 Found 16 hits with Last Name = 'sartorelli' and Initial = 'ac'
Found 16 hits with Last Name = 'sartorelli' and Initial = 'ac' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Methylated-DNA--protein-cysteine methyltransferase
(Homo sapiens (Human)) | BDBM50397045

(CHEMBL485756)Show InChI InChI=1S/C13H14N6O/c14-5-8-2-1-3-9(4-8)6-20-12-10-11(17-7-16-10)18-13(15)19-12/h1-4,7H,5-6,14H2,(H3,15,16,17,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibition of AGT in human HL60 cells assessed as AGT levels using [3H]AGT after 2 hrs |
Bioorg Med Chem Lett 22: 6242-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.008
BindingDB Entry DOI: 10.7270/Q2F47Q7H |
More data for this
Ligand-Target Pair | |
Methylated-DNA--protein-cysteine methyltransferase
(Homo sapiens (Human)) | BDBM50397044

(CHEMBL2171205)Show InChI InChI=1S/C15H18N6O/c1-21(2)7-10-4-3-5-11(6-10)8-22-14-12-13(18-9-17-12)19-15(16)20-14/h3-6,9H,7-8H2,1-2H3,(H3,16,17,18,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibition of AGT in human HL60 cells assessed as AGT levels using [3H]AGT after 2 hrs |
Bioorg Med Chem Lett 22: 6242-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.008
BindingDB Entry DOI: 10.7270/Q2F47Q7H |
More data for this
Ligand-Target Pair | |
Methylated-DNA--protein-cysteine methyltransferase
(Homo sapiens (Human)) | BDBM5491

(6-(benzyloxy)-9H-purin-2-amine | CHEMBL407874 | O6...)Show InChI InChI=1S/C12H11N5O/c13-12-16-10-9(14-7-15-10)11(17-12)18-6-8-4-2-1-3-5-8/h1-5,7H,6H2,(H3,13,14,15,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibition of AGT in human HL60 cells assessed as AGT levels using [3H]AGT after 2 hrs |
Bioorg Med Chem Lett 22: 6242-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.008
BindingDB Entry DOI: 10.7270/Q2F47Q7H |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Methylated-DNA--protein-cysteine methyltransferase
(Homo sapiens (Human)) | BDBM50397043

(CHEMBL2171202)Show InChI InChI=1S/C15H16N6O2/c1-9(22)17-6-10-3-2-4-11(5-10)7-23-14-12-13(19-8-18-12)20-15(16)21-14/h2-5,8H,6-7H2,1H3,(H,17,22)(H3,16,18,19,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibition of AGT in human HL60 cells assessed as AGT levels using [3H]AGT after 2 hrs |
Bioorg Med Chem Lett 22: 6242-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.008
BindingDB Entry DOI: 10.7270/Q2F47Q7H |
More data for this
Ligand-Target Pair | |
Ribonucleoside-diphosphate reductase subunit M2
(Homo sapiens (Human)) | BDBM50051772
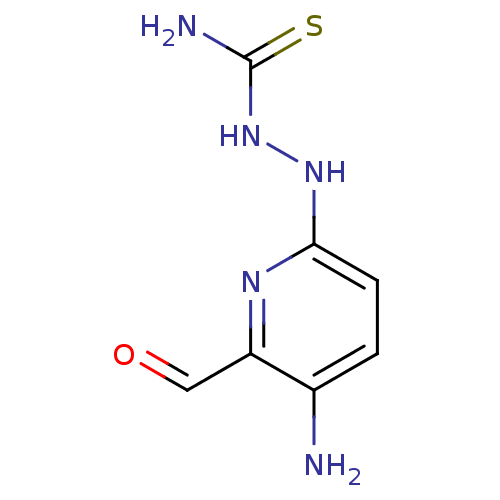
((Z)-N'-(5-amino-6-formylpyridin-2-yl)carbamohydraz...)Show InChI InChI=1S/C7H9N5OS/c8-4-1-2-6(10-5(4)3-13)11-12-7(9)14/h1-3H,8H2,(H,10,11)(H3,9,12,14) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 820 | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibitory activity against CDP reductase. |
J Med Chem 39: 2586-93 (1996)
Article DOI: 10.1021/jm9600454
BindingDB Entry DOI: 10.7270/Q2KS6QN9 |
More data for this
Ligand-Target Pair | |
Ribonucleoside-diphosphate reductase subunit M2
(Homo sapiens (Human)) | BDBM50051768
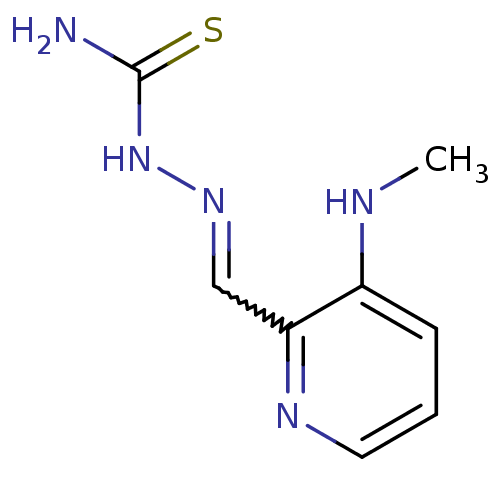
((1Z,N'E)-N'-((3-(methylamino)pyridin-2-yl)methylen...)Show InChI InChI=1S/C8H11N5S/c1-10-6-3-2-4-11-7(6)5-12-13-8(9)14/h2-5,10H,1H3,(H3,9,13,14) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibitory activity against CDP reductase. |
J Med Chem 39: 2586-93 (1996)
Article DOI: 10.1021/jm9600454
BindingDB Entry DOI: 10.7270/Q2KS6QN9 |
More data for this
Ligand-Target Pair | |
Ribonucleoside-diphosphate reductase subunit M2
(Homo sapiens (Human)) | BDBM50051776
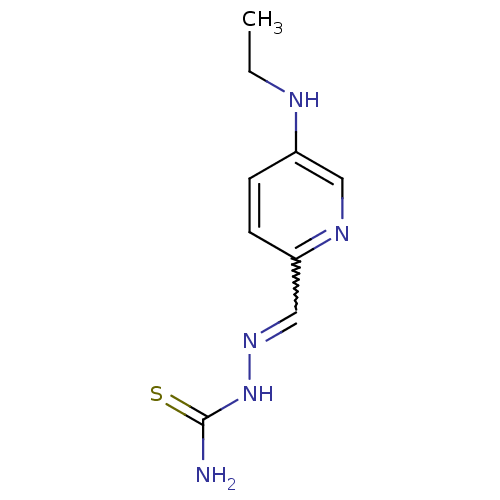
((1Z,N'E)-N'-((5-(ethylamino)pyridin-2-yl)methylene...)Show InChI InChI=1S/C9H13N5S/c1-2-11-7-3-4-8(12-5-7)6-13-14-9(10)15/h3-6,11H,2H2,1H3,(H3,10,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibitory activity against CDP reductase. |
J Med Chem 39: 2586-93 (1996)
Article DOI: 10.1021/jm9600454
BindingDB Entry DOI: 10.7270/Q2KS6QN9 |
More data for this
Ligand-Target Pair | |
Ribonucleoside-diphosphate reductase subunit M2
(Homo sapiens (Human)) | BDBM50051769

((1Z,N'E)-N'-((5-(methylamino)pyridin-2-yl)methylen...)Show InChI InChI=1S/C8H11N5S/c1-10-6-2-3-7(11-4-6)5-12-13-8(9)14/h2-5,10H,1H3,(H3,9,13,14) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibitory activity against CDP reductase. |
J Med Chem 39: 2586-93 (1996)
Article DOI: 10.1021/jm9600454
BindingDB Entry DOI: 10.7270/Q2KS6QN9 |
More data for this
Ligand-Target Pair | |
Ribonucleoside-diphosphate reductase subunit M2
(Homo sapiens (Human)) | BDBM50051767
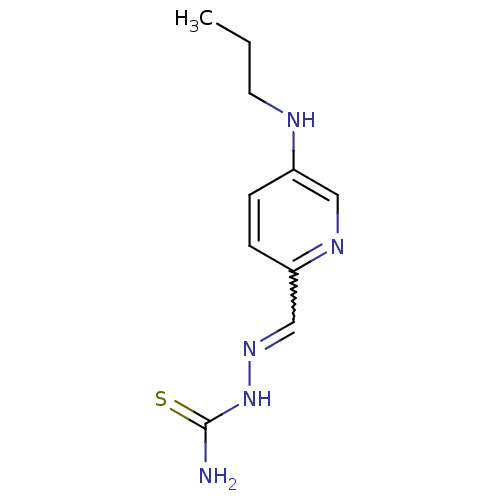
((1Z,N'E)-N'-((5-(propylamino)pyridin-2-yl)methylen...)Show InChI InChI=1S/C10H15N5S/c1-2-5-12-8-3-4-9(13-6-8)7-14-15-10(11)16/h3-4,6-7,12H,2,5H2,1H3,(H3,11,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibitory activity against CDP reductase. |
J Med Chem 39: 2586-93 (1996)
Article DOI: 10.1021/jm9600454
BindingDB Entry DOI: 10.7270/Q2KS6QN9 |
More data for this
Ligand-Target Pair | |
Ribonucleoside-diphosphate reductase subunit M2
(Homo sapiens (Human)) | BDBM50051775
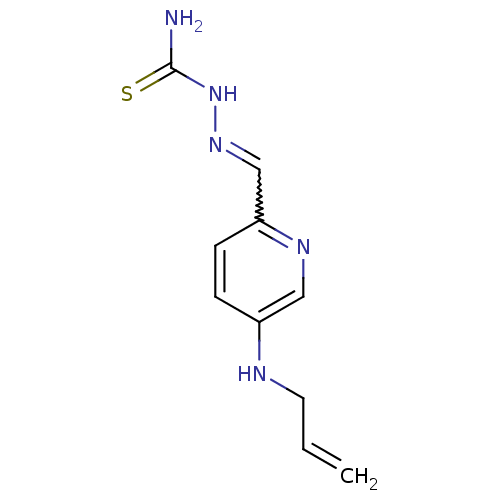
((1Z,N'E)-N'-((5-(allylamino)pyridin-2-yl)methylene...)Show InChI InChI=1S/C10H13N5S/c1-2-5-12-8-3-4-9(13-6-8)7-14-15-10(11)16/h2-4,6-7,12H,1,5H2,(H3,11,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibitory activity against CDP reductase. |
J Med Chem 39: 2586-93 (1996)
Article DOI: 10.1021/jm9600454
BindingDB Entry DOI: 10.7270/Q2KS6QN9 |
More data for this
Ligand-Target Pair | |
Ribonucleoside-diphosphate reductase subunit M2
(Homo sapiens (Human)) | BDBM50051774
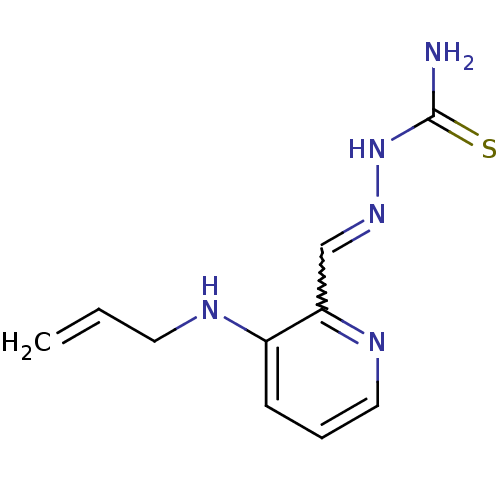
((1Z,N'E)-N'-((3-(allylamino)pyridin-2-yl)methylene...)Show InChI InChI=1S/C10H13N5S/c1-2-5-12-8-4-3-6-13-9(8)7-14-15-10(11)16/h2-4,6-7,12H,1,5H2,(H3,11,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibitory activity against CDP reductase. |
J Med Chem 39: 2586-93 (1996)
Article DOI: 10.1021/jm9600454
BindingDB Entry DOI: 10.7270/Q2KS6QN9 |
More data for this
Ligand-Target Pair | |
Ribonucleoside-diphosphate reductase subunit M2
(Homo sapiens (Human)) | BDBM50051766
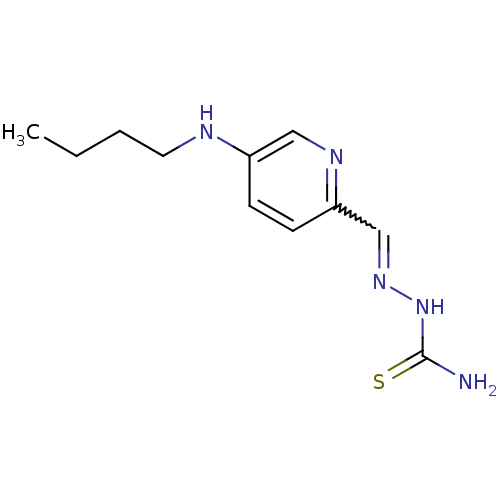
((1Z,N'E)-N'-((5-(butylamino)pyridin-2-yl)methylene...)Show InChI InChI=1S/C11H17N5S/c1-2-3-6-13-9-4-5-10(14-7-9)8-15-16-11(12)17/h4-5,7-8,13H,2-3,6H2,1H3,(H3,12,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibitory activity against CDP reductase. |
J Med Chem 39: 2586-93 (1996)
Article DOI: 10.1021/jm9600454
BindingDB Entry DOI: 10.7270/Q2KS6QN9 |
More data for this
Ligand-Target Pair | |
Ribonucleoside-diphosphate reductase subunit M2
(Homo sapiens (Human)) | BDBM50051777
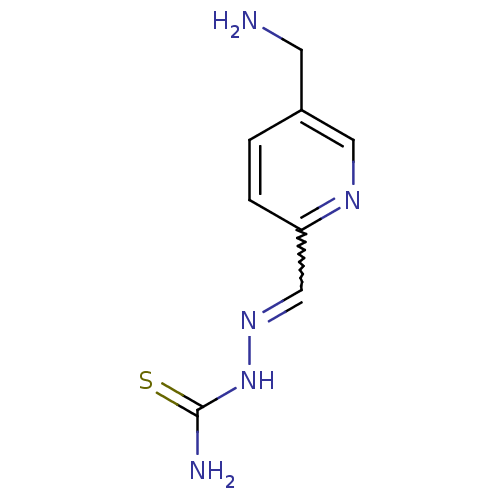
((1Z,N'E)-N'-((5-(aminomethyl)pyridin-2-yl)methylen...)Show InChI InChI=1S/C8H11N5S/c9-3-6-1-2-7(11-4-6)5-12-13-8(10)14/h1-2,4-5H,3,9H2,(H3,10,13,14) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibitory activity against CDP reductase. |
J Med Chem 39: 2586-93 (1996)
Article DOI: 10.1021/jm9600454
BindingDB Entry DOI: 10.7270/Q2KS6QN9 |
More data for this
Ligand-Target Pair | |
Ribonucleoside-diphosphate reductase subunit M2
(Homo sapiens (Human)) | BDBM50051773
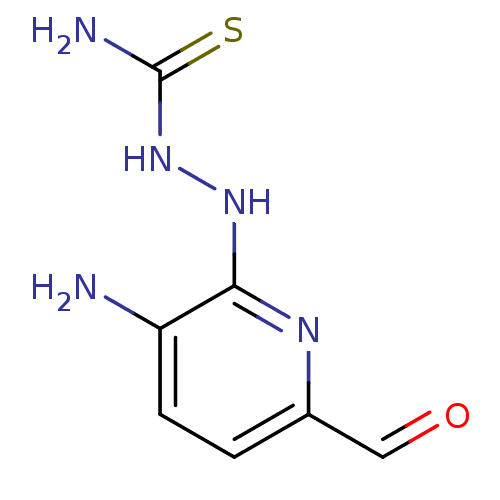
((Z)-N'-(3-amino-6-formylpyridin-2-yl)carbamohydraz...)Show InChI InChI=1S/C7H9N5OS/c8-5-2-1-4(3-13)10-6(5)11-12-7(9)14/h1-3H,8H2,(H,10,11)(H3,9,12,14) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibitory activity against CDP reductase. |
J Med Chem 39: 2586-93 (1996)
Article DOI: 10.1021/jm9600454
BindingDB Entry DOI: 10.7270/Q2KS6QN9 |
More data for this
Ligand-Target Pair | |
Ribonucleoside-diphosphate reductase subunit M2
(Homo sapiens (Human)) | BDBM50051771
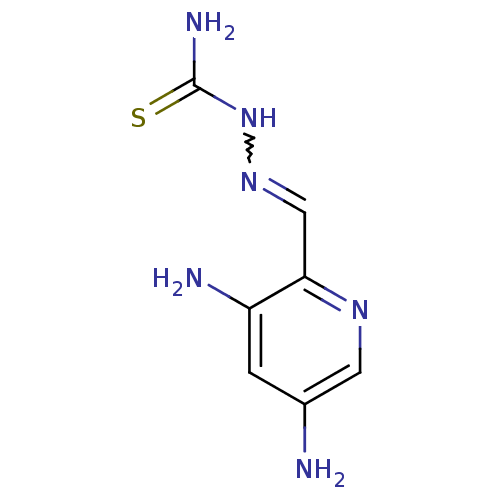
((1Z,N'E)-N'-((3,5-diaminopyridin-2-yl)methylene)ca...)Show InChI InChI=1S/C7H10N6S/c8-4-1-5(9)6(11-2-4)3-12-13-7(10)14/h1-3H,8-9H2,(H3,10,13,14) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibitory activity against CDP reductase. |
J Med Chem 39: 2586-93 (1996)
Article DOI: 10.1021/jm9600454
BindingDB Entry DOI: 10.7270/Q2KS6QN9 |
More data for this
Ligand-Target Pair | |
Ribonucleoside-diphosphate reductase subunit M2
(Homo sapiens (Human)) | BDBM50051770
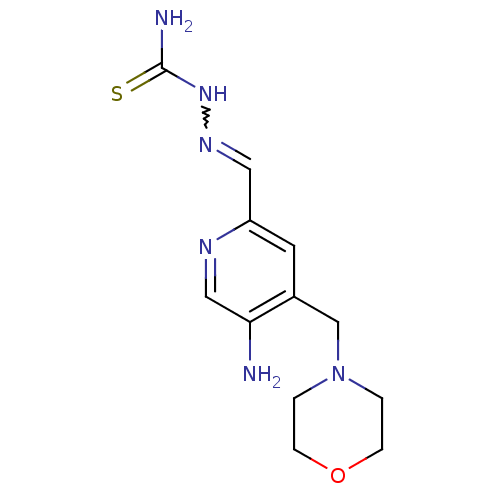
((1Z,N'E)-N'-((5-amino-4-(morpholinomethyl)pyridin-...)Show InChI InChI=1S/C12H18N6OS/c13-11-7-15-10(6-16-17-12(14)20)5-9(11)8-18-1-3-19-4-2-18/h5-7H,1-4,8,13H2,(H3,14,17,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibitory activity against CDP reductase. |
J Med Chem 39: 2586-93 (1996)
Article DOI: 10.1021/jm9600454
BindingDB Entry DOI: 10.7270/Q2KS6QN9 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data