 Found 177 hits with Last Name = 'pommery' and Initial = 'n'
Found 177 hits with Last Name = 'pommery' and Initial = 'n' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50477671
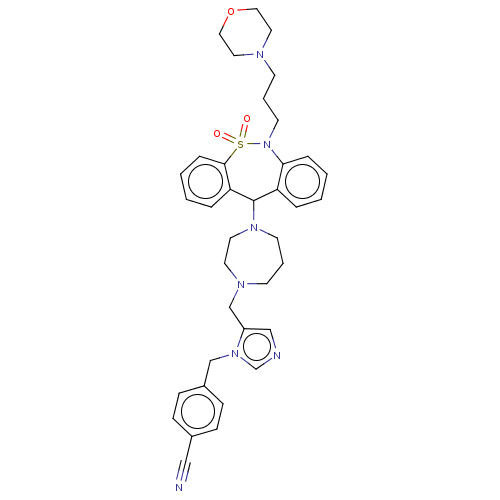
(CHEMBL250092)Show SMILES O=S1(=O)N(CCCN2CCOCC2)c2ccccc2C(N2CCCN(Cc3cncn3Cc3ccc(cc3)C#N)CC2)c2ccccc12 Show InChI InChI=1S/C37H43N7O3S/c38-25-30-11-13-31(14-12-30)27-43-29-39-26-32(43)28-41-16-5-17-42(20-19-41)37-33-7-1-3-9-35(33)44(18-6-15-40-21-23-47-24-22-40)48(45,46)36-10-4-2-8-34(36)37/h1-4,7-14,26,29,37H,5-6,15-24,27-28H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lille 2
Curated by ChEMBL
| Assay Description
Inhibition of FTase by fluorescence spectrometry technique |
Bioorg Med Chem Lett 17: 5465-71 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.002
BindingDB Entry DOI: 10.7270/Q2154KTZ |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50000829
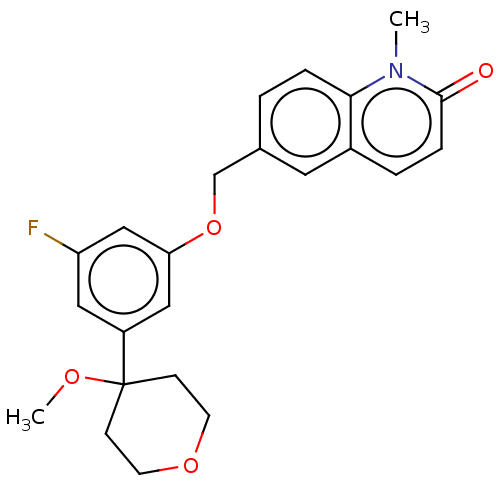
(6-((3-fluoro-5-(4-methoxytetrahydro-2H-pyran-4-yl)...)Show SMILES COC1(CCOCC1)c1cc(F)cc(OCc2ccc3n(C)c(=O)ccc3c2)c1 Show InChI InChI=1S/C23H24FNO4/c1-25-21-5-3-16(11-17(21)4-6-22(25)26)15-29-20-13-18(12-19(24)14-20)23(27-2)7-9-28-10-8-23/h3-6,11-14H,7-10,15H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Lille-Nord de France
Curated by ChEMBL
| Assay Description
Inhibition of 5-lipoxygenase in human whole blood |
Bioorg Med Chem 18: 3910-24 (2010)
Article DOI: 10.1016/j.bmc.2010.04.034
BindingDB Entry DOI: 10.7270/Q28K7B16 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50477675
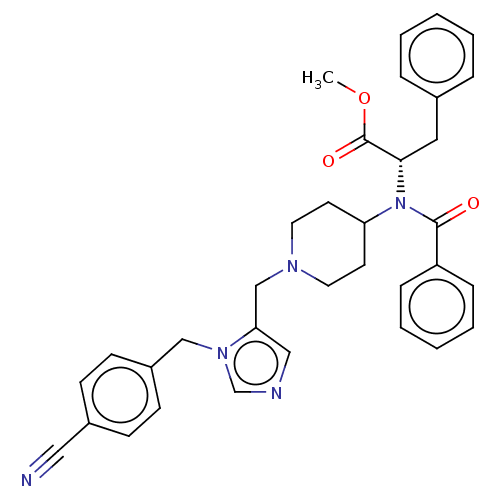
(CHEMBL376337)Show SMILES COC(=O)[C@H](Cc1ccccc1)N(C1CCN(Cc2cncn2Cc2ccc(cc2)C#N)CC1)C(=O)c1ccccc1 |r| Show InChI InChI=1S/C34H35N5O3/c1-42-34(41)32(20-26-8-4-2-5-9-26)39(33(40)29-10-6-3-7-11-29)30-16-18-37(19-17-30)24-31-22-36-25-38(31)23-28-14-12-27(21-35)13-15-28/h2-15,22,25,30,32H,16-20,23-24H2,1H3/t32-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lille 2
Curated by ChEMBL
| Assay Description
Inhibition of FTase by fluorescence spectrometry technique |
Bioorg Med Chem Lett 17: 5465-71 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.002
BindingDB Entry DOI: 10.7270/Q2154KTZ |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50477678
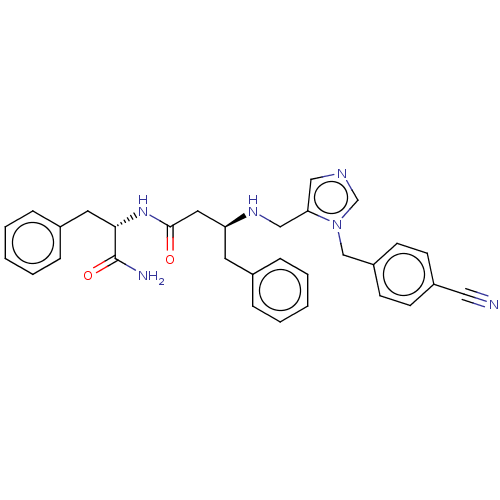
(CHEMBL251295)Show SMILES NC(=O)[C@H](Cc1ccccc1)NC(=O)C[C@H](Cc1ccccc1)NCc1cncn1Cc1ccc(cc1)C#N Show InChI InChI=1S/C31H32N6O2/c32-18-25-11-13-26(14-12-25)21-37-22-34-19-28(37)20-35-27(15-23-7-3-1-4-8-23)17-30(38)36-29(31(33)39)16-24-9-5-2-6-10-24/h1-14,19,22,27,29,35H,15-17,20-21H2,(H2,33,39)(H,36,38)/t27-,29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lille 2
Curated by ChEMBL
| Assay Description
Inhibition of FTase by fluorescence spectrometry technique |
Bioorg Med Chem Lett 17: 5465-71 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.002
BindingDB Entry DOI: 10.7270/Q2154KTZ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50156581
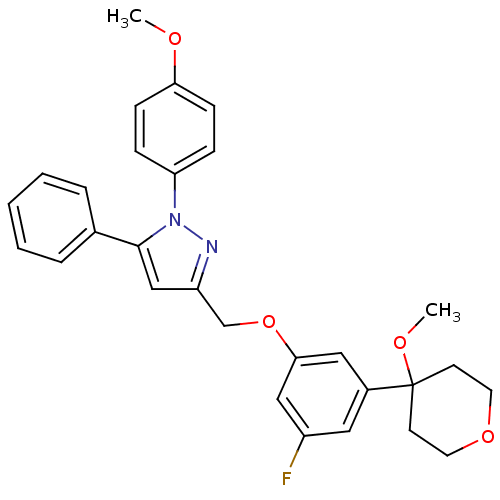
(3-[3-fluoro-5-(4-methoxytetrahydropyran-4-yl)pheno...)Show SMILES COc1ccc(cc1)-n1nc(COc2cc(F)cc(c2)C2(CCOCC2)OC)cc1-c1ccccc1 Show InChI InChI=1S/C29H29FN2O4/c1-33-26-10-8-25(9-11-26)32-28(21-6-4-3-5-7-21)19-24(31-32)20-36-27-17-22(16-23(30)18-27)29(34-2)12-14-35-15-13-29/h3-11,16-19H,12-15,20H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille 2
Curated by ChEMBL
| Assay Description
Inhibition of COX1 expressed in CHO cells assessed as inhibition of arachidonic acid-stimulated PGE2 production by enzyme immunoassay |
J Med Chem 47: 6195-206 (2004)
Article DOI: 10.1021/jm0407761
BindingDB Entry DOI: 10.7270/Q2DR2V01 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille 2
Curated by ChEMBL
| Assay Description
Inhibition of COX2 expressed in CHO cells assessed as inhibition of arachidonic acid-stimulated PGE2 production by enzyme immunoassay |
J Med Chem 47: 6195-206 (2004)
Article DOI: 10.1021/jm0407761
BindingDB Entry DOI: 10.7270/Q2DR2V01 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50477674
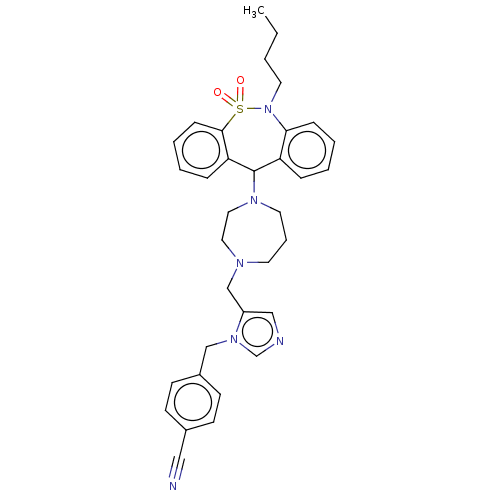
(CHEMBL400918)Show SMILES CCCCN1c2ccccc2C(N2CCCN(Cc3cncn3Cc3ccc(cc3)C#N)CC2)c2ccccc2S1(=O)=O Show InChI InChI=1S/C34H38N6O2S/c1-2-3-19-40-32-11-6-4-9-30(32)34(31-10-5-7-12-33(31)43(40,41)42)38-18-8-17-37(20-21-38)25-29-23-36-26-39(29)24-28-15-13-27(22-35)14-16-28/h4-7,9-16,23,26,34H,2-3,8,17-21,24-25H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lille 2
Curated by ChEMBL
| Assay Description
Inhibition of FTase by fluorescence spectrometry technique |
Bioorg Med Chem Lett 17: 5465-71 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.002
BindingDB Entry DOI: 10.7270/Q2154KTZ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50110484

(3-((3-fluoro-5-(1-methoxycyclohexyl)phenoxy)methyl...)Show SMILES COC1(CCOCC1)c1cc(F)cc(OCc2cc(-c3ccccc3)n(n2)-c2ccc(cc2)S(C)(=O)=O)c1 Show InChI InChI=1S/C29H29FN2O5S/c1-35-29(12-14-36-15-13-29)22-16-23(30)18-26(17-22)37-20-24-19-28(21-6-4-3-5-7-21)32(31-24)25-8-10-27(11-9-25)38(2,33)34/h3-11,16-19H,12-15,20H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille 2
Curated by ChEMBL
| Assay Description
Inhibition of COX2 expressed in CHO cells assessed as inhibition of arachidonic acid-stimulated PGE2 production by enzyme immunoassay |
J Med Chem 47: 6195-206 (2004)
Article DOI: 10.1021/jm0407761
BindingDB Entry DOI: 10.7270/Q2DR2V01 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50477670
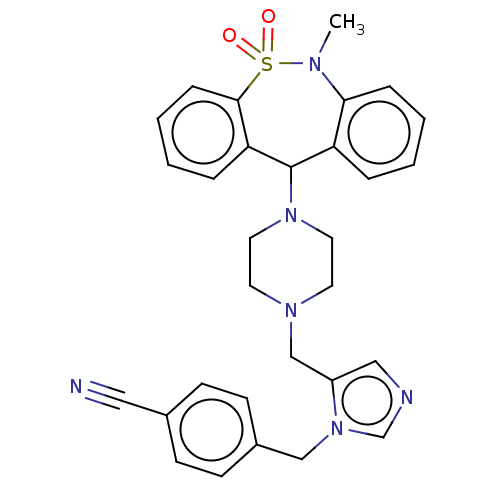
(CHEMBL400385)Show SMILES CN1c2ccccc2C(N2CCN(Cc3cncn3Cc3ccc(cc3)C#N)CC2)c2ccccc2S1(=O)=O Show InChI InChI=1S/C30H30N6O2S/c1-33-28-8-4-2-6-26(28)30(27-7-3-5-9-29(27)39(33,37)38)35-16-14-34(15-17-35)21-25-19-32-22-36(25)20-24-12-10-23(18-31)11-13-24/h2-13,19,22,30H,14-17,20-21H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lille 2
Curated by ChEMBL
| Assay Description
Inhibition of FTase by fluorescence spectrometry technique |
Bioorg Med Chem Lett 17: 5465-71 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.002
BindingDB Entry DOI: 10.7270/Q2154KTZ |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50477676
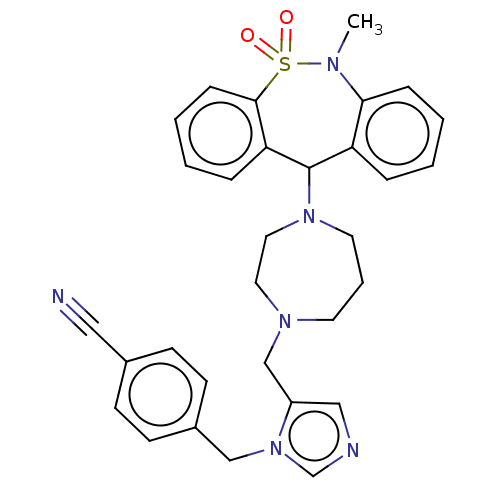
(CHEMBL251503)Show SMILES CN1c2ccccc2C(N2CCCN(Cc3cncn3Cc3ccc(cc3)C#N)CC2)c2ccccc2S1(=O)=O Show InChI InChI=1S/C31H32N6O2S/c1-34-29-9-4-2-7-27(29)31(28-8-3-5-10-30(28)40(34,38)39)36-16-6-15-35(17-18-36)22-26-20-33-23-37(26)21-25-13-11-24(19-32)12-14-25/h2-5,7-14,20,23,31H,6,15-18,21-22H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lille 2
Curated by ChEMBL
| Assay Description
Inhibition of FTase by fluorescence spectrometry technique |
Bioorg Med Chem Lett 17: 5465-71 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.002
BindingDB Entry DOI: 10.7270/Q2154KTZ |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50477673
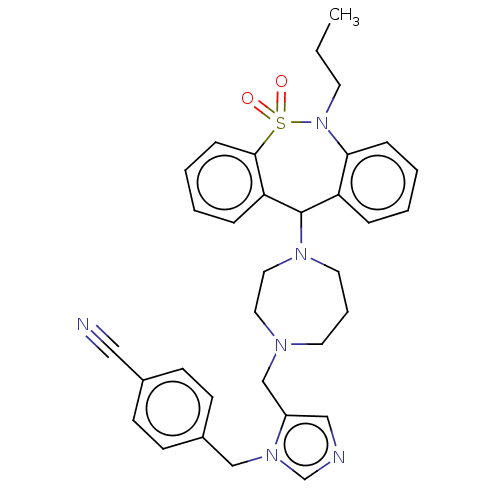
(CHEMBL400415)Show SMILES CCCN1c2ccccc2C(N2CCCN(Cc3cncn3Cc3ccc(cc3)C#N)CC2)c2ccccc2S1(=O)=O Show InChI InChI=1S/C33H36N6O2S/c1-2-16-39-31-10-5-3-8-29(31)33(30-9-4-6-11-32(30)42(39,40)41)37-18-7-17-36(19-20-37)24-28-22-35-25-38(28)23-27-14-12-26(21-34)13-15-27/h3-6,8-15,22,25,33H,2,7,16-20,23-24H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lille 2
Curated by ChEMBL
| Assay Description
Inhibition of FTase by fluorescence spectrometry technique |
Bioorg Med Chem Lett 17: 5465-71 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.002
BindingDB Entry DOI: 10.7270/Q2154KTZ |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50140147
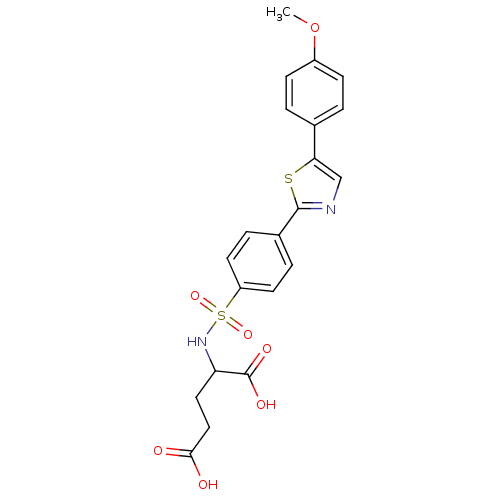
(2-{4-[5-(4-Methoxy-phenyl)-thiazol-2-yl]-benzenesu...)Show SMILES COc1ccc(cc1)-c1cnc(s1)-c1ccc(cc1)S(=O)(=O)NC(CCC(O)=O)C(O)=O Show InChI InChI=1S/C21H20N2O7S2/c1-30-15-6-2-13(3-7-15)18-12-22-20(31-18)14-4-8-16(9-5-14)32(28,29)23-17(21(26)27)10-11-19(24)25/h2-9,12,17,23H,10-11H2,1H3,(H,24,25)(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ des Sciences et Technologies de Lille
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloprotease-9 |
Bioorg Med Chem Lett 14: 1119-21 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.076
BindingDB Entry DOI: 10.7270/Q20002NZ |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50140147

(2-{4-[5-(4-Methoxy-phenyl)-thiazol-2-yl]-benzenesu...)Show SMILES COc1ccc(cc1)-c1cnc(s1)-c1ccc(cc1)S(=O)(=O)NC(CCC(O)=O)C(O)=O Show InChI InChI=1S/C21H20N2O7S2/c1-30-15-6-2-13(3-7-15)18-12-22-20(31-18)14-4-8-16(9-5-14)32(28,29)23-17(21(26)27)10-11-19(24)25/h2-9,12,17,23H,10-11H2,1H3,(H,24,25)(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ des Sciences et Technologies de Lille
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloprotease-2 |
Bioorg Med Chem Lett 14: 1119-21 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.076
BindingDB Entry DOI: 10.7270/Q20002NZ |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50000829

(6-((3-fluoro-5-(4-methoxytetrahydro-2H-pyran-4-yl)...)Show SMILES COC1(CCOCC1)c1cc(F)cc(OCc2ccc3n(C)c(=O)ccc3c2)c1 Show InChI InChI=1S/C23H24FNO4/c1-25-21-5-3-16(11-17(21)4-6-22(25)26)15-29-20-13-18(12-19(24)14-20)23(27-2)7-9-28-10-8-23/h3-6,11-14H,7-10,15H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille 2
Curated by ChEMBL
| Assay Description
Inhibition of 5LOX in human whole blood assessed as inhibition of calcium ionophore A 23187-stimulated 5HETE production by HPLC analysis |
J Med Chem 47: 6195-206 (2004)
Article DOI: 10.1021/jm0407761
BindingDB Entry DOI: 10.7270/Q2DR2V01 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50156577
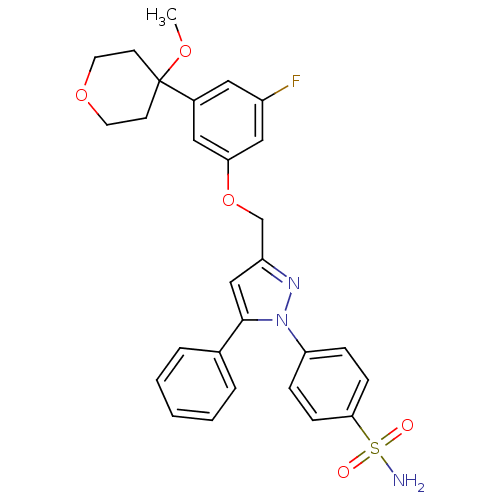
(1-(4-aminosulfonylphenyl)-3-[3-fluoro-5-(4-methoxy...)Show SMILES COC1(CCOCC1)c1cc(F)cc(OCc2cc(-c3ccccc3)n(n2)-c2ccc(cc2)S(N)(=O)=O)c1 Show InChI InChI=1S/C28H28FN3O5S/c1-35-28(11-13-36-14-12-28)21-15-22(29)17-25(16-21)37-19-23-18-27(20-5-3-2-4-6-20)32(31-23)24-7-9-26(10-8-24)38(30,33)34/h2-10,15-18H,11-14,19H2,1H3,(H2,30,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille 2
Curated by ChEMBL
| Assay Description
Inhibition of COX2 expressed in CHO cells assessed as inhibition of arachidonic acid-stimulated PGE2 production by enzyme immunoassay |
J Med Chem 47: 6195-206 (2004)
Article DOI: 10.1021/jm0407761
BindingDB Entry DOI: 10.7270/Q2DR2V01 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Lille-Nord de France
Curated by ChEMBL
| Assay Description
Inhibition of COX1 in human whole blood |
Bioorg Med Chem 18: 3910-24 (2010)
Article DOI: 10.1016/j.bmc.2010.04.034
BindingDB Entry DOI: 10.7270/Q28K7B16 |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50110484

(3-((3-fluoro-5-(1-methoxycyclohexyl)phenoxy)methyl...)Show SMILES COC1(CCOCC1)c1cc(F)cc(OCc2cc(-c3ccccc3)n(n2)-c2ccc(cc2)S(C)(=O)=O)c1 Show InChI InChI=1S/C29H29FN2O5S/c1-35-29(12-14-36-15-13-29)22-16-23(30)18-26(17-22)37-20-24-19-28(21-6-4-3-5-7-21)32(31-24)25-8-10-27(11-9-25)38(2,33)34/h3-11,16-19H,12-15,20H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Lille-Nord de France
Curated by ChEMBL
| Assay Description
Inhibition of 5-lipoxygenase in human whole blood |
Bioorg Med Chem 18: 3910-24 (2010)
Article DOI: 10.1016/j.bmc.2010.04.034
BindingDB Entry DOI: 10.7270/Q28K7B16 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50140149
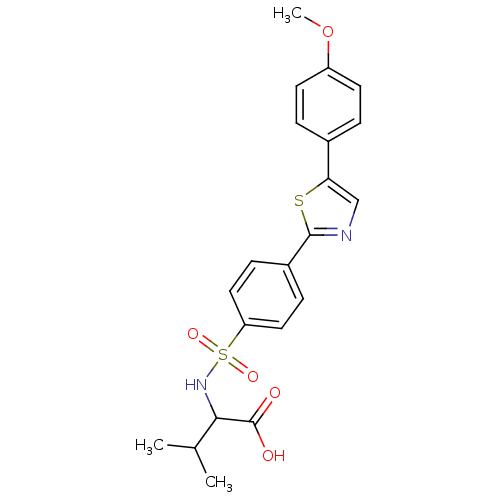
(2-{4-[5-(4-Methoxy-phenyl)-thiazol-2-yl]-benzenesu...)Show SMILES COc1ccc(cc1)-c1cnc(s1)-c1ccc(cc1)S(=O)(=O)NC(C(C)C)C(O)=O Show InChI InChI=1S/C21H22N2O5S2/c1-13(2)19(21(24)25)23-30(26,27)17-10-6-15(7-11-17)20-22-12-18(29-20)14-4-8-16(28-3)9-5-14/h4-13,19,23H,1-3H3,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ des Sciences et Technologies de Lille
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloprotease-2 |
Bioorg Med Chem Lett 14: 1119-21 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.076
BindingDB Entry DOI: 10.7270/Q20002NZ |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50140146
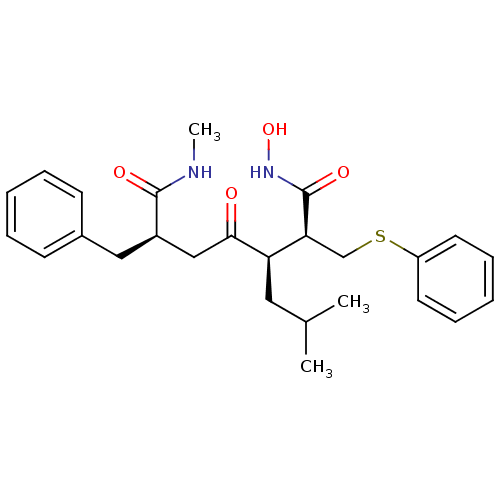
((S)-3-((R)-Isobutyl)-4-oxo-6-((R)-phenylmethyl)-2-...)Show SMILES CNC(=O)[C@@H](CC(=O)[C@H](CC(C)C)[C@H](CSc1ccccc1)C(=O)NO)Cc1ccccc1 Show InChI InChI=1S/C26H34N2O4S/c1-18(2)14-22(23(26(31)28-32)17-33-21-12-8-5-9-13-21)24(29)16-20(25(30)27-3)15-19-10-6-4-7-11-19/h4-13,18,20,22-23,32H,14-17H2,1-3H3,(H,27,30)(H,28,31)/t20-,22-,23+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ des Sciences et Technologies de Lille
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloprotease-9 |
Bioorg Med Chem Lett 14: 1119-21 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.076
BindingDB Entry DOI: 10.7270/Q20002NZ |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50140146

((S)-3-((R)-Isobutyl)-4-oxo-6-((R)-phenylmethyl)-2-...)Show SMILES CNC(=O)[C@@H](CC(=O)[C@H](CC(C)C)[C@H](CSc1ccccc1)C(=O)NO)Cc1ccccc1 Show InChI InChI=1S/C26H34N2O4S/c1-18(2)14-22(23(26(31)28-32)17-33-21-12-8-5-9-13-21)24(29)16-20(25(30)27-3)15-19-10-6-4-7-11-19/h4-13,18,20,22-23,32H,14-17H2,1-3H3,(H,27,30)(H,28,31)/t20-,22-,23+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ des Sciences et Technologies de Lille
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloprotease-2 |
Bioorg Med Chem Lett 14: 1119-21 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.076
BindingDB Entry DOI: 10.7270/Q20002NZ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50156584
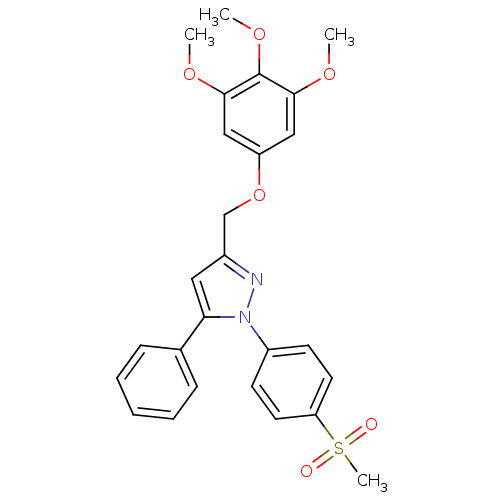
(1-(4-methanesulfonylphenyl)-3-[(3,4,5-trimethoxy)p...)Show SMILES COc1cc(OCc2cc(-c3ccccc3)n(n2)-c2ccc(cc2)S(C)(=O)=O)cc(OC)c1OC Show InChI InChI=1S/C26H26N2O6S/c1-31-24-15-21(16-25(32-2)26(24)33-3)34-17-19-14-23(18-8-6-5-7-9-18)28(27-19)20-10-12-22(13-11-20)35(4,29)30/h5-16H,17H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille 2
Curated by ChEMBL
| Assay Description
Inhibition of COX2 expressed in CHO cells assessed as inhibition of arachidonic acid-stimulated PGE2 production by enzyme immunoassay |
J Med Chem 47: 6195-206 (2004)
Article DOI: 10.1021/jm0407761
BindingDB Entry DOI: 10.7270/Q2DR2V01 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50477677
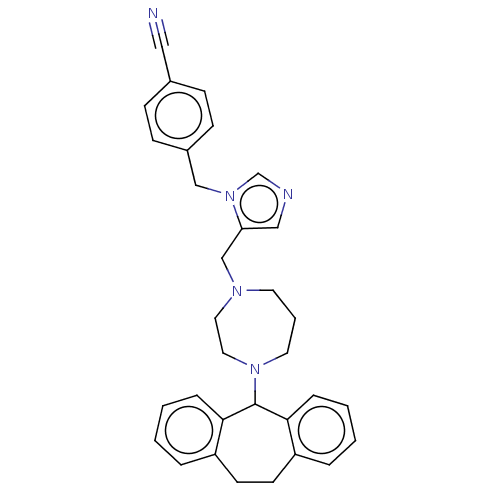
(CHEMBL251711)Show SMILES N#Cc1ccc(Cn2cncc2CN2CCCN(CC2)C2c3ccccc3CCc3ccccc23)cc1 Show InChI InChI=1S/C32H33N5/c33-20-25-10-12-26(13-11-25)22-37-24-34-21-29(37)23-35-16-5-17-36(19-18-35)32-30-8-3-1-6-27(30)14-15-28-7-2-4-9-31(28)32/h1-4,6-13,21,24,32H,5,14-19,22-23H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 285 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lille 2
Curated by ChEMBL
| Assay Description
Inhibition of FTase by fluorescence spectrometry technique |
Bioorg Med Chem Lett 17: 5465-71 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.002
BindingDB Entry DOI: 10.7270/Q2154KTZ |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50110484

(3-((3-fluoro-5-(1-methoxycyclohexyl)phenoxy)methyl...)Show SMILES COC1(CCOCC1)c1cc(F)cc(OCc2cc(-c3ccccc3)n(n2)-c2ccc(cc2)S(C)(=O)=O)c1 Show InChI InChI=1S/C29H29FN2O5S/c1-35-29(12-14-36-15-13-29)22-16-23(30)18-26(17-22)37-20-24-19-28(21-6-4-3-5-7-21)32(31-24)25-8-10-27(11-9-25)38(2,33)34/h3-11,16-19H,12-15,20H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille 2
Curated by ChEMBL
| Assay Description
Inhibition of 5LOX in human whole blood assessed as inhibition of calcium ionophore A 23187-stimulated 5HETE production by HPLC analysis |
J Med Chem 47: 6195-206 (2004)
Article DOI: 10.1021/jm0407761
BindingDB Entry DOI: 10.7270/Q2DR2V01 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50156581

(3-[3-fluoro-5-(4-methoxytetrahydropyran-4-yl)pheno...)Show SMILES COc1ccc(cc1)-n1nc(COc2cc(F)cc(c2)C2(CCOCC2)OC)cc1-c1ccccc1 Show InChI InChI=1S/C29H29FN2O4/c1-33-26-10-8-25(9-11-26)32-28(21-6-4-3-5-7-21)19-24(31-32)20-36-27-17-22(16-23(30)18-27)29(34-2)12-14-35-15-13-29/h3-11,16-19H,12-15,20H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille 2
Curated by ChEMBL
| Assay Description
Inhibition of COX2 expressed in CHO cells assessed as inhibition of arachidonic acid-stimulated PGE2 production by enzyme immunoassay |
J Med Chem 47: 6195-206 (2004)
Article DOI: 10.1021/jm0407761
BindingDB Entry DOI: 10.7270/Q2DR2V01 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50477672
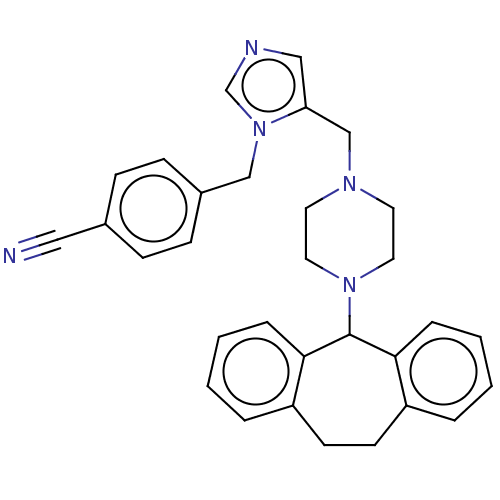
(CHEMBL248869)Show SMILES N#Cc1ccc(Cn2cncc2CN2CCN(CC2)C2c3ccccc3CCc3ccccc23)cc1 Show InChI InChI=1S/C31H31N5/c32-19-24-9-11-25(12-10-24)21-36-23-33-20-28(36)22-34-15-17-35(18-16-34)31-29-7-3-1-5-26(29)13-14-27-6-2-4-8-30(27)31/h1-12,20,23,31H,13-18,21-22H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lille 2
Curated by ChEMBL
| Assay Description
Inhibition of FTase by fluorescence spectrometry technique |
Bioorg Med Chem Lett 17: 5465-71 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.002
BindingDB Entry DOI: 10.7270/Q2154KTZ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50156594

(3-[(3-fluoro-5-methoxy)phenoxymethyl]-1-(4-methane...)Show SMILES COc1cc(F)cc(OCc2cc(-c3ccccc3)n(n2)-c2ccc(cc2)S(C)(=O)=O)c1 Show InChI InChI=1S/C24H21FN2O4S/c1-30-21-12-18(25)13-22(15-21)31-16-19-14-24(17-6-4-3-5-7-17)27(26-19)20-8-10-23(11-9-20)32(2,28)29/h3-15H,16H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille 2
Curated by ChEMBL
| Assay Description
Inhibition of COX2 expressed in CHO cells assessed as inhibition of arachidonic acid-stimulated PGE2 production by enzyme immunoassay |
J Med Chem 47: 6195-206 (2004)
Article DOI: 10.1021/jm0407761
BindingDB Entry DOI: 10.7270/Q2DR2V01 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50022271
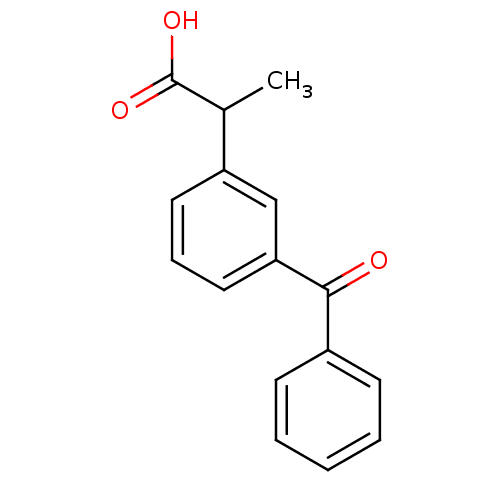
(2-(3-Benzoylphenyl)propionic acid | 2-(3-benzoylph...)Show InChI InChI=1S/C16H14O3/c1-11(16(18)19)13-8-5-9-14(10-13)15(17)12-6-3-2-4-7-12/h2-11H,1H3,(H,18,19) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille 2
Curated by ChEMBL
| Assay Description
Inhibition of COX1 in human whole blood |
Bioorg Med Chem Lett 18: 4655-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.018
BindingDB Entry DOI: 10.7270/Q2G73DH6 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50140150
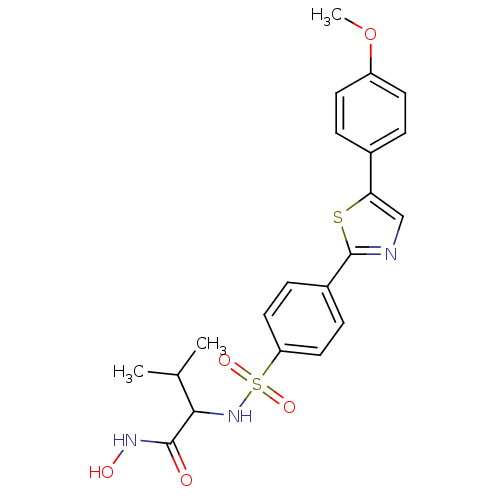
(CHEMBL13974 | N-Hydroxy-2-{4-[5-(4-methoxy-phenyl)...)Show SMILES COc1ccc(cc1)-c1cnc(s1)-c1ccc(cc1)S(=O)(=O)NC(C(C)C)C(=O)NO Show InChI InChI=1S/C21H23N3O5S2/c1-13(2)19(20(25)23-26)24-31(27,28)17-10-6-15(7-11-17)21-22-12-18(30-21)14-4-8-16(29-3)9-5-14/h4-13,19,24,26H,1-3H3,(H,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ des Sciences et Technologies de Lille
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloprotease-2 |
Bioorg Med Chem Lett 14: 1119-21 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.076
BindingDB Entry DOI: 10.7270/Q20002NZ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50156580
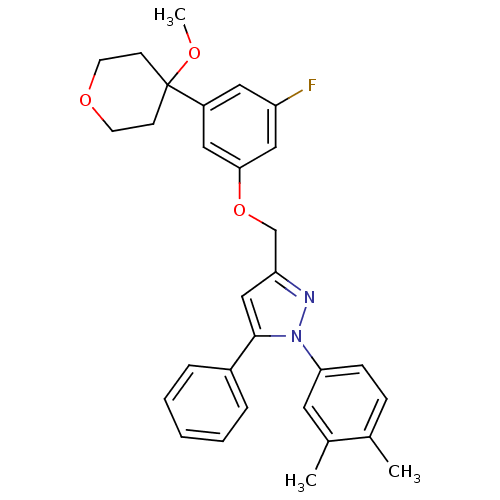
(3-[3-fluoro-5-(4-methoxytetrahydropyran-4-yl)pheno...)Show SMILES COC1(CCOCC1)c1cc(F)cc(OCc2cc(-c3ccccc3)n(n2)-c2ccc(C)c(C)c2)c1 Show InChI InChI=1S/C30H31FN2O3/c1-21-9-10-27(15-22(21)2)33-29(23-7-5-4-6-8-23)19-26(32-33)20-36-28-17-24(16-25(31)18-28)30(34-3)11-13-35-14-12-30/h4-10,15-19H,11-14,20H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille 2
Curated by ChEMBL
| Assay Description
Inhibition of COX2 expressed in CHO cells assessed as inhibition of arachidonic acid-stimulated PGE2 production by enzyme immunoassay |
J Med Chem 47: 6195-206 (2004)
Article DOI: 10.1021/jm0407761
BindingDB Entry DOI: 10.7270/Q2DR2V01 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50272731
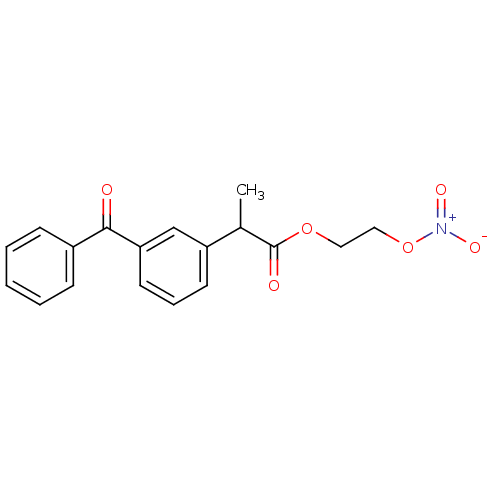
(2-(3-Benzoyl-phenyl)-propionic acid 2-nitrooxy-eth...)Show SMILES CC(C(=O)OCCO[N+]([O-])=O)c1cccc(c1)C(=O)c1ccccc1 Show InChI InChI=1S/C18H17NO6/c1-13(18(21)24-10-11-25-19(22)23)15-8-5-9-16(12-15)17(20)14-6-3-2-4-7-14/h2-9,12-13H,10-11H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille 2
Curated by ChEMBL
| Assay Description
Inhibition of COX1 in human whole blood |
Bioorg Med Chem Lett 18: 4655-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.018
BindingDB Entry DOI: 10.7270/Q2G73DH6 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50156590
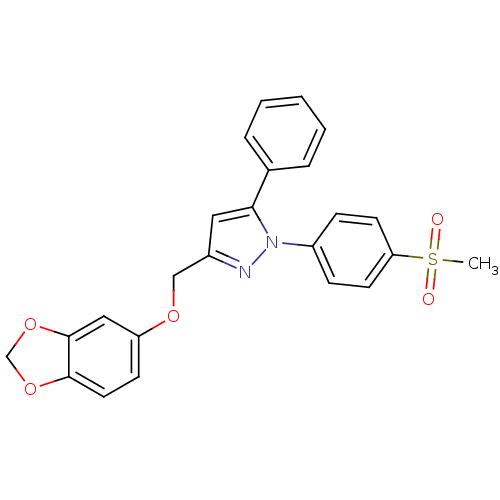
(1-(4-methanesulfonylphenyl)-3-[(3,4-methylenedioxy...)Show SMILES CS(=O)(=O)c1ccc(cc1)-n1nc(COc2ccc3OCOc3c2)cc1-c1ccccc1 Show InChI InChI=1S/C24H20N2O5S/c1-32(27,28)21-10-7-19(8-11-21)26-22(17-5-3-2-4-6-17)13-18(25-26)15-29-20-9-12-23-24(14-20)31-16-30-23/h2-14H,15-16H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille 2
Curated by ChEMBL
| Assay Description
Inhibition of COX2 expressed in CHO cells assessed as inhibition of arachidonic acid-stimulated PGE2 production by enzyme immunoassay |
J Med Chem 47: 6195-206 (2004)
Article DOI: 10.1021/jm0407761
BindingDB Entry DOI: 10.7270/Q2DR2V01 |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50156585
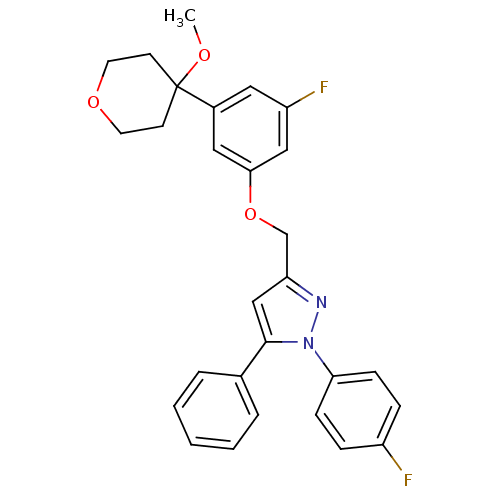
(3-[3-fluoro-5-(4-methoxytetrahydropyran-4-yl)pheno...)Show SMILES COC1(CCOCC1)c1cc(F)cc(OCc2cc(-c3ccccc3)n(n2)-c2ccc(F)cc2)c1 Show InChI InChI=1S/C28H26F2N2O3/c1-33-28(11-13-34-14-12-28)21-15-23(30)17-26(16-21)35-19-24-18-27(20-5-3-2-4-6-20)32(31-24)25-9-7-22(29)8-10-25/h2-10,15-18H,11-14,19H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille 2
Curated by ChEMBL
| Assay Description
Inhibition of 5LOX in human whole blood assessed as inhibition of calcium ionophore A 23187-stimulated 5HETE production by HPLC analysis |
J Med Chem 47: 6195-206 (2004)
Article DOI: 10.1021/jm0407761
BindingDB Entry DOI: 10.7270/Q2DR2V01 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50272677
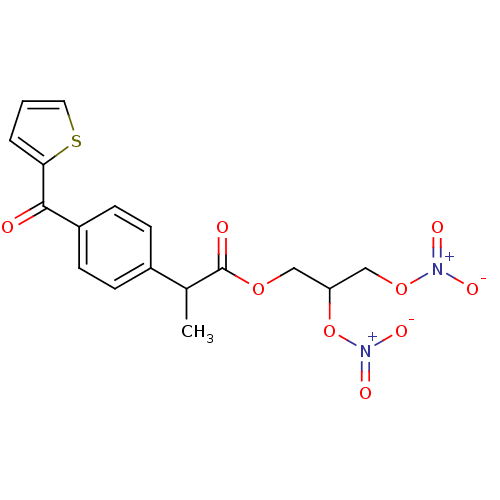
(2-[4-(Thiophene-2-carbonyl)-phenyl]-propionic acid...)Show SMILES CC(C(=O)OCC(CO[N+]([O-])=O)O[N+]([O-])=O)c1ccc(cc1)C(=O)c1cccs1 Show InChI InChI=1S/C17H16N2O9S/c1-11(17(21)26-9-14(28-19(24)25)10-27-18(22)23)12-4-6-13(7-5-12)16(20)15-3-2-8-29-15/h2-8,11,14H,9-10H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille 2
Curated by ChEMBL
| Assay Description
Inhibition of COX1 in human whole blood |
Bioorg Med Chem Lett 18: 4655-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.018
BindingDB Entry DOI: 10.7270/Q2G73DH6 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Lille-Nord de France
Curated by ChEMBL
| Assay Description
Inhibition of COX2 in human whole blood |
Bioorg Med Chem 18: 3910-24 (2010)
Article DOI: 10.1016/j.bmc.2010.04.034
BindingDB Entry DOI: 10.7270/Q28K7B16 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Lille-Nord de France
Curated by ChEMBL
| Assay Description
Inhibition of COX2 in human whole blood assessed as inhibition of 12-hydroxyheptadecatrienoic acid production by HPLC method |
Bioorg Med Chem 18: 3910-24 (2010)
Article DOI: 10.1016/j.bmc.2010.04.034
BindingDB Entry DOI: 10.7270/Q28K7B16 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50090676
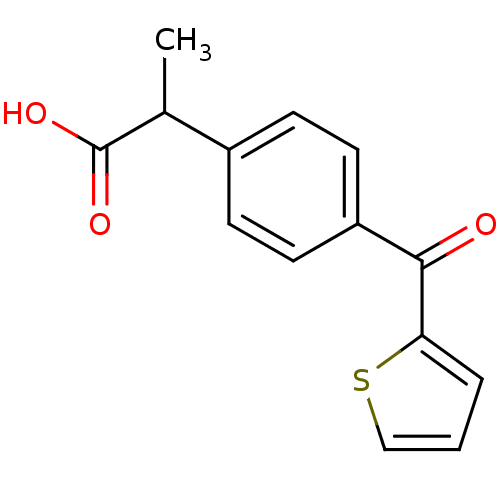
((+-)-2-(p-(2-thenoyl)phenyl)propionic acid | 2-(4-...)Show InChI InChI=1S/C14H12O3S/c1-9(14(16)17)10-4-6-11(7-5-10)13(15)12-3-2-8-18-12/h2-9H,1H3,(H,16,17) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille 2
Curated by ChEMBL
| Assay Description
Inhibition of COX1 in human whole blood |
Bioorg Med Chem Lett 18: 4655-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.018
BindingDB Entry DOI: 10.7270/Q2G73DH6 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50156587
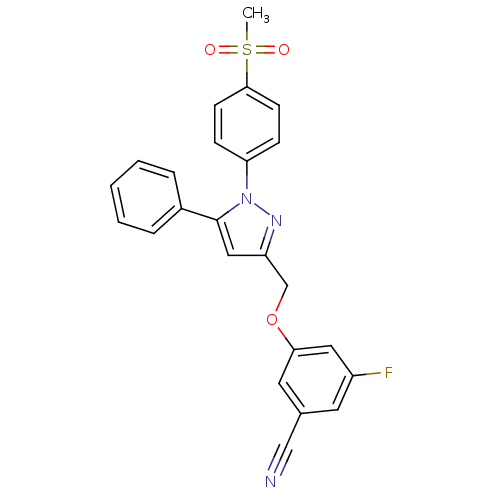
(3-[(5-cyano-3-fluoro)phenoxymethyl]-1-(4-methanesu...)Show SMILES CS(=O)(=O)c1ccc(cc1)-n1nc(COc2cc(F)cc(c2)C#N)cc1-c1ccccc1 Show InChI InChI=1S/C24H18FN3O3S/c1-32(29,30)23-9-7-21(8-10-23)28-24(18-5-3-2-4-6-18)14-20(27-28)16-31-22-12-17(15-26)11-19(25)13-22/h2-14H,16H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille 2
Curated by ChEMBL
| Assay Description
Inhibition of COX2 expressed in CHO cells assessed as inhibition of arachidonic acid-stimulated PGE2 production by enzyme immunoassay |
J Med Chem 47: 6195-206 (2004)
Article DOI: 10.1021/jm0407761
BindingDB Entry DOI: 10.7270/Q2DR2V01 |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50156582
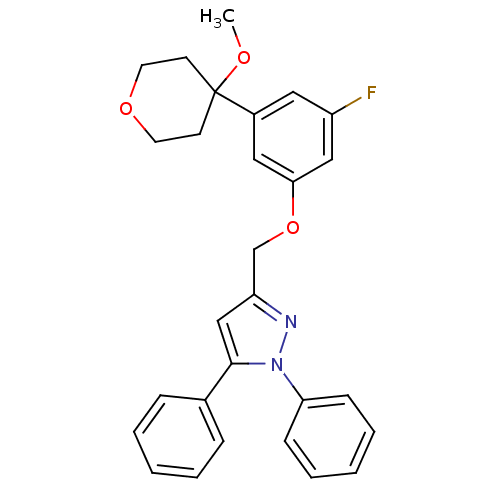
(3-[3-fluoro-5-(4-methoxytetrahydropyran-4-yl)pheno...)Show SMILES COC1(CCOCC1)c1cc(F)cc(OCc2cc(-c3ccccc3)n(n2)-c2ccccc2)c1 Show InChI InChI=1S/C28H27FN2O3/c1-32-28(12-14-33-15-13-28)22-16-23(29)18-26(17-22)34-20-24-19-27(21-8-4-2-5-9-21)31(30-24)25-10-6-3-7-11-25/h2-11,16-19H,12-15,20H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille 2
Curated by ChEMBL
| Assay Description
Inhibition of 5LOX in human whole blood assessed as inhibition of calcium ionophore A 23187-stimulated 5HETE production by HPLC analysis |
J Med Chem 47: 6195-206 (2004)
Article DOI: 10.1021/jm0407761
BindingDB Entry DOI: 10.7270/Q2DR2V01 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50272676
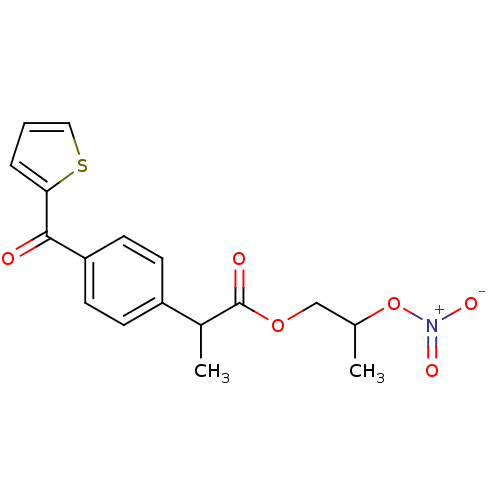
(2-[4-(Thiophene-2-carbonyl)-phenyl]-propionic acid...)Show SMILES CC(COC(=O)C(C)c1ccc(cc1)C(=O)c1cccs1)O[N+]([O-])=O Show InChI InChI=1S/C17H17NO6S/c1-11(24-18(21)22)10-23-17(20)12(2)13-5-7-14(8-6-13)16(19)15-4-3-9-25-15/h3-9,11-12H,10H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille 2
Curated by ChEMBL
| Assay Description
Inhibition of COX1 in human whole blood |
Bioorg Med Chem Lett 18: 4655-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.018
BindingDB Entry DOI: 10.7270/Q2G73DH6 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50272675
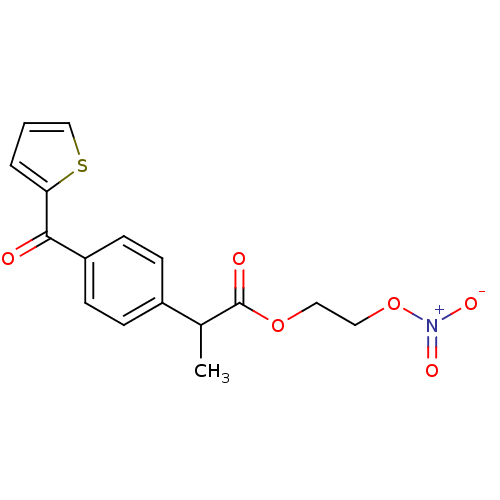
(2-[4-(Thiophene-2-carbonyl)-phenyl]-propionic acid...)Show SMILES CC(C(=O)OCCO[N+]([O-])=O)c1ccc(cc1)C(=O)c1cccs1 Show InChI InChI=1S/C16H15NO6S/c1-11(16(19)22-8-9-23-17(20)21)12-4-6-13(7-5-12)15(18)14-3-2-10-24-14/h2-7,10-11H,8-9H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 580 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille 2
Curated by ChEMBL
| Assay Description
Inhibition of COX1 in human whole blood |
Bioorg Med Chem Lett 18: 4655-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.018
BindingDB Entry DOI: 10.7270/Q2G73DH6 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50272733
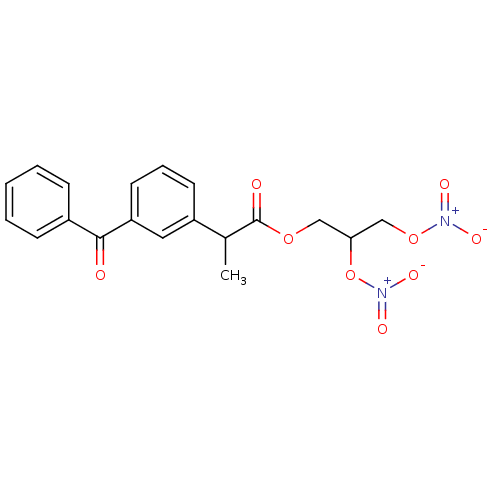
(2-(3-Benzoyl-phenyl)-propionic acid 2,3-bis-nitroo...)Show SMILES CC(C(=O)OCC(CO[N+]([O-])=O)O[N+]([O-])=O)c1cccc(c1)C(=O)c1ccccc1 Show InChI InChI=1S/C19H18N2O9/c1-13(19(23)28-11-17(30-21(26)27)12-29-20(24)25)15-8-5-9-16(10-15)18(22)14-6-3-2-4-7-14/h2-10,13,17H,11-12H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 580 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille 2
Curated by ChEMBL
| Assay Description
Inhibition of COX1 in human whole blood |
Bioorg Med Chem Lett 18: 4655-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.018
BindingDB Entry DOI: 10.7270/Q2G73DH6 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50272732
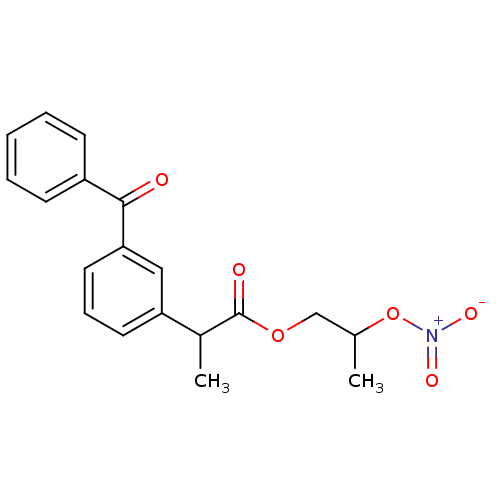
(2-(3-Benzoyl-phenyl)-propionic acid 2-nitrooxy-pro...)Show SMILES CC(COC(=O)C(C)c1cccc(c1)C(=O)c1ccccc1)O[N+]([O-])=O Show InChI InChI=1S/C19H19NO6/c1-13(26-20(23)24)12-25-19(22)14(2)16-9-6-10-17(11-16)18(21)15-7-4-3-5-8-15/h3-11,13-14H,12H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille 2
Curated by ChEMBL
| Assay Description
Inhibition of COX1 in human whole blood |
Bioorg Med Chem Lett 18: 4655-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.018
BindingDB Entry DOI: 10.7270/Q2G73DH6 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50156582

(3-[3-fluoro-5-(4-methoxytetrahydropyran-4-yl)pheno...)Show SMILES COC1(CCOCC1)c1cc(F)cc(OCc2cc(-c3ccccc3)n(n2)-c2ccccc2)c1 Show InChI InChI=1S/C28H27FN2O3/c1-32-28(12-14-33-15-13-28)22-16-23(29)18-26(17-22)34-20-24-19-27(21-8-4-2-5-9-21)31(30-24)25-10-6-3-7-11-25/h2-11,16-19H,12-15,20H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 610 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille 2
Curated by ChEMBL
| Assay Description
Inhibition of COX2 expressed in CHO cells assessed as inhibition of arachidonic acid-stimulated PGE2 production by enzyme immunoassay |
J Med Chem 47: 6195-206 (2004)
Article DOI: 10.1021/jm0407761
BindingDB Entry DOI: 10.7270/Q2DR2V01 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50022271

(2-(3-Benzoylphenyl)propionic acid | 2-(3-benzoylph...)Show InChI InChI=1S/C16H14O3/c1-11(16(18)19)13-8-5-9-14(10-13)15(17)12-6-3-2-4-7-12/h2-11H,1H3,(H,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 690 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille 2
Curated by ChEMBL
| Assay Description
Inhibition of COX2 in human whole blood |
Bioorg Med Chem Lett 18: 4655-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.018
BindingDB Entry DOI: 10.7270/Q2G73DH6 |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50000541

((+-)-1-(1-Benzo[b]thien-2-ylethyl)-1-hydroxyurea |...)Show InChI InChI=1S/C11H12N2O2S/c1-7(13(15)11(12)14)10-6-8-4-2-3-5-9(8)16-10/h2-7,15H,1H3,(H2,12,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Lille-Nord de France
Curated by ChEMBL
| Assay Description
Inhibition of 5-lipoxygenase in human whole blood assessed as inhibition of LTB4 production by HPLC method |
Bioorg Med Chem 18: 3910-24 (2010)
Article DOI: 10.1016/j.bmc.2010.04.034
BindingDB Entry DOI: 10.7270/Q28K7B16 |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50000541

((+-)-1-(1-Benzo[b]thien-2-ylethyl)-1-hydroxyurea |...)Show InChI InChI=1S/C11H12N2O2S/c1-7(13(15)11(12)14)10-6-8-4-2-3-5-9(8)16-10/h2-7,15H,1H3,(H2,12,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Lille-Nord de France
Curated by ChEMBL
| Assay Description
Inhibition of 5-lipoxygenase in human whole blood |
Bioorg Med Chem 18: 3910-24 (2010)
Article DOI: 10.1016/j.bmc.2010.04.034
BindingDB Entry DOI: 10.7270/Q28K7B16 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50140149

(2-{4-[5-(4-Methoxy-phenyl)-thiazol-2-yl]-benzenesu...)Show SMILES COc1ccc(cc1)-c1cnc(s1)-c1ccc(cc1)S(=O)(=O)NC(C(C)C)C(O)=O Show InChI InChI=1S/C21H22N2O5S2/c1-13(2)19(21(24)25)23-30(26,27)17-10-6-15(7-11-17)20-22-12-18(29-20)14-4-8-16(28-3)9-5-14/h4-13,19,23H,1-3H3,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ des Sciences et Technologies de Lille
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloprotease-9 |
Bioorg Med Chem Lett 14: 1119-21 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.076
BindingDB Entry DOI: 10.7270/Q20002NZ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50156579
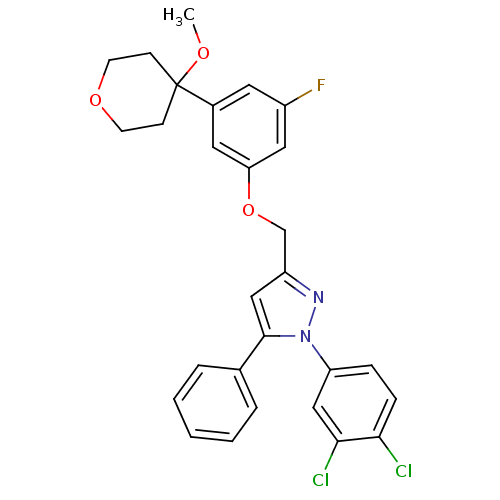
(1-(3,4-dichlorophenyl)-3-[3-fluoro-5-(4-methoxytet...)Show SMILES COC1(CCOCC1)c1cc(F)cc(OCc2cc(-c3ccccc3)n(n2)-c2ccc(Cl)c(Cl)c2)c1 Show InChI InChI=1S/C28H25Cl2FN2O3/c1-34-28(9-11-35-12-10-28)20-13-21(31)15-24(14-20)36-18-22-16-27(19-5-3-2-4-6-19)33(32-22)23-7-8-25(29)26(30)17-23/h2-8,13-17H,9-12,18H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille 2
Curated by ChEMBL
| Assay Description
Inhibition of COX2 expressed in CHO cells assessed as inhibition of arachidonic acid-stimulated PGE2 production by enzyme immunoassay |
J Med Chem 47: 6195-206 (2004)
Article DOI: 10.1021/jm0407761
BindingDB Entry DOI: 10.7270/Q2DR2V01 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50156583
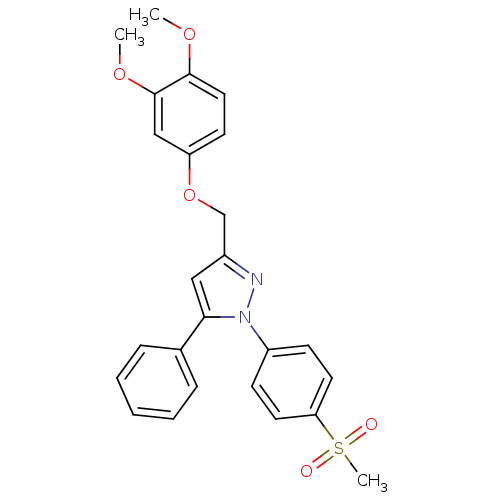
(1-(4-methanesulfonylphenyl)-3-[(3,4-dimethoxy)phen...)Show SMILES COc1ccc(OCc2cc(-c3ccccc3)n(n2)-c2ccc(cc2)S(C)(=O)=O)cc1OC Show InChI InChI=1S/C25H24N2O5S/c1-30-24-14-11-21(16-25(24)31-2)32-17-19-15-23(18-7-5-4-6-8-18)27(26-19)20-9-12-22(13-10-20)33(3,28)29/h4-16H,17H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille 2
Curated by ChEMBL
| Assay Description
Inhibition of COX2 expressed in CHO cells assessed as inhibition of arachidonic acid-stimulated PGE2 production by enzyme immunoassay |
J Med Chem 47: 6195-206 (2004)
Article DOI: 10.1021/jm0407761
BindingDB Entry DOI: 10.7270/Q2DR2V01 |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50156586
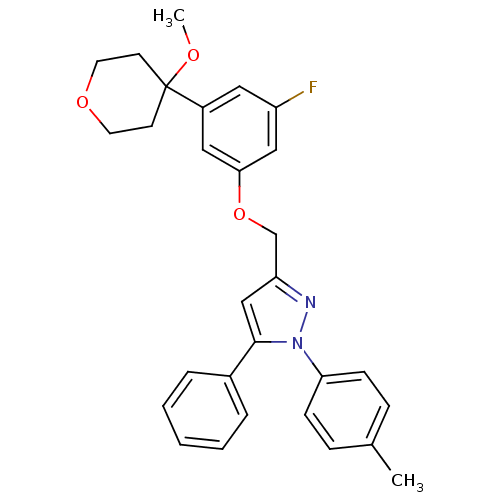
(3-[3-fluoro-5-(4-methoxytetrahydropyran-4-yl)pheno...)Show SMILES COC1(CCOCC1)c1cc(F)cc(OCc2cc(-c3ccccc3)n(n2)-c2ccc(C)cc2)c1 Show InChI InChI=1S/C29H29FN2O3/c1-21-8-10-26(11-9-21)32-28(22-6-4-3-5-7-22)19-25(31-32)20-35-27-17-23(16-24(30)18-27)29(33-2)12-14-34-15-13-29/h3-11,16-19H,12-15,20H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 720 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille 2
Curated by ChEMBL
| Assay Description
Inhibition of 5LOX in human whole blood assessed as inhibition of calcium ionophore A 23187-stimulated 5HETE production by HPLC analysis |
J Med Chem 47: 6195-206 (2004)
Article DOI: 10.1021/jm0407761
BindingDB Entry DOI: 10.7270/Q2DR2V01 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data