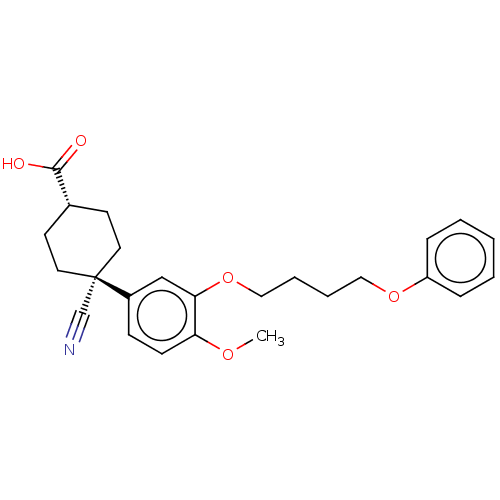 (CHEMBL3323128) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of human PDE4B expressed in Sf21 cells using cAMP substrate by scintillation proximity assay | Bioorg Med Chem Lett 24: 4084-9 (2014) Article DOI: 10.1016/j.bmcl.2014.07.063 BindingDB Entry DOI: 10.7270/Q26D5VNC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8197 (4-{6-methyl-2-phenylpyrazolo[1,5-a]pyridazin-3-yl}...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of human GSK3beta using biotinylated-aminohexyl-Ala-Ala-Ala-Lys-Arg-Arg-Glu-Ile-Leu-Ser-Arg-Arg-Pro-Ser(PO3)-Tyr-Arg-amide as substrate af... | J Med Chem 63: 756-783 (2020) Article DOI: 10.1021/acs.jmedchem.9b01741 BindingDB Entry DOI: 10.7270/Q2PR809G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8189 (N-(2,3-dihydro-1,4-benzodioxin-6-yl)-4-{2-[3-(trif...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of human GSK3beta using biotinylated-aminohexyl-Ala-Ala-Ala-Lys-Arg-Arg-Glu-Ile-Leu-Ser-Arg-Arg-Pro-Ser(PO3)-Tyr-Arg-amide as substrate af... | J Med Chem 63: 756-783 (2020) Article DOI: 10.1021/acs.jmedchem.9b01741 BindingDB Entry DOI: 10.7270/Q2PR809G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8133 (N-(4-tert-butylphenyl)-4-{pyrazolo[1,5-a]pyridazin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of human GSK3beta using biotinylated-aminohexyl-Ala-Ala-Ala-Lys-Arg-Arg-Glu-Ile-Leu-Ser-Arg-Arg-Pro-Ser(PO3)-Tyr-Arg-amide as substrate af... | J Med Chem 63: 756-783 (2020) Article DOI: 10.1021/acs.jmedchem.9b01741 BindingDB Entry DOI: 10.7270/Q2PR809G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8152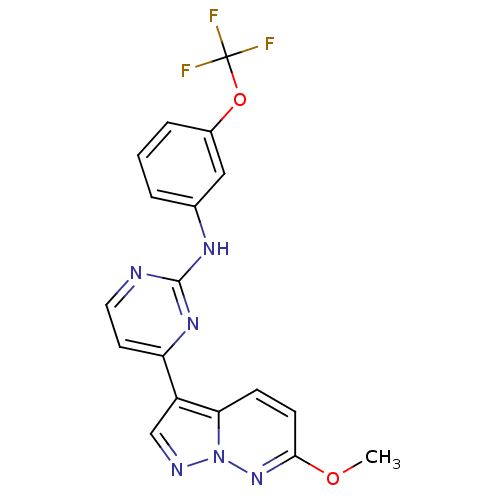 (4-{6-methoxypyrazolo[1,5-a]pyridazin-3-yl}-N-[3-(t...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of human GSK3beta using biotinylated-aminohexyl-Ala-Ala-Ala-Lys-Arg-Arg-Glu-Ile-Leu-Ser-Arg-Arg-Pro-Ser(PO3)-Tyr-Arg-amide as substrate af... | J Med Chem 63: 756-783 (2020) Article DOI: 10.1021/acs.jmedchem.9b01741 BindingDB Entry DOI: 10.7270/Q2PR809G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4 (Homo sapiens (Human)) | BDBM8128 (N-(3-methoxyphenyl)-4-{pyrazolo[1,5-a]pyridazin-3-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of human CDK4 using biotinylated-aminohexyl-Ala-Ala-Ala-Lys-Arg-Arg-Glu-Ile-Leu-Ser-Arg-Arg-Pro-Ser(PO3)-Tyr-Arg-amide as substrate after ... | J Med Chem 63: 756-783 (2020) Article DOI: 10.1021/acs.jmedchem.9b01741 BindingDB Entry DOI: 10.7270/Q2PR809G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM92862 (US9284315, BEZ-235 | mTOR Inhibitor, BEZ235) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of human PI3K-gamma assessed as inhibition of Ptdlns(3,4,5)P3 phosphorylation after 1 hr by TR-FRET analysis | J Med Chem 57: 4834-48 (2014) Article DOI: 10.1021/jm500361r BindingDB Entry DOI: 10.7270/Q2CJ8G1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM50018265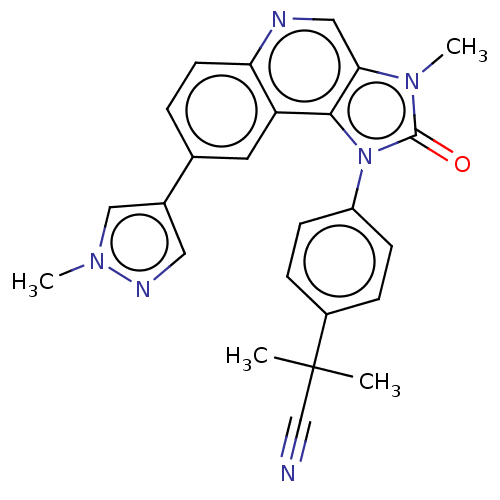 (CHEMBL3290293) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 126 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of human PI3K-gamma assessed as inhibition of Ptdlns(3,4,5)P3 phosphorylation after 1 hr by TR-FRET analysis | J Med Chem 57: 4834-48 (2014) Article DOI: 10.1021/jm500361r BindingDB Entry DOI: 10.7270/Q2CJ8G1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4 (Homo sapiens (Human)) | BDBM8126 (N-phenyl-4-{pyrazolo[1,5-a]pyridazin-3-yl}pyrimidi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 158 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of human CDK4 using biotinylated-aminohexyl-Ala-Ala-Ala-Lys-Arg-Arg-Glu-Ile-Leu-Ser-Arg-Arg-Pro-Ser(PO3)-Tyr-Arg-amide as substrate after ... | J Med Chem 63: 756-783 (2020) Article DOI: 10.1021/acs.jmedchem.9b01741 BindingDB Entry DOI: 10.7270/Q2PR809G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM8133 (N-(4-tert-butylphenyl)-4-{pyrazolo[1,5-a]pyridazin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 158 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of human CDK2 using biotinylated-aminohexyl-Ala-Ala-Ala-Lys-Arg-Arg-Glu-Ile-Leu-Ser-Arg-Arg-Pro-Ser(PO3)-Tyr-Arg-amide as substrate after ... | J Med Chem 63: 756-783 (2020) Article DOI: 10.1021/acs.jmedchem.9b01741 BindingDB Entry DOI: 10.7270/Q2PR809G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM8152 (4-{6-methoxypyrazolo[1,5-a]pyridazin-3-yl}-N-[3-(t...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of human CDK2 using biotinylated-aminohexyl-Ala-Ala-Ala-Lys-Arg-Arg-Glu-Ile-Leu-Ser-Arg-Arg-Pro-Ser(PO3)-Tyr-Arg-amide as substrate after ... | J Med Chem 63: 756-783 (2020) Article DOI: 10.1021/acs.jmedchem.9b01741 BindingDB Entry DOI: 10.7270/Q2PR809G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM50018267 (CHEMBL3290305) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 251 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of human PI3K-gamma assessed as inhibition of Ptdlns(3,4,5)P3 phosphorylation after 1 hr by TR-FRET analysis | J Med Chem 57: 4834-48 (2014) Article DOI: 10.1021/jm500361r BindingDB Entry DOI: 10.7270/Q2CJ8G1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphodiesterase (Trypanosoma brucei) | BDBM50441528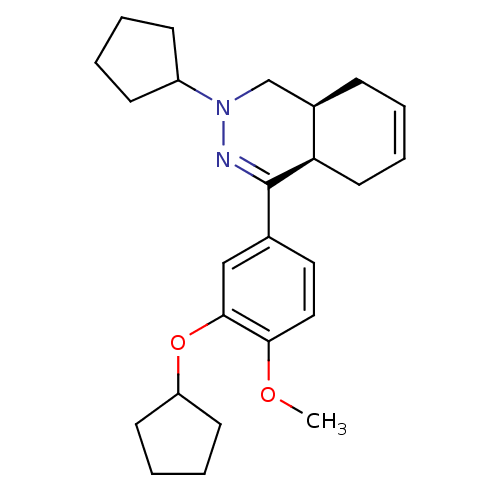 (CHEMBL2436772) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 278 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of Trypanosoma brucei PDEB1 | Bioorg Med Chem Lett 23: 5971-4 (2013) Article DOI: 10.1016/j.bmcl.2013.08.057 BindingDB Entry DOI: 10.7270/Q22B90GC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM50018268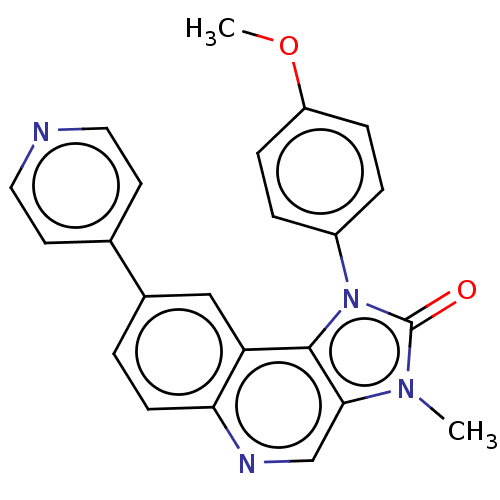 (CHEMBL3290307) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 316 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of human PI3K-gamma assessed as inhibition of Ptdlns(3,4,5)P3 phosphorylation after 1 hr by TR-FRET analysis | J Med Chem 57: 4834-48 (2014) Article DOI: 10.1021/jm500361r BindingDB Entry DOI: 10.7270/Q2CJ8G1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform (Homo sapiens (Human)) | BDBM92862 (US9284315, BEZ-235 | mTOR Inhibitor, BEZ235) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 316 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of human PI3K-beta assessed as inhibition of Ptdlns(3,4,5)P3 phosphorylation after 1 hr by TR-FRET analysis | J Med Chem 57: 4834-48 (2014) Article DOI: 10.1021/jm500361r BindingDB Entry DOI: 10.7270/Q2CJ8G1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4 (Homo sapiens (Human)) | BDBM8136 (N-(2,3-dihydro-1,4-benzodioxin-6-yl)-4-{pyrazolo[1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 316 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of human CDK4 using biotinylated-aminohexyl-Ala-Ala-Ala-Lys-Arg-Arg-Glu-Ile-Leu-Ser-Arg-Arg-Pro-Ser(PO3)-Tyr-Arg-amide as substrate after ... | J Med Chem 63: 756-783 (2020) Article DOI: 10.1021/acs.jmedchem.9b01741 BindingDB Entry DOI: 10.7270/Q2PR809G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM50018268 (CHEMBL3290307) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of human recombinant mTOR assessed as inhibition of 4EBP1 phosphorylation after 30 mins by TR-FRET analysis | J Med Chem 57: 4834-48 (2014) Article DOI: 10.1021/jm500361r BindingDB Entry DOI: 10.7270/Q2CJ8G1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM50018265 (CHEMBL3290293) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 398 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of human PI3K-alpha assessed as inhibition of Ptdlns(3,4,5)P3 phosphorylation after 1 hr by TR-FRET analysis | J Med Chem 57: 4834-48 (2014) Article DOI: 10.1021/jm500361r BindingDB Entry DOI: 10.7270/Q2CJ8G1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM50018267 (CHEMBL3290305) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 468 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of human recombinant mTOR assessed as inhibition of 4EBP1 phosphorylation after 30 mins by TR-FRET analysis | J Med Chem 57: 4834-48 (2014) Article DOI: 10.1021/jm500361r BindingDB Entry DOI: 10.7270/Q2CJ8G1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM50018268 (CHEMBL3290307) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 501 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of human PI3K-alpha assessed as inhibition of Ptdlns(3,4,5)P3 phosphorylation after 1 hr by TR-FRET analysis | J Med Chem 57: 4834-48 (2014) Article DOI: 10.1021/jm500361r BindingDB Entry DOI: 10.7270/Q2CJ8G1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM50018267 (CHEMBL3290305) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 631 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of human PI3K-alpha assessed as inhibition of Ptdlns(3,4,5)P3 phosphorylation after 1 hr by TR-FRET analysis | J Med Chem 57: 4834-48 (2014) Article DOI: 10.1021/jm500361r BindingDB Entry DOI: 10.7270/Q2CJ8G1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM50018265 (CHEMBL3290293) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 794 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of human PI3K-delta assessed as inhibition of Ptdlns(3,4,5)P3 phosphorylation after 1 hr by TR-FRET analysis | J Med Chem 57: 4834-48 (2014) Article DOI: 10.1021/jm500361r BindingDB Entry DOI: 10.7270/Q2CJ8G1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphodiesterase (Trypanosoma brucei) | BDBM50058062 (CHEMBL3323128) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 950 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of Trypanosoma brucei recombinant PDEB1 pre-incubated for 5 mins prior to substrate addition | Bioorg Med Chem Lett 24: 4084-9 (2014) Article DOI: 10.1016/j.bmcl.2014.07.063 BindingDB Entry DOI: 10.7270/Q26D5VNC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM50018265 (CHEMBL3290293) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of human recombinant mTOR assessed as inhibition of 4EBP1 phosphorylation after 30 mins by TR-FRET analysis | J Med Chem 57: 4834-48 (2014) Article DOI: 10.1021/jm500361r BindingDB Entry DOI: 10.7270/Q2CJ8G1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4 (Homo sapiens (Human)) | BDBM8146 (N-(3,5-dimethylphenyl)-4-{pyrazolo[1,5-a]pyridazin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of human CDK4 using biotinylated-aminohexyl-Ala-Ala-Ala-Lys-Arg-Arg-Glu-Ile-Leu-Ser-Arg-Arg-Pro-Ser(PO3)-Tyr-Arg-amide as substrate after ... | J Med Chem 63: 756-783 (2020) Article DOI: 10.1021/acs.jmedchem.9b01741 BindingDB Entry DOI: 10.7270/Q2PR809G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM50018266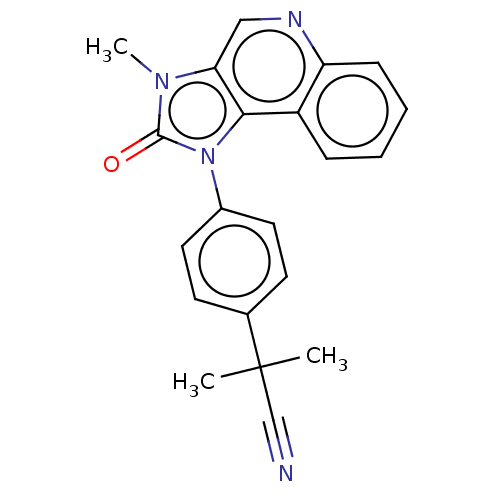 (CHEMBL3290300) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of human recombinant mTOR assessed as inhibition of 4EBP1 phosphorylation after 30 mins by TR-FRET analysis | J Med Chem 57: 4834-48 (2014) Article DOI: 10.1021/jm500361r BindingDB Entry DOI: 10.7270/Q2CJ8G1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4 (Homo sapiens (Human)) | BDBM8133 (N-(4-tert-butylphenyl)-4-{pyrazolo[1,5-a]pyridazin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of human CDK4 using biotinylated-aminohexyl-Ala-Ala-Ala-Lys-Arg-Arg-Glu-Ile-Leu-Ser-Arg-Arg-Pro-Ser(PO3)-Tyr-Arg-amide as substrate after ... | J Med Chem 63: 756-783 (2020) Article DOI: 10.1021/acs.jmedchem.9b01741 BindingDB Entry DOI: 10.7270/Q2PR809G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM50018266 (CHEMBL3290300) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of human PI3K-gamma assessed as inhibition of Ptdlns(3,4,5)P3 phosphorylation after 1 hr by TR-FRET analysis | J Med Chem 57: 4834-48 (2014) Article DOI: 10.1021/jm500361r BindingDB Entry DOI: 10.7270/Q2CJ8G1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM50018267 (CHEMBL3290305) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of human PI3K-delta assessed as inhibition of Ptdlns(3,4,5)P3 phosphorylation after 1 hr by TR-FRET analysis | J Med Chem 57: 4834-48 (2014) Article DOI: 10.1021/jm500361r BindingDB Entry DOI: 10.7270/Q2CJ8G1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM50018268 (CHEMBL3290307) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of human PI3K-delta assessed as inhibition of Ptdlns(3,4,5)P3 phosphorylation after 1 hr by TR-FRET analysis | J Med Chem 57: 4834-48 (2014) Article DOI: 10.1021/jm500361r BindingDB Entry DOI: 10.7270/Q2CJ8G1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphodiesterase (Trypanosoma brucei) | BDBM153391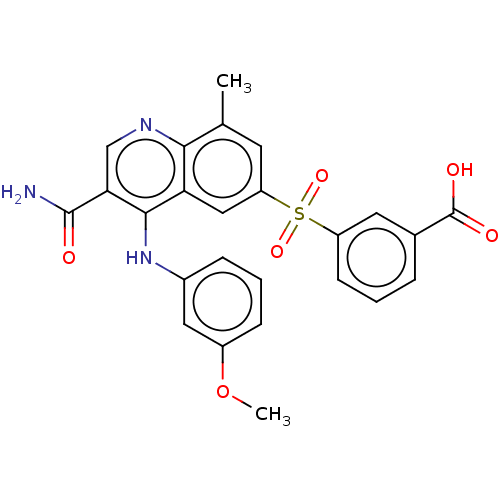 (3-(3-carbamoyl-4-(3-methoxyphenylamino)-8-methylqu...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Northeastern University | Assay Description In short, recombinant TbrPDEB1 and TbrPDEB2 were assayed in 10 mM Tris pH 7.4, bovine serum albumin (0.2 mg/mL), 10 mM MgCl2, 25ÁM cAMP (Enzo Lifesci... | Chem Biol Drug Des 85: 549-64 (2015) Article DOI: 10.1111/cbdd.12443 BindingDB Entry DOI: 10.7270/Q2FF3R28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphodiesterase (Trypanosoma brucei) | BDBM153402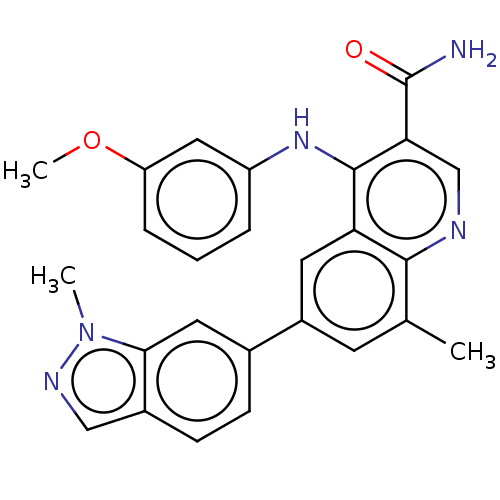 (4-((3-Methoxyphenyl)amino)-8-methyl-6-(1-methyl-1H...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Northeastern University | Assay Description In short, recombinant TbrPDEB1 and TbrPDEB2 were assayed in 10 mM Tris pH 7.4, bovine serum albumin (0.2 mg/mL), 10 mM MgCl2, 25ÁM cAMP (Enzo Lifesci... | Chem Biol Drug Des 85: 549-64 (2015) Article DOI: 10.1111/cbdd.12443 BindingDB Entry DOI: 10.7270/Q2FF3R28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphodiesterase (Trypanosoma brucei) | BDBM153408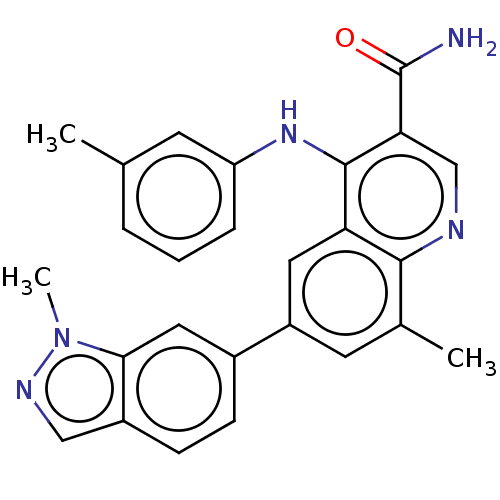 (8-Methyl-6-(1-methyl-1H-indazol-6-yl)-4-(m-tolylam...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Northeastern University | Assay Description In short, recombinant TbrPDEB1 and TbrPDEB2 were assayed in 10 mM Tris pH 7.4, bovine serum albumin (0.2 mg/mL), 10 mM MgCl2, 25ÁM cAMP (Enzo Lifesci... | Chem Biol Drug Des 85: 549-64 (2015) Article DOI: 10.1111/cbdd.12443 BindingDB Entry DOI: 10.7270/Q2FF3R28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 99 total ) | Next | Last >> |