

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM228279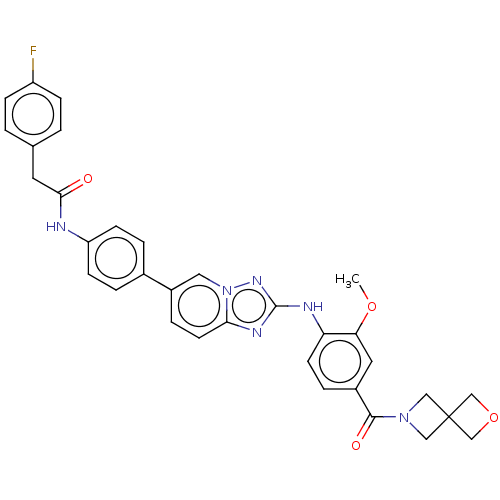 (US9555022, Example 02.17) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Inhibition of GST-tagged recombinant human MPS1 expressed in baculovirus expression system using biotin-Ahx-PWDPDDADITEILG as substrate preincubated ... | J Med Chem 63: 8025-8042 (2020) Article DOI: 10.1021/acs.jmedchem.9b02035 BindingDB Entry DOI: 10.7270/Q2MP56VP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM228278 (US9555022, Example 02.16) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Inhibition of GST-tagged recombinant human MPS1 expressed in baculovirus expression system using biotin-Ahx-PWDPDDADITEILG as substrate preincubated ... | J Med Chem 63: 8025-8042 (2020) Article DOI: 10.1021/acs.jmedchem.9b02035 BindingDB Entry DOI: 10.7270/Q2MP56VP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM50542581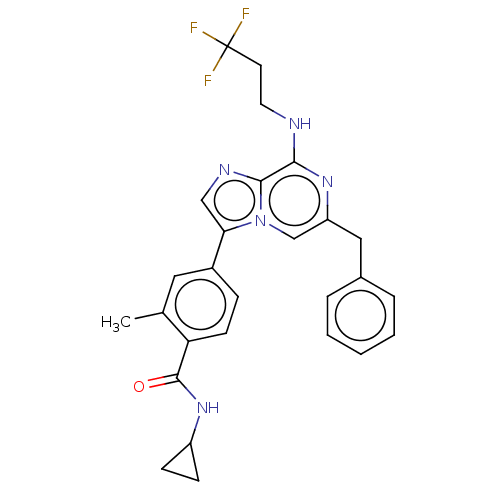 (CHEMBL4642174) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Inhibition of GST-tagged recombinant human MPS1 expressed in baculovirus expression system using biotin-Ahx-PWDPDDADITEILG as substrate preincubated ... | J Med Chem 63: 8025-8042 (2020) Article DOI: 10.1021/acs.jmedchem.9b02035 BindingDB Entry DOI: 10.7270/Q2MP56VP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM228270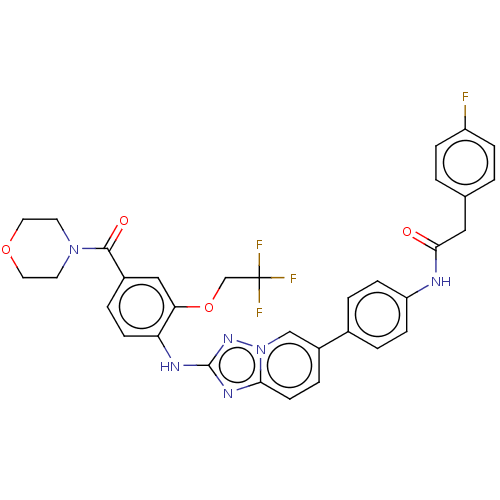 (US9555022, Example 02.09) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Inhibition of GST-tagged recombinant human MPS1 expressed in baculovirus expression system using biotin-Ahx-PWDPDDADITEILG as substrate preincubated ... | J Med Chem 63: 8025-8042 (2020) Article DOI: 10.1021/acs.jmedchem.9b02035 BindingDB Entry DOI: 10.7270/Q2MP56VP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM227802 (US10047096, 65 | rac-4-[(2-Fluoro-5-hydroxy-4-meth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.246 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description The kinase used was a recombinant fusion protein composed of N-terminal GST and a C-terminal fragment of human KDR (amino acids D807-V1356), expresse... | US Patent US10047096 (2018) BindingDB Entry DOI: 10.7270/Q23R0VW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM258444 (US9512130, 2) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Inhibition of MPS1 in human HeLa cells assessed as reduction in spindle assembly checkpoint incubated for 4 hrs by p-histone H3/Hoechst 33342 stainin... | J Med Chem 63: 8025-8042 (2020) Article DOI: 10.1021/acs.jmedchem.9b02035 BindingDB Entry DOI: 10.7270/Q2MP56VP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM227931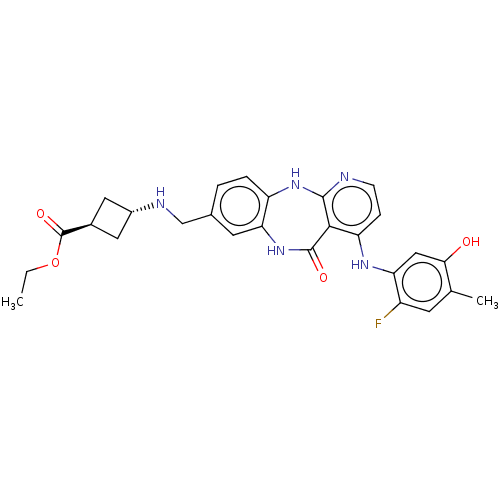 (US10047096, 192 | trans-Ethyl 3-[({4-[(2-fluoro-5-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.274 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description The kinase used was a recombinant fusion protein composed of N-terminal GST and a C-terminal fragment of human KDR (amino acids D807-V1356), expresse... | US Patent US10047096 (2018) BindingDB Entry DOI: 10.7270/Q23R0VW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM227801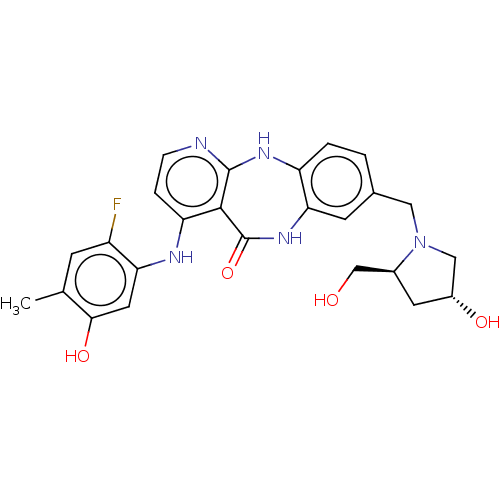 (4-[(2-Fluoro-5-hydroxy-4-methylphenyl)amino]-8-{[(...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.287 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description The kinase used was a recombinant fusion protein composed of N-terminal GST and a C-terminal fragment of human KDR (amino acids D807-V1356), expresse... | US Patent US10047096 (2018) BindingDB Entry DOI: 10.7270/Q23R0VW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM246227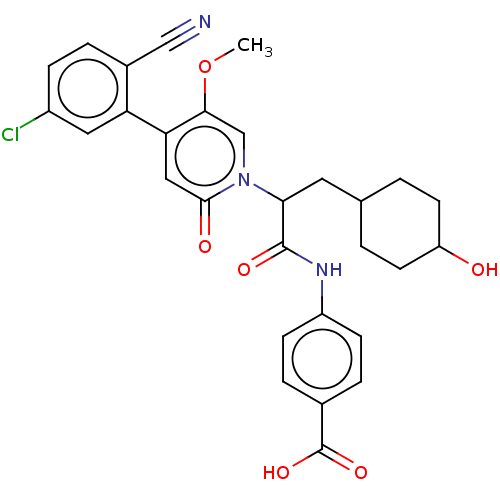 (US10183932, Example 135 | US9434690, 132 | US94346...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the factor XIa inhibition of the substances according to the invention, a biochemical test system is used which utilizes the reaction of... | US Patent US10183932 (2019) BindingDB Entry DOI: 10.7270/Q23T9K99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM246227 (US10183932, Example 135 | US9434690, 132 | US94346...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description Test substances are dissolved in dimethyl sulphoxide and serially diluted in dimethyl sulphoxide (3000 μM to 0.0078 μM; resulting final con... | US Patent US9822102 (2017) BindingDB Entry DOI: 10.7270/Q2FN18G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM246227 (US10183932, Example 135 | US9434690, 132 | US94346...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description Test substances are dissolved in dimethyl sulphoxide and serially diluted in dimethyl sulphoxide (3000 μM to 0.0078 μM; resulting final con... | US Patent US9434690 (2016) BindingDB Entry DOI: 10.7270/Q2K936FF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM50542592 (CHEMBL4645348) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Inhibition of GST-tagged recombinant human MPS1 expressed in baculovirus expression system using biotin-Ahx-PWDPDDADITEILG as substrate preincubated ... | J Med Chem 63: 8025-8042 (2020) Article DOI: 10.1021/acs.jmedchem.9b02035 BindingDB Entry DOI: 10.7270/Q2MP56VP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM259464 (US9512126, 1) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Inhibition of MPS1 in human HeLa cells assessed as reduction in spindle assembly checkpoint incubated for 4 hrs by p-histone H3/Hoechst 33342 stainin... | J Med Chem 63: 8025-8042 (2020) Article DOI: 10.1021/acs.jmedchem.9b02035 BindingDB Entry DOI: 10.7270/Q2MP56VP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM227926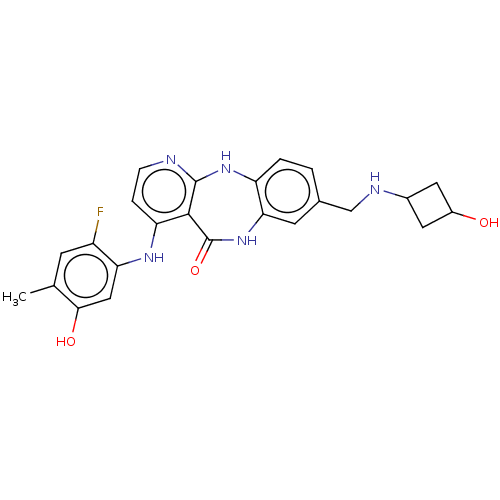 (4-[(2-Fluoro-5-hydroxy-4-methylphenyl)amino]-8-{[(...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.325 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description The kinase used was a recombinant fusion protein composed of N-terminal GST and a C-terminal fragment of human KDR (amino acids D807-V1356), expresse... | US Patent US10047096 (2018) BindingDB Entry DOI: 10.7270/Q23R0VW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM227794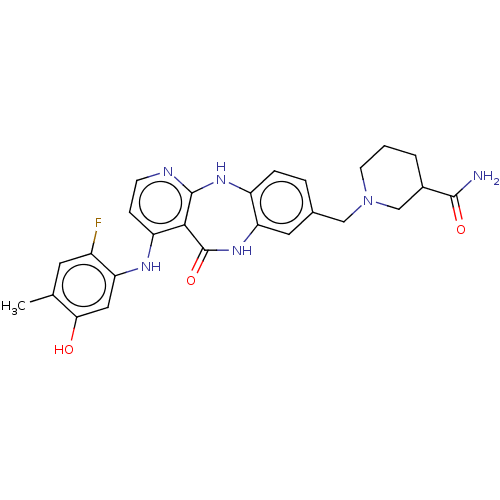 (US10047096, 57 | rac-1-({4-[(2-Fluoro-5-hydroxy-4-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description The kinase used was a recombinant fusion protein composed of N-terminal GST and a C-terminal fragment of human KDR (amino acids D807-V1356), expresse... | US Patent US10047096 (2018) BindingDB Entry DOI: 10.7270/Q23R0VW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM227785 (4-[(2-Fluoro-5-hydroxy-4-methylphenyl)amino]-8-[(i...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.363 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description The kinase used was a recombinant fusion protein composed of N-terminal GST and a C-terminal fragment of human KDR (amino acids D807-V1356), expresse... | US Patent US10047096 (2018) BindingDB Entry DOI: 10.7270/Q23R0VW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM227814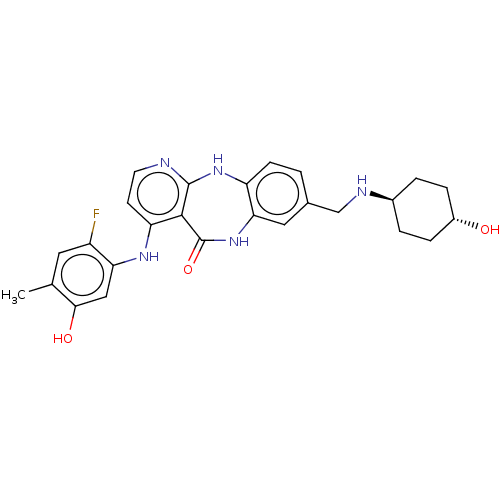 (4-[(2-Fluoro-5-hydroxy-4-methylphenyl)amino]-8-{[(...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.372 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description The kinase used was a recombinant fusion protein composed of N-terminal GST and a C-terminal fragment of human KDR (amino acids D807-V1356), expresse... | US Patent US10047096 (2018) BindingDB Entry DOI: 10.7270/Q23R0VW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM227902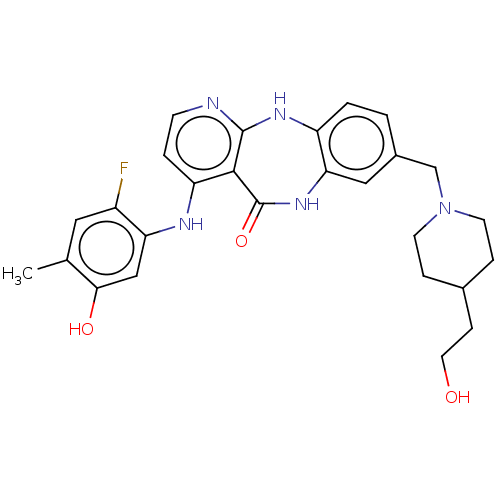 (4-[(2-Fluoro-5-hydroxy-4-methylphenyl)amino]-8-{[4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.388 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description The kinase used was a recombinant fusion protein composed of N-terminal GST and a C-terminal fragment of human KDR (amino acids D807-V1356), expresse... | US Patent US10047096 (2018) BindingDB Entry DOI: 10.7270/Q23R0VW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM227786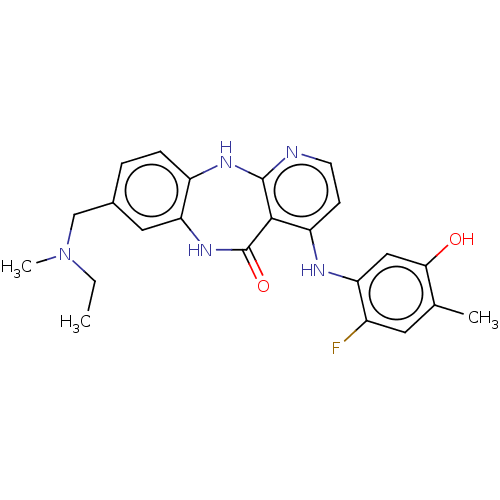 (8-{[Ethyl(methyl)amino]methyl}-4-[(2-fluoro-5-hydr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.388 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description The kinase used was a recombinant fusion protein composed of N-terminal GST and a C-terminal fragment of human KDR (amino acids D807-V1356), expresse... | US Patent US10047096 (2018) BindingDB Entry DOI: 10.7270/Q23R0VW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM227799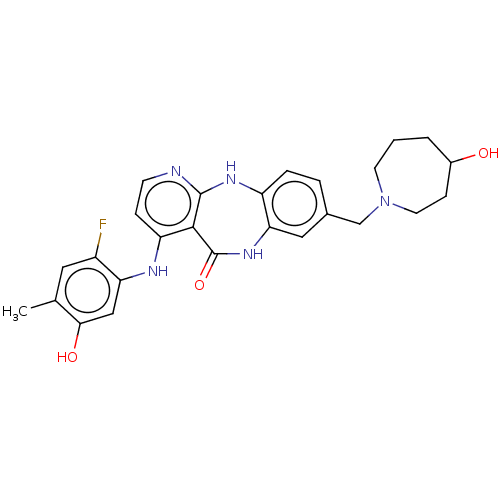 (US10047096, 62 | rac-4-[(2-Fluoro-5-hydroxy-4-meth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.394 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description The kinase used was a recombinant fusion protein composed of N-terminal GST and a C-terminal fragment of human KDR (amino acids D807-V1356), expresse... | US Patent US10047096 (2018) BindingDB Entry DOI: 10.7270/Q23R0VW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM50542591 (CHEMBL4640247) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Inhibition of MPS1 in human HeLa cells assessed as reduction in spindle assembly checkpoint incubated for 4 hrs by p-histone H3/Hoechst 33342 stainin... | J Med Chem 63: 8025-8042 (2020) Article DOI: 10.1021/acs.jmedchem.9b02035 BindingDB Entry DOI: 10.7270/Q2MP56VP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM227765 (8-[(Diethylamino)methyl]-4-[(2-fluoro-5-hydroxy-4-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.401 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description The kinase used was a recombinant fusion protein composed of N-terminal GST and a C-terminal fragment of human KDR (amino acids D807-V1356), expresse... | US Patent US10047096 (2018) BindingDB Entry DOI: 10.7270/Q23R0VW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM227906 (4-[(2-Fluoro-5-hydroxy-4-methylphenyl)amino]-8-{[(...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.407 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description The kinase used was a recombinant fusion protein composed of N-terminal GST and a C-terminal fragment of human KDR (amino acids D807-V1356), expresse... | US Patent US10047096 (2018) BindingDB Entry DOI: 10.7270/Q23R0VW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM227930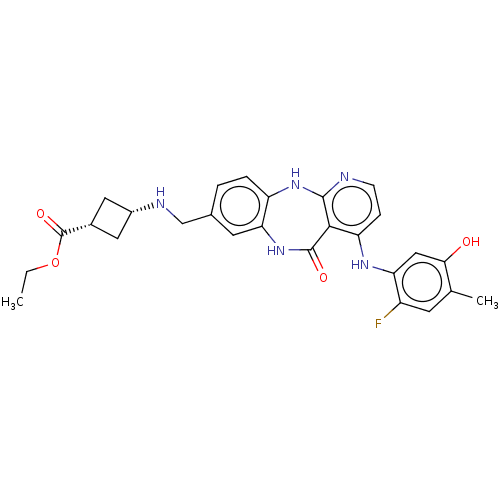 (US10047096, 191 | cis-Ethyl 3-[({4-[(2-fluoro-5-hy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.419 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description The kinase used was a recombinant fusion protein composed of N-terminal GST and a C-terminal fragment of human KDR (amino acids D807-V1356), expresse... | US Patent US10047096 (2018) BindingDB Entry DOI: 10.7270/Q23R0VW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM227816 (8-{[(1,3-Dihydroxypropan-2-yl)amino]methyl}-4-[(2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.429 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description The kinase used was a recombinant fusion protein composed of N-terminal GST and a C-terminal fragment of human KDR (amino acids D807-V1356), expresse... | US Patent US10047096 (2018) BindingDB Entry DOI: 10.7270/Q23R0VW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM227796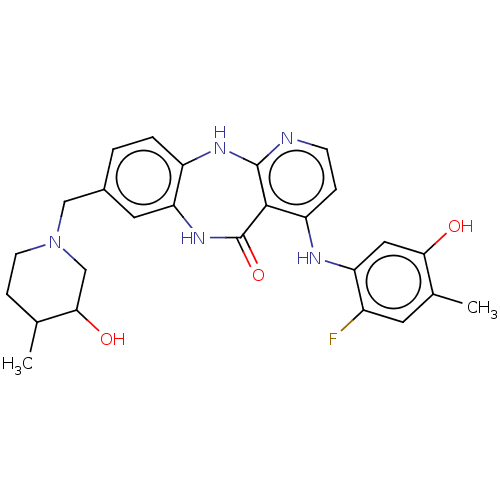 (4-[(2-Fluoro-5-hydroxy-4-methylphenyl)amino]-8-[(3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.432 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description The kinase used was a recombinant fusion protein composed of N-terminal GST and a C-terminal fragment of human KDR (amino acids D807-V1356), expresse... | US Patent US10047096 (2018) BindingDB Entry DOI: 10.7270/Q23R0VW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM227768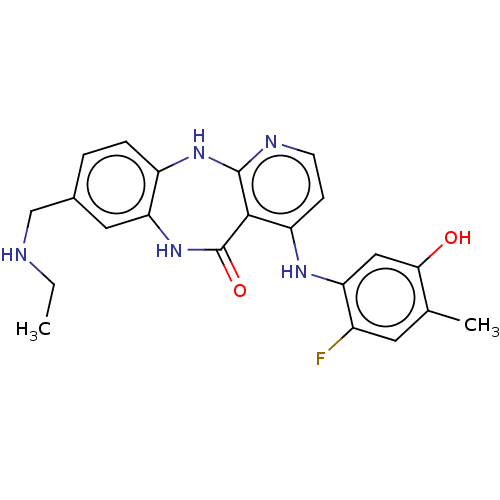 (8-[(Ethylamino)methyl]-4-[(2-fluoro-5-hydroxy-4-me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.448 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description The kinase used was a recombinant fusion protein composed of N-terminal GST and a C-terminal fragment of human KDR (amino acids D807-V1356), expresse... | US Patent US10047096 (2018) BindingDB Entry DOI: 10.7270/Q23R0VW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM227812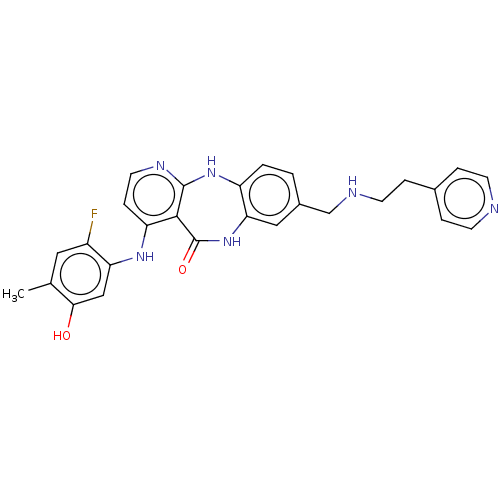 (4-[(2-Fluoro-5-hydroxy-4-methylphenyl)amino]-8-({[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.459 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description The kinase used was a recombinant fusion protein composed of N-terminal GST and a C-terminal fragment of human KDR (amino acids D807-V1356), expresse... | US Patent US10047096 (2018) BindingDB Entry DOI: 10.7270/Q23R0VW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM227771 (8-[(Cyclopentylamino)methyl]-4-[(2-fluoro-5-hydrox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.472 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description The kinase used was a recombinant fusion protein composed of N-terminal GST and a C-terminal fragment of human KDR (amino acids D807-V1356), expresse... | US Patent US10047096 (2018) BindingDB Entry DOI: 10.7270/Q23R0VW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM227792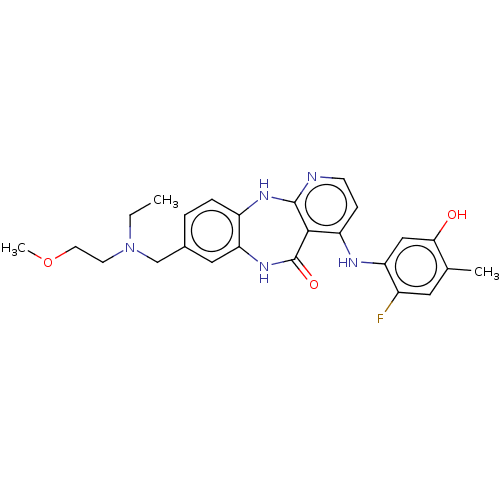 (8-{[Ethyl(2-methoxyethyl)amino]methyl}-4-[(2-fluor...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.473 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description The kinase used was a recombinant fusion protein composed of N-terminal GST and a C-terminal fragment of human KDR (amino acids D807-V1356), expresse... | US Patent US10047096 (2018) BindingDB Entry DOI: 10.7270/Q23R0VW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM227812 (4-[(2-Fluoro-5-hydroxy-4-methylphenyl)amino]-8-({[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.475 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description The kinase used was a recombinant fusion protein composed of N-terminal GST and a C-terminal fragment of human PDGFRβ (amino acids R561-L1106), expr... | US Patent US10047096 (2018) BindingDB Entry DOI: 10.7270/Q23R0VW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM227786 (8-{[Ethyl(methyl)amino]methyl}-4-[(2-fluoro-5-hydr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.487 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description The kinase used was a recombinant fusion protein composed of N-terminal GST and a C-terminal fragment of human PDGFRβ (amino acids R561-L1106), expr... | US Patent US10047096 (2018) BindingDB Entry DOI: 10.7270/Q23R0VW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM227807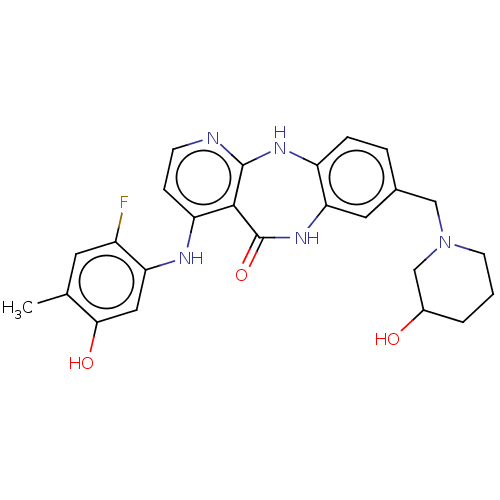 (US10047096, 69 | US10047096, 70 | US10047096, 71 |...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.496 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description The kinase used was a recombinant fusion protein composed of N-terminal GST and a C-terminal fragment of human KDR (amino acids D807-V1356), expresse... | US Patent US10047096 (2018) BindingDB Entry DOI: 10.7270/Q23R0VW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM227782 (4-[(2-Fluoro-5-hydroxy-4-methylphenyl)amino]-8-[(4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.498 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description The kinase used was a recombinant fusion protein composed of N-terminal GST and a C-terminal fragment of human KDR (amino acids D807-V1356), expresse... | US Patent US10047096 (2018) BindingDB Entry DOI: 10.7270/Q23R0VW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM246196 (US10183932, Example 105 | US9434690, 101 | US94346...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the factor XIa inhibition of the substances according to the invention, a biochemical test system is used which utilizes the reaction of... | US Patent US10183932 (2019) BindingDB Entry DOI: 10.7270/Q23T9K99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM341292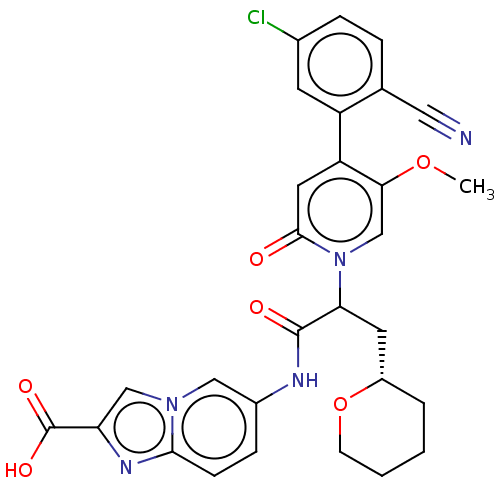 (6-({2-[4-(5-Chloro-2-cyanophenyl)-5-methoxy-2-oxop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description Test substances are dissolved in dimethyl sulphoxide and serially diluted in dimethyl sulphoxide (3000 μM to 0.0078 μM; resulting final con... | US Patent US9765070 (2017) BindingDB Entry DOI: 10.7270/Q25D8TZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM246196 (US10183932, Example 105 | US9434690, 101 | US94346...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description Test substances are dissolved in dimethyl sulphoxide and serially diluted in dimethyl sulphoxide (3000 μM to 0.0078 μM; resulting final con... | US Patent US9822102 (2017) BindingDB Entry DOI: 10.7270/Q2FN18G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM329340 ((2R)—N-{4-[2-({4-[(3-fluoroazetidin-1-yl)carb...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Inhibition of GST-tagged recombinant human MPS1 expressed in baculovirus expression system using biotin-Ahx-PWDPDDADITEILG as substrate preincubated ... | J Med Chem 63: 8025-8042 (2020) Article DOI: 10.1021/acs.jmedchem.9b02035 BindingDB Entry DOI: 10.7270/Q2MP56VP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM50542581 (CHEMBL4642174) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Inhibition of GST-tagged recombinant human MPS1 expressed in baculovirus expression system using biotin-Ahx-PWDPDDADITEILG as substrate preincubated ... | J Med Chem 63: 8025-8042 (2020) Article DOI: 10.1021/acs.jmedchem.9b02035 BindingDB Entry DOI: 10.7270/Q2MP56VP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM50542583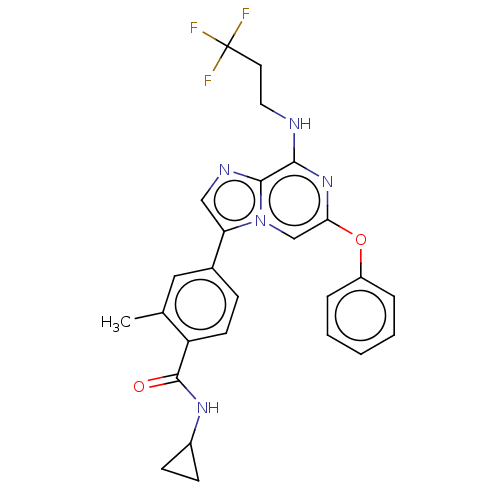 (CHEMBL4634949) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Inhibition of GST-tagged recombinant human MPS1 expressed in baculovirus expression system using biotin-Ahx-PWDPDDADITEILG as substrate preincubated ... | J Med Chem 63: 8025-8042 (2020) Article DOI: 10.1021/acs.jmedchem.9b02035 BindingDB Entry DOI: 10.7270/Q2MP56VP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM246196 (US10183932, Example 105 | US9434690, 101 | US94346...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description Test substances are dissolved in dimethyl sulphoxide and serially diluted in dimethyl sulphoxide (3000 μM to 0.0078 μM; resulting final con... | US Patent US9434690 (2016) BindingDB Entry DOI: 10.7270/Q2K936FF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM259464 (US9512126, 1) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Inhibition of GST-tagged recombinant human MPS1 expressed in baculovirus expression system using biotin-Ahx-PWDPDDADITEILG as substrate preincubated ... | J Med Chem 63: 8025-8042 (2020) Article DOI: 10.1021/acs.jmedchem.9b02035 BindingDB Entry DOI: 10.7270/Q2MP56VP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM50542591 (CHEMBL4640247) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Inhibition of GST-tagged recombinant human MPS1 expressed in baculovirus expression system using biotin-Ahx-PWDPDDADITEILG as substrate preincubated ... | J Med Chem 63: 8025-8042 (2020) Article DOI: 10.1021/acs.jmedchem.9b02035 BindingDB Entry DOI: 10.7270/Q2MP56VP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM50542592 (CHEMBL4645348) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Inhibition of GST-tagged recombinant human MPS1 expressed in baculovirus expression system using biotin-Ahx-PWDPDDADITEILG as substrate preincubated ... | J Med Chem 63: 8025-8042 (2020) Article DOI: 10.1021/acs.jmedchem.9b02035 BindingDB Entry DOI: 10.7270/Q2MP56VP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM227925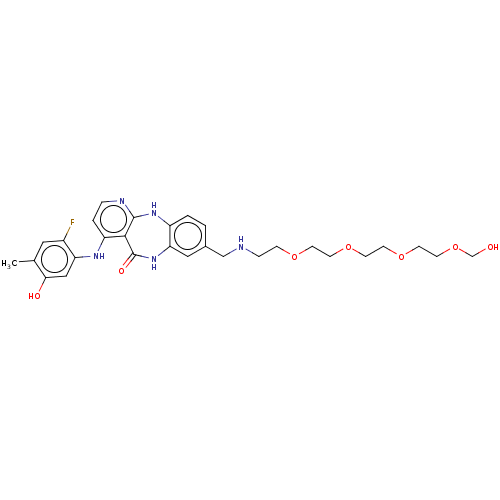 (4-[(2-Fluoro-5-hydroxy-4-methylphenyl)amino]-8-(13...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.503 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description The kinase used was a recombinant fusion protein composed of N-terminal GST and a C-terminal fragment of human KDR (amino acids D807-V1356), expresse... | US Patent US10047096 (2018) BindingDB Entry DOI: 10.7270/Q23R0VW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM227929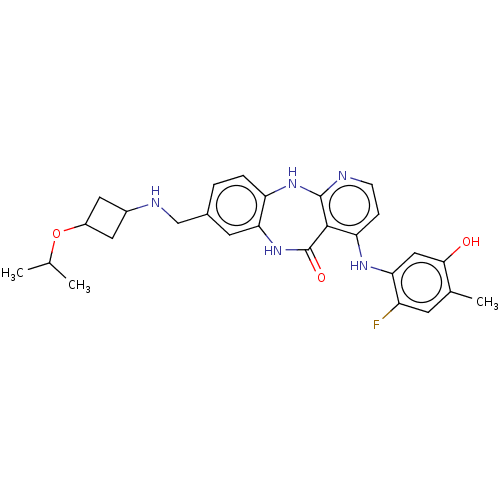 (4-[(2-Fluoro-5-hydroxy-4-methylphenyl)amino]-8-{[(...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.503 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description The kinase used was a recombinant fusion protein composed of N-terminal GST and a C-terminal fragment of human KDR (amino acids D807-V1356), expresse... | US Patent US10047096 (2018) BindingDB Entry DOI: 10.7270/Q23R0VW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM227830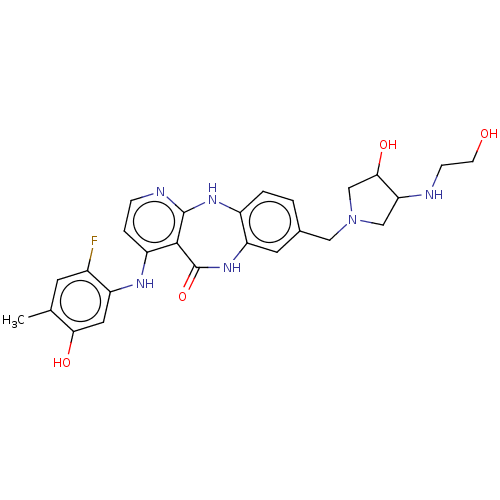 (4-[(2-Fluoro-5-hydroxy-4-methylphenyl)amino]-8-({3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.522 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description The kinase used was a recombinant fusion protein composed of N-terminal GST and a C-terminal fragment of human KDR (amino acids D807-V1356), expresse... | US Patent US10047096 (2018) BindingDB Entry DOI: 10.7270/Q23R0VW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM227790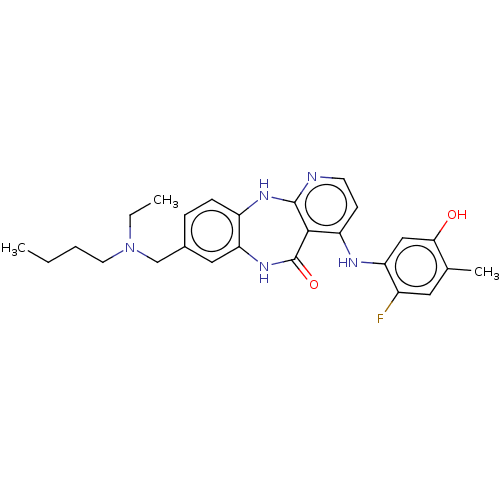 (8-{[Butyl(ethyl)amino]methyl}-4-[(2-fluoro-5-hydro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.525 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description The kinase used was a recombinant fusion protein composed of N-terminal GST and a C-terminal fragment of human KDR (amino acids D807-V1356), expresse... | US Patent US10047096 (2018) BindingDB Entry DOI: 10.7270/Q23R0VW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM227795 (BDBM227897 | US10047096, 58 | rac-4-[(2-Fluoro-5-h...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.548 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description The kinase used was a recombinant fusion protein composed of N-terminal GST and a C-terminal fragment of human KDR (amino acids D807-V1356), expresse... | US Patent US10047096 (2018) BindingDB Entry DOI: 10.7270/Q23R0VW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM227934 (US10047096, 195 | trans-3-[({4-[(2-Fluoro-5-hydrox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.573 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description The kinase used was a recombinant fusion protein composed of N-terminal GST and a C-terminal fragment of human KDR (amino acids D807-V1356), expresse... | US Patent US10047096 (2018) BindingDB Entry DOI: 10.7270/Q23R0VW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2979 total ) | Next | Last >> |