 Found 470 hits with Last Name = 'rotstein' and Initial = 'd'
Found 470 hits with Last Name = 'rotstein' and Initial = 'd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Aromatase
(Homo sapiens (Human)) | BDBM50044424
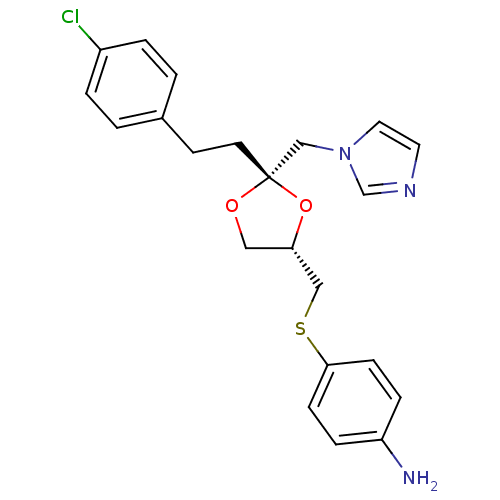
(4-(((2S,4S)-2-((1H-imidazol-1-yl)methyl)-2-(4-chlo...)Show SMILES Nc1ccc(SC[C@@H]2CO[C@](CCc3ccc(Cl)cc3)(Cn3ccnc3)O2)cc1 Show InChI InChI=1S/C22H24ClN3O2S/c23-18-3-1-17(2-4-18)9-10-22(15-26-12-11-25-16-26)27-13-20(28-22)14-29-21-7-5-19(24)6-8-21/h1-8,11-12,16,20H,9-10,13-15,24H2/t20-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 7.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Discovery Research
Curated by ChEMBL
| Assay Description
Binding affinity for Cytochrome P450 19A1 |
J Med Chem 36: 2235-7 (1993)
BindingDB Entry DOI: 10.7270/Q2ZW1K0P |
More data for this
Ligand-Target Pair | |
Lanosterol 14-alpha demethylase
(Rattus norvegicus) | BDBM50161586

(4-{(2S,4R)-2-[2-(4-Chloro-phenyl)-ethyl]-2-imidazo...)Show SMILES Nc1ccc(SC[C@H]2CO[C@](CCc3ccc(Cl)cc3)(Cn3ccnc3)O2)cc1 Show InChI InChI=1S/C22H24ClN3O2S/c23-18-3-1-17(2-4-18)9-10-22(15-26-12-11-25-16-26)27-13-20(28-22)14-29-21-7-5-19(24)6-8-21/h1-8,11-12,16,20H,9-10,13-15,24H2/t20-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Discovery Research
Curated by ChEMBL
| Assay Description
Apparent Ki for rat Lanosterol 14-alpha demethylase |
J Med Chem 36: 2235-7 (1993)
BindingDB Entry DOI: 10.7270/Q2ZW1K0P |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50044425

(4-{(2R,4R)-2-[2-(4-Chloro-phenyl)-ethyl]-2-imidazo...)Show SMILES Nc1ccc(SC[C@H]2CO[C@@](CCc3ccc(Cl)cc3)(Cn3ccnc3)O2)cc1 Show InChI InChI=1S/C22H24ClN3O2S/c23-18-3-1-17(2-4-18)9-10-22(15-26-12-11-25-16-26)27-13-20(28-22)14-29-21-7-5-19(24)6-8-21/h1-8,11-12,16,20H,9-10,13-15,24H2/t20-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Discovery Research
Curated by ChEMBL
| Assay Description
Binding affinity for Cytochrome P450 19A1 |
J Med Chem 36: 2235-7 (1993)
BindingDB Entry DOI: 10.7270/Q2ZW1K0P |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B1, mitochondrial
(Homo sapiens (Human)) | BDBM50044425

(4-{(2R,4R)-2-[2-(4-Chloro-phenyl)-ethyl]-2-imidazo...)Show SMILES Nc1ccc(SC[C@H]2CO[C@@](CCc3ccc(Cl)cc3)(Cn3ccnc3)O2)cc1 Show InChI InChI=1S/C22H24ClN3O2S/c23-18-3-1-17(2-4-18)9-10-22(15-26-12-11-25-16-26)27-13-20(28-22)14-29-21-7-5-19(24)6-8-21/h1-8,11-12,16,20H,9-10,13-15,24H2/t20-,22-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Discovery Research
Curated by ChEMBL
| Assay Description
Binding affinity for corticoid 11-beta-hydroxylase |
J Med Chem 36: 2235-7 (1993)
BindingDB Entry DOI: 10.7270/Q2ZW1K0P |
More data for this
Ligand-Target Pair | |
Lanosterol 14-alpha demethylase
(Homo sapiens (Human)) | BDBM31768

(CHEMBL295698 | Ketoconazole | Nizoral | Panfungol)Show SMILES CC(=O)N1CCN(CC1)c1ccc(OC[C@@H]2CO[C@](Cn3ccnc3)(O2)c2ccc(Cl)cc2Cl)cc1 |r| Show InChI InChI=1S/C26H28Cl2N4O4/c1-19(33)31-10-12-32(13-11-31)21-3-5-22(6-4-21)34-15-23-16-35-26(36-23,17-30-9-8-29-18-30)24-7-2-20(27)14-25(24)28/h2-9,14,18,23H,10-13,15-17H2,1H3/t23-,26-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Discovery Research
Curated by ChEMBL
| Assay Description
Inhibition of lanosterol 14-alpha-demethylase in hamster hepatic microsomes |
J Med Chem 36: 2235-7 (1993)
BindingDB Entry DOI: 10.7270/Q2ZW1K0P |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50044424

(4-(((2S,4S)-2-((1H-imidazol-1-yl)methyl)-2-(4-chlo...)Show SMILES Nc1ccc(SC[C@@H]2CO[C@](CCc3ccc(Cl)cc3)(Cn3ccnc3)O2)cc1 Show InChI InChI=1S/C22H24ClN3O2S/c23-18-3-1-17(2-4-18)9-10-22(15-26-12-11-25-16-26)27-13-20(28-22)14-29-21-7-5-19(24)6-8-21/h1-8,11-12,16,20H,9-10,13-15,24H2/t20-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Discovery Research
Curated by ChEMBL
| Assay Description
Binding affinity for progesterone 6-beta-hydroxylase of hepatic microsomes |
J Med Chem 36: 2235-7 (1993)
BindingDB Entry DOI: 10.7270/Q2ZW1K0P |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50044425

(4-{(2R,4R)-2-[2-(4-Chloro-phenyl)-ethyl]-2-imidazo...)Show SMILES Nc1ccc(SC[C@H]2CO[C@@](CCc3ccc(Cl)cc3)(Cn3ccnc3)O2)cc1 Show InChI InChI=1S/C22H24ClN3O2S/c23-18-3-1-17(2-4-18)9-10-22(15-26-12-11-25-16-26)27-13-20(28-22)14-29-21-7-5-19(24)6-8-21/h1-8,11-12,16,20H,9-10,13-15,24H2/t20-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Discovery Research
Curated by ChEMBL
| Assay Description
Binding affinity for progesterone 6-beta-hydroxylase of hepatic microsomes |
J Med Chem 36: 2235-7 (1993)
BindingDB Entry DOI: 10.7270/Q2ZW1K0P |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B1, mitochondrial
(Homo sapiens (Human)) | BDBM50044424

(4-(((2S,4S)-2-((1H-imidazol-1-yl)methyl)-2-(4-chlo...)Show SMILES Nc1ccc(SC[C@@H]2CO[C@](CCc3ccc(Cl)cc3)(Cn3ccnc3)O2)cc1 Show InChI InChI=1S/C22H24ClN3O2S/c23-18-3-1-17(2-4-18)9-10-22(15-26-12-11-25-16-26)27-13-20(28-22)14-29-21-7-5-19(24)6-8-21/h1-8,11-12,16,20H,9-10,13-15,24H2/t20-,22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Discovery Research
Curated by ChEMBL
| Assay Description
Binding affinity for corticoid 11-beta-hydroxylase |
J Med Chem 36: 2235-7 (1993)
BindingDB Entry DOI: 10.7270/Q2ZW1K0P |
More data for this
Ligand-Target Pair | |
Lanosterol 14-alpha demethylase
(Rattus norvegicus) | BDBM50044425

(4-{(2R,4R)-2-[2-(4-Chloro-phenyl)-ethyl]-2-imidazo...)Show SMILES Nc1ccc(SC[C@H]2CO[C@@](CCc3ccc(Cl)cc3)(Cn3ccnc3)O2)cc1 Show InChI InChI=1S/C22H24ClN3O2S/c23-18-3-1-17(2-4-18)9-10-22(15-26-12-11-25-16-26)27-13-20(28-22)14-29-21-7-5-19(24)6-8-21/h1-8,11-12,16,20H,9-10,13-15,24H2/t20-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Discovery Research
Curated by ChEMBL
| Assay Description
Inhibition of lanosterol 14-alpha-demethylase of rat hepatic microsomes |
J Med Chem 36: 2235-7 (1993)
BindingDB Entry DOI: 10.7270/Q2ZW1K0P |
More data for this
Ligand-Target Pair | |
Lanosterol 14-alpha demethylase
(Homo sapiens (Human)) | BDBM50044425

(4-{(2R,4R)-2-[2-(4-Chloro-phenyl)-ethyl]-2-imidazo...)Show SMILES Nc1ccc(SC[C@H]2CO[C@@](CCc3ccc(Cl)cc3)(Cn3ccnc3)O2)cc1 Show InChI InChI=1S/C22H24ClN3O2S/c23-18-3-1-17(2-4-18)9-10-22(15-26-12-11-25-16-26)27-13-20(28-22)14-29-21-7-5-19(24)6-8-21/h1-8,11-12,16,20H,9-10,13-15,24H2/t20-,22-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Discovery Research
Curated by ChEMBL
| Assay Description
Inhibition of lanosterol 14-alpha-demethylase in hamster hepatic microsomes |
J Med Chem 36: 2235-7 (1993)
BindingDB Entry DOI: 10.7270/Q2ZW1K0P |
More data for this
Ligand-Target Pair | |
Lanosterol 14-alpha demethylase
(Homo sapiens (Human)) | BDBM50044425

(4-{(2R,4R)-2-[2-(4-Chloro-phenyl)-ethyl]-2-imidazo...)Show SMILES Nc1ccc(SC[C@H]2CO[C@@](CCc3ccc(Cl)cc3)(Cn3ccnc3)O2)cc1 Show InChI InChI=1S/C22H24ClN3O2S/c23-18-3-1-17(2-4-18)9-10-22(15-26-12-11-25-16-26)27-13-20(28-22)14-29-21-7-5-19(24)6-8-21/h1-8,11-12,16,20H,9-10,13-15,24H2/t20-,22-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 40.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Discovery Research
Curated by ChEMBL
| Assay Description
Inhibition of lanosterol 14-alpha-demethylase in hamster hepatic microsomes |
J Med Chem 36: 2235-7 (1993)
BindingDB Entry DOI: 10.7270/Q2ZW1K0P |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50044425

(4-{(2R,4R)-2-[2-(4-Chloro-phenyl)-ethyl]-2-imidazo...)Show SMILES Nc1ccc(SC[C@H]2CO[C@@](CCc3ccc(Cl)cc3)(Cn3ccnc3)O2)cc1 Show InChI InChI=1S/C22H24ClN3O2S/c23-18-3-1-17(2-4-18)9-10-22(15-26-12-11-25-16-26)27-13-20(28-22)14-29-21-7-5-19(24)6-8-21/h1-8,11-12,16,20H,9-10,13-15,24H2/t20-,22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Discovery Research
Curated by ChEMBL
| Assay Description
Binding affinity for progesterone 17alpha,20-lyase |
J Med Chem 36: 2235-7 (1993)
BindingDB Entry DOI: 10.7270/Q2ZW1K0P |
More data for this
Ligand-Target Pair | |
Lanosterol 14-alpha demethylase
(Homo sapiens (Human)) | BDBM31768

(CHEMBL295698 | Ketoconazole | Nizoral | Panfungol)Show SMILES CC(=O)N1CCN(CC1)c1ccc(OC[C@@H]2CO[C@](Cn3ccnc3)(O2)c2ccc(Cl)cc2Cl)cc1 |r| Show InChI InChI=1S/C26H28Cl2N4O4/c1-19(33)31-10-12-32(13-11-31)21-3-5-22(6-4-21)34-15-23-16-35-26(36-23,17-30-9-8-29-18-30)24-7-2-20(27)14-25(24)28/h2-9,14,18,23H,10-13,15-17H2,1H3/t23-,26-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Discovery Research
Curated by ChEMBL
| Assay Description
Inhibition of lanosterol 14-alpha-demethylase in hamster hepatic microsomes |
J Med Chem 36: 2235-7 (1993)
BindingDB Entry DOI: 10.7270/Q2ZW1K0P |
More data for this
Ligand-Target Pair | |
Lanosterol 14-alpha demethylase
(Rattus norvegicus) | BDBM31768

(CHEMBL295698 | Ketoconazole | Nizoral | Panfungol)Show SMILES CC(=O)N1CCN(CC1)c1ccc(OC[C@@H]2CO[C@](Cn3ccnc3)(O2)c2ccc(Cl)cc2Cl)cc1 |r| Show InChI InChI=1S/C26H28Cl2N4O4/c1-19(33)31-10-12-32(13-11-31)21-3-5-22(6-4-21)34-15-23-16-35-26(36-23,17-30-9-8-29-18-30)24-7-2-20(27)14-25(24)28/h2-9,14,18,23H,10-13,15-17H2,1H3/t23-,26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Discovery Research
Curated by ChEMBL
| Assay Description
Inhibition of lanosterol 14-alpha-demethylase of rat hepatic microsomes |
J Med Chem 36: 2235-7 (1993)
BindingDB Entry DOI: 10.7270/Q2ZW1K0P |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50044425

(4-{(2R,4R)-2-[2-(4-Chloro-phenyl)-ethyl]-2-imidazo...)Show SMILES Nc1ccc(SC[C@H]2CO[C@@](CCc3ccc(Cl)cc3)(Cn3ccnc3)O2)cc1 Show InChI InChI=1S/C22H24ClN3O2S/c23-18-3-1-17(2-4-18)9-10-22(15-26-12-11-25-16-26)27-13-20(28-22)14-29-21-7-5-19(24)6-8-21/h1-8,11-12,16,20H,9-10,13-15,24H2/t20-,22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 109 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Discovery Research
Curated by ChEMBL
| Assay Description
Binding affinity for cholesterol 17-alpha-hydroxylase |
J Med Chem 36: 2235-7 (1993)
BindingDB Entry DOI: 10.7270/Q2ZW1K0P |
More data for this
Ligand-Target Pair | |
Lanosterol 14-alpha demethylase
(Rattus norvegicus) | BDBM31653

(CHEMBL421109 | imidazole-dioxolane, 5)Show SMILES Nc1ccc(SC[C@@H]2CO[C@@](CCc3ccc(Cl)cc3)(Cn3ccnc3)O2)cc1 |r| Show InChI InChI=1S/C22H24ClN3O2S/c23-18-3-1-17(2-4-18)9-10-22(15-26-12-11-25-16-26)27-13-20(28-22)14-29-21-7-5-19(24)6-8-21/h1-8,11-12,16,20H,9-10,13-15,24H2/t20-,22+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 117 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Discovery Research
Curated by ChEMBL
| Assay Description
Apparent Ki for rat Lanosterol 14-alpha demethylase |
J Med Chem 36: 2235-7 (1993)
BindingDB Entry DOI: 10.7270/Q2ZW1K0P |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50044424

(4-(((2S,4S)-2-((1H-imidazol-1-yl)methyl)-2-(4-chlo...)Show SMILES Nc1ccc(SC[C@@H]2CO[C@](CCc3ccc(Cl)cc3)(Cn3ccnc3)O2)cc1 Show InChI InChI=1S/C22H24ClN3O2S/c23-18-3-1-17(2-4-18)9-10-22(15-26-12-11-25-16-26)27-13-20(28-22)14-29-21-7-5-19(24)6-8-21/h1-8,11-12,16,20H,9-10,13-15,24H2/t20-,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 447 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Discovery Research
Curated by ChEMBL
| Assay Description
Binding affinity for progesterone 17-alpha,20-lyase |
J Med Chem 36: 2235-7 (1993)
BindingDB Entry DOI: 10.7270/Q2ZW1K0P |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50044424

(4-(((2S,4S)-2-((1H-imidazol-1-yl)methyl)-2-(4-chlo...)Show SMILES Nc1ccc(SC[C@@H]2CO[C@](CCc3ccc(Cl)cc3)(Cn3ccnc3)O2)cc1 Show InChI InChI=1S/C22H24ClN3O2S/c23-18-3-1-17(2-4-18)9-10-22(15-26-12-11-25-16-26)27-13-20(28-22)14-29-21-7-5-19(24)6-8-21/h1-8,11-12,16,20H,9-10,13-15,24H2/t20-,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Discovery Research
Curated by ChEMBL
| Assay Description
Binding affinity for cholesterol 17-alpha-hydroxylase |
J Med Chem 36: 2235-7 (1993)
BindingDB Entry DOI: 10.7270/Q2ZW1K0P |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50341350
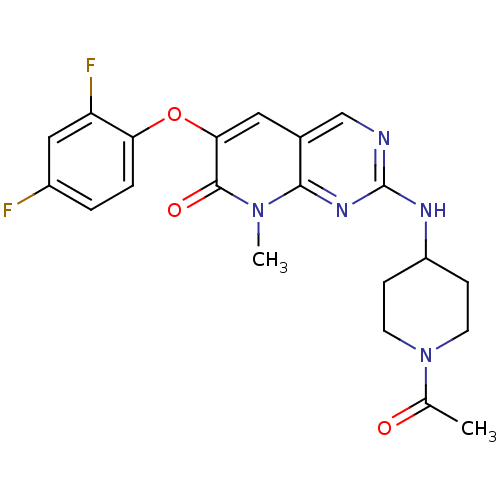
(2-(1-Acetyl-piperidin-4-ylamino)-6-(2,4-difluoroph...)Show SMILES CC(=O)N1CCC(CC1)Nc1ncc2cc(Oc3ccc(F)cc3F)c(=O)n(C)c2n1 Show InChI InChI=1S/C21H21F2N5O3/c1-12(29)28-7-5-15(6-8-28)25-21-24-11-13-9-18(20(30)27(2)19(13)26-21)31-17-4-3-14(22)10-16(17)23/h3-4,9-11,15H,5-8H2,1-2H3,(H,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant p38alpha assessed as incorporation of 33P from gamma-[33P]ATP into myelin basic protein after 30 mins by scintillatio... |
J Med Chem 54: 2255-65 (2011)
Article DOI: 10.1021/jm101423y
BindingDB Entry DOI: 10.7270/Q20P109X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50341361
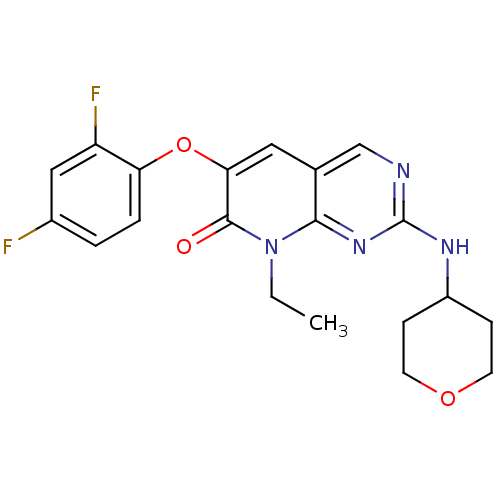
(6-(2,4-Difluorophenoxy)-8-ethyl-2-(tetrahydro-2H-p...)Show SMILES CCn1c2nc(NC3CCOCC3)ncc2cc(Oc2ccc(F)cc2F)c1=O Show InChI InChI=1S/C20H20F2N4O3/c1-2-26-18-12(11-23-20(25-18)24-14-5-7-28-8-6-14)9-17(19(26)27)29-16-4-3-13(21)10-15(16)22/h3-4,9-11,14H,2,5-8H2,1H3,(H,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant p38alpha assessed as incorporation of 33P from gamma-[33P]ATP into myelin basic protein after 30 mins by scintillatio... |
J Med Chem 54: 2255-65 (2011)
Article DOI: 10.1021/jm101423y
BindingDB Entry DOI: 10.7270/Q20P109X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50341366

(6-(2,4-Difluorophenoxy)-8-(1,1-dioxo-hexahydro-thi...)Show SMILES Fc1ccc(Oc2cc3cnc(NC4CCOCC4)nc3n(C3CCS(=O)(=O)CC3)c2=O)c(F)c1 Show InChI InChI=1S/C23H24F2N4O5S/c24-15-1-2-19(18(25)12-15)34-20-11-14-13-26-23(27-16-3-7-33-8-4-16)28-21(14)29(22(20)30)17-5-9-35(31,32)10-6-17/h1-2,11-13,16-17H,3-10H2,(H,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant p38alpha assessed as incorporation of 33P from gamma-[33P]ATP into myelin basic protein after 30 mins by scintillatio... |
J Med Chem 54: 2255-65 (2011)
Article DOI: 10.1021/jm101423y
BindingDB Entry DOI: 10.7270/Q20P109X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50341370
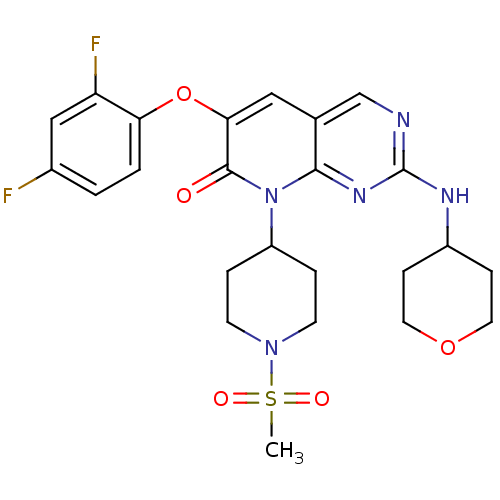
(6-(2,4-Difluorophenoxy)-8-(1-methanesulfonyl-piper...)Show SMILES CS(=O)(=O)N1CCC(CC1)n1c2nc(NC3CCOCC3)ncc2cc(Oc2ccc(F)cc2F)c1=O Show InChI InChI=1S/C24H27F2N5O5S/c1-37(33,34)30-8-4-18(5-9-30)31-22-15(14-27-24(29-22)28-17-6-10-35-11-7-17)12-21(23(31)32)36-20-3-2-16(25)13-19(20)26/h2-3,12-14,17-18H,4-11H2,1H3,(H,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant p38alpha assessed as incorporation of 33P from gamma-[33P]ATP into myelin basic protein after 30 mins by scintillatio... |
J Med Chem 54: 2255-65 (2011)
Article DOI: 10.1021/jm101423y
BindingDB Entry DOI: 10.7270/Q20P109X |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50305799

(5-((3aR,6aS)-5-(2-(1-(3,3-difluorocyclobutanecarbo...)Show SMILES Cc1cc(nc(C)c1C(=O)N1C[C@@H]2CN(CCC3(CN(C3)C(=O)C3CC(F)(F)C3)c3ccccc3)C[C@@H]2C1)C#N |r| Show InChI InChI=1S/C31H35F2N5O2/c1-20-10-26(13-34)35-21(2)27(20)29(40)37-16-23-14-36(15-24(23)17-37)9-8-30(25-6-4-3-5-7-25)18-38(19-30)28(39)22-11-31(32,33)12-22/h3-7,10,22-24H,8-9,11-12,14-19H2,1-2H3/t23-,24+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant CCR5 by cell-cell fusion assay |
Bioorg Med Chem Lett 20: 704-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.072
BindingDB Entry DOI: 10.7270/Q22R3RSC |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50329256
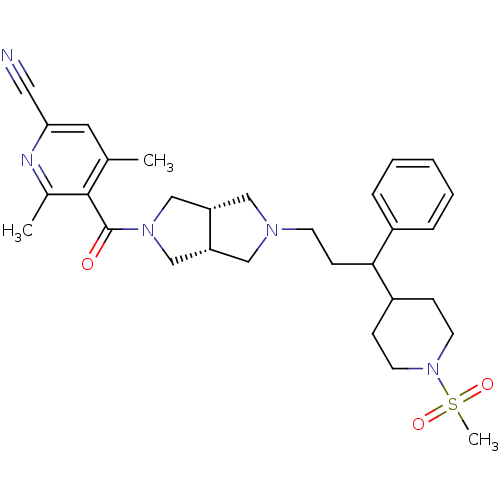
(4,6-dimethyl-5-((3aR,6aS)-5-(3-(1-(methylsulfonyl)...)Show SMILES Cc1cc(nc(C)c1C(=O)N1C[C@@H]2CN(CCC(C3CCN(CC3)S(C)(=O)=O)c3ccccc3)C[C@@H]2C1)C#N |r| Show InChI InChI=1S/C30H39N5O3S/c1-21-15-27(16-31)32-22(2)29(21)30(36)34-19-25-17-33(18-26(25)20-34)12-11-28(23-7-5-4-6-8-23)24-9-13-35(14-10-24)39(3,37)38/h4-8,15,24-26,28H,9-14,17-20H2,1-3H3/t25-,26+,28? | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Inhibition of CCR5 by cell-cell fusion inhibition assay |
Bioorg Med Chem Lett 20: 6802-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.118
BindingDB Entry DOI: 10.7270/Q29G5N1F |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50334986

(4,4-Difluoro-cyclohexanecarboxylic acid {(S)-3-[(1...)Show SMILES CC(C)c1nnc(C)n1[C@H]1C[C@@H]2CC[C@H](C1)N2CC[C@H](NC(=O)C1CCC(F)(F)CC1)c1ccccc1 |r,TLB:17:16:9.15.10:13.12| Show InChI InChI=1S/C29H41F2N5O/c1-19(2)27-34-33-20(3)36(27)25-17-23-9-10-24(18-25)35(23)16-13-26(21-7-5-4-6-8-21)32-28(37)22-11-14-29(30,31)15-12-22/h4-8,19,22-26H,9-18H2,1-3H3,(H,32,37)/t23-,24+,25-,26-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Displacement of [128I]RANTES from human CCR5 receptor coexpressed with Galphai6 in CHO cells after 2 hrs by scintillation counting |
Bioorg Med Chem Lett 20: 1674-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.080
BindingDB Entry DOI: 10.7270/Q23B6131 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50341356

(6-(2,4-Difluorophenoxy)-8-methyl-2-((S)-1-methyl-2...)Show SMILES C[C@@H](Cn1ncnn1)Nc1ncc2cc(Oc3ccc(F)cc3F)c(=O)n(C)c2n1 |r| Show InChI InChI=1S/C18H16F2N8O2/c1-10(8-28-23-9-22-26-28)24-18-21-7-11-5-15(17(29)27(2)16(11)25-18)30-14-4-3-12(19)6-13(14)20/h3-7,9-10H,8H2,1-2H3,(H,21,24,25)/t10-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant p38alpha assessed as incorporation of 33P from gamma-[33P]ATP into myelin basic protein after 30 mins by scintillatio... |
J Med Chem 54: 2255-65 (2011)
Article DOI: 10.1021/jm101423y
BindingDB Entry DOI: 10.7270/Q20P109X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50341363

(6-(2,4-Difluorophenoxy)-8-(3-hydroxy-propyl)-2-(te...)Show SMILES OCCCn1c2nc(NC3CCOCC3)ncc2cc(Oc2ccc(F)cc2F)c1=O Show InChI InChI=1S/C21H22F2N4O4/c22-14-2-3-17(16(23)11-14)31-18-10-13-12-24-21(25-15-4-8-30-9-5-15)26-19(13)27(20(18)29)6-1-7-28/h2-3,10-12,15,28H,1,4-9H2,(H,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant p38alpha assessed as incorporation of 33P from gamma-[33P]ATP into myelin basic protein after 30 mins by scintillatio... |
J Med Chem 54: 2255-65 (2011)
Article DOI: 10.1021/jm101423y
BindingDB Entry DOI: 10.7270/Q20P109X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50341369
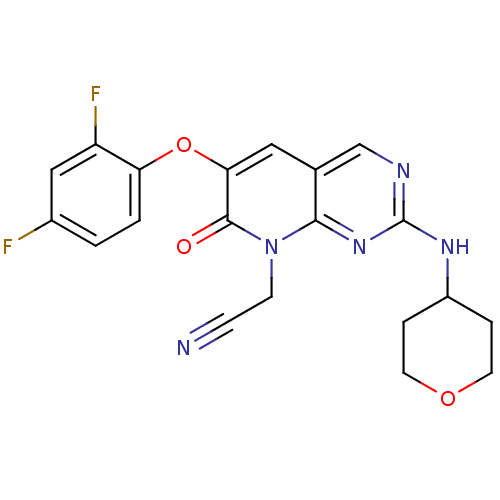
(CHEMBL1766506 | [6-(2,4-Difluorophenoxy)-7-oxo-2-(...)Show SMILES Fc1ccc(Oc2cc3cnc(NC4CCOCC4)nc3n(CC#N)c2=O)c(F)c1 Show InChI InChI=1S/C20H17F2N5O3/c21-13-1-2-16(15(22)10-13)30-17-9-12-11-24-20(25-14-3-7-29-8-4-14)26-18(12)27(6-5-23)19(17)28/h1-2,9-11,14H,3-4,6-8H2,(H,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant p38alpha assessed as incorporation of 33P from gamma-[33P]ATP into myelin basic protein after 30 mins by scintillatio... |
J Med Chem 54: 2255-65 (2011)
Article DOI: 10.1021/jm101423y
BindingDB Entry DOI: 10.7270/Q20P109X |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50305800
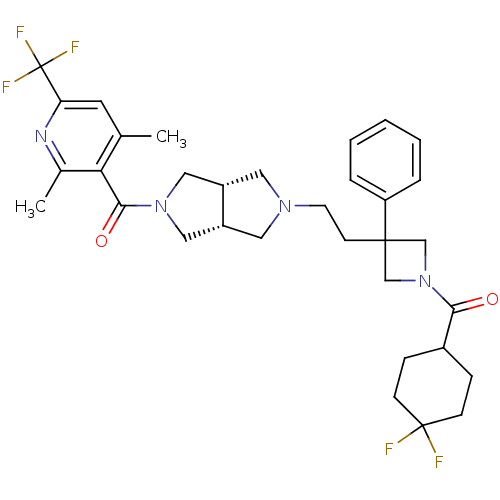
(((3aR,6aS)-5-(2-(1-(4,4-difluorocyclohexanecarbony...)Show SMILES Cc1cc(nc(C)c1C(=O)N1C[C@@H]2CN(CCC3(CN(C3)C(=O)C3CCC(F)(F)CC3)c3ccccc3)C[C@@H]2C1)C(F)(F)F |r| Show InChI InChI=1S/C33H39F5N4O2/c1-21-14-27(33(36,37)38)39-22(2)28(21)30(44)41-17-24-15-40(16-25(24)18-41)13-12-31(26-6-4-3-5-7-26)19-42(20-31)29(43)23-8-10-32(34,35)11-9-23/h3-7,14,23-25H,8-13,15-20H2,1-2H3/t24-,25+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant CCR5 by cell-cell fusion assay |
Bioorg Med Chem Lett 20: 704-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.072
BindingDB Entry DOI: 10.7270/Q22R3RSC |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50318447
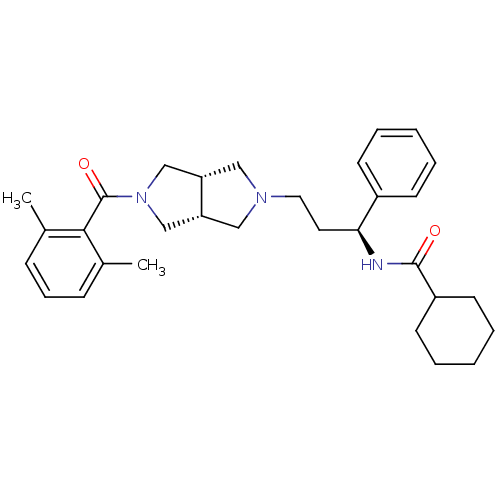
(CHEMBL1096764 | N-((S)-3-((3aR,6aS)-5-(2,6-dimethy...)Show SMILES Cc1cccc(C)c1C(=O)N1C[C@@H]2CN(CC[C@H](NC(=O)C3CCCCC3)c3ccccc3)C[C@@H]2C1 |r| Show InChI InChI=1S/C31H41N3O2/c1-22-10-9-11-23(2)29(22)31(36)34-20-26-18-33(19-27(26)21-34)17-16-28(24-12-5-3-6-13-24)32-30(35)25-14-7-4-8-15-25/h3,5-6,9-13,25-28H,4,7-8,14-21H2,1-2H3,(H,32,35)/t26-,27+,28-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Binding affinity at CCR5 receptor by radiolabeled RANTES binding assay |
Bioorg Med Chem Lett 20: 3116-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.095
BindingDB Entry DOI: 10.7270/Q27S7PQ2 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM102563
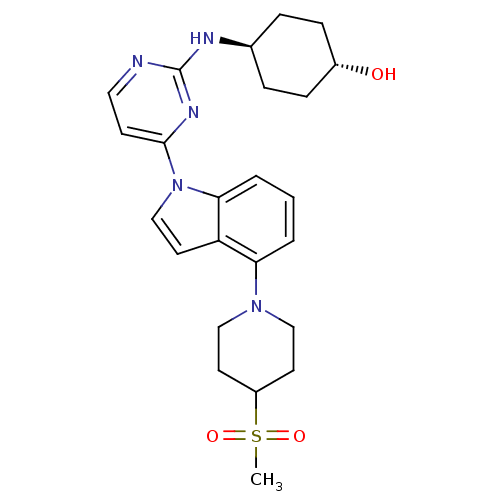
(US8536172, I-12)Show SMILES CS(=O)(=O)C1CCN(CC1)c1cccc2n(ccc12)-c1ccnc(N[C@H]2CC[C@H](O)CC2)n1 |r,wU:25.27,wD:28.31,(5.57,-4.44,;6.66,-3.35,;7.75,-2.27,;7.06,-4.84,;5.17,-2.96,;4.08,-4.04,;2.59,-3.65,;2.19,-2.16,;3.28,-1.07,;4.77,-1.47,;.71,-1.76,;-.44,-2.79,;-1.9,-2.31,;-2.22,-.81,;-1.08,.22,;-1.08,1.76,;.39,2.24,;1.29,.99,;.39,-.25,;-2.41,2.53,;-2.41,4.07,;-3.75,4.84,;-5.08,4.07,;-5.08,2.53,;-6.41,1.76,;-6.41,.22,;-5.08,-.55,;-5.08,-2.09,;-6.41,-2.86,;-6.41,-4.4,;-7.75,-2.09,;-7.75,-.55,;-3.75,1.76,)| Show InChI InChI=1S/C24H31N5O3S/c1-33(31,32)19-10-14-28(15-11-19)21-3-2-4-22-20(21)12-16-29(22)23-9-13-25-24(27-23)26-17-5-7-18(30)8-6-17/h2-4,9,12-13,16-19,30H,5-8,10-11,14-15H2,1H3,(H,25,26,27)/t17-,18- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Inhibition of human JNK1alpha1 using GST-tagged ATF2 as substrate preincubated for 10 mins prior to substrate addition measured after 30 mins by micr... |
Bioorg Med Chem Lett 23: 3565-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.029
BindingDB Entry DOI: 10.7270/Q2MG7SF1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM102557
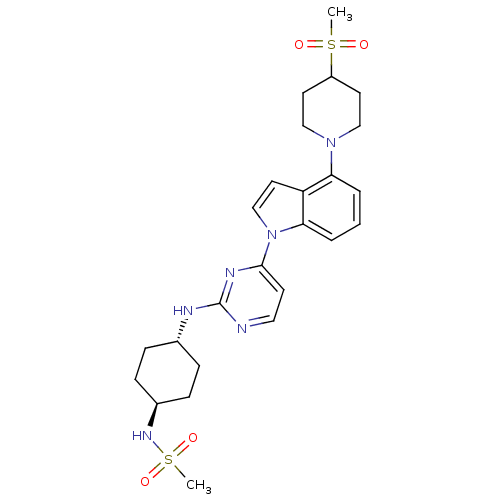
(US8536172, I-6)Show SMILES CS(=O)(=O)N[C@H]1CC[C@@H](CC1)Nc1nccc(n1)-n1ccc2c(cccc12)N1CCC(CC1)S(C)(=O)=O |r,wU:8.11,wD:5.4,(-6.41,-5.01,;-5.08,-4.24,;-5.08,-5.78,;-3.75,-3.47,;-6.41,-3.47,;-6.41,-1.93,;-5.08,-1.16,;-5.08,.38,;-6.41,1.15,;-7.75,.38,;-7.75,-1.16,;-6.41,2.69,;-5.08,3.46,;-5.08,5,;-3.75,5.78,;-2.41,5,;-2.41,3.46,;-3.75,2.69,;-1.08,2.69,;.39,3.17,;1.29,1.92,;.39,.68,;.71,-.83,;-.44,-1.86,;-1.9,-1.38,;-2.22,.12,;-1.08,1.15,;2.19,-1.23,;2.59,-2.71,;4.08,-3.11,;5.17,-2.02,;4.77,-.54,;3.28,-.14,;6.66,-2.42,;5.57,-3.51,;7.75,-1.33,;7.06,-3.91,)| Show InChI InChI=1S/C25H34N6O4S2/c1-36(32,33)20-11-15-30(16-12-20)22-4-3-5-23-21(22)13-17-31(23)24-10-14-26-25(28-24)27-18-6-8-19(9-7-18)29-37(2,34)35/h3-5,10,13-14,17-20,29H,6-9,11-12,15-16H2,1-2H3,(H,26,27,28)/t18-,19- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Inhibition of human JNK1alpha1 using GST-tagged ATF2 as substrate preincubated for 10 mins prior to substrate addition measured after 30 mins by micr... |
Bioorg Med Chem Lett 23: 3565-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.029
BindingDB Entry DOI: 10.7270/Q2MG7SF1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50305801
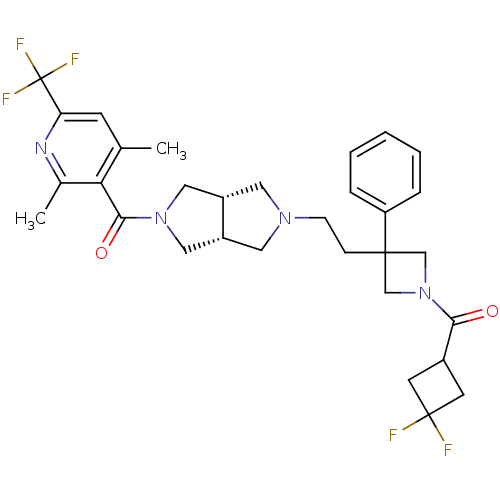
(((3aR,6aS)-5-(2-(1-(3,3-difluorocyclobutanecarbony...)Show SMILES Cc1cc(nc(C)c1C(=O)N1C[C@@H]2CN(CCC3(CN(C3)C(=O)C3CC(F)(F)C3)c3ccccc3)C[C@@H]2C1)C(F)(F)F |r| Show InChI InChI=1S/C31H35F5N4O2/c1-19-10-25(31(34,35)36)37-20(2)26(19)28(42)39-15-22-13-38(14-23(22)16-39)9-8-29(24-6-4-3-5-7-24)17-40(18-29)27(41)21-11-30(32,33)12-21/h3-7,10,21-23H,8-9,11-18H2,1-2H3/t22-,23+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant CCR5 by cell-cell fusion assay |
Bioorg Med Chem Lett 20: 704-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.072
BindingDB Entry DOI: 10.7270/Q22R3RSC |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50318446
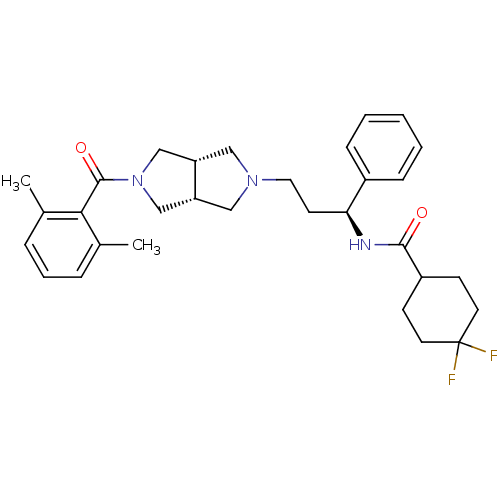
(CHEMBL1096765 | N-((S)-3-((3aR,6aS)-5-(2,6-dimethy...)Show SMILES Cc1cccc(C)c1C(=O)N1C[C@@H]2CN(CC[C@H](NC(=O)C3CCC(F)(F)CC3)c3ccccc3)C[C@@H]2C1 |r| Show InChI InChI=1S/C31H39F2N3O2/c1-21-7-6-8-22(2)28(21)30(38)36-19-25-17-35(18-26(25)20-36)16-13-27(23-9-4-3-5-10-23)34-29(37)24-11-14-31(32,33)15-12-24/h3-10,24-27H,11-20H2,1-2H3,(H,34,37)/t25-,26+,27-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Binding affinity at CCR5 receptor by radiolabeled RANTES binding assay |
Bioorg Med Chem Lett 20: 3116-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.095
BindingDB Entry DOI: 10.7270/Q27S7PQ2 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50341352
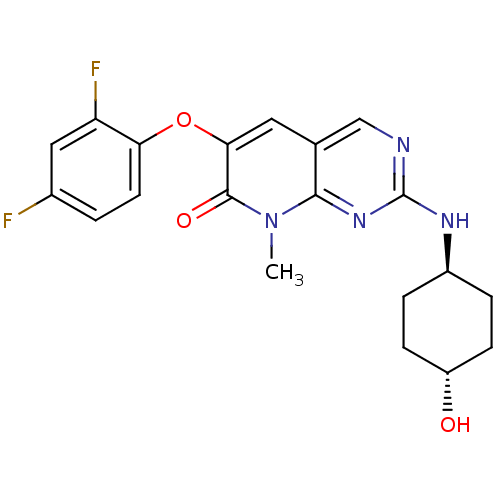
(CHEMBL1766580 | trans-6-(2,4-Difluorophenoxy)-2-(t...)Show SMILES Cn1c2nc(N[C@H]3CC[C@H](O)CC3)ncc2cc(Oc2ccc(F)cc2F)c1=O |r,wU:9.9,wD:6.5,(28.23,-9.5,;28.24,-7.96,;26.92,-7.19,;25.59,-7.95,;24.26,-7.19,;22.93,-7.96,;22.93,-9.5,;21.59,-10.26,;21.58,-11.79,;22.9,-12.57,;22.89,-14.11,;24.24,-11.81,;24.25,-10.27,;24.26,-5.65,;25.59,-4.87,;26.92,-5.65,;28.25,-4.88,;29.58,-5.66,;30.92,-4.91,;32.25,-5.69,;32.22,-7.23,;33.55,-8.01,;34.89,-7.26,;36.22,-8.04,;34.9,-5.71,;33.58,-4.93,;33.59,-3.39,;29.58,-7.2,;30.91,-7.98,)| Show InChI InChI=1S/C20H20F2N4O3/c1-26-18-11(10-23-20(25-18)24-13-3-5-14(27)6-4-13)8-17(19(26)28)29-16-7-2-12(21)9-15(16)22/h2,7-10,13-14,27H,3-6H2,1H3,(H,23,24,25)/t13-,14- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant p38alpha assessed as incorporation of 33P from gamma-[33P]ATP into myelin basic protein after 30 mins by scintillatio... |
J Med Chem 54: 2255-65 (2011)
Article DOI: 10.1021/jm101423y
BindingDB Entry DOI: 10.7270/Q20P109X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50341372
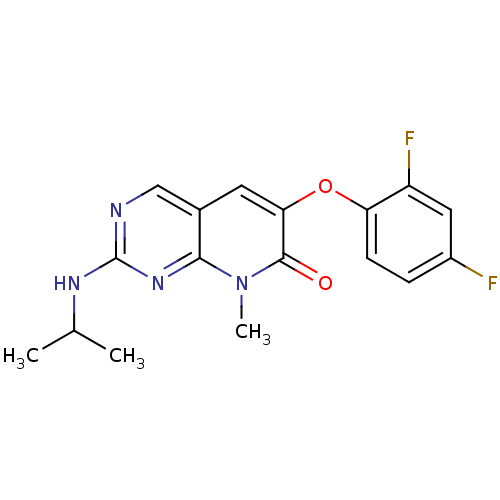
(6-(2,4-Difluorophenoxy)-2-isopropylamino-8-methyl-...)Show InChI InChI=1S/C17H16F2N4O2/c1-9(2)21-17-20-8-10-6-14(16(24)23(3)15(10)22-17)25-13-5-4-11(18)7-12(13)19/h4-9H,1-3H3,(H,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant p38alpha assessed as incorporation of 33P from gamma-[33P]ATP into myelin basic protein after 30 mins by scintillatio... |
J Med Chem 54: 2255-65 (2011)
Article DOI: 10.1021/jm101423y
BindingDB Entry DOI: 10.7270/Q20P109X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50310739
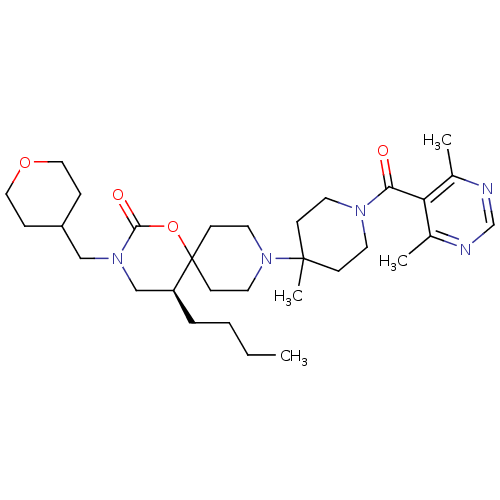
((S)-5-butyl-9-(1-(4,6-dimethylpyrimidine-5-carbony...)Show SMILES CCCC[C@H]1CN(CC2CCOCC2)C(=O)OC11CCN(CC1)C1(C)CCN(CC1)C(=O)c1c(C)ncnc1C |r| Show InChI InChI=1S/C31H49N5O4/c1-5-6-7-26-21-35(20-25-8-18-39-19-9-25)29(38)40-31(26)12-16-36(17-13-31)30(4)10-14-34(15-11-30)28(37)27-23(2)32-22-33-24(27)3/h22,25-26H,5-21H2,1-4H3/t26-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Displacement of [128I]-RANTES from CCR5 receptor receptors expressed in CHO cells |
Bioorg Med Chem Lett 20: 1830-3 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.004
BindingDB Entry DOI: 10.7270/Q25Q4W7T |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM102560

(US8536172, I-9)Show SMILES CS(=O)(=O)N[C@H]1CC[C@@H](CC1)Nc1nccc(n1)-n1ccc2c(cccc12)N1CCN(CC1)C(=O)CC#N |r,wU:8.11,wD:5.4,(-9.56,-3.47,;-8.22,-4.24,;-8.22,-5.78,;-6.89,-5.01,;-6.89,-3.47,;-6.89,-1.93,;-5.55,-1.16,;-5.55,.38,;-6.89,1.15,;-8.22,.38,;-8.22,-1.16,;-6.89,2.69,;-5.55,3.46,;-5.55,5,;-4.22,5.78,;-2.89,5,;-2.89,3.46,;-4.22,2.69,;-1.55,2.69,;-.09,3.17,;.82,1.92,;-.09,.68,;.23,-.83,;-.91,-1.86,;-2.38,-1.38,;-2.7,.12,;-1.55,1.15,;1.72,-1.23,;2.12,-2.71,;3.61,-3.11,;4.69,-2.02,;4.3,-.54,;2.81,-.14,;6.18,-2.42,;7.27,-1.33,;6.58,-3.91,;8.07,-4.31,;9.56,-4.71,)| Show InChI InChI=1S/C26H32N8O3S/c1-38(36,37)31-20-7-5-19(6-8-20)29-26-28-13-10-24(30-26)34-14-11-21-22(3-2-4-23(21)34)32-15-17-33(18-16-32)25(35)9-12-27/h2-4,10-11,13-14,19-20,31H,5-9,15-18H2,1H3,(H,28,29,30)/t19-,20- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Inhibition of human JNK1alpha1 using GST-tagged ATF2 as substrate preincubated for 10 mins prior to substrate addition measured after 30 mins by micr... |
Bioorg Med Chem Lett 23: 3565-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.029
BindingDB Entry DOI: 10.7270/Q2MG7SF1 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50341346
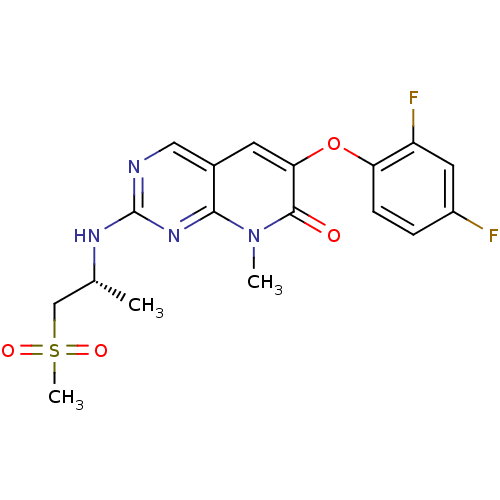
((R)-6-(2,4-difluorophenoxy)-8-methyl-2-(1-(methyls...)Show SMILES C[C@H](CS(C)(=O)=O)Nc1ncc2cc(Oc3ccc(F)cc3F)c(=O)n(C)c2n1 |r| Show InChI InChI=1S/C18H18F2N4O4S/c1-10(9-29(3,26)27)22-18-21-8-11-6-15(17(25)24(2)16(11)23-18)28-14-5-4-12(19)7-13(14)20/h4-8,10H,9H2,1-3H3,(H,21,22,23)/t10-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant p38alpha assessed as incorporation of 33P from gamma-[33P]ATP into myelin basic protein after 30 mins by scintillatio... |
J Med Chem 54: 2255-65 (2011)
Article DOI: 10.1021/jm101423y
BindingDB Entry DOI: 10.7270/Q20P109X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50312846
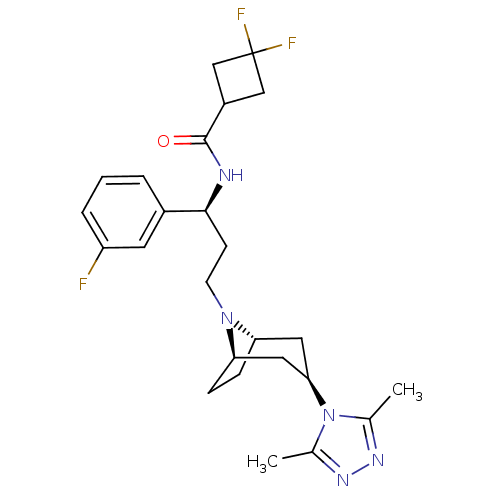
(CHEMBL1087535 | N-((S)-3-((1R,3S,5S)-3-(3,5-dimeth...)Show SMILES Cc1nnc(C)n1[C@@H]1C[C@@H]2CC[C@H](C1)N2CC[C@H](NC(=O)C1CC(F)(F)C1)c1cccc(F)c1 |r,THB:15:14:13.7.8:11.10| Show InChI InChI=1S/C25H32F3N5O/c1-15-30-31-16(2)33(15)22-11-20-6-7-21(12-22)32(20)9-8-23(17-4-3-5-19(26)10-17)29-24(34)18-13-25(27,28)14-18/h3-5,10,18,20-23H,6-9,11-14H2,1-2H3,(H,29,34)/t20-,21+,22+,23-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Displacement of [128I]RANTES from human CCR5 receptor coexpressed with Galphai6 in CHO cells after 2 hrs by scintillation counting |
Bioorg Med Chem Lett 20: 1674-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.080
BindingDB Entry DOI: 10.7270/Q23B6131 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
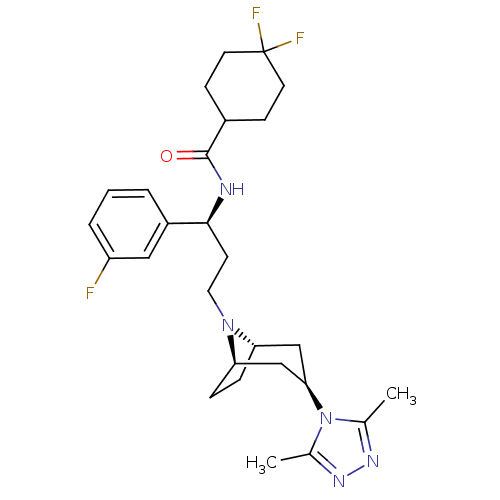
(Homo sapiens (Human)) | BDBM50312848
(CHEMBL1087153 | N-((S)-3-((1R,3S,5S)-3-(3,5-dimeth...)Show SMILES Cc1nnc(C)n1[C@@H]1C[C@@H]2CC[C@H](C1)N2CC[C@H](NC(=O)C1CCC(F)(F)CC1)c1cccc(F)c1 |r,THB:15:14:13.7.8:11.10| Show InChI InChI=1S/C27H36F3N5O/c1-17-32-33-18(2)35(17)24-15-22-6-7-23(16-24)34(22)13-10-25(20-4-3-5-21(28)14-20)31-26(36)19-8-11-27(29,30)12-9-19/h3-5,14,19,22-25H,6-13,15-16H2,1-2H3,(H,31,36)/t22-,23+,24+,25-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Displacement of [128I]RANTES from human CCR5 receptor coexpressed with Galphai6 in CHO cells after 2 hrs by scintillation counting |
Bioorg Med Chem Lett 20: 1674-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.080
BindingDB Entry DOI: 10.7270/Q23B6131 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50305794

(((3aR,6aS)-5-(2-(1-(4,4-difluorocyclohexanecarbony...)Show SMILES Cc1nc(nc(C)c1C(=O)N1C[C@@H]2CN(CCC3(CN(C3)C(=O)C3CCC(F)(F)CC3)c3ccccc3)C[C@@H]2C1)C(F)(F)F |r| Show InChI InChI=1S/C32H38F5N5O2/c1-20-26(21(2)39-29(38-20)32(35,36)37)28(44)41-16-23-14-40(15-24(23)17-41)13-12-30(25-6-4-3-5-7-25)18-42(19-30)27(43)22-8-10-31(33,34)11-9-22/h3-7,22-24H,8-19H2,1-2H3/t23-,24+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant CCR5 by cell-cell fusion assay |
Bioorg Med Chem Lett 20: 704-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.072
BindingDB Entry DOI: 10.7270/Q22R3RSC |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50318451

(CHEMBL1096445 | N-((S)-3-((3aR,6aS)-5-(2,6-dimethy...)Show SMILES Cc1cccc(C)c1C(=O)N1C[C@@H]2CN(CC[C@H](NC(=O)C3CCCC3)c3ccccc3)C[C@@H]2C1 |r| Show InChI InChI=1S/C30H39N3O2/c1-21-9-8-10-22(2)28(21)30(35)33-19-25-17-32(18-26(25)20-33)16-15-27(23-11-4-3-5-12-23)31-29(34)24-13-6-7-14-24/h3-5,8-12,24-27H,6-7,13-20H2,1-2H3,(H,31,34)/t25-,26+,27-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Binding affinity at CCR5 receptor by radiolabeled RANTES binding assay |
Bioorg Med Chem Lett 20: 3116-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.095
BindingDB Entry DOI: 10.7270/Q27S7PQ2 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM102617
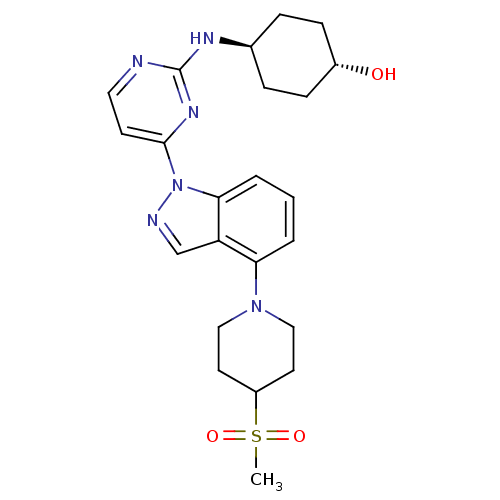
(US8536172, I-66)Show SMILES CS(=O)(=O)C1CCN(CC1)c1cccc2n(ncc12)-c1ccnc(N[C@H]2CC[C@H](O)CC2)n1 |r,wU:25.27,wD:28.31,(10.53,3.6,;9.19,2.83,;8.42,4.17,;9.96,1.5,;7.86,2.06,;7.86,.52,;6.52,-.25,;5.19,.52,;5.19,2.06,;6.52,2.83,;3.86,-.25,;3.86,-1.79,;2.52,-2.56,;1.19,-1.79,;1.19,-.25,;.04,.78,;.67,2.19,;2.2,2.03,;2.52,.52,;-1.5,.78,;-2.27,2.12,;-3.81,2.12,;-4.58,.78,;-3.81,-.55,;-4.58,-1.88,;-6.06,-2.28,;-6.46,-3.77,;-7.95,-4.17,;-9.04,-3.08,;-10.53,-3.48,;-8.64,-1.59,;-7.15,-1.19,;-2.27,-.55,)| Show InChI InChI=1S/C23H30N6O3S/c1-33(31,32)18-10-13-28(14-11-18)20-3-2-4-21-19(20)15-25-29(21)22-9-12-24-23(27-22)26-16-5-7-17(30)8-6-16/h2-4,9,12,15-18,30H,5-8,10-11,13-14H2,1H3,(H,24,26,27)/t16-,17- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Inhibition of human JNK1alpha1 using GST-tagged ATF2 as substrate preincubated for 10 mins prior to substrate addition measured after 30 mins by micr... |
Bioorg Med Chem Lett 23: 3565-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.029
BindingDB Entry DOI: 10.7270/Q2MG7SF1 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50341365

(6-(2,4-Difluorophenoxy)-8-(3-methanesulfonyl-propy...)Show SMILES CS(=O)(=O)CCCn1c2nc(NC3CCOCC3)ncc2cc(Oc2ccc(F)cc2F)c1=O Show InChI InChI=1S/C22H24F2N4O5S/c1-34(30,31)10-2-7-28-20-14(13-25-22(27-20)26-16-5-8-32-9-6-16)11-19(21(28)29)33-18-4-3-15(23)12-17(18)24/h3-4,11-13,16H,2,5-10H2,1H3,(H,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant p38alpha assessed as incorporation of 33P from gamma-[33P]ATP into myelin basic protein after 30 mins by scintillatio... |
J Med Chem 54: 2255-65 (2011)
Article DOI: 10.1021/jm101423y
BindingDB Entry DOI: 10.7270/Q20P109X |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
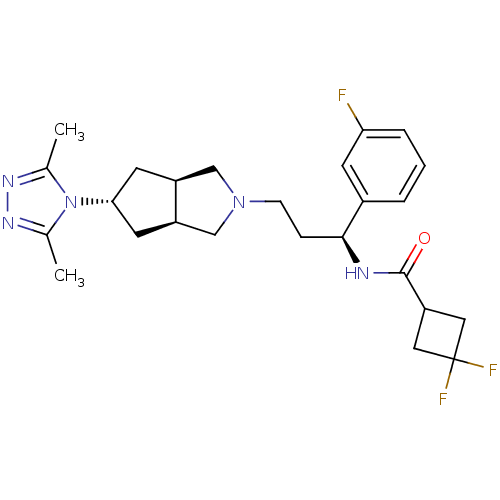
(Homo sapiens (Human)) | BDBM50312852
(CHEMBL1081476 | Exo-N-((S)-3-(5-(3,5-dimethyl-4H-1...)Show SMILES Cc1nnc(C)n1[C@@H]1C[C@H]2CN(CC[C@H](NC(=O)C3CC(F)(F)C3)c3cccc(F)c3)C[C@H]2C1 |r| Show InChI InChI=1S/C25H32F3N5O/c1-15-30-31-16(2)33(15)22-9-18-13-32(14-19(18)10-22)7-6-23(17-4-3-5-21(26)8-17)29-24(34)20-11-25(27,28)12-20/h3-5,8,18-20,22-23H,6-7,9-14H2,1-2H3,(H,29,34)/t18-,19+,22+,23-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Displacement of [128I]RANTES from human CCR5 receptor coexpressed with Galphai6 in CHO cells after 2 hrs by scintillation counting |
Bioorg Med Chem Lett 20: 1674-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.080
BindingDB Entry DOI: 10.7270/Q23B6131 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
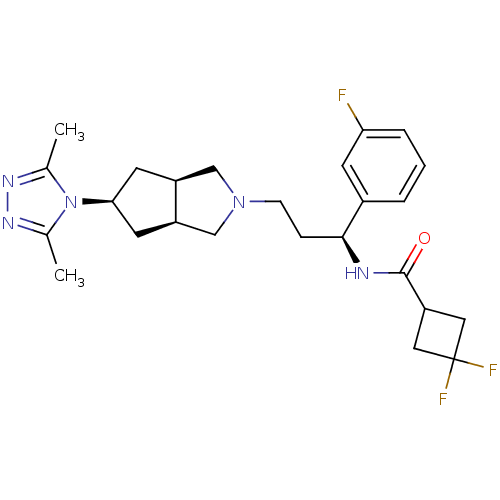
(Homo sapiens (Human)) | BDBM50312850
(CHEMBL1082191 | Endo-N-((S)-3-(5-(3,5-dimethyl-4H-...)Show SMILES Cc1nnc(C)n1[C@H]1C[C@H]2CN(CC[C@H](NC(=O)C3CC(F)(F)C3)c3cccc(F)c3)C[C@H]2C1 |r| Show InChI InChI=1S/C25H32F3N5O/c1-15-30-31-16(2)33(15)22-9-18-13-32(14-19(18)10-22)7-6-23(17-4-3-5-21(26)8-17)29-24(34)20-11-25(27,28)12-20/h3-5,8,18-20,22-23H,6-7,9-14H2,1-2H3,(H,29,34)/t18-,19+,22-,23-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Displacement of [128I]RANTES from human CCR5 receptor coexpressed with Galphai6 in CHO cells after 2 hrs by scintillation counting |
Bioorg Med Chem Lett 20: 1674-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.080
BindingDB Entry DOI: 10.7270/Q23B6131 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
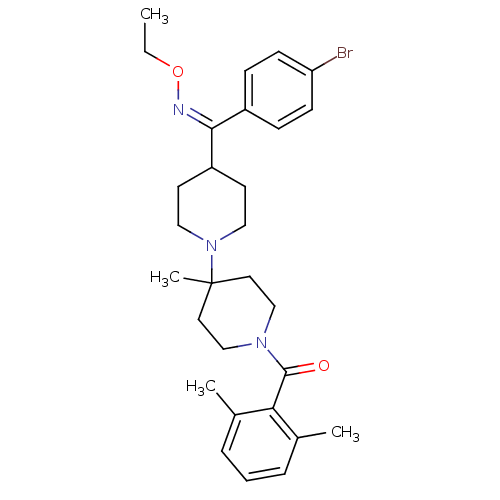
(Homo sapiens (Human)) | BDBM50115528
((Z)-(4-((4-bromophenyl)(ethoxyimino)methyl)-4'-met...)Show SMILES CCO\N=C(\C1CCN(CC1)C1(C)CCN(CC1)C(=O)c1c(C)cccc1C)c1ccc(Br)cc1 Show InChI InChI=1S/C29H38BrN3O2/c1-5-35-31-27(23-9-11-25(30)12-10-23)24-13-17-33(18-14-24)29(4)15-19-32(20-16-29)28(34)26-21(2)7-6-8-22(26)3/h6-12,24H,5,13-20H2,1-4H3/b31-27+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Binding affinity at CCR5 receptor by radiolabeled RANTES binding assay |
Bioorg Med Chem Lett 20: 3116-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.095
BindingDB Entry DOI: 10.7270/Q27S7PQ2 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50318439

(CHEMBL1097815 | N-((S)-3-((3aR,6aS)-5-(4-fluoro-2,...)Show SMILES Cc1cc(F)cc(C)c1C(=O)N1C[C@@H]2CN(CC[C@H](NC(=O)C3CCCC3)c3ccccc3)C[C@@H]2C1 |r| Show InChI InChI=1S/C30H38FN3O2/c1-20-14-26(31)15-21(2)28(20)30(36)34-18-24-16-33(17-25(24)19-34)13-12-27(22-8-4-3-5-9-22)32-29(35)23-10-6-7-11-23/h3-5,8-9,14-15,23-25,27H,6-7,10-13,16-19H2,1-2H3,(H,32,35)/t24-,25+,27-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Binding affinity at CCR5 receptor by radiolabeled RANTES binding assay |
Bioorg Med Chem Lett 20: 3116-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.095
BindingDB Entry DOI: 10.7270/Q27S7PQ2 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
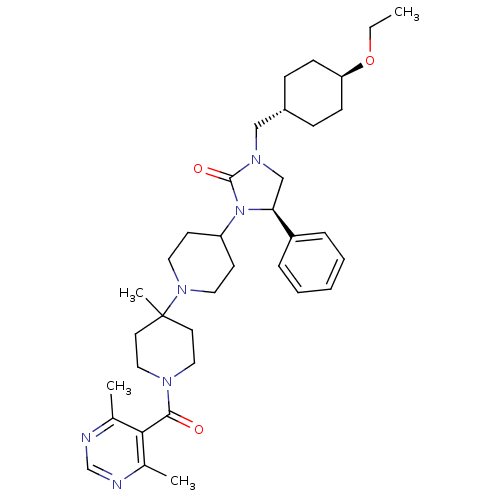
(Homo sapiens (Human)) | BDBM50319434
((R)-3-(1'-(4,6-dimethylpyrimidine-5-carbonyl)-4'-m...)Show SMILES CCO[C@H]1CC[C@H](CN2C[C@H](N(C3CCN(CC3)C3(C)CCN(CC3)C(=O)c3c(C)ncnc3C)C2=O)c2ccccc2)CC1 |r,wU:10.40,6.6,wD:3.2,(13.9,-8.06,;13.92,-6.52,;15.26,-5.76,;15.27,-4.22,;16.61,-3.46,;16.63,-1.92,;15.29,-1.14,;15.3,.4,;16.64,1.16,;16.57,2.7,;18.01,3.24,;18.97,2.04,;20.51,2.11,;21.23,3.48,;22.77,3.56,;23.6,2.25,;22.89,.87,;21.34,.81,;25.13,2.32,;24.35,3.65,;25.96,1.01,;27.49,1.07,;28.21,2.44,;27.38,3.74,;25.84,3.68,;29.75,2.5,;30.46,3.86,;30.57,1.19,;32.1,1.26,;32.81,2.63,;32.92,-.04,;32.21,-1.41,;30.67,-1.47,;29.85,-.17,;28.31,-.22,;18.12,.75,;18.67,-.69,;18.79,4.57,;20.33,4.56,;21.11,5.89,;20.34,7.22,;18.8,7.23,;18.03,5.9,;13.96,-1.9,;13.95,-3.44,)| Show InChI InChI=1S/C36H52N6O3/c1-5-45-31-13-11-28(12-14-31)23-40-24-32(29-9-7-6-8-10-29)42(35(40)44)30-15-19-41(20-16-30)36(4)17-21-39(22-18-36)34(43)33-26(2)37-25-38-27(33)3/h6-10,25,28,30-32H,5,11-24H2,1-4H3/t28-,31-,32-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Binding affinity at CCR5 receptor by radiolabelled RANTES binding assay |
Bioorg Med Chem Lett 20: 3219-22 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.077
BindingDB Entry DOI: 10.7270/Q2FT8M63 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data