 Found 4639 hits with Last Name = 'ravi' and Initial = 'm'
Found 4639 hits with Last Name = 'ravi' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50182163
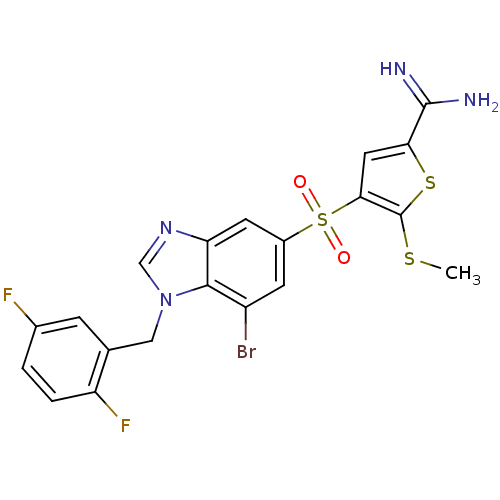
(4-[7-bromo-1-(2,5-difluoro-benzyl)-1H-benzoimidazo...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cc(Br)c2n(Cc3cc(F)ccc3F)cnc2c1)C(N)=N Show InChI InChI=1S/C20H15BrF2N4O2S3/c1-30-20-17(7-16(31-20)19(24)25)32(28,29)12-5-13(21)18-15(6-12)26-9-27(18)8-10-4-11(22)2-3-14(10)23/h2-7,9H,8H2,1H3,(H3,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of C1S |
Bioorg Med Chem Lett 16: 2200-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.036
BindingDB Entry DOI: 10.7270/Q2251HSV |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50233679
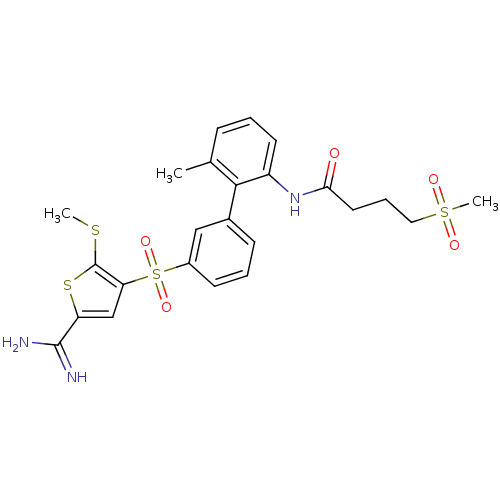
(CHEMBL399284 | N-[3'-(5-carbamimidoyl-2-methylsulf...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cccc(c1)-c1c(C)cccc1NC(=O)CCCS(C)(=O)=O)C(N)=N Show InChI InChI=1S/C24H27N3O5S4/c1-15-7-4-10-18(27-21(28)11-6-12-35(3,29)30)22(15)16-8-5-9-17(13-16)36(31,32)20-14-19(23(25)26)34-24(20)33-2/h4-5,7-10,13-14H,6,11-12H2,1-3H3,(H3,25,26)(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Complement C1s subcomponent |
Bioorg Med Chem Lett 18: 1603-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.064
BindingDB Entry DOI: 10.7270/Q2K35TD4 |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50182160
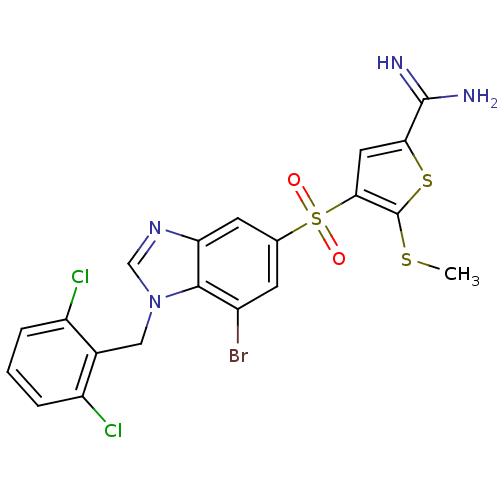
(4-[7-bromo-1-(2,6-dichloro-benzyl)-1H-benzoimidazo...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cc(Br)c2n(Cc3c(Cl)cccc3Cl)cnc2c1)C(N)=N Show InChI InChI=1S/C20H15BrCl2N4O2S3/c1-30-20-17(7-16(31-20)19(24)25)32(28,29)10-5-12(21)18-15(6-10)26-9-27(18)8-11-13(22)3-2-4-14(11)23/h2-7,9H,8H2,1H3,(H3,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of C1S |
Bioorg Med Chem Lett 16: 2200-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.036
BindingDB Entry DOI: 10.7270/Q2251HSV |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50233691

(4-(2'-amino-6'-methyl-biphenyl-3-sulfonyl)-5-methy...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cccc(c1)-c1c(C)cccc1N)C(N)=N Show InChI InChI=1S/C19H19N3O2S3/c1-11-5-3-8-14(20)17(11)12-6-4-7-13(9-12)27(23,24)16-10-15(18(21)22)26-19(16)25-2/h3-10H,20H2,1-2H3,(H3,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Complement C1s subcomponent |
Bioorg Med Chem Lett 18: 1603-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.064
BindingDB Entry DOI: 10.7270/Q2K35TD4 |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50233674
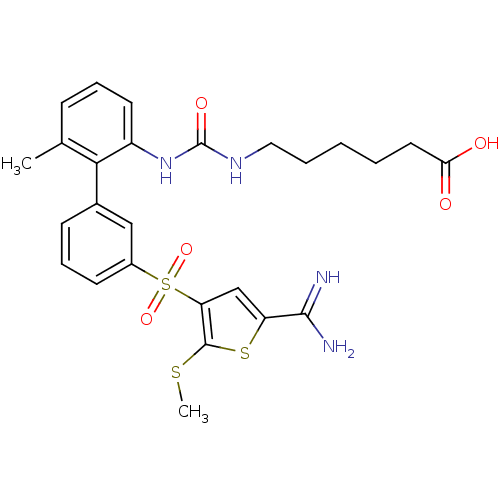
(6-{3-[3'-(5-carbamimidoyl-2-methylsulfanyl-thiophe...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cccc(c1)-c1c(C)cccc1NC(=O)NCCCCCC(O)=O)C(N)=N Show InChI InChI=1S/C26H30N4O5S3/c1-16-8-6-11-19(30-26(33)29-13-5-3-4-12-22(31)32)23(16)17-9-7-10-18(14-17)38(34,35)21-15-20(24(27)28)37-25(21)36-2/h6-11,14-15H,3-5,12-13H2,1-2H3,(H3,27,28)(H,31,32)(H2,29,30,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Complement C1s subcomponent |
Bioorg Med Chem Lett 18: 1603-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.064
BindingDB Entry DOI: 10.7270/Q2K35TD4 |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50182171
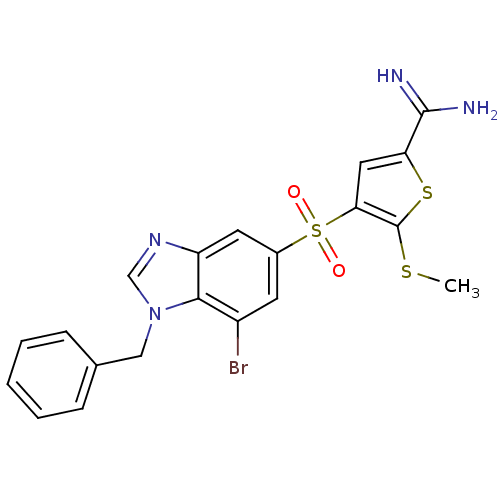
(4-(1-benzyl-7-bromo-1H-benzoimidazole-5-sulfonyl)-...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cc(Br)c2n(Cc3ccccc3)cnc2c1)C(N)=N Show InChI InChI=1S/C20H17BrN4O2S3/c1-28-20-17(9-16(29-20)19(22)23)30(26,27)13-7-14(21)18-15(8-13)24-11-25(18)10-12-5-3-2-4-6-12/h2-9,11H,10H2,1H3,(H3,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of C1S |
Bioorg Med Chem Lett 16: 2200-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.036
BindingDB Entry DOI: 10.7270/Q2251HSV |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50182170
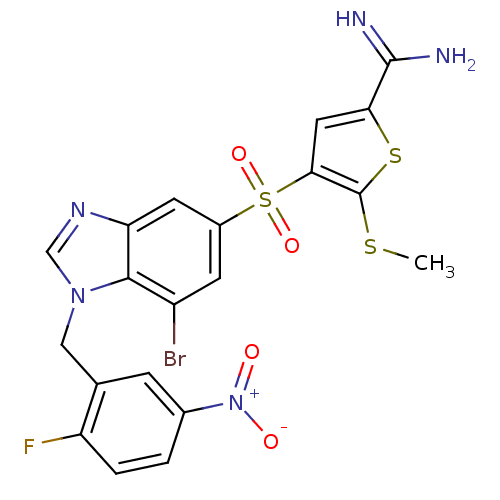
(4-[7-bromo-1-(2-fluoro-5-nitro-benzyl)-1H-benzoimi...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cc(Br)c2n(Cc3cc(ccc3F)[N+]([O-])=O)cnc2c1)C(N)=N Show InChI InChI=1S/C20H15BrFN5O4S3/c1-32-20-17(7-16(33-20)19(23)24)34(30,31)12-5-13(21)18-15(6-12)25-9-26(18)8-10-4-11(27(28)29)2-3-14(10)22/h2-7,9H,8H2,1H3,(H3,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of C1S |
Bioorg Med Chem Lett 16: 2200-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.036
BindingDB Entry DOI: 10.7270/Q2251HSV |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50233686

(4-(2'-methyl-biphenyl-3-sulfonyl)-5-methylsulfanyl...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cccc(c1)-c1ccccc1C)C(N)=N Show InChI InChI=1S/C19H18N2O2S3/c1-12-6-3-4-9-15(12)13-7-5-8-14(10-13)26(22,23)17-11-16(18(20)21)25-19(17)24-2/h3-11H,1-2H3,(H3,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Complement C1s subcomponent |
Bioorg Med Chem Lett 18: 1603-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.064
BindingDB Entry DOI: 10.7270/Q2K35TD4 |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50233688

(4-(2'-chloro-biphenyl-3-sulfonyl)-5-methylsulfanyl...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cccc(c1)-c1ccccc1Cl)C(N)=N Show InChI InChI=1S/C18H15ClN2O2S3/c1-24-18-16(10-15(25-18)17(20)21)26(22,23)12-6-4-5-11(9-12)13-7-2-3-8-14(13)19/h2-10H,1H3,(H3,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Complement C1s subcomponent |
Bioorg Med Chem Lett 18: 1603-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.064
BindingDB Entry DOI: 10.7270/Q2K35TD4 |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50233692

(4-[3-(6-methyl-pyridin-2-yl)-benzenesulfonyl]-5-me...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cccc(c1)-c1cccc(C)n1)C(N)=N Show InChI InChI=1S/C18H17N3O2S3/c1-11-5-3-8-14(21-11)12-6-4-7-13(9-12)26(22,23)16-10-15(17(19)20)25-18(16)24-2/h3-10H,1-2H3,(H3,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Complement C1s subcomponent |
Bioorg Med Chem Lett 18: 1603-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.064
BindingDB Entry DOI: 10.7270/Q2K35TD4 |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50182159
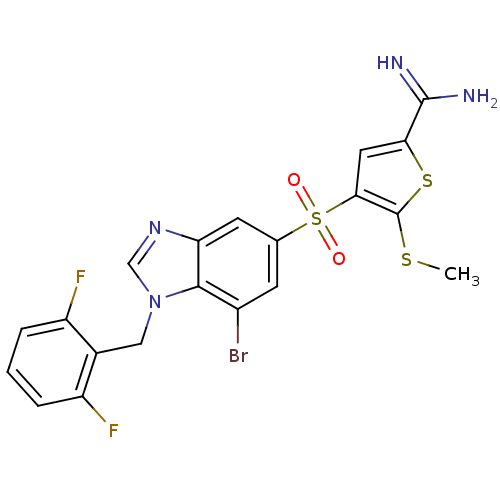
(4-[7-bromo-1-(2,6-difluoro-benzyl)-1H-benzoimidazo...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cc(Br)c2n(Cc3c(F)cccc3F)cnc2c1)C(N)=N Show InChI InChI=1S/C20H15BrF2N4O2S3/c1-30-20-17(7-16(31-20)19(24)25)32(28,29)10-5-12(21)18-15(6-10)26-9-27(18)8-11-13(22)3-2-4-14(11)23/h2-7,9H,8H2,1H3,(H3,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of C1S |
Bioorg Med Chem Lett 16: 2200-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.036
BindingDB Entry DOI: 10.7270/Q2251HSV |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50233694

(5-[3'-(5-carbamimidoyl-2-methylsulfanyl-thiophene-...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cccc(c1)-c1c(C)cccc1NC(=O)CCCCC(O)=O)C(N)=N Show InChI InChI=1S/C25H27N3O5S3/c1-15-7-5-10-18(28-21(29)11-3-4-12-22(30)31)23(15)16-8-6-9-17(13-16)36(32,33)20-14-19(24(26)27)35-25(20)34-2/h5-10,13-14H,3-4,11-12H2,1-2H3,(H3,26,27)(H,28,29)(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Complement C1s subcomponent |
Bioorg Med Chem Lett 18: 1603-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.064
BindingDB Entry DOI: 10.7270/Q2K35TD4 |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50233677

(4-(2'-hydroxymethyl-6'-methyl-biphenyl-3-sulfonyl)...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cccc(c1)-c1c(C)cccc1CO)C(N)=N Show InChI InChI=1S/C20H20N2O3S3/c1-12-5-3-7-14(11-23)18(12)13-6-4-8-15(9-13)28(24,25)17-10-16(19(21)22)27-20(17)26-2/h3-10,23H,11H2,1-2H3,(H3,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Complement C1s subcomponent |
Bioorg Med Chem Lett 18: 1603-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.064
BindingDB Entry DOI: 10.7270/Q2K35TD4 |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50182176
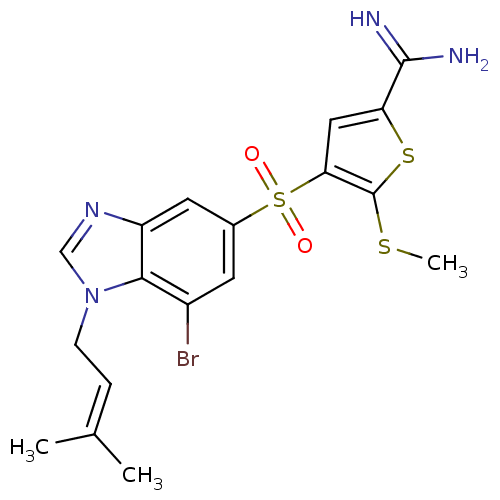
(4-[7-bromo-1-(3-methyl-but-2-enyl)-1H-benzoimidazo...)Show SMILES [#6]-[#16]-c1sc(cc1S(=O)(=O)c1cc(Br)c2n(-[#6]\[#6]=[#6](/[#6])-[#6])cnc2c1)-[#6](-[#7])=[#7] Show InChI InChI=1S/C18H19BrN4O2S3/c1-10(2)4-5-23-9-22-13-7-11(6-12(19)16(13)23)28(24,25)15-8-14(17(20)21)27-18(15)26-3/h4,6-9H,5H2,1-3H3,(H3,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of C1S |
Bioorg Med Chem Lett 16: 2200-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.036
BindingDB Entry DOI: 10.7270/Q2251HSV |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50233689
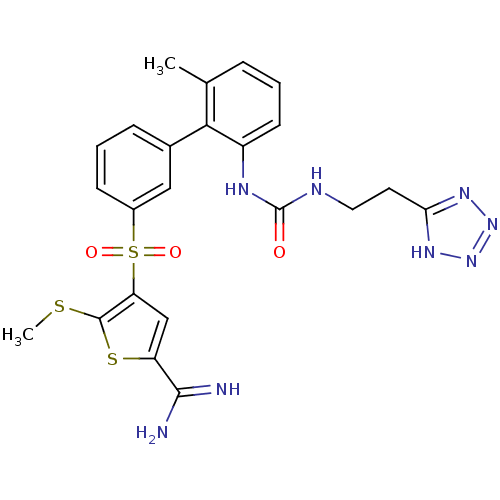
(5-methylsulfanyl-4-(6'-methyl-2'-{3-[2-(2H-tetrazo...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cccc(c1)-c1c(C)cccc1NC(=O)NCCc1nnn[nH]1)C(N)=N Show InChI InChI=1S/C23H24N8O3S3/c1-13-5-3-8-16(27-23(32)26-10-9-19-28-30-31-29-19)20(13)14-6-4-7-15(11-14)37(33,34)18-12-17(21(24)25)36-22(18)35-2/h3-8,11-12H,9-10H2,1-2H3,(H3,24,25)(H2,26,27,32)(H,28,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Complement C1s subcomponent |
Bioorg Med Chem Lett 18: 1603-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.064
BindingDB Entry DOI: 10.7270/Q2K35TD4 |
More data for this
Ligand-Target Pair | |
Proton-coupled folate transporter
(Homo sapiens (Human)) | BDBM50503240
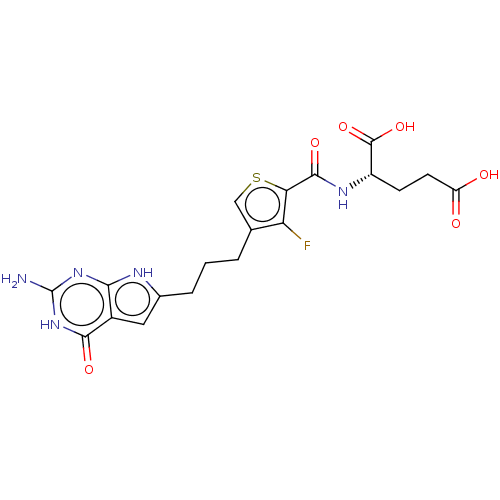
(CHEMBL4445651)Show SMILES Nc1nc2[nH]c(CCCc3csc(C(=O)N[C@@H](CCC(O)=O)C(O)=O)c3F)cc2c(=O)[nH]1 |r| Show InChI InChI=1S/C19H20FN5O6S/c20-13-8(2-1-3-9-6-10-15(22-9)24-19(21)25-16(10)28)7-32-14(13)17(29)23-11(18(30)31)4-5-12(26)27/h6-7,11H,1-5H2,(H,23,29)(H,26,27)(H,30,31)(H4,21,22,24,25,28)/t11-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of human PCFT expressed in Chinese hamster R2/PCFT4 cells assessed as reduction in [3H]MTX uptake at pH 5.5 measured over 2 mins by Dixon ... |
J Med Chem 61: 4228-4248 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00408
BindingDB Entry DOI: 10.7270/Q2M048QF |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50182185
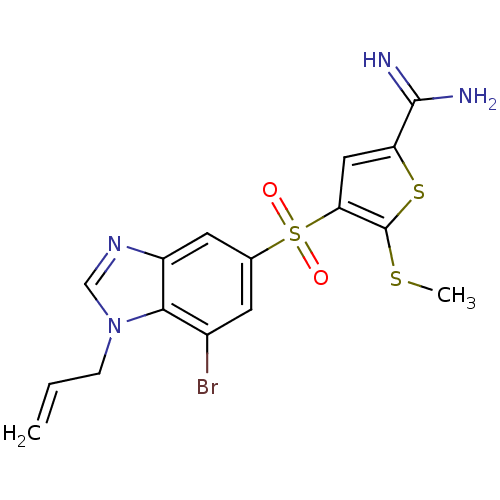
(4-(1-allyl-7-bromo-1H-benzoimidazole-5-sulfonyl)-5...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cc(Br)c2n(CC=C)cnc2c1)C(N)=N Show InChI InChI=1S/C16H15BrN4O2S3/c1-3-4-21-8-20-11-6-9(5-10(17)14(11)21)26(22,23)13-7-12(15(18)19)25-16(13)24-2/h3,5-8H,1,4H2,2H3,(H3,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of C1S |
Bioorg Med Chem Lett 16: 2200-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.036
BindingDB Entry DOI: 10.7270/Q2251HSV |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50182173
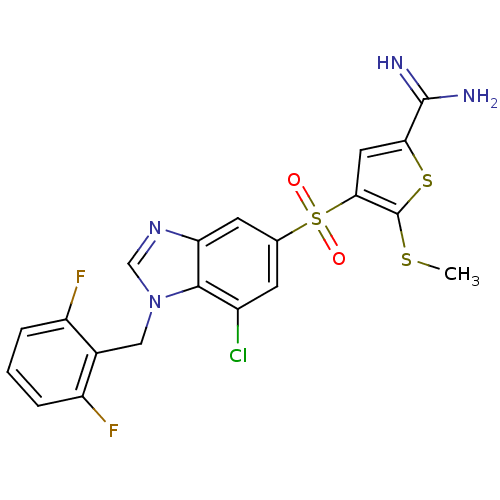
(4-[7-chloro-1-(2,6-difluoro-benzyl)-1H-benzoimidaz...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cc(Cl)c2n(Cc3c(F)cccc3F)cnc2c1)C(N)=N Show InChI InChI=1S/C20H15ClF2N4O2S3/c1-30-20-17(7-16(31-20)19(24)25)32(28,29)10-5-12(21)18-15(6-10)26-9-27(18)8-11-13(22)3-2-4-14(11)23/h2-7,9H,8H2,1H3,(H3,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of C1S |
Bioorg Med Chem Lett 16: 2200-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.036
BindingDB Entry DOI: 10.7270/Q2251HSV |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50182183

(4-[7-chloro-3-(2,6-difluoro-benzyl)-3H-benzoimidaz...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cc(Cl)c2ncn(Cc3c(F)cccc3F)c2c1)C(N)=N Show InChI InChI=1S/C20H15ClF2N4O2S3/c1-30-20-17(7-16(31-20)19(24)25)32(28,29)10-5-12(21)18-15(6-10)27(9-26-18)8-11-13(22)3-2-4-14(11)23/h2-7,9H,8H2,1H3,(H3,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of C1S |
Bioorg Med Chem Lett 16: 2200-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.036
BindingDB Entry DOI: 10.7270/Q2251HSV |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50233693

(CHEMBL252619 | N-[3'-(5-carbamimidoyl-2-methylsulf...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cccc(c1)-c1c(C)cccc1NC(=O)CS(C)(=O)=O)C(N)=N Show InChI InChI=1S/C22H23N3O5S4/c1-13-6-4-9-16(25-19(26)12-33(3,27)28)20(13)14-7-5-8-15(10-14)34(29,30)18-11-17(21(23)24)32-22(18)31-2/h4-11H,12H2,1-3H3,(H3,23,24)(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Complement C1s subcomponent |
Bioorg Med Chem Lett 18: 1603-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.064
BindingDB Entry DOI: 10.7270/Q2K35TD4 |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50233680

(4-(2'-hydroxymethyl-biphenyl-3-sulfonyl)-5-methyls...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cccc(c1)-c1ccccc1CO)C(N)=N Show InChI InChI=1S/C19H18N2O3S3/c1-25-19-17(10-16(26-19)18(20)21)27(23,24)14-7-4-6-12(9-14)15-8-3-2-5-13(15)11-22/h2-10,22H,11H2,1H3,(H3,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Complement C1s subcomponent |
Bioorg Med Chem Lett 18: 1603-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.064
BindingDB Entry DOI: 10.7270/Q2K35TD4 |
More data for this
Ligand-Target Pair | |
Proton-coupled folate transporter
(Homo sapiens (Human)) | BDBM50027656
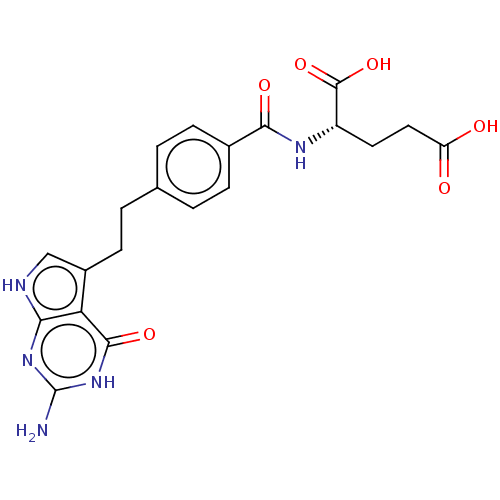
(CHEBI:63616 | LY-2315 | LY-231514 | PEMETREXED | U...)Show SMILES Nc1nc2[nH]cc(CCc3ccc(cc3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)c2c(=O)[nH]1 Show InChI InChI=1S/C20H21N5O6/c21-20-24-16-15(18(29)25-20)12(9-22-16)6-3-10-1-4-11(5-2-10)17(28)23-13(19(30)31)7-8-14(26)27/h1-2,4-5,9,13H,3,6-8H2,(H,23,28)(H,26,27)(H,30,31)(H4,21,22,24,25,29)/t13-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of human PCFT expressed in Chinese hamster R2/PCFT4 cells assessed as reduction in [3H]MTX uptake at pH 5.5 measured over 2 mins by Dixon ... |
J Med Chem 61: 4228-4248 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00408
BindingDB Entry DOI: 10.7270/Q2M048QF |
More data for this
Ligand-Target Pair | |
Proton-coupled folate transporter
(Homo sapiens (Human)) | BDBM50354833

(AGF94 | CHEMBL1834488)Show SMILES Nc1nc2[nH]c(CCCc3ccc(s3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)cc2c(=O)[nH]1 |r| Show InChI InChI=1S/C19H21N5O6S/c20-19-23-15-11(16(27)24-19)8-9(21-15)2-1-3-10-4-6-13(31-10)17(28)22-12(18(29)30)5-7-14(25)26/h4,6,8,12H,1-3,5,7H2,(H,22,28)(H,25,26)(H,29,30)(H4,20,21,23,24,27)/t12-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of human PCFT expressed in Chinese hamster R2/PCFT4 cells assessed as reduction in [3H]MTX uptake at pH 5.5 measured over 2 mins by Dixon ... |
J Med Chem 61: 4228-4248 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00408
BindingDB Entry DOI: 10.7270/Q2M048QF |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50233681
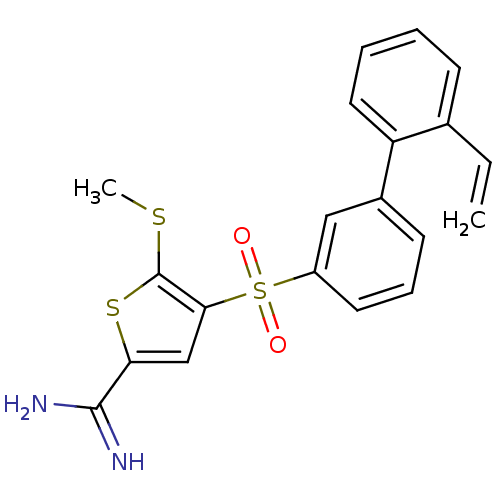
(5-methylsulfanyl-4-(2'-vinyl-biphenyl-3-sulfonyl)-...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cccc(c1)-c1ccccc1C=C)C(N)=N Show InChI InChI=1S/C20H18N2O2S3/c1-3-13-7-4-5-10-16(13)14-8-6-9-15(11-14)27(23,24)18-12-17(19(21)22)26-20(18)25-2/h3-12H,1H2,2H3,(H3,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Complement C1s subcomponent |
Bioorg Med Chem Lett 18: 1603-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.064
BindingDB Entry DOI: 10.7270/Q2K35TD4 |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50233676

(3'-(5-carbamimidoyl-2-methylsulfanyl-thiophene-3-s...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cccc(c1)-c1c(C)cccc1C(O)=O)C(N)=N Show InChI InChI=1S/C20H18N2O4S3/c1-11-5-3-8-14(19(23)24)17(11)12-6-4-7-13(9-12)29(25,26)16-10-15(18(21)22)28-20(16)27-2/h3-10H,1-2H3,(H3,21,22)(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Complement C1s subcomponent |
Bioorg Med Chem Lett 18: 1603-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.064
BindingDB Entry DOI: 10.7270/Q2K35TD4 |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50233683
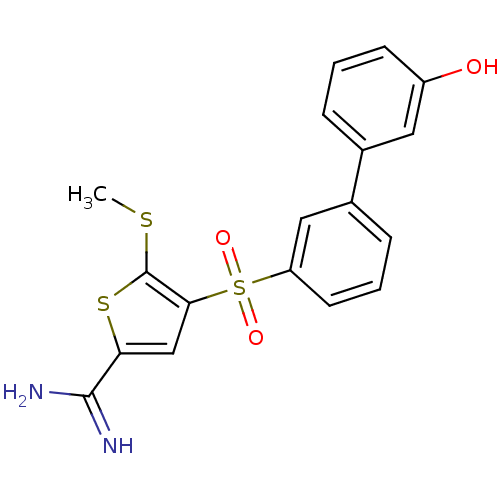
(4-(3'-hydroxy-biphenyl-3-sulfonyl)-5-methylsulfany...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cccc(c1)-c1cccc(O)c1)C(N)=N Show InChI InChI=1S/C18H16N2O3S3/c1-24-18-16(10-15(25-18)17(19)20)26(22,23)14-7-3-5-12(9-14)11-4-2-6-13(21)8-11/h2-10,21H,1H3,(H3,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Complement C1s subcomponent |
Bioorg Med Chem Lett 18: 1603-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.064
BindingDB Entry DOI: 10.7270/Q2K35TD4 |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50182164

(4-[7-bromo-3-(2,6-dichloro-benzyl)-3H-benzoimidazo...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cc(Br)c2ncn(Cc3c(Cl)cccc3Cl)c2c1)C(N)=N Show InChI InChI=1S/C20H15BrCl2N4O2S3/c1-30-20-17(7-16(31-20)19(24)25)32(28,29)10-5-12(21)18-15(6-10)27(9-26-18)8-11-13(22)3-2-4-14(11)23/h2-7,9H,8H2,1H3,(H3,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of C1S |
Bioorg Med Chem Lett 16: 2200-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.036
BindingDB Entry DOI: 10.7270/Q2251HSV |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50182174
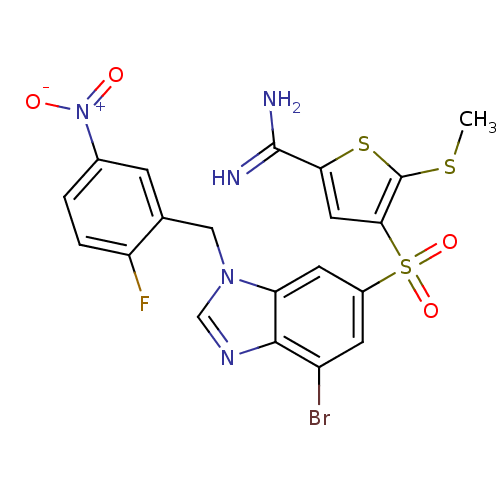
(4-[7-bromo-3-(2-fluoro-5-nitro-benzyl)-3H-benzoimi...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cc(Br)c2ncn(Cc3cc(ccc3F)[N+]([O-])=O)c2c1)C(N)=N Show InChI InChI=1S/C20H15BrFN5O4S3/c1-32-20-17(7-16(33-20)19(23)24)34(30,31)12-5-13(21)18-15(6-12)26(9-25-18)8-10-4-11(27(28)29)2-3-14(10)22/h2-7,9H,8H2,1H3,(H3,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of C1S |
Bioorg Med Chem Lett 16: 2200-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.036
BindingDB Entry DOI: 10.7270/Q2251HSV |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50182181
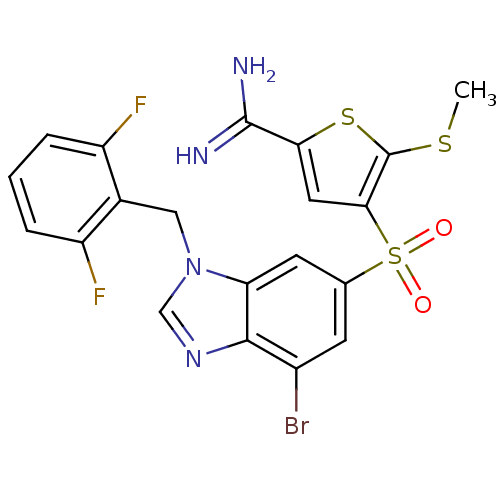
(4-[7-bromo-3-(2,6-difluoro-benzyl)-3H-benzoimidazo...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cc(Br)c2ncn(Cc3c(F)cccc3F)c2c1)C(N)=N Show InChI InChI=1S/C20H15BrF2N4O2S3/c1-30-20-17(7-16(31-20)19(24)25)32(28,29)10-5-12(21)18-15(6-10)27(9-26-18)8-11-13(22)3-2-4-14(11)23/h2-7,9H,8H2,1H3,(H3,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of C1S |
Bioorg Med Chem Lett 16: 2200-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.036
BindingDB Entry DOI: 10.7270/Q2251HSV |
More data for this
Ligand-Target Pair | |
Proton-coupled folate transporter
(Homo sapiens (Human)) | BDBM50503237

(CHEMBL4540298)Show SMILES Nc1nc2[nH]c(CCCCc3ccc(C(=O)N[C@@H](CCC(O)=O)C(O)=O)c(F)c3)cc2c(=O)[nH]1 |r| Show InChI InChI=1S/C22H24FN5O6/c23-15-9-11(5-6-13(15)19(31)26-16(21(33)34)7-8-17(29)30)3-1-2-4-12-10-14-18(25-12)27-22(24)28-20(14)32/h5-6,9-10,16H,1-4,7-8H2,(H,26,31)(H,29,30)(H,33,34)(H4,24,25,27,28,32)/t16-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of human PCFT expressed in Chinese hamster R2/PCFT4 cells assessed as reduction in [3H]MTX uptake at pH 5.5 measured over 2 mins by Dixon ... |
J Med Chem 61: 4228-4248 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00408
BindingDB Entry DOI: 10.7270/Q2M048QF |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50182180
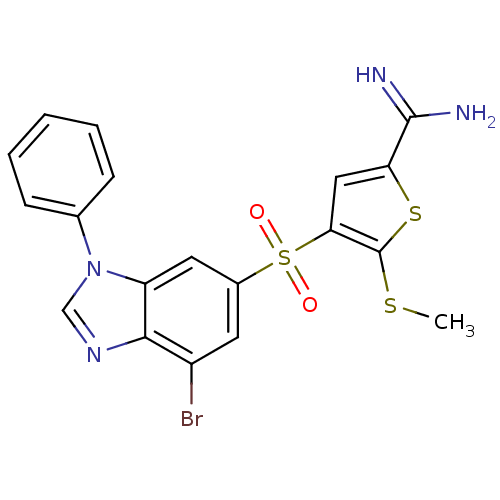
(4-(7-bromo-3-phenyl-3H-benzoimidazole-5-sulfonyl)-...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cc(Br)c2ncn(-c3ccccc3)c2c1)C(N)=N Show InChI InChI=1S/C19H15BrN4O2S3/c1-27-19-16(9-15(28-19)18(21)22)29(25,26)12-7-13(20)17-14(8-12)24(10-23-17)11-5-3-2-4-6-11/h2-10H,1H3,(H3,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of C1S |
Bioorg Med Chem Lett 16: 2200-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.036
BindingDB Entry DOI: 10.7270/Q2251HSV |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50233678

(4-(4'-hydroxy-biphenyl-3-sulfonyl)-5-methylsulfany...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cccc(c1)-c1ccc(O)cc1)C(N)=N Show InChI InChI=1S/C18H16N2O3S3/c1-24-18-16(10-15(25-18)17(19)20)26(22,23)14-4-2-3-12(9-14)11-5-7-13(21)8-6-11/h2-10,21H,1H3,(H3,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Complement C1s subcomponent |
Bioorg Med Chem Lett 18: 1603-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.064
BindingDB Entry DOI: 10.7270/Q2K35TD4 |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50182153
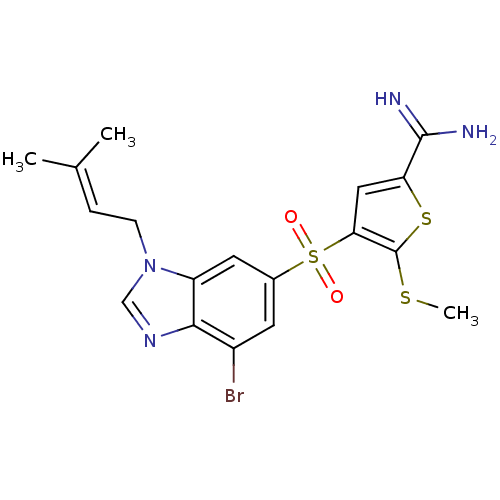
(4-[7-bromo-3-(3-methyl-but-2-enyl)-3H-benzoimidazo...)Show SMILES [#6]-[#16]-c1sc(cc1S(=O)(=O)c1cc(Br)c2ncn(-[#6]\[#6]=[#6](\[#6])-[#6])c2c1)-[#6](-[#7])=[#7] Show InChI InChI=1S/C18H19BrN4O2S3/c1-10(2)4-5-23-9-22-16-12(19)6-11(7-13(16)23)28(24,25)15-8-14(17(20)21)27-18(15)26-3/h4,6-9H,5H2,1-3H3,(H3,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of C1S |
Bioorg Med Chem Lett 16: 2200-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.036
BindingDB Entry DOI: 10.7270/Q2251HSV |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50182182
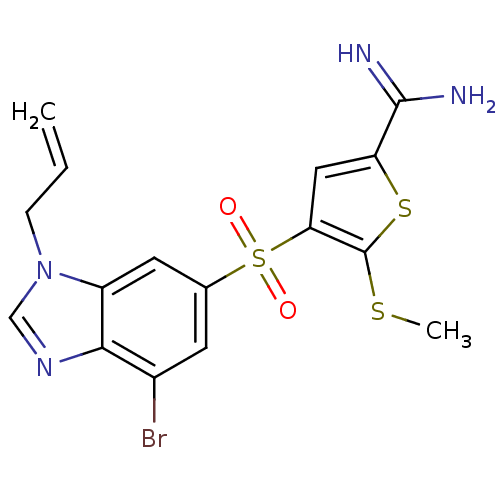
(4-(3-allyl-7-bromo-3H-benzoimidazole-5-sulfonyl)-5...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cc(Br)c2ncn(CC=C)c2c1)C(N)=N Show InChI InChI=1S/C16H15BrN4O2S3/c1-3-4-21-8-20-14-10(17)5-9(6-11(14)21)26(22,23)13-7-12(15(18)19)25-16(13)24-2/h3,5-8H,1,4H2,2H3,(H3,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of C1S |
Bioorg Med Chem Lett 16: 2200-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.036
BindingDB Entry DOI: 10.7270/Q2251HSV |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50182169
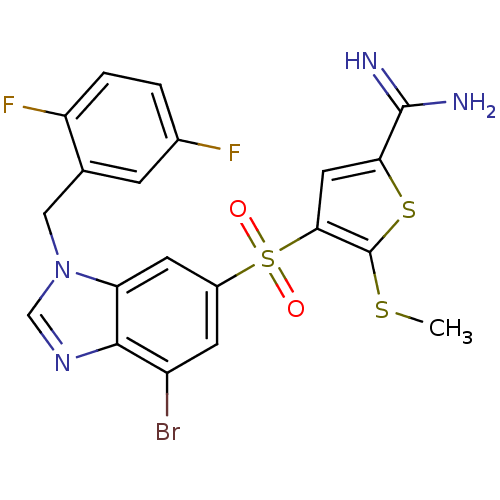
(4-[7-bromo-3-(2,5-difluoro-benzyl)-3H-benzoimidazo...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cc(Br)c2ncn(Cc3cc(F)ccc3F)c2c1)C(N)=N Show InChI InChI=1S/C20H15BrF2N4O2S3/c1-30-20-17(7-16(31-20)19(24)25)32(28,29)12-5-13(21)18-15(6-12)27(9-26-18)8-10-4-11(22)2-3-14(10)23/h2-7,9H,8H2,1H3,(H3,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of C1S |
Bioorg Med Chem Lett 16: 2200-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.036
BindingDB Entry DOI: 10.7270/Q2251HSV |
More data for this
Ligand-Target Pair | |
Proton-coupled folate transporter
(Homo sapiens (Human)) | BDBM50457435
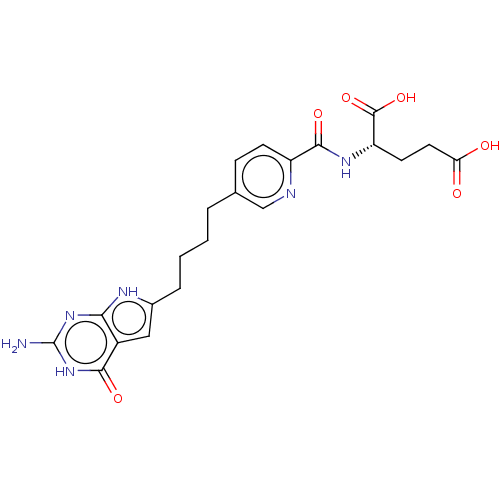
(CHEMBL4214638)Show SMILES Nc1nc2[nH]c(CCCCc3ccc(nc3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)cc2c(=O)[nH]1 |r| Show InChI InChI=1S/C21H24N6O6/c22-21-26-17-13(18(30)27-21)9-12(24-17)4-2-1-3-11-5-6-14(23-10-11)19(31)25-15(20(32)33)7-8-16(28)29/h5-6,9-10,15H,1-4,7-8H2,(H,25,31)(H,28,29)(H,32,33)(H4,22,24,26,27,30)/t15-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of human PCFT expressed in Chinese hamster R2/PCFT4 cells assessed as reduction in [3H]MTX uptake at pH 5.5 measured over 2 mins by Dixon ... |
J Med Chem 61: 4228-4248 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00408
BindingDB Entry DOI: 10.7270/Q2M048QF |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50233687

(4-(4'-methyl-biphenyl-3-sulfonyl)-5-methylsulfanyl...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cccc(c1)-c1ccc(C)cc1)C(N)=N Show InChI InChI=1S/C19H18N2O2S3/c1-12-6-8-13(9-7-12)14-4-3-5-15(10-14)26(22,23)17-11-16(18(20)21)25-19(17)24-2/h3-11H,1-2H3,(H3,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Complement C1s subcomponent |
Bioorg Med Chem Lett 18: 1603-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.064
BindingDB Entry DOI: 10.7270/Q2K35TD4 |
More data for this
Ligand-Target Pair | |
Proton-coupled folate transporter
(Homo sapiens (Human)) | BDBM50503238
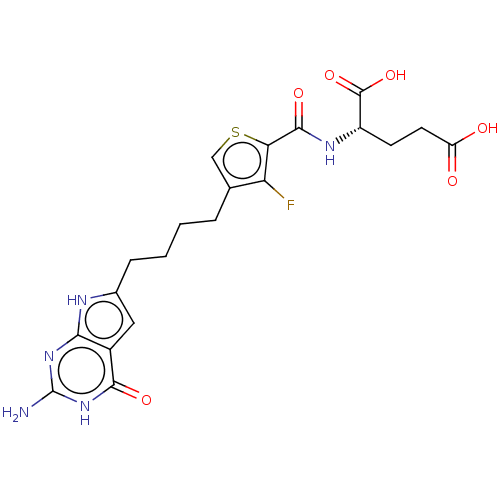
(CHEMBL4465095)Show SMILES Nc1nc2[nH]c(CCCCc3csc(C(=O)N[C@@H](CCC(O)=O)C(O)=O)c3F)cc2c(=O)[nH]1 |r| Show InChI InChI=1S/C20H22FN5O6S/c21-14-9(8-33-15(14)18(30)24-12(19(31)32)5-6-13(27)28)3-1-2-4-10-7-11-16(23-10)25-20(22)26-17(11)29/h7-8,12H,1-6H2,(H,24,30)(H,27,28)(H,31,32)(H4,22,23,25,26,29)/t12-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of human PCFT expressed in Chinese hamster R2/PCFT4 cells assessed as reduction in [3H]MTX uptake at pH 5.5 measured over 2 mins by Dixon ... |
J Med Chem 61: 4228-4248 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00408
BindingDB Entry DOI: 10.7270/Q2M048QF |
More data for this
Ligand-Target Pair | |
Proton-coupled folate transporter
(Homo sapiens (Human)) | BDBM50027656

(CHEBI:63616 | LY-2315 | LY-231514 | PEMETREXED | U...)Show SMILES Nc1nc2[nH]cc(CCc3ccc(cc3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)c2c(=O)[nH]1 Show InChI InChI=1S/C20H21N5O6/c21-20-24-16-15(18(29)25-20)12(9-22-16)6-3-10-1-4-11(5-2-10)17(28)23-13(19(30)31)7-8-14(26)27/h1-2,4-5,9,13H,3,6-8H2,(H,23,28)(H,26,27)(H,30,31)(H4,21,22,24,25,29)/t13-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 259 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]MTX uptake at human PCFT expressed in Chinese hamster R2/PCFT4 cells at pH 5.5 measured after 2 mins by Dixon plot analysis |
J Med Chem 61: 2027-2040 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01708
BindingDB Entry DOI: 10.7270/Q2ST7SF1 |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50233675
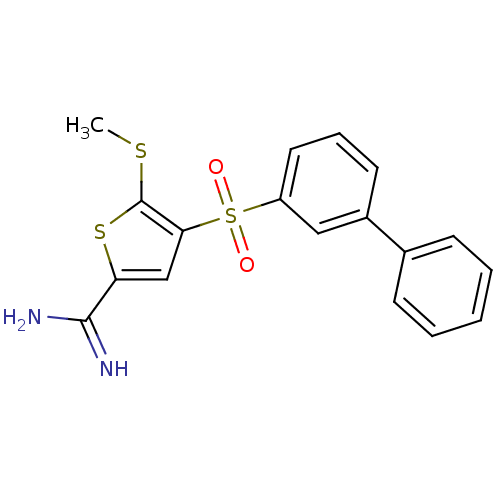
(4-(biphenyl-3-sulfonyl)-5-methylsulfanyl-thiophene...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cccc(c1)-c1ccccc1)C(N)=N Show InChI InChI=1S/C18H16N2O2S3/c1-23-18-16(11-15(24-18)17(19)20)25(21,22)14-9-5-8-13(10-14)12-6-3-2-4-7-12/h2-11H,1H3,(H3,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Complement C1s subcomponent |
Bioorg Med Chem Lett 18: 1603-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.064
BindingDB Entry DOI: 10.7270/Q2K35TD4 |
More data for this
Ligand-Target Pair | |
Proton-coupled folate transporter
(Homo sapiens (Human)) | BDBM50503236
(CHEMBL4538151)Show SMILES Nc1nc2[nH]c(CCCc3ccc(C(=O)N[C@@H](CCC(O)=O)C(O)=O)c(F)c3)cc2c(=O)[nH]1 |r| Show InChI InChI=1S/C21H22FN5O6/c22-14-8-10(2-1-3-11-9-13-17(24-11)26-21(23)27-19(13)31)4-5-12(14)18(30)25-15(20(32)33)6-7-16(28)29/h4-5,8-9,15H,1-3,6-7H2,(H,25,30)(H,28,29)(H,32,33)(H4,23,24,26,27,31)/t15-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of human PCFT expressed in Chinese hamster R2/PCFT4 cells assessed as reduction in [3H]MTX uptake at pH 5.5 measured over 2 mins by Dixon ... |
J Med Chem 61: 4228-4248 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00408
BindingDB Entry DOI: 10.7270/Q2M048QF |
More data for this
Ligand-Target Pair | |
Proton-coupled folate transporter
(Homo sapiens (Human)) | BDBM50126172
(CHEMBL3628344)Show SMILES Nc1nc2[nH]c(CCCc3csc(c3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)cc2c(=O)[nH]1 |r| Show InChI InChI=1S/C19H21N5O6S/c20-19-23-15-11(16(27)24-19)7-10(21-15)3-1-2-9-6-13(31-8-9)17(28)22-12(18(29)30)4-5-14(25)26/h6-8,12H,1-5H2,(H,22,28)(H,25,26)(H,29,30)(H4,20,21,23,24,27)/t12-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of human PCFT expressed in Chinese hamster R2/PCFT4 cells assessed as reduction in [3H]MTX uptake at pH 5.5 measured over 2 mins by Dixon ... |
J Med Chem 61: 4228-4248 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00408
BindingDB Entry DOI: 10.7270/Q2M048QF |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50182186
(4-(7-bromo-1-phenyl-1H-benzoimidazole-5-sulfonyl)-...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cc(Br)c2n(cnc2c1)-c1ccccc1)C(N)=N Show InChI InChI=1S/C19H15BrN4O2S3/c1-27-19-16(9-15(28-19)18(21)22)29(25,26)12-7-13(20)17-14(8-12)23-10-24(17)11-5-3-2-4-6-11/h2-10H,1H3,(H3,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of C1S |
Bioorg Med Chem Lett 16: 2200-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.036
BindingDB Entry DOI: 10.7270/Q2251HSV |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50182156
(4-[7-bromo-1-((S)-1-phenyl-ethyl)-1H-benzoimidazol...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cc(Br)c2n(cnc2c1)[C@@H](C)c1ccccc1)C(N)=N Show InChI InChI=1S/C21H19BrN4O2S3/c1-12(13-6-4-3-5-7-13)26-11-25-16-9-14(8-15(22)19(16)26)31(27,28)18-10-17(20(23)24)30-21(18)29-2/h3-12H,1-2H3,(H3,23,24)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of C1S |
Bioorg Med Chem Lett 16: 2200-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.036
BindingDB Entry DOI: 10.7270/Q2251HSV |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50182161
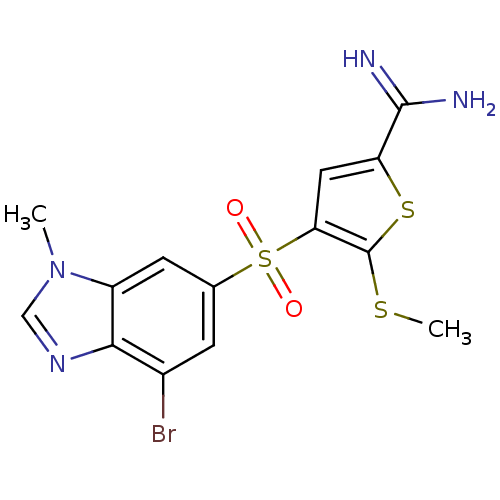
(4-(7-bromo-3-methyl-3H-benzoimidazole-5-sulfonyl)-...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cc(Br)c2ncn(C)c2c1)C(N)=N Show InChI InChI=1S/C14H13BrN4O2S3/c1-19-6-18-12-8(15)3-7(4-9(12)19)24(20,21)11-5-10(13(16)17)23-14(11)22-2/h3-6H,1-2H3,(H3,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of C1S |
Bioorg Med Chem Lett 16: 2200-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.036
BindingDB Entry DOI: 10.7270/Q2251HSV |
More data for this
Ligand-Target Pair | |
Proton-coupled folate transporter
(Homo sapiens (Human)) | BDBM50457435

(CHEMBL4214638)Show SMILES Nc1nc2[nH]c(CCCCc3ccc(nc3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)cc2c(=O)[nH]1 |r| Show InChI InChI=1S/C21H24N6O6/c22-21-26-17-13(18(30)27-21)9-12(24-17)4-2-1-3-11-5-6-14(23-10-11)19(31)25-15(20(32)33)7-8-16(28)29/h5-6,9-10,15H,1-4,7-8H2,(H,25,31)(H,28,29)(H,32,33)(H4,22,24,26,27,30)/t15-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 332 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]MTX uptake at human PCFT expressed in Chinese hamster R2/PCFT4 cells at pH 5.5 measured after 2 mins by Dixon plot analysis |
J Med Chem 61: 2027-2040 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01708
BindingDB Entry DOI: 10.7270/Q2ST7SF1 |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50233695

(4-(4-methoxy-biphenyl-3-sulfonyl)-5-methylsulfanyl...)Show SMILES COc1ccc(cc1)-c1cccc(c1)S(=O)(=O)c1cc(sc1SC)C(N)=N Show InChI InChI=1S/C19H18N2O3S3/c1-24-14-8-6-12(7-9-14)13-4-3-5-15(10-13)27(22,23)17-11-16(18(20)21)26-19(17)25-2/h3-11H,1-2H3,(H3,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Complement C1s subcomponent |
Bioorg Med Chem Lett 18: 1603-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.064
BindingDB Entry DOI: 10.7270/Q2K35TD4 |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50233690
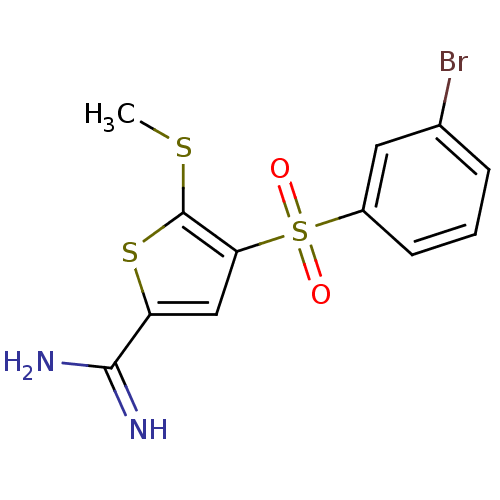
(4-(3-bromo-benzenesulfonyl)-5-methylsulfanyl-thiop...)Show InChI InChI=1S/C12H11BrN2O2S3/c1-18-12-10(6-9(19-12)11(14)15)20(16,17)8-4-2-3-7(13)5-8/h2-6H,1H3,(H3,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Complement C1s subcomponent |
Bioorg Med Chem Lett 18: 1603-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.064
BindingDB Entry DOI: 10.7270/Q2K35TD4 |
More data for this
Ligand-Target Pair | |
Proton-coupled folate transporter
(Homo sapiens (Human)) | BDBM50393640
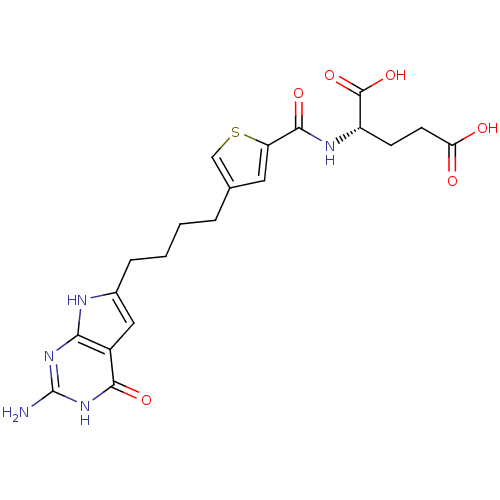
(CHEMBL2158681)Show SMILES Nc1nc2[nH]c(CCCCc3csc(c3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)cc2c(=O)[nH]1 |r| Show InChI InChI=1S/C20H23N5O6S/c21-20-24-16-12(17(28)25-20)8-11(22-16)4-2-1-3-10-7-14(32-9-10)18(29)23-13(19(30)31)5-6-15(26)27/h7-9,13H,1-6H2,(H,23,29)(H,26,27)(H,30,31)(H4,21,22,24,25,28)/t13-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of human PCFT expressed in Chinese hamster R2/PCFT4 cells assessed as reduction in [3H]MTX uptake at pH 5.5 measured over 2 mins by Dixon ... |
J Med Chem 61: 4228-4248 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00408
BindingDB Entry DOI: 10.7270/Q2M048QF |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50182172
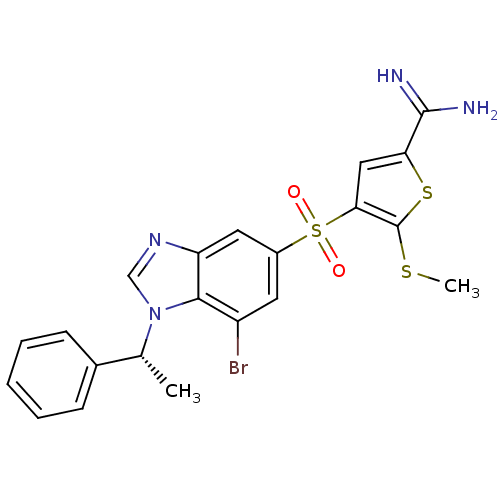
(4-[7-bromo-1-((R)-1-phenyl-ethyl)-1H-benzoimidazol...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cc(Br)c2n(cnc2c1)[C@H](C)c1ccccc1)C(N)=N Show InChI InChI=1S/C21H19BrN4O2S3/c1-12(13-6-4-3-5-7-13)26-11-25-16-9-14(8-15(22)19(16)26)31(27,28)18-10-17(20(23)24)30-21(18)29-2/h3-12H,1-2H3,(H3,23,24)/t12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of C1S |
Bioorg Med Chem Lett 16: 2200-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.036
BindingDB Entry DOI: 10.7270/Q2251HSV |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data