

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4690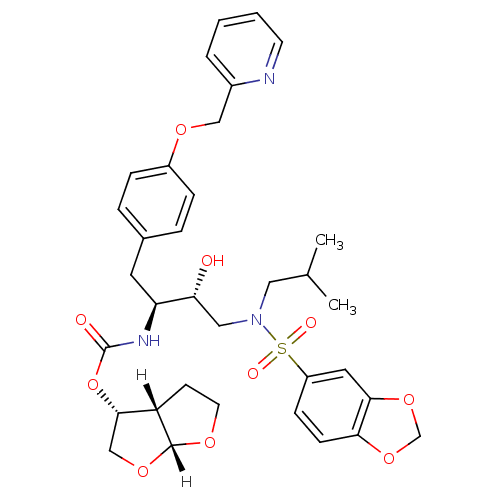 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00000600 | -82.5 | n/a | n/a | n/a | n/a | n/a | 5.5 | 30 |
GlaxoSmithKline | Assay Description The method was a competitive displacement assay used to determine binding affinities of other inhibitors relative to that of GW0385. The inhibitor of... | Biochemistry 43: 14500-7 (2004) Article DOI: 10.1021/bi0488799 BindingDB Entry DOI: 10.7270/Q25M63WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4689 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0000130 | -80.6 | n/a | n/a | n/a | n/a | n/a | 5.5 | 30 |
GlaxoSmithKline | Assay Description The method was a competitive displacement assay used to determine binding affinities of other inhibitors relative to that of GW0385. The inhibitor of... | Biochemistry 43: 14500-7 (2004) Article DOI: 10.1021/bi0488799 BindingDB Entry DOI: 10.7270/Q25M63WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4685 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0000150 | -80.2 | n/a | n/a | n/a | n/a | n/a | 5.5 | 30 |
GlaxoSmithKline | Assay Description The assay method employed kinetic determinations of values for k1 and k-1, from which value of inhibition constant (Ki ) was determined (k-1/k1). The... | Biochemistry 43: 14500-7 (2004) Article DOI: 10.1021/bi0488799 BindingDB Entry DOI: 10.7270/Q25M63WJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4688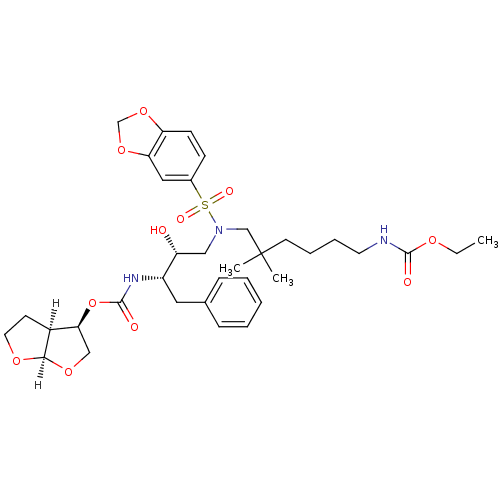 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.000165 | -74.2 | n/a | n/a | n/a | n/a | n/a | 5.5 | 30 |
GlaxoSmithKline | Assay Description The method was a competitive displacement assay used to determine binding affinities of other inhibitors relative to that of GW0385. The inhibitor of... | Biochemistry 43: 14500-7 (2004) Article DOI: 10.1021/bi0488799 BindingDB Entry DOI: 10.7270/Q25M63WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,M535I,L552P,A560V,V571F,I573V] (Human immunodeficiency virus type 1) | BDBM4689 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.000220 | -73.5 | n/a | n/a | n/a | n/a | n/a | 5.5 | 30 |
GlaxoSmithKline | Assay Description Enzymatic activity was determined with fluorogenic substrate in the presence and absence of inhibitor. The fluorescence increase due to hydrolysis of... | Biochemistry 43: 14500-7 (2004) Article DOI: 10.1021/bi0488799 BindingDB Entry DOI: 10.7270/Q25M63WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4687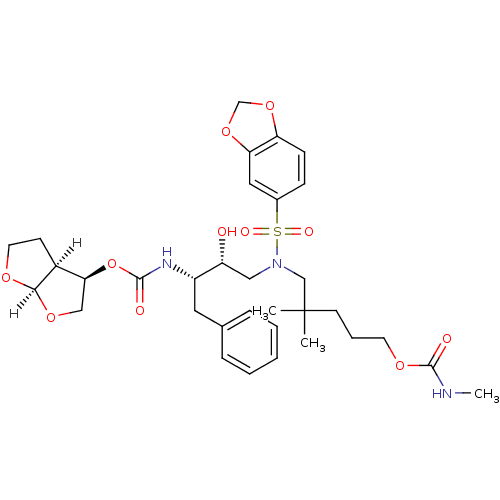 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.000240 | -73.2 | n/a | n/a | n/a | n/a | n/a | 5.5 | 30 |
GlaxoSmithKline | Assay Description The method was a competitive displacement assay used to determine binding affinities of other inhibitors relative to that of GW0385. The inhibitor of... | Biochemistry 43: 14500-7 (2004) Article DOI: 10.1021/bi0488799 BindingDB Entry DOI: 10.7270/Q25M63WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,M535I,L552P,A560V,V571F,I573V] (Human immunodeficiency virus type 1) | BDBM4690 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.000420 | -71.8 | n/a | n/a | n/a | n/a | n/a | 5.5 | 30 |
GlaxoSmithKline | Assay Description Enzymatic activity was determined with fluorogenic substrate in the presence and absence of inhibitor. The fluorescence increase due to hydrolysis of... | Biochemistry 43: 14500-7 (2004) Article DOI: 10.1021/bi0488799 BindingDB Entry DOI: 10.7270/Q25M63WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,M535I,L552P,A560V,V571F,I573V] (Human immunodeficiency virus type 1) | BDBM4685 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.000750 | -70.4 | n/a | n/a | n/a | n/a | n/a | 5.5 | 30 |
GlaxoSmithKline | Assay Description The assay method employed kinetic determinations of values for k1 and k-1, from which value of inhibition constant (Ki ) was determined (k-1/k1). The... | Biochemistry 43: 14500-7 (2004) Article DOI: 10.1021/bi0488799 BindingDB Entry DOI: 10.7270/Q25M63WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,L499I,L508Q,K509R,E524D,M525I,S526N,M535I,I539V,I543V,I551V,L552P,A560V,V571A,L579M] (Human immunodeficiency virus type 1) | BDBM4688 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.00120 | -69.2 | n/a | n/a | n/a | n/a | n/a | 5.5 | 30 |
GlaxoSmithKline | Assay Description Enzymatic activity was determined with fluorogenic substrate in the presence and absence of inhibitor. The fluorescence increase due to hydrolysis of... | Biochemistry 43: 14500-7 (2004) Article DOI: 10.1021/bi0488799 BindingDB Entry DOI: 10.7270/Q25M63WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,L499I,L508Q,K509R,E524D,M525I,S526N,M535I,I539V,I543V,I551V,L552P,A560V,V571A,L579M] (Human immunodeficiency virus type 1) | BDBM4690 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00170 | -68.3 | n/a | n/a | n/a | n/a | n/a | 5.5 | 30 |
GlaxoSmithKline | Assay Description Enzymatic activity was determined with fluorogenic substrate in the presence and absence of inhibitor. The fluorescence increase due to hydrolysis of... | Biochemistry 43: 14500-7 (2004) Article DOI: 10.1021/bi0488799 BindingDB Entry DOI: 10.7270/Q25M63WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,I539V] (Human immunodeficiency virus type 1) | BDBM4685 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.00200 | -67.9 | n/a | n/a | n/a | n/a | n/a | 5.5 | 30 |
GlaxoSmithKline | Assay Description The assay method employed kinetic determinations of values for k1 and k-1, from which value of inhibition constant (Ki ) was determined (k-1/k1). The... | Biochemistry 43: 14500-7 (2004) Article DOI: 10.1021/bi0488799 BindingDB Entry DOI: 10.7270/Q25M63WJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,L499I,L508Q,K509R,E524D,M525I,S526N,M535I,I539V,I543V,I551V,L552P,A560V,V571A,L579M] (Human immunodeficiency virus type 1) | BDBM4689 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.00240 | -67.4 | n/a | n/a | n/a | n/a | n/a | 5.5 | 30 |
GlaxoSmithKline | Assay Description Enzymatic activity was determined with fluorogenic substrate in the presence and absence of inhibitor. The fluorescence increase due to hydrolysis of... | Biochemistry 43: 14500-7 (2004) Article DOI: 10.1021/bi0488799 BindingDB Entry DOI: 10.7270/Q25M63WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,I539V] (Human immunodeficiency virus type 1) | BDBM4690 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00260 | -67.2 | n/a | n/a | n/a | n/a | n/a | 5.5 | 30 |
GlaxoSmithKline | Assay Description The method was a competitive displacement assay used to determine binding affinities of other inhibitors relative to that of GW0385. The inhibitor of... | Biochemistry 43: 14500-7 (2004) Article DOI: 10.1021/bi0488799 BindingDB Entry DOI: 10.7270/Q25M63WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,L499I,L508Q,K509R,E524D,M525I,S526N,M535I,I539V,I543V,I551V,L552P,A560V,V571A,L579M] (Human immunodeficiency virus type 1) | BDBM4685 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.00340 | -66.6 | n/a | n/a | n/a | n/a | n/a | 5.5 | 30 |
GlaxoSmithKline | Assay Description The assay method employed kinetic determinations of values for k1 and k-1, from which value of inhibition constant (Ki ) was determined (k-1/k1). The... | Biochemistry 43: 14500-7 (2004) Article DOI: 10.1021/bi0488799 BindingDB Entry DOI: 10.7270/Q25M63WJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,I539V] (Human immunodeficiency virus type 1) | BDBM4689 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.00390 | -66.2 | n/a | n/a | n/a | n/a | n/a | 5.5 | 30 |
GlaxoSmithKline | Assay Description The method was a competitive displacement assay used to determine binding affinities of other inhibitors relative to that of GW0385. The inhibitor of... | Biochemistry 43: 14500-7 (2004) Article DOI: 10.1021/bi0488799 BindingDB Entry DOI: 10.7270/Q25M63WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,M535I,L552P,A560V,V571F,I573V] (Human immunodeficiency virus type 1) | BDBM4688 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.00430 | -66.0 | n/a | n/a | n/a | n/a | n/a | 5.5 | 30 |
GlaxoSmithKline | Assay Description Enzymatic activity was determined with fluorogenic substrate in the presence and absence of inhibitor. The fluorescence increase due to hydrolysis of... | Biochemistry 43: 14500-7 (2004) Article DOI: 10.1021/bi0488799 BindingDB Entry DOI: 10.7270/Q25M63WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,I539V] (Human immunodeficiency virus type 1) | BDBM4688 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.00460 | -65.8 | n/a | n/a | n/a | n/a | n/a | 5.5 | 30 |
GlaxoSmithKline | Assay Description The method was a competitive displacement assay used to determine binding affinities of other inhibitors relative to that of GW0385. The inhibitor of... | Biochemistry 43: 14500-7 (2004) Article DOI: 10.1021/bi0488799 BindingDB Entry DOI: 10.7270/Q25M63WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,M535I,L552P,A560V,V571F,I573V] (Human immunodeficiency virus type 1) | BDBM4687 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0250 | -61.5 | n/a | n/a | n/a | n/a | n/a | 5.5 | 30 |
GlaxoSmithKline | Assay Description Enzymatic activity was determined with fluorogenic substrate in the presence and absence of inhibitor. The fluorescence increase due to hydrolysis of... | Biochemistry 43: 14500-7 (2004) Article DOI: 10.1021/bi0488799 BindingDB Entry DOI: 10.7270/Q25M63WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,I539V] (Human immunodeficiency virus type 1) | BDBM4687 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0270 | -61.3 | n/a | n/a | n/a | n/a | n/a | 5.5 | 30 |
GlaxoSmithKline | Assay Description The method was a competitive displacement assay used to determine binding affinities of other inhibitors relative to that of GW0385. The inhibitor of... | Biochemistry 43: 14500-7 (2004) Article DOI: 10.1021/bi0488799 BindingDB Entry DOI: 10.7270/Q25M63WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,L499I,L508Q,K509R,E524D,M525I,S526N,M535I,I539V,I543V,I551V,L552P,A560V,V571A,L579M] (Human immunodeficiency virus type 1) | BDBM4687 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0544 | -59.6 | n/a | n/a | n/a | n/a | n/a | 5.5 | 30 |
GlaxoSmithKline | Assay Description Enzymatic activity was determined with fluorogenic substrate in the presence and absence of inhibitor. The fluorescence increase due to hydrolysis of... | Biochemistry 43: 14500-7 (2004) Article DOI: 10.1021/bi0488799 BindingDB Entry DOI: 10.7270/Q25M63WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM577 ((3S)-oxolan-3-yl N-[(2S,3R)-4-[(4-aminobenzene)(2-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0570 | -59.5 | n/a | n/a | n/a | n/a | n/a | 5.5 | 30 |
GlaxoSmithKline | Assay Description Enzymatic activity was determined with fluorogenic substrate in the presence and absence of inhibitor. The fluorescence increase due to hydrolysis of... | Biochemistry 43: 14500-7 (2004) Article DOI: 10.1021/bi0488799 BindingDB Entry DOI: 10.7270/Q25M63WJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,M535I,L552P,A560V,V571F,I573V] (Human immunodeficiency virus type 1) | BDBM577 ((3S)-oxolan-3-yl N-[(2S,3R)-4-[(4-aminobenzene)(2-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.30 | -50.1 | n/a | n/a | n/a | n/a | n/a | 5.5 | 30 |
GlaxoSmithKline | Assay Description Enzymatic activity was determined with fluorogenic substrate in the presence and absence of inhibitor. The fluorescence increase due to hydrolysis of... | Biochemistry 43: 14500-7 (2004) Article DOI: 10.1021/bi0488799 BindingDB Entry DOI: 10.7270/Q25M63WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,I539V] (Human immunodeficiency virus type 1) | BDBM577 ((3S)-oxolan-3-yl N-[(2S,3R)-4-[(4-aminobenzene)(2-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 4.90 | -48.2 | n/a | n/a | n/a | n/a | n/a | 5.5 | 30 |
GlaxoSmithKline | Assay Description Enzymatic activity was determined with fluorogenic substrate in the presence and absence of inhibitor. The fluorescence increase due to hydrolysis of... | Biochemistry 43: 14500-7 (2004) Article DOI: 10.1021/bi0488799 BindingDB Entry DOI: 10.7270/Q25M63WJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,L499I,L508Q,K509R,E524D,M525I,S526N,M535I,I539V,I543V,I551V,L552P,A560V,V571A,L579M] (Human immunodeficiency virus type 1) | BDBM577 ((3S)-oxolan-3-yl N-[(2S,3R)-4-[(4-aminobenzene)(2-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 58 | -42.0 | n/a | n/a | n/a | n/a | n/a | 5.5 | 30 |
GlaxoSmithKline | Assay Description Enzymatic activity was determined with fluorogenic substrate in the presence and absence of inhibitor. The fluorescence increase due to hydrolysis of... | Biochemistry 43: 14500-7 (2004) Article DOI: 10.1021/bi0488799 BindingDB Entry DOI: 10.7270/Q25M63WJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50400413 (CHEMBL2177777) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of CYP2C9 | J Med Chem 55: 10972-94 (2012) Article DOI: 10.1021/jm301404a BindingDB Entry DOI: 10.7270/Q2RJ4KMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-dependent insulinotropic receptor (Homo sapiens (Human)) | BDBM50400367 (CHEMBL2181688) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 501 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Agonist activity at human GPR119 receptor expressed in CHO-K1 cells co-expressing 6CRE-luciferase gene after 5 hrs by luciferase reporter gene assay | J Med Chem 55: 10972-94 (2012) Article DOI: 10.1021/jm301404a BindingDB Entry DOI: 10.7270/Q2RJ4KMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-dependent insulinotropic receptor (Homo sapiens (Human)) | BDBM50400368 (CHEMBL2181687) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 158 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Agonist activity at human GPR119 receptor expressed in CHO-K1 cells co-expressing 6CRE-luciferase gene after 5 hrs by luciferase reporter gene assay | J Med Chem 55: 10972-94 (2012) Article DOI: 10.1021/jm301404a BindingDB Entry DOI: 10.7270/Q2RJ4KMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-dependent insulinotropic receptor (Homo sapiens (Human)) | BDBM50400369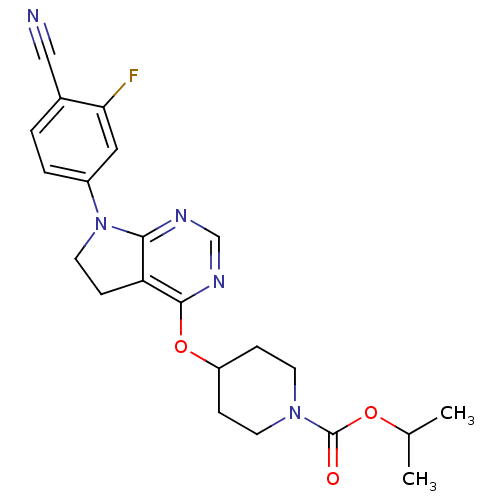 (CHEMBL2181686) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 398 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Agonist activity at human GPR119 receptor expressed in CHO-K1 cells co-expressing 6CRE-luciferase gene after 5 hrs by luciferase reporter gene assay | J Med Chem 55: 10972-94 (2012) Article DOI: 10.1021/jm301404a BindingDB Entry DOI: 10.7270/Q2RJ4KMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-dependent insulinotropic receptor (Homo sapiens (Human)) | BDBM50400370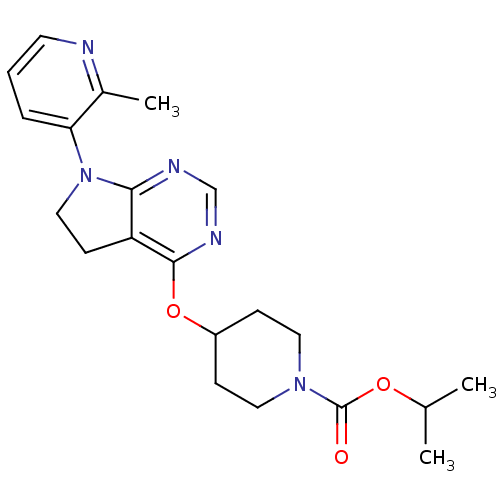 (CHEMBL2181685) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Agonist activity at human GPR119 receptor expressed in CHO-K1 cells co-expressing 6CRE-luciferase gene after 5 hrs by luciferase reporter gene assay | J Med Chem 55: 10972-94 (2012) Article DOI: 10.1021/jm301404a BindingDB Entry DOI: 10.7270/Q2RJ4KMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-dependent insulinotropic receptor (Homo sapiens (Human)) | BDBM50400371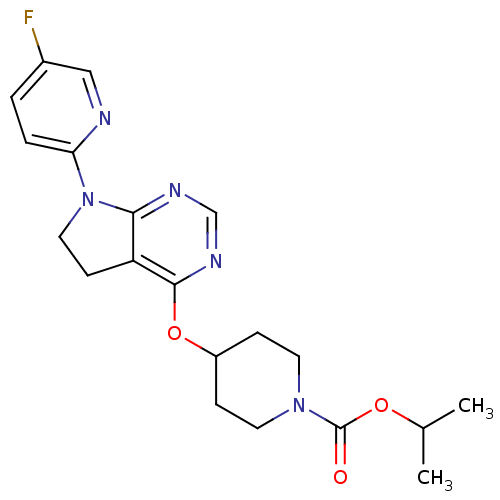 (CHEMBL2181684) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Agonist activity at human GPR119 receptor expressed in CHO-K1 cells co-expressing 6CRE-luciferase gene after 5 hrs by luciferase reporter gene assay | J Med Chem 55: 10972-94 (2012) Article DOI: 10.1021/jm301404a BindingDB Entry DOI: 10.7270/Q2RJ4KMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-dependent insulinotropic receptor (Homo sapiens (Human)) | BDBM50400372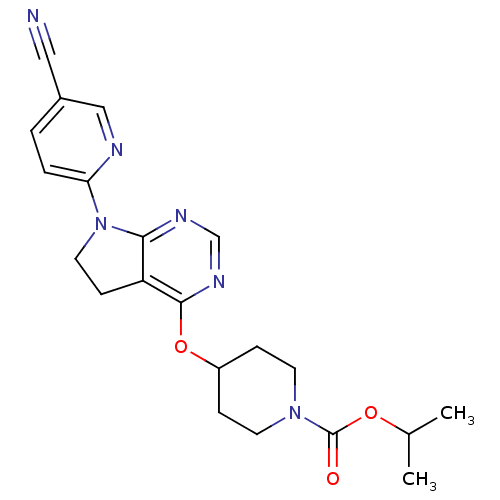 (CHEMBL2181683) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 794 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Agonist activity at human GPR119 receptor expressed in CHO-K1 cells co-expressing 6CRE-luciferase gene after 5 hrs by luciferase reporter gene assay | J Med Chem 55: 10972-94 (2012) Article DOI: 10.1021/jm301404a BindingDB Entry DOI: 10.7270/Q2RJ4KMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-dependent insulinotropic receptor (Homo sapiens (Human)) | BDBM50400373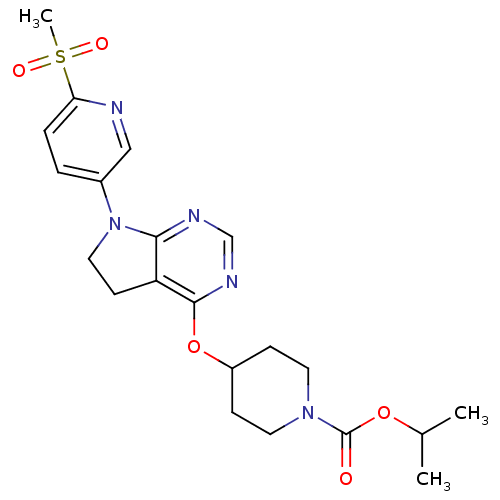 (CHEMBL2181682) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 251 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Agonist activity at human GPR119 receptor expressed in CHO-K1 cells co-expressing 6CRE-luciferase gene after 5 hrs by luciferase reporter gene assay | J Med Chem 55: 10972-94 (2012) Article DOI: 10.1021/jm301404a BindingDB Entry DOI: 10.7270/Q2RJ4KMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-dependent insulinotropic receptor (Homo sapiens (Human)) | BDBM50400374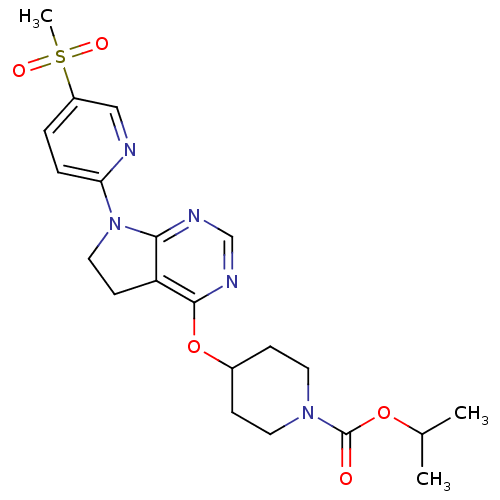 (CHEMBL2181681) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 126 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Agonist activity at human GPR119 receptor expressed in CHO-K1 cells co-expressing 6CRE-luciferase gene after 5 hrs by luciferase reporter gene assay | J Med Chem 55: 10972-94 (2012) Article DOI: 10.1021/jm301404a BindingDB Entry DOI: 10.7270/Q2RJ4KMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-dependent insulinotropic receptor (Homo sapiens (Human)) | BDBM50400375 (CHEMBL2181680) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Agonist activity at human GPR119 receptor expressed in CHO-K1 cells co-expressing 6CRE-luciferase gene after 5 hrs by luciferase reporter gene assay | J Med Chem 55: 10972-94 (2012) Article DOI: 10.1021/jm301404a BindingDB Entry DOI: 10.7270/Q2RJ4KMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-dependent insulinotropic receptor (Homo sapiens (Human)) | BDBM50400377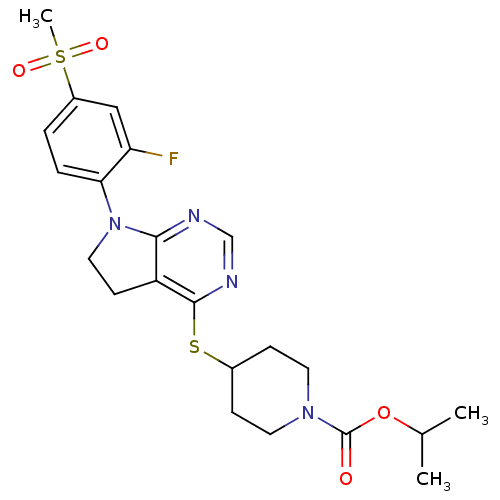 (CHEMBL2181678) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 79.4 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Agonist activity at human GPR119 receptor expressed in CHO-K1 cells co-expressing 6CRE-luciferase gene after 5 hrs by luciferase reporter gene assay | J Med Chem 55: 10972-94 (2012) Article DOI: 10.1021/jm301404a BindingDB Entry DOI: 10.7270/Q2RJ4KMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-dependent insulinotropic receptor (Homo sapiens (Human)) | BDBM50400378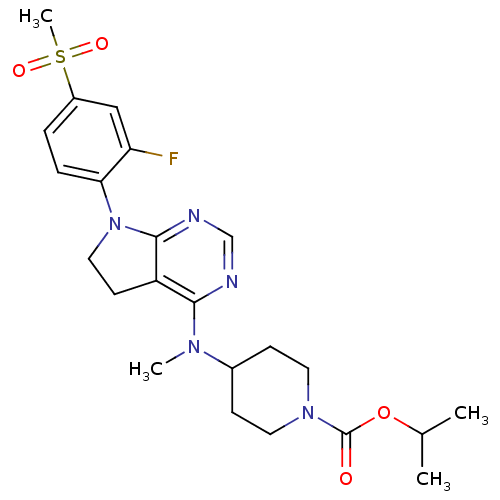 (CHEMBL2181677) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 631 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Agonist activity at human GPR119 receptor expressed in CHO-K1 cells co-expressing 6CRE-luciferase gene after 5 hrs by luciferase reporter gene assay | J Med Chem 55: 10972-94 (2012) Article DOI: 10.1021/jm301404a BindingDB Entry DOI: 10.7270/Q2RJ4KMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-dependent insulinotropic receptor (Homo sapiens (Human)) | BDBM50400379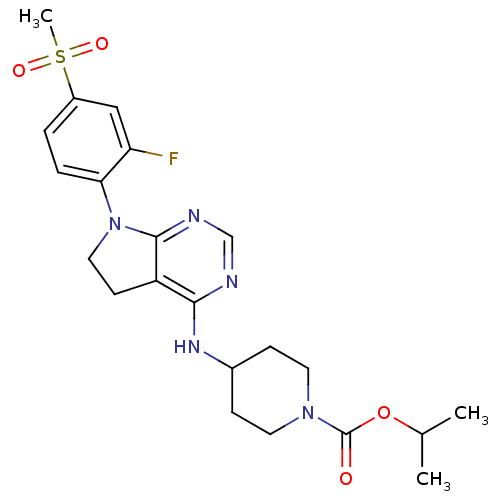 (CHEMBL2181676) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Agonist activity at human GPR119 receptor expressed in CHO-K1 cells co-expressing 6CRE-luciferase gene after 5 hrs by luciferase reporter gene assay | J Med Chem 55: 10972-94 (2012) Article DOI: 10.1021/jm301404a BindingDB Entry DOI: 10.7270/Q2RJ4KMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-dependent insulinotropic receptor (Homo sapiens (Human)) | BDBM50400380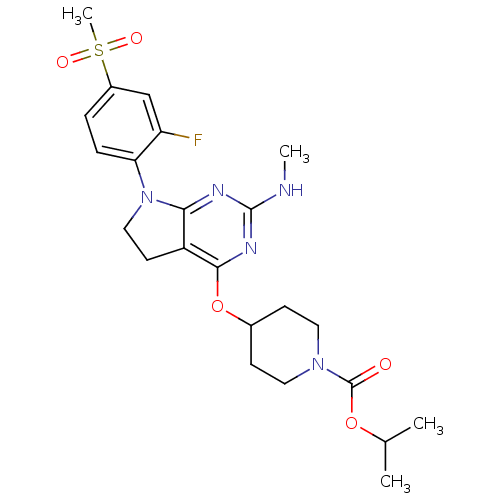 (CHEMBL2181675) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Agonist activity at human GPR119 receptor expressed in CHO-K1 cells co-expressing 6CRE-luciferase gene after 5 hrs by luciferase reporter gene assay | J Med Chem 55: 10972-94 (2012) Article DOI: 10.1021/jm301404a BindingDB Entry DOI: 10.7270/Q2RJ4KMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-dependent insulinotropic receptor (Homo sapiens (Human)) | BDBM50400381 (CHEMBL2177775) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 794 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Agonist activity at human GPR119 receptor expressed in CHO-K1 cells co-expressing 6CRE-luciferase gene after 5 hrs by luciferase reporter gene assay | J Med Chem 55: 10972-94 (2012) Article DOI: 10.1021/jm301404a BindingDB Entry DOI: 10.7270/Q2RJ4KMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-dependent insulinotropic receptor (Homo sapiens (Human)) | BDBM50400382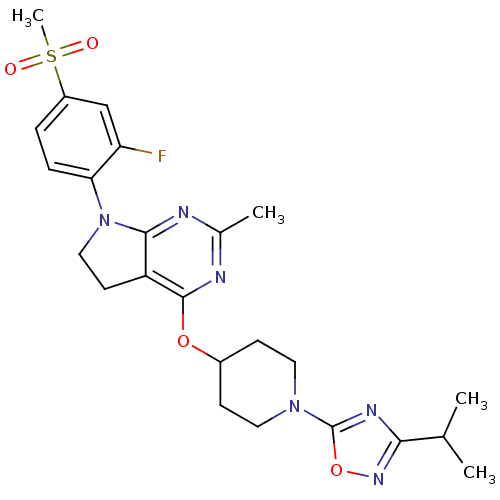 (CHEMBL2177774) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 251 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Agonist activity at human GPR119 receptor expressed in CHO-K1 cells co-expressing 6CRE-luciferase gene after 5 hrs by luciferase reporter gene assay | J Med Chem 55: 10972-94 (2012) Article DOI: 10.1021/jm301404a BindingDB Entry DOI: 10.7270/Q2RJ4KMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-dependent insulinotropic receptor (Homo sapiens (Human)) | BDBM50400383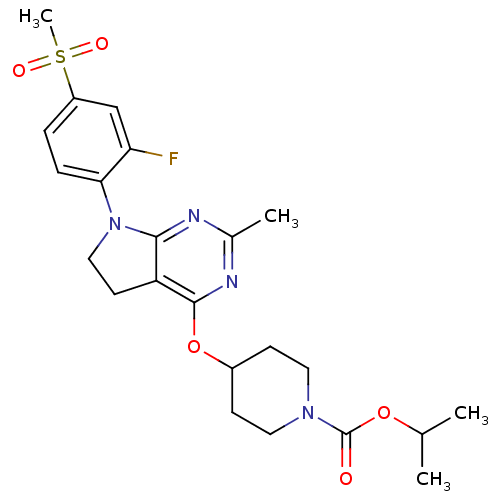 (CHEMBL2177773) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 100 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Agonist activity at human GPR119 receptor expressed in CHO-K1 cells co-expressing 6CRE-luciferase gene after 5 hrs by luciferase reporter gene assay | J Med Chem 55: 10972-94 (2012) Article DOI: 10.1021/jm301404a BindingDB Entry DOI: 10.7270/Q2RJ4KMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-dependent insulinotropic receptor (Homo sapiens (Human)) | BDBM50400384 (CHEMBL2177772) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 126 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Agonist activity at human GPR119 receptor expressed in CHO-K1 cells co-expressing 6CRE-luciferase gene after 5 hrs by luciferase reporter gene assay | J Med Chem 55: 10972-94 (2012) Article DOI: 10.1021/jm301404a BindingDB Entry DOI: 10.7270/Q2RJ4KMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-dependent insulinotropic receptor (Homo sapiens (Human)) | BDBM50400385 (CHEMBL2177771) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Agonist activity at human GPR119 receptor expressed in CHO-K1 cells co-expressing 6CRE-luciferase gene after 5 hrs by luciferase reporter gene assay | J Med Chem 55: 10972-94 (2012) Article DOI: 10.1021/jm301404a BindingDB Entry DOI: 10.7270/Q2RJ4KMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-dependent insulinotropic receptor (Homo sapiens (Human)) | BDBM50400386 (CHEMBL2177770) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 79.4 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Agonist activity at human GPR119 receptor expressed in CHO-K1 cells co-expressing 6CRE-luciferase gene after 5 hrs by luciferase reporter gene assay | J Med Chem 55: 10972-94 (2012) Article DOI: 10.1021/jm301404a BindingDB Entry DOI: 10.7270/Q2RJ4KMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-dependent insulinotropic receptor (Homo sapiens (Human)) | BDBM50400387 (CHEMBL2177769) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 50.1 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Agonist activity at human GPR119 receptor expressed in CHO-K1 cells co-expressing 6CRE-luciferase gene after 5 hrs by luciferase reporter gene assay | J Med Chem 55: 10972-94 (2012) Article DOI: 10.1021/jm301404a BindingDB Entry DOI: 10.7270/Q2RJ4KMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-dependent insulinotropic receptor (Homo sapiens (Human)) | BDBM50400388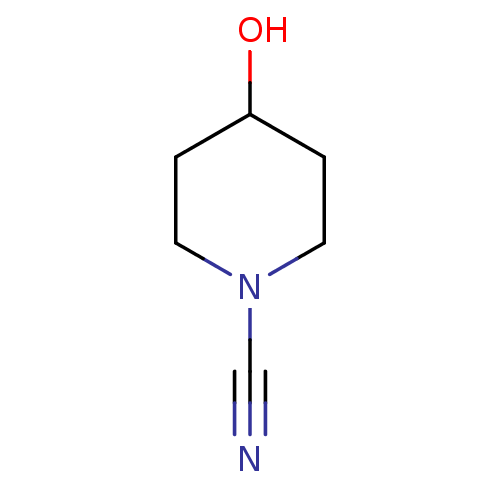 (CHEMBL2177768) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 316 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Agonist activity at human GPR119 receptor expressed in CHO-K1 cells co-expressing 6CRE-luciferase gene after 5 hrs by luciferase reporter gene assay | J Med Chem 55: 10972-94 (2012) Article DOI: 10.1021/jm301404a BindingDB Entry DOI: 10.7270/Q2RJ4KMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-dependent insulinotropic receptor (Homo sapiens (Human)) | BDBM50400389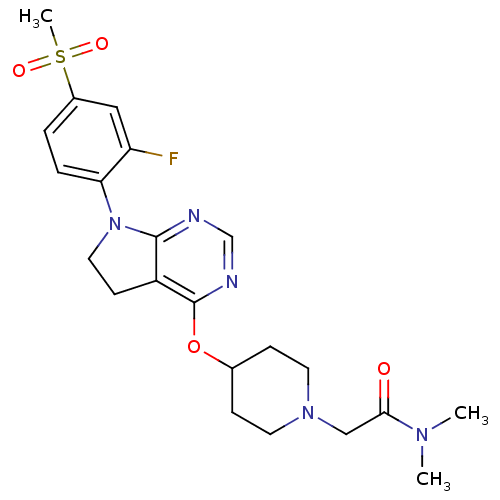 (CHEMBL2177767) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Agonist activity at human GPR119 receptor expressed in CHO-K1 cells co-expressing 6CRE-luciferase gene after 5 hrs by luciferase reporter gene assay | J Med Chem 55: 10972-94 (2012) Article DOI: 10.1021/jm301404a BindingDB Entry DOI: 10.7270/Q2RJ4KMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-dependent insulinotropic receptor (Homo sapiens (Human)) | BDBM50400376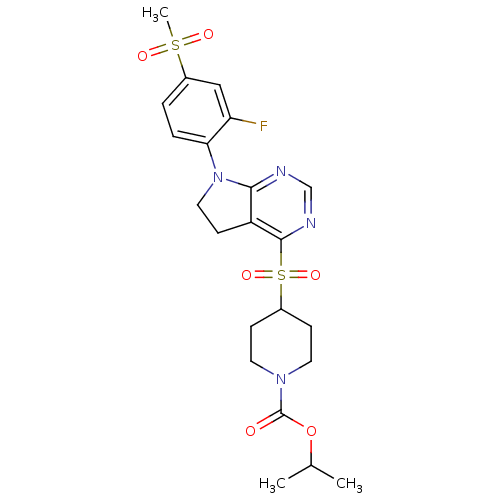 (CHEMBL2181679) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Agonist activity at human GPR119 receptor expressed in CHO-K1 cells co-expressing 6CRE-luciferase gene after 5 hrs by luciferase reporter gene assay | J Med Chem 55: 10972-94 (2012) Article DOI: 10.1021/jm301404a BindingDB Entry DOI: 10.7270/Q2RJ4KMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-dependent insulinotropic receptor (Homo sapiens (Human)) | BDBM50400391 (CHEMBL2177765) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.26E+3 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Agonist activity at human GPR119 receptor expressed in CHO-K1 cells co-expressing 6CRE-luciferase gene after 5 hrs by luciferase reporter gene assay | J Med Chem 55: 10972-94 (2012) Article DOI: 10.1021/jm301404a BindingDB Entry DOI: 10.7270/Q2RJ4KMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-dependent insulinotropic receptor (Homo sapiens (Human)) | BDBM50400392 (CHEMBL2177764) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.58E+3 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Agonist activity at human GPR119 receptor expressed in CHO-K1 cells co-expressing 6CRE-luciferase gene after 5 hrs by luciferase reporter gene assay | J Med Chem 55: 10972-94 (2012) Article DOI: 10.1021/jm301404a BindingDB Entry DOI: 10.7270/Q2RJ4KMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 80 total ) | Next | Last >> |