

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Substance-K receptor (Homo sapiens (Human)) | BDBM50404016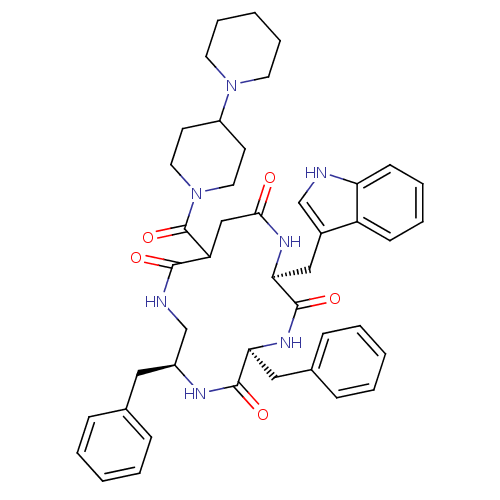 (CHEMBL2112922) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche S.p.A. Laboratori di Firenze Curated by ChEMBL | Assay Description In vitro functional activity towards Neurokinin NK2 receptor on isolated rabbit urinary bladder (RUB) | Bioorg Med Chem Lett 12: 693-6 (2002) BindingDB Entry DOI: 10.7270/Q22N53FR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50404016 (CHEMBL2112922) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche S.p.A. Laboratori di Firenze Curated by ChEMBL | Assay Description In vitro functional activity towards Neurokinin NK2 receptor on isolated rabbit urinary bladder (RUB) | Bioorg Med Chem Lett 12: 693-6 (2002) BindingDB Entry DOI: 10.7270/Q22N53FR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50472916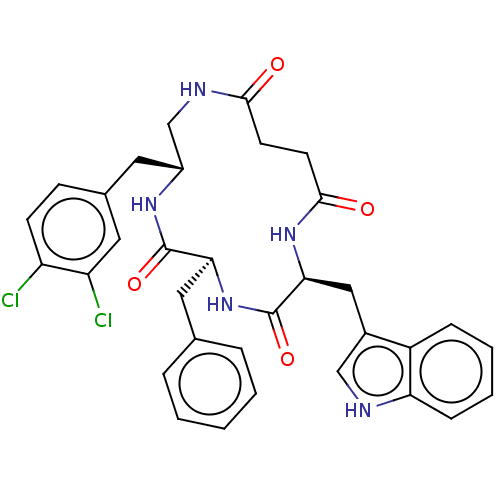 (CHEMBL223221 | MEN-11690) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche S.p.A. Curated by ChEMBL | Assay Description Binding affinity towards Tachykinin receptor 2 | J Med Chem 43: 4041-4 (2000) Article DOI: 10.1021/jm0010217 BindingDB Entry DOI: 10.7270/Q2Z322CV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50404025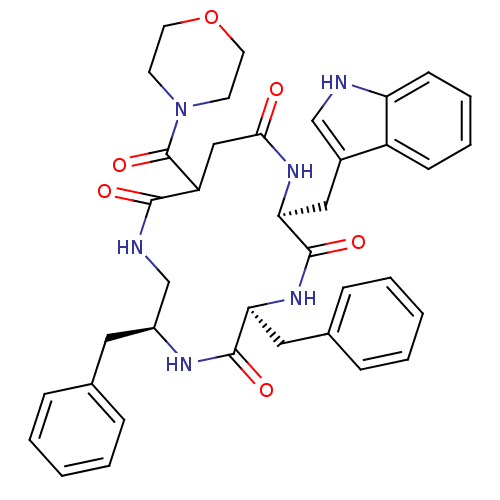 (CHEMBL2112925) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche S.p.A. Laboratori di Firenze Curated by ChEMBL | Assay Description In vitro functional activity towards Neurokinin NK2 receptor on isolated rabbit urinary bladder (RUB) | Bioorg Med Chem Lett 12: 693-6 (2002) BindingDB Entry DOI: 10.7270/Q22N53FR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50366377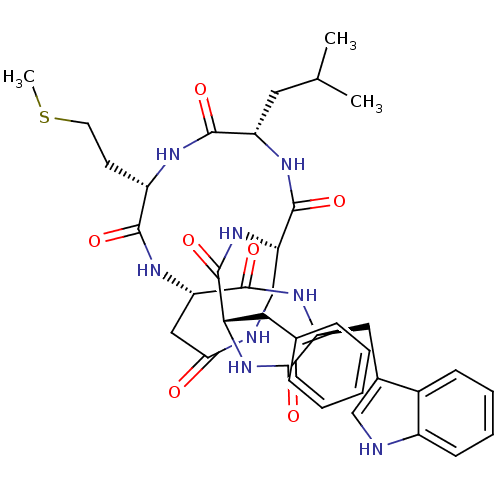 (CHEMBL334721 | MEN-10627) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche S.p.A. Curated by ChEMBL | Assay Description Binding affinity towards Tachykinin receptor 2 | J Med Chem 43: 4041-4 (2000) Article DOI: 10.1021/jm0010217 BindingDB Entry DOI: 10.7270/Q2Z322CV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50404017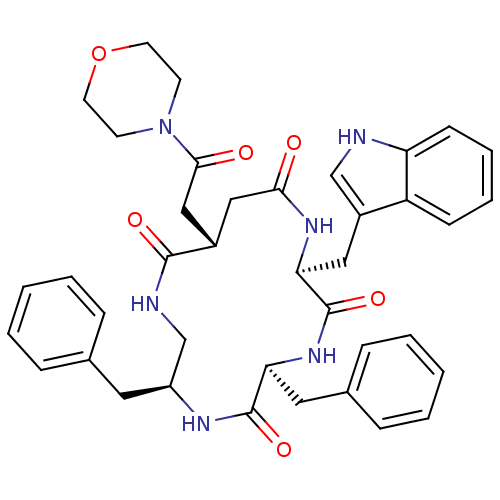 (CHEMBL2112923) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche S.p.A. Laboratori di Firenze Curated by ChEMBL | Assay Description In vitro functional activity towards Neurokinin NK2 receptor on isolated rabbit urinary bladder (RUB) | Bioorg Med Chem Lett 12: 693-6 (2002) BindingDB Entry DOI: 10.7270/Q22N53FR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50404023 (CHEMBL159449) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche S.p.A. Laboratori di Firenze Curated by ChEMBL | Assay Description In vitro functional activity towards Neurokinin NK2 receptor on isolated rabbit urinary bladder (RUB) | Bioorg Med Chem Lett 12: 693-6 (2002) BindingDB Entry DOI: 10.7270/Q22N53FR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50404021 (CHEMBL161242) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche S.p.A. Laboratori di Firenze Curated by ChEMBL | Assay Description In vitro functional activity towards Neurokinin NK2 receptor on isolated rabbit urinary bladder (RUB) | Bioorg Med Chem Lett 12: 693-6 (2002) BindingDB Entry DOI: 10.7270/Q22N53FR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50472917 (CHEMBL323884 | MEN-11749) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche S.p.A. Curated by ChEMBL | Assay Description Binding affinity towards Tachykinin receptor 2 | J Med Chem 43: 4041-4 (2000) Article DOI: 10.1021/jm0010217 BindingDB Entry DOI: 10.7270/Q2Z322CV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50404020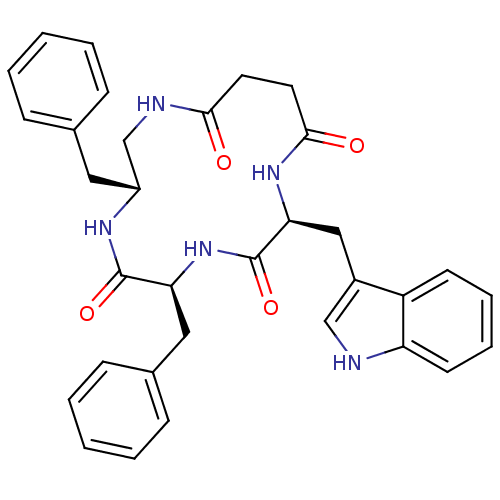 (CHEMBL435324 | MEN-11558) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche S.p.A. Laboratori di Firenze Curated by ChEMBL | Assay Description Displacement of [125I]NKA from human Tachykinin receptor 2 expressed in CHO cells | Bioorg Med Chem Lett 12: 693-6 (2002) BindingDB Entry DOI: 10.7270/Q22N53FR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50404020 (CHEMBL435324 | MEN-11558) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche S.p.A. Curated by ChEMBL | Assay Description Binding affinity towards Tachykinin receptor 2 | J Med Chem 43: 4041-4 (2000) Article DOI: 10.1021/jm0010217 BindingDB Entry DOI: 10.7270/Q2Z322CV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50472911 (CHEMBL407782 | MEN-11420) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche S.p.A. Curated by ChEMBL | Assay Description Binding affinity towards Tachykinin receptor 2 | J Med Chem 43: 4041-4 (2000) Article DOI: 10.1021/jm0010217 BindingDB Entry DOI: 10.7270/Q2Z322CV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50404019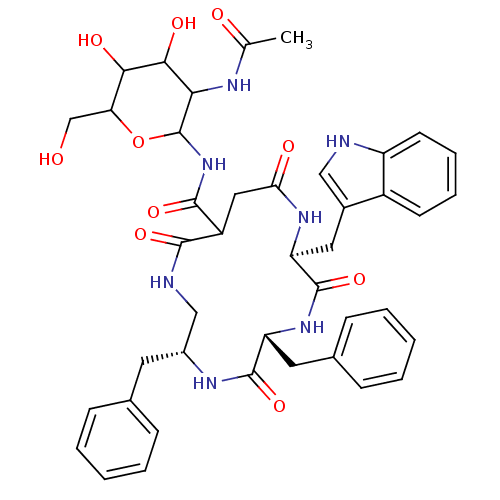 (CHEMBL345402) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche S.p.A. Laboratori di Firenze Curated by ChEMBL | Assay Description In vitro functional activity towards Neurokinin NK2 receptor on isolated rabbit urinary bladder (RUB) | Bioorg Med Chem Lett 12: 693-6 (2002) BindingDB Entry DOI: 10.7270/Q22N53FR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50470323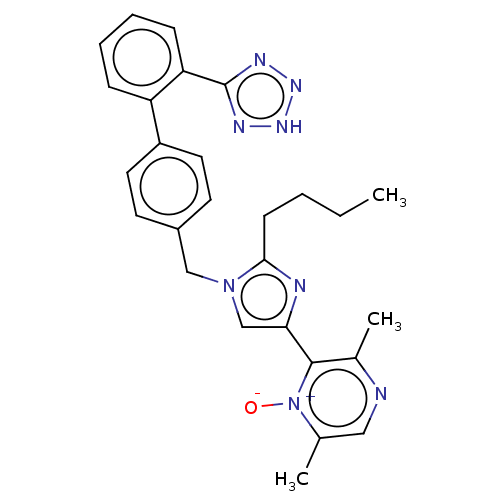 (CHEMBL327505) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori Guidotti S.p.A. Curated by ChEMBL | Assay Description Inhibition of [3H]angiotensin II binding to rat adrenal cortex membrane containing angiotensin II receptor, type 1 | J Med Chem 38: 2925-37 (1995) Article DOI: 10.1021/jm00015a015 BindingDB Entry DOI: 10.7270/Q2697689 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50029895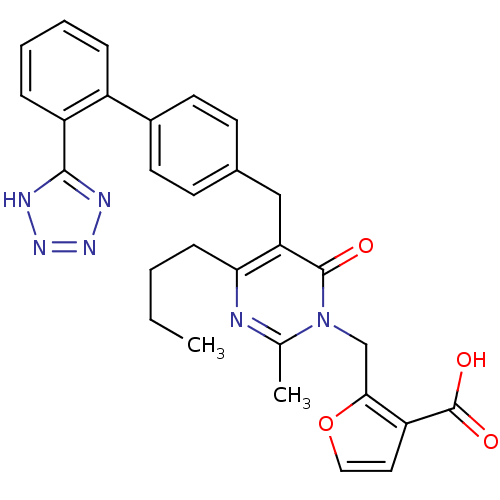 (2-{4-Butyl-2-methyl-6-oxo-5-[2'-(1H-tetrazol-5-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Lusofarmaco Curated by ChEMBL | Assay Description Binding affinity towards Angiotensin II type 2 receptor in bovine cerebellar cortical membranes; Inactive | J Med Chem 38: 4806-20 (1996) BindingDB Entry DOI: 10.7270/Q2JQ101N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50029905 (4'-(2-Butyl-4-methyl-6-oxo-6H-pyrimidin-1-ylmethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Lusofarmaco Curated by ChEMBL | Assay Description Binding affinity towards Angiotensin II type 2 receptor in bovine cerebellar cortical membranes; Inactive | J Med Chem 38: 4806-20 (1996) BindingDB Entry DOI: 10.7270/Q2JQ101N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50029899 (3-{4-Butyl-2-methyl-6-oxo-5-[2'-(1H-tetrazol-5-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Lusofarmaco Curated by ChEMBL | Assay Description Binding affinity towards Angiotensin II type 2 receptor in bovine cerebellar cortical membranes; Inactive | J Med Chem 38: 4806-20 (1996) BindingDB Entry DOI: 10.7270/Q2JQ101N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50029908 (4'-(4-Butyl-6-oxo-1-thiophen-2-ylmethyl-1,6-dihydr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Lusofarmaco Curated by ChEMBL | Assay Description Binding affinity towards Angiotensin II type 2 receptor in bovine cerebellar cortical membranes; Inactive | J Med Chem 38: 4806-20 (1996) BindingDB Entry DOI: 10.7270/Q2JQ101N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50029893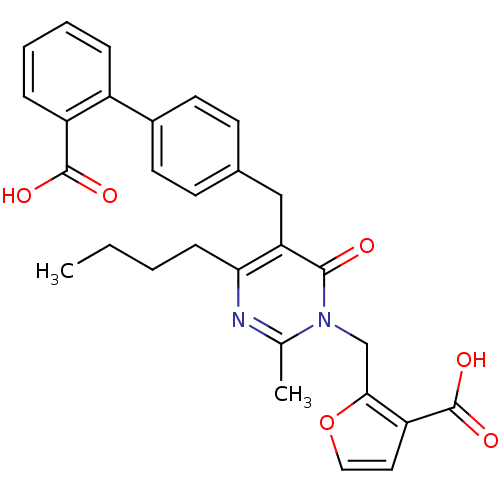 (2-[4-Butyl-5-(2'-carboxy-biphenyl-4-ylmethyl)-2-me...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Lusofarmaco Curated by ChEMBL | Assay Description Binding affinity towards Angiotensin II type 2 receptor in bovine cerebellar cortical membranes; Inactive | J Med Chem 38: 4806-20 (1996) BindingDB Entry DOI: 10.7270/Q2JQ101N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50029906 (2-[4-Butyl-5-(2'-carboxy-biphenyl-4-ylmethyl)-2-me...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Lusofarmaco Curated by ChEMBL | Assay Description Binding affinity towards Angiotensin II type 2 receptor in bovine cerebellar cortical membranes; Inactive | J Med Chem 38: 4806-20 (1996) BindingDB Entry DOI: 10.7270/Q2JQ101N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50029909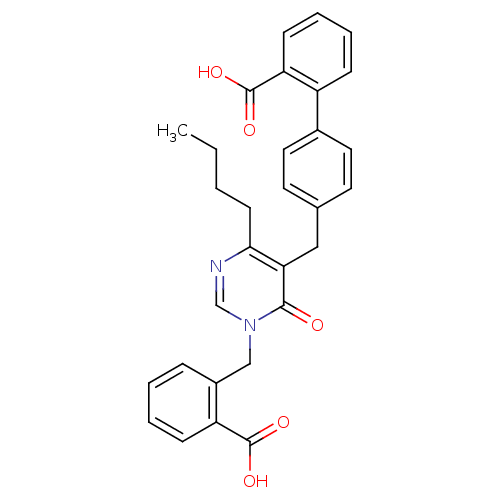 (4'-[4-Butyl-1-(2-carboxy-benzyl)-6-oxo-1,6-dihydro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Lusofarmaco Curated by ChEMBL | Assay Description Binding affinity towards Angiotensin II type 2 receptor in bovine cerebellar cortical membranes; Inactive | J Med Chem 38: 4806-20 (1996) BindingDB Entry DOI: 10.7270/Q2JQ101N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50029907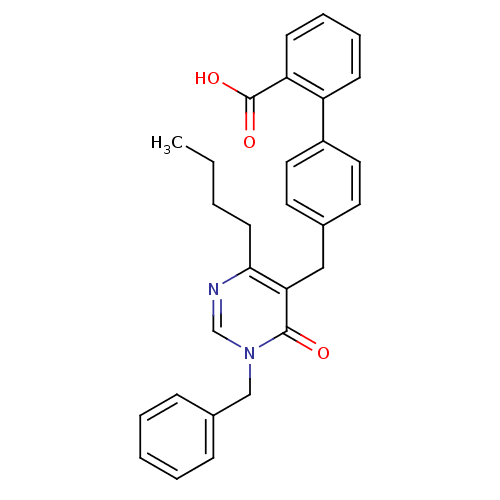 (4'-(1-Benzyl-4-butyl-6-oxo-1,6-dihydro-pyrimidin-5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Lusofarmaco Curated by ChEMBL | Assay Description Binding affinity towards Angiotensin II type 2 receptor in bovine cerebellar cortical membranes; Inactive | J Med Chem 38: 4806-20 (1996) BindingDB Entry DOI: 10.7270/Q2JQ101N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50029910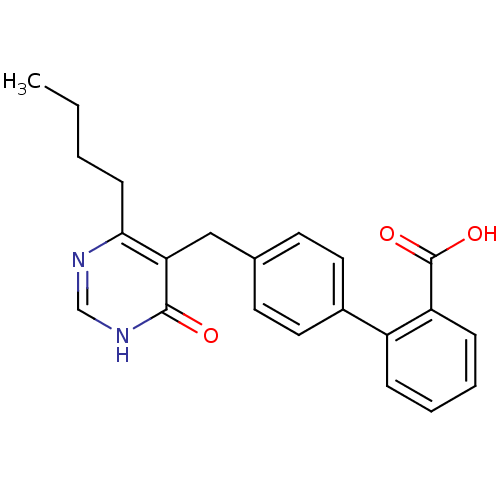 (4'-(4-Butyl-6-oxo-1,6-dihydro-pyrimidin-5-ylmethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Lusofarmaco Curated by ChEMBL | Assay Description Binding affinity towards Angiotensin II type 2 receptor in bovine cerebellar cortical membranes; Inactive | J Med Chem 38: 4806-20 (1996) BindingDB Entry DOI: 10.7270/Q2JQ101N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50029911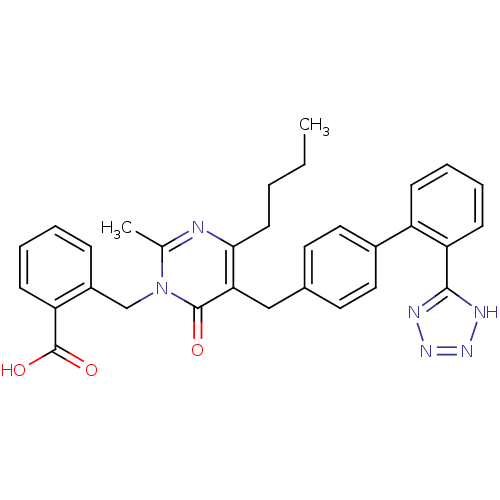 (2-{4-Butyl-2-methyl-6-oxo-5-[2'-(1H-tetrazol-5-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Lusofarmaco Curated by ChEMBL | Assay Description Binding affinity towards Angiotensin II type 2 receptor in bovine cerebellar cortical membranes; Inactive | J Med Chem 38: 4806-20 (1996) BindingDB Entry DOI: 10.7270/Q2JQ101N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50029903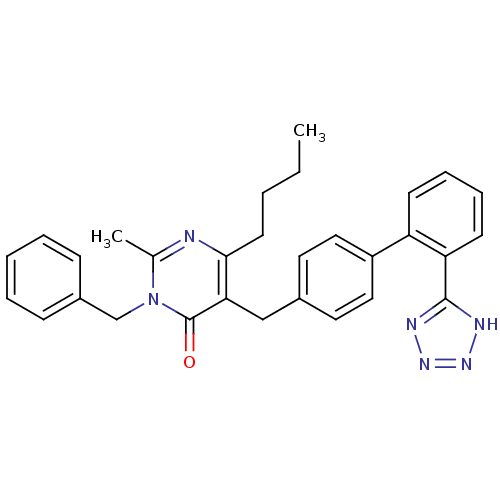 (3-Benzyl-6-butyl-2-methyl-5-[2'-(1H-tetrazol-5-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Lusofarmaco Curated by ChEMBL | Assay Description Binding affinity towards Angiotensin II type 2 receptor in bovine cerebellar cortical membranes; Inactive | J Med Chem 38: 4806-20 (1996) BindingDB Entry DOI: 10.7270/Q2JQ101N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50404022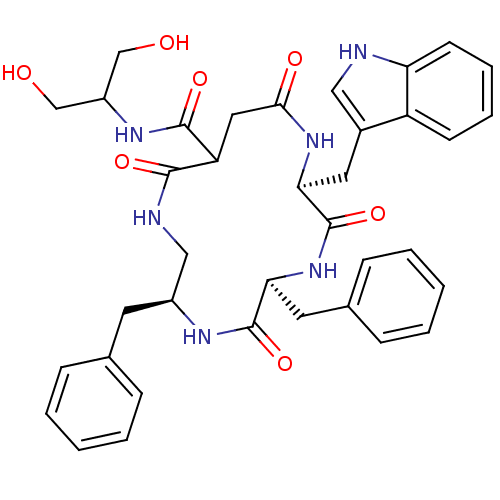 (CHEMBL2112926) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche S.p.A. Laboratori di Firenze Curated by ChEMBL | Assay Description In vitro functional activity towards Neurokinin NK2 receptor on isolated rabbit urinary bladder (RUB) | Bioorg Med Chem Lett 12: 693-6 (2002) BindingDB Entry DOI: 10.7270/Q22N53FR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50029894 (4'-(4-Butyl-2-methyl-6-oxo-1,6-dihydro-pyrimidin-5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Lusofarmaco Curated by ChEMBL | Assay Description Binding affinity towards Angiotensin II type 2 receptor in bovine cerebellar cortical membranes; Inactive | J Med Chem 38: 4806-20 (1996) BindingDB Entry DOI: 10.7270/Q2JQ101N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50029904 (4'-[4-Butyl-1-(2-carboxy-benzyl)-2-methyl-6-oxo-1,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Lusofarmaco Curated by ChEMBL | Assay Description Binding affinity towards Angiotensin II type 2 receptor in bovine cerebellar cortical membranes; Inactive | J Med Chem 38: 4806-20 (1996) BindingDB Entry DOI: 10.7270/Q2JQ101N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50029902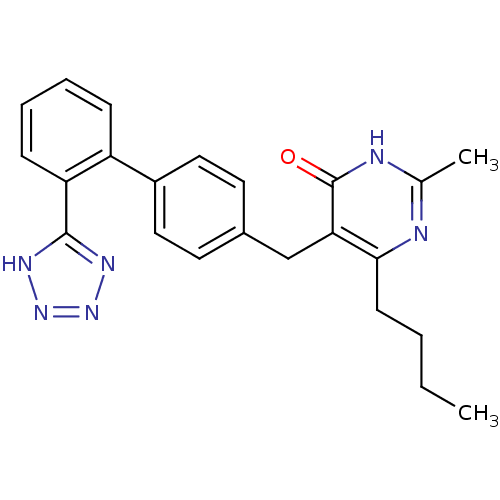 (6-Butyl-2-methyl-5-[2'-(1H-tetrazol-5-yl)-biphenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | >4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Lusofarmaco Curated by ChEMBL | Assay Description Binding affinity towards Angiotensin II type 2 receptor in bovine cerebellar cortical membranes; Inactive | J Med Chem 38: 4806-20 (1996) BindingDB Entry DOI: 10.7270/Q2JQ101N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50029897 (4'-[4-Butyl-1-(4-carboxy-benzyl)-6-oxo-1,6-dihydro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Lusofarmaco Curated by ChEMBL | Assay Description Binding affinity towards Angiotensin II type 2 receptor in bovine cerebellar cortical membranes; Inactive | J Med Chem 38: 4806-20 (1996) BindingDB Entry DOI: 10.7270/Q2JQ101N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50029901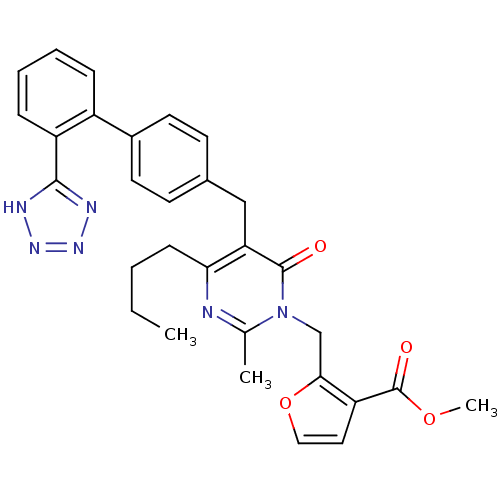 (2-{4-Butyl-2-methyl-6-oxo-5-[2'-(1H-tetrazol-5-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Lusofarmaco Curated by ChEMBL | Assay Description Binding affinity towards Angiotensin II type 2 receptor in bovine cerebellar cortical membranes; Inactive | J Med Chem 38: 4806-20 (1996) BindingDB Entry DOI: 10.7270/Q2JQ101N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50029892 (2-{4-Butyl-2-methyl-6-oxo-5-[2'-(1H-tetrazol-5-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Lusofarmaco Curated by ChEMBL | Assay Description Binding affinity towards Angiotensin II type 2 receptor in bovine cerebellar cortical membranes; Inactive | J Med Chem 38: 4806-20 (1996) BindingDB Entry DOI: 10.7270/Q2JQ101N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50241501 (6-Butyl-2-methyl-5-[2'-(1H-tetrazol-5-yl)-biphenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Lusofarmaco Curated by ChEMBL | Assay Description Binding affinity towards Angiotensin II type 2 receptor in bovine cerebellar cortical membranes; Inactive | J Med Chem 38: 4806-20 (1996) BindingDB Entry DOI: 10.7270/Q2JQ101N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50241491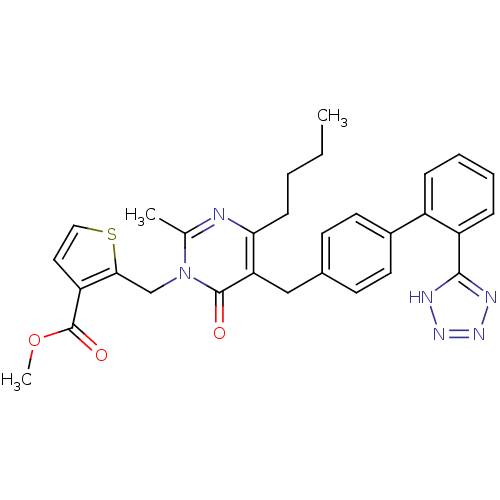 (2-{4-Butyl-2-methyl-6-oxo-5-[2'-(1H-tetrazol-5-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Lusofarmaco Curated by ChEMBL | Assay Description Binding affinity towards Angiotensin II type 2 receptor in bovine cerebellar cortical membranes; Inactive | J Med Chem 38: 4806-20 (1996) BindingDB Entry DOI: 10.7270/Q2JQ101N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50029896 (4'-(2-Butyl-6-oxo-6H-pyrimidin-1-ylmethyl)-bipheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Lusofarmaco Curated by ChEMBL | Assay Description Binding affinity towards Angiotensin II type 2 receptor in bovine cerebellar cortical membranes; Inactive | J Med Chem 38: 4806-20 (1996) BindingDB Entry DOI: 10.7270/Q2JQ101N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50470328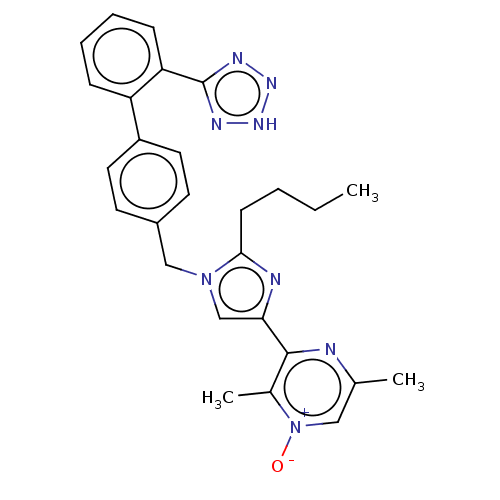 (CHEMBL317300) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori Guidotti S.p.A. Curated by ChEMBL | Assay Description Inhibition of [3H]angiotensin II binding to rat adrenal cortex membrane containing angiotensin II receptor, type 1 | J Med Chem 38: 2925-37 (1995) Article DOI: 10.1021/jm00015a015 BindingDB Entry DOI: 10.7270/Q2697689 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50470310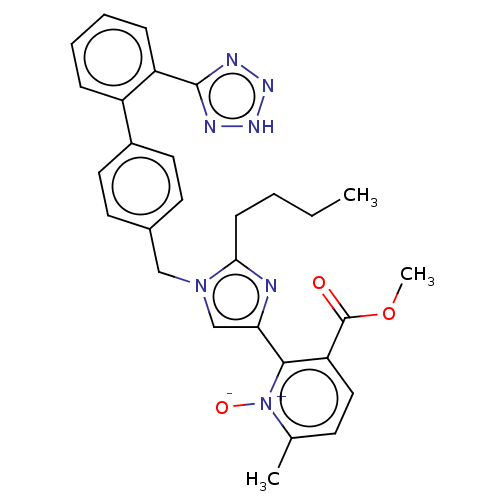 (CHEMBL98592) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori Guidotti S.p.A. Curated by ChEMBL | Assay Description Inhibition of [3H]angiotensin II binding to rat adrenal cortex membrane containing angiotensin II receptor, type 1 | J Med Chem 38: 2925-37 (1995) Article DOI: 10.1021/jm00015a015 BindingDB Entry DOI: 10.7270/Q2697689 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50404014 (CHEMBL159585) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche S.p.A. Laboratori di Firenze Curated by ChEMBL | Assay Description In vitro functional activity towards Neurokinin NK2 receptor on isolated rabbit urinary bladder (RUB) | Bioorg Med Chem Lett 12: 693-6 (2002) BindingDB Entry DOI: 10.7270/Q22N53FR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50404026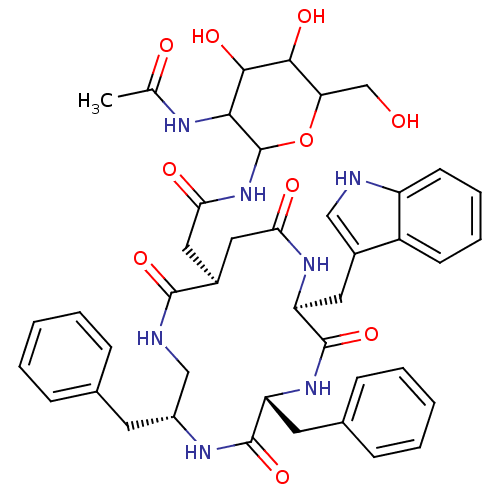 (CHEMBL350596) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche S.p.A. Laboratori di Firenze Curated by ChEMBL | Assay Description In vitro functional activity towards Neurokinin NK2 receptor on isolated rabbit urinary bladder (RUB) | Bioorg Med Chem Lett 12: 693-6 (2002) BindingDB Entry DOI: 10.7270/Q22N53FR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50404024 (CHEMBL346285) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche S.p.A. Laboratori di Firenze Curated by ChEMBL | Assay Description In vitro functional activity towards Neurokinin NK2 receptor on isolated rabbit urinary bladder (RUB) | Bioorg Med Chem Lett 12: 693-6 (2002) BindingDB Entry DOI: 10.7270/Q22N53FR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50470321 (CHEMBL99008) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori Guidotti S.p.A. Curated by ChEMBL | Assay Description Inhibition of [3H]angiotensin II binding to rat adrenal cortex membrane containing angiotensin II receptor, type 1 | J Med Chem 38: 2925-37 (1995) Article DOI: 10.1021/jm00015a015 BindingDB Entry DOI: 10.7270/Q2697689 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50472912 (CHEMBL116564 | MEN-11712) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche S.p.A. Curated by ChEMBL | Assay Description Binding affinity towards Tachykinin receptor 2 | J Med Chem 43: 4041-4 (2000) Article DOI: 10.1021/jm0010217 BindingDB Entry DOI: 10.7270/Q2Z322CV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50404015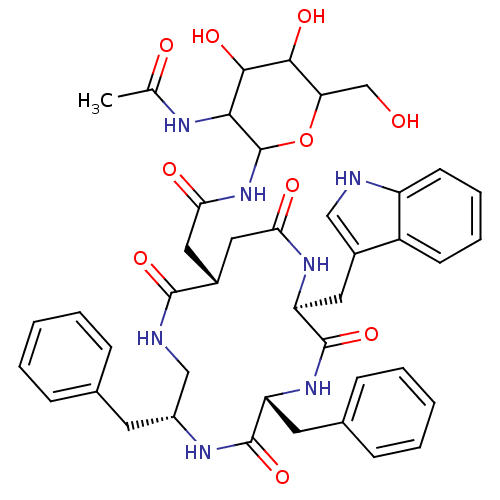 (CHEMBL161288) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche S.p.A. Laboratori di Firenze Curated by ChEMBL | Assay Description In vitro functional activity towards Neurokinin NK2 receptor on isolated rabbit urinary bladder (RUB) | Bioorg Med Chem Lett 12: 693-6 (2002) BindingDB Entry DOI: 10.7270/Q22N53FR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50470340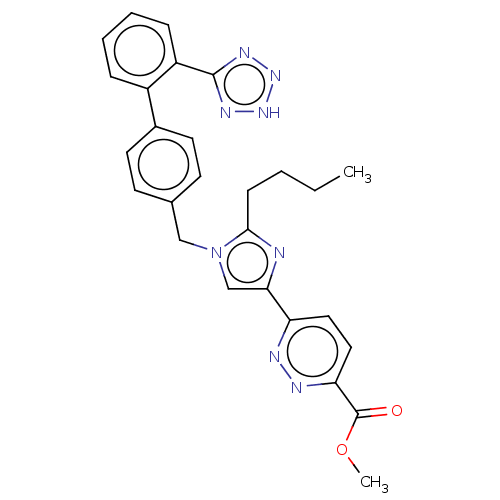 (CHEMBL98370) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori Guidotti S.p.A. Curated by ChEMBL | Assay Description Inhibition of [3H]angiotensin II binding to rat adrenal cortex membrane containing angiotensin II receptor, type 1 | J Med Chem 38: 2925-37 (1995) Article DOI: 10.1021/jm00015a015 BindingDB Entry DOI: 10.7270/Q2697689 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50470326 (CHEMBL319990) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 9.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori Guidotti S.p.A. Curated by ChEMBL | Assay Description Inhibition of [3H]angiotensin II binding to rat adrenal cortex membrane containing angiotensin II receptor, type 1 | J Med Chem 38: 2925-37 (1995) Article DOI: 10.1021/jm00015a015 BindingDB Entry DOI: 10.7270/Q2697689 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50472910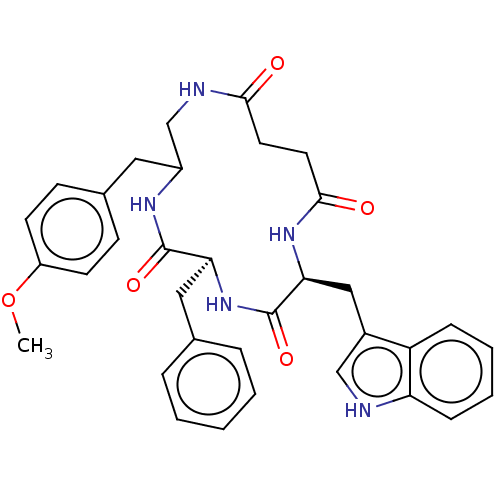 (CHEMBL263194) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche S.p.A. Curated by ChEMBL | Assay Description Binding affinity towards Tachykinin receptor 2 | J Med Chem 43: 4041-4 (2000) Article DOI: 10.1021/jm0010217 BindingDB Entry DOI: 10.7270/Q2Z322CV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50404018 (CHEMBL2112924) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche S.p.A. Laboratori di Firenze Curated by ChEMBL | Assay Description In vitro functional activity towards Neurokinin NK2 receptor on isolated rabbit urinary bladder (RUB) | Bioorg Med Chem Lett 12: 693-6 (2002) BindingDB Entry DOI: 10.7270/Q22N53FR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50472908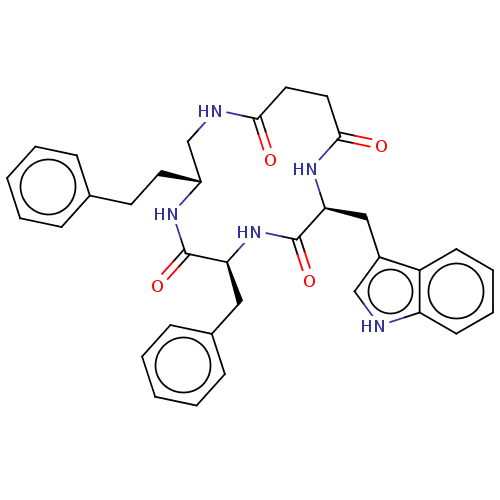 (CHEMBL336053) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche S.p.A. Curated by ChEMBL | Assay Description Binding affinity towards Tachykinin receptor 2 | J Med Chem 43: 4041-4 (2000) Article DOI: 10.1021/jm0010217 BindingDB Entry DOI: 10.7270/Q2Z322CV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50470313 (CHEMBL441640) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori Guidotti S.p.A. Curated by ChEMBL | Assay Description Inhibition of [3H]angiotensin II binding to rat adrenal cortex membrane containing angiotensin II receptor, type 1 | J Med Chem 38: 2925-37 (1995) Article DOI: 10.1021/jm00015a015 BindingDB Entry DOI: 10.7270/Q2697689 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50470330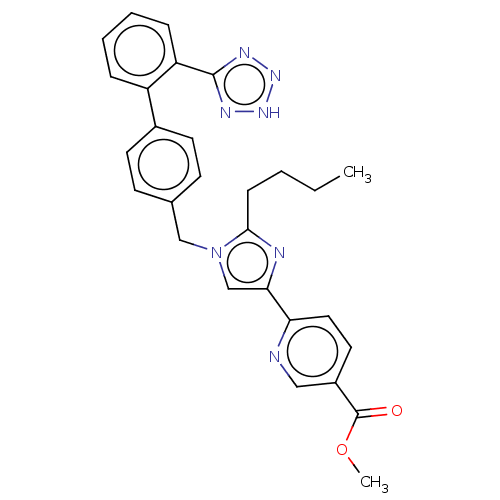 (CHEMBL318194) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori Guidotti S.p.A. Curated by ChEMBL | Assay Description Inhibition of [3H]angiotensin II binding to rat adrenal cortex membrane containing angiotensin II receptor, type 1 | J Med Chem 38: 2925-37 (1995) Article DOI: 10.1021/jm00015a015 BindingDB Entry DOI: 10.7270/Q2697689 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 152 total ) | Next | Last >> |