

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Delta-type opioid receptor (MOUSE) | BDBM50370067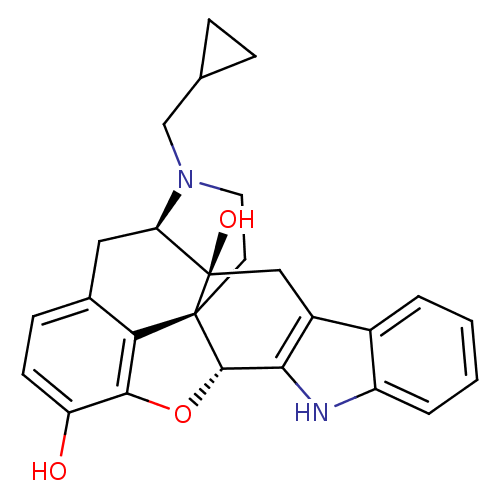 (CHEMBL1237164) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | PDB PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Antagonist activity of compound on agonist stimulated [35S]GTP-gamma-S binding on delta-opioid receptor | J Med Chem 44: 1471-4 (2001) BindingDB Entry DOI: 10.7270/Q2PK0GVP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM21864 ((21R)-22-(cyclopropylmethyl)-14-oxa-11,22-diazahep...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace [3H]p-CI-DPDPE in C6 glioma cells expressing the cloned Opioid receptor delta 1 | Bioorg Med Chem Lett 10: 2449-51 (2001) BindingDB Entry DOI: 10.7270/Q20K2939 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM21864 ((21R)-22-(cyclopropylmethyl)-14-oxa-11,22-diazahep...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PubMed | 0.0620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Ability to inhibit binding of SNC80 to delta opioid receptor | Bioorg Med Chem Lett 9: 3435-8 (2000) BindingDB Entry DOI: 10.7270/Q2GT5NPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM21864 ((21R)-22-(cyclopropylmethyl)-14-oxa-11,22-diazahep...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 0.0620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Ability to inhibit GTP-gamma-S binding to Opioid receptor delta 1 of guinea pig caudate. | J Med Chem 42: 1673-9 (1999) Article DOI: 10.1021/jm9807003 BindingDB Entry DOI: 10.7270/Q24M957P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2/delta/gamma (Homo sapiens (Human)) | BDBM50218667 (APC-1390 | CHEMBL46809) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Binding affinity to 5-HT3 serotonin receptor in NG 108-15 neuroblastoma glioma cells using [3H]-GR-65,630 radioligand. | Bioorg Med Chem Lett 11: 1629-33 (2001) BindingDB Entry DOI: 10.7270/Q2R78DHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2 (Homo sapiens (Human)) | BDBM50093142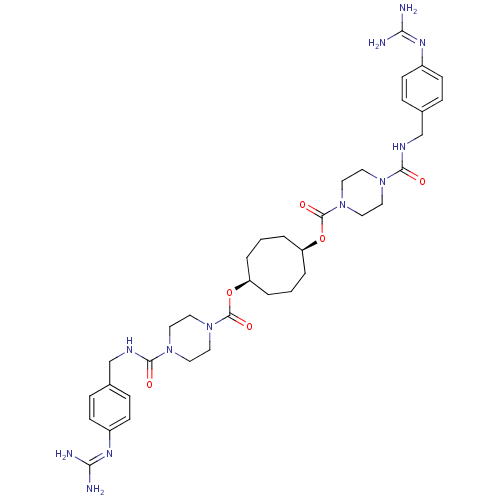 (1,5-di{4-[4-amino(imino)methylaminobenzylcarbamoyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against human tryptase enzyme | Bioorg Med Chem Lett 11: 2325-30 (2001) BindingDB Entry DOI: 10.7270/Q21835S6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50256671 (CHEMBL4085431) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Drug Design and Synthesis Section, Molecular Targets and Medications Discovery Branch, Intramural Research Program, National Institute on Drug Abuse and the National Institute on Alcohol Abuse and Al Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in rat brain after 60 mins by liquid scintillation counting method | Bioorg Med Chem 25: 2406-2422 (2017) Article DOI: 10.1016/j.bmc.2017.02.064 BindingDB Entry DOI: 10.7270/Q2765HS8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2/delta/gamma (Homo sapiens (Human)) | BDBM50093175 (CHEMBL311655 | Derivative of APC-2059) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Evaluated for its inhibitory potency against tryptase | Bioorg Med Chem Lett 10: 2361-6 (2001) BindingDB Entry DOI: 10.7270/Q2HX1BWR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2/delta/gamma (Homo sapiens (Human)) | BDBM50093157 (CHEMBL431969 | Derivative of piperazine-1-carboxyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Evaluated for its inhibitory potency against tryptase | Bioorg Med Chem Lett 10: 2361-6 (2001) BindingDB Entry DOI: 10.7270/Q2HX1BWR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50217952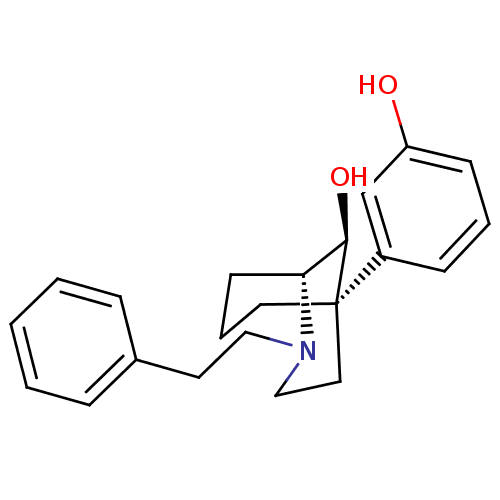 ((1R,5R,9S)-(-)-9-hydroxy-5-(3-hydroxyphenyl-2-phen...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Displacement of [125I]IOXY from human mu opioid receptor expressed in CHO cells | J Med Chem 50: 3765-76 (2007) Article DOI: 10.1021/jm061325e BindingDB Entry DOI: 10.7270/Q2D21XB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50217956 ((1R,5S)-(+)-5-(3-hydroxyphenyl)-9-methylene-2-phen...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Displacement of [125I]IOXY from human mu opioid receptor expressed in CHO cells | J Med Chem 50: 3765-76 (2007) Article DOI: 10.1021/jm061325e BindingDB Entry DOI: 10.7270/Q2D21XB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2/delta/gamma (Homo sapiens (Human)) | BDBM50093192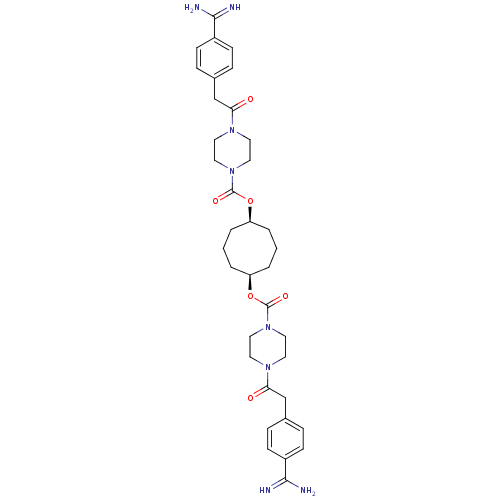 (CHEMBL311482 | Derivative of piperazine-1-carboxyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Evaluated for its inhibitory potency against tryptase | Bioorg Med Chem Lett 10: 2361-6 (2001) BindingDB Entry DOI: 10.7270/Q2HX1BWR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM21864 ((21R)-22-(cyclopropylmethyl)-14-oxa-11,22-diazahep...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PubMed | 0.217 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Compound was tested for its ability to displace [3H]DADLE from delta receptor in rat brain. | Bioorg Med Chem Lett 8: 799-804 (1999) BindingDB Entry DOI: 10.7270/Q2SF2WPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM21864 ((21R)-22-(cyclopropylmethyl)-14-oxa-11,22-diazahep...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Ability to displace [3H]U-69593 from kappa opioid receptor | Bioorg Med Chem Lett 9: 3435-8 (2000) BindingDB Entry DOI: 10.7270/Q2GT5NPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM21864 ((21R)-22-(cyclopropylmethyl)-14-oxa-11,22-diazahep...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK Curated by ChEMBL | Assay Description Displacement of [3H]DADLE from Opioid receptor delta 1 | Bioorg Med Chem Lett 11: 2883-5 (2001) BindingDB Entry DOI: 10.7270/Q2D79BZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50370067 (CHEMBL1237164) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | PDB PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]DADLE at delta-opioid receptor | J Med Chem 44: 1471-4 (2001) BindingDB Entry DOI: 10.7270/Q2PK0GVP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM21864 ((21R)-22-(cyclopropylmethyl)-14-oxa-11,22-diazahep...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Binding affinity towards Opioid receptor delta 1 by the displacement of [3H]DADLE radioligand in rat brain membranes | J Med Chem 42: 1673-9 (1999) Article DOI: 10.1021/jm9807003 BindingDB Entry DOI: 10.7270/Q24M957P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM21864 ((21R)-22-(cyclopropylmethyl)-14-oxa-11,22-diazahep...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]DADLE from morphine-insensitive Opioid receptor delta 1 (presence of 50 nM morphine) of rat brain membranes | J Med Chem 41: 2872-81 (1998) Article DOI: 10.1021/jm980083i BindingDB Entry DOI: 10.7270/Q24F1RFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50217952 ((1R,5R,9S)-(-)-9-hydroxy-5-(3-hydroxyphenyl-2-phen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.265 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Drug Design and Synthesis Section, Molecular Targets and Medications Discovery Branch, Intramural Research Program, National Institute on Drug Abuse and the National Institute on Alcohol Abuse and Al Curated by ChEMBL | Assay Description Agonist activity at rat mu opioid receptor expressed in rat C6 cell membranes assessed as stimulation of [35S]GTPgammaS binding incubated for 1 hr by... | Bioorg Med Chem 25: 2406-2422 (2017) Article DOI: 10.1016/j.bmc.2017.02.064 BindingDB Entry DOI: 10.7270/Q2765HS8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50017698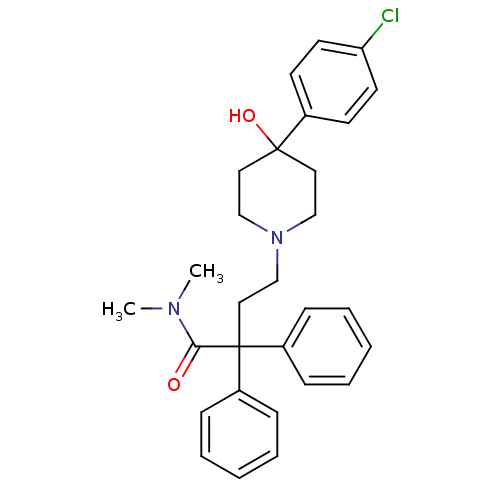 (4-(4-(4-chlorophenyl)-4-hydroxypiperidin-1-yl)-N,N...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 0.268 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in HEK293 cell membrane incubated for 60 mins by radioligand binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00611 BindingDB Entry DOI: 10.7270/Q2N301V5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50001054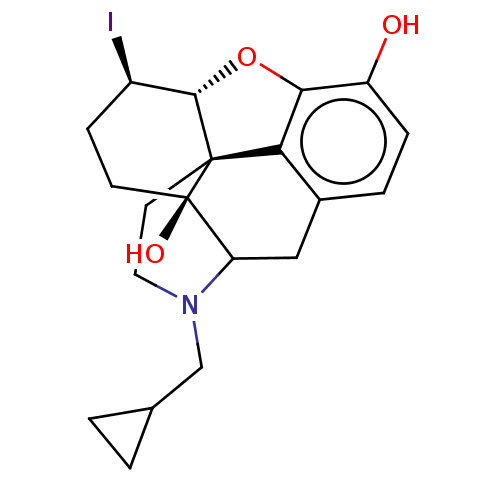 (4-cyclopropylmethyl-14-iodo-(14R,17S)-12-oxa-4-aza...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description In vito concentration required to displace [3H]DAGO (Kd = 0.7 nM and concentration is 1.7 nM) from opioid receptor mu in rat brain membranes. | J Med Chem 35: 2826-35 (1992) BindingDB Entry DOI: 10.7270/Q2639QB3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM21864 ((21R)-22-(cyclopropylmethyl)-14-oxa-11,22-diazahep...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]DADLE from Opioid receptor delta 1 of rat brain membranes (DAMGO quenching mu receptor) | J Med Chem 41: 2872-81 (1998) Article DOI: 10.1021/jm980083i BindingDB Entry DOI: 10.7270/Q24F1RFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50021214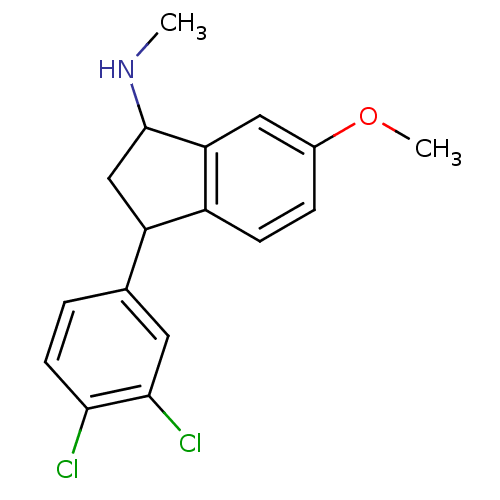 (CHEMBL300019 | CHEMBL537996 | [3-(3,4-Dichloro-phe...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [125I]RTI-55 from serotonin transporter of frozen rat caudate membranes | J Med Chem 47: 2624-34 (2004) Article DOI: 10.1021/jm0305873 BindingDB Entry DOI: 10.7270/Q2MK6DF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50001053 (14-bromo-4-cyclopropylmethyl-(13R,14R,17S)-12-oxa-...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description In vito concentration required to displace 9 (Kd = 1.6 nM and concentration is 1.8 nM) from opioid receptor kappa 1 in guinea brain membranes. | J Med Chem 35: 2826-35 (1992) BindingDB Entry DOI: 10.7270/Q2639QB3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50068455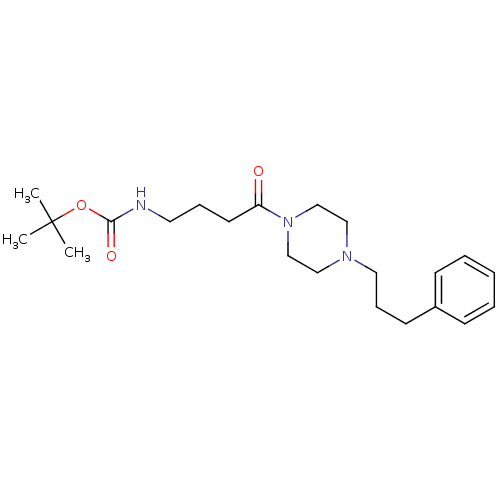 (CHEMBL342809 | {4-Oxo-4-[4-(3-phenyl-propyl)-piper...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity was tested against sigma-1 receptor in guinea brain membranes. | J Med Chem 41: 4950-7 (1999) Article DOI: 10.1021/jm980143k BindingDB Entry DOI: 10.7270/Q2NG4PR0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50585129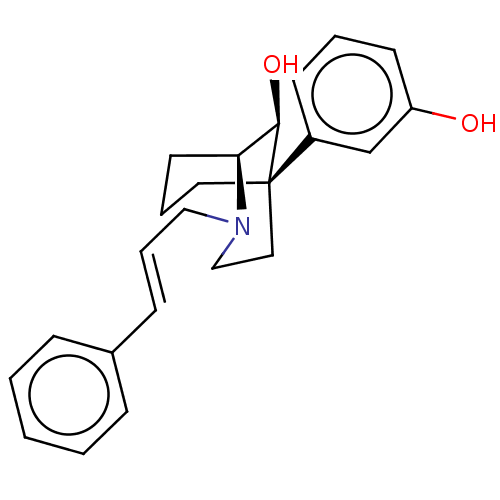 (CHEMBL5077645) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.377 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in HEK293 cell membrane incubated for 60 mins by radioligand binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00611 BindingDB Entry DOI: 10.7270/Q2N301V5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50021226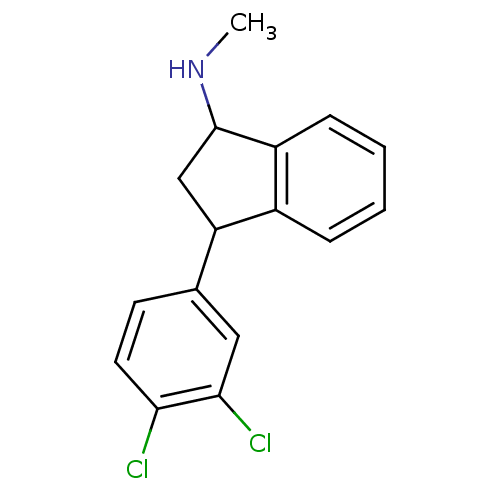 (CHEMBL296602 | Indatraline | [3-(3,4-Dichloro-phen...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [125I]RTI-55 from serotonin transporter of frozen rat caudate membranes | J Med Chem 47: 2624-34 (2004) Article DOI: 10.1021/jm0305873 BindingDB Entry DOI: 10.7270/Q2MK6DF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2 (Homo sapiens (Human)) | BDBM50101018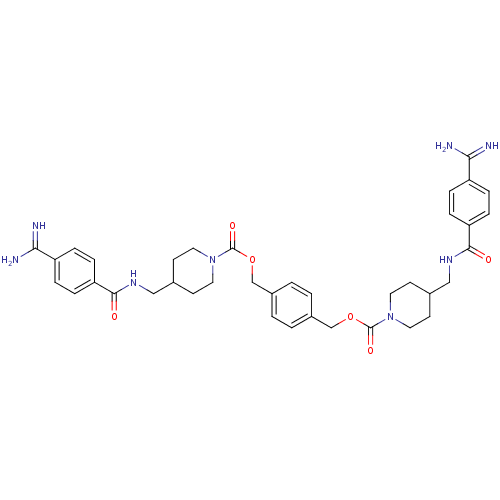 (1,4-di{4-[4-amino(imino)methylphenylcarboxamidomet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against human tryptase enzyme | Bioorg Med Chem Lett 11: 2325-30 (2001) BindingDB Entry DOI: 10.7270/Q21835S6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2/delta/gamma (Homo sapiens (Human)) | BDBM50093156 (CHEMBL432172 | Derivative of APC-2059) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Evaluated for its inhibitory potency against tryptase | Bioorg Med Chem Lett 10: 2361-6 (2001) BindingDB Entry DOI: 10.7270/Q2HX1BWR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2/delta/gamma (Homo sapiens (Human)) | BDBM50093167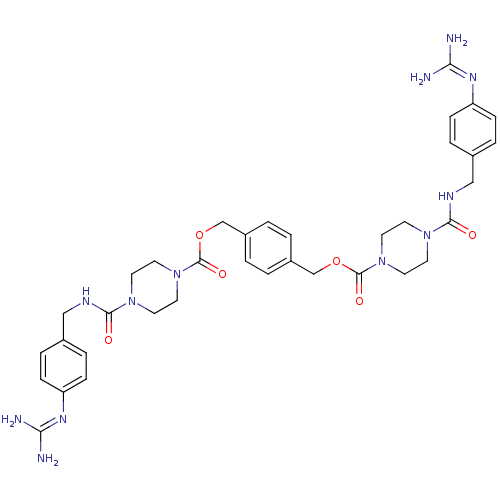 (CHEMBL75750 | Derivative of APC-2059) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Evaluated for its inhibitory potency against tryptase | Bioorg Med Chem Lett 10: 2361-6 (2001) BindingDB Entry DOI: 10.7270/Q2HX1BWR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2/delta/gamma (Homo sapiens (Human)) | BDBM50093154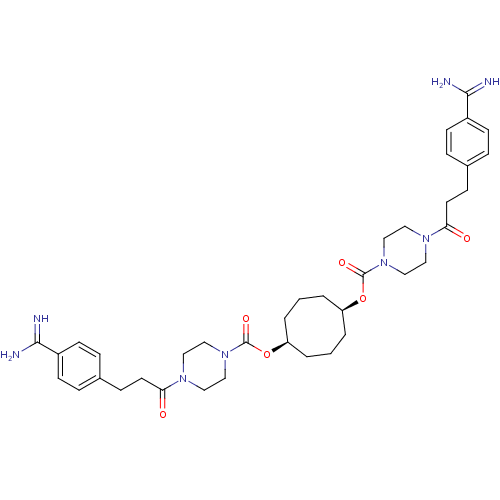 (CHEMBL448786 | Derivative of piperazine-1-carboxyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Evaluated for its inhibitory potency against tryptase | Bioorg Med Chem Lett 10: 2361-6 (2001) BindingDB Entry DOI: 10.7270/Q2HX1BWR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2/delta/gamma (Homo sapiens (Human)) | BDBM50101018 (1,4-di{4-[4-amino(imino)methylphenylcarboxamidomet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Binding affinity to 5-HT3 serotonin receptor in NG 108-15 neuroblastoma glioma cells using [3H]-GR-65,630 radioligand. | Bioorg Med Chem Lett 11: 1629-33 (2001) BindingDB Entry DOI: 10.7270/Q2R78DHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50183549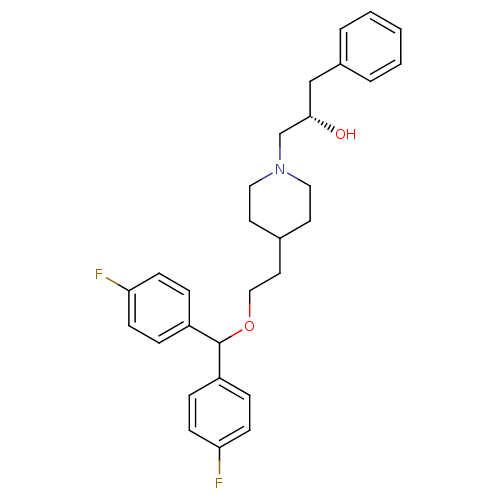 ((S)-(+)-4-[2-[bis-(4-fluorophenyl)methoxy]ethyl]-1...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK Curated by ChEMBL | Assay Description Displacement of [125I]RTI-55 from DAT | J Med Chem 49: 1766-72 (2006) Article DOI: 10.1021/jm050766f BindingDB Entry DOI: 10.7270/Q2KS6R5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM21864 ((21R)-22-(cyclopropylmethyl)-14-oxa-11,22-diazahep...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Binding affinity for Opioid receptor delta 1 was determined by inhibition of binding of [3H]DADLE (1.3-2.0 nM) to rat brain membranes | J Med Chem 42: 3527-38 (1999) Article DOI: 10.1021/jm990039i BindingDB Entry DOI: 10.7270/Q2QR4XSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50001054 (4-cyclopropylmethyl-14-iodo-(14R,17S)-12-oxa-4-aza...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description In vito concentration required to displace 9 (Kd = 1.6 nM and concentration is 1.8 nM) from opioid receptor kappa 1 in guinea brain membranes. | J Med Chem 35: 2826-35 (1992) BindingDB Entry DOI: 10.7270/Q2639QB3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50118598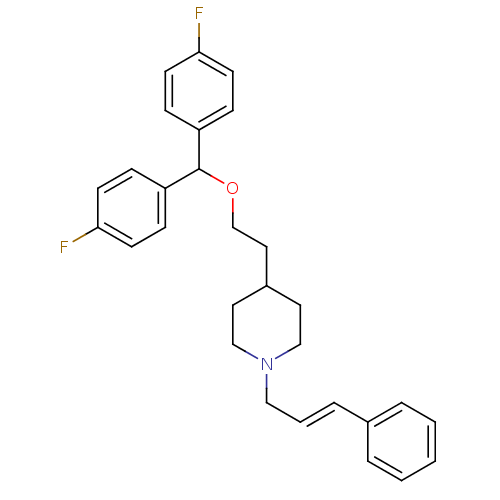 (4-{2-[Bis-(4-fluoro-phenyl)-methoxy]-ethyl}-1-(3-p...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK Curated by ChEMBL | Assay Description Binding affinity of the compound towards dopamine transporter (DAT) by using [125I]-RTI-55 radioligand | J Med Chem 45: 4371-4 (2002) BindingDB Entry DOI: 10.7270/Q2RF5TCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50065862 (22-cyclopropylmethyl-9-phenyl-14-oxa-11,22-diazahe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]DADLE from morphine-insensitive Opioid receptor delta 1 (presence of 50 nM morphine) of rat brain membranes | J Med Chem 41: 2872-81 (1998) Article DOI: 10.1021/jm980083i BindingDB Entry DOI: 10.7270/Q24F1RFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2/delta/gamma (Homo sapiens (Human)) | BDBM50093199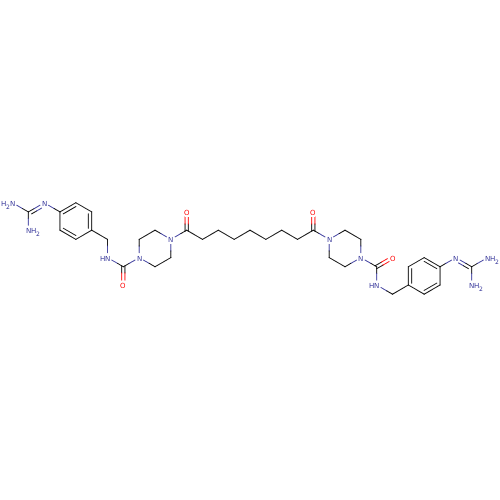 (CHEMBL75972 | Derivative of APC-2059) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Evaluated for its inhibitory potency against tryptase | Bioorg Med Chem Lett 10: 2361-6 (2001) BindingDB Entry DOI: 10.7270/Q2HX1BWR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2/delta/gamma (Homo sapiens (Human)) | BDBM50218668 (CHEMBL42900) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Binding affinity to 5-HT3 serotonin receptor in NG 108-15 neuroblastoma glioma cells using [3H]-GR-65,630 radioligand. | Bioorg Med Chem Lett 11: 1629-33 (2001) BindingDB Entry DOI: 10.7270/Q2R78DHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2/delta/gamma (Homo sapiens (Human)) | BDBM50093158 (1-{4-[4-amino(imino)methylaminobenzylcarbamoyl]hex...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Evaluated for its inhibitory potency against tryptase | Bioorg Med Chem Lett 10: 2361-6 (2001) BindingDB Entry DOI: 10.7270/Q2HX1BWR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2/delta/gamma (Homo sapiens (Human)) | BDBM50093173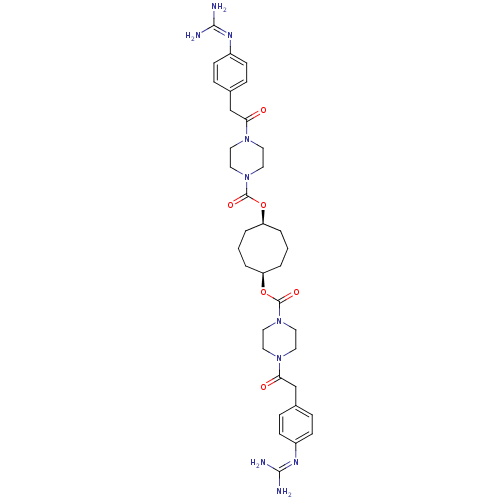 (CHEMBL309830 | Derivative of piperazine-1-carboxyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Evaluated for its inhibitory potency against tryptase | Bioorg Med Chem Lett 10: 2361-6 (2001) BindingDB Entry DOI: 10.7270/Q2HX1BWR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50056543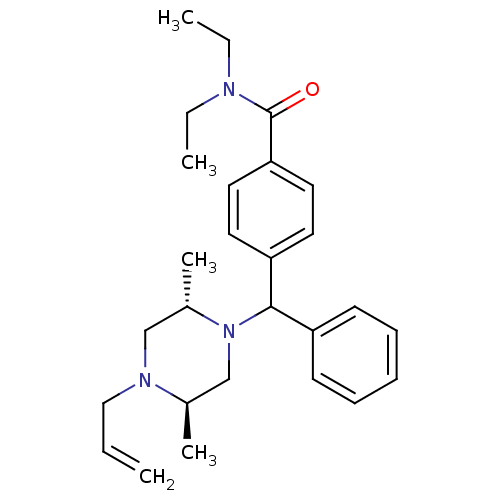 (4-[((1R,2S,5R)-4-Allyl-2,5-dimethyl-piperazin-1-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description In vitro binding affinity to Opioid receptor delta 1 in rat brain membranes by [3H]DADLE (Tyr-D-Ala-Gly-Phe-D-Leu) displacement. | J Med Chem 42: 5455-63 (2000) BindingDB Entry DOI: 10.7270/Q2QJ7J0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50183539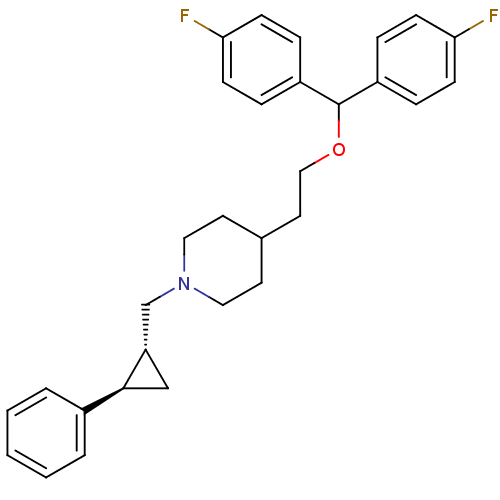 (CHEMBL381256 | CHEMBL429492 | trans-(R,R)-4-[2-[bi...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK Curated by ChEMBL | Assay Description Displacement of [125I]RTI-55 from DAT | J Med Chem 49: 1766-72 (2006) Article DOI: 10.1021/jm050766f BindingDB Entry DOI: 10.7270/Q2KS6R5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50366775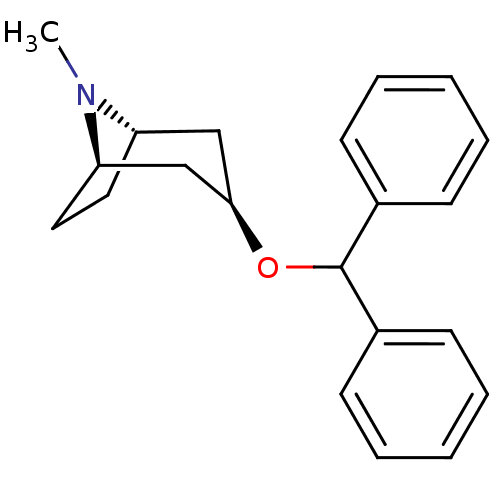 (BENZTROPINE | Benzatropine) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | PubMed | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity against Muscarinic receptor from rat brain membranes using [3H]pirenzepine | J Med Chem 44: 3937-45 (2001) BindingDB Entry DOI: 10.7270/Q26Q1XZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2 (Homo sapiens (Human)) | BDBM50084616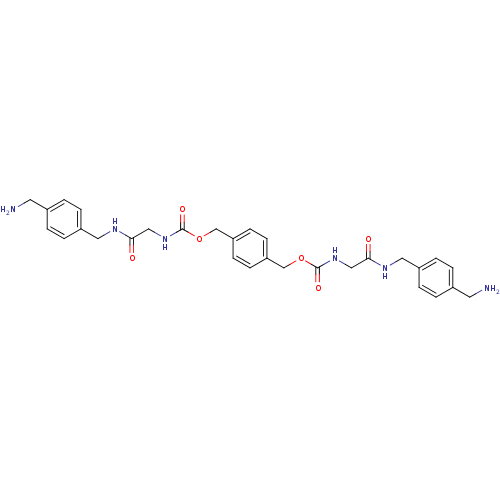 (CHEMBL310290 | [(4-Aminomethyl-benzylcarbamoyl)-me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory potency against tryptase | Bioorg Med Chem Lett 10: 2357-60 (2001) BindingDB Entry DOI: 10.7270/Q2NP23NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50217950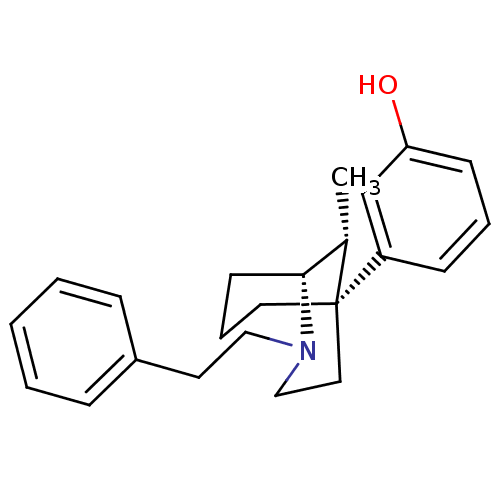 ((1R,5S,9R)-(+)-5-(3-hydroxyphenyl)-9-methyl-2-phen...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Displacement of [125I]IOXY from human mu opioid receptor expressed in CHO cells | J Med Chem 50: 3765-76 (2007) Article DOI: 10.1021/jm061325e BindingDB Entry DOI: 10.7270/Q2D21XB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50065854 (22-cyclopropylmethyl-9-phenoxy-14-oxa-11,22-diazah...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]DADLE from morphine-insensitive Opioid receptor delta 1 (presence of 50 nM morphine) of rat brain membranes | J Med Chem 41: 2872-81 (1998) Article DOI: 10.1021/jm980083i BindingDB Entry DOI: 10.7270/Q24F1RFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50585128 (CHEMBL5080654) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.633 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in HEK293 cell membrane incubated for 60 mins by radioligand binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00611 BindingDB Entry DOI: 10.7270/Q2N301V5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50217956 ((1R,5S)-(+)-5-(3-hydroxyphenyl)-9-methylene-2-phen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Displacement of [125I]IOXY from human kappa opioid receptor expressed in CHO cells | J Med Chem 50: 3765-76 (2007) Article DOI: 10.1021/jm061325e BindingDB Entry DOI: 10.7270/Q2D21XB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50098674 ((E)-17-(2-butenyl)-6,7-dehydro-4,5alpha-epoxy-3,14...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Antagonist activity of compound on agonist stimulated [35S]GTP-gamma-S binding on delta-opioid receptor | J Med Chem 44: 1471-4 (2001) BindingDB Entry DOI: 10.7270/Q2PK0GVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 5706 total ) | Next | Last >> |