

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coagulation factor X (Homo sapiens (Human)) | BDBM17298 (4-[({1-methyl-5-[1-(pyrrolidin-1-ylcarbonyl)cyclop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 15 | -46.5 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Boehringer Ingelheim Pharma KG | Assay Description For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con... | Structure 9: 29-37 (2001) Article DOI: 10.1016/s0969-2126(00)00551-7 BindingDB Entry DOI: 10.7270/Q2028PTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM17298 (4-[({1-methyl-5-[1-(pyrrolidin-1-ylcarbonyl)cyclop...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 20 | -45.7 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Boehringer Ingelheim Pharma KG | Assay Description For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con... | Structure 9: 29-37 (2001) Article DOI: 10.1016/s0969-2126(00)00551-7 BindingDB Entry DOI: 10.7270/Q2028PTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17297 (4-[({1-methyl-5-[(2-methyl-1H-1,3-benzodiazol-1-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Patents | PDB Article PubMed | 40 | -43.9 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Boehringer Ingelheim Pharma KG | Assay Description For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con... | Structure 9: 29-37 (2001) Article DOI: 10.1016/s0969-2126(00)00551-7 BindingDB Entry DOI: 10.7270/Q2028PTC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17295 (BIBT0871 | ethyl 2-{[(E)-{[1-(2-{[(4-carbamimidoyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem | PDB Article PubMed | 57 | -43.0 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Boehringer Ingelheim Pharma KG | Assay Description For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con... | Structure 9: 29-37 (2001) Article DOI: 10.1016/s0969-2126(00)00551-7 BindingDB Entry DOI: 10.7270/Q2028PTC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM17297 (4-[({1-methyl-5-[(2-methyl-1H-1,3-benzodiazol-1-yl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Patents | DrugBank MMDB PDB Article PubMed | 67 | -42.6 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Boehringer Ingelheim Pharma KG | Assay Description For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con... | Structure 9: 29-37 (2001) Article DOI: 10.1016/s0969-2126(00)00551-7 BindingDB Entry DOI: 10.7270/Q2028PTC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM17298 (4-[({1-methyl-5-[1-(pyrrolidin-1-ylcarbonyl)cyclop...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 102 | -41.5 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Boehringer Ingelheim Pharma KG | Assay Description For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con... | Structure 9: 29-37 (2001) Article DOI: 10.1016/s0969-2126(00)00551-7 BindingDB Entry DOI: 10.7270/Q2028PTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM17295 (BIBT0871 | ethyl 2-{[(E)-{[1-(2-{[(4-carbamimidoyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem | DrugBank MMDB PDB Article PubMed | 110 | -41.3 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Boehringer Ingelheim Pharma KG | Assay Description For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con... | Structure 9: 29-37 (2001) Article DOI: 10.1016/s0969-2126(00)00551-7 BindingDB Entry DOI: 10.7270/Q2028PTC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM17295 (BIBT0871 | ethyl 2-{[(E)-{[1-(2-{[(4-carbamimidoyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem | PDB Article PubMed | 140 | -40.7 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Boehringer Ingelheim Pharma KG | Assay Description For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con... | Structure 9: 29-37 (2001) Article DOI: 10.1016/s0969-2126(00)00551-7 BindingDB Entry DOI: 10.7270/Q2028PTC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM17297 (4-[({1-methyl-5-[(2-methyl-1H-1,3-benzodiazol-1-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Patents | PDB Article PubMed | 780 | -36.3 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Boehringer Ingelheim Pharma KG | Assay Description For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con... | Structure 9: 29-37 (2001) Article DOI: 10.1016/s0969-2126(00)00551-7 BindingDB Entry DOI: 10.7270/Q2028PTC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM17297 (4-[({1-methyl-5-[(2-methyl-1H-1,3-benzodiazol-1-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Patents | Article PubMed | 4.10E+3 | -32.0 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Boehringer Ingelheim Pharma KG | Assay Description For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con... | Structure 9: 29-37 (2001) Article DOI: 10.1016/s0969-2126(00)00551-7 BindingDB Entry DOI: 10.7270/Q2028PTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM17298 (4-[({1-methyl-5-[1-(pyrrolidin-1-ylcarbonyl)cyclop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 6.50E+3 | -30.8 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Boehringer Ingelheim Pharma KG | Assay Description For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con... | Structure 9: 29-37 (2001) Article DOI: 10.1016/s0969-2126(00)00551-7 BindingDB Entry DOI: 10.7270/Q2028PTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM17295 (BIBT0871 | ethyl 2-{[(E)-{[1-(2-{[(4-carbamimidoyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem | Article PubMed | 6.80E+3 | -30.7 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Boehringer Ingelheim Pharma KG | Assay Description For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con... | Structure 9: 29-37 (2001) Article DOI: 10.1016/s0969-2126(00)00551-7 BindingDB Entry DOI: 10.7270/Q2028PTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM17298 (4-[({1-methyl-5-[1-(pyrrolidin-1-ylcarbonyl)cyclop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 8.20E+3 | -30.2 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Boehringer Ingelheim Pharma KG | Assay Description For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con... | Structure 9: 29-37 (2001) Article DOI: 10.1016/s0969-2126(00)00551-7 BindingDB Entry DOI: 10.7270/Q2028PTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM17295 (BIBT0871 | ethyl 2-{[(E)-{[1-(2-{[(4-carbamimidoyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem | Article PubMed | 9.00E+3 | -30.0 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Boehringer Ingelheim Pharma KG | Assay Description For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con... | Structure 9: 29-37 (2001) Article DOI: 10.1016/s0969-2126(00)00551-7 BindingDB Entry DOI: 10.7270/Q2028PTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM17297 (4-[({1-methyl-5-[(2-methyl-1H-1,3-benzodiazol-1-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Patents | Article PubMed | 9.20E+3 | -29.9 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Boehringer Ingelheim Pharma KG | Assay Description For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con... | Structure 9: 29-37 (2001) Article DOI: 10.1016/s0969-2126(00)00551-7 BindingDB Entry DOI: 10.7270/Q2028PTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM17298 (4-[({1-methyl-5-[1-(pyrrolidin-1-ylcarbonyl)cyclop...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.30E+4 | -29.0 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Boehringer Ingelheim Pharma KG | Assay Description For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con... | Structure 9: 29-37 (2001) Article DOI: 10.1016/s0969-2126(00)00551-7 BindingDB Entry DOI: 10.7270/Q2028PTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM17297 (4-[({1-methyl-5-[(2-methyl-1H-1,3-benzodiazol-1-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Patents | Article PubMed | 1.60E+4 | -28.5 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Boehringer Ingelheim Pharma KG | Assay Description For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con... | Structure 9: 29-37 (2001) Article DOI: 10.1016/s0969-2126(00)00551-7 BindingDB Entry DOI: 10.7270/Q2028PTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM17298 (4-[({1-methyl-5-[1-(pyrrolidin-1-ylcarbonyl)cyclop...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | >3.00E+4 | >-26.9 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Boehringer Ingelheim Pharma KG | Assay Description For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con... | Structure 9: 29-37 (2001) Article DOI: 10.1016/s0969-2126(00)00551-7 BindingDB Entry DOI: 10.7270/Q2028PTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM17297 (4-[({1-methyl-5-[(2-methyl-1H-1,3-benzodiazol-1-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Patents | Article PubMed | >4.00E+4 | >-26.1 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Boehringer Ingelheim Pharma KG | Assay Description For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con... | Structure 9: 29-37 (2001) Article DOI: 10.1016/s0969-2126(00)00551-7 BindingDB Entry DOI: 10.7270/Q2028PTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM17295 (BIBT0871 | ethyl 2-{[(E)-{[1-(2-{[(4-carbamimidoyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem | Article PubMed | >4.00E+4 | >-26.1 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Boehringer Ingelheim Pharma KG | Assay Description For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con... | Structure 9: 29-37 (2001) Article DOI: 10.1016/s0969-2126(00)00551-7 BindingDB Entry DOI: 10.7270/Q2028PTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM17298 (4-[({1-methyl-5-[1-(pyrrolidin-1-ylcarbonyl)cyclop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | >4.00E+4 | >-26.1 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Boehringer Ingelheim Pharma KG | Assay Description For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con... | Structure 9: 29-37 (2001) Article DOI: 10.1016/s0969-2126(00)00551-7 BindingDB Entry DOI: 10.7270/Q2028PTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM17295 (BIBT0871 | ethyl 2-{[(E)-{[1-(2-{[(4-carbamimidoyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem | Article PubMed | 4.40E+4 | -25.9 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Boehringer Ingelheim Pharma KG | Assay Description For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con... | Structure 9: 29-37 (2001) Article DOI: 10.1016/s0969-2126(00)00551-7 BindingDB Entry DOI: 10.7270/Q2028PTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM17295 (BIBT0871 | ethyl 2-{[(E)-{[1-(2-{[(4-carbamimidoyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem | Article PubMed | >5.00E+4 | >-25.5 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Boehringer Ingelheim Pharma KG | Assay Description For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con... | Structure 9: 29-37 (2001) Article DOI: 10.1016/s0969-2126(00)00551-7 BindingDB Entry DOI: 10.7270/Q2028PTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM17297 (4-[({1-methyl-5-[(2-methyl-1H-1,3-benzodiazol-1-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Patents | Article PubMed | >5.00E+4 | >-25.5 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Boehringer Ingelheim Pharma KG | Assay Description For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con... | Structure 9: 29-37 (2001) Article DOI: 10.1016/s0969-2126(00)00551-7 BindingDB Entry DOI: 10.7270/Q2028PTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM310019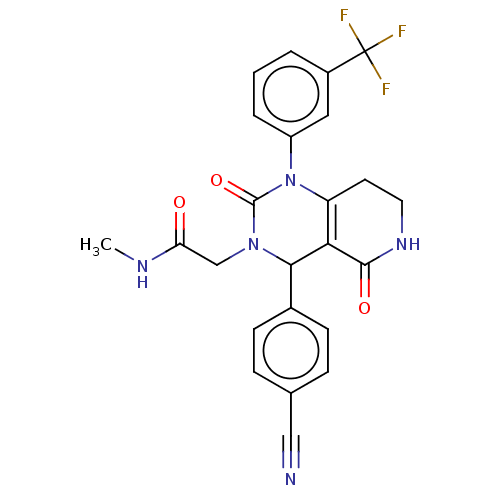 (US9657015, Example 12 | US9657015, Example 12.1 | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Materials: Human neutrophil elastase was purchased from Calbiochem (Cat. No.: 324681) and the elastase substrate MeOSuc-Ala-Ala-Pro-Val-AMC from Bach... | US Patent US9657015 (2017) BindingDB Entry DOI: 10.7270/Q28G8NRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM310046 (US9657015, Example 15 | US9657015, Example 15.1 | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Materials: Human neutrophil elastase was purchased from Calbiochem (Cat. No.: 324681) and the elastase substrate MeOSuc-Ala-Ala-Pro-Val-AMC from Bach... | US Patent US9657015 (2017) BindingDB Entry DOI: 10.7270/Q28G8NRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM310046 (US9657015, Example 15 | US9657015, Example 15.1 | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Materials: Human neutrophil elastase was purchased from Calbiochem (Cat. No.: 324681) and the elastase substrate MeOSuc-Ala-Ala-Pro-Val-AMC from Bach... | US Patent US9657015 (2017) BindingDB Entry DOI: 10.7270/Q28G8NRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM310046 (US9657015, Example 15 | US9657015, Example 15.1 | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Materials: Human neutrophil elastase was purchased from Calbiochem (Cat. No.: 324681) and the elastase substrate MeOSuc-Ala-Ala-Pro-Val-AMC from Bach... | US Patent US9657015 (2017) BindingDB Entry DOI: 10.7270/Q28G8NRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM310046 (US9657015, Example 15 | US9657015, Example 15.1 | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Materials: Human neutrophil elastase was purchased from Calbiochem (Cat. No.: 324681) and the elastase substrate MeOSuc-Ala-Ala-Pro-Val-AMC from Bach... | US Patent US9657015 (2017) BindingDB Entry DOI: 10.7270/Q28G8NRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM310053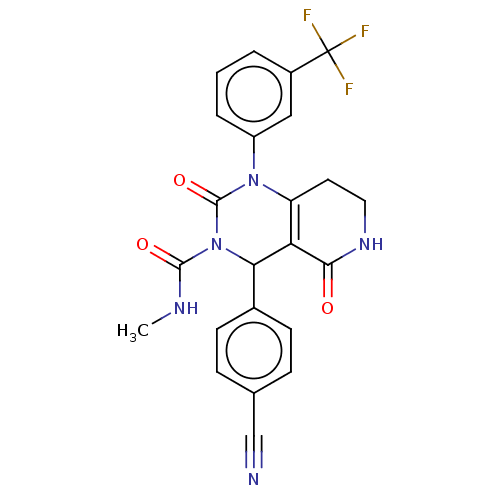 (US9657015, Example 16) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Materials: Human neutrophil elastase was purchased from Calbiochem (Cat. No.: 324681) and the elastase substrate MeOSuc-Ala-Ala-Pro-Val-AMC from Bach... | US Patent US9657015 (2017) BindingDB Entry DOI: 10.7270/Q28G8NRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM310054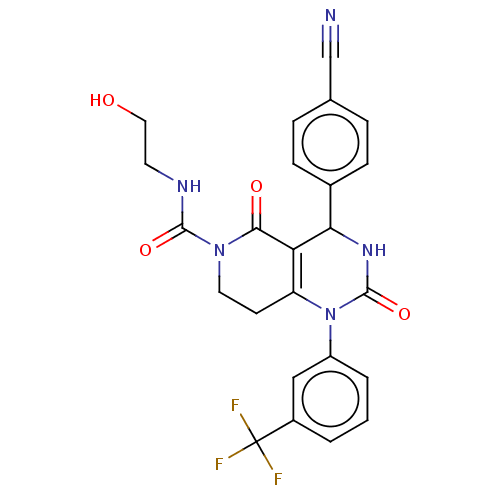 (BDBM310055 | US9657015, Example 17) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Materials: Human neutrophil elastase was purchased from Calbiochem (Cat. No.: 324681) and the elastase substrate MeOSuc-Ala-Ala-Pro-Val-AMC from Bach... | US Patent US9657015 (2017) BindingDB Entry DOI: 10.7270/Q28G8NRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM310059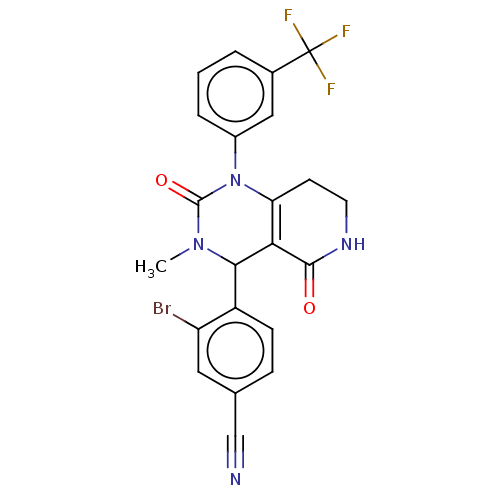 (US9657015, Example 22) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Materials: Human neutrophil elastase was purchased from Calbiochem (Cat. No.: 324681) and the elastase substrate MeOSuc-Ala-Ala-Pro-Val-AMC from Bach... | US Patent US9657015 (2017) BindingDB Entry DOI: 10.7270/Q28G8NRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM310060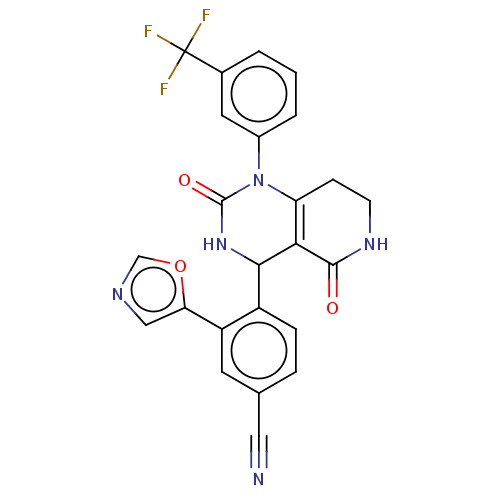 (US9657015, Example 23) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Materials: Human neutrophil elastase was purchased from Calbiochem (Cat. No.: 324681) and the elastase substrate MeOSuc-Ala-Ala-Pro-Val-AMC from Bach... | US Patent US9657015 (2017) BindingDB Entry DOI: 10.7270/Q28G8NRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM310061 (US9657015, Example 24) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Materials: Human neutrophil elastase was purchased from Calbiochem (Cat. No.: 324681) and the elastase substrate MeOSuc-Ala-Ala-Pro-Val-AMC from Bach... | US Patent US9657015 (2017) BindingDB Entry DOI: 10.7270/Q28G8NRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM310062 (US9657015, Example 25 | US9657015, Example 30A) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Materials: Human neutrophil elastase was purchased from Calbiochem (Cat. No.: 324681) and the elastase substrate MeOSuc-Ala-Ala-Pro-Val-AMC from Bach... | US Patent US9657015 (2017) BindingDB Entry DOI: 10.7270/Q28G8NRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM310063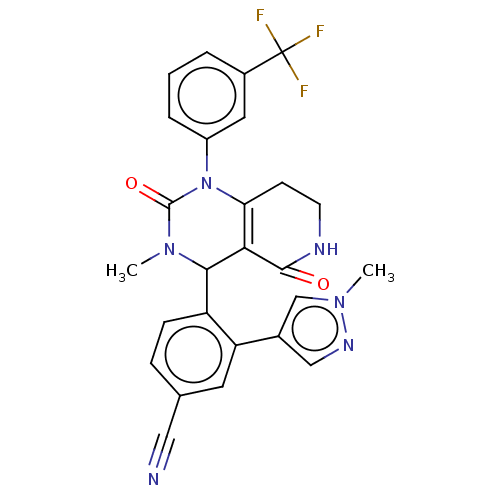 (US9657015, Example 26) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Materials: Human neutrophil elastase was purchased from Calbiochem (Cat. No.: 324681) and the elastase substrate MeOSuc-Ala-Ala-Pro-Val-AMC from Bach... | US Patent US9657015 (2017) BindingDB Entry DOI: 10.7270/Q28G8NRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM310064 (US9657015, Example 27) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Materials: Human neutrophil elastase was purchased from Calbiochem (Cat. No.: 324681) and the elastase substrate MeOSuc-Ala-Ala-Pro-Val-AMC from Bach... | US Patent US9657015 (2017) BindingDB Entry DOI: 10.7270/Q28G8NRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM310065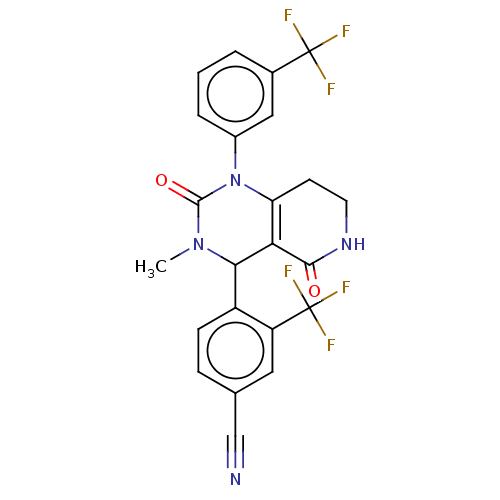 (US9657015, Example 28) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Materials: Human neutrophil elastase was purchased from Calbiochem (Cat. No.: 324681) and the elastase substrate MeOSuc-Ala-Ala-Pro-Val-AMC from Bach... | US Patent US9657015 (2017) BindingDB Entry DOI: 10.7270/Q28G8NRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM310066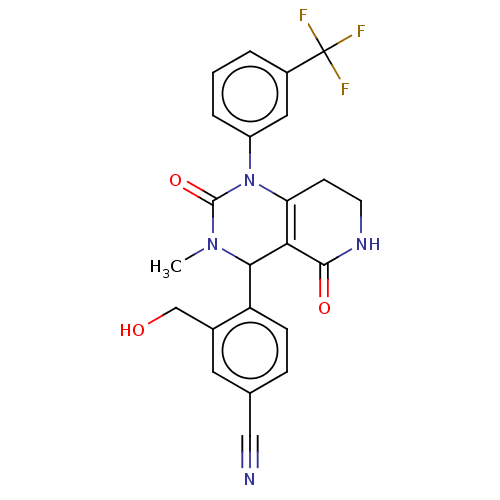 (US9657015, Example 29) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Materials: Human neutrophil elastase was purchased from Calbiochem (Cat. No.: 324681) and the elastase substrate MeOSuc-Ala-Ala-Pro-Val-AMC from Bach... | US Patent US9657015 (2017) BindingDB Entry DOI: 10.7270/Q28G8NRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM310062 (US9657015, Example 25 | US9657015, Example 30A) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Materials: Human neutrophil elastase was purchased from Calbiochem (Cat. No.: 324681) and the elastase substrate MeOSuc-Ala-Ala-Pro-Val-AMC from Bach... | US Patent US9657015 (2017) BindingDB Entry DOI: 10.7270/Q28G8NRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM310069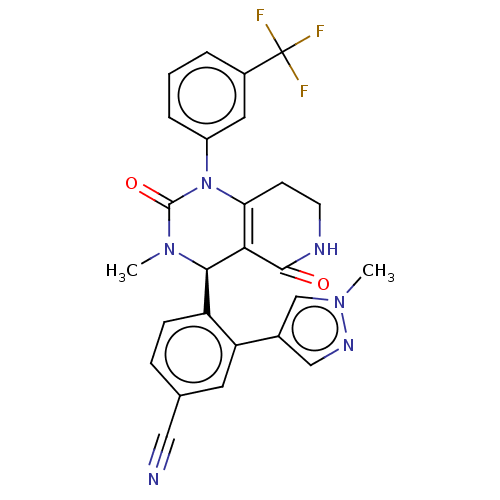 (US9657015, Example 31A) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Materials: Human neutrophil elastase was purchased from Calbiochem (Cat. No.: 324681) and the elastase substrate MeOSuc-Ala-Ala-Pro-Val-AMC from Bach... | US Patent US9657015 (2017) BindingDB Entry DOI: 10.7270/Q28G8NRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM310071 (US9657015, Example 32A) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Materials: Human neutrophil elastase was purchased from Calbiochem (Cat. No.: 324681) and the elastase substrate MeOSuc-Ala-Ala-Pro-Val-AMC from Bach... | US Patent US9657015 (2017) BindingDB Entry DOI: 10.7270/Q28G8NRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM310073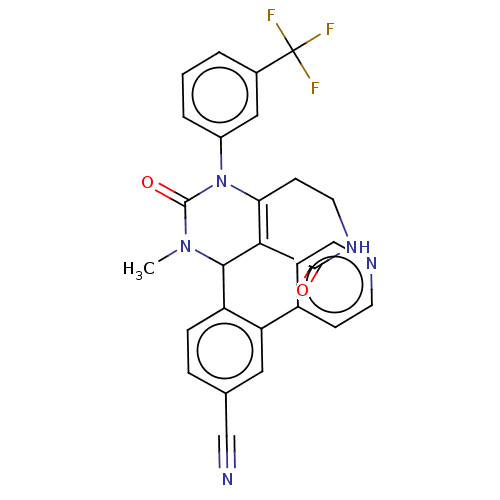 (US9657015, Example 33) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Materials: Human neutrophil elastase was purchased from Calbiochem (Cat. No.: 324681) and the elastase substrate MeOSuc-Ala-Ala-Pro-Val-AMC from Bach... | US Patent US9657015 (2017) BindingDB Entry DOI: 10.7270/Q28G8NRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM310074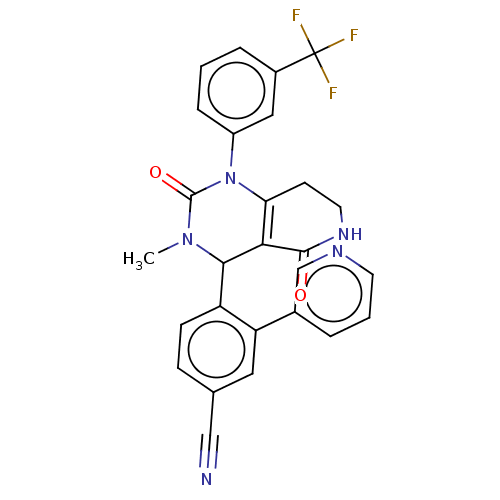 (US9657015, Example 34) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Materials: Human neutrophil elastase was purchased from Calbiochem (Cat. No.: 324681) and the elastase substrate MeOSuc-Ala-Ala-Pro-Val-AMC from Bach... | US Patent US9657015 (2017) BindingDB Entry DOI: 10.7270/Q28G8NRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM310075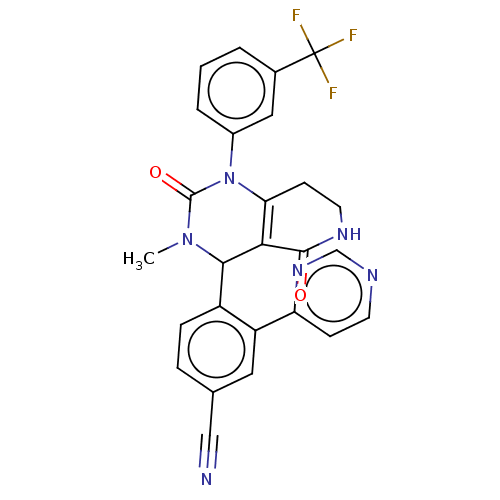 (US9657015, Example 35) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Materials: Human neutrophil elastase was purchased from Calbiochem (Cat. No.: 324681) and the elastase substrate MeOSuc-Ala-Ala-Pro-Val-AMC from Bach... | US Patent US9657015 (2017) BindingDB Entry DOI: 10.7270/Q28G8NRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM310076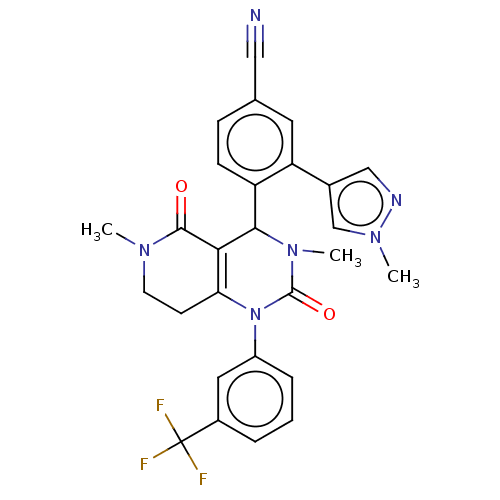 (US9657015, Example 36 | US9657015, Example 36.1 | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Materials: Human neutrophil elastase was purchased from Calbiochem (Cat. No.: 324681) and the elastase substrate MeOSuc-Ala-Ala-Pro-Val-AMC from Bach... | US Patent US9657015 (2017) BindingDB Entry DOI: 10.7270/Q28G8NRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM310080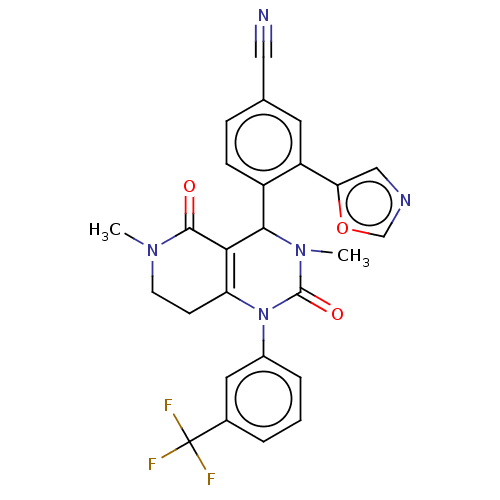 (US9657015, Example 37) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Materials: Human neutrophil elastase was purchased from Calbiochem (Cat. No.: 324681) and the elastase substrate MeOSuc-Ala-Ala-Pro-Val-AMC from Bach... | US Patent US9657015 (2017) BindingDB Entry DOI: 10.7270/Q28G8NRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM310081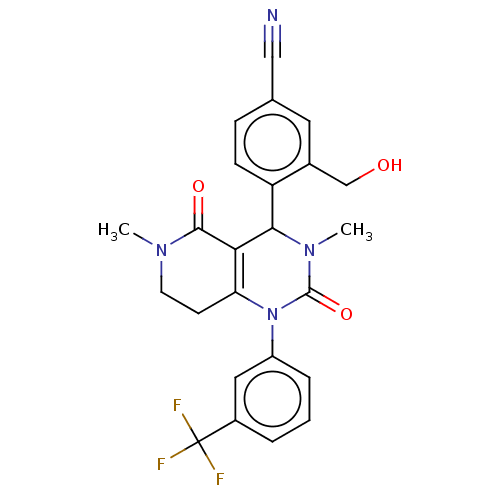 (US9657015, Example 38) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Materials: Human neutrophil elastase was purchased from Calbiochem (Cat. No.: 324681) and the elastase substrate MeOSuc-Ala-Ala-Pro-Val-AMC from Bach... | US Patent US9657015 (2017) BindingDB Entry DOI: 10.7270/Q28G8NRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM310082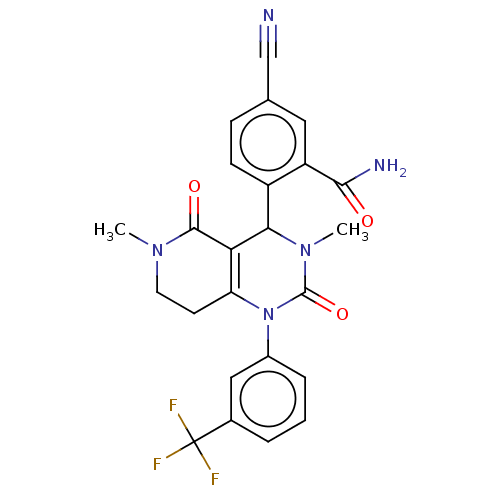 (US9657015, Example 39 | US9657015, Example 39.1 | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Materials: Human neutrophil elastase was purchased from Calbiochem (Cat. No.: 324681) and the elastase substrate MeOSuc-Ala-Ala-Pro-Val-AMC from Bach... | US Patent US9657015 (2017) BindingDB Entry DOI: 10.7270/Q28G8NRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM310082 (US9657015, Example 39 | US9657015, Example 39.1 | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Materials: Human neutrophil elastase was purchased from Calbiochem (Cat. No.: 324681) and the elastase substrate MeOSuc-Ala-Ala-Pro-Val-AMC from Bach... | US Patent US9657015 (2017) BindingDB Entry DOI: 10.7270/Q28G8NRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1133 total ) | Next | Last >> |