

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50066461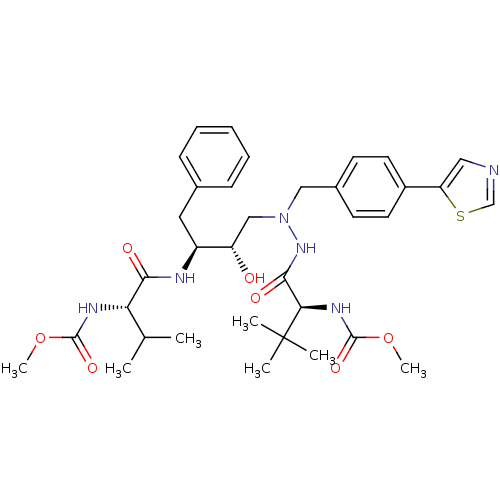 (CHEMBL326408 | {(S)-1-[N'-[(2S,3S)-2-Hydroxy-3-((S...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba-Geigy AG Curated by ChEMBL | Assay Description The percent reduction of the reverse transcriptase (RT) activity in HIV-1/MN-infected MT-2 cells. | J Med Chem 41: 3387-401 (1998) Article DOI: 10.1021/jm970873c BindingDB Entry DOI: 10.7270/Q2H1315D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50066474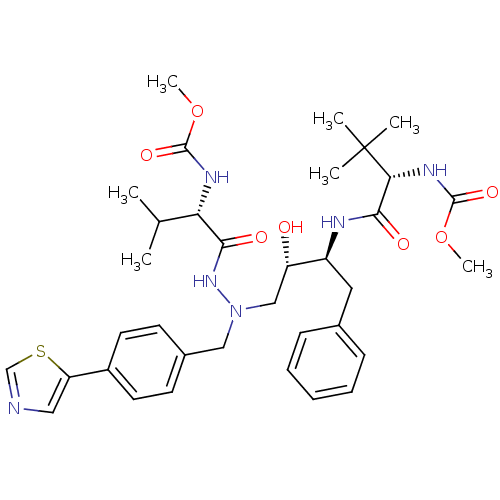 (((S)-1-{(1S,2S)-1-Benzyl-2-hydroxy-3-[N'-((S)-2-me...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba-Geigy AG Curated by ChEMBL | Assay Description The percent reduction of the reverse transcriptase (RT) activity in HIV-1/MN-infected MT-2 cells. | J Med Chem 41: 3387-401 (1998) Article DOI: 10.1021/jm970873c BindingDB Entry DOI: 10.7270/Q2H1315D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50066462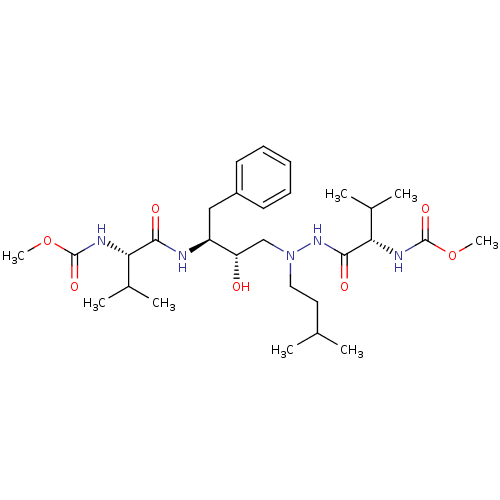 (CHEMBL326347 | {(S)-1-[N'-[(2S,3S)-2-Hydroxy-3-((S...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba-Geigy AG Curated by ChEMBL | Assay Description The percent reduction of the reverse transcriptase (RT) activity in HIV-1/MN-infected MT-2 cells. | J Med Chem 41: 3387-401 (1998) Article DOI: 10.1021/jm970873c BindingDB Entry DOI: 10.7270/Q2H1315D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50066473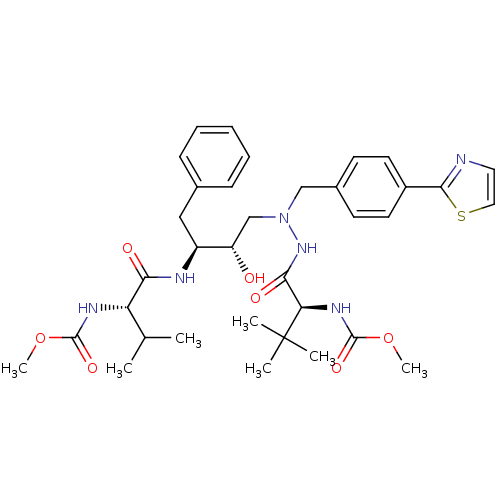 (CHEMBL325900 | {(S)-1-[N'-[(2S,3S)-2-Hydroxy-3-((S...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba-Geigy AG Curated by ChEMBL | Assay Description The percent reduction of the reverse transcriptase (RT) activity in HIV-1/MN-infected MT-2 cells. | J Med Chem 41: 3387-401 (1998) Article DOI: 10.1021/jm970873c BindingDB Entry DOI: 10.7270/Q2H1315D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50066476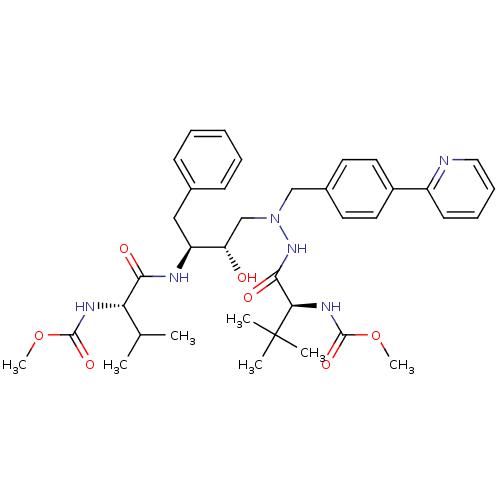 (CHEMBL333386 | {(S)-1-[N'-[(2S,3S)-2-Hydroxy-3-((S...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba-Geigy AG Curated by ChEMBL | Assay Description The percent reduction of the reverse transcriptase (RT) activity in HIV-1/MN-infected MT-2 cells. | J Med Chem 41: 3387-401 (1998) Article DOI: 10.1021/jm970873c BindingDB Entry DOI: 10.7270/Q2H1315D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50066459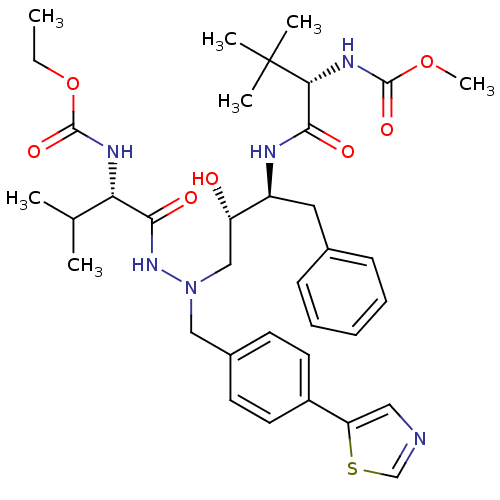 (((S)-1-{(1S,2S)-1-Benzyl-3-[N'-((S)-2-ethoxycarbon...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba-Geigy AG Curated by ChEMBL | Assay Description The percent reduction of the reverse transcriptase (RT) activity in HIV-1/MN-infected MT-2 cells. | J Med Chem 41: 3387-401 (1998) Article DOI: 10.1021/jm970873c BindingDB Entry DOI: 10.7270/Q2H1315D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM13934 (Atazanavir | BMS 232632 | CGP 73547 | CHEMBL1163 |...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba-Geigy AG Curated by ChEMBL | Assay Description The percent reduction of the reverse transcriptase (RT) activity in HIV-1/MN-infected MT-2 cells. | J Med Chem 41: 3387-401 (1998) Article DOI: 10.1021/jm970873c BindingDB Entry DOI: 10.7270/Q2H1315D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50066475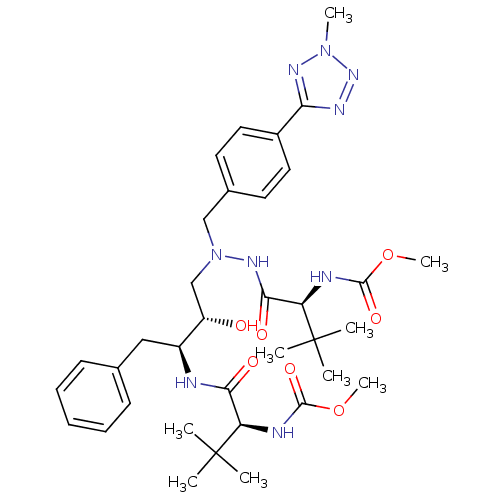 (((S)-1-{N'-[(2S,3S)-2-Hydroxy-3-((S)-2-methoxycarb...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba-Geigy AG Curated by ChEMBL | Assay Description The percent reduction of the reverse transcriptase (RT) activity in HIV-1/MN-infected MT-2 cells. | J Med Chem 41: 3387-401 (1998) Article DOI: 10.1021/jm970873c BindingDB Entry DOI: 10.7270/Q2H1315D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50066472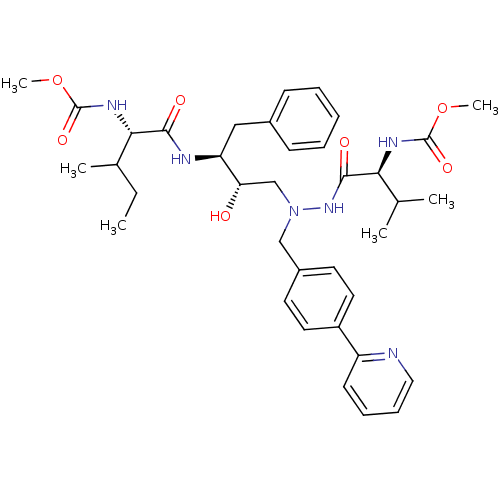 (((S)-1-{(1S,2S)-1-Benzyl-2-hydroxy-3-[N'-((S)-2-me...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba-Geigy AG Curated by ChEMBL | Assay Description The percent reduction of the reverse transcriptase (RT) activity in HIV-1/MN-infected MT-2 cells. | J Med Chem 41: 3387-401 (1998) Article DOI: 10.1021/jm970873c BindingDB Entry DOI: 10.7270/Q2H1315D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50066468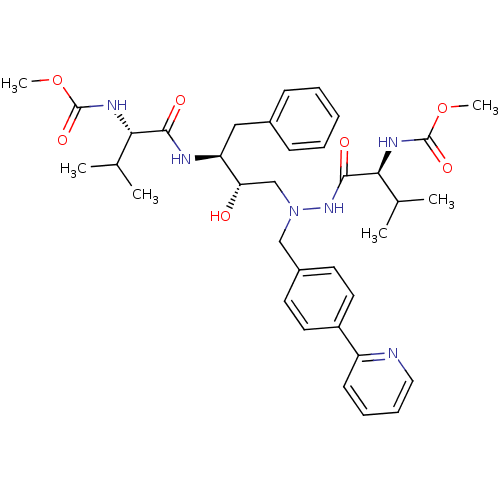 (CHEMBL324521 | {(S)-1-[N'-[(2S,3S)-2-Hydroxy-3-((S...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba-Geigy AG Curated by ChEMBL | Assay Description The percent reduction of the reverse transcriptase (RT) activity in HIV-1/MN-infected MT-2 cells. | J Med Chem 41: 3387-401 (1998) Article DOI: 10.1021/jm970873c BindingDB Entry DOI: 10.7270/Q2H1315D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50066463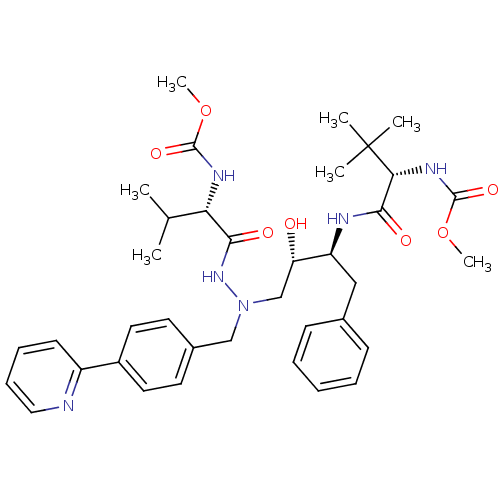 (((S)-1-{(1S,2S)-1-Benzyl-2-hydroxy-3-[N'-((S)-2-me...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba-Geigy AG Curated by ChEMBL | Assay Description The percent reduction of the reverse transcriptase (RT) activity in HIV-1/MN-infected MT-2 cells. | J Med Chem 41: 3387-401 (1998) Article DOI: 10.1021/jm970873c BindingDB Entry DOI: 10.7270/Q2H1315D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50066465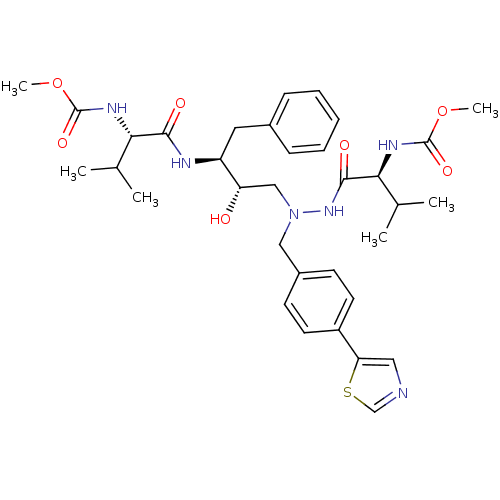 (CHEMBL442013 | {(S)-1-[N'-[(2S,3S)-2-Hydroxy-3-((S...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba-Geigy AG Curated by ChEMBL | Assay Description The percent reduction of the reverse transcriptase (RT) activity in HIV-1/MN-infected MT-2 cells. | J Med Chem 41: 3387-401 (1998) Article DOI: 10.1021/jm970873c BindingDB Entry DOI: 10.7270/Q2H1315D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50066467 (CHEMBL114039 | {(S)-1-[N'-[(2S,3S)-2-Hydroxy-3-((S...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba-Geigy AG Curated by ChEMBL | Assay Description The percent reduction of the reverse transcriptase (RT) activity in HIV-1/MN-infected MT-2 cells. | J Med Chem 41: 3387-401 (1998) Article DOI: 10.1021/jm970873c BindingDB Entry DOI: 10.7270/Q2H1315D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50066469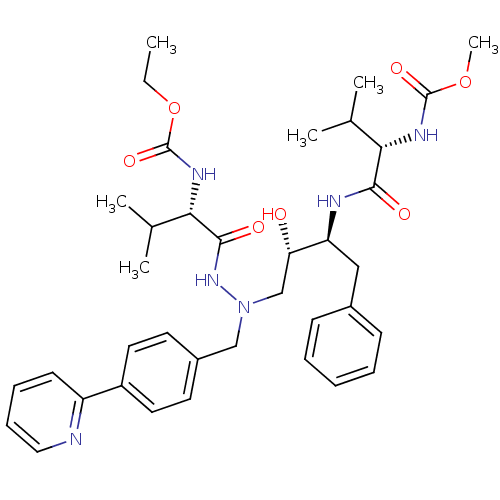 (CHEMBL113943 | {(S)-1-[N'-[(2S,3S)-2-Hydroxy-3-((S...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba-Geigy AG Curated by ChEMBL | Assay Description The percent reduction of the reverse transcriptase (RT) activity in HIV-1/MN-infected MT-2 cells. | J Med Chem 41: 3387-401 (1998) Article DOI: 10.1021/jm970873c BindingDB Entry DOI: 10.7270/Q2H1315D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50066471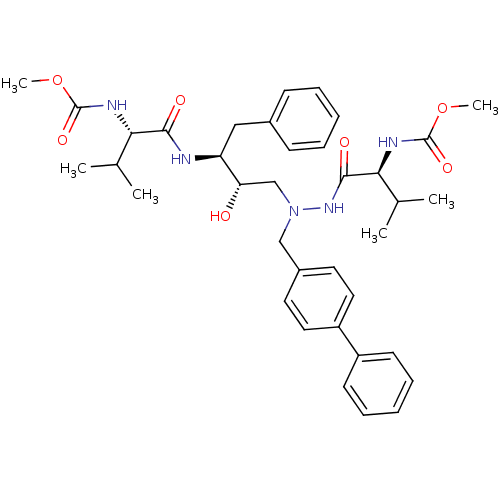 (((S)-1-{N'-Biphenyl-4-ylmethyl-N'-[(2S,3S)-2-hydro...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba-Geigy AG Curated by ChEMBL | Assay Description The percent reduction of the reverse transcriptase (RT) activity in HIV-1/MN-infected MT-2 cells. | J Med Chem 41: 3387-401 (1998) Article DOI: 10.1021/jm970873c BindingDB Entry DOI: 10.7270/Q2H1315D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50066464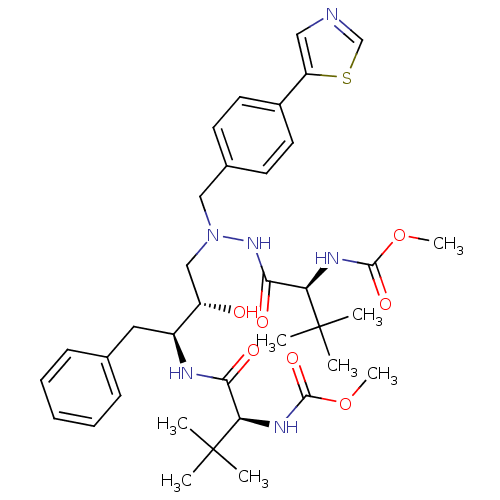 (CHEMBL116165 | {(S)-1-[N'-[(2S,3S)-2-Hydroxy-3-((S...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba-Geigy AG Curated by ChEMBL | Assay Description The percent reduction of the reverse transcriptase (RT) activity in HIV-1/MN-infected MT-2 cells. | J Med Chem 41: 3387-401 (1998) Article DOI: 10.1021/jm970873c BindingDB Entry DOI: 10.7270/Q2H1315D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50066477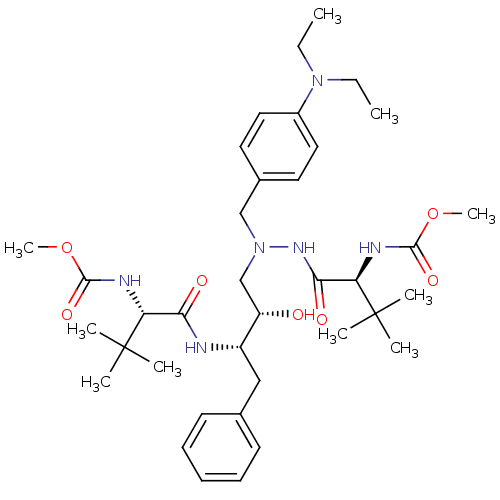 (((S)-1-{N'-(4-Diethylamino-benzyl)-N'-[(2S,3S)-2-h...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba-Geigy AG Curated by ChEMBL | Assay Description The percent reduction of the reverse transcriptase (RT) activity in HIV-1/MN-infected MT-2 cells. | J Med Chem 41: 3387-401 (1998) Article DOI: 10.1021/jm970873c BindingDB Entry DOI: 10.7270/Q2H1315D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50066460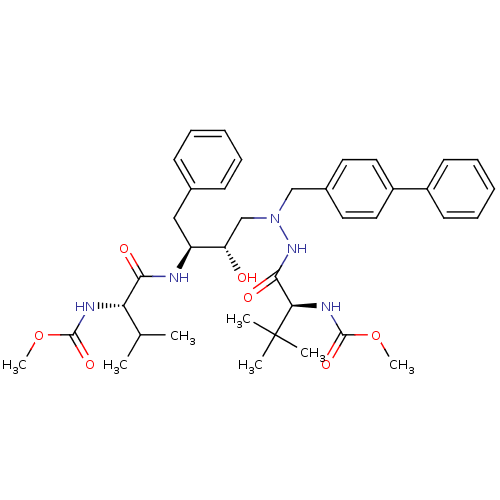 (((S)-1-{N'-Biphenyl-4-ylmethyl-N'-[(2S,3S)-2-hydro...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba-Geigy AG Curated by ChEMBL | Assay Description The percent reduction of the reverse transcriptase (RT) activity in HIV-1/MN-infected MT-2 cells. | J Med Chem 41: 3387-401 (1998) Article DOI: 10.1021/jm970873c BindingDB Entry DOI: 10.7270/Q2H1315D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50066466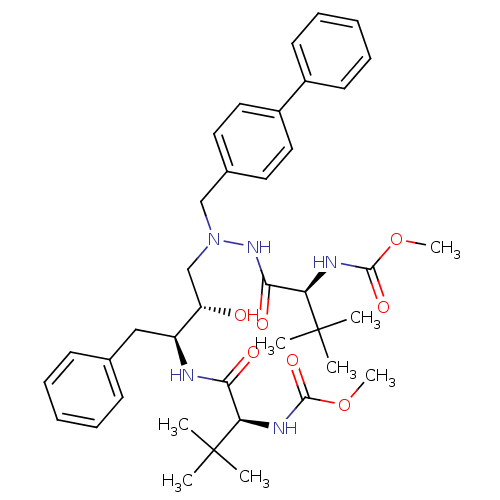 (((S)-1-{N'-Biphenyl-4-ylmethyl-N'-[(2S,3S)-2-hydro...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba-Geigy AG Curated by ChEMBL | Assay Description The percent reduction of the reverse transcriptase (RT) activity in HIV-1/MN-infected MT-2 cells. | J Med Chem 41: 3387-401 (1998) Article DOI: 10.1021/jm970873c BindingDB Entry DOI: 10.7270/Q2H1315D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50066470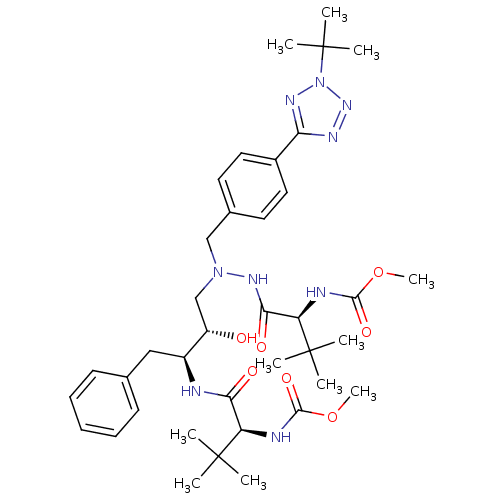 (((S)-1-{N'-[4-(2-tert-Butyl-2H-tetrazol-5-yl)-benz...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba-Geigy AG Curated by ChEMBL | Assay Description The percent reduction of the reverse transcriptase (RT) activity in HIV-1/MN-infected MT-2 cells. | J Med Chem 41: 3387-401 (1998) Article DOI: 10.1021/jm970873c BindingDB Entry DOI: 10.7270/Q2H1315D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50066458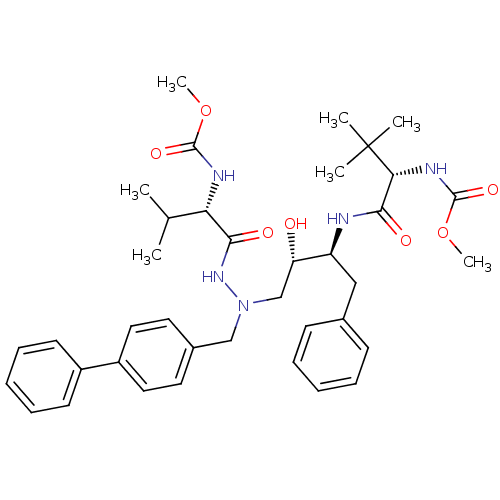 (((S)-1-{(1S,2S)-1-Benzyl-3-[N-biphenyl-4-ylmethyl-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba-Geigy AG Curated by ChEMBL | Assay Description The percent reduction of the reverse transcriptase (RT) activity in HIV-1/MN-infected MT-2 cells. | J Med Chem 41: 3387-401 (1998) Article DOI: 10.1021/jm970873c BindingDB Entry DOI: 10.7270/Q2H1315D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM202 (CGP 53820 analog | CHEMBL324572 | ethyl N-[(1S)-1-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 177 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba-Geigy AG Curated by ChEMBL | Assay Description The percent reduction of the reverse transcriptase (RT) activity in HIV-1/MN-infected MT-2 cells. | J Med Chem 41: 3387-401 (1998) Article DOI: 10.1021/jm970873c BindingDB Entry DOI: 10.7270/Q2H1315D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50031643 (1-(4-Benzyloxy-phenyl)-2-dimethylaminomethyl-prope...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA Limited Curated by ChEMBL | Assay Description In vitro inhibition of the Epidermal growth factor receptor activity in A431 membranes | J Med Chem 38: 2441-8 (1995) BindingDB Entry DOI: 10.7270/Q2MS3TDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50031651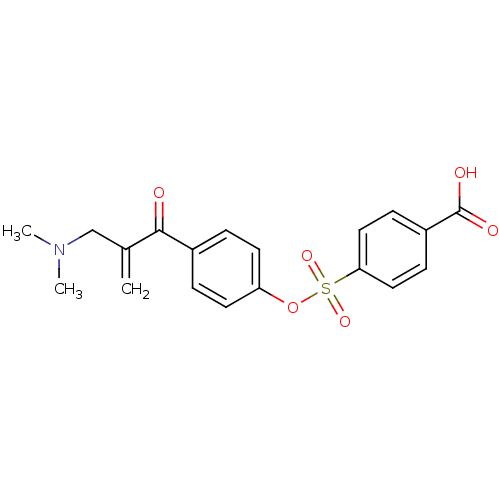 (4-[4-(2-Dimethylaminomethyl-acryloyl)-phenoxysulfo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA Limited Curated by ChEMBL | Assay Description In vitro inhibition of the Epidermal growth factor receptor activity in A431 membranes | J Med Chem 38: 2441-8 (1995) BindingDB Entry DOI: 10.7270/Q2MS3TDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50031655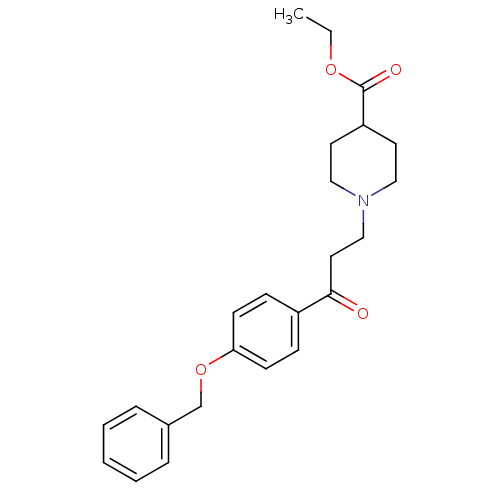 (1-[3-(4-Benzyloxy-phenyl)-3-oxo-propyl]-piperidine...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA Limited Curated by ChEMBL | Assay Description In vitro inhibition of the Epidermal growth factor receptor activity in A431 membranes | J Med Chem 38: 2441-8 (1995) BindingDB Entry DOI: 10.7270/Q2MS3TDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50031657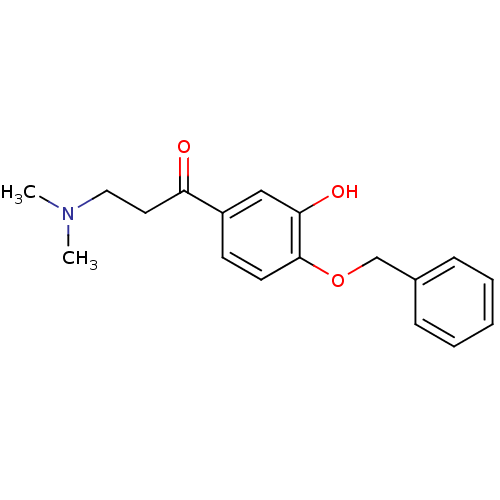 (1-(4-Benzyloxy-3-hydroxy-phenyl)-3-dimethylamino-p...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA Limited Curated by ChEMBL | Assay Description In vitro inhibition of the Epidermal growth factor receptor activity in A431 membranes | J Med Chem 38: 2441-8 (1995) BindingDB Entry DOI: 10.7270/Q2MS3TDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50031659 (4-[4-(2-Dimethylaminomethyl-acryloyl)-phenoxysulfo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA Limited Curated by ChEMBL | Assay Description In vitro inhibition of the Epidermal growth factor receptor activity in A431 membranes | J Med Chem 38: 2441-8 (1995) BindingDB Entry DOI: 10.7270/Q2MS3TDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50031648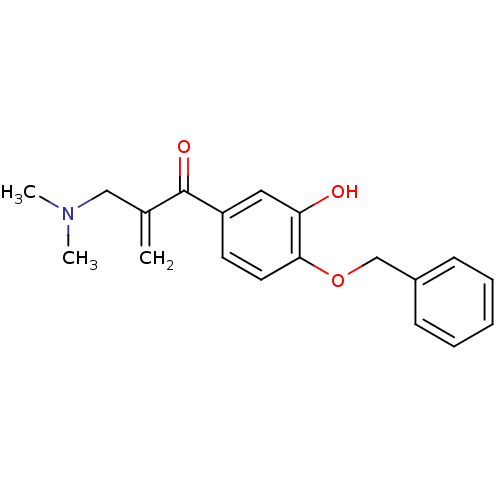 (1-(4-Benzyloxy-3-hydroxy-phenyl)-2-dimethylaminome...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 670 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA Limited Curated by ChEMBL | Assay Description In vitro inhibition of the Epidermal growth factor receptor activity in A431 membranes | J Med Chem 38: 2441-8 (1995) BindingDB Entry DOI: 10.7270/Q2MS3TDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50031642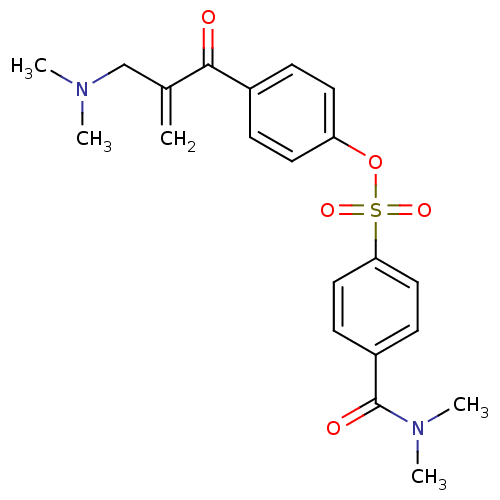 (4-Dimethylcarbamoyl-benzenesulfonic acid 4-(2-dime...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA Limited Curated by ChEMBL | Assay Description In vitro inhibition of the Epidermal growth factor receptor activity in A431 membranes | J Med Chem 38: 2441-8 (1995) BindingDB Entry DOI: 10.7270/Q2MS3TDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM19459 (5,7-dihydroxy-3-(4-hydroxyphenyl)-4H-chromen-4-one...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA Limited Curated by ChEMBL | Assay Description In vitro inhibition of the Epidermal growth factor receptor activity in A431 membranes | J Med Chem 38: 2441-8 (1995) BindingDB Entry DOI: 10.7270/Q2MS3TDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50031647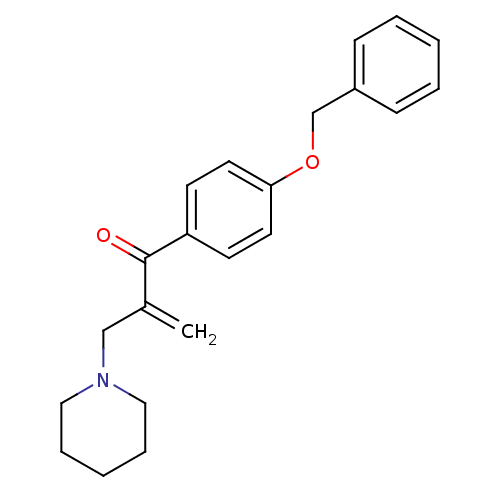 (1-(4-Benzyloxy-phenyl)-2-piperidin-1-ylmethyl-prop...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA Limited Curated by ChEMBL | Assay Description In vitro inhibition of the Epidermal growth factor receptor activity in A431 membranes | J Med Chem 38: 2441-8 (1995) BindingDB Entry DOI: 10.7270/Q2MS3TDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50031662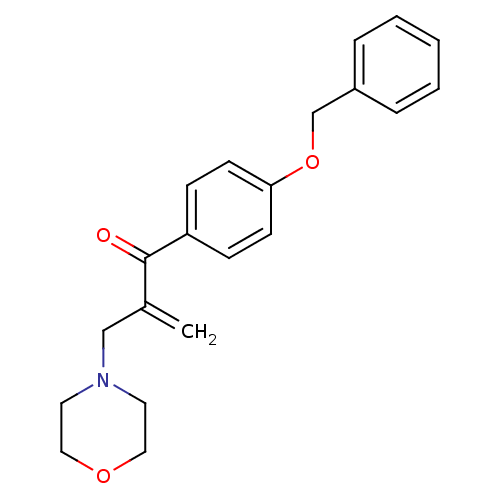 (1-(4-Benzyloxy-phenyl)-2-morpholin-4-ylmethyl-prop...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA Limited Curated by ChEMBL | Assay Description In vitro inhibition of the Epidermal growth factor receptor activity in A431 membranes | J Med Chem 38: 2441-8 (1995) BindingDB Entry DOI: 10.7270/Q2MS3TDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50031660 (1-(4-Benzyloxy-phenyl)-3-dimethylamino-propan-1-on...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA Limited Curated by ChEMBL | Assay Description In vitro inhibition of the Epidermal growth factor receptor activity in A431 membranes | J Med Chem 38: 2441-8 (1995) BindingDB Entry DOI: 10.7270/Q2MS3TDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50031646 (1-(4-Benzyloxy-phenyl)-2-piperazin-1-ylmethyl-prop...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA Limited Curated by ChEMBL | Assay Description In vitro inhibition of the Epidermal growth factor receptor activity in A431 membranes | J Med Chem 38: 2441-8 (1995) BindingDB Entry DOI: 10.7270/Q2MS3TDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50031661 (1-(4-Benzyloxy-phenyl)-3-morpholin-4-yl-propan-1-o...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA Limited Curated by ChEMBL | Assay Description In vitro inhibition of the Epidermal growth factor receptor activity in A431 membranes | J Med Chem 38: 2441-8 (1995) BindingDB Entry DOI: 10.7270/Q2MS3TDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50031658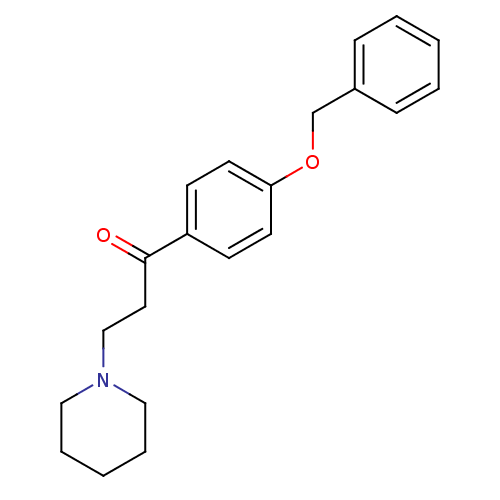 (1-(4-Benzyloxy-phenyl)-3-piperidin-1-yl-propan-1-o...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA Limited Curated by ChEMBL | Assay Description In vitro inhibition of the Epidermal growth factor receptor activity in A431 membranes | J Med Chem 38: 2441-8 (1995) BindingDB Entry DOI: 10.7270/Q2MS3TDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50031644 (1-(4-Benzyloxy-phenyl)-3-dimethylamino-2-(3,4-dime...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA Limited Curated by ChEMBL | Assay Description In vitro inhibition of the Epidermal growth factor receptor activity in A431 membranes | J Med Chem 38: 2441-8 (1995) BindingDB Entry DOI: 10.7270/Q2MS3TDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50031649 (2-{3-(4-benzyloxy)-2-[(dimethylamino)methyl]-3-oxo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA Limited Curated by ChEMBL | Assay Description In vitro inhibition of the Epidermal growth factor receptor activity in A431 membranes | J Med Chem 38: 2441-8 (1995) BindingDB Entry DOI: 10.7270/Q2MS3TDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50369028 (CHEMBL468975) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA Limited Curated by ChEMBL | Assay Description In vitro inhibition of the Epidermal growth factor receptor activity in A431 membranes | J Med Chem 38: 2441-8 (1995) BindingDB Entry DOI: 10.7270/Q2MS3TDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50031656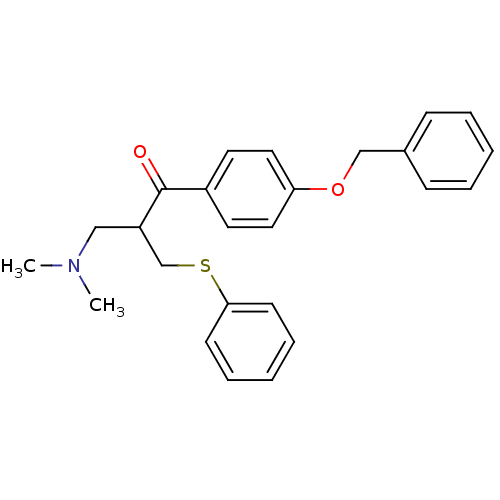 (1-(4-Benzyloxy-phenyl)-3-dimethylamino-2-phenylsul...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA Limited Curated by ChEMBL | Assay Description In vitro inhibition of the Epidermal growth factor receptor activity in A431 membranes | J Med Chem 38: 2441-8 (1995) BindingDB Entry DOI: 10.7270/Q2MS3TDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50031650 (2-[3-(4-Benzyloxy-phenyl)-2-dimethylaminomethyl-3-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA Limited Curated by ChEMBL | Assay Description In vitro inhibition of the Epidermal growth factor receptor activity in A431 membranes | J Med Chem 38: 2441-8 (1995) BindingDB Entry DOI: 10.7270/Q2MS3TDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50031653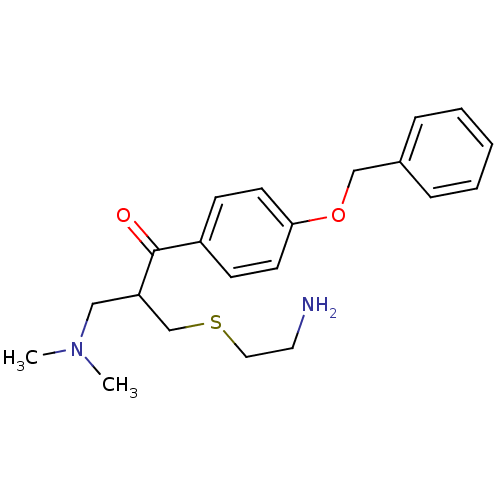 (2-(2-Amino-ethylsulfanylmethyl)-1-(4-benzyloxy-phe...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 8.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA Limited Curated by ChEMBL | Assay Description In vitro inhibition of the Epidermal growth factor receptor activity in A431 membranes | J Med Chem 38: 2441-8 (1995) BindingDB Entry DOI: 10.7270/Q2MS3TDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM19459 (5,7-dihydroxy-3-(4-hydroxyphenyl)-4H-chromen-4-one...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA Limited Curated by ChEMBL | Assay Description In vitro inhibition of the v-abl tyrosine kinase activity in A431 membranes using angiotensin II as phosphate acceptor as substrate | J Med Chem 38: 2441-8 (1995) BindingDB Entry DOI: 10.7270/Q2MS3TDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50031642 (4-Dimethylcarbamoyl-benzenesulfonic acid 4-(2-dime...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA Limited Curated by ChEMBL | Assay Description In vitro inhibition of the c-src tyrosine kinase activity in A431 membranes using angiotensin II as phosphate acceptor as substrate | J Med Chem 38: 2441-8 (1995) BindingDB Entry DOI: 10.7270/Q2MS3TDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50031659 (4-[4-(2-Dimethylaminomethyl-acryloyl)-phenoxysulfo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA Limited Curated by ChEMBL | Assay Description In vitro inhibition of the c-src tyrosine kinase activity in A431 membranes using angiotensin II as phosphate acceptor as substrate | J Med Chem 38: 2441-8 (1995) BindingDB Entry DOI: 10.7270/Q2MS3TDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM50031659 (4-[4-(2-Dimethylaminomethyl-acryloyl)-phenoxysulfo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA Limited Curated by ChEMBL | Assay Description In vitro inhibition of the v-abl tyrosine kinase activity in A431 membranes using angiotensin II as phosphate acceptor as substrate | J Med Chem 38: 2441-8 (1995) BindingDB Entry DOI: 10.7270/Q2MS3TDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM50031653 (2-(2-Amino-ethylsulfanylmethyl)-1-(4-benzyloxy-phe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA Limited Curated by ChEMBL | Assay Description In vitro inhibition of the v-abl tyrosine kinase activity in A431 membranes using angiotensin II as phosphate acceptor as substrate | J Med Chem 38: 2441-8 (1995) BindingDB Entry DOI: 10.7270/Q2MS3TDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM50031651 (4-[4-(2-Dimethylaminomethyl-acryloyl)-phenoxysulfo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA Limited Curated by ChEMBL | Assay Description In vitro inhibition of the v-abl tyrosine kinase activity in A431 membranes using angiotensin II as phosphate acceptor as substrate | J Med Chem 38: 2441-8 (1995) BindingDB Entry DOI: 10.7270/Q2MS3TDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM50031648 (1-(4-Benzyloxy-3-hydroxy-phenyl)-2-dimethylaminome...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA Limited Curated by ChEMBL | Assay Description In vitro inhibition of the v-abl tyrosine kinase activity in A431 membranes using angiotensin II as phosphate acceptor as substrate | J Med Chem 38: 2441-8 (1995) BindingDB Entry DOI: 10.7270/Q2MS3TDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50031657 (1-(4-Benzyloxy-3-hydroxy-phenyl)-3-dimethylamino-p...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA Limited Curated by ChEMBL | Assay Description In vitro inhibition of the c-src tyrosine kinase activity in A431 membranes using angiotensin II as phosphate acceptor as substrate | J Med Chem 38: 2441-8 (1995) BindingDB Entry DOI: 10.7270/Q2MS3TDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 88 total ) | Next | Last >> |