 Found 214 hits with Last Name = 'rubenstein' and Initial = 's'
Found 214 hits with Last Name = 'rubenstein' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM28681

(5-[(4-{2-[methyl(pyridin-2-yl)amino]ethoxy}phenyl)...)Show InChI InChI=1S/C18H19N3O3S/c1-21(16-4-2-3-9-19-16)10-11-24-14-7-5-13(6-8-14)12-15-17(22)20-18(23)25-15/h2-9,22H,10-12H2,1H3,(H,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-rosiglitazone from GST-tagged PPARgammaLBD (unknown origin) after 1 hr by scintillation proximity assay |
Bioorg Med Chem 21: 979-92 (2013)
Article DOI: 10.1016/j.bmc.2012.11.058
BindingDB Entry DOI: 10.7270/Q23F4R13 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50428877
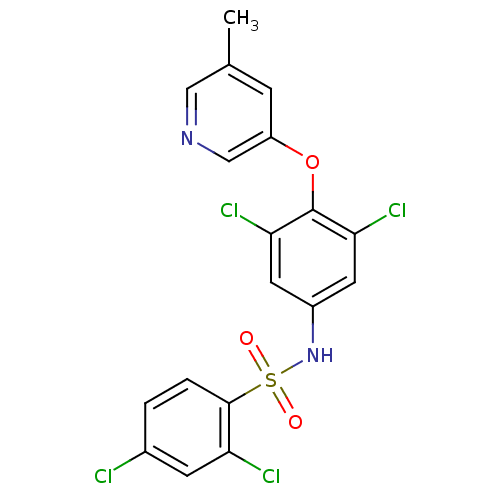
(CHEMBL2338480)Show SMILES Cc1cncc(Oc2c(Cl)cc(NS(=O)(=O)c3ccc(Cl)cc3Cl)cc2Cl)c1 Show InChI InChI=1S/C18H12Cl4N2O3S/c1-10-4-13(9-23-8-10)27-18-15(21)6-12(7-16(18)22)24-28(25,26)17-3-2-11(19)5-14(17)20/h2-9,24H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem 21: 979-92 (2013)
Article DOI: 10.1016/j.bmc.2012.11.058
BindingDB Entry DOI: 10.7270/Q23F4R13 |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50003815
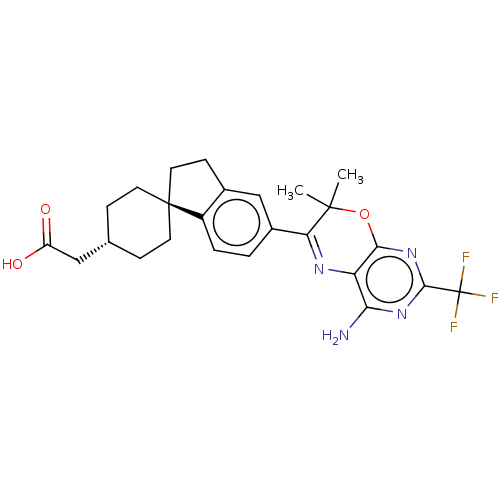
(CHEMBL3235321)Show SMILES CC1(C)Oc2nc(nc(N)c2N=C1c1ccc2c(CC[C@@]22CC[C@H](CC(O)=O)CC2)c1)C(F)(F)F |r,wU:20.22,wD:23.26,c:12,(27.23,-21.22,;25.69,-21.22,;26.46,-22.55,;24.36,-22,;23.03,-21.23,;21.69,-22.01,;20.36,-21.24,;20.36,-19.69,;21.69,-18.92,;21.68,-17.38,;23.02,-19.69,;24.35,-18.91,;25.69,-19.67,;27.02,-18.9,;27,-17.37,;28.32,-16.58,;29.67,-17.35,;29.68,-18.88,;31.15,-19.35,;32.04,-18.1,;31.12,-16.86,;30.02,-15.78,;30.42,-14.29,;31.91,-13.88,;32.3,-12.39,;33.79,-11.99,;34.89,-13.08,;34.19,-10.5,;33,-14.97,;32.61,-16.45,;28.35,-19.66,;19.02,-22.01,;17.69,-21.24,;19.02,-23.55,;17.68,-22.77,)| Show InChI InChI=1S/C25H27F3N4O3/c1-23(2)19(30-18-20(29)31-22(25(26,27)28)32-21(18)35-23)15-3-4-16-14(12-15)7-10-24(16)8-5-13(6-9-24)11-17(33)34/h3-4,12-13H,5-11H2,1-2H3,(H,33,34)(H2,29,31,32)/t13-,24- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of DGAT1 (unknown origin) by cell-based assay |
J Med Chem 57: 3464-83 (2014)
Article DOI: 10.1021/jm500135c
BindingDB Entry DOI: 10.7270/Q23X886W |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM27947
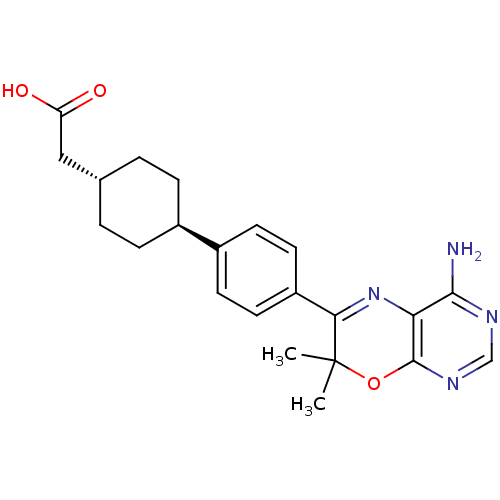
(2-[4-(4-{4-amino-7,7-dimethyl-7H-pyrimido[4,5-b][1...)Show SMILES CC1(C)Oc2ncnc(N)c2N=C1c1ccc(cc1)[C@H]1CC[C@H](CC(O)=O)CC1 |r,wU:19.21,wD:22.25,c:12,(1.74,.99,;.97,2.32,;2.46,2.72,;-.37,1.55,;-1.7,2.32,;-3.03,1.55,;-4.37,2.32,;-4.37,3.86,;-3.03,4.63,;-3.03,6.17,;-1.7,3.86,;-.37,4.63,;.97,3.86,;2.3,4.63,;2.3,6.17,;3.63,6.94,;4.97,6.17,;4.97,4.63,;3.63,3.86,;6.19,6.75,;6.19,8.3,;7.53,9.07,;8.86,8.3,;10.19,9.07,;11.53,8.3,;12.86,9.07,;11.53,6.76,;8.86,6.75,;7.53,5.98,)| Show InChI InChI=1S/C22H26N4O3/c1-22(2)19(26-18-20(23)24-12-25-21(18)29-22)16-9-7-15(8-10-16)14-5-3-13(4-6-14)11-17(27)28/h7-10,12-14H,3-6,11H2,1-2H3,(H,27,28)(H2,23,24,25)/t13-,14- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of DGAT1 (unknown origin) by cell-based assay |
J Med Chem 57: 3464-83 (2014)
Article DOI: 10.1021/jm500135c
BindingDB Entry DOI: 10.7270/Q23X886W |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Diacylglycerol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50003815

(CHEMBL3235321)Show SMILES CC1(C)Oc2nc(nc(N)c2N=C1c1ccc2c(CC[C@@]22CC[C@H](CC(O)=O)CC2)c1)C(F)(F)F |r,wU:20.22,wD:23.26,c:12,(27.23,-21.22,;25.69,-21.22,;26.46,-22.55,;24.36,-22,;23.03,-21.23,;21.69,-22.01,;20.36,-21.24,;20.36,-19.69,;21.69,-18.92,;21.68,-17.38,;23.02,-19.69,;24.35,-18.91,;25.69,-19.67,;27.02,-18.9,;27,-17.37,;28.32,-16.58,;29.67,-17.35,;29.68,-18.88,;31.15,-19.35,;32.04,-18.1,;31.12,-16.86,;30.02,-15.78,;30.42,-14.29,;31.91,-13.88,;32.3,-12.39,;33.79,-11.99,;34.89,-13.08,;34.19,-10.5,;33,-14.97,;32.61,-16.45,;28.35,-19.66,;19.02,-22.01,;17.69,-21.24,;19.02,-23.55,;17.68,-22.77,)| Show InChI InChI=1S/C25H27F3N4O3/c1-23(2)19(30-18-20(29)31-22(25(26,27)28)32-21(18)35-23)15-3-4-16-14(12-15)7-10-24(16)8-5-13(6-9-24)11-17(33)34/h3-4,12-13H,5-11H2,1-2H3,(H,33,34)(H2,29,31,32)/t13-,24- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human liver flag-tagged DGAT1 expressed in baculovirus infected insect cells after 2 hrs by SPA |
J Med Chem 57: 3464-83 (2014)
Article DOI: 10.1021/jm500135c
BindingDB Entry DOI: 10.7270/Q23X886W |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50003860

(CHEMBL3235317)Show SMILES CC1(C)Oc2nc(nc(N)c2N=C1c1ccc(cc1)[C@H]1CC[C@H](CC(O)=O)CC1)C(F)(F)F |r,wU:19.21,wD:22.25,c:12,(27.64,-8.52,;26.1,-8.52,;26.87,-9.85,;24.77,-9.3,;23.44,-8.53,;22.1,-9.31,;20.77,-8.54,;20.77,-6.99,;22.1,-6.22,;22.1,-4.68,;23.44,-6.99,;24.76,-6.21,;26.1,-6.97,;27.43,-6.19,;28.76,-6.96,;30.09,-6.19,;30.08,-4.64,;28.73,-3.88,;27.41,-4.66,;31.41,-3.86,;31.39,-2.32,;32.72,-1.55,;34.06,-2.31,;35.39,-1.54,;36.73,-2.3,;36.74,-3.84,;38.06,-1.53,;34.06,-3.85,;32.74,-4.63,;19.44,-9.31,;18.1,-8.53,;19.43,-10.85,;18.09,-10.06,)| Show InChI InChI=1S/C23H25F3N4O3/c1-22(2)18(28-17-19(27)29-21(23(24,25)26)30-20(17)33-22)15-9-7-14(8-10-15)13-5-3-12(4-6-13)11-16(31)32/h7-10,12-13H,3-6,11H2,1-2H3,(H,31,32)(H2,27,29,30)/t12-,13- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human liver flag-tagged DGAT1 expressed in baculovirus infected insect cells after 2 hrs by SPA |
J Med Chem 57: 3464-83 (2014)
Article DOI: 10.1021/jm500135c
BindingDB Entry DOI: 10.7270/Q23X886W |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50003857
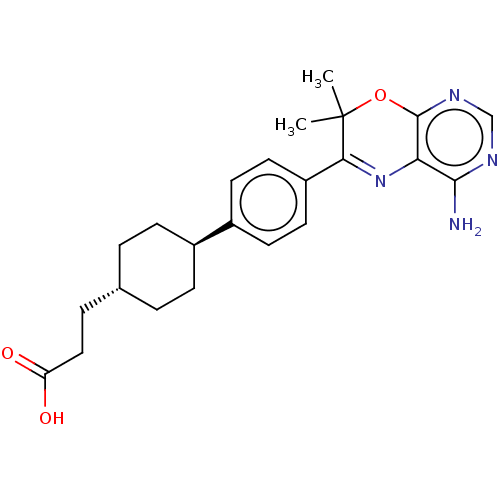
(CHEMBL3235314)Show SMILES CC1(C)Oc2ncnc(N)c2N=C1c1ccc(cc1)[C@H]1CC[C@H](CCC(O)=O)CC1 |r,wU:19.21,wD:22.25,c:12,(25.63,-58.39,;24.09,-58.39,;24.86,-59.72,;22.76,-59.17,;21.42,-58.4,;20.09,-59.18,;18.75,-58.41,;18.76,-56.86,;20.09,-56.09,;20.08,-54.55,;21.42,-56.86,;22.75,-56.08,;24.08,-56.84,;25.41,-56.06,;26.75,-56.83,;28.07,-56.06,;28.07,-54.51,;26.72,-53.75,;25.4,-54.53,;29.39,-53.73,;29.37,-52.2,;30.71,-51.42,;32.05,-52.18,;33.38,-51.41,;34.71,-52.17,;34.72,-53.71,;33.39,-54.49,;36.06,-54.48,;32.05,-53.72,;30.73,-54.5,)| Show InChI InChI=1S/C23H28N4O3/c1-23(2)20(27-19-21(24)25-13-26-22(19)30-23)17-10-8-16(9-11-17)15-6-3-14(4-7-15)5-12-18(28)29/h8-11,13-15H,3-7,12H2,1-2H3,(H,28,29)(H2,24,25,26)/t14-,15- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human liver flag-tagged DGAT1 expressed in baculovirus infected insect cells after 2 hrs by SPA |
J Med Chem 57: 3464-83 (2014)
Article DOI: 10.1021/jm500135c
BindingDB Entry DOI: 10.7270/Q23X886W |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM27947

(2-[4-(4-{4-amino-7,7-dimethyl-7H-pyrimido[4,5-b][1...)Show SMILES CC1(C)Oc2ncnc(N)c2N=C1c1ccc(cc1)[C@H]1CC[C@H](CC(O)=O)CC1 |r,wU:19.21,wD:22.25,c:12,(1.74,.99,;.97,2.32,;2.46,2.72,;-.37,1.55,;-1.7,2.32,;-3.03,1.55,;-4.37,2.32,;-4.37,3.86,;-3.03,4.63,;-3.03,6.17,;-1.7,3.86,;-.37,4.63,;.97,3.86,;2.3,4.63,;2.3,6.17,;3.63,6.94,;4.97,6.17,;4.97,4.63,;3.63,3.86,;6.19,6.75,;6.19,8.3,;7.53,9.07,;8.86,8.3,;10.19,9.07,;11.53,8.3,;12.86,9.07,;11.53,6.76,;8.86,6.75,;7.53,5.98,)| Show InChI InChI=1S/C22H26N4O3/c1-22(2)19(26-18-20(23)24-12-25-21(18)29-22)16-9-7-15(8-10-16)14-5-3-13(4-6-14)11-17(27)28/h7-10,12-14H,3-6,11H2,1-2H3,(H,27,28)(H2,23,24,25)/t13-,14- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human liver flag-tagged DGAT1 expressed in baculovirus infected insect cells after 2 hrs by SPA |
J Med Chem 57: 3464-83 (2014)
Article DOI: 10.1021/jm500135c
BindingDB Entry DOI: 10.7270/Q23X886W |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Diacylglycerol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50003858
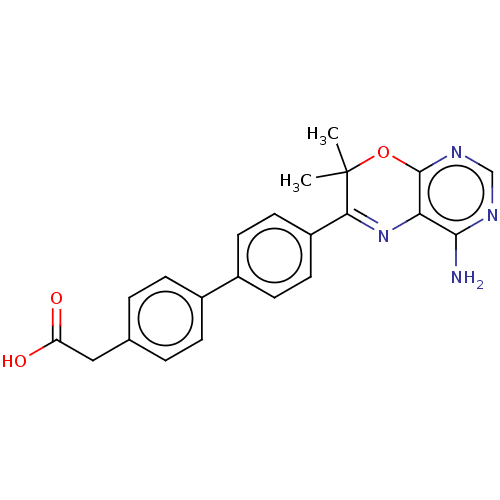
(CHEMBL3235315)Show SMILES CC1(C)Oc2ncnc(N)c2N=C1c1ccc(cc1)-c1ccc(CC(O)=O)cc1 |c:12| Show InChI InChI=1S/C22H20N4O3/c1-22(2)19(26-18-20(23)24-12-25-21(18)29-22)16-9-7-15(8-10-16)14-5-3-13(4-6-14)11-17(27)28/h3-10,12H,11H2,1-2H3,(H,27,28)(H2,23,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human liver flag-tagged DGAT1 expressed in baculovirus infected insect cells after 2 hrs by SPA |
J Med Chem 57: 3464-83 (2014)
Article DOI: 10.1021/jm500135c
BindingDB Entry DOI: 10.7270/Q23X886W |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50003862

(CHEMBL3235319)Show SMILES CC1(C)Oc2ncnc(N)c2N=C1c1ccc(cc1)[C@H]1CC[C@H](Cc2nnn[nH]2)CC1 |r,wU:19.21,wD:22.25,c:12,(58.66,-9.38,;57.12,-9.38,;57.89,-10.71,;55.79,-10.16,;54.46,-9.39,;53.12,-10.17,;51.79,-9.4,;51.79,-7.86,;53.12,-7.08,;53.11,-5.54,;54.45,-7.85,;55.78,-7.08,;57.12,-7.84,;58.45,-7.06,;59.78,-7.83,;61.11,-7.05,;61.1,-5.51,;59.75,-4.75,;58.43,-5.53,;62.43,-4.73,;62.4,-3.19,;63.74,-2.41,;65.08,-3.18,;66.41,-2.4,;67.75,-3.17,;69.14,-2.53,;70.18,-3.67,;69.41,-5.01,;67.9,-4.7,;65.08,-4.72,;63.76,-5.49,)| Show InChI InChI=1S/C22H26N8O/c1-22(2)19(26-18-20(23)24-12-25-21(18)31-22)16-9-7-15(8-10-16)14-5-3-13(4-6-14)11-17-27-29-30-28-17/h7-10,12-14H,3-6,11H2,1-2H3,(H2,23,24,25)(H,27,28,29,30)/t13-,14- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human liver flag-tagged DGAT1 expressed in baculovirus infected insect cells after 2 hrs by SPA |
J Med Chem 57: 3464-83 (2014)
Article DOI: 10.1021/jm500135c
BindingDB Entry DOI: 10.7270/Q23X886W |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50003864

(CHEMBL3235322)Show SMILES CC1(C)Oc2ncnc(N)c2C=C1c1ccc(cc1)[C@H]1CC[C@H](CC(O)=O)CC1 |r,wU:19.21,wD:22.25,c:12,(42.97,-20.87,;41.43,-20.87,;42.2,-22.2,;40.1,-21.65,;38.77,-20.88,;37.43,-21.66,;36.1,-20.89,;36.1,-19.34,;37.43,-18.57,;37.43,-17.03,;38.77,-19.34,;40.09,-18.56,;41.43,-19.32,;42.76,-18.55,;44.09,-19.31,;45.42,-18.54,;45.41,-17,;44.06,-16.23,;42.74,-17.02,;46.74,-16.22,;46.72,-14.68,;48.05,-13.9,;49.39,-14.66,;50.72,-13.89,;52.06,-14.65,;52.07,-16.19,;53.39,-13.88,;49.39,-16.2,;48.07,-16.98,)| Show InChI InChI=1S/C23H27N3O3/c1-23(2)19(12-18-21(24)25-13-26-22(18)29-23)17-9-7-16(8-10-17)15-5-3-14(4-6-15)11-20(27)28/h7-10,12-15H,3-6,11H2,1-2H3,(H,27,28)(H2,24,25,26)/t14-,15- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human liver flag-tagged DGAT1 expressed in baculovirus infected insect cells after 2 hrs by SPA |
J Med Chem 57: 3464-83 (2014)
Article DOI: 10.1021/jm500135c
BindingDB Entry DOI: 10.7270/Q23X886W |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50003859
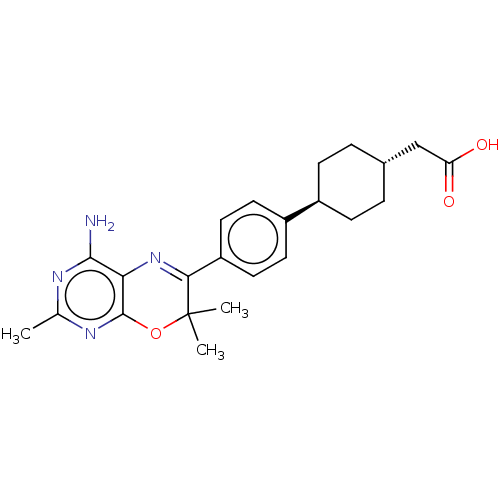
(CHEMBL3235316)Show SMILES Cc1nc(N)c2N=C(c3ccc(cc3)[C@H]3CC[C@H](CC(O)=O)CC3)C(C)(C)Oc2n1 |r,wU:14.14,wD:17.18,t:6,(.98,-9.8,;2.31,-9.03,;2.32,-7.49,;3.64,-6.72,;3.64,-5.18,;4.98,-7.48,;6.31,-6.71,;7.64,-7.47,;8.97,-6.69,;10.3,-7.46,;11.63,-6.68,;11.62,-5.14,;10.28,-4.38,;8.95,-5.16,;12.95,-4.36,;12.93,-2.82,;14.26,-2.05,;15.6,-2.81,;16.93,-2.04,;18.27,-2.8,;18.28,-4.34,;19.61,-2.02,;15.61,-4.35,;14.28,-5.12,;7.65,-9.01,;9.19,-9.01,;8.42,-10.35,;6.32,-9.79,;4.98,-9.03,;3.65,-9.8,)| Show InChI InChI=1S/C23H28N4O3/c1-13-25-21(24)19-22(26-13)30-23(2,3)20(27-19)17-10-8-16(9-11-17)15-6-4-14(5-7-15)12-18(28)29/h8-11,14-15H,4-7,12H2,1-3H3,(H,28,29)(H2,24,25,26)/t14-,15- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human liver flag-tagged DGAT1 expressed in baculovirus infected insect cells after 2 hrs by SPA |
J Med Chem 57: 3464-83 (2014)
Article DOI: 10.1021/jm500135c
BindingDB Entry DOI: 10.7270/Q23X886W |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50003713
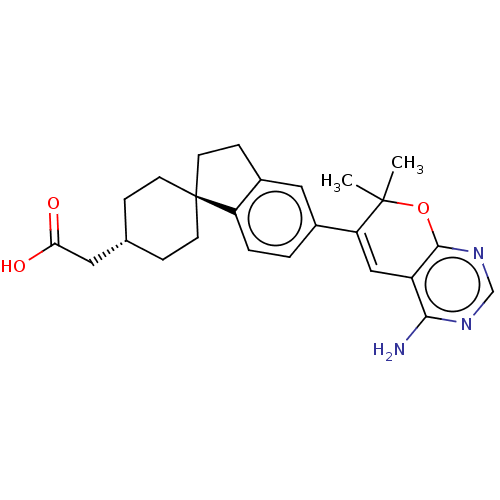
(CHEMBL3235323)Show SMILES CC1(C)Oc2ncnc(N)c2C=C1c1ccc2c(CC[C@@]22CC[C@H](CC(O)=O)CC2)c1 |r,wU:20.22,wD:23.26,c:12,(61.12,-21.75,;59.58,-21.75,;60.35,-23.09,;58.25,-22.53,;56.91,-21.76,;55.58,-22.54,;54.24,-21.77,;54.25,-20.23,;55.57,-19.46,;55.57,-17.92,;56.91,-20.22,;58.24,-19.45,;59.57,-20.21,;60.9,-19.43,;60.88,-17.9,;62.21,-17.12,;63.56,-17.88,;63.57,-19.42,;65.03,-19.88,;65.93,-18.63,;65.01,-17.39,;63.91,-16.31,;64.31,-14.82,;65.79,-14.42,;66.19,-12.93,;67.68,-12.52,;68.77,-13.61,;68.08,-11.03,;66.88,-15.5,;66.49,-16.98,;62.24,-20.2,)| Show InChI InChI=1S/C25H29N3O3/c1-24(2)20(13-18-22(26)27-14-28-23(18)31-24)16-3-4-19-17(12-16)7-10-25(19)8-5-15(6-9-25)11-21(29)30/h3-4,12-15H,5-11H2,1-2H3,(H,29,30)(H2,26,27,28)/t15-,25- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of DGAT1 (unknown origin) by cell-based assay |
J Med Chem 57: 3464-83 (2014)
Article DOI: 10.1021/jm500135c
BindingDB Entry DOI: 10.7270/Q23X886W |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50428878

(CHEMBL2338479)Show SMILES Clc1ccc(c(Cl)c1)S(=O)(=O)Nc1cc(Cl)c(Oc2cncc(c2)C#N)c(Cl)c1 Show InChI InChI=1S/C18H9Cl4N3O3S/c19-11-1-2-17(14(20)4-11)29(26,27)25-12-5-15(21)18(16(22)6-12)28-13-3-10(7-23)8-24-9-13/h1-6,8-9,25H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem 21: 979-92 (2013)
Article DOI: 10.1016/j.bmc.2012.11.058
BindingDB Entry DOI: 10.7270/Q23F4R13 |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50003856
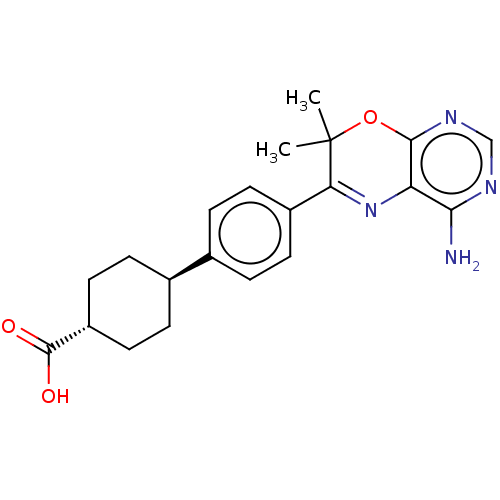
(CHEMBL3235313)Show SMILES CC1(C)Oc2ncnc(N)c2N=C1c1ccc(cc1)[C@H]1CC[C@@H](CC1)C(O)=O |r,wU:19.21,wD:22.28,c:12,(6.26,-59.02,;4.72,-59.02,;5.49,-60.36,;3.39,-59.8,;2.06,-59.04,;.72,-59.82,;-.62,-59.04,;-.62,-57.5,;.72,-56.73,;.71,-55.19,;2.05,-57.49,;3.38,-56.72,;4.72,-57.48,;6.05,-56.7,;7.38,-57.47,;8.71,-56.69,;8.7,-55.15,;7.35,-54.39,;6.03,-55.17,;10.03,-54.37,;10,-52.83,;11.34,-52.06,;12.68,-52.82,;12.68,-54.36,;11.36,-55.13,;14.01,-52.05,;15.35,-52.81,;14.01,-50.51,)| Show InChI InChI=1S/C21H24N4O3/c1-21(2)17(25-16-18(22)23-11-24-19(16)28-21)14-7-3-12(4-8-14)13-5-9-15(10-6-13)20(26)27/h3-4,7-8,11,13,15H,5-6,9-10H2,1-2H3,(H,26,27)(H2,22,23,24)/t13-,15- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human liver flag-tagged DGAT1 expressed in baculovirus infected insect cells after 2 hrs by SPA |
J Med Chem 57: 3464-83 (2014)
Article DOI: 10.1021/jm500135c
BindingDB Entry DOI: 10.7270/Q23X886W |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50428881
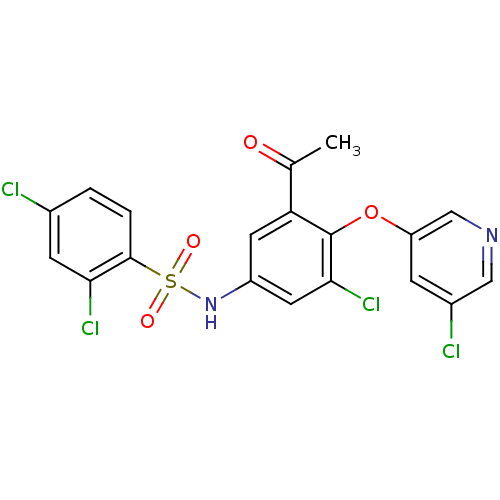
(CHEMBL2331773)Show SMILES CC(=O)c1cc(NS(=O)(=O)c2ccc(Cl)cc2Cl)cc(Cl)c1Oc1cncc(Cl)c1 Show InChI InChI=1S/C19H12Cl4N2O4S/c1-10(26)15-6-13(25-30(27,28)18-3-2-11(20)5-16(18)22)7-17(23)19(15)29-14-4-12(21)8-24-9-14/h2-9,25H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-rosiglitazone from GST-tagged PPARgammaLBD (unknown origin) after 1 hr by scintillation proximity assay |
Bioorg Med Chem 21: 979-92 (2013)
Article DOI: 10.1016/j.bmc.2012.11.058
BindingDB Entry DOI: 10.7270/Q23F4R13 |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50003713

(CHEMBL3235323)Show SMILES CC1(C)Oc2ncnc(N)c2C=C1c1ccc2c(CC[C@@]22CC[C@H](CC(O)=O)CC2)c1 |r,wU:20.22,wD:23.26,c:12,(61.12,-21.75,;59.58,-21.75,;60.35,-23.09,;58.25,-22.53,;56.91,-21.76,;55.58,-22.54,;54.24,-21.77,;54.25,-20.23,;55.57,-19.46,;55.57,-17.92,;56.91,-20.22,;58.24,-19.45,;59.57,-20.21,;60.9,-19.43,;60.88,-17.9,;62.21,-17.12,;63.56,-17.88,;63.57,-19.42,;65.03,-19.88,;65.93,-18.63,;65.01,-17.39,;63.91,-16.31,;64.31,-14.82,;65.79,-14.42,;66.19,-12.93,;67.68,-12.52,;68.77,-13.61,;68.08,-11.03,;66.88,-15.5,;66.49,-16.98,;62.24,-20.2,)| Show InChI InChI=1S/C25H29N3O3/c1-24(2)20(13-18-22(26)27-14-28-23(18)31-24)16-3-4-19-17(12-16)7-10-25(19)8-5-15(6-9-25)11-21(29)30/h3-4,12-15H,5-11H2,1-2H3,(H,29,30)(H2,26,27,28)/t15-,25- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human liver flag-tagged DGAT1 expressed in baculovirus infected insect cells after 2 hrs by SPA |
J Med Chem 57: 3464-83 (2014)
Article DOI: 10.1021/jm500135c
BindingDB Entry DOI: 10.7270/Q23X886W |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50003861
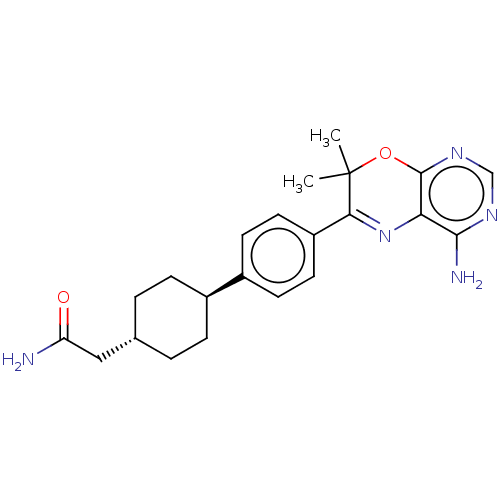
(CHEMBL3235318)Show SMILES CC1(C)Oc2ncnc(N)c2N=C1c1ccc(cc1)[C@H]1CC[C@H](CC(N)=O)CC1 |r,wU:19.21,wD:22.25,c:12,(42.45,-9.21,;40.91,-9.21,;41.68,-10.54,;39.58,-9.99,;38.25,-9.22,;36.91,-10,;35.58,-9.23,;35.58,-7.68,;36.91,-6.91,;36.9,-5.37,;38.24,-7.68,;39.57,-6.9,;40.91,-7.66,;42.24,-6.89,;43.57,-7.65,;44.9,-6.88,;44.89,-5.34,;43.54,-4.58,;42.22,-5.36,;46.22,-4.56,;46.19,-3.02,;47.53,-2.24,;48.87,-3,;50.2,-2.23,;51.54,-2.99,;51.55,-4.53,;52.87,-2.22,;48.87,-4.55,;47.55,-5.32,)| Show InChI InChI=1S/C22H27N5O2/c1-22(2)19(27-18-20(24)25-12-26-21(18)29-22)16-9-7-15(8-10-16)14-5-3-13(4-6-14)11-17(23)28/h7-10,12-14H,3-6,11H2,1-2H3,(H2,23,28)(H2,24,25,26)/t13-,14- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human liver flag-tagged DGAT1 expressed in baculovirus infected insect cells after 2 hrs by SPA |
J Med Chem 57: 3464-83 (2014)
Article DOI: 10.1021/jm500135c
BindingDB Entry DOI: 10.7270/Q23X886W |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50428885
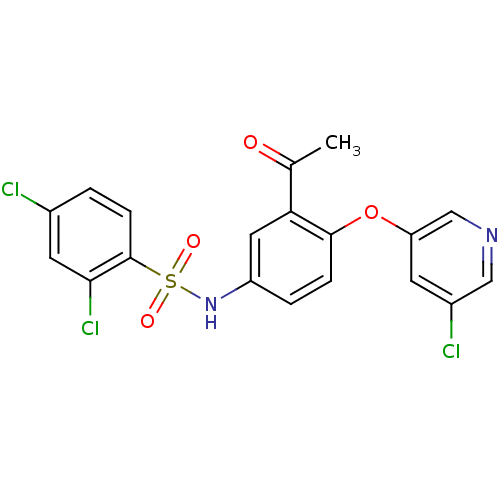
(CHEMBL2331781)Show SMILES CC(=O)c1cc(NS(=O)(=O)c2ccc(Cl)cc2Cl)ccc1Oc1cncc(Cl)c1 Show InChI InChI=1S/C19H13Cl3N2O4S/c1-11(25)16-8-14(3-4-18(16)28-15-6-13(21)9-23-10-15)24-29(26,27)19-5-2-12(20)7-17(19)22/h2-10,24H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-rosiglitazone from GST-tagged PPARgammaLBD (unknown origin) after 1 hr by scintillation proximity assay |
Bioorg Med Chem 21: 979-92 (2013)
Article DOI: 10.1016/j.bmc.2012.11.058
BindingDB Entry DOI: 10.7270/Q23F4R13 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50428884
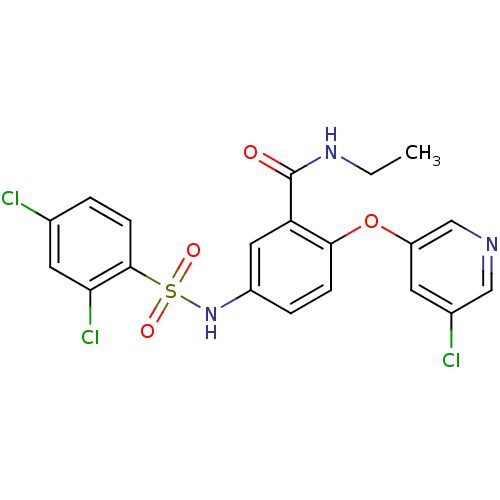
(CHEMBL2331786)Show SMILES CCNC(=O)c1cc(NS(=O)(=O)c2ccc(Cl)cc2Cl)ccc1Oc1cncc(Cl)c1 Show InChI InChI=1S/C20H16Cl3N3O4S/c1-2-25-20(27)16-9-14(4-5-18(16)30-15-7-13(22)10-24-11-15)26-31(28,29)19-6-3-12(21)8-17(19)23/h3-11,26H,2H2,1H3,(H,25,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-rosiglitazone from GST-tagged PPARgammaLBD (unknown origin) after 1 hr by scintillation proximity assay |
Bioorg Med Chem 21: 979-92 (2013)
Article DOI: 10.1016/j.bmc.2012.11.058
BindingDB Entry DOI: 10.7270/Q23F4R13 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50428854
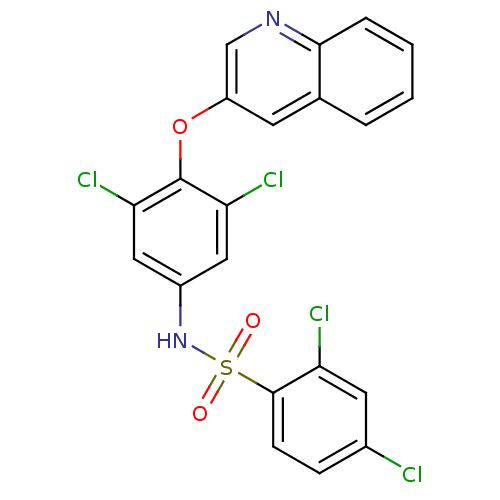
(CHEMBL1236924)Show SMILES Clc1ccc(c(Cl)c1)S(=O)(=O)Nc1cc(Cl)c(Oc2cnc3ccccc3c2)c(Cl)c1 Show InChI InChI=1S/C21H12Cl4N2O3S/c22-13-5-6-20(16(23)8-13)31(28,29)27-14-9-17(24)21(18(25)10-14)30-15-7-12-3-1-2-4-19(12)26-11-15/h1-11,27H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-rosiglitazone from GST-tagged PPARgammaLBD (unknown origin) after 1 hr by scintillation proximity assay |
Bioorg Med Chem 21: 979-92 (2013)
Article DOI: 10.1016/j.bmc.2012.11.058
BindingDB Entry DOI: 10.7270/Q23F4R13 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Lysophosphatidic acid receptor 2
(Homo sapiens (Human)) | BDBM50373826

(CHEMBL256470)Show SMILES C[C@@H](CN1CCN(CC1)S(=O)(=O)c1ccc(Cl)c(Cl)c1)Nc1ncnc2c(C)csc12 Show InChI InChI=1S/C20H23Cl2N5O2S2/c1-13-11-30-19-18(13)23-12-24-20(19)25-14(2)10-26-5-7-27(8-6-26)31(28,29)15-3-4-16(21)17(22)9-15/h3-4,9,11-12,14H,5-8,10H2,1-2H3,(H,23,24,25)/t14-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at LPA2 expressed in RH7777 cells with Gi4-protein and aequorin by calcium mobilization assay |
Bioorg Med Chem Lett 18: 1037-41 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.024
BindingDB Entry DOI: 10.7270/Q2JQ11W8 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50428883
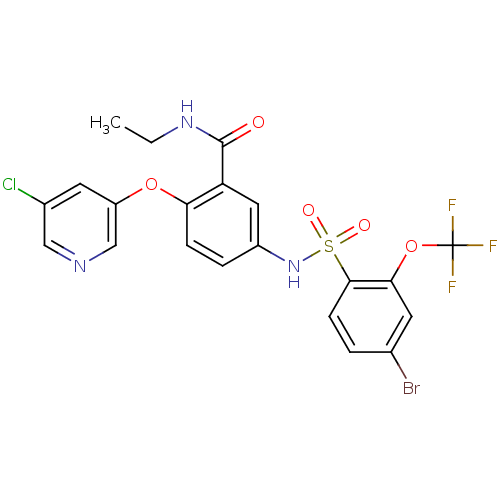
(CHEMBL2331774)Show SMILES CCNC(=O)c1cc(NS(=O)(=O)c2ccc(Br)cc2OC(F)(F)F)ccc1Oc1cncc(Cl)c1 Show InChI InChI=1S/C21H16BrClF3N3O5S/c1-2-28-20(30)16-9-14(4-5-17(16)33-15-8-13(23)10-27-11-15)29-35(31,32)19-6-3-12(22)7-18(19)34-21(24,25)26/h3-11,29H,2H2,1H3,(H,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-rosiglitazone from GST-tagged PPARgammaLBD (unknown origin) after 1 hr by scintillation proximity assay |
Bioorg Med Chem 21: 979-92 (2013)
Article DOI: 10.1016/j.bmc.2012.11.058
BindingDB Entry DOI: 10.7270/Q23F4R13 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50428882
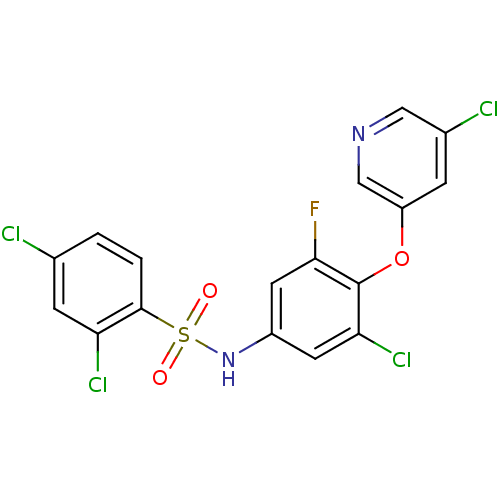
(CHEMBL2331771)Show SMILES Fc1cc(NS(=O)(=O)c2ccc(Cl)cc2Cl)cc(Cl)c1Oc1cncc(Cl)c1 Show InChI InChI=1S/C17H9Cl4FN2O3S/c18-9-1-2-16(13(20)4-9)28(25,26)24-11-5-14(21)17(15(22)6-11)27-12-3-10(19)7-23-8-12/h1-8,24H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-rosiglitazone from GST-tagged PPARgammaLBD (unknown origin) after 1 hr by scintillation proximity assay |
Bioorg Med Chem 21: 979-92 (2013)
Article DOI: 10.1016/j.bmc.2012.11.058
BindingDB Entry DOI: 10.7270/Q23F4R13 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50428857
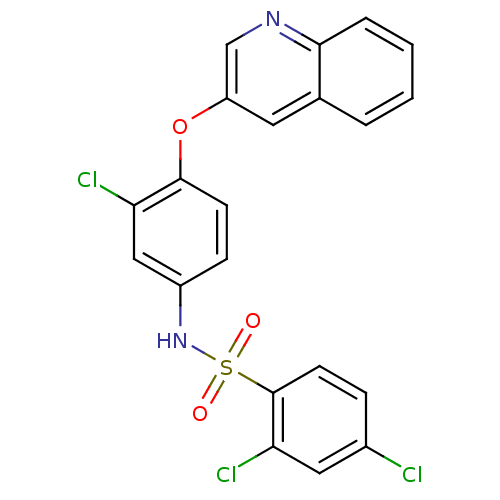
(CHEMBL2338472)Show SMILES Clc1ccc(c(Cl)c1)S(=O)(=O)Nc1ccc(Oc2cnc3ccccc3c2)c(Cl)c1 Show InChI InChI=1S/C21H13Cl3N2O3S/c22-14-5-8-21(18(24)10-14)30(27,28)26-15-6-7-20(17(23)11-15)29-16-9-13-3-1-2-4-19(13)25-12-16/h1-12,26H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-rosiglitazone from GST-tagged PPARgammaLBD (unknown origin) after 1 hr by scintillation proximity assay |
Bioorg Med Chem 21: 979-92 (2013)
Article DOI: 10.1016/j.bmc.2012.11.058
BindingDB Entry DOI: 10.7270/Q23F4R13 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50428877

(CHEMBL2338480)Show SMILES Cc1cncc(Oc2c(Cl)cc(NS(=O)(=O)c3ccc(Cl)cc3Cl)cc2Cl)c1 Show InChI InChI=1S/C18H12Cl4N2O3S/c1-10-4-13(9-23-8-10)27-18-15(21)6-12(7-16(18)22)24-28(25,26)17-3-2-11(19)5-14(17)20/h2-9,24H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-rosiglitazone from GST-tagged PPARgammaLBD (unknown origin) after 1 hr by scintillation proximity assay |
Bioorg Med Chem 21: 979-92 (2013)
Article DOI: 10.1016/j.bmc.2012.11.058
BindingDB Entry DOI: 10.7270/Q23F4R13 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50428887
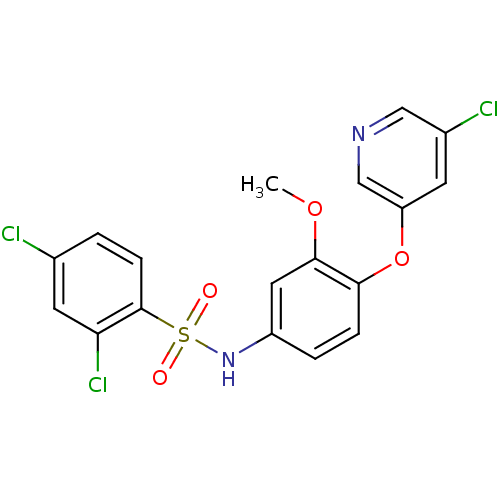
(CHEMBL2338487)Show SMILES COc1cc(NS(=O)(=O)c2ccc(Cl)cc2Cl)ccc1Oc1cncc(Cl)c1 Show InChI InChI=1S/C18H13Cl3N2O4S/c1-26-17-8-13(3-4-16(17)27-14-6-12(20)9-22-10-14)23-28(24,25)18-5-2-11(19)7-15(18)21/h2-10,23H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-rosiglitazone from GST-tagged PPARgammaLBD (unknown origin) after 1 hr by scintillation proximity assay |
Bioorg Med Chem 21: 979-92 (2013)
Article DOI: 10.1016/j.bmc.2012.11.058
BindingDB Entry DOI: 10.7270/Q23F4R13 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50428865
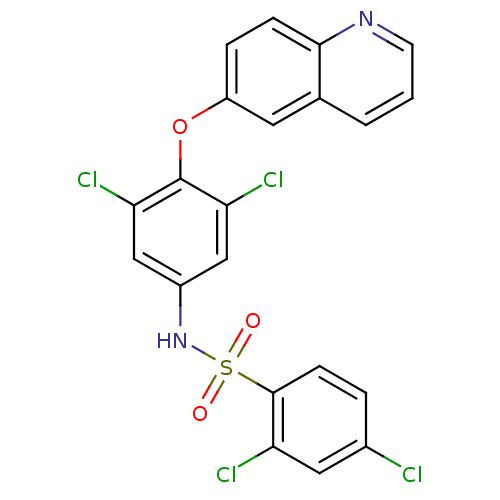
(CHEMBL2338477)Show SMILES Clc1ccc(c(Cl)c1)S(=O)(=O)Nc1cc(Cl)c(Oc2ccc3ncccc3c2)c(Cl)c1 Show InChI InChI=1S/C21H12Cl4N2O3S/c22-13-3-6-20(16(23)9-13)31(28,29)27-14-10-17(24)21(18(25)11-14)30-15-4-5-19-12(8-15)2-1-7-26-19/h1-11,27H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-rosiglitazone from GST-tagged PPARgammaLBD (unknown origin) after 1 hr by scintillation proximity assay |
Bioorg Med Chem 21: 979-92 (2013)
Article DOI: 10.1016/j.bmc.2012.11.058
BindingDB Entry DOI: 10.7270/Q23F4R13 |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50003863

(CHEMBL3235320)Show SMILES CC1(C)Oc2ncnc(N)c2N=C1c1ccc(cc1)[C@H]1CC[C@H](CS(N)(=O)=O)CC1 |r,wU:19.21,wD:22.25,c:12,(8.17,-20.46,;6.63,-20.46,;7.4,-21.8,;5.3,-21.24,;3.97,-20.48,;2.64,-21.25,;1.3,-20.48,;1.3,-18.94,;2.63,-18.17,;2.63,-16.63,;3.97,-18.93,;5.29,-18.16,;6.63,-18.92,;7.96,-18.14,;9.29,-18.91,;10.62,-18.13,;10.61,-16.59,;9.26,-15.83,;7.94,-16.61,;11.94,-15.81,;11.92,-14.27,;13.25,-13.5,;14.59,-14.26,;15.92,-13.48,;17.26,-14.25,;17.27,-15.79,;18.02,-12.91,;18.8,-14.24,;14.6,-15.8,;13.27,-16.57,)| Show InChI InChI=1S/C21H27N5O3S/c1-21(2)18(26-17-19(22)24-12-25-20(17)29-21)16-9-7-15(8-10-16)14-5-3-13(4-6-14)11-30(23,27)28/h7-10,12-14H,3-6,11H2,1-2H3,(H2,22,24,25)(H2,23,27,28)/t13-,14- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human liver flag-tagged DGAT1 expressed in baculovirus infected insect cells after 2 hrs by SPA |
J Med Chem 57: 3464-83 (2014)
Article DOI: 10.1021/jm500135c
BindingDB Entry DOI: 10.7270/Q23X886W |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 14
(Homo sapiens (Human)) | BDBM50429614

(CHEMBL2334589)Show SMILES COCCOc1cnc(N)nc1-c1c[nH]c2ccc(cc12)C#CC(C)(C)O Show InChI InChI=1S/C20H22N4O3/c1-20(2,25)7-6-13-4-5-16-14(10-13)15(11-22-16)18-17(27-9-8-26-3)12-23-19(21)24-18/h4-5,10-12,22,25H,8-9H2,1-3H3,(H2,21,23,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of NIK in human HT-29 cells assessed as LTalpha/beta2-induced p100 processing to NFkappaB2 preincubated for 30 mins before LTalpha/beta2 s... |
Bioorg Med Chem Lett 23: 1238-44 (2013)
Article DOI: 10.1016/j.bmcl.2013.01.012
BindingDB Entry DOI: 10.7270/Q2J967RQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50428864
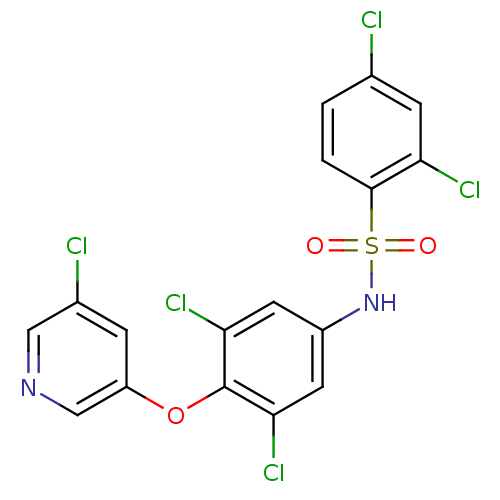
(CHEMBL2331772)Show SMILES Clc1ccc(c(Cl)c1)S(=O)(=O)Nc1cc(Cl)c(Oc2cncc(Cl)c2)c(Cl)c1 Show InChI InChI=1S/C17H9Cl5N2O3S/c18-9-1-2-16(13(20)4-9)28(25,26)24-11-5-14(21)17(15(22)6-11)27-12-3-10(19)7-23-8-12/h1-8,24H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-rosiglitazone from GST-tagged PPARgammaLBD (unknown origin) after 1 hr by scintillation proximity assay |
Bioorg Med Chem 21: 979-92 (2013)
Article DOI: 10.1016/j.bmc.2012.11.058
BindingDB Entry DOI: 10.7270/Q23F4R13 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50428868
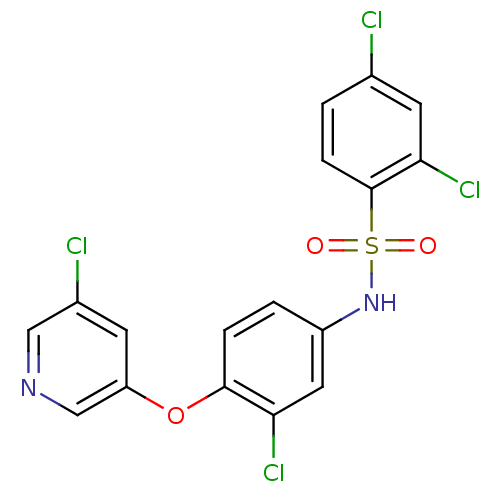
(CHEMBL2331782)Show SMILES Clc1ccc(c(Cl)c1)S(=O)(=O)Nc1ccc(Oc2cncc(Cl)c2)c(Cl)c1 Show InChI InChI=1S/C17H10Cl4N2O3S/c18-10-1-4-17(15(21)6-10)27(24,25)23-12-2-3-16(14(20)7-12)26-13-5-11(19)8-22-9-13/h1-9,23H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-rosiglitazone from GST-tagged PPARgammaLBD (unknown origin) after 1 hr by scintillation proximity assay |
Bioorg Med Chem 21: 979-92 (2013)
Article DOI: 10.1016/j.bmc.2012.11.058
BindingDB Entry DOI: 10.7270/Q23F4R13 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50428858
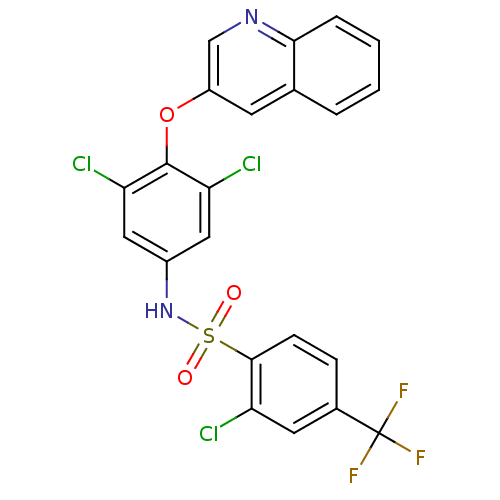
(CHEMBL2338474)Show SMILES FC(F)(F)c1ccc(c(Cl)c1)S(=O)(=O)Nc1cc(Cl)c(Oc2cnc3ccccc3c2)c(Cl)c1 Show InChI InChI=1S/C22H12Cl3F3N2O3S/c23-16-8-13(22(26,27)28)5-6-20(16)34(31,32)30-14-9-17(24)21(18(25)10-14)33-15-7-12-3-1-2-4-19(12)29-11-15/h1-11,30H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-rosiglitazone from GST-tagged PPARgammaLBD (unknown origin) after 1 hr by scintillation proximity assay |
Bioorg Med Chem 21: 979-92 (2013)
Article DOI: 10.1016/j.bmc.2012.11.058
BindingDB Entry DOI: 10.7270/Q23F4R13 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50428871
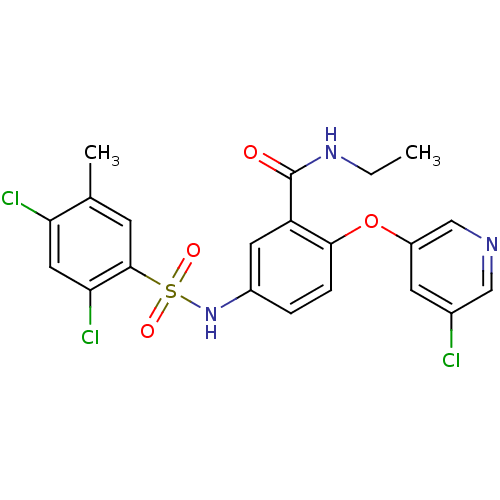
(CHEMBL2331785)Show SMILES CCNC(=O)c1cc(NS(=O)(=O)c2cc(C)c(Cl)cc2Cl)ccc1Oc1cncc(Cl)c1 Show InChI InChI=1S/C21H18Cl3N3O4S/c1-3-26-21(28)16-8-14(4-5-19(16)31-15-7-13(22)10-25-11-15)27-32(29,30)20-6-12(2)17(23)9-18(20)24/h4-11,27H,3H2,1-2H3,(H,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-rosiglitazone from GST-tagged PPARgammaLBD (unknown origin) after 1 hr by scintillation proximity assay |
Bioorg Med Chem 21: 979-92 (2013)
Article DOI: 10.1016/j.bmc.2012.11.058
BindingDB Entry DOI: 10.7270/Q23F4R13 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50428874
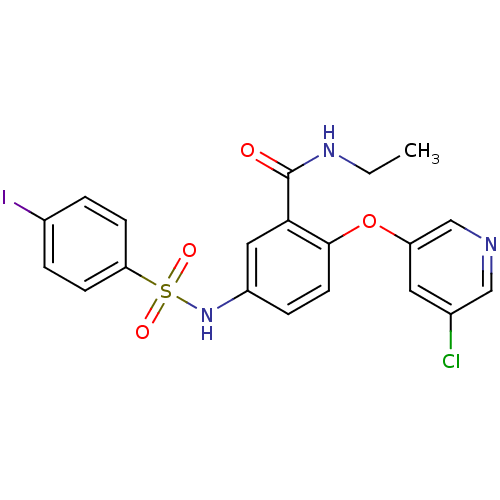
(CHEMBL2331777)Show SMILES CCNC(=O)c1cc(NS(=O)(=O)c2ccc(I)cc2)ccc1Oc1cncc(Cl)c1 Show InChI InChI=1S/C20H17ClIN3O4S/c1-2-24-20(26)18-10-15(25-30(27,28)17-6-3-14(22)4-7-17)5-8-19(18)29-16-9-13(21)11-23-12-16/h3-12,25H,2H2,1H3,(H,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-rosiglitazone from GST-tagged PPARgammaLBD (unknown origin) after 1 hr by scintillation proximity assay |
Bioorg Med Chem 21: 979-92 (2013)
Article DOI: 10.1016/j.bmc.2012.11.058
BindingDB Entry DOI: 10.7270/Q23F4R13 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50428878

(CHEMBL2338479)Show SMILES Clc1ccc(c(Cl)c1)S(=O)(=O)Nc1cc(Cl)c(Oc2cncc(c2)C#N)c(Cl)c1 Show InChI InChI=1S/C18H9Cl4N3O3S/c19-11-1-2-17(14(20)4-11)29(26,27)25-12-5-15(21)18(16(22)6-12)28-13-3-10(7-23)8-24-9-13/h1-6,8-9,25H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-rosiglitazone from GST-tagged PPARgammaLBD (unknown origin) after 1 hr by scintillation proximity assay |
Bioorg Med Chem 21: 979-92 (2013)
Article DOI: 10.1016/j.bmc.2012.11.058
BindingDB Entry DOI: 10.7270/Q23F4R13 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50428856
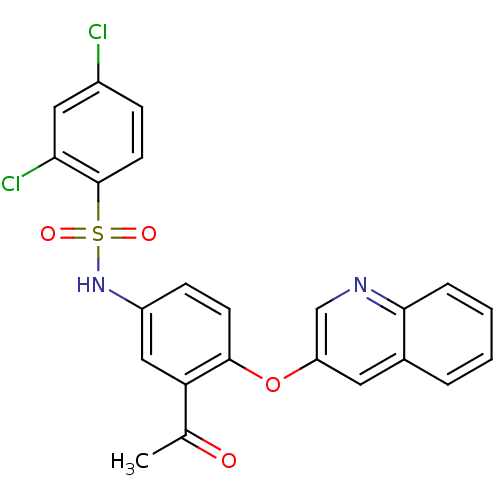
(CHEMBL2338475)Show SMILES CC(=O)c1cc(NS(=O)(=O)c2ccc(Cl)cc2Cl)ccc1Oc1cnc2ccccc2c1 Show InChI InChI=1S/C23H16Cl2N2O4S/c1-14(28)19-12-17(27-32(29,30)23-9-6-16(24)11-20(23)25)7-8-22(19)31-18-10-15-4-2-3-5-21(15)26-13-18/h2-13,27H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-rosiglitazone from GST-tagged PPARgammaLBD (unknown origin) after 1 hr by scintillation proximity assay |
Bioorg Med Chem 21: 979-92 (2013)
Article DOI: 10.1016/j.bmc.2012.11.058
BindingDB Entry DOI: 10.7270/Q23F4R13 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50428873
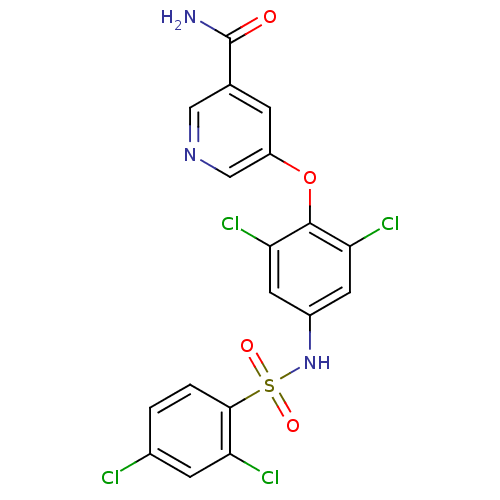
(CHEMBL2338481)Show SMILES NC(=O)c1cncc(Oc2c(Cl)cc(NS(=O)(=O)c3ccc(Cl)cc3Cl)cc2Cl)c1 Show InChI InChI=1S/C18H11Cl4N3O4S/c19-10-1-2-16(13(20)4-10)30(27,28)25-11-5-14(21)17(15(22)6-11)29-12-3-9(18(23)26)7-24-8-12/h1-8,25H,(H2,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem 21: 979-92 (2013)
Article DOI: 10.1016/j.bmc.2012.11.058
BindingDB Entry DOI: 10.7270/Q23F4R13 |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50003859

(CHEMBL3235316)Show SMILES Cc1nc(N)c2N=C(c3ccc(cc3)[C@H]3CC[C@H](CC(O)=O)CC3)C(C)(C)Oc2n1 |r,wU:14.14,wD:17.18,t:6,(.98,-9.8,;2.31,-9.03,;2.32,-7.49,;3.64,-6.72,;3.64,-5.18,;4.98,-7.48,;6.31,-6.71,;7.64,-7.47,;8.97,-6.69,;10.3,-7.46,;11.63,-6.68,;11.62,-5.14,;10.28,-4.38,;8.95,-5.16,;12.95,-4.36,;12.93,-2.82,;14.26,-2.05,;15.6,-2.81,;16.93,-2.04,;18.27,-2.8,;18.28,-4.34,;19.61,-2.02,;15.61,-4.35,;14.28,-5.12,;7.65,-9.01,;9.19,-9.01,;8.42,-10.35,;6.32,-9.79,;4.98,-9.03,;3.65,-9.8,)| Show InChI InChI=1S/C23H28N4O3/c1-13-25-21(24)19-22(26-13)30-23(2,3)20(27-19)17-10-8-16(9-11-17)15-6-4-14(5-7-15)12-18(28)29/h8-11,14-15H,4-7,12H2,1-3H3,(H,28,29)(H2,24,25,26)/t14-,15- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of DGAT1 (unknown origin) by cell-based assay |
J Med Chem 57: 3464-83 (2014)
Article DOI: 10.1021/jm500135c
BindingDB Entry DOI: 10.7270/Q23X886W |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50428876
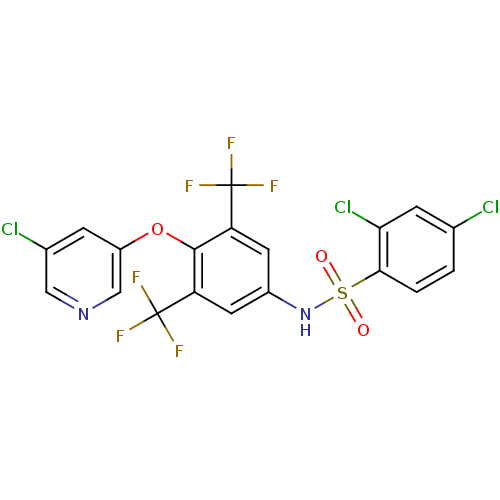
(CHEMBL2338478)Show SMILES FC(F)(F)c1cc(NS(=O)(=O)c2ccc(Cl)cc2Cl)cc(c1Oc1cncc(Cl)c1)C(F)(F)F Show InChI InChI=1S/C19H9Cl3F6N2O3S/c20-9-1-2-16(15(22)4-9)34(31,32)30-11-5-13(18(23,24)25)17(14(6-11)19(26,27)28)33-12-3-10(21)7-29-8-12/h1-8,30H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-rosiglitazone from GST-tagged PPARgammaLBD (unknown origin) after 1 hr by scintillation proximity assay |
Bioorg Med Chem 21: 979-92 (2013)
Article DOI: 10.1016/j.bmc.2012.11.058
BindingDB Entry DOI: 10.7270/Q23F4R13 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50428873

(CHEMBL2338481)Show SMILES NC(=O)c1cncc(Oc2c(Cl)cc(NS(=O)(=O)c3ccc(Cl)cc3Cl)cc2Cl)c1 Show InChI InChI=1S/C18H11Cl4N3O4S/c19-10-1-2-16(13(20)4-10)30(27,28)25-11-5-14(21)17(15(22)6-11)29-12-3-9(18(23)26)7-24-8-12/h1-8,25H,(H2,23,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-rosiglitazone from GST-tagged PPARgammaLBD (unknown origin) after 1 hr by scintillation proximity assay |
Bioorg Med Chem 21: 979-92 (2013)
Article DOI: 10.1016/j.bmc.2012.11.058
BindingDB Entry DOI: 10.7270/Q23F4R13 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50428870
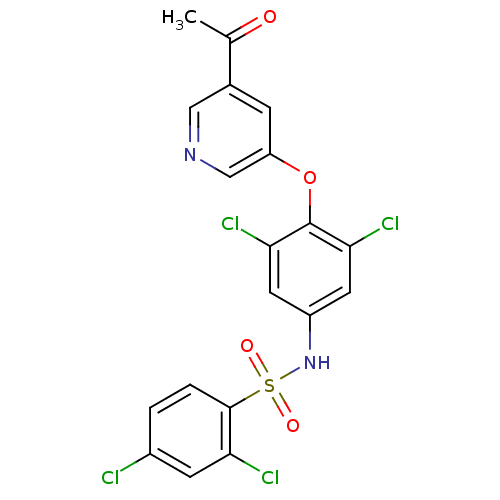
(CHEMBL2338482)Show SMILES CC(=O)c1cncc(Oc2c(Cl)cc(NS(=O)(=O)c3ccc(Cl)cc3Cl)cc2Cl)c1 Show InChI InChI=1S/C19H12Cl4N2O4S/c1-10(26)11-4-14(9-24-8-11)29-19-16(22)6-13(7-17(19)23)25-30(27,28)18-3-2-12(20)5-15(18)21/h2-9,25H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-rosiglitazone from GST-tagged PPARgammaLBD (unknown origin) after 1 hr by scintillation proximity assay |
Bioorg Med Chem 21: 979-92 (2013)
Article DOI: 10.1016/j.bmc.2012.11.058
BindingDB Entry DOI: 10.7270/Q23F4R13 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50428880
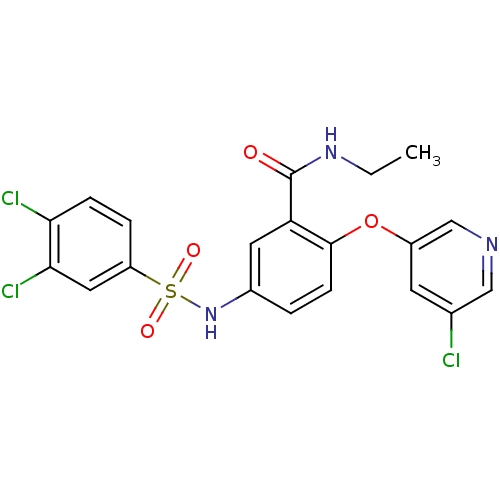
(CHEMBL2331775)Show SMILES CCNC(=O)c1cc(NS(=O)(=O)c2ccc(Cl)c(Cl)c2)ccc1Oc1cncc(Cl)c1 Show InChI InChI=1S/C20H16Cl3N3O4S/c1-2-25-20(27)16-8-13(3-6-19(16)30-14-7-12(21)10-24-11-14)26-31(28,29)15-4-5-17(22)18(23)9-15/h3-11,26H,2H2,1H3,(H,25,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-rosiglitazone from GST-tagged PPARgammaLBD (unknown origin) after 1 hr by scintillation proximity assay |
Bioorg Med Chem 21: 979-92 (2013)
Article DOI: 10.1016/j.bmc.2012.11.058
BindingDB Entry DOI: 10.7270/Q23F4R13 |
More data for this
Ligand-Target Pair | |
Lysophosphatidic acid receptor 2
(Homo sapiens (Human)) | BDBM50373827
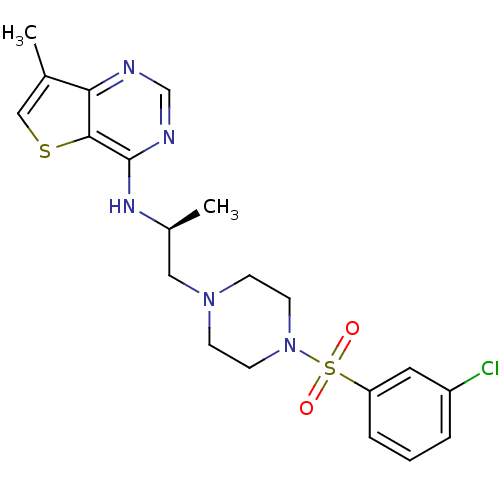
(CHEMBL270865)Show SMILES C[C@@H](CN1CCN(CC1)S(=O)(=O)c1cccc(Cl)c1)Nc1ncnc2c(C)csc12 Show InChI InChI=1S/C20H24ClN5O2S2/c1-14-12-29-19-18(14)22-13-23-20(19)24-15(2)11-25-6-8-26(9-7-25)30(27,28)17-5-3-4-16(21)10-17/h3-5,10,12-13,15H,6-9,11H2,1-2H3,(H,22,23,24)/t15-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at LPA2 expressed in RH7777 cells with Gi4-protein and aequorin by calcium mobilization assay |
Bioorg Med Chem Lett 18: 1037-41 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.024
BindingDB Entry DOI: 10.7270/Q2JQ11W8 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50428866
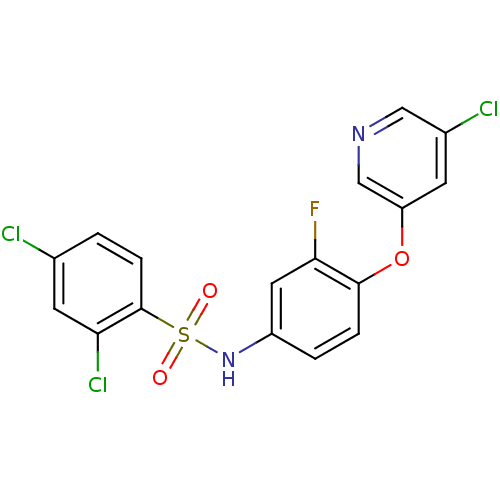
(CHEMBL2331783)Show SMILES Fc1cc(NS(=O)(=O)c2ccc(Cl)cc2Cl)ccc1Oc1cncc(Cl)c1 Show InChI InChI=1S/C17H10Cl3FN2O3S/c18-10-1-4-17(14(20)6-10)27(24,25)23-12-2-3-16(15(21)7-12)26-13-5-11(19)8-22-9-13/h1-9,23H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-rosiglitazone from GST-tagged PPARgammaLBD (unknown origin) after 1 hr by scintillation proximity assay |
Bioorg Med Chem 21: 979-92 (2013)
Article DOI: 10.1016/j.bmc.2012.11.058
BindingDB Entry DOI: 10.7270/Q23F4R13 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 14
(Homo sapiens (Human)) | BDBM50429614

(CHEMBL2334589)Show SMILES COCCOc1cnc(N)nc1-c1c[nH]c2ccc(cc12)C#CC(C)(C)O Show InChI InChI=1S/C20H22N4O3/c1-20(2,25)7-6-13-4-5-16-14(10-13)15(11-22-16)18-17(27-9-8-26-3)12-23-19(21)24-18/h4-5,10-12,22,25H,8-9H2,1-3H3,(H2,21,23,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of NIK (unknown origin) autophosphorylation after 1 hr by chemiluminescent assay |
Bioorg Med Chem Lett 23: 1238-44 (2013)
Article DOI: 10.1016/j.bmcl.2013.01.012
BindingDB Entry DOI: 10.7270/Q2J967RQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Lysophosphatidic acid receptor 3
(Homo sapiens (Human)) | BDBM50373831
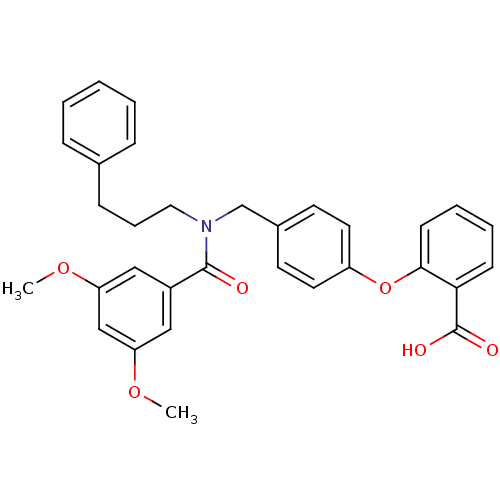
(CHEMBL272087)Show SMILES COc1cc(OC)cc(c1)C(=O)N(CCCc1ccccc1)Cc1ccc(Oc2ccccc2C(O)=O)cc1 Show InChI InChI=1S/C32H31NO6/c1-37-27-19-25(20-28(21-27)38-2)31(34)33(18-8-11-23-9-4-3-5-10-23)22-24-14-16-26(17-15-24)39-30-13-7-6-12-29(30)32(35)36/h3-7,9-10,12-17,19-21H,8,11,18,22H2,1-2H3,(H,35,36) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at LPA3 expressed in RH7777 cells with Gi4-protein and aequorin by calcium mobilization assay |
Bioorg Med Chem Lett 18: 1037-41 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.024
BindingDB Entry DOI: 10.7270/Q2JQ11W8 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50428855
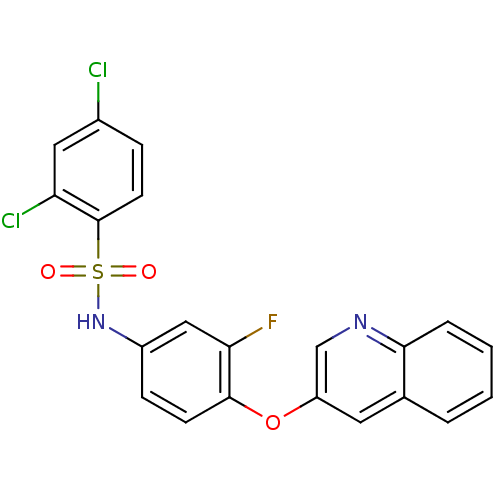
(CHEMBL2338473)Show SMILES Fc1cc(NS(=O)(=O)c2ccc(Cl)cc2Cl)ccc1Oc1cnc2ccccc2c1 Show InChI InChI=1S/C21H13Cl2FN2O3S/c22-14-5-8-21(17(23)10-14)30(27,28)26-15-6-7-20(18(24)11-15)29-16-9-13-3-1-2-4-19(13)25-12-16/h1-12,26H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-rosiglitazone from GST-tagged PPARgammaLBD (unknown origin) after 1 hr by scintillation proximity assay |
Bioorg Med Chem 21: 979-92 (2013)
Article DOI: 10.1016/j.bmc.2012.11.058
BindingDB Entry DOI: 10.7270/Q23F4R13 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 14
(Homo sapiens (Human)) | BDBM50429615
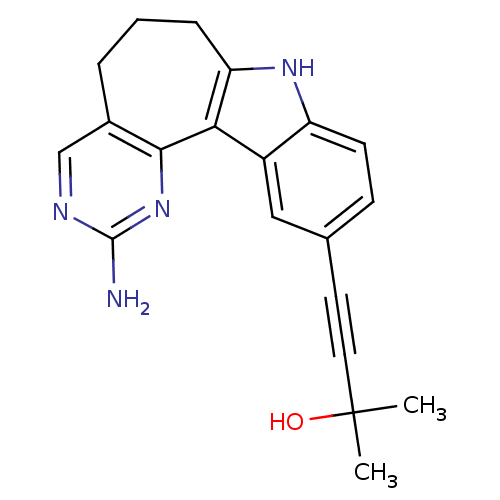
(CHEMBL2334586)Show SMILES CC(C)(O)C#Cc1ccc2[nH]c3CCCc4cnc(N)nc4-c3c2c1 Show InChI InChI=1S/C20H20N4O/c1-20(2,25)9-8-12-6-7-15-14(10-12)17-16(23-15)5-3-4-13-11-22-19(21)24-18(13)17/h6-7,10-11,23,25H,3-5H2,1-2H3,(H2,21,22,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of NIK (unknown origin) autophosphorylation after 1 hr by chemiluminescent assay |
Bioorg Med Chem Lett 23: 1238-44 (2013)
Article DOI: 10.1016/j.bmcl.2013.01.012
BindingDB Entry DOI: 10.7270/Q2J967RQ |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50428869
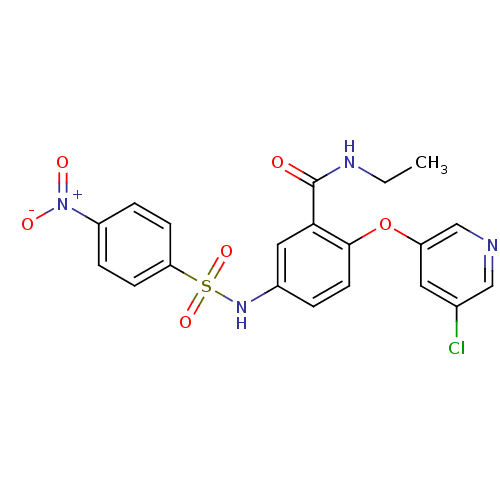
(CHEMBL2331778)Show SMILES CCNC(=O)c1cc(NS(=O)(=O)c2ccc(cc2)[N+]([O-])=O)ccc1Oc1cncc(Cl)c1 Show InChI InChI=1S/C20H17ClN4O6S/c1-2-23-20(26)18-10-14(3-8-19(18)31-16-9-13(21)11-22-12-16)24-32(29,30)17-6-4-15(5-7-17)25(27)28/h3-12,24H,2H2,1H3,(H,23,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-rosiglitazone from GST-tagged PPARgammaLBD (unknown origin) after 1 hr by scintillation proximity assay |
Bioorg Med Chem 21: 979-92 (2013)
Article DOI: 10.1016/j.bmc.2012.11.058
BindingDB Entry DOI: 10.7270/Q23F4R13 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data