

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM8336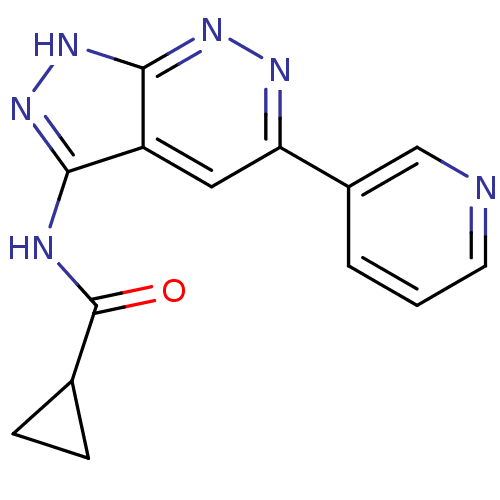 (N-[5-(pyridin-3-yl)-1H-pyrazolo[3,4-c]pyridazin-3-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0800 | -57.1 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 10 uM ATP/ [gamma-33P] ATP. Aft... | Bioorg Med Chem Lett 13: 1581-4 (2003) Article DOI: 10.1016/s0960-894x(03)00135-5 BindingDB Entry DOI: 10.7270/Q2BK19JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM8337 (N-[5-(2,3-difluorophenyl)-1H-pyrazolo[3,4-c]pyrida...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.110 | -56.3 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 10 uM ATP/ [gamma-33P] ATP. Aft... | Bioorg Med Chem Lett 13: 1581-4 (2003) Article DOI: 10.1016/s0960-894x(03)00135-5 BindingDB Entry DOI: 10.7270/Q2BK19JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM8339 (N-[5-(2,3-difluorophenyl)-1H-pyrazolo[3,4-c]pyrida...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.190 | -54.9 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 10 uM ATP/ [gamma-33P] ATP. Aft... | Bioorg Med Chem Lett 13: 1581-4 (2003) Article DOI: 10.1016/s0960-894x(03)00135-5 BindingDB Entry DOI: 10.7270/Q2BK19JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM8338 (N-[5-(2,3-difluorophenyl)-1H-pyrazolo[3,4-c]pyrida...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.950 | -51.0 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 10 uM ATP/ [gamma-33P] ATP. Aft... | Bioorg Med Chem Lett 13: 1581-4 (2003) Article DOI: 10.1016/s0960-894x(03)00135-5 BindingDB Entry DOI: 10.7270/Q2BK19JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM8337 (N-[5-(2,3-difluorophenyl)-1H-pyrazolo[3,4-c]pyrida...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5 | -46.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 21 |
GlaxoSmithKline | Assay Description In vitro kinase assay using purified CDK2/Cyclin A was incubated at room temperature with substrate, and test compounds in the presence of 100 uM ATP... | Bioorg Med Chem Lett 13: 1581-4 (2003) Article DOI: 10.1016/s0960-894x(03)00135-5 BindingDB Entry DOI: 10.7270/Q2BK19JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM8336 (N-[5-(pyridin-3-yl)-1H-pyrazolo[3,4-c]pyridazin-3-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5 | -46.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 21 |
GlaxoSmithKline | Assay Description In vitro kinase assay using purified CDK2/Cyclin A was incubated at room temperature with substrate, and test compounds in the presence of 100 uM ATP... | Bioorg Med Chem Lett 13: 1581-4 (2003) Article DOI: 10.1016/s0960-894x(03)00135-5 BindingDB Entry DOI: 10.7270/Q2BK19JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50096383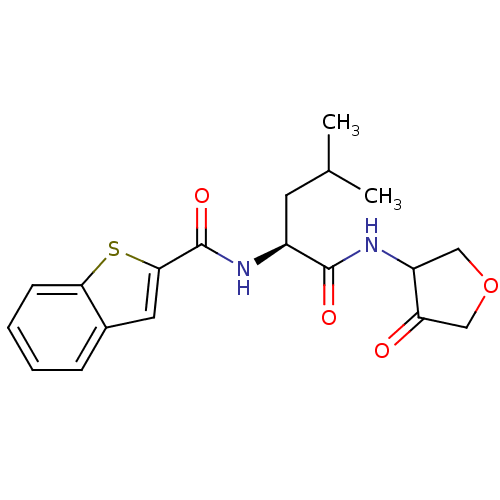 (Benzo[b]thiophene-2-carboxylic acid [(S)-3-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against human cysteine protease, cathepsin K. | Bioorg Med Chem Lett 11: 195-8 (2001) BindingDB Entry DOI: 10.7270/Q2ZP45CQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50096371 (4-tert-Butyl-N-[(S)-3-methyl-1-(4-oxo-tetrahydro-f...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against human cysteine protease, cathepsin K. | Bioorg Med Chem Lett 11: 195-8 (2001) BindingDB Entry DOI: 10.7270/Q2ZP45CQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50096373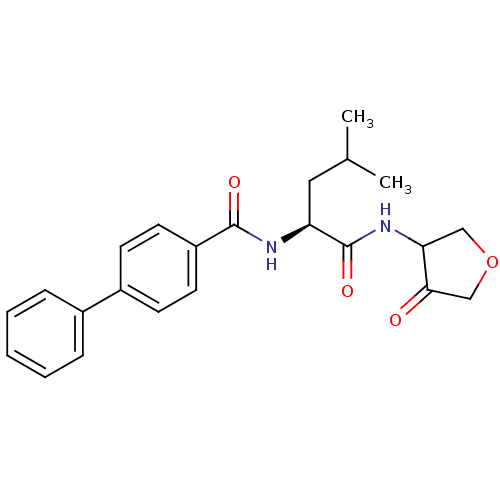 (Biphenyl-4-carboxylic acid [(S)-3-methyl-1-(4-oxo-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against human cysteine protease, cathepsin K. | Bioorg Med Chem Lett 11: 195-8 (2001) BindingDB Entry DOI: 10.7270/Q2ZP45CQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50096380 (1H-Indole-6-carboxylic acid [(S)-3-methyl-1-(4-oxo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against human cysteine protease, cathepsin K. | Bioorg Med Chem Lett 11: 195-8 (2001) BindingDB Entry DOI: 10.7270/Q2ZP45CQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19804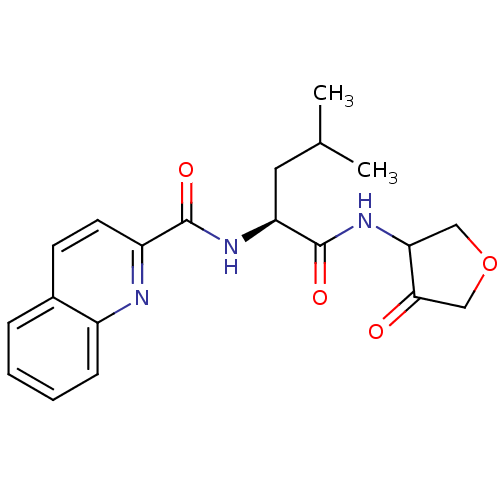 ((2S)-4-methyl-N-(4-oxooxolan-3-yl)-2-(quinolin-2-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against human cysteine protease, cathepsin K. | Bioorg Med Chem Lett 11: 195-8 (2001) BindingDB Entry DOI: 10.7270/Q2ZP45CQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50096366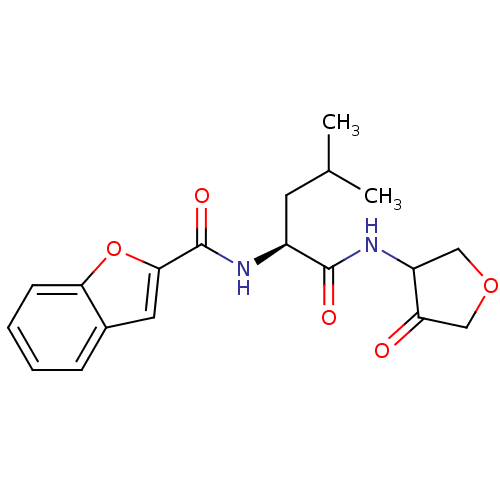 (Benzofuran-2-carboxylic acid [(S)-3-methyl-1-(4-ox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against human cysteine protease, cathepsin K. | Bioorg Med Chem Lett 11: 195-8 (2001) BindingDB Entry DOI: 10.7270/Q2ZP45CQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19797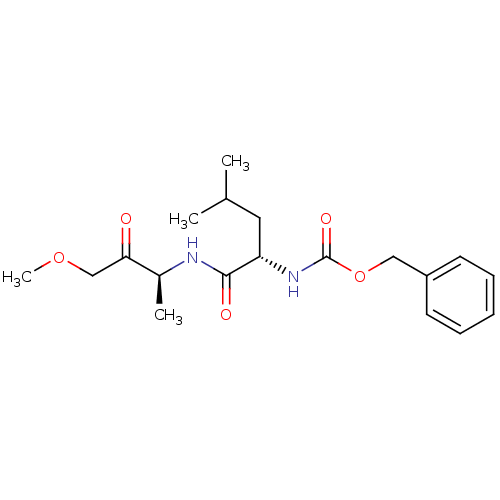 (CHEMBL301683 | acyclic alkoxymethyl ketone inhibit...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against human cysteine protease, cathepsin K. | Bioorg Med Chem Lett 11: 195-8 (2001) BindingDB Entry DOI: 10.7270/Q2ZP45CQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50096370 (CHEMBL59115 | Naphthalene-2-carboxylic acid [(S)-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against human cysteine protease, cathepsin K. | Bioorg Med Chem Lett 11: 195-8 (2001) BindingDB Entry DOI: 10.7270/Q2ZP45CQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50096374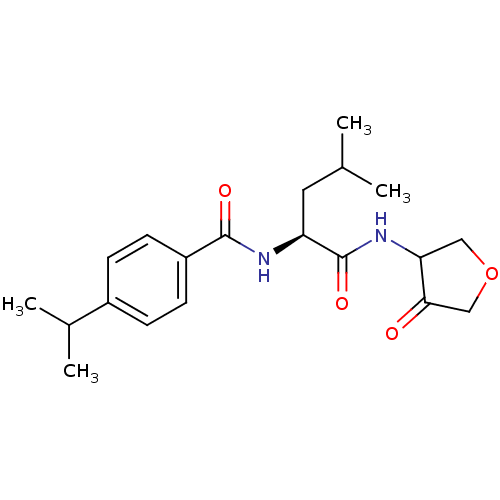 (4-Isopropyl-N-[(S)-3-methyl-1-(4-oxo-tetrahydro-fu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against human cysteine protease, cathepsin K. | Bioorg Med Chem Lett 11: 195-8 (2001) BindingDB Entry DOI: 10.7270/Q2ZP45CQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19798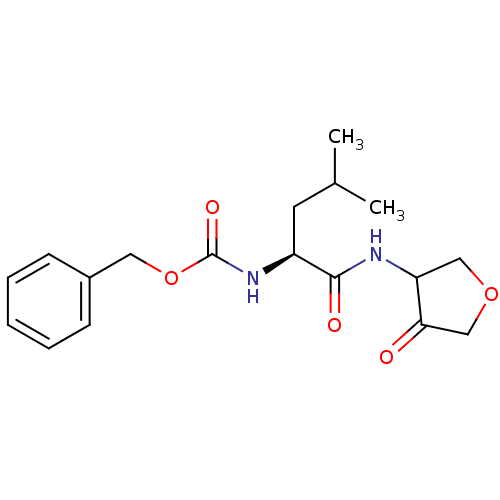 (3-amidotetrahydrofuran-4-one, 3 | CHEMBL61805 | be...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against human cysteine protease, cathepsin K. | Bioorg Med Chem Lett 11: 195-8 (2001) BindingDB Entry DOI: 10.7270/Q2ZP45CQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50096391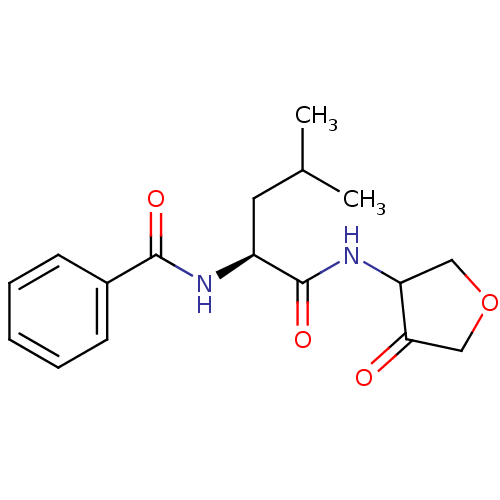 (CHEMBL58567 | N-[(S)-3-Methyl-1-(4-oxo-tetrahydro-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against human cysteine protease, cathepsin K. | Bioorg Med Chem Lett 11: 195-8 (2001) BindingDB Entry DOI: 10.7270/Q2ZP45CQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50096389 (Benzo[b]thiophene-2-carboxylic acid [(S)-1-(4-oxo-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against human cysteine protease, cathepsin K. | Bioorg Med Chem Lett 11: 195-8 (2001) BindingDB Entry DOI: 10.7270/Q2ZP45CQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50096368 (CHEMBL59170 | Pyridine-2-carboxylic acid [(S)-3-me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against human cysteine protease, cathepsin K. | Bioorg Med Chem Lett 11: 195-8 (2001) BindingDB Entry DOI: 10.7270/Q2ZP45CQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM8338 (N-[5-(2,3-difluorophenyl)-1H-pyrazolo[3,4-c]pyrida...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 450 | -35.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 21 |
GlaxoSmithKline | Assay Description In vitro kinase assay using purified CDK2/Cyclin A was incubated at room temperature with substrate, and test compounds in the presence of 100 uM ATP... | Bioorg Med Chem Lett 13: 1581-4 (2003) Article DOI: 10.1016/s0960-894x(03)00135-5 BindingDB Entry DOI: 10.7270/Q2BK19JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM8339 (N-[5-(2,3-difluorophenyl)-1H-pyrazolo[3,4-c]pyrida...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 540 | -35.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 21 |
GlaxoSmithKline | Assay Description In vitro kinase assay using purified CDK2/Cyclin A was incubated at room temperature with substrate, and test compounds in the presence of 100 uM ATP... | Bioorg Med Chem Lett 13: 1581-4 (2003) Article DOI: 10.1016/s0960-894x(03)00135-5 BindingDB Entry DOI: 10.7270/Q2BK19JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50096369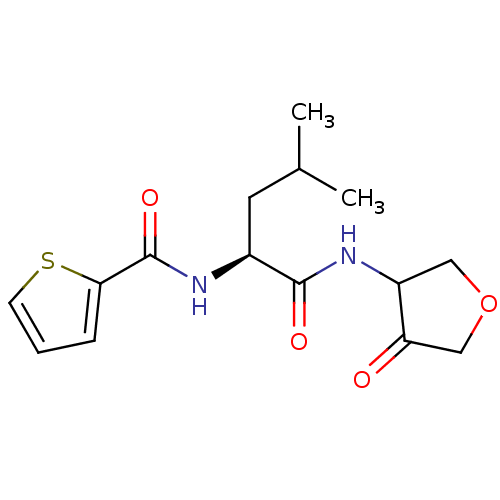 (CHEMBL61482 | Thiophene-2-carboxylic acid [(S)-3-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against human cysteine protease, cathepsin K. | Bioorg Med Chem Lett 11: 195-8 (2001) BindingDB Entry DOI: 10.7270/Q2ZP45CQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50096381 (4-Methanesulfonyl-N-[(S)-3-methyl-1-(4-oxo-tetrahy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against human cysteine protease, cathepsin K. | Bioorg Med Chem Lett 11: 195-8 (2001) BindingDB Entry DOI: 10.7270/Q2ZP45CQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50096376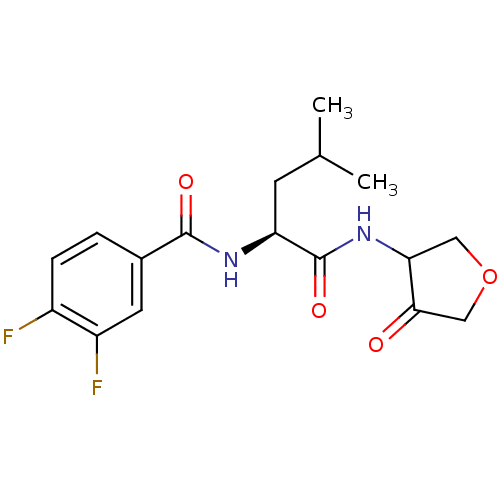 (3,4-Difluoro-N-[(S)-3-methyl-1-(4-oxo-tetrahydro-f...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against human cysteine protease, cathepsin K. | Bioorg Med Chem Lett 11: 195-8 (2001) BindingDB Entry DOI: 10.7270/Q2ZP45CQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50096378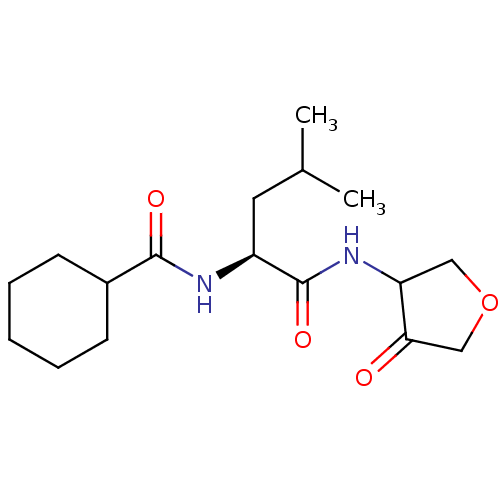 (CHEMBL61797 | Cyclohexanecarboxylic acid [(S)-3-me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against human cysteine protease, cathepsin K. | Bioorg Med Chem Lett 11: 195-8 (2001) BindingDB Entry DOI: 10.7270/Q2ZP45CQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50096384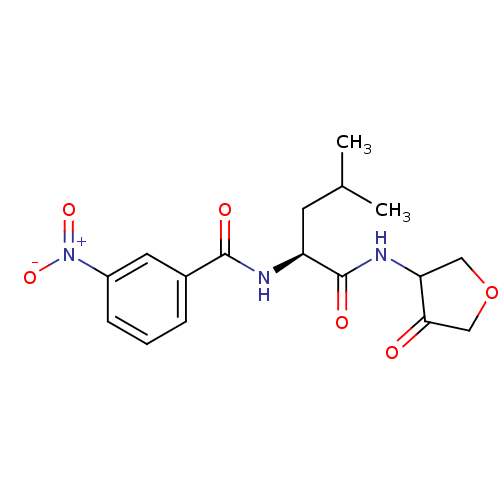 (CHEMBL62042 | N-[(S)-3-Methyl-1-(4-oxo-tetrahydro-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against human cysteine protease, cathepsin K. | Bioorg Med Chem Lett 11: 195-8 (2001) BindingDB Entry DOI: 10.7270/Q2ZP45CQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50096382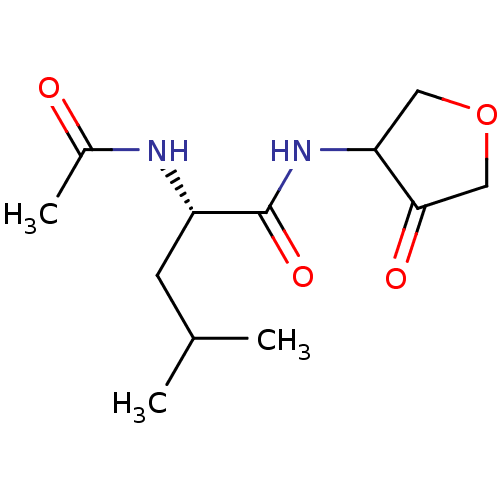 ((S)-2-Acetylamino-4-methyl-pentanoic acid (4-oxo-t...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against human cysteine protease, cathepsin K. | Bioorg Med Chem Lett 11: 195-8 (2001) BindingDB Entry DOI: 10.7270/Q2ZP45CQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM8327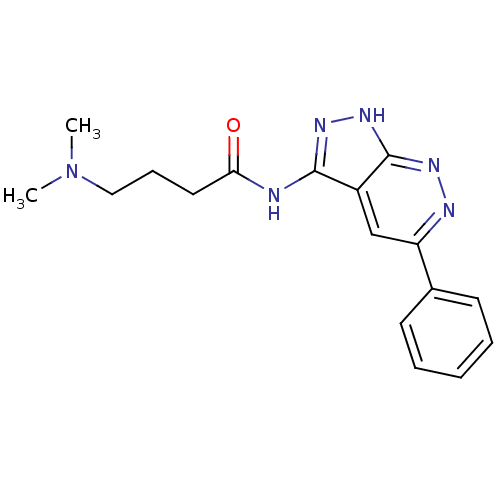 (4-(dimethylamino)-N-{5-phenyl-1H-pyrazolo[3,4-c]py...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 1.70E+3 | -32.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 21 |
GlaxoSmithKline | Assay Description In vitro kinase assay using purified CDK2/Cyclin A was incubated at room temperature with substrate, and test compounds in the presence of 100 uM ATP... | Bioorg Med Chem Lett 13: 1581-4 (2003) Article DOI: 10.1016/s0960-894x(03)00135-5 BindingDB Entry DOI: 10.7270/Q2BK19JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50096367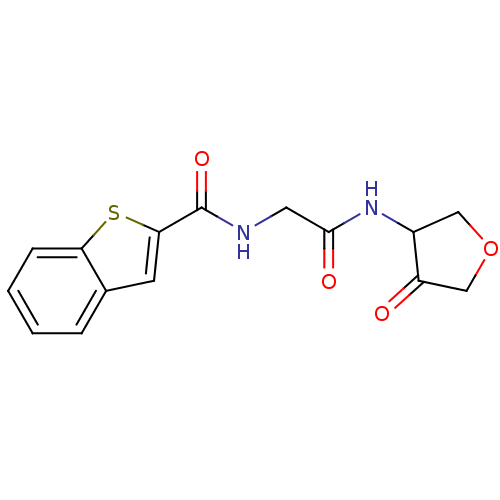 (Benzo[b]thiophene-2-carboxylic acid [(4-oxo-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against human cysteine protease, cathepsin K. | Bioorg Med Chem Lett 11: 195-8 (2001) BindingDB Entry DOI: 10.7270/Q2ZP45CQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50096375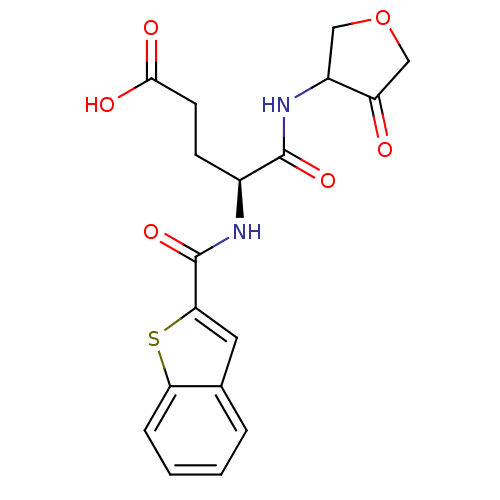 ((S)-4-[(Benzo[b]thiophene-2-carbonyl)-amino]-4-(4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against human cysteine protease, cathepsin K. | Bioorg Med Chem Lett 11: 195-8 (2001) BindingDB Entry DOI: 10.7270/Q2ZP45CQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50096390 (Benzo[b]thiophene-2-carboxylic acid [(S)-2-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against human cysteine protease, cathepsin K. | Bioorg Med Chem Lett 11: 195-8 (2001) BindingDB Entry DOI: 10.7270/Q2ZP45CQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50096388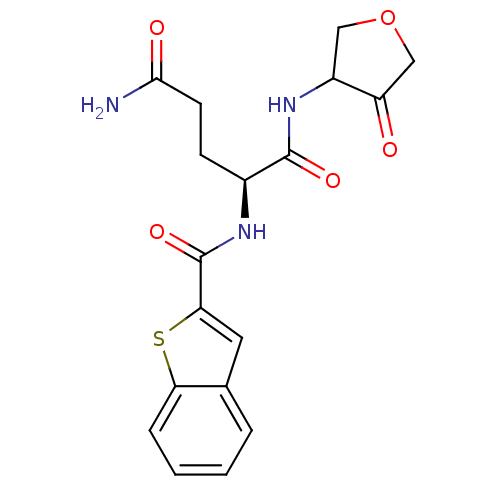 ((S)-2-[(Benzo[b]thiophene-2-carbonyl)-amino]-penta...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against human cysteine protease, cathepsin K. | Bioorg Med Chem Lett 11: 195-8 (2001) BindingDB Entry DOI: 10.7270/Q2ZP45CQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50096386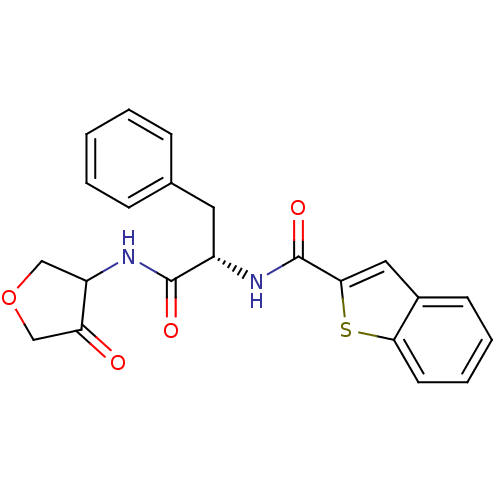 (Benzo[b]thiophene-2-carboxylic acid [(S)-1-(4-oxo-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against human cysteine protease, cathepsin K. | Bioorg Med Chem Lett 11: 195-8 (2001) BindingDB Entry DOI: 10.7270/Q2ZP45CQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50096387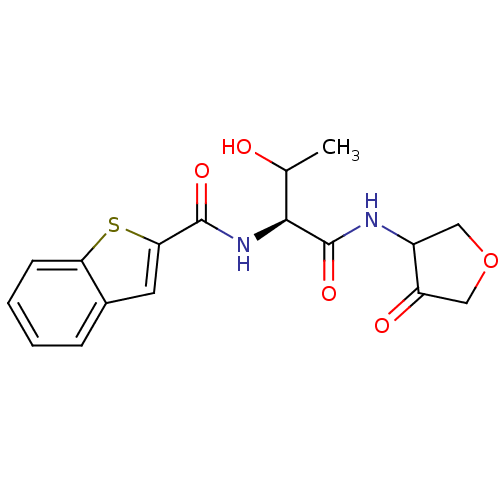 (Benzo[b]thiophene-2-carboxylic acid [(S)-2-hydroxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against human cysteine protease, cathepsin K. | Bioorg Med Chem Lett 11: 195-8 (2001) BindingDB Entry DOI: 10.7270/Q2ZP45CQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50096385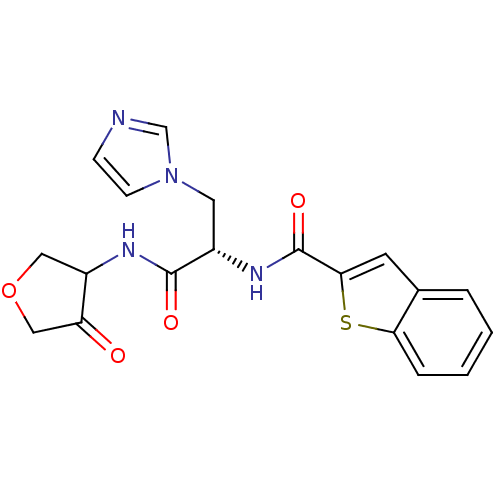 (Benzo[b]thiophene-2-carboxylic acid [(S)-2-imidazo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against human cysteine protease, cathepsin K. | Bioorg Med Chem Lett 11: 195-8 (2001) BindingDB Entry DOI: 10.7270/Q2ZP45CQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50096379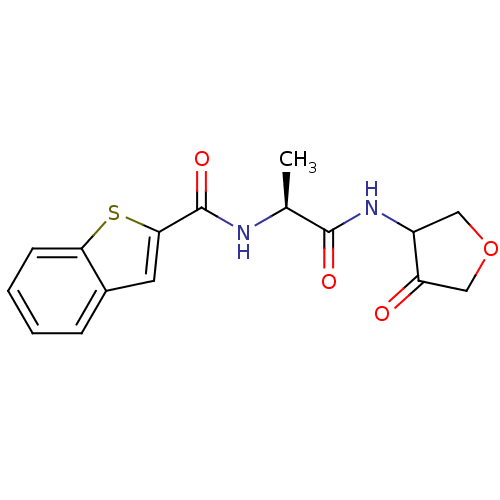 (Benzo[b]thiophene-2-carboxylic acid [(S)-1-(4-oxo-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against human cysteine protease, cathepsin K. | Bioorg Med Chem Lett 11: 195-8 (2001) BindingDB Entry DOI: 10.7270/Q2ZP45CQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50096377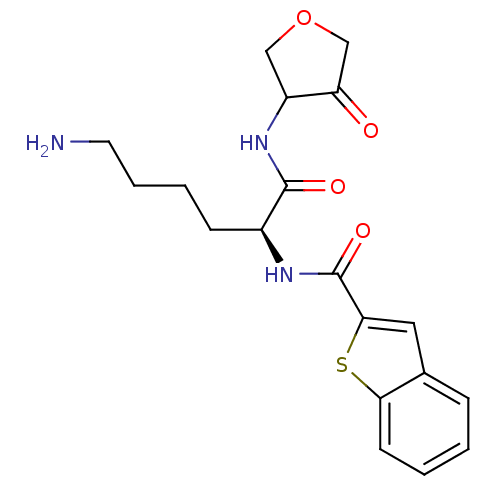 (Benzo[b]thiophene-2-carboxylic acid [(S)-5-amino-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against human cysteine protease, cathepsin K. | Bioorg Med Chem Lett 11: 195-8 (2001) BindingDB Entry DOI: 10.7270/Q2ZP45CQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50096372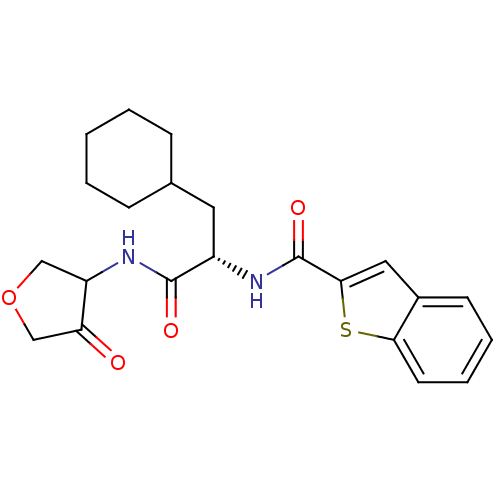 (Benzo[b]thiophene-2-carboxylic acid [(S)-2-cyclohe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against human cysteine protease, cathepsin K. | Bioorg Med Chem Lett 11: 195-8 (2001) BindingDB Entry DOI: 10.7270/Q2ZP45CQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM8349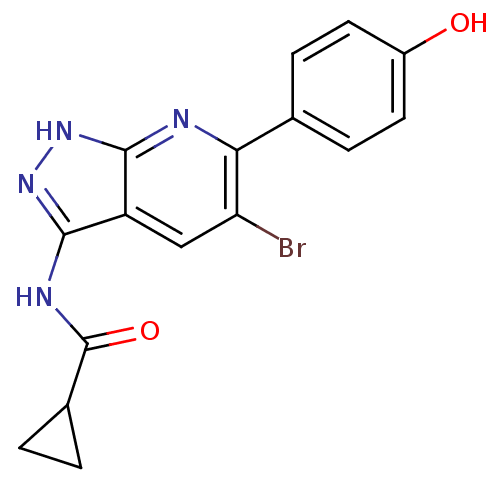 (6-aryl-pyrazolo[3,4-b]pyridine analogue 13 | CHEMB...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | 7.0 | 22 |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 10 uM ATP/ [gamma-33P] ATP. Aft... | Bioorg Med Chem Lett 13: 3055-7 (2003) Article DOI: 10.1016/s0960-894x(03)00645-0 BindingDB Entry DOI: 10.7270/Q26T0JVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM8359 (6-aryl-pyrazolo[3,4-b]pyridine analogue 23 | N-[5-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 10 uM ATP/ [gamma-33P] ATP. Aft... | Bioorg Med Chem Lett 13: 3055-7 (2003) Article DOI: 10.1016/s0960-894x(03)00645-0 BindingDB Entry DOI: 10.7270/Q26T0JVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM8350 (6-aryl-pyrazolo[3,4-b]pyridine analogue 14 | N-[5-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.0 | 22 |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 10 uM ATP/ [gamma-33P] ATP. Aft... | Bioorg Med Chem Lett 13: 3055-7 (2003) Article DOI: 10.1016/s0960-894x(03)00645-0 BindingDB Entry DOI: 10.7270/Q26T0JVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM8364 (CHEMBL260416 | N-[6-(3-bromo-4-hydroxyphenyl)-1H-p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description In vitro kinase assay using purified CDK2/Cyclin A was incubated at room temperature with substrate, and test compounds in the presence of 100 uM ATP... | Bioorg Med Chem Lett 13: 3059-62 (2003) Article DOI: 10.1016/s0960-894x(03)00646-2 BindingDB Entry DOI: 10.7270/Q2348HKS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM8365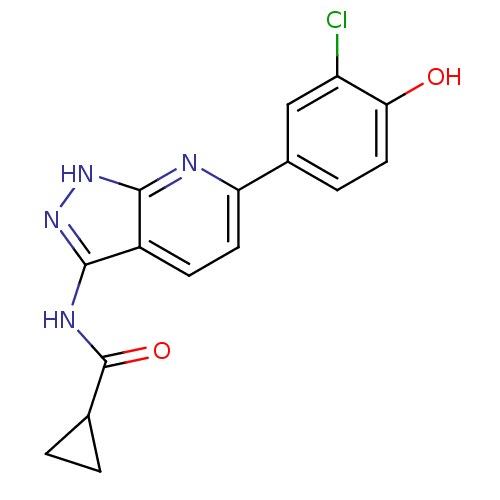 (N-[6-(3-chloro-4-hydroxyphenyl)-1H-pyrazolo[3,4-b]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description In vitro kinase assay using purified CDK2/Cyclin A was incubated at room temperature with substrate, and test compounds in the presence of 100 uM ATP... | Bioorg Med Chem Lett 13: 3059-62 (2003) Article DOI: 10.1016/s0960-894x(03)00646-2 BindingDB Entry DOI: 10.7270/Q2348HKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM8357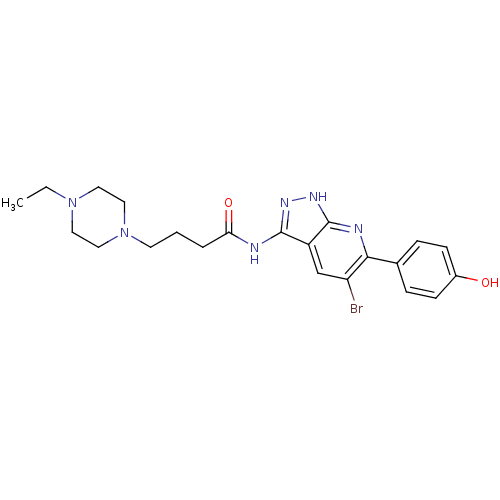 (6-aryl-pyrazolo[3,4-b]pyridine analogue 21 | CHEMB...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 10 uM ATP/ [gamma-33P] ATP. Aft... | Bioorg Med Chem Lett 13: 3055-7 (2003) Article DOI: 10.1016/s0960-894x(03)00645-0 BindingDB Entry DOI: 10.7270/Q26T0JVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM8357 (6-aryl-pyrazolo[3,4-b]pyridine analogue 21 | CHEMB...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 10 uM ATP/ [gamma-33P] ATP. Aft... | Bioorg Med Chem Lett 13: 3055-7 (2003) Article DOI: 10.1016/s0960-894x(03)00645-0 BindingDB Entry DOI: 10.7270/Q26T0JVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM8330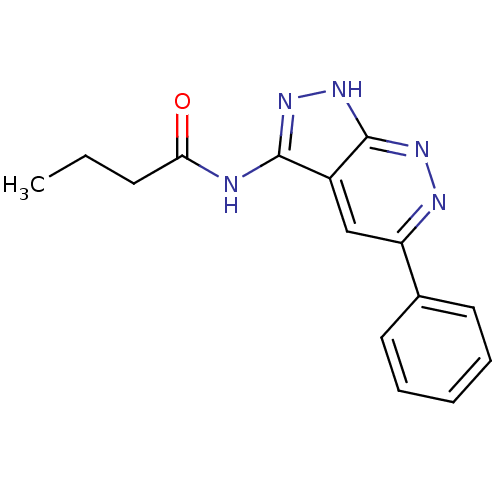 (N-{5-phenyl-1H-pyrazolo[3,4-c]pyridazin-3-yl}butan...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.0 | 22 |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 10 uM ATP/ [gamma-33P] ATP. Aft... | Bioorg Med Chem Lett 13: 1581-4 (2003) Article DOI: 10.1016/s0960-894x(03)00135-5 BindingDB Entry DOI: 10.7270/Q2BK19JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM8334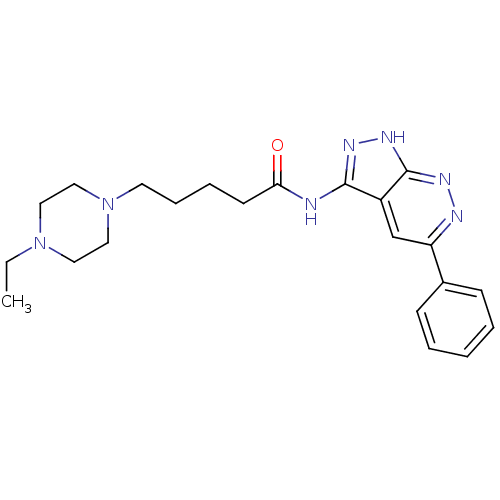 (5-(4-ethylpiperazin-1-yl)-N-{5-phenyl-1H-pyrazolo[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.0 | 22 |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 10 uM ATP/ [gamma-33P] ATP. Aft... | Bioorg Med Chem Lett 13: 1581-4 (2003) Article DOI: 10.1016/s0960-894x(03)00135-5 BindingDB Entry DOI: 10.7270/Q2BK19JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM8349 (6-aryl-pyrazolo[3,4-b]pyridine analogue 13 | CHEMB...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description In vitro kinase assay using purified CDK2/Cyclin A was incubated at room temperature with substrate, and test compounds in the presence of 100 uM ATP... | Bioorg Med Chem Lett 13: 3059-62 (2003) Article DOI: 10.1016/s0960-894x(03)00646-2 BindingDB Entry DOI: 10.7270/Q2348HKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM8348 (6-aryl-pyrazolo[3,4-b]pyridine analogue 12 | CHEMB...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description In vitro kinase assay using purified CDK2/Cyclin A was incubated at room temperature with substrate, and test compounds in the presence of 100 uM ATP... | Bioorg Med Chem Lett 13: 3059-62 (2003) Article DOI: 10.1016/s0960-894x(03)00646-2 BindingDB Entry DOI: 10.7270/Q2348HKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM8370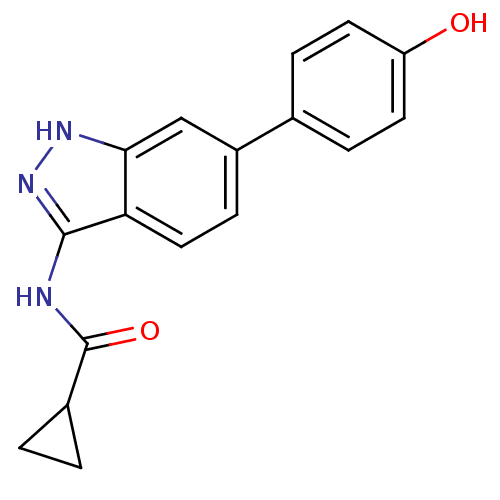 (N-[6-(4-hydroxyphenyl)-1H-indazol-3-yl]cyclopropan...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description In vitro kinase assay using purified CDK2/Cyclin A was incubated at room temperature with substrate, and test compounds in the presence of 100 uM ATP... | Bioorg Med Chem Lett 13: 3059-62 (2003) Article DOI: 10.1016/s0960-894x(03)00646-2 BindingDB Entry DOI: 10.7270/Q2348HKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 164 total ) | Next | Last >> |