 Found 73 hits with Last Name = 'riou' and Initial = 'jf'
Found 73 hits with Last Name = 'riou' and Initial = 'jf' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM50088001
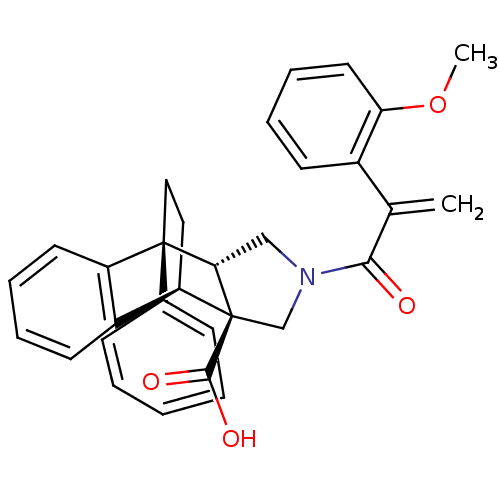
((+/-)-11-[2-(2-methoxyphenyl)acryloyl]-1-phenyl-(1...)Show SMILES COc1ccccc1C(=C)C(=O)N1C[C@@H]2[C@](C1)([C@@H]1CC[C@@]2(c2ccccc2)c2ccccc12)C(O)=O |THB:28:27:15.14:19.18,31:32:15.14:19.18| Show InChI InChI=1S/C31H29NO4/c1-20(22-12-7-9-15-26(22)36-2)28(33)32-18-27-30(21-10-4-3-5-11-21)17-16-25(31(27,19-32)29(34)35)23-13-6-8-14-24(23)30/h3-15,25,27H,1,16-19H2,2H3,(H,34,35)/t25-,27+,30-,31+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer S.A.
Curated by ChEMBL
| Assay Description
Inhibition of farnesyltransferase by Ras/SPA assay |
J Med Chem 43: 1807-16 (2000)
BindingDB Entry DOI: 10.7270/Q23N22MR |
More data for this
Ligand-Target Pair | |
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM50088009

((+/-)-11-[2-(2-methoxyphenyl)acetyl]-1-phenyl-(1R,...)Show SMILES COc1ccccc1CC(=O)N1C[C@@H]2[C@](C1)([C@@H]1CC[C@@]2(c2ccccc2)c2ccccc12)C(O)=O |THB:30:31:14.13:18.17,27:26:14.13:18.17| Show InChI InChI=1S/C30H29NO4/c1-35-25-14-8-5-9-20(25)17-27(32)31-18-26-29(21-10-3-2-4-11-21)16-15-24(30(26,19-31)28(33)34)22-12-6-7-13-23(22)29/h2-14,24,26H,15-19H2,1H3,(H,33,34)/t24-,26+,29-,30+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer S.A.
Curated by ChEMBL
| Assay Description
Inhibition of farnesyltransferase by Ras/SPA assay |
J Med Chem 43: 1807-16 (2000)
BindingDB Entry DOI: 10.7270/Q23N22MR |
More data for this
Ligand-Target Pair | |
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM50088004
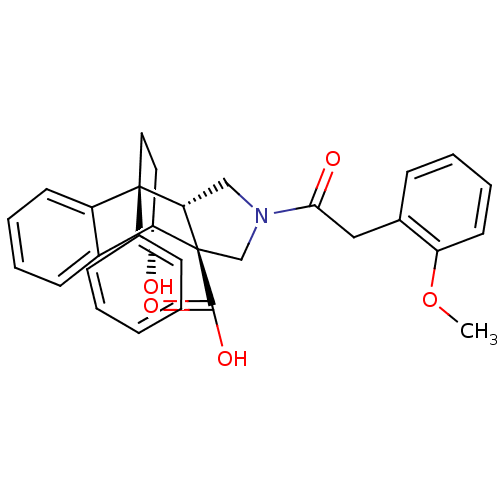
(8-hydroxy-11-[2-(2-methoxyphenyl)acetyl]-1-phenyl-...)Show SMILES COc1ccccc1CC(=O)N1C[C@@H]2[C@@](C1)(C(O)=O)[C@]1(O)CC[C@@]2(c2ccccc2)c2ccccc12 Show InChI InChI=1S/C30H29NO5/c1-36-24-14-8-5-9-20(24)17-26(32)31-18-25-28(21-10-3-2-4-11-21)15-16-30(35,29(25,19-31)27(33)34)23-13-7-6-12-22(23)28/h2-14,25,35H,15-19H2,1H3,(H,33,34)/t25-,28+,29-,30-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer S.A.
Curated by ChEMBL
| Assay Description
Inhibition of farnesyltransferase by Ras/SPA assay |
J Med Chem 43: 1807-16 (2000)
BindingDB Entry DOI: 10.7270/Q23N22MR |
More data for this
Ligand-Target Pair | |
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM50088014

(1-(2,3-dihydrobenzo[b]furan-5-yl)-11-[2-(2-methoxy...)Show SMILES COc1ccccc1C(=C)C(=O)N1C[C@@H]2[C@](C1)([C@@H]1CC[C@@]2(c2ccc3OCCc3c2)c2ccccc12)C(O)=O |THB:31:30:15.14:19.18,34:35:15.14:19.18| Show InChI InChI=1S/C33H31NO5/c1-20(23-7-4-6-10-28(23)38-2)30(35)34-18-29-32(22-11-12-27-21(17-22)14-16-39-27)15-13-26(33(29,19-34)31(36)37)24-8-3-5-9-25(24)32/h3-12,17,26,29H,1,13-16,18-19H2,2H3,(H,36,37)/t26-,29+,32-,33+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer S.A.
Curated by ChEMBL
| Assay Description
Inhibition of farnesyltransferase by Ras/SPA assay |
J Med Chem 43: 1807-16 (2000)
BindingDB Entry DOI: 10.7270/Q23N22MR |
More data for this
Ligand-Target Pair | |
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM50088008
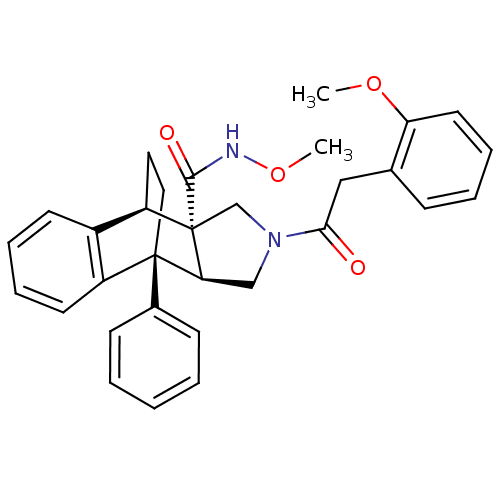
(11-[2-(2-methoxyphenyl)acetyl]-1-phenyl-(1R,8R,9S,...)Show SMILES CONC(=O)[C@]12CN(C[C@H]1[C@]1(CC[C@@H]2c2ccccc12)c1ccccc1)C(=O)Cc1ccccc1OC |THB:15:14:5.9:11.12,18:19:5.9:11.12| Show InChI InChI=1S/C31H32N2O4/c1-36-26-15-9-6-10-21(26)18-28(34)33-19-27-30(22-11-4-3-5-12-22)17-16-25(23-13-7-8-14-24(23)30)31(27,20-33)29(35)32-37-2/h3-15,25,27H,16-20H2,1-2H3,(H,32,35)/t25-,27+,30-,31+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer S.A.
Curated by ChEMBL
| Assay Description
Inhibition of farnesyltransferase by Ras/SPA assay |
J Med Chem 43: 1807-16 (2000)
BindingDB Entry DOI: 10.7270/Q23N22MR |
More data for this
Ligand-Target Pair | |
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM50088013
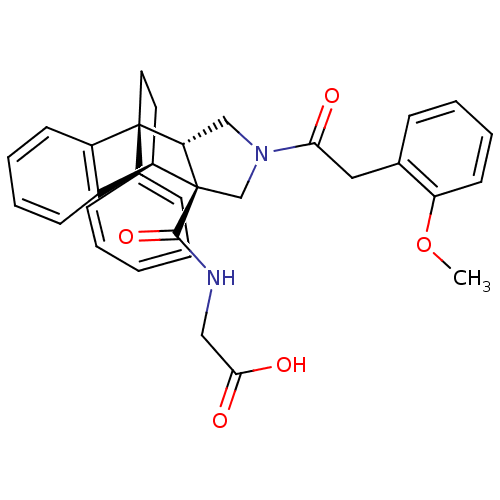
(2-[11-[2-(2-methoxyphenyl)acetyl]-1-phenyl-(1R,8R,...)Show SMILES COc1ccccc1CC(=O)N1C[C@@H]2[C@](C1)([C@@H]1CC[C@@]2(c2ccccc2)c2ccccc12)C(=O)NCC(O)=O |THB:27:26:14.13:18.17,30:31:14.13:18.17| Show InChI InChI=1S/C32H32N2O5/c1-39-26-14-8-5-9-21(26)17-28(35)34-19-27-31(22-10-3-2-4-11-22)16-15-25(23-12-6-7-13-24(23)31)32(27,20-34)30(38)33-18-29(36)37/h2-14,25,27H,15-20H2,1H3,(H,33,38)(H,36,37)/t25-,27+,31-,32+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 738 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer S.A.
Curated by ChEMBL
| Assay Description
Inhibition of farnesyltransferase by Ras/SPA assay |
J Med Chem 43: 1807-16 (2000)
BindingDB Entry DOI: 10.7270/Q23N22MR |
More data for this
Ligand-Target Pair | |
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM50088002

(1-(4-carboxymethoxyphenyl)-11-[2-(2-methoxyphenyl)...)Show SMILES COc1ccccc1C(=C)C(=O)N1C[C@@H]2[C@](C1)([C@@H]1CC[C@@]2(c2ccc(OCC(O)=O)cc2)c2ccccc12)C(O)=O |THB:36:37:15.14:19.18,33:32:15.14:19.18| Show InChI InChI=1S/C33H31NO7/c1-20(23-7-4-6-10-27(23)40-2)30(37)34-17-28-32(21-11-13-22(14-12-21)41-18-29(35)36)16-15-26(33(28,19-34)31(38)39)24-8-3-5-9-25(24)32/h3-14,26,28H,1,15-19H2,2H3,(H,35,36)(H,38,39)/t26-,28+,32-,33+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.55E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer S.A.
Curated by ChEMBL
| Assay Description
Inhibition of farnesyltransferase by Ras/SPA assay |
J Med Chem 43: 1807-16 (2000)
BindingDB Entry DOI: 10.7270/Q23N22MR |
More data for this
Ligand-Target Pair | |
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM50088003
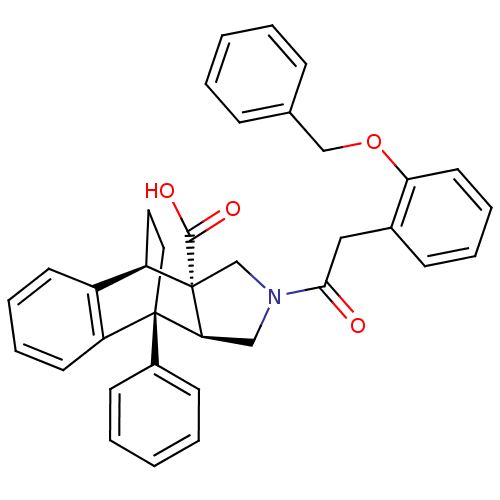
(11-[2-(2-benzyloxyphenyl)acetyl]-1-phenyl-(1R,8R,9...)Show SMILES OC(=O)[C@]12CN(C[C@H]1[C@]1(CC[C@@H]2c2ccccc12)c1ccccc1)C(=O)Cc1ccccc1OCc1ccccc1 |THB:13:12:3.7:9.10,16:17:3.7:9.10| Show InChI InChI=1S/C36H33NO4/c38-33(21-26-13-7-10-18-31(26)41-23-25-11-3-1-4-12-25)37-22-32-35(27-14-5-2-6-15-27)20-19-30(36(32,24-37)34(39)40)28-16-8-9-17-29(28)35/h1-18,30,32H,19-24H2,(H,39,40)/t30-,32+,35-,36+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer S.A.
Curated by ChEMBL
| Assay Description
Inhibition of farnesyltransferase by Ras/SPA assay |
J Med Chem 43: 1807-16 (2000)
BindingDB Entry DOI: 10.7270/Q23N22MR |
More data for this
Ligand-Target Pair | |
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM50088005
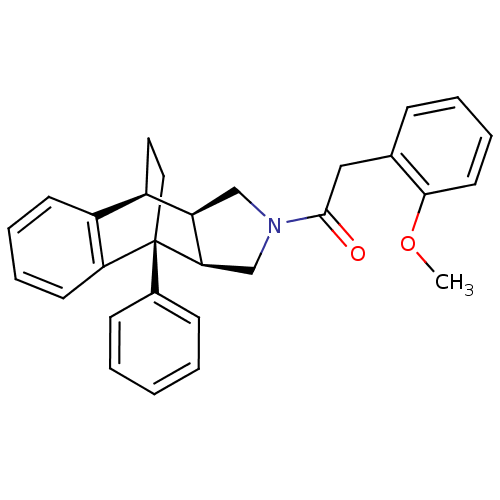
(2-(2-methoxyphenyl)-1-[1-phenyl-(1R,8S,9R,13R)-11-...)Show SMILES COc1ccccc1CC(=O)N1C[C@H]2[C@@H](C1)[C@]1(CC[C@@H]2c2ccccc12)c1ccccc1 Show InChI InChI=1S/C29H29NO2/c1-32-27-14-8-5-9-20(27)17-28(31)30-18-24-22-15-16-29(26(24)19-30,21-10-3-2-4-11-21)25-13-7-6-12-23(22)25/h2-14,22,24,26H,15-19H2,1H3/t22-,24-,26-,29-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer S.A.
Curated by ChEMBL
| Assay Description
Inhibition of farnesyltransferase by Ras/SPA assay |
J Med Chem 43: 1807-16 (2000)
BindingDB Entry DOI: 10.7270/Q23N22MR |
More data for this
Ligand-Target Pair | |
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM50088006

(6-hydroxy-11-[2-(2-methoxyphenyl)acetyl]-1-phenyl-...)Show SMILES COc1ccccc1CC(=O)N1C[C@@H]2[C@](C1)([C@@H]1CC[C@@]2(c2ccccc2)c2cccc(O)c12)C(O)=O |THB:30:32:14.13:18.17,27:26:14.13:18.17| Show InChI InChI=1S/C30H29NO5/c1-36-24-13-6-5-8-19(24)16-26(33)31-17-25-29(20-9-3-2-4-10-20)15-14-22(30(25,18-31)28(34)35)27-21(29)11-7-12-23(27)32/h2-13,22,25,32H,14-18H2,1H3,(H,34,35)/t22-,25+,29-,30+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer S.A.
Curated by ChEMBL
| Assay Description
Inhibition of farnesyltransferase by Ras/SPA assay |
J Med Chem 43: 1807-16 (2000)
BindingDB Entry DOI: 10.7270/Q23N22MR |
More data for this
Ligand-Target Pair | |
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM50088000
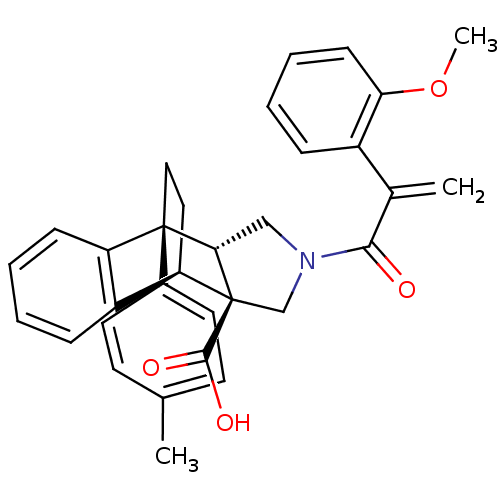
((+/-)-11-[2-(2-methoxyphenyl)acryloyl]-1-(4-methyl...)Show SMILES COc1ccccc1C(=C)C(=O)N1C[C@@H]2[C@](C1)([C@@H]1CC[C@@]2(c2ccc(C)cc2)c2ccccc12)C(O)=O |THB:29:28:15.14:19.18,32:33:15.14:19.18| Show InChI InChI=1S/C32H31NO4/c1-20-12-14-22(15-13-20)31-17-16-26(24-9-4-6-10-25(24)31)32(30(35)36)19-33(18-28(31)32)29(34)21(2)23-8-5-7-11-27(23)37-3/h4-15,26,28H,2,16-19H2,1,3H3,(H,35,36)/t26-,28+,31-,32+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer S.A.
Curated by ChEMBL
| Assay Description
Inhibition of Farnesyltransferase |
J Med Chem 43: 1807-16 (2000)
BindingDB Entry DOI: 10.7270/Q23N22MR |
More data for this
Ligand-Target Pair | |
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM50088001

((+/-)-11-[2-(2-methoxyphenyl)acryloyl]-1-phenyl-(1...)Show SMILES COc1ccccc1C(=C)C(=O)N1C[C@@H]2[C@](C1)([C@@H]1CC[C@@]2(c2ccccc2)c2ccccc12)C(O)=O |THB:28:27:15.14:19.18,31:32:15.14:19.18| Show InChI InChI=1S/C31H29NO4/c1-20(22-12-7-9-15-26(22)36-2)28(33)32-18-27-30(21-10-4-3-5-11-21)17-16-25(31(27,19-32)29(34)35)23-13-6-8-14-24(23)30/h3-15,25,27H,1,16-19H2,2H3,(H,34,35)/t25-,27+,30-,31+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer S.A.
Curated by ChEMBL
| Assay Description
Inhibition of Farnesyltransferase |
J Med Chem 43: 1807-16 (2000)
BindingDB Entry DOI: 10.7270/Q23N22MR |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50134030
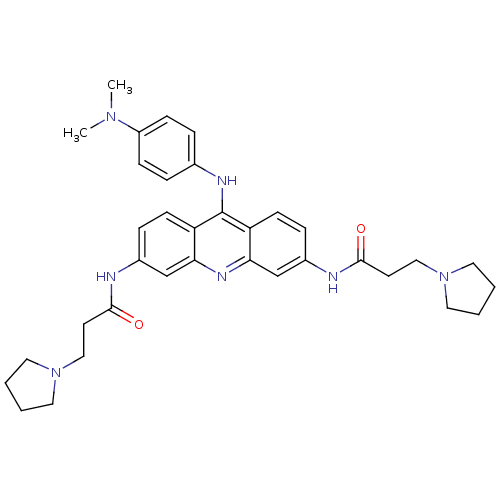
(9-[4-(N,N-dimethylamino)phenylamino]-3,6-bis(3-pyr...)Show SMILES CN(C)c1ccc(Nc2c3ccc(NC(=O)CCN4CCCC4)cc3nc3cc(NC(=O)CCN4CCCC4)ccc23)cc1 Show InChI InChI=1S/C35H43N7O2/c1-40(2)28-11-7-25(8-12-28)38-35-29-13-9-26(36-33(43)15-21-41-17-3-4-18-41)23-31(29)39-32-24-27(10-14-30(32)35)37-34(44)16-22-42-19-5-6-20-42/h7-14,23-24H,3-6,15-22H2,1-2H3,(H,36,43)(H,37,44)(H,38,39) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Reims-Champagne-Ardenne
Curated by ChEMBL
| Assay Description
Inhibition of human telomerase in A549 cells by TRAP assay |
Eur J Med Chem 44: 3880-8 (2009)
Article DOI: 10.1016/j.ejmech.2009.04.021
BindingDB Entry DOI: 10.7270/Q2W37WDH |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50298376
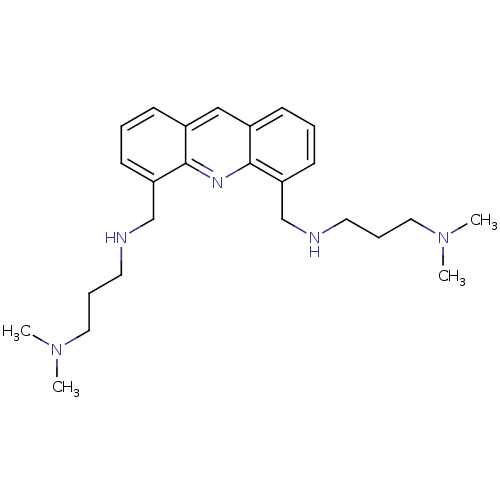
(4,5-Bis[3-(N,N-dimethylamino)propylaminomethyl]acr...)Show InChI InChI=1S/C25H37N5/c1-29(2)15-7-13-26-18-22-11-5-9-20-17-21-10-6-12-23(25(21)28-24(20)22)19-27-14-8-16-30(3)4/h5-6,9-12,17,26-27H,7-8,13-16,18-19H2,1-4H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Reims-Champagne-Ardenne
Curated by ChEMBL
| Assay Description
Inhibition of human telomerase in A549 cells by TRAP assay |
Eur J Med Chem 44: 3880-8 (2009)
Article DOI: 10.1016/j.ejmech.2009.04.021
BindingDB Entry DOI: 10.7270/Q2W37WDH |
More data for this
Ligand-Target Pair | |
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM50088009

((+/-)-11-[2-(2-methoxyphenyl)acetyl]-1-phenyl-(1R,...)Show SMILES COc1ccccc1CC(=O)N1C[C@@H]2[C@](C1)([C@@H]1CC[C@@]2(c2ccccc2)c2ccccc12)C(O)=O |THB:30:31:14.13:18.17,27:26:14.13:18.17| Show InChI InChI=1S/C30H29NO4/c1-35-25-14-8-5-9-20(25)17-27(32)31-18-26-29(21-10-3-2-4-11-21)16-15-24(30(26,19-31)28(33)34)22-12-6-7-13-23(22)29/h2-14,24,26H,15-19H2,1H3,(H,33,34)/t24-,26+,29-,30+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer S.A.
Curated by ChEMBL
| Assay Description
Inhibition of Farnesyltransferase |
J Med Chem 43: 1807-16 (2000)
BindingDB Entry DOI: 10.7270/Q23N22MR |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM2581

(3,13,23-triazahexacyclo[14.7.0.0^{2,10}.0^{4,9}.0^...)Show SMILES O=C1NCc2c1c1c3ccccc3[nH]c1c1[nH]c3ccccc3c21 Show InChI InChI=1S/C20H13N3O/c24-20-17-12(9-21-20)15-10-5-1-3-7-13(10)22-18(15)19-16(17)11-6-2-4-8-14(11)23-19/h1-8,22-23H,9H2,(H,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Blaise Pascal
Curated by ChEMBL
| Assay Description
Inhibition of Protein Kinase C(PKC) |
J Med Chem 39: 4471-7 (1996)
Article DOI: 10.1021/jm9603779
BindingDB Entry DOI: 10.7270/Q2S181MM |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50298371

(2,2'-[4,5-Acridindiylbis(methyleneimino)]bis(N,N,N...)Show SMILES C[N+](C)(C)CCC(=O)NCc1cccc2cc3cccc(CNC(=O)CC[N+](C)(C)C)c3nc12 Show InChI InChI=1S/C27H37N5O2/c1-31(2,3)15-13-24(33)28-18-22-11-7-9-20-17-21-10-8-12-23(27(21)30-26(20)22)19-29-25(34)14-16-32(4,5)6/h7-12,17H,13-16,18-19H2,1-6H3/p+2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Reims-Champagne-Ardenne
Curated by ChEMBL
| Assay Description
Inhibition of human telomerase in A549 cells by TRAP assay |
Eur J Med Chem 44: 3880-8 (2009)
Article DOI: 10.1016/j.ejmech.2009.04.021
BindingDB Entry DOI: 10.7270/Q2W37WDH |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50054428
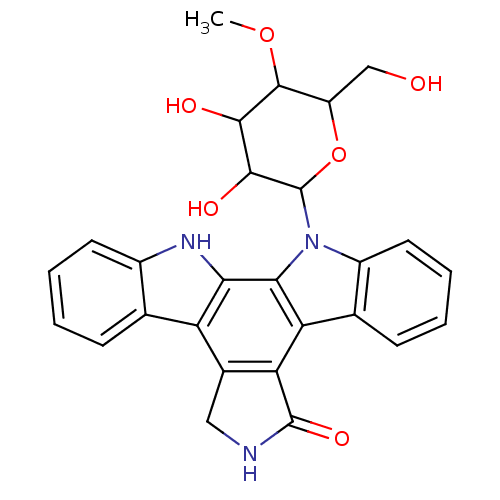
(13-(3,4-dihydroxy-6-hydroxymethyl-5-methoxytetrahy...)Show SMILES COC1C(CO)OC(C(O)C1O)n1c2ccccc2c2c3C(=O)NCc3c3c4ccccc4[nH]c3c12 Show InChI InChI=1S/C27H25N3O6/c1-35-25-17(11-31)36-27(24(33)23(25)32)30-16-9-5-3-7-13(16)19-20-14(10-28-26(20)34)18-12-6-2-4-8-15(12)29-21(18)22(19)30/h2-9,17,23-25,27,29,31-33H,10-11H2,1H3,(H,28,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Blaise Pascal
Curated by ChEMBL
| Assay Description
Inhibition of Protein Kinase C(PKC) |
J Med Chem 39: 4471-7 (1996)
Article DOI: 10.1021/jm9603779
BindingDB Entry DOI: 10.7270/Q2S181MM |
More data for this
Ligand-Target Pair | |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM50471649

(CHEMBL3350555)Show SMILES CO[C@@H]1[C@@H](CO)O[C@H]([C@H](O)[C@H]1O)n1c2ccccc2c2c3CNC(=O)c3c3c4ccccc4[nH]c3c12.CO[C@@H]1[C@@H](CO)O[C@H]([C@H](O)[C@H]1O)n1c2ccccc2c2c3C(=O)NCc3c3c4ccccc4[nH]c3c12 |r| Show InChI InChI=1S/2C27H25N3O6/c1-35-25-17(11-31)36-27(24(33)23(25)32)30-16-9-5-3-7-13(16)18-14-10-28-26(34)20(14)19-12-6-2-4-8-15(12)29-21(19)22(18)30;1-35-25-17(11-31)36-27(24(33)23(25)32)30-16-9-5-3-7-13(16)19-20-14(10-28-26(20)34)18-12-6-2-4-8-15(12)29-21(18)22(19)30/h2*2-9,17,23-25,27,29,31-33H,10-11H2,1H3,(H,28,34)/t2*17-,23-,24-,25-,27-/m11/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Blaise Pascal
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C |
J Med Chem 41: 1631-40 (1998)
Article DOI: 10.1021/jm970843+
BindingDB Entry DOI: 10.7270/Q2RV0RF0 |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50298372
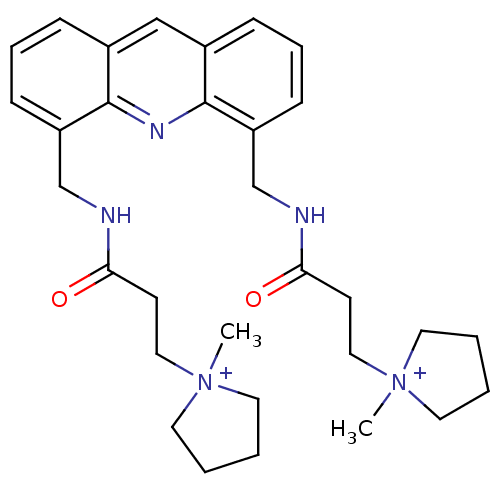
(2,2'-[4,5-Acridindiylbis(methyleneimino)]bis[N-met...)Show SMILES C[N+]1(CCC(=O)NCc2cccc3cc4cccc(CNC(=O)CC[N+]5(C)CCCC5)c4nc23)CCCC1 Show InChI InChI=1S/C31H41N5O2/c1-35(15-3-4-16-35)19-13-28(37)32-22-26-11-7-9-24-21-25-10-8-12-27(31(25)34-30(24)26)23-33-29(38)14-20-36(2)17-5-6-18-36/h7-12,21H,3-6,13-20,22-23H2,1-2H3/p+2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Reims-Champagne-Ardenne
Curated by ChEMBL
| Assay Description
Inhibition of human telomerase in A549 cells by TRAP assay |
Eur J Med Chem 44: 3880-8 (2009)
Article DOI: 10.1016/j.ejmech.2009.04.021
BindingDB Entry DOI: 10.7270/Q2W37WDH |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50298373
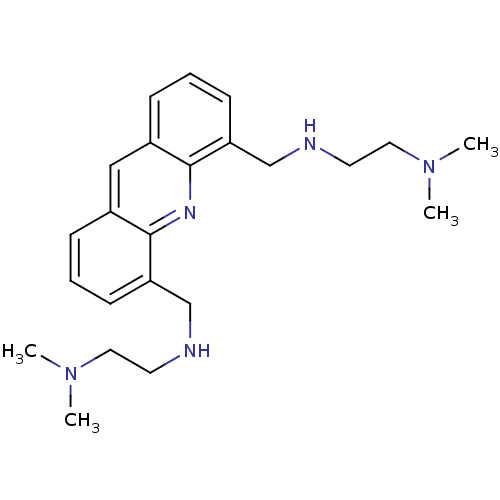
(4,5-Bis[2-(N,N-dimethylamino)ethylaminomethyl]acri...)Show InChI InChI=1S/C23H33N5/c1-27(2)13-11-24-16-20-9-5-7-18-15-19-8-6-10-21(23(19)26-22(18)20)17-25-12-14-28(3)4/h5-10,15,24-25H,11-14,16-17H2,1-4H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Reims-Champagne-Ardenne
Curated by ChEMBL
| Assay Description
Inhibition of human telomerase in A549 cells by TRAP assay |
Eur J Med Chem 44: 3880-8 (2009)
Article DOI: 10.1016/j.ejmech.2009.04.021
BindingDB Entry DOI: 10.7270/Q2W37WDH |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50298374
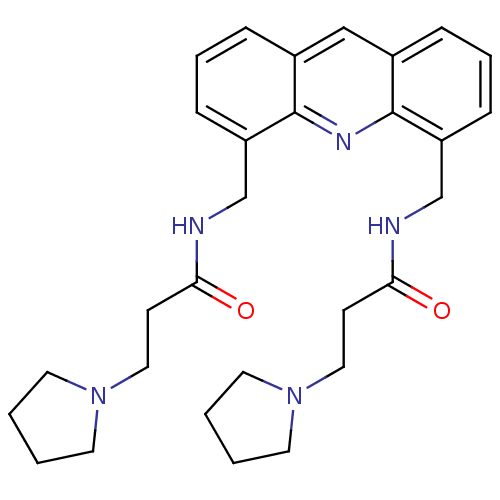
(CHEMBL577150 | N,N'-[4,5-Acridindiylbis(methylene)...)Show SMILES O=C(CCN1CCCC1)NCc1cccc2cc3cccc(CNC(=O)CCN4CCCC4)c3nc12 Show InChI InChI=1S/C29H37N5O2/c35-26(11-17-33-13-1-2-14-33)30-20-24-9-5-7-22-19-23-8-6-10-25(29(23)32-28(22)24)21-31-27(36)12-18-34-15-3-4-16-34/h5-10,19H,1-4,11-18,20-21H2,(H,30,35)(H,31,36) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Reims-Champagne-Ardenne
Curated by ChEMBL
| Assay Description
Inhibition of human telomerase in A549 cells by TRAP assay |
Eur J Med Chem 44: 3880-8 (2009)
Article DOI: 10.1016/j.ejmech.2009.04.021
BindingDB Entry DOI: 10.7270/Q2W37WDH |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50298375
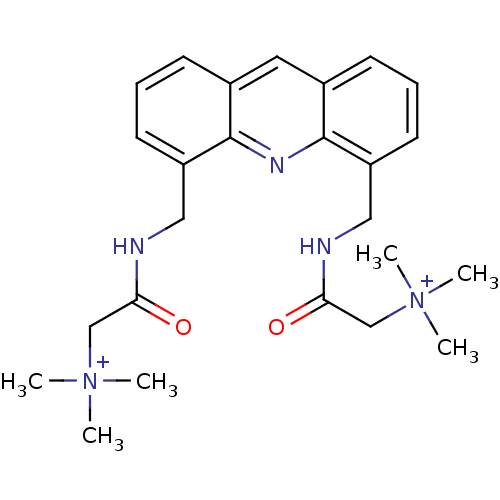
(2,2'-[4,5-Acridindiylbis(methyleneimino)]bis(N,N,N...)Show SMILES C[N+](C)(C)CC(=O)NCc1cccc2cc3cccc(CNC(=O)C[N+](C)(C)C)c3nc12 Show InChI InChI=1S/C25H33N5O2/c1-29(2,3)16-22(31)26-14-20-11-7-9-18-13-19-10-8-12-21(25(19)28-24(18)20)15-27-23(32)17-30(4,5)6/h7-13H,14-17H2,1-6H3/p+2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Reims-Champagne-Ardenne
Curated by ChEMBL
| Assay Description
Inhibition of human telomerase in A549 cells by TRAP assay |
Eur J Med Chem 44: 3880-8 (2009)
Article DOI: 10.1016/j.ejmech.2009.04.021
BindingDB Entry DOI: 10.7270/Q2W37WDH |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50298377
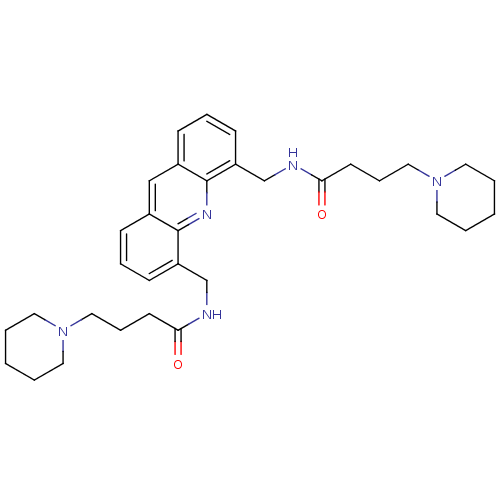
(CHEMBL576188 | N,N'-[4,5-Acridindiylbis(methylene)...)Show SMILES O=C(CCCN1CCCCC1)NCc1cccc2cc3cccc(CNC(=O)CCCN4CCCCC4)c3nc12 Show InChI InChI=1S/C33H45N5O2/c39-30(15-9-21-37-17-3-1-4-18-37)34-24-28-13-7-11-26-23-27-12-8-14-29(33(27)36-32(26)28)25-35-31(40)16-10-22-38-19-5-2-6-20-38/h7-8,11-14,23H,1-6,9-10,15-22,24-25H2,(H,34,39)(H,35,40) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Reims-Champagne-Ardenne
Curated by ChEMBL
| Assay Description
Inhibition of human telomerase in A549 cells by TRAP assay |
Eur J Med Chem 44: 3880-8 (2009)
Article DOI: 10.1016/j.ejmech.2009.04.021
BindingDB Entry DOI: 10.7270/Q2W37WDH |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50298378
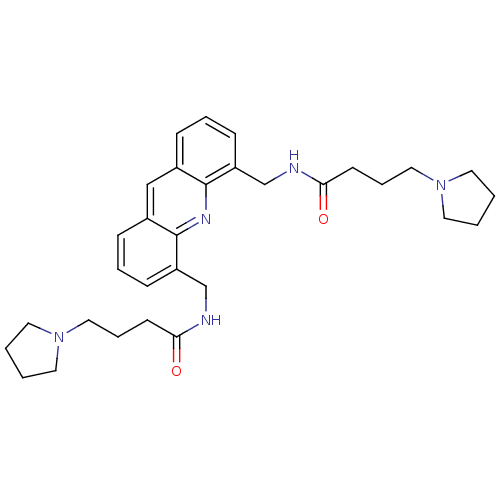
(CHEMBL573120 | N,N'-[4,5-Acridindiylbis(methylene)...)Show SMILES O=C(CCCN1CCCC1)NCc1cccc2cc3cccc(CNC(=O)CCCN4CCCC4)c3nc12 Show InChI InChI=1S/C31H41N5O2/c37-28(13-7-19-35-15-1-2-16-35)32-22-26-11-5-9-24-21-25-10-6-12-27(31(25)34-30(24)26)23-33-29(38)14-8-20-36-17-3-4-18-36/h5-6,9-12,21H,1-4,7-8,13-20,22-23H2,(H,32,37)(H,33,38) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Reims-Champagne-Ardenne
Curated by ChEMBL
| Assay Description
Inhibition of human telomerase in A549 cells by TRAP assay |
Eur J Med Chem 44: 3880-8 (2009)
Article DOI: 10.1016/j.ejmech.2009.04.021
BindingDB Entry DOI: 10.7270/Q2W37WDH |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50054426
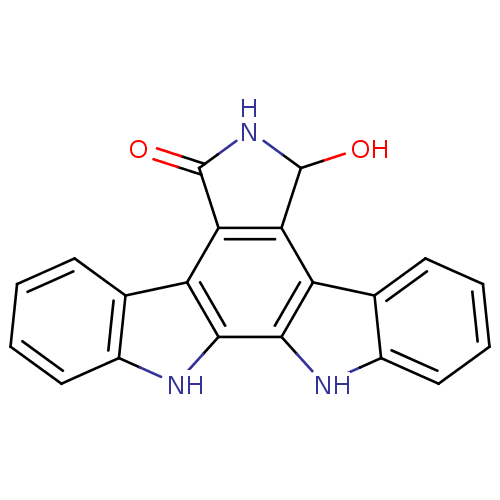
(7-hydroxy-6,7,12,13-tetrahydro-5H-indolo[2,3-a]pyr...)Show SMILES OC1NC(=O)c2c1c1c3ccccc3[nH]c1c1[nH]c3ccccc3c21 Show InChI InChI=1S/C20H13N3O2/c24-19-15-13-9-5-1-3-7-11(9)21-17(13)18-14(16(15)20(25)23-19)10-6-2-4-8-12(10)22-18/h1-8,19,21-22,24H,(H,23,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.21E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Blaise Pascal
Curated by ChEMBL
| Assay Description
Inhibition of Protein Kinase C(PKC) |
J Med Chem 39: 4471-7 (1996)
Article DOI: 10.1021/jm9603779
BindingDB Entry DOI: 10.7270/Q2S181MM |
More data for this
Ligand-Target Pair | |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM50472524

(CHEMBL345881)Show SMILES COC1C(O)C(OC(=O)CBr)C(OC1CO)n1c2c(Cl)cccc2c2c3C(=O)NC(=O)c3c3c4cccc(Cl)c4[nH]c3c12 Show InChI InChI=1S/C29H22BrCl2N3O8/c1-41-25-14(9-36)42-29(26(24(25)38)43-15(37)8-30)35-22-11(5-3-7-13(22)32)17-19-18(27(39)34-28(19)40)16-10-4-2-6-12(31)20(10)33-21(16)23(17)35/h2-7,14,24-26,29,33,36,38H,8-9H2,1H3,(H,34,39,40) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Blaise Pascal
Curated by ChEMBL
| Assay Description
Inhibition of protein kinase C (PKC) |
J Med Chem 42: 584-92 (1999)
Article DOI: 10.1021/jm980396d
BindingDB Entry DOI: 10.7270/Q2SF2ZXP |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50298379
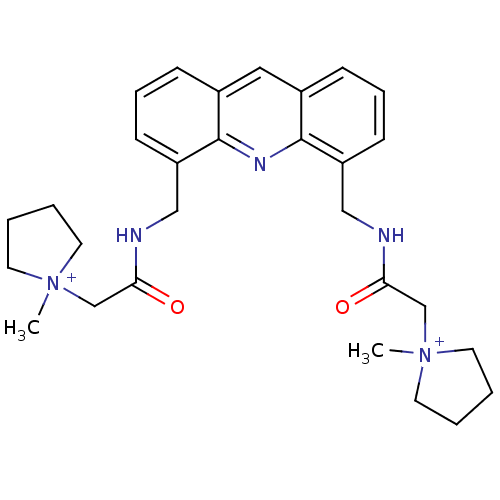
(2,2'-[4,5-Acridindiylbis(methyleneimino)]bis[N-met...)Show SMILES C[N+]1(CC(=O)NCc2cccc3cc4cccc(CNC(=O)C[N+]5(C)CCCC5)c4nc23)CCCC1 Show InChI InChI=1S/C29H37N5O2/c1-33(13-3-4-14-33)20-26(35)30-18-24-11-7-9-22-17-23-10-8-12-25(29(23)32-28(22)24)19-31-27(36)21-34(2)15-5-6-16-34/h7-12,17H,3-6,13-16,18-21H2,1-2H3/p+2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Reims-Champagne-Ardenne
Curated by ChEMBL
| Assay Description
Inhibition of human telomerase in A549 cells by TRAP assay |
Eur J Med Chem 44: 3880-8 (2009)
Article DOI: 10.1016/j.ejmech.2009.04.021
BindingDB Entry DOI: 10.7270/Q2W37WDH |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50054423
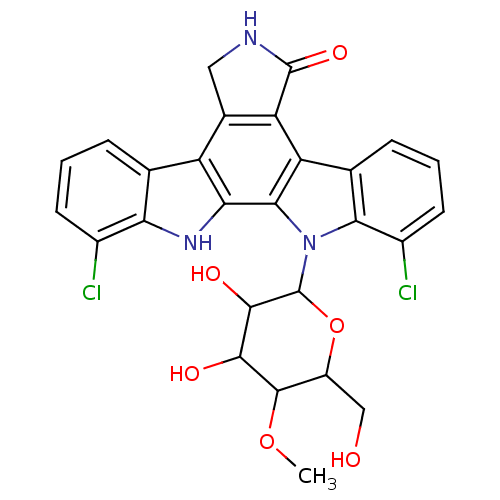
(1,11-dichloro-13-(3,4-dihydroxy-6-hydroxymethyl-5-...)Show SMILES COC1C(O)C(O)C(OC1CO)n1c2c(Cl)cccc2c2c3C(=O)NCc3c3c4cccc(Cl)c4[nH]c3c12 Show InChI InChI=1S/C27H23Cl2N3O6/c1-37-25-15(9-33)38-27(24(35)23(25)34)32-21-11(5-3-7-14(21)29)17-18-12(8-30-26(18)36)16-10-4-2-6-13(28)19(10)31-20(16)22(17)32/h2-7,15,23-25,27,31,33-35H,8-9H2,1H3,(H,30,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.88E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Blaise Pascal
Curated by ChEMBL
| Assay Description
Inhibition of Protein Kinase C(PKC) |
J Med Chem 39: 4471-7 (1996)
Article DOI: 10.1021/jm9603779
BindingDB Entry DOI: 10.7270/Q2S181MM |
More data for this
Ligand-Target Pair | |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM50471652
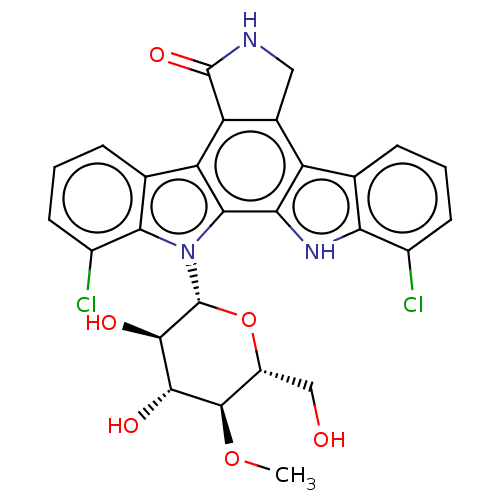
(CHEMBL3350556)Show SMILES CO[C@H]1[C@H](O)[C@@H](O)[C@@H](O[C@@H]1CO)n1c2c(Cl)cccc2c2c3CNC(=O)c3c3c4cccc(Cl)c4[nH]c3c12.CO[C@H]1[C@H](O)[C@@H](O)[C@@H](O[C@@H]1CO)n1c2c(Cl)cccc2c2c3C(=O)NCc3c3c4cccc(Cl)c4[nH]c3c12 |r| Show InChI InChI=1S/2C27H23Cl2N3O6/c1-37-25-15(9-33)38-27(24(35)23(25)34)32-21-11(5-3-7-14(21)29)16-12-8-30-26(36)18(12)17-10-4-2-6-13(28)19(10)31-20(17)22(16)32;1-37-25-15(9-33)38-27(24(35)23(25)34)32-21-11(5-3-7-14(21)29)17-18-12(8-30-26(18)36)16-10-4-2-6-13(28)19(10)31-20(16)22(17)32/h2*2-7,15,23-25,27,31,33-35H,8-9H2,1H3,(H,30,36)/t2*15-,23-,24-,25-,27-/m11/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.88E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Blaise Pascal
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C |
J Med Chem 41: 1631-40 (1998)
Article DOI: 10.1021/jm970843+
BindingDB Entry DOI: 10.7270/Q2RV0RF0 |
More data for this
Ligand-Target Pair | |
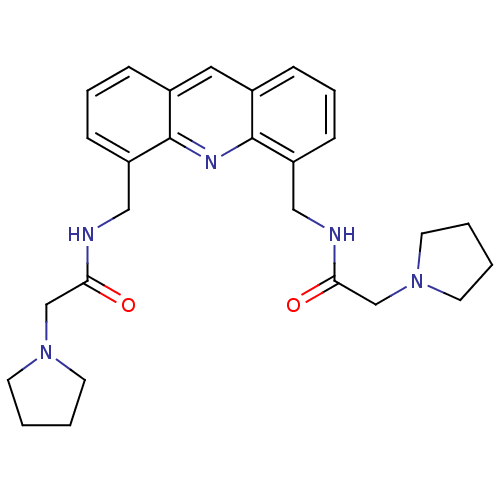
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50298382
(CHEMBL573353 | N,N'-[4,5-Acridindiylbis(methylene)...)Show SMILES O=C(CN1CCCC1)NCc1cccc2cc3cccc(CNC(=O)CN4CCCC4)c3nc12 Show InChI InChI=1S/C27H33N5O2/c33-24(18-31-11-1-2-12-31)28-16-22-9-5-7-20-15-21-8-6-10-23(27(21)30-26(20)22)17-29-25(34)19-32-13-3-4-14-32/h5-10,15H,1-4,11-14,16-19H2,(H,28,33)(H,29,34) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Reims-Champagne-Ardenne
Curated by ChEMBL
| Assay Description
Inhibition of human telomerase in A549 cells by TRAP assay |
Eur J Med Chem 44: 3880-8 (2009)
Article DOI: 10.1016/j.ejmech.2009.04.021
BindingDB Entry DOI: 10.7270/Q2W37WDH |
More data for this
Ligand-Target Pair | |
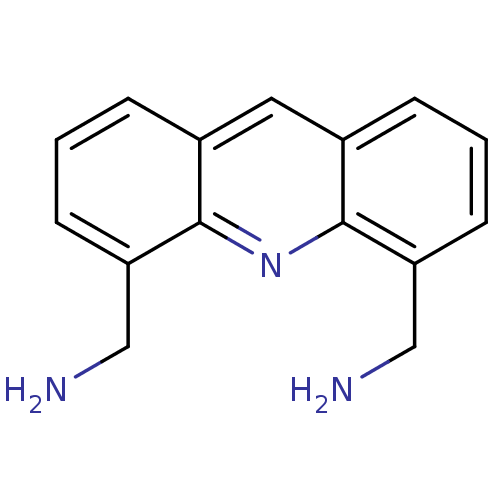
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50298386
(4,5-Bis(aminomethyl)acridine dihydrochloride salt ...)Show InChI InChI=1S/C15H15N3/c16-8-12-5-1-3-10-7-11-4-2-6-13(9-17)15(11)18-14(10)12/h1-7H,8-9,16-17H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Reims-Champagne-Ardenne
Curated by ChEMBL
| Assay Description
Inhibition of human telomerase in A549 cells by TRAP assay |
Eur J Med Chem 44: 3880-8 (2009)
Article DOI: 10.1016/j.ejmech.2009.04.021
BindingDB Entry DOI: 10.7270/Q2W37WDH |
More data for this
Ligand-Target Pair | |
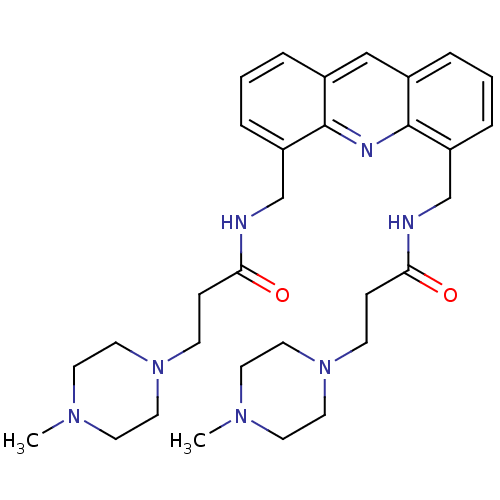
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50298385
(CHEMBL573861 | N,N'-[4,5-Acridindiylbis(methylene)...)Show SMILES CN1CCN(CCC(=O)NCc2cccc3cc4cccc(CNC(=O)CCN5CCN(C)CC5)c4nc23)CC1 Show InChI InChI=1S/C31H43N7O2/c1-35-13-17-37(18-14-35)11-9-28(39)32-22-26-7-3-5-24-21-25-6-4-8-27(31(25)34-30(24)26)23-33-29(40)10-12-38-19-15-36(2)16-20-38/h3-8,21H,9-20,22-23H2,1-2H3,(H,32,39)(H,33,40) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Reims-Champagne-Ardenne
Curated by ChEMBL
| Assay Description
Inhibition of human telomerase in A549 cells by TRAP assay |
Eur J Med Chem 44: 3880-8 (2009)
Article DOI: 10.1016/j.ejmech.2009.04.021
BindingDB Entry DOI: 10.7270/Q2W37WDH |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50298384

(CHEMBL573387 | N,N'-[4,5-Acridindiylbis(methylene)...)Show SMILES O=C(CCN1CCCCC1)NCc1cccc2cc3cccc(CNC(=O)CCN4CCCCC4)c3nc12 Show InChI InChI=1S/C31H41N5O2/c37-28(13-19-35-15-3-1-4-16-35)32-22-26-11-7-9-24-21-25-10-8-12-27(31(25)34-30(24)26)23-33-29(38)14-20-36-17-5-2-6-18-36/h7-12,21H,1-6,13-20,22-23H2,(H,32,37)(H,33,38) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Reims-Champagne-Ardenne
Curated by ChEMBL
| Assay Description
Inhibition of human telomerase in A549 cells by TRAP assay |
Eur J Med Chem 44: 3880-8 (2009)
Article DOI: 10.1016/j.ejmech.2009.04.021
BindingDB Entry DOI: 10.7270/Q2W37WDH |
More data for this
Ligand-Target Pair | |
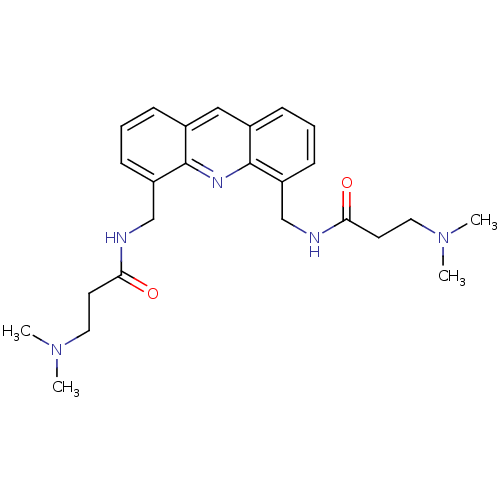
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50298383
(CHEMBL575850 | N,N'-[4,5-Acridindiylbis(methylene)...)Show SMILES CN(C)CCC(=O)NCc1cccc2cc3cccc(CNC(=O)CCN(C)C)c3nc12 Show InChI InChI=1S/C25H33N5O2/c1-29(2)13-11-22(31)26-16-20-9-5-7-18-15-19-8-6-10-21(25(19)28-24(18)20)17-27-23(32)12-14-30(3)4/h5-10,15H,11-14,16-17H2,1-4H3,(H,26,31)(H,27,32) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Reims-Champagne-Ardenne
Curated by ChEMBL
| Assay Description
Inhibition of human telomerase in A549 cells by TRAP assay |
Eur J Med Chem 44: 3880-8 (2009)
Article DOI: 10.1016/j.ejmech.2009.04.021
BindingDB Entry DOI: 10.7270/Q2W37WDH |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50298381
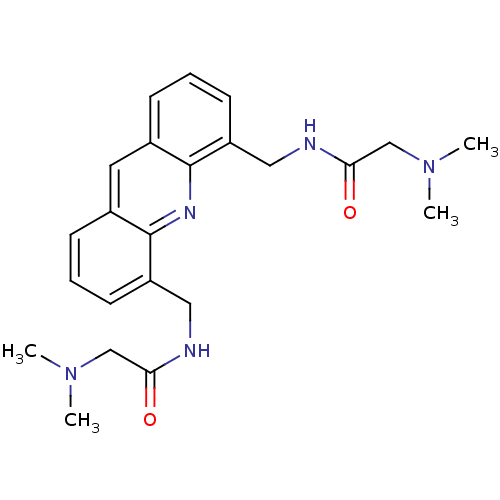
(CHEMBL573386 | N,N'-[4,5-Acridindiylbis(methylene)...)Show SMILES CN(C)CC(=O)NCc1cccc2cc3cccc(CNC(=O)CN(C)C)c3nc12 Show InChI InChI=1S/C23H29N5O2/c1-27(2)14-20(29)24-12-18-9-5-7-16-11-17-8-6-10-19(23(17)26-22(16)18)13-25-21(30)15-28(3)4/h5-11H,12-15H2,1-4H3,(H,24,29)(H,25,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Reims-Champagne-Ardenne
Curated by ChEMBL
| Assay Description
Inhibition of human telomerase in A549 cells by TRAP assay |
Eur J Med Chem 44: 3880-8 (2009)
Article DOI: 10.1016/j.ejmech.2009.04.021
BindingDB Entry DOI: 10.7270/Q2W37WDH |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50298380
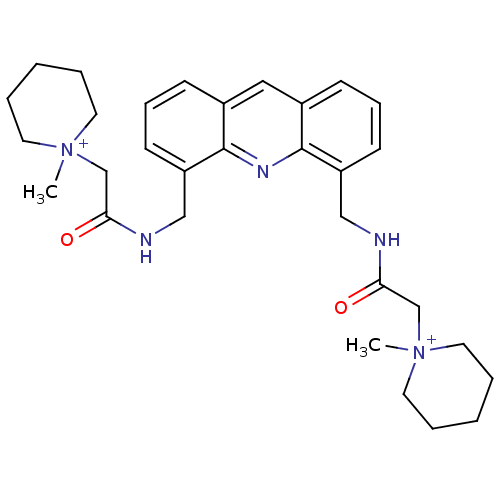
(2,2'-[4,5-Acridindiylbis(methyleneimino)]bis[N-met...)Show SMILES C[N+]1(CC(=O)NCc2cccc3cc4cccc(CNC(=O)C[N+]5(C)CCCCC5)c4nc23)CCCCC1 Show InChI InChI=1S/C31H41N5O2/c1-35(15-5-3-6-16-35)22-28(37)32-20-26-13-9-11-24-19-25-12-10-14-27(31(25)34-30(24)26)21-33-29(38)23-36(2)17-7-4-8-18-36/h9-14,19H,3-8,15-18,20-23H2,1-2H3/p+2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Reims-Champagne-Ardenne
Curated by ChEMBL
| Assay Description
Inhibition of human telomerase in A549 cells by TRAP assay |
Eur J Med Chem 44: 3880-8 (2009)
Article DOI: 10.1016/j.ejmech.2009.04.021
BindingDB Entry DOI: 10.7270/Q2W37WDH |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50054429
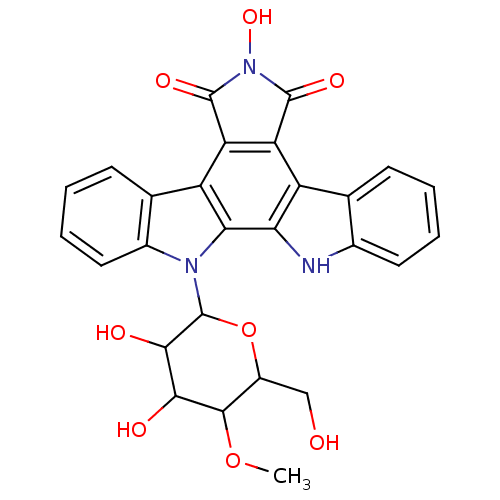
(6-Hydroxy-12-(4-O-methyl-beta-D-glucopyrannosyl) -...)Show SMILES COC1C(CO)OC(C(O)C1O)n1c2ccccc2c2c3C(=O)N(O)C(=O)c3c3c4ccccc4[nH]c3c12 Show InChI InChI=1S/C27H23N3O8/c1-37-24-15(10-31)38-27(23(33)22(24)32)29-14-9-5-3-7-12(14)17-19-18(25(34)30(36)26(19)35)16-11-6-2-4-8-13(11)28-20(16)21(17)29/h2-9,15,22-24,27-28,31-33,36H,10H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Blaise Pascal
Curated by ChEMBL
| Assay Description
Inhibition of Protein Kinase C(PKC) |
J Med Chem 39: 4471-7 (1996)
Article DOI: 10.1021/jm9603779
BindingDB Entry DOI: 10.7270/Q2S181MM |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50054425
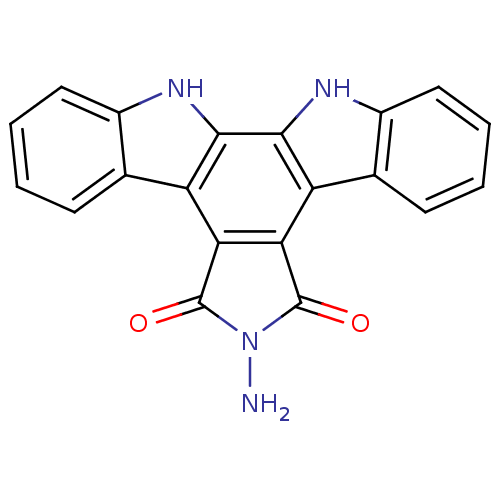
(6-amino-6,7,12,13-tetrahydro-5H-indolo[2,3-a]pyrro...)Show SMILES NN1C(=O)c2c(C1=O)c1c3ccccc3[nH]c1c1[nH]c3ccccc3c21 Show InChI InChI=1S/C20H12N4O2/c21-24-19(25)15-13-9-5-1-3-7-11(9)22-17(13)18-14(16(15)20(24)26)10-6-2-4-8-12(10)23-18/h1-8,22-23H,21H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.35E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Blaise Pascal
Curated by ChEMBL
| Assay Description
Inhibition of Protein Kinase C(PKC) |
J Med Chem 39: 4471-7 (1996)
Article DOI: 10.1021/jm9603779
BindingDB Entry DOI: 10.7270/Q2S181MM |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM2672
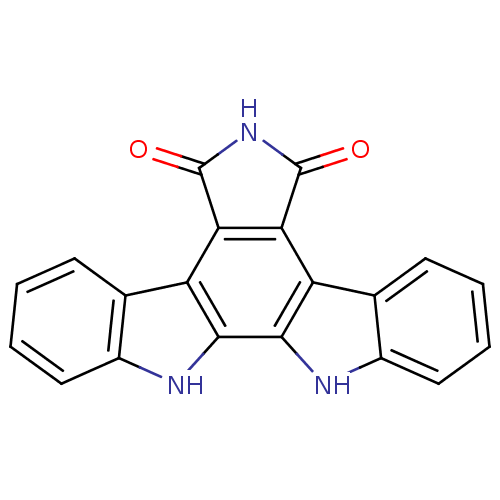
(3,13,23-triazahexacyclo[14.7.0.0^{2,10}.0^{4,9}.0^...)Show SMILES O=C1NC(=O)c2c1c1c3ccccc3[nH]c1c1[nH]c3ccccc3c21 Show InChI InChI=1S/C20H11N3O2/c24-19-15-13-9-5-1-3-7-11(9)21-17(13)18-14(16(15)20(25)23-19)10-6-2-4-8-12(10)22-18/h1-8,21-22H,(H,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.47E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Blaise Pascal
Curated by ChEMBL
| Assay Description
Inhibition of Protein Kinase C(PKC) |
J Med Chem 39: 4471-7 (1996)
Article DOI: 10.1021/jm9603779
BindingDB Entry DOI: 10.7270/Q2S181MM |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50060400
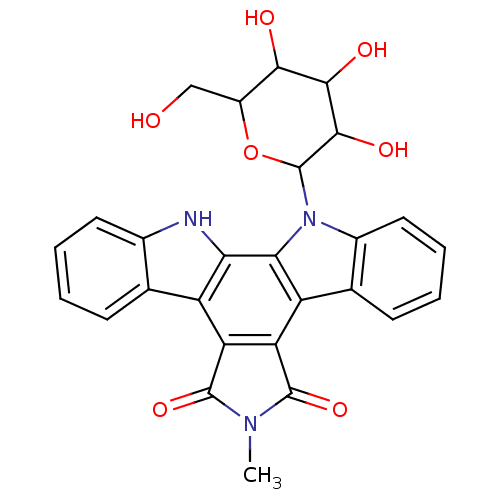
(6-methyl-12-(3,4,5-trihydroxy-6-hydroxymethyltetra...)Show SMILES CN1C(=O)c2c(C1=O)c1c3ccccc3n(C3OC(CO)C(O)C(O)C3O)c1c1[nH]c3ccccc3c21 Show InChI InChI=1S/C27H23N3O7/c1-29-25(35)18-16-11-6-2-4-8-13(11)28-20(16)21-17(19(18)26(29)36)12-7-3-5-9-14(12)30(21)27-24(34)23(33)22(32)15(10-31)37-27/h2-9,15,22-24,27-28,31-34H,10H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Blaise Pascal
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C alpha |
J Med Chem 40: 3456-65 (1997)
Article DOI: 10.1021/jm9702084
BindingDB Entry DOI: 10.7270/Q2T72GJ6 |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50054432
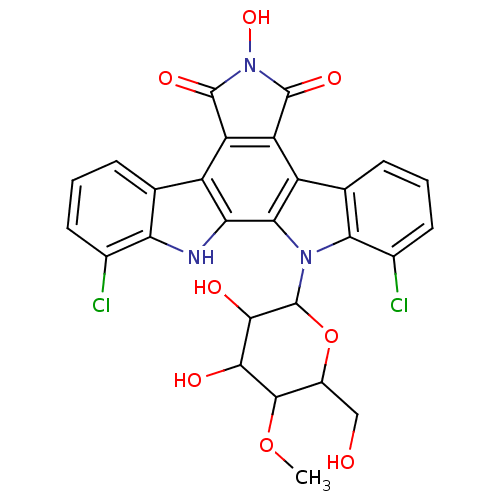
(1,11-Dichloro-6-hydroxy-12-(4-O-methyl-beta-D-gluc...)Show SMILES COC1C(CO)OC(C(O)C1O)n1c2c(Cl)cccc2c2c3C(=O)N(O)C(=O)c3c3c4cccc(Cl)c4[nH]c3c12 Show InChI InChI=1S/C27H21Cl2N3O8/c1-39-24-13(8-33)40-27(23(35)22(24)34)31-20-10(5-3-7-12(20)29)15-17-16(25(36)32(38)26(17)37)14-9-4-2-6-11(28)18(9)30-19(14)21(15)31/h2-7,13,22-24,27,30,33-35,38H,8H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Blaise Pascal
Curated by ChEMBL
| Assay Description
Inhibition of Protein Kinase C(PKC) |
J Med Chem 39: 4471-7 (1996)
Article DOI: 10.1021/jm9603779
BindingDB Entry DOI: 10.7270/Q2S181MM |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50054434
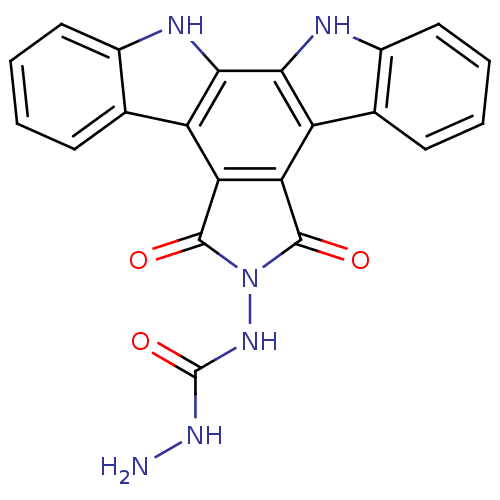
(6-(4-Semicarbazido)-6,7,12,13-tetrahydro-5,7-dioxo...)Show SMILES NNC(=O)NN1C(=O)c2c(C1=O)c1c3ccccc3[nH]c1c1[nH]c3ccccc3c21 Show InChI InChI=1S/C21H14N6O3/c22-25-21(30)26-27-19(28)15-13-9-5-1-3-7-11(9)23-17(13)18-14(16(15)20(27)29)10-6-2-4-8-12(10)24-18/h1-8,23-24H,22H2,(H2,25,26,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Blaise Pascal
Curated by ChEMBL
| Assay Description
Inhibition of Protein Kinase C(PKC) |
J Med Chem 39: 4471-7 (1996)
Article DOI: 10.1021/jm9603779
BindingDB Entry DOI: 10.7270/Q2S181MM |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50054422
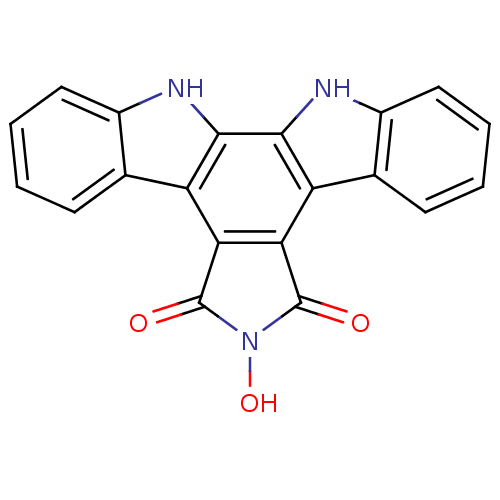
(6-hydroxy-6,7,12,13-tetrahydro-5H-indolo[2,3-a]pyr...)Show SMILES ON1C(=O)c2c(C1=O)c1c3ccccc3[nH]c1c1[nH]c3ccccc3c21 Show InChI InChI=1S/C20H11N3O3/c24-19-15-13-9-5-1-3-7-11(9)21-17(13)18-14(16(15)20(25)23(19)26)10-6-2-4-8-12(10)22-18/h1-8,21-22,26H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.92E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Blaise Pascal
Curated by ChEMBL
| Assay Description
Inhibition of Protein Kinase C(PKC) |
J Med Chem 39: 4471-7 (1996)
Article DOI: 10.1021/jm9603779
BindingDB Entry DOI: 10.7270/Q2S181MM |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50060402
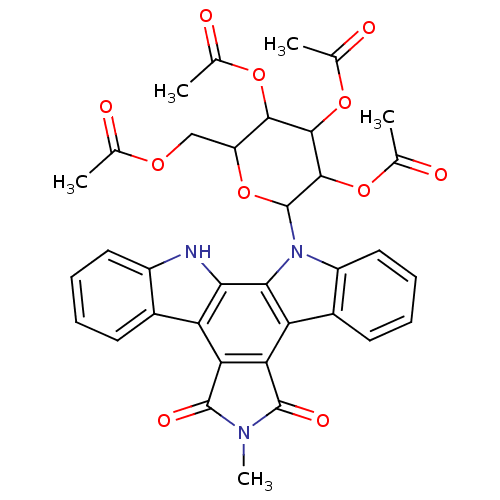
(3,5-di(methylcarbonyloxy)-2-methylcarbonyloxymethy...)Show SMILES CN1C(=O)c2c(C1=O)c1c3ccccc3n(C3OC(COC(C)=O)C(OC(C)=O)C(OC(C)=O)C3OC(C)=O)c1c1[nH]c3ccccc3c21 Show InChI InChI=1S/C35H31N3O11/c1-15(39)45-14-23-30(46-16(2)40)31(47-17(3)41)32(48-18(4)42)35(49-23)38-22-13-9-7-11-20(22)25-27-26(33(43)37(5)34(27)44)24-19-10-6-8-12-21(19)36-28(24)29(25)38/h6-13,23,30-32,35-36H,14H2,1-5H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Blaise Pascal
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C alpha |
J Med Chem 40: 3456-65 (1997)
Article DOI: 10.1021/jm9702084
BindingDB Entry DOI: 10.7270/Q2T72GJ6 |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50060403

(1,11-dichloro-12-(3,4-dihydroxy-6-hydroxymethyl-5-...)Show SMILES COC1C(CO)OC(C(O)C1O)n1c2c(Cl)cccc2c2c3C(=O)N(C)C(=O)c3c3c4cccc(Cl)c4[nH]c3c12 Show InChI InChI=1S/C28H23Cl2N3O7/c1-32-26(37)17-15-10-5-3-7-12(29)19(10)31-20(15)22-16(18(17)27(32)38)11-6-4-8-13(30)21(11)33(22)28-24(36)23(35)25(39-2)14(9-34)40-28/h3-8,14,23-25,28,31,34-36H,9H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Blaise Pascal
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C alpha |
J Med Chem 40: 3456-65 (1997)
Article DOI: 10.1021/jm9702084
BindingDB Entry DOI: 10.7270/Q2T72GJ6 |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50054420
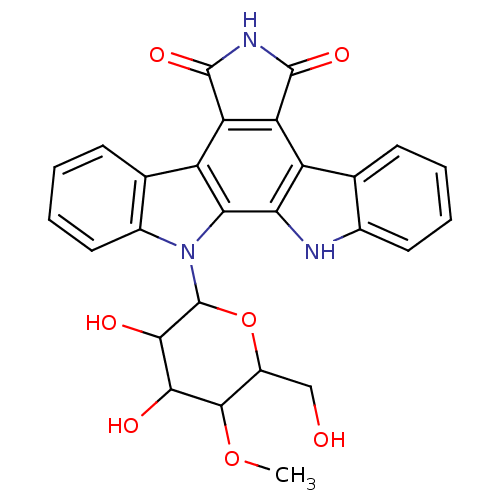
(12-(3,4-dihydroxy-6-hydroxymethyl-5-methoxytetrahy...)Show SMILES COC1C(CO)OC(C(O)C1O)n1c2ccccc2c2c3C(=O)NC(=O)c3c3c4ccccc4[nH]c3c12 Show InChI InChI=1S/C27H23N3O7/c1-36-24-15(10-31)37-27(23(33)22(24)32)30-14-9-5-3-7-12(14)17-19-18(25(34)29-26(19)35)16-11-6-2-4-8-13(11)28-20(16)21(17)30/h2-9,15,22-24,27-28,31-33H,10H2,1H3,(H,29,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Blaise Pascal
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C alpha |
J Med Chem 40: 3456-65 (1997)
Article DOI: 10.1021/jm9702084
BindingDB Entry DOI: 10.7270/Q2T72GJ6 |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50162287
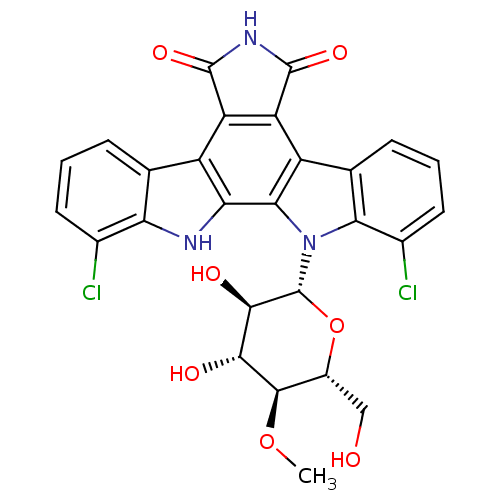
((Rebeccamycin)1,11-dichloro-12-(3,4-dihydroxy-6-hy...)Show SMILES CO[C@@H]1[C@@H](CO)O[C@H]([C@H](O)[C@H]1O)n1c2c(Cl)cccc2c2c3C(=O)NC(=O)c3c3c4cccc(Cl)c4[nH]c3c12 |r| Show InChI InChI=1S/C27H21Cl2N3O7/c1-38-24-13(8-33)39-27(23(35)22(24)34)32-20-10(5-3-7-12(20)29)15-17-16(25(36)31-26(17)37)14-9-4-2-6-11(28)18(9)30-19(14)21(15)32/h2-7,13,22-24,27,30,33-35H,8H2,1H3,(H,31,36,37)/t13-,22-,23-,24-,27-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Blaise Pascal
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C alpha |
J Med Chem 40: 3456-65 (1997)
Article DOI: 10.1021/jm9702084
BindingDB Entry DOI: 10.7270/Q2T72GJ6 |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50060401
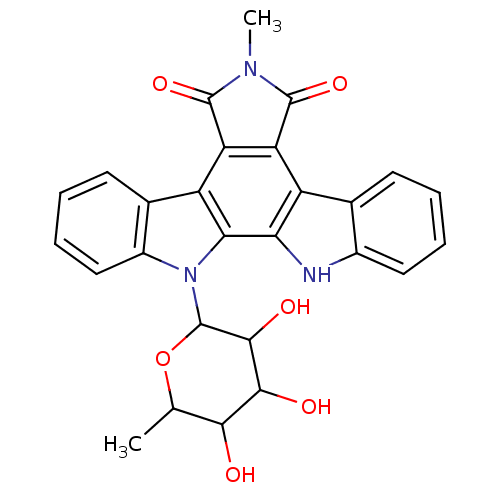
(6-methyl-12-(3,4,5-trihydroxy-6-methyltetrahydro-2...)Show SMILES CC1OC(C(O)C(O)C1O)n1c2ccccc2c2c3C(=O)N(C)C(=O)c3c3c4ccccc4[nH]c3c12 Show InChI InChI=1S/C27H23N3O6/c1-11-22(31)23(32)24(33)27(36-11)30-15-10-6-4-8-13(15)17-19-18(25(34)29(2)26(19)35)16-12-7-3-5-9-14(12)28-20(16)21(17)30/h3-11,22-24,27-28,31-33H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Blaise Pascal
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C alpha |
J Med Chem 40: 3456-65 (1997)
Article DOI: 10.1021/jm9702084
BindingDB Entry DOI: 10.7270/Q2T72GJ6 |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50060404
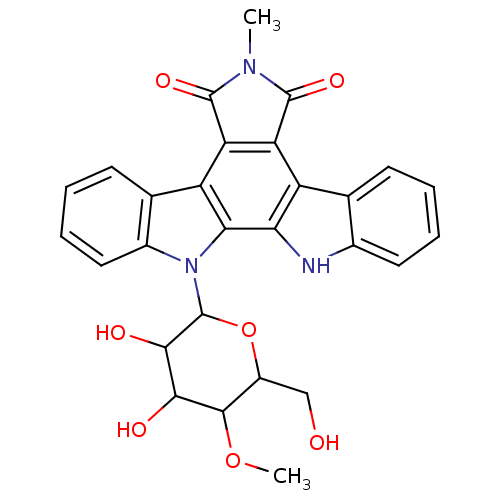
(12-(3,4-dihydroxy-6-hydroxymethyl-5-methoxytetrahy...)Show SMILES COC1C(CO)OC(C(O)C1O)n1c2ccccc2c2c3C(=O)N(C)C(=O)c3c3c4ccccc4[nH]c3c12 Show InChI InChI=1S/C28H25N3O7/c1-30-26(35)19-17-12-7-3-5-9-14(12)29-21(17)22-18(20(19)27(30)36)13-8-4-6-10-15(13)31(22)28-24(34)23(33)25(37-2)16(11-32)38-28/h3-10,16,23-25,28-29,32-34H,11H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Blaise Pascal
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C alpha |
J Med Chem 40: 3456-65 (1997)
Article DOI: 10.1021/jm9702084
BindingDB Entry DOI: 10.7270/Q2T72GJ6 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data