

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50166735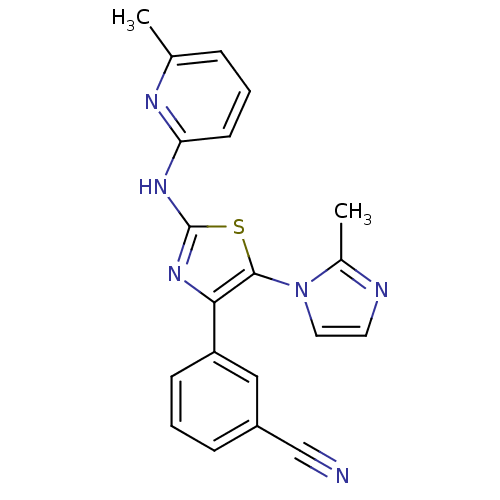 (3-[5-(2-Methyl-imidazol-1-yl)-2-(pyrazin-2-ylamino...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of luciferase production elicited by NECA by compound in CHO cells transfected with human adenosine A2b receptor and a luciferase expressi... | Bioorg Med Chem Lett 15: 3081-5 (2005) Article DOI: 10.1016/j.bmcl.2005.04.021 BindingDB Entry DOI: 10.7270/Q2X63NQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50166742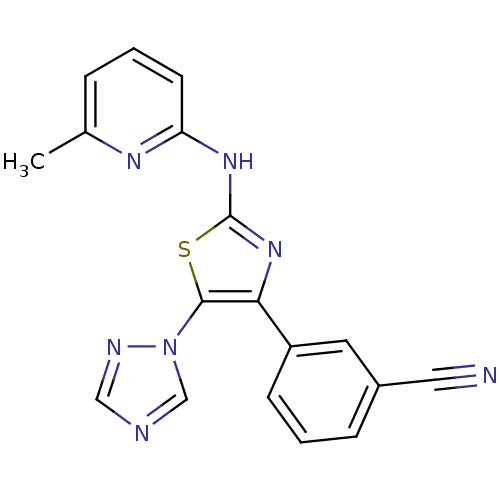 (3-[2-(3-Methyl-pyridin-2-ylamino)-5-[1,2,4]triazol...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of luciferase production elicited by NECA by compound in CHO cells transfected with human adenosine A2b receptor and a luciferase expressi... | Bioorg Med Chem Lett 15: 3081-5 (2005) Article DOI: 10.1016/j.bmcl.2005.04.021 BindingDB Entry DOI: 10.7270/Q2X63NQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50166739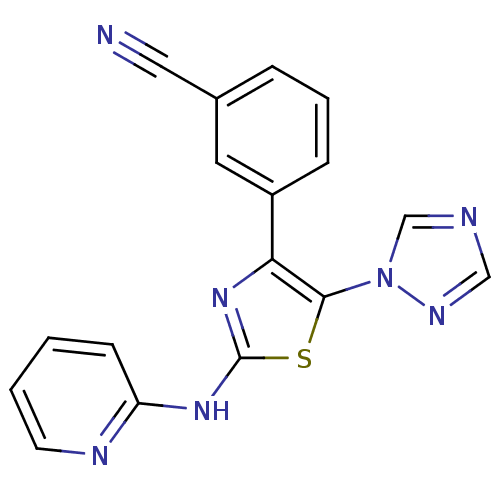 (3-[2-(Pyridin-2-ylamino)-5-[1,2,4]triazol-1-yl-thi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Displacement of [125I]-AB-MECA from human adenosine A3 receptors transfected in CHO cells | Bioorg Med Chem Lett 15: 3081-5 (2005) Article DOI: 10.1016/j.bmcl.2005.04.021 BindingDB Entry DOI: 10.7270/Q2X63NQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50166742 (3-[2-(3-Methyl-pyridin-2-ylamino)-5-[1,2,4]triazol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Displacement of [125I]-AB-MECA from human adenosine A3 receptors transfected in CHO cells | Bioorg Med Chem Lett 15: 3081-5 (2005) Article DOI: 10.1016/j.bmcl.2005.04.021 BindingDB Entry DOI: 10.7270/Q2X63NQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50166739 (3-[2-(Pyridin-2-ylamino)-5-[1,2,4]triazol-1-yl-thi...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of luciferase production elicited by NECA by compound in CHO cells transfected with human adenosine A2b receptor and a luciferase expressi... | Bioorg Med Chem Lett 15: 3081-5 (2005) Article DOI: 10.1016/j.bmcl.2005.04.021 BindingDB Entry DOI: 10.7270/Q2X63NQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50166745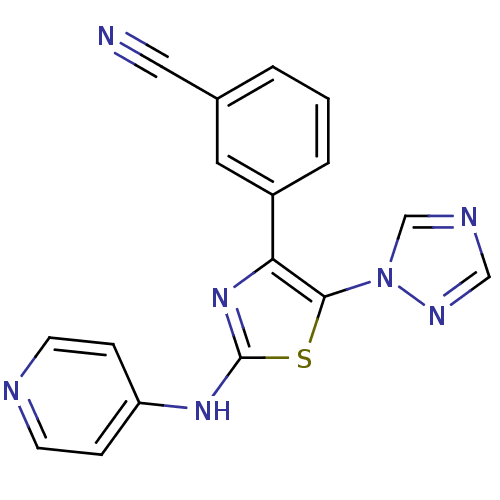 (3-[2-(Pyridin-4-ylamino)-5-[1,2,4]triazol-1-yl-thi...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of luciferase production elicited by NECA by compound in CHO cells transfected with human adenosine A2b receptor and a luciferase expressi... | Bioorg Med Chem Lett 15: 3081-5 (2005) Article DOI: 10.1016/j.bmcl.2005.04.021 BindingDB Entry DOI: 10.7270/Q2X63NQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50166737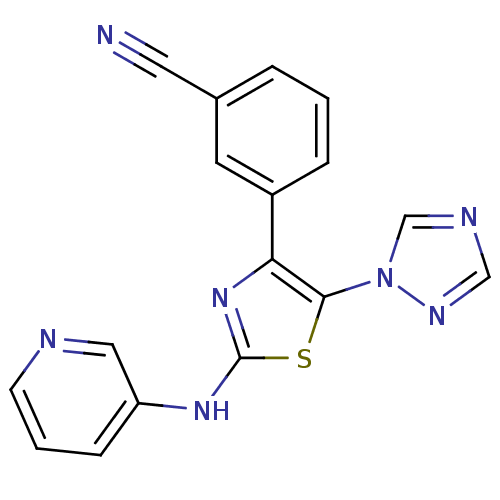 (3-[2-(Pyridin-3-ylamino)-5-[1,2,4]triazol-1-yl-thi...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 86 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of luciferase production elicited by NECA by compound in CHO cells transfected with human adenosine A2b receptor and a luciferase expressi... | Bioorg Med Chem Lett 15: 3081-5 (2005) Article DOI: 10.1016/j.bmcl.2005.04.021 BindingDB Entry DOI: 10.7270/Q2X63NQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50166743 (3-[5-Imidazol-1-yl-2-(pyridin-3-ylamino)-thiazol-4...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 92 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of luciferase production elicited by NECA by compound in CHO cells transfected with human adenosine A2b receptor and a luciferase expressi... | Bioorg Med Chem Lett 15: 3081-5 (2005) Article DOI: 10.1016/j.bmcl.2005.04.021 BindingDB Entry DOI: 10.7270/Q2X63NQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50166743 (3-[5-Imidazol-1-yl-2-(pyridin-3-ylamino)-thiazol-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 124 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Displacement of [125I]-AB-MECA from human adenosine A3 receptors transfected in CHO cells | Bioorg Med Chem Lett 15: 3081-5 (2005) Article DOI: 10.1016/j.bmcl.2005.04.021 BindingDB Entry DOI: 10.7270/Q2X63NQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50166741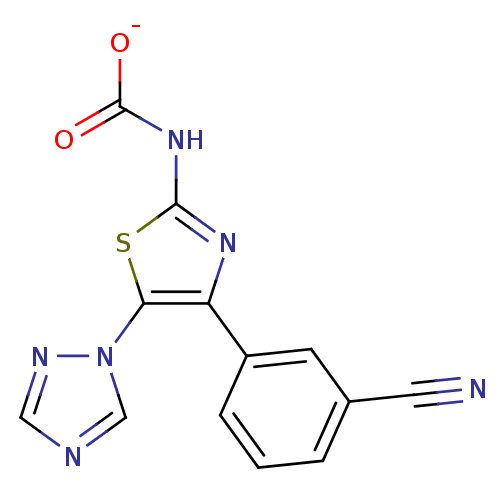 (4-(3-cyanophenyl)-5-(1H-1,2,4-triazol-1-yl)-1,3-th...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 134 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Displacement of [125I]-AB-MECA from human adenosine A3 receptors transfected in CHO cells | Bioorg Med Chem Lett 15: 3081-5 (2005) Article DOI: 10.1016/j.bmcl.2005.04.021 BindingDB Entry DOI: 10.7270/Q2X63NQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50166735 (3-[5-(2-Methyl-imidazol-1-yl)-2-(pyrazin-2-ylamino...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 175 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Displacement of [3H]ZM-241,385 from human adenosine A2a receptors transfected in HEK 293 cells | Bioorg Med Chem Lett 15: 3081-5 (2005) Article DOI: 10.1016/j.bmcl.2005.04.021 BindingDB Entry DOI: 10.7270/Q2X63NQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50166735 (3-[5-(2-Methyl-imidazol-1-yl)-2-(pyrazin-2-ylamino...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 224 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Displacement of [3H]DPCPX from human adenosine A1 receptors transfected in CHO cells | Bioorg Med Chem Lett 15: 3081-5 (2005) Article DOI: 10.1016/j.bmcl.2005.04.021 BindingDB Entry DOI: 10.7270/Q2X63NQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50166745 (3-[2-(Pyridin-4-ylamino)-5-[1,2,4]triazol-1-yl-thi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 304 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Displacement of [125I]-AB-MECA from human adenosine A3 receptors transfected in CHO cells | Bioorg Med Chem Lett 15: 3081-5 (2005) Article DOI: 10.1016/j.bmcl.2005.04.021 BindingDB Entry DOI: 10.7270/Q2X63NQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50166741 (4-(3-cyanophenyl)-5-(1H-1,2,4-triazol-1-yl)-1,3-th...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 365 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of luciferase production elicited by NECA by compound in CHO cells transfected with human adenosine A2b receptor and a luciferase expressi... | Bioorg Med Chem Lett 15: 3081-5 (2005) Article DOI: 10.1016/j.bmcl.2005.04.021 BindingDB Entry DOI: 10.7270/Q2X63NQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50166735 (3-[5-(2-Methyl-imidazol-1-yl)-2-(pyrazin-2-ylamino...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 419 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Displacement of [125I]-AB-MECA from human adenosine A3 receptors transfected in CHO cells | Bioorg Med Chem Lett 15: 3081-5 (2005) Article DOI: 10.1016/j.bmcl.2005.04.021 BindingDB Entry DOI: 10.7270/Q2X63NQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50166743 (3-[5-Imidazol-1-yl-2-(pyridin-3-ylamino)-thiazol-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Displacement of [3H]DPCPX from human adenosine A1 receptors transfected in CHO cells | Bioorg Med Chem Lett 15: 3081-5 (2005) Article DOI: 10.1016/j.bmcl.2005.04.021 BindingDB Entry DOI: 10.7270/Q2X63NQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50166737 (3-[2-(Pyridin-3-ylamino)-5-[1,2,4]triazol-1-yl-thi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 698 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Displacement of [125I]-AB-MECA from human adenosine A3 receptors transfected in CHO cells | Bioorg Med Chem Lett 15: 3081-5 (2005) Article DOI: 10.1016/j.bmcl.2005.04.021 BindingDB Entry DOI: 10.7270/Q2X63NQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50166737 (3-[2-(Pyridin-3-ylamino)-5-[1,2,4]triazol-1-yl-thi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Displacement of [3H]DPCPX from human adenosine A1 receptors transfected in CHO cells | Bioorg Med Chem Lett 15: 3081-5 (2005) Article DOI: 10.1016/j.bmcl.2005.04.021 BindingDB Entry DOI: 10.7270/Q2X63NQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50166739 (3-[2-(Pyridin-2-ylamino)-5-[1,2,4]triazol-1-yl-thi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Displacement of [3H]DPCPX from human adenosine A1 receptors transfected in CHO cells | Bioorg Med Chem Lett 15: 3081-5 (2005) Article DOI: 10.1016/j.bmcl.2005.04.021 BindingDB Entry DOI: 10.7270/Q2X63NQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50166739 (3-[2-(Pyridin-2-ylamino)-5-[1,2,4]triazol-1-yl-thi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Displacement of [3H]ZM-241,385 from human adenosine A2a receptors transfected in HEK 293 cells | Bioorg Med Chem Lett 15: 3081-5 (2005) Article DOI: 10.1016/j.bmcl.2005.04.021 BindingDB Entry DOI: 10.7270/Q2X63NQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50166737 (3-[2-(Pyridin-3-ylamino)-5-[1,2,4]triazol-1-yl-thi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.57E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Displacement of [3H]ZM-241,385 from human adenosine A2a receptors transfected in HEK 293 cells | Bioorg Med Chem Lett 15: 3081-5 (2005) Article DOI: 10.1016/j.bmcl.2005.04.021 BindingDB Entry DOI: 10.7270/Q2X63NQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50166743 (3-[5-Imidazol-1-yl-2-(pyridin-3-ylamino)-thiazol-4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Displacement of [3H]ZM-241,385 from human adenosine A2a receptors transfected in HEK 293 cells | Bioorg Med Chem Lett 15: 3081-5 (2005) Article DOI: 10.1016/j.bmcl.2005.04.021 BindingDB Entry DOI: 10.7270/Q2X63NQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50166741 (4-(3-cyanophenyl)-5-(1H-1,2,4-triazol-1-yl)-1,3-th...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.84E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Displacement of [3H]DPCPX from human adenosine A1 receptors transfected in CHO cells | Bioorg Med Chem Lett 15: 3081-5 (2005) Article DOI: 10.1016/j.bmcl.2005.04.021 BindingDB Entry DOI: 10.7270/Q2X63NQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50166745 (3-[2-(Pyridin-4-ylamino)-5-[1,2,4]triazol-1-yl-thi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Displacement of [3H]DPCPX from human adenosine A1 receptors transfected in CHO cells | Bioorg Med Chem Lett 15: 3081-5 (2005) Article DOI: 10.1016/j.bmcl.2005.04.021 BindingDB Entry DOI: 10.7270/Q2X63NQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50166745 (3-[2-(Pyridin-4-ylamino)-5-[1,2,4]triazol-1-yl-thi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Displacement of [3H]ZM-241,385 from human adenosine A2a receptors transfected in HEK 293 cells | Bioorg Med Chem Lett 15: 3081-5 (2005) Article DOI: 10.1016/j.bmcl.2005.04.021 BindingDB Entry DOI: 10.7270/Q2X63NQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50166741 (4-(3-cyanophenyl)-5-(1H-1,2,4-triazol-1-yl)-1,3-th...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5.61E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Displacement of [3H]ZM-241,385 from human adenosine A2a receptors transfected in HEK 293 cells | Bioorg Med Chem Lett 15: 3081-5 (2005) Article DOI: 10.1016/j.bmcl.2005.04.021 BindingDB Entry DOI: 10.7270/Q2X63NQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50166742 (3-[2-(3-Methyl-pyridin-2-ylamino)-5-[1,2,4]triazol...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Displacement of [3H]ZM-241,385 from human adenosine A2a receptors transfected in HEK 293 cells | Bioorg Med Chem Lett 15: 3081-5 (2005) Article DOI: 10.1016/j.bmcl.2005.04.021 BindingDB Entry DOI: 10.7270/Q2X63NQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50166742 (3-[2-(3-Methyl-pyridin-2-ylamino)-5-[1,2,4]triazol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Displacement of [3H]DPCPX from human adenosine A1 receptors transfected in CHO cells | Bioorg Med Chem Lett 15: 3081-5 (2005) Article DOI: 10.1016/j.bmcl.2005.04.021 BindingDB Entry DOI: 10.7270/Q2X63NQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50108504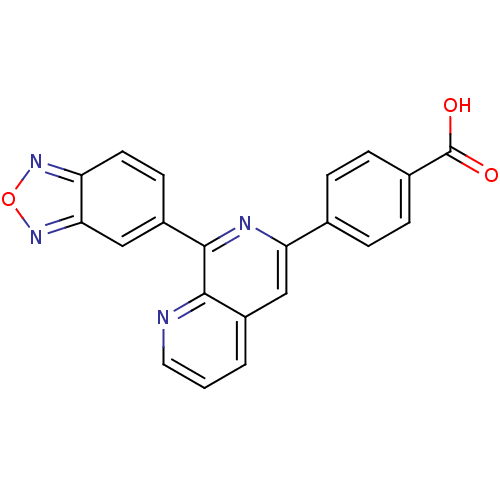 (4-(8-(benzo[c][1,2,5]oxadiazol-5-yl)-1,7-naphthyri...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of human PDE4D | J Med Chem 55: 7472-9 (2012) Article DOI: 10.1021/jm300459a BindingDB Entry DOI: 10.7270/Q2RN390V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50108504 (4-(8-(benzo[c][1,2,5]oxadiazol-5-yl)-1,7-naphthyri...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of human PDE4D | J Med Chem 58: 6747-52 (2015) Article DOI: 10.1021/acs.jmedchem.5b00902 BindingDB Entry DOI: 10.7270/Q2319XPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50113973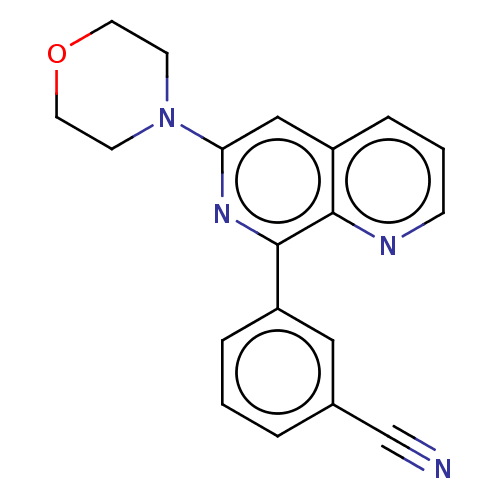 (CHEMBL3605513) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of human PDE4D | J Med Chem 58: 6747-52 (2015) Article DOI: 10.1021/acs.jmedchem.5b00902 BindingDB Entry DOI: 10.7270/Q2319XPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50048182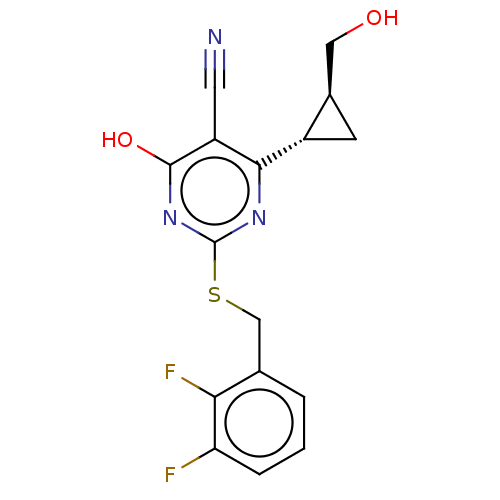 (CHEMBL3310787) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity against human recombinant CXCR2 receptor expressed in CHO cell membranes by SPA based [35S]GTPgammaS binding assay | Bioorg Med Chem Lett 24: 3285-90 (2014) Article DOI: 10.1016/j.bmcl.2014.06.011 BindingDB Entry DOI: 10.7270/Q2930VTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50396765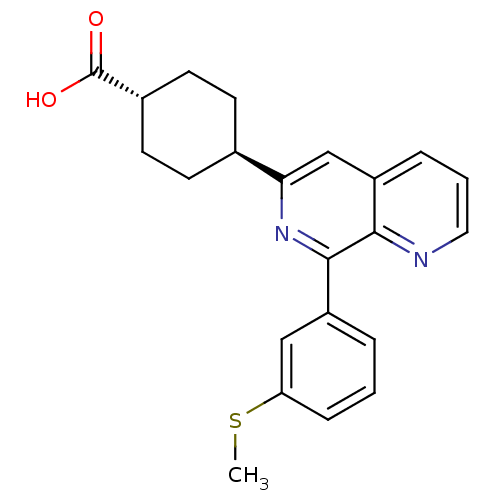 (CHEMBL2172376) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of human PDE4D-mediated [3H]CAMP hydrolysis after 30 to 60 mins by scintillation proximity assay | J Med Chem 55: 7472-9 (2012) Article DOI: 10.1021/jm300459a BindingDB Entry DOI: 10.7270/Q2RN390V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50048183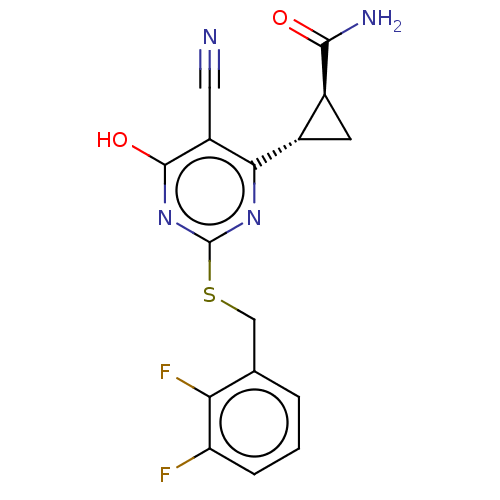 (CHEMBL3310788) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity against human recombinant CXCR2 receptor expressed in CHO cell membranes by SPA based [35S]GTPgammaS binding assay | Bioorg Med Chem Lett 24: 3285-90 (2014) Article DOI: 10.1016/j.bmcl.2014.06.011 BindingDB Entry DOI: 10.7270/Q2930VTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50113976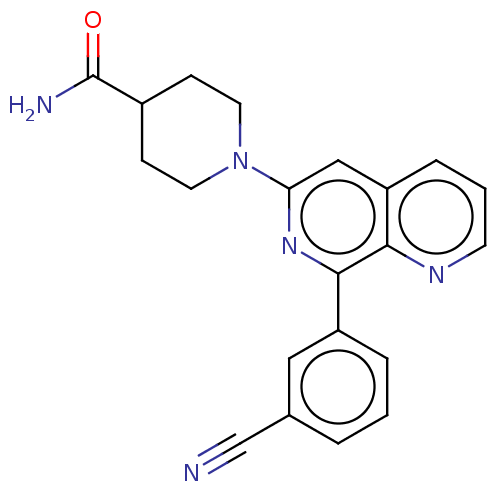 (CHEMBL3605510) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of human PDE4D | J Med Chem 58: 6747-52 (2015) Article DOI: 10.1021/acs.jmedchem.5b00902 BindingDB Entry DOI: 10.7270/Q2319XPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50048181 (CHEMBL3310786) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity against human recombinant CXCR2 receptor expressed in CHO cell membranes by SPA based [35S]GTPgammaS binding assay | Bioorg Med Chem Lett 24: 3285-90 (2014) Article DOI: 10.1016/j.bmcl.2014.06.011 BindingDB Entry DOI: 10.7270/Q2930VTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50113975 (CHEMBL3605511) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of human PDE4D | J Med Chem 58: 6747-52 (2015) Article DOI: 10.1021/acs.jmedchem.5b00902 BindingDB Entry DOI: 10.7270/Q2319XPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM50396765 (CHEMBL2172376) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of human PDE4B-mediated [3H]CAMP hydrolysis after 30 to 60 mins by scintillation proximity assay | J Med Chem 55: 7472-9 (2012) Article DOI: 10.1021/jm300459a BindingDB Entry DOI: 10.7270/Q2RN390V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50396759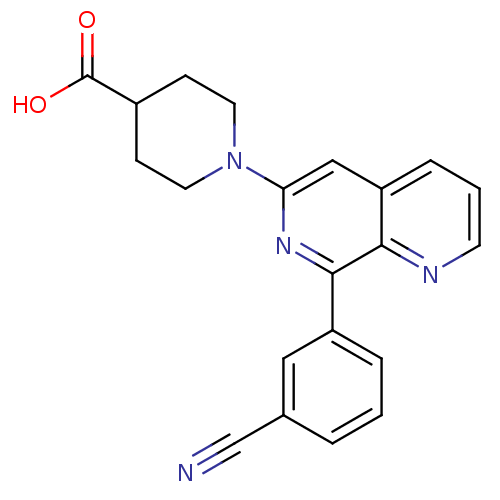 (CHEMBL2172370) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of human PDE4D | J Med Chem 58: 6747-52 (2015) Article DOI: 10.1021/acs.jmedchem.5b00902 BindingDB Entry DOI: 10.7270/Q2319XPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50396759 (CHEMBL2172370) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of human PDE4D-mediated [3H]CAMP hydrolysis after 30 to 60 mins by scintillation proximity assay | J Med Chem 55: 7472-9 (2012) Article DOI: 10.1021/jm300459a BindingDB Entry DOI: 10.7270/Q2RN390V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50113974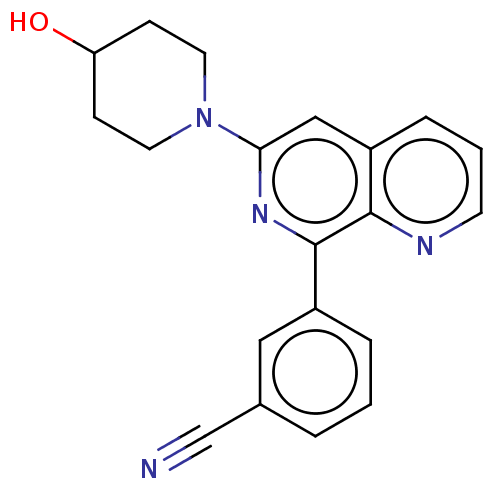 (CHEMBL3605512) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of human PDE4D | J Med Chem 58: 6747-52 (2015) Article DOI: 10.1021/acs.jmedchem.5b00902 BindingDB Entry DOI: 10.7270/Q2319XPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50396762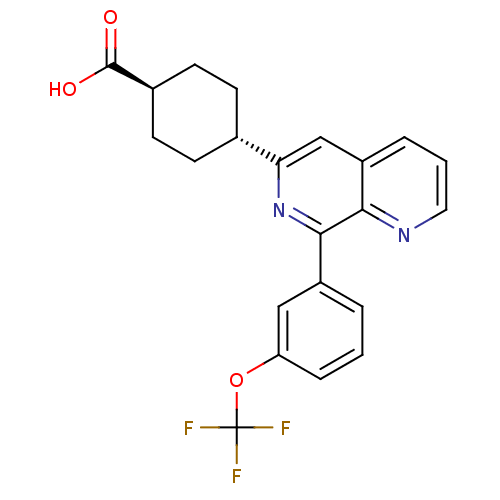 (CHEMBL2172373) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of human PDE4D-mediated [3H]CAMP hydrolysis after 30 to 60 mins by scintillation proximity assay | J Med Chem 55: 7472-9 (2012) Article DOI: 10.1021/jm300459a BindingDB Entry DOI: 10.7270/Q2RN390V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A (Homo sapiens (Human)) | BDBM50396765 (CHEMBL2172376) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of human PDE4A-mediated [3H]CAMP hydrolysis after 30 to 60 mins by scintillation proximity assay | J Med Chem 55: 7472-9 (2012) Article DOI: 10.1021/jm300459a BindingDB Entry DOI: 10.7270/Q2RN390V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50113977 (CHEMBL3605509) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of human PDE4D | J Med Chem 58: 6747-52 (2015) Article DOI: 10.1021/acs.jmedchem.5b00902 BindingDB Entry DOI: 10.7270/Q2319XPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50396758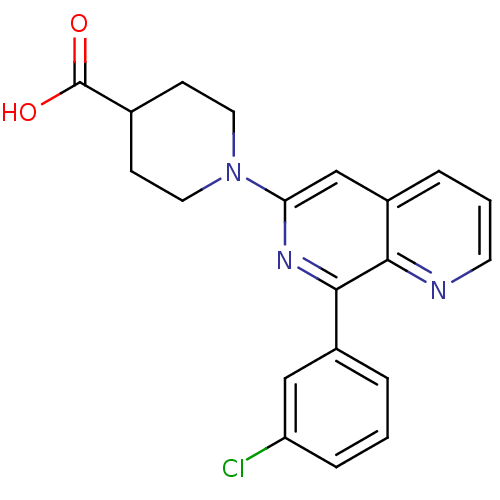 (CHEMBL2172369) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of human PDE4D-mediated [3H]CAMP hydrolysis after 30 to 60 mins by scintillation proximity assay | J Med Chem 55: 7472-9 (2012) Article DOI: 10.1021/jm300459a BindingDB Entry DOI: 10.7270/Q2RN390V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50396756 (CHEMBL2172367) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of human PDE4D-mediated [3H]CAMP hydrolysis after 30 to 60 mins by scintillation proximity assay | J Med Chem 55: 7472-9 (2012) Article DOI: 10.1021/jm300459a BindingDB Entry DOI: 10.7270/Q2RN390V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50396763 (CHEMBL2172374) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of human PDE4D | J Med Chem 58: 6747-52 (2015) Article DOI: 10.1021/acs.jmedchem.5b00902 BindingDB Entry DOI: 10.7270/Q2319XPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50396763 (CHEMBL2172374) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of human PDE4D-mediated [3H]CAMP hydrolysis after 30 to 60 mins by scintillation proximity assay | J Med Chem 55: 7472-9 (2012) Article DOI: 10.1021/jm300459a BindingDB Entry DOI: 10.7270/Q2RN390V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM50108504 (4-(8-(benzo[c][1,2,5]oxadiazol-5-yl)-1,7-naphthyri...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of human PDE4B | J Med Chem 55: 7472-9 (2012) Article DOI: 10.1021/jm300459a BindingDB Entry DOI: 10.7270/Q2RN390V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM50108504 (4-(8-(benzo[c][1,2,5]oxadiazol-5-yl)-1,7-naphthyri...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of PDE4B (unknown origin) | J Med Chem 58: 6747-52 (2015) Article DOI: 10.1021/acs.jmedchem.5b00902 BindingDB Entry DOI: 10.7270/Q2319XPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 151 total ) | Next | Last >> |