

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-lactamase (Enterobacter cloacae) | BDBM50339868 (4-(1-hydroxy-1,3-dihydrobenzo[c][1,2]oxaborol-6-yl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 21: 2533-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.024 BindingDB Entry DOI: 10.7270/Q2G44QK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50339869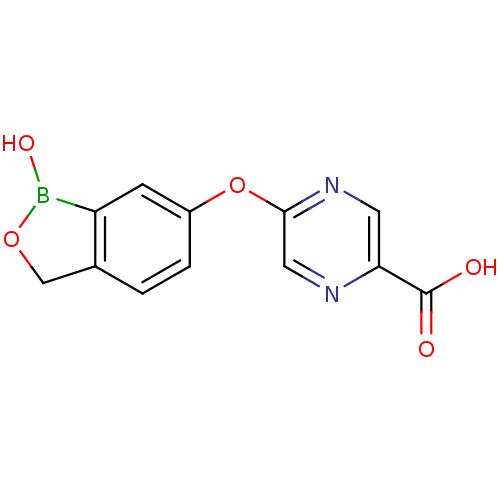 (5-(1-hydroxy-1,3-dihydrobenzo[c][1,2]oxaborol-6-yl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 21: 2533-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.024 BindingDB Entry DOI: 10.7270/Q2G44QK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50339870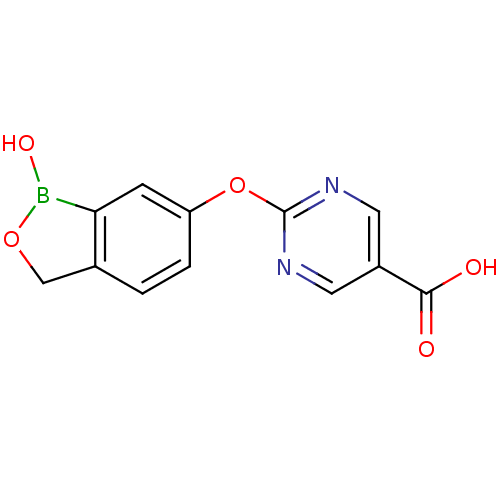 (2-(1-hydroxy-1,3-dihydrobenzo[c][1,2]oxaborol-6-yl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 21: 2533-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.024 BindingDB Entry DOI: 10.7270/Q2G44QK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50339846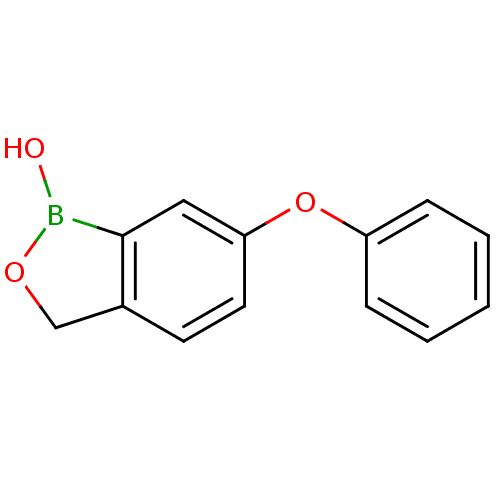 (6-phenoxybenzo[c][1,2]oxaborol-1(3H)-ol | CHEMBL17...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 21: 2533-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.024 BindingDB Entry DOI: 10.7270/Q2G44QK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50339856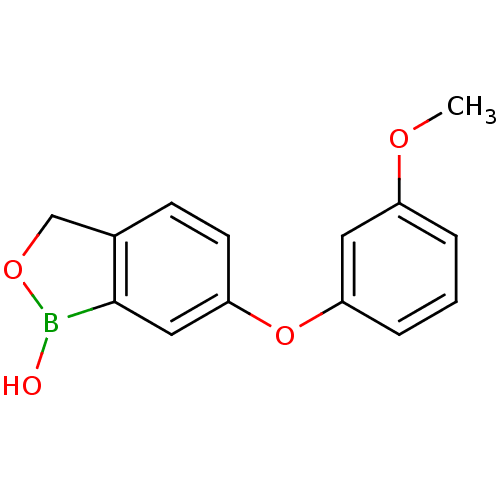 (6-(3-methoxyphenoxy)benzo[c][1,2]oxaborol-1(3H)-ol...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 21: 2533-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.024 BindingDB Entry DOI: 10.7270/Q2G44QK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50339862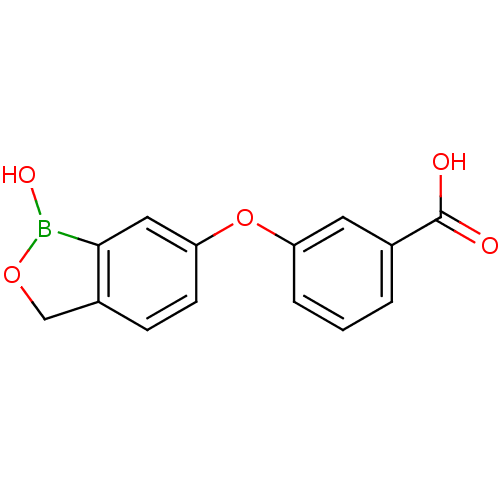 (3-(1-hydroxy-1,3-dihydrobenzo[c][1,2]oxaborol-6-yl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.44E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 21: 2533-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.024 BindingDB Entry DOI: 10.7270/Q2G44QK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine--tRNA ligase, cytoplasmic (Saccharomyces cerevisiae S288c) | BDBM50370987 (TAVABOROLE) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem | Article PubMed | 1.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Inhibition of Saccharomyces cerevisiae cytoplasmic leucyl-tRNA synthetase after 20 mins | Science 316: 1759-1761 (2007) Article DOI: 10.1126/science.1142189 BindingDB Entry DOI: 10.7270/Q2P84CQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50339860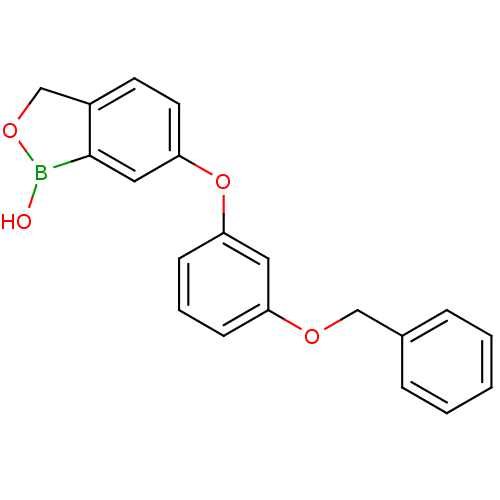 (6-(3-(benzyloxy)phenoxy)benzo[c][1,2]oxaborol-1(3H...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 21: 2533-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.024 BindingDB Entry DOI: 10.7270/Q2G44QK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50339857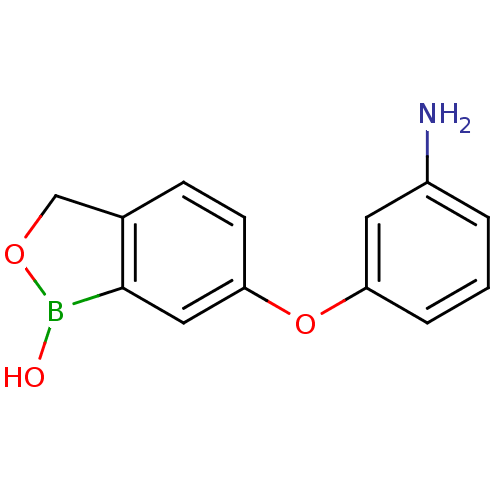 (6-(3-aminophenoxy)benzo[c][1,2]oxaborol-1(3H)-ol |...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 21: 2533-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.024 BindingDB Entry DOI: 10.7270/Q2G44QK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50339859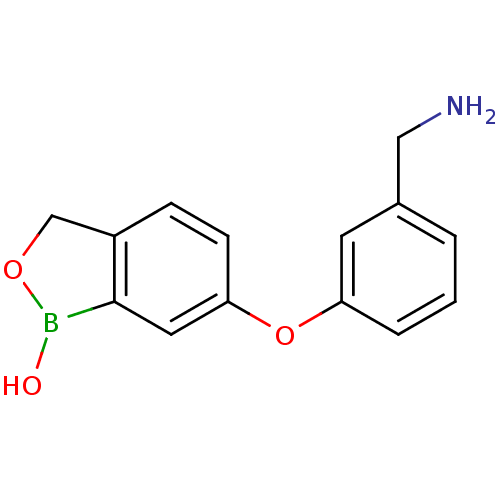 (6-(3-(aminomethyl)phenoxy)benzo[c][1,2]oxaborol-1(...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 21: 2533-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.024 BindingDB Entry DOI: 10.7270/Q2G44QK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50339855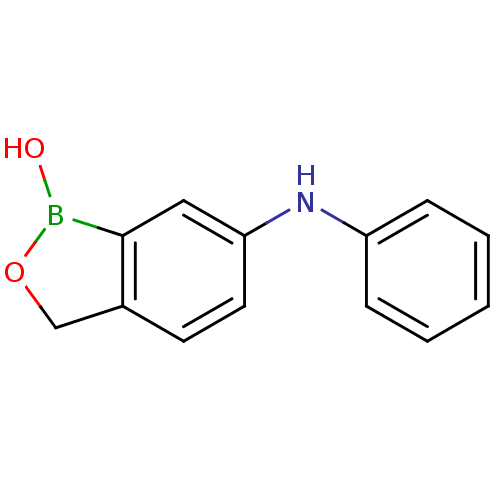 (6-(phenylamino)benzo[c][1,2]oxaborol-1(3H)-ol | CH...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 4.73E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 21: 2533-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.024 BindingDB Entry DOI: 10.7270/Q2G44QK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50339867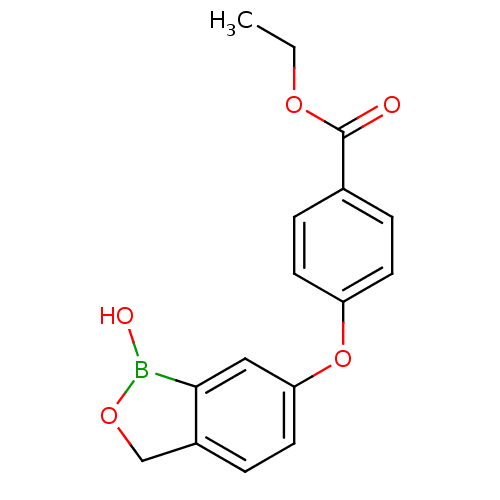 (CHEMBL1761273 | ethyl 4-(1-hydroxy-1,3-dihydrobenz...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 21: 2533-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.024 BindingDB Entry DOI: 10.7270/Q2G44QK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50339863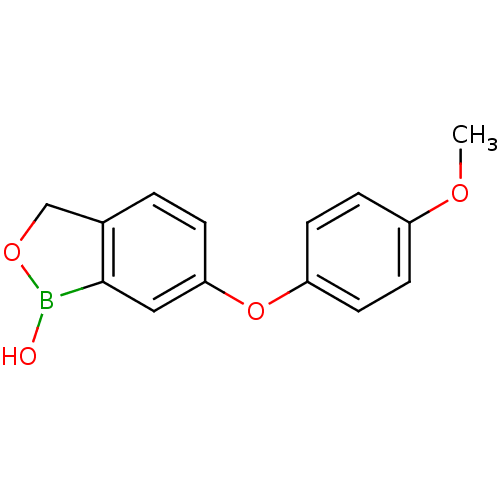 (6-(4-methoxyphenoxy)benzo[c][1,2]oxaborol-1(3H)-ol...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 21: 2533-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.024 BindingDB Entry DOI: 10.7270/Q2G44QK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50339850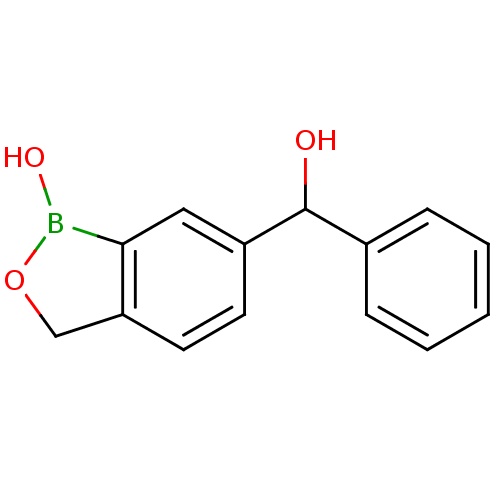 (6-(hydroxy(phenyl)methyl)benzo[c][1,2]oxaborol-1(3...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 7.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 21: 2533-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.024 BindingDB Entry DOI: 10.7270/Q2G44QK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50339861 (6-(3-((dimethylamino)methyl)phenoxy)benzo[c][1,2]o...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.72E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 21: 2533-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.024 BindingDB Entry DOI: 10.7270/Q2G44QK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50339858 (6-(3-(hydroxymethyl)phenoxy)benzo[c][1,2]oxaborol-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 21: 2533-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.024 BindingDB Entry DOI: 10.7270/Q2G44QK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50339864 (6-(4-aminophenoxy)benzo[c][1,2]oxaborol-1(3H)-ol |...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 8.73E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 21: 2533-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.024 BindingDB Entry DOI: 10.7270/Q2G44QK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50339847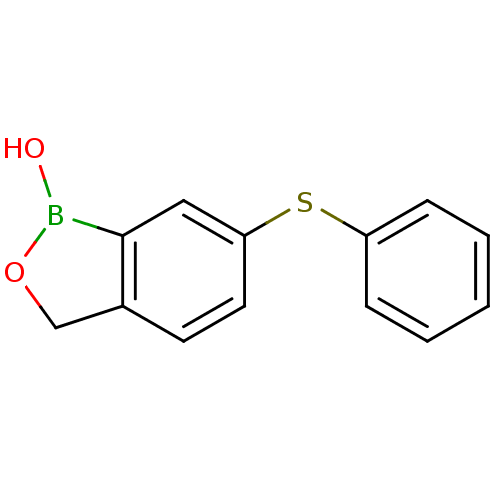 (6-(phenylthio)benzo[c][1,2]oxaborol-1(3H)-ol | CHE...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 9.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 21: 2533-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.024 BindingDB Entry DOI: 10.7270/Q2G44QK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50339865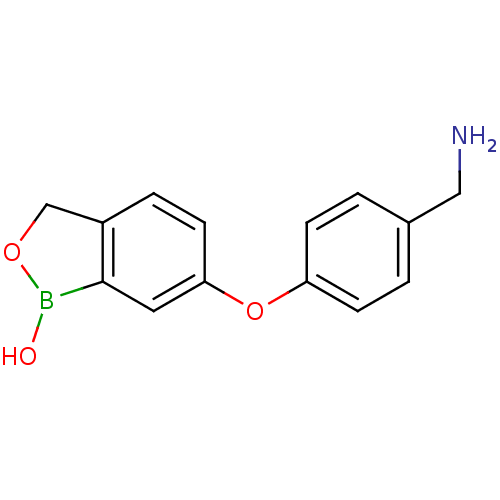 (6-(4-(aminomethyl)phenoxy)benzo[c][1,2]oxaborol-1(...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.33E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 21: 2533-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.024 BindingDB Entry DOI: 10.7270/Q2G44QK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50339854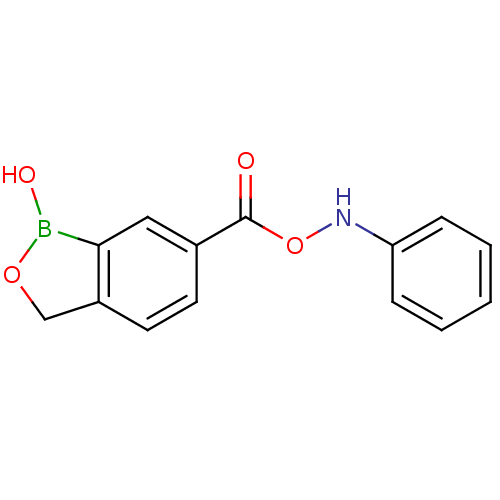 (6-((phenylaminooxy)carbonyl)benzo[c][1,2]oxaborol-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 21: 2533-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.024 BindingDB Entry DOI: 10.7270/Q2G44QK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50339848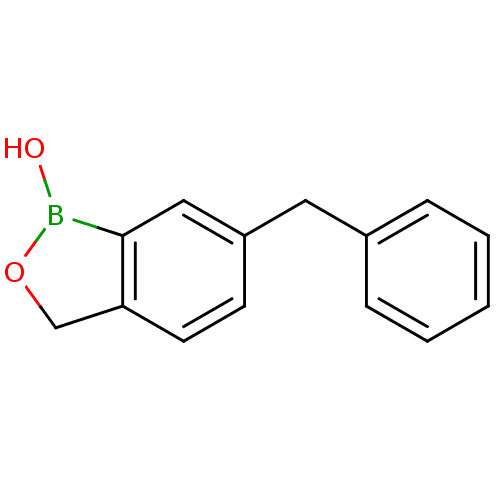 (6-benzylbenzo[c][1,2]oxaborol-1(3H)-ol | CHEMBL176...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.98E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 21: 2533-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.024 BindingDB Entry DOI: 10.7270/Q2G44QK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50339849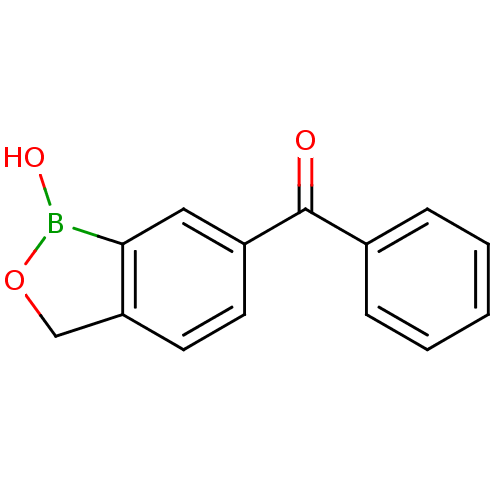 ((1-hydroxy-1,3-dihydrobenzo[c][1,2]oxaborol-6-yl)(...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.85E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 21: 2533-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.024 BindingDB Entry DOI: 10.7270/Q2G44QK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine--tRNA ligase, cytoplasmic (Saccharomyces cerevisiae S288c) | BDBM50370987 (TAVABOROLE) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem | Article PubMed | 3.14E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Inhibition of Saccharomyces cerevisiae cytoplasmic leucyl-tRNA synthetase after 2 mins | Science 316: 1759-1761 (2007) Article DOI: 10.1126/science.1142189 BindingDB Entry DOI: 10.7270/Q2P84CQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50339866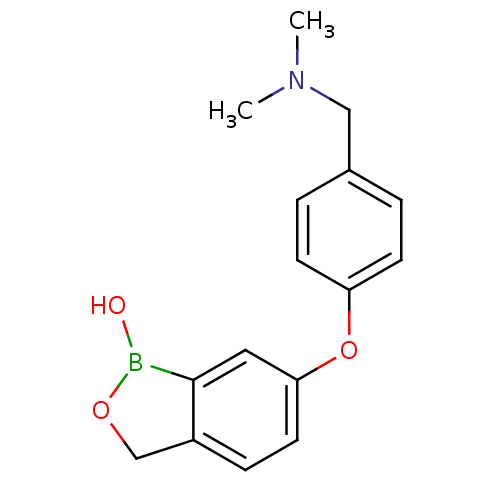 (6-(4-((dimethylamino)methyl)phenoxy)benzo[c][1,2]o...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.06E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 21: 2533-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.024 BindingDB Entry DOI: 10.7270/Q2G44QK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50339853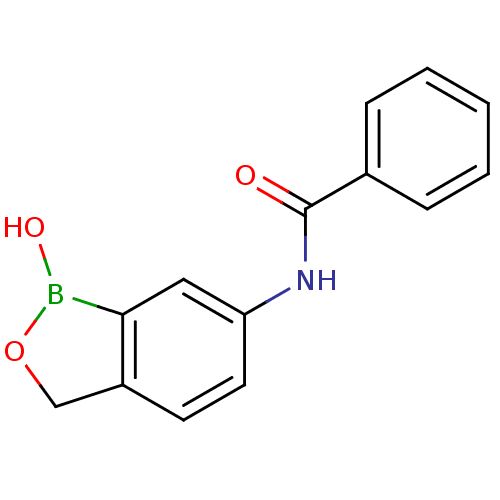 (CHEMBL1761258 | N-(1-hydroxy-1,3-dihydrobenzo[c][1...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 4.66E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 21: 2533-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.024 BindingDB Entry DOI: 10.7270/Q2G44QK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50339851 (6-(phenylsulfinyl)benzo[c][1,2]oxaborol-1(3H)-ol |...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 21: 2533-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.024 BindingDB Entry DOI: 10.7270/Q2G44QK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50339852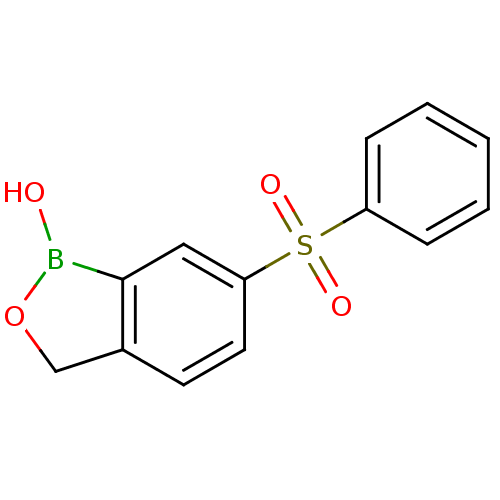 (6-(phenylsulfonyl)benzo[c][1,2]oxaborol-1(3H)-ol |...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6.32E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 21: 2533-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.024 BindingDB Entry DOI: 10.7270/Q2G44QK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50228403 ((R)-8-(3-aminopiperidin-1-yl)-7-(but-2-ynyl)-3-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd. Curated by ChEMBL | Assay Description Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system | Bioorg Med Chem Lett 22: 1464-8 (2012) Article DOI: 10.1016/j.bmcl.2011.11.054 BindingDB Entry DOI: 10.7270/Q2SF2WN8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50095261 ((3-Chloro-phenyl)-(6,7-diethoxy-quinazolin-4-yl)-a...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
Lawrence Berkeley National Laboratory Curated by ChEMBL | Assay Description Displacement of [125I]4-(3-iodoanilino)-6,7-dimethoxyquinazoline from EGFR tyrosine kinase in human A431 cell membranes | J Med Chem 48: 7445-56 (2005) Article DOI: 10.1021/jm050607w BindingDB Entry DOI: 10.7270/Q26D5TT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3556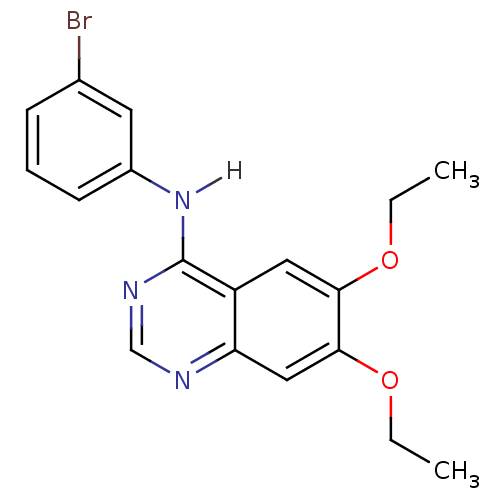 (4-[(3-Bromophenyl)amino]-6,7-diethoxyquinazoline |...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a |
Lawrence Berkeley National Laboratory Curated by ChEMBL | Assay Description Displacement of [125I]4-(3-iodoanilino)-6,7-dimethoxyquinazoline from EGFR tyrosine kinase in human A431 cell membranes | J Med Chem 48: 7445-56 (2005) Article DOI: 10.1021/jm050607w BindingDB Entry DOI: 10.7270/Q26D5TT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50364171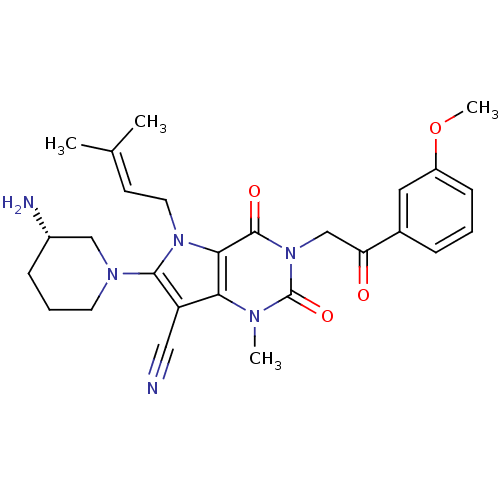 (CHEMBL1951598) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd. Curated by ChEMBL | Assay Description Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system | Bioorg Med Chem Lett 22: 1464-8 (2012) Article DOI: 10.1016/j.bmcl.2011.11.054 BindingDB Entry DOI: 10.7270/Q2SF2WN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50364156 (CHEMBL1951432) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd. Curated by ChEMBL | Assay Description Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system | Bioorg Med Chem Lett 22: 1464-8 (2012) Article DOI: 10.1016/j.bmcl.2011.11.054 BindingDB Entry DOI: 10.7270/Q2SF2WN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50095273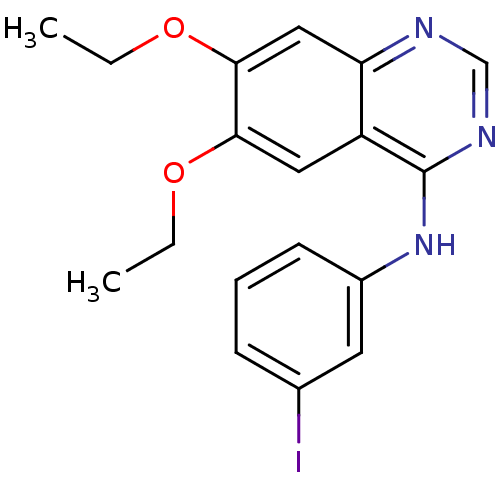 ((6,7-Diethoxy-quinazolin-4-yl)-(3-iodo-phenyl)-ami...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a |
Lawrence Berkeley National Laboratory Curated by ChEMBL | Assay Description Displacement of [125I]4-(3-iodoanilino)-6,7-dimethoxyquinazoline from EGFR tyrosine kinase in human A431 cell membranes | J Med Chem 48: 7445-56 (2005) Article DOI: 10.1021/jm050607w BindingDB Entry DOI: 10.7270/Q26D5TT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3032 (CHEMBL1204168 | CHEMBL29197 | N-(3-bromophenyl)-6,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a |
Lawrence Berkeley National Laboratory Curated by ChEMBL | Assay Description Displacement of [125I]4-(3-iodoanilino)-6,7-dimethoxyquinazoline from EGFR tyrosine kinase in human A431 cell membranes | J Med Chem 48: 7445-56 (2005) Article DOI: 10.1021/jm050607w BindingDB Entry DOI: 10.7270/Q26D5TT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3532 (CHEMBL540068 | CHEMBL7917 | N-(3-chlorophenyl)-6,7...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Lawrence Berkeley National Laboratory Curated by ChEMBL | Assay Description Inhibition of human EGFR tyrosine kinase phosphorylation expressed in mouse BaF3 cells | J Med Chem 48: 7445-56 (2005) Article DOI: 10.1021/jm050607w BindingDB Entry DOI: 10.7270/Q26D5TT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50364184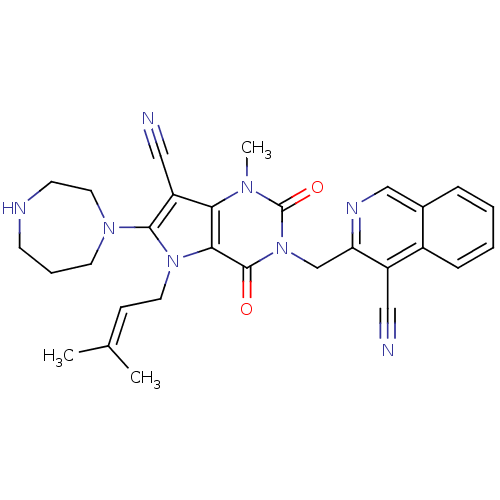 (CHEMBL1951611) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd. Curated by ChEMBL | Assay Description Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system | Bioorg Med Chem Lett 22: 1464-8 (2012) Article DOI: 10.1016/j.bmcl.2011.11.054 BindingDB Entry DOI: 10.7270/Q2SF2WN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50364158 (CHEMBL1951430) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd. Curated by ChEMBL | Assay Description Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system | Bioorg Med Chem Lett 22: 1464-8 (2012) Article DOI: 10.1016/j.bmcl.2011.11.054 BindingDB Entry DOI: 10.7270/Q2SF2WN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cobra venom factor/Complement factor B/Complement factor D (Naja kaouthia (Monocled cobra) (Naja siamensis)-Ho...) | BDBM251408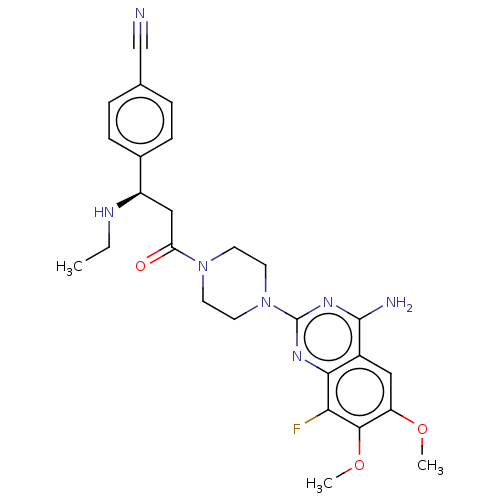 (US9452990, 90) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.4 | 25 |
NOVARTIS AG US Patent | Assay Description CVF-Bb complex prepared from purified cobra venom factor (1 μM), recombinant human complement factor B (expressed in drosophila cells and purifi... | US Patent US9452990 (2016) BindingDB Entry DOI: 10.7270/Q2H130Z9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50364173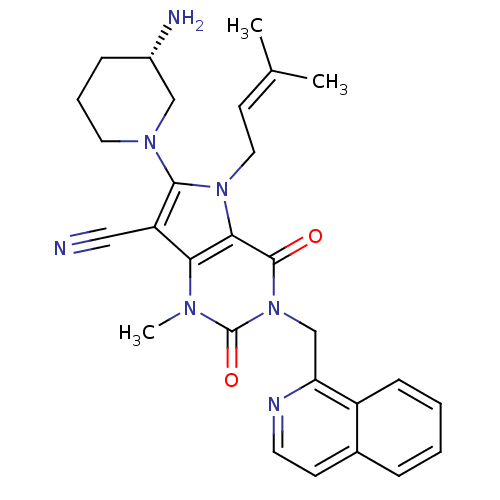 (CHEMBL1951599) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd. Curated by ChEMBL | Assay Description Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system | Bioorg Med Chem Lett 22: 1464-8 (2012) Article DOI: 10.1016/j.bmcl.2011.11.054 BindingDB Entry DOI: 10.7270/Q2SF2WN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cobra venom factor/Complement factor B/Complement factor D (Naja kaouthia (Monocled cobra) (Naja siamensis)-Ho...) | BDBM251420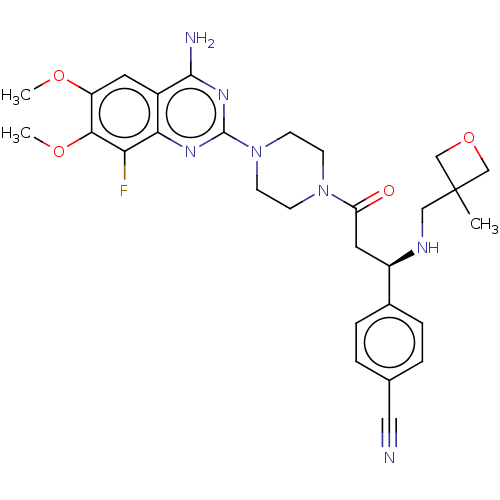 (US9452990, 102) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.4 | 25 |
NOVARTIS AG US Patent | Assay Description CVF-Bb complex prepared from purified cobra venom factor (1 μM), recombinant human complement factor B (expressed in drosophila cells and purifi... | US Patent US9452990 (2016) BindingDB Entry DOI: 10.7270/Q2H130Z9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50364187 (CHEMBL1951416) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd. Curated by ChEMBL | Assay Description Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system | Bioorg Med Chem Lett 22: 1464-8 (2012) Article DOI: 10.1016/j.bmcl.2011.11.054 BindingDB Entry DOI: 10.7270/Q2SF2WN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3533 (CHEMBL1204305 | CHEMBL96065 | N-(3-iodophenyl)-6,7...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.05 | n/a | n/a | n/a | n/a | n/a | n/a |
Lawrence Berkeley National Laboratory Curated by ChEMBL | Assay Description Displacement of [125I]4-(3-iodoanilino)-6,7-dimethoxyquinazoline from EGFR tyrosine kinase in human A431 cell membranes | J Med Chem 48: 7445-56 (2005) Article DOI: 10.1021/jm050607w BindingDB Entry DOI: 10.7270/Q26D5TT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50095261 ((3-Chloro-phenyl)-(6,7-diethoxy-quinazolin-4-yl)-a...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Lawrence Berkeley National Laboratory Curated by ChEMBL | Assay Description Inhibition of human EGFR tyrosine kinase phosphorylation expressed in mouse BaF3 cells | J Med Chem 48: 7445-56 (2005) Article DOI: 10.1021/jm050607w BindingDB Entry DOI: 10.7270/Q26D5TT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3532 (CHEMBL540068 | CHEMBL7917 | N-(3-chlorophenyl)-6,7...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.26 | n/a | n/a | n/a | n/a | n/a | n/a |
Lawrence Berkeley National Laboratory Curated by ChEMBL | Assay Description Displacement of [125I]4-(3-iodoanilino)-6,7-dimethoxyquinazoline from EGFR tyrosine kinase in human A431 cell membranes | J Med Chem 48: 7445-56 (2005) Article DOI: 10.1021/jm050607w BindingDB Entry DOI: 10.7270/Q26D5TT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50364147 (CHEMBL1951595) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd. Curated by ChEMBL | Assay Description Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system | Bioorg Med Chem Lett 22: 1464-8 (2012) Article DOI: 10.1016/j.bmcl.2011.11.054 BindingDB Entry DOI: 10.7270/Q2SF2WN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50364186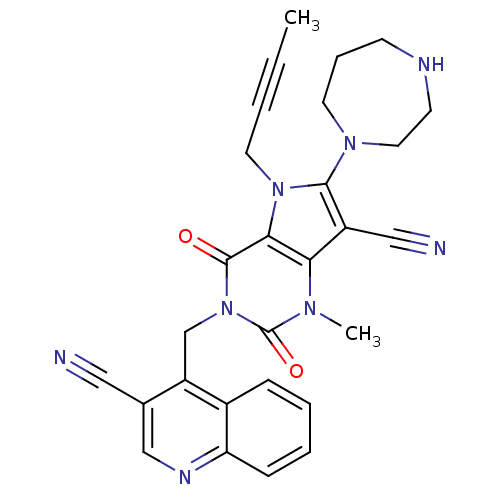 (CHEMBL1951614) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd. Curated by ChEMBL | Assay Description Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system | Bioorg Med Chem Lett 22: 1464-8 (2012) Article DOI: 10.1016/j.bmcl.2011.11.054 BindingDB Entry DOI: 10.7270/Q2SF2WN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus 14) | BDBM50455115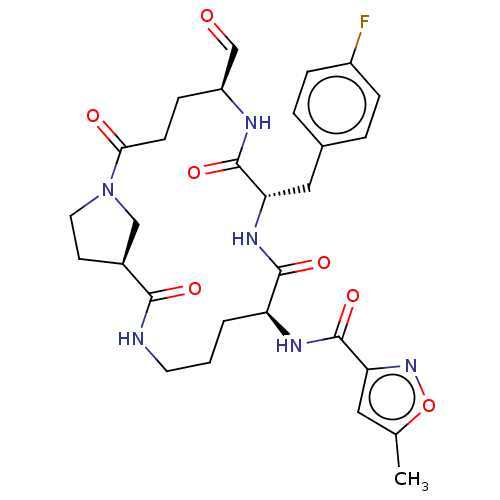 (CHEMBL4202932) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Human rhinovirus serotype 14 3C protease preincubated for 1 hr followed by Cys(PT14M)-Ala-Ile-Phe-Gln'Gly-Pro-Asp-Phe(4-NH2)-OH substra... | Bioorg Med Chem Lett 28: 906-909 (2018) Article DOI: 10.1016/j.bmcl.2018.01.064 BindingDB Entry DOI: 10.7270/Q2TF00Z3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus 14) | BDBM50455107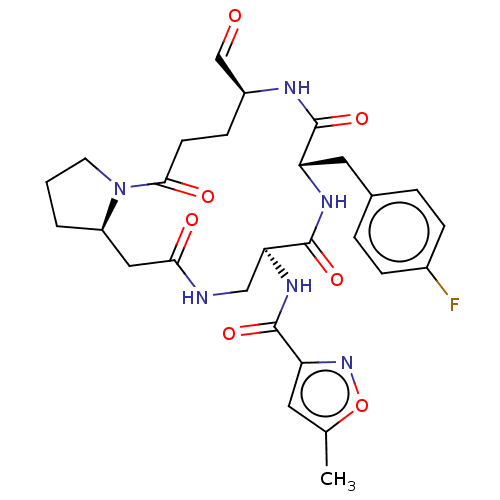 (CHEMBL4212167) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Human rhinovirus serotype 14 3C protease preincubated for 1 hr followed by Cys(PT14M)-Ala-Ile-Phe-Gln'Gly-Pro-Asp-Phe(4-NH2)-OH substra... | Bioorg Med Chem Lett 28: 906-909 (2018) Article DOI: 10.1016/j.bmcl.2018.01.064 BindingDB Entry DOI: 10.7270/Q2TF00Z3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus 14) | BDBM50455106 (CHEMBL4216095) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Human rhinovirus serotype 14 3C protease preincubated for 1 hr followed by Cys(PT14M)-Ala-Ile-Phe-Gln'Gly-Pro-Asp-Phe(4-NH2)-OH substra... | Bioorg Med Chem Lett 28: 906-909 (2018) Article DOI: 10.1016/j.bmcl.2018.01.064 BindingDB Entry DOI: 10.7270/Q2TF00Z3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50364183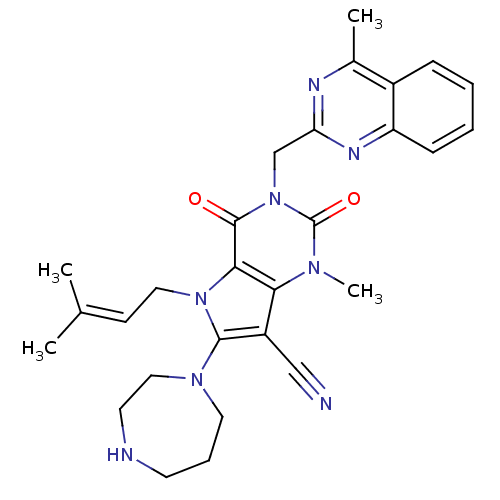 (CHEMBL1951609) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd. Curated by ChEMBL | Assay Description Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system | Bioorg Med Chem Lett 22: 1464-8 (2012) Article DOI: 10.1016/j.bmcl.2011.11.054 BindingDB Entry DOI: 10.7270/Q2SF2WN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 632 total ) | Next | Last >> |