

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50405400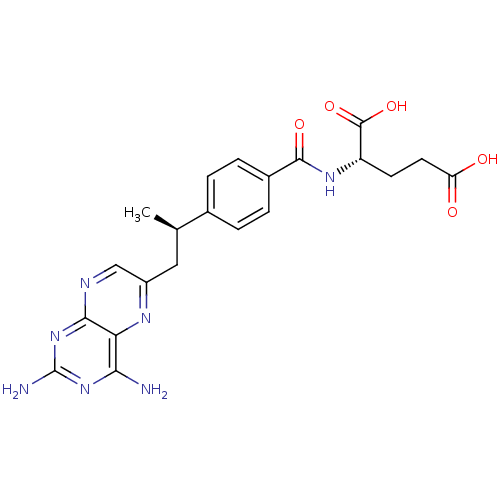 (CHEMBL2051987) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for binding affinity against Dihydrofolate reductase of L1210 cells | J Med Chem 29: 1056-61 (1986) BindingDB Entry DOI: 10.7270/Q2P55MHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50405400 (CHEMBL2051987) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for binding affinity against Dihydrofolate reductase of L1210 cells | J Med Chem 29: 1056-61 (1986) BindingDB Entry DOI: 10.7270/Q2P55MHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50226274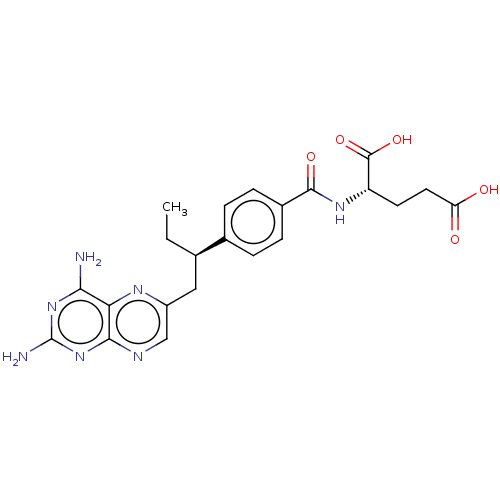 (CHEMBL3349020) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | PubMed | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for binding affinity against Dihydrofolate reductase of L1210 cells | J Med Chem 29: 1056-61 (1986) BindingDB Entry DOI: 10.7270/Q2P55MHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for binding affinity against Dihydrofolate reductase of L1210 cells | J Med Chem 29: 1056-61 (1986) BindingDB Entry DOI: 10.7270/Q2P55MHM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50026389 (2-{4-[(2,4-Diamino-pteridin-6-ylmethyl)-methyl-ami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against Dihydrofolate reductase in L1210 cell in mice | J Med Chem 28: 1016-25 (1985) BindingDB Entry DOI: 10.7270/Q2BP01TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50026392 (2-[3-(2-Carboxy-2-{4-[(2,4-diamino-pteridin-6-ylme...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00471 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against Dihydrofolate reductase in L1210 cell in mice | J Med Chem 28: 1016-25 (1985) BindingDB Entry DOI: 10.7270/Q2BP01TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50026399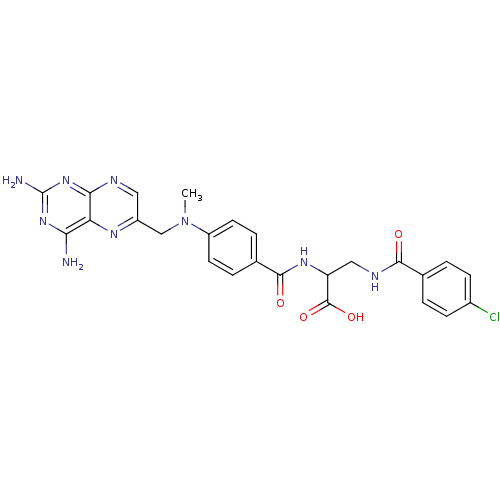 (3-(4-Chloro-benzoylamino)-2-{4-[(2,4-diamino-pteri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00498 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against Dihydrofolate reductase in L1210 cell in mice | J Med Chem 28: 1016-25 (1985) BindingDB Entry DOI: 10.7270/Q2BP01TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50026391 (5-(4-Chloro-benzoylamino)-2-{4-[(2,4-diamino-pteri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00531 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against Dihydrofolate reductase in L1210 cell in mice | J Med Chem 28: 1016-25 (1985) BindingDB Entry DOI: 10.7270/Q2BP01TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | 0.00581 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against Dihydrofolate reductase in L1210 cell in mice | J Med Chem 28: 1016-25 (1985) BindingDB Entry DOI: 10.7270/Q2BP01TS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against Dihydrofolate reductase in L1210 cell in mice | J Med Chem 28: 1016-25 (1985) BindingDB Entry DOI: 10.7270/Q2BP01TS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50026402 (4-(4-Chloro-benzoylamino)-2-{4-[(2,4-diamino-pteri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00642 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against Dihydrofolate reductase in L1210 cell in mice | J Med Chem 28: 1016-25 (1985) BindingDB Entry DOI: 10.7270/Q2BP01TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50405401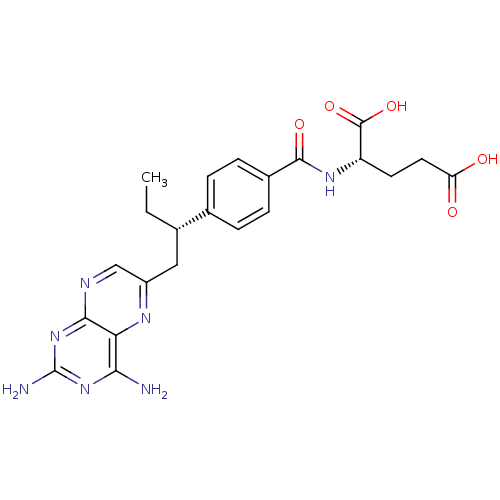 (CHEMBL2051990) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for binding affinity against Dihydrofolate reductase of L1210 cells | J Med Chem 29: 1056-61 (1986) BindingDB Entry DOI: 10.7270/Q2P55MHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50130172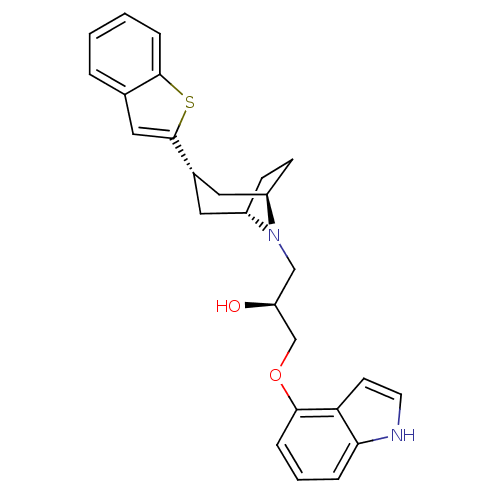 (1-(3-Benzo[b]thiophen-2-yl-8-aza-bicyclo[3.2.1]oct...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro affinity of the compound at the 5-HT reuptake site using [3H]-paroxetine as radioligand in rat frontal cortex membranes | Bioorg Med Chem Lett 13: 2393-7 (2003) BindingDB Entry DOI: 10.7270/Q2Z31Z1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 2 (Homo sapiens (Human)) | BDBM85212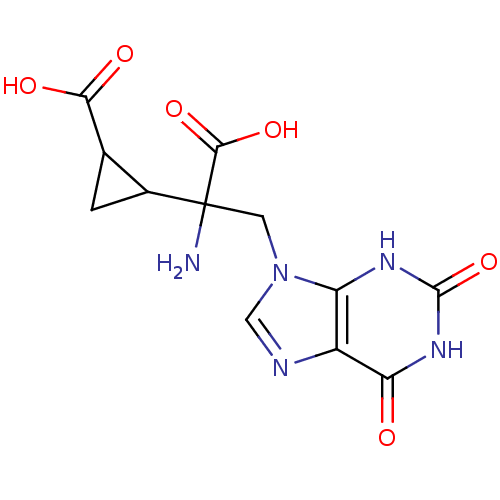 (CAS_5311260 | LY341495 | NSC_5311260) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 0.0780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by PDSP Ki Database | J Neurochem 71: 2558-64 (1998) Article DOI: 10.1046/j.1471-4159.1998.71062558.x BindingDB Entry DOI: 10.7270/Q25D8QCZ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Rattus norvegicus (rat)) | BDBM21392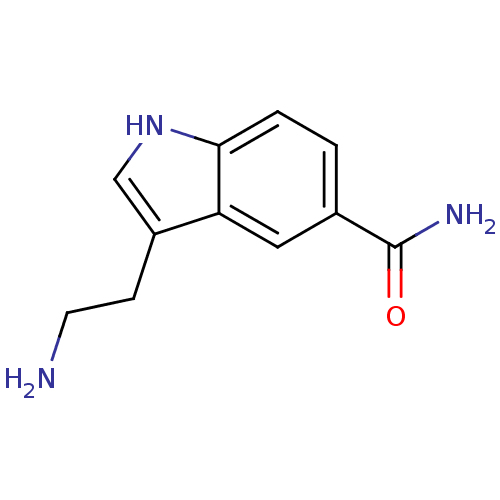 (3-(2-aminoethyl)-1H-indole-5-carboxamide | 5-CT | ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
State University of Groningen Curated by ChEMBL | Assay Description Binding affinity at rat 5-hydroxytryptamine 7 receptor. | J Med Chem 46: 5365-74 (2003) Article DOI: 10.1021/jm030826m BindingDB Entry DOI: 10.7270/Q2K93B8V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398598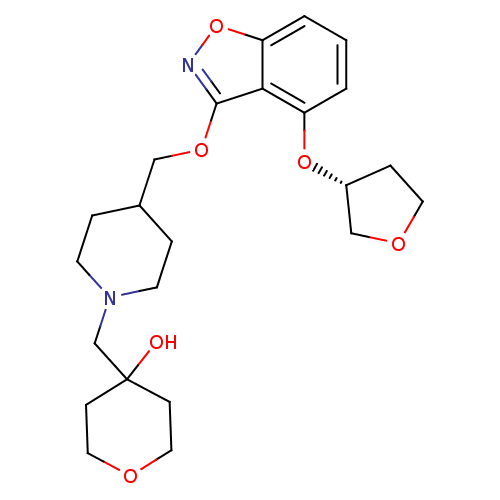 (CHEMBL2152922) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from human 5HT4D receptor expressed in HEK293 cells after 30 mins by liquid scintillation counting | J Med Chem 55: 9240-54 (2012) Article DOI: 10.1021/jm300953p BindingDB Entry DOI: 10.7270/Q2FQ9XRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50130170 ((S)-1-(1H-Indol-4-yloxy)-3-[(1R,3S,5S)-3-(4-methox...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity at 5-hydroxytryptamine 1A receptor by [3H]-8-OH-DPAT displacement. | Bioorg Med Chem Lett 13: 2393-7 (2003) BindingDB Entry DOI: 10.7270/Q2Z31Z1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50130170 ((S)-1-(1H-Indol-4-yloxy)-3-[(1R,3S,5S)-3-(4-methox...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro affinity of the compound at the 5-HT reuptake site using [3H]-paroxetine as radioligand in rat frontal cortex membranes | Bioorg Med Chem Lett 13: 2393-7 (2003) BindingDB Entry DOI: 10.7270/Q2Z31Z1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50135254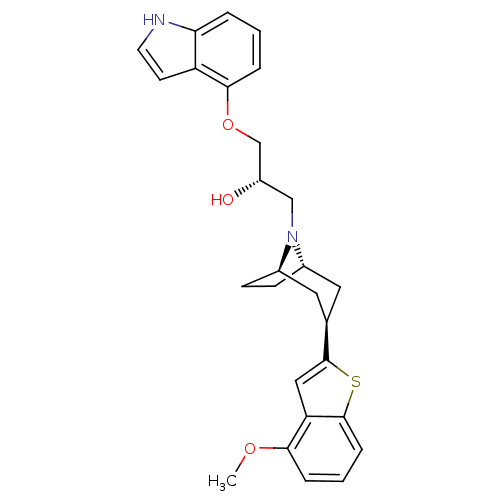 ((S)-1-(1H-Indol-4-yloxy)-3-[(1S,3R,5R)-3-(4-methox...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Affinity at the 5-HT reuptake site labeled with [3H]-paroxetine using rat frontal cortex membranes | Bioorg Med Chem Lett 13: 3939-42 (2003) BindingDB Entry DOI: 10.7270/Q24T6HSX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50135249 ((S)-1-((2S,4R)-4-(5-fluorobenzo[b]thiophen-2-yl)-2...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Affinity at the 5-HT reuptake site labeled with [3H]-paroxetine using rat frontal cortex membranes | Bioorg Med Chem Lett 13: 3939-42 (2003) BindingDB Entry DOI: 10.7270/Q24T6HSX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50135249 ((S)-1-((2S,4R)-4-(5-fluorobenzo[b]thiophen-2-yl)-2...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]paroxetine from 5HT reuptake site | Bioorg Med Chem Lett 16: 2347-51 (2006) Article DOI: 10.1016/j.bmcl.2005.11.007 BindingDB Entry DOI: 10.7270/Q2W958RV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Rattus norvegicus (rat)) | BDBM50474418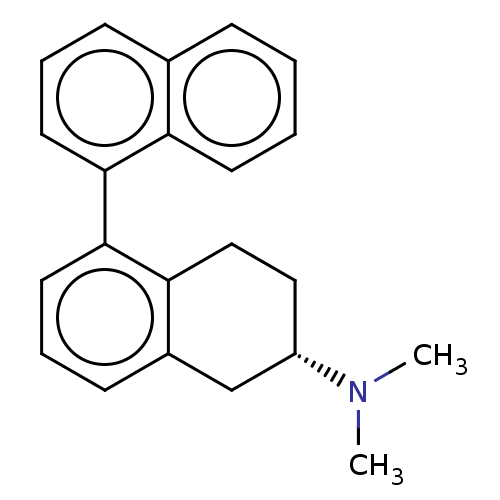 (CHEMBL611472) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
State University of Groningen Curated by ChEMBL | Assay Description Binding affinity at rat 5-hydroxytryptamine 7 receptor. | J Med Chem 46: 5365-74 (2003) Article DOI: 10.1021/jm030826m BindingDB Entry DOI: 10.7270/Q2K93B8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50130163 ((S)-1-(1H-Indol-4-yloxy)-3-[(4S,6R)-4-(4-methoxy-b...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro affinity of the compound at the 5-HT reuptake site using [3H]-paroxetine as radioligand in rat frontal cortex membranes | Bioorg Med Chem Lett 13: 2393-7 (2003) BindingDB Entry DOI: 10.7270/Q2Z31Z1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398597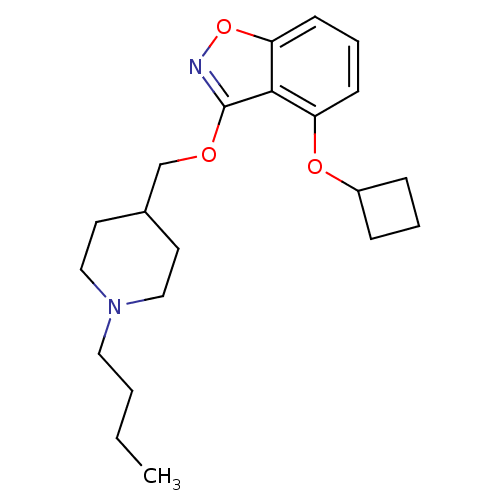 (CHEMBL2179584) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from human 5HT4D receptor expressed in HEK293 cells after 30 mins by liquid scintillation counting | J Med Chem 55: 9240-54 (2012) Article DOI: 10.1021/jm300953p BindingDB Entry DOI: 10.7270/Q2FQ9XRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50130163 ((S)-1-(1H-Indol-4-yloxy)-3-[(4S,6R)-4-(4-methoxy-b...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]paroxetine from 5HT reuptake site | Bioorg Med Chem Lett 16: 2347-51 (2006) Article DOI: 10.1016/j.bmcl.2005.11.007 BindingDB Entry DOI: 10.7270/Q2W958RV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398593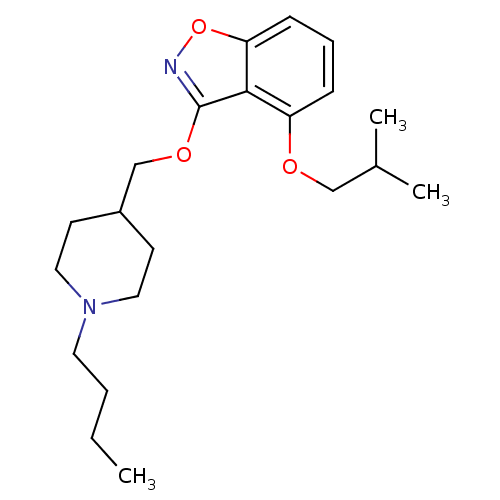 (CHEMBL2179587) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from human 5HT4D receptor expressed in HEK293 cells after 30 mins by liquid scintillation counting | J Med Chem 55: 9240-54 (2012) Article DOI: 10.1021/jm300953p BindingDB Entry DOI: 10.7270/Q2FQ9XRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (RAT) | BDBM50398598 (CHEMBL2152922) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from 5HT4 receptor in rat striatal membrane after 30 mins by liquid scintillation counting | J Med Chem 55: 9240-54 (2012) Article DOI: 10.1021/jm300953p BindingDB Entry DOI: 10.7270/Q2FQ9XRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50145598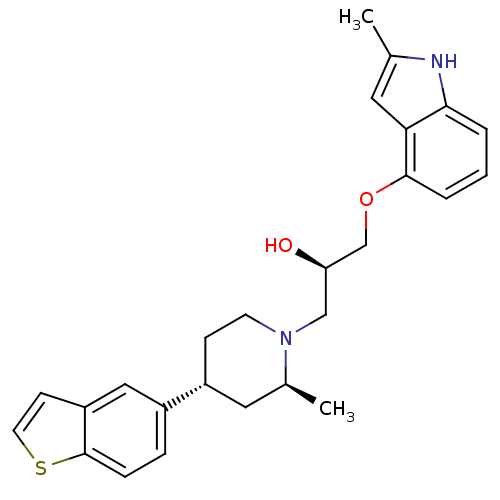 ((R)-1-((3S,4R)-4-Benzo[b]thiophen-5-yl-2-methyl-pi...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity at 5-HT reuptake site labeled with [3H]-paroxetine | Bioorg Med Chem Lett 14: 2653-6 (2004) Article DOI: 10.1016/j.bmcl.2004.02.088 BindingDB Entry DOI: 10.7270/Q2GF0SZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50130152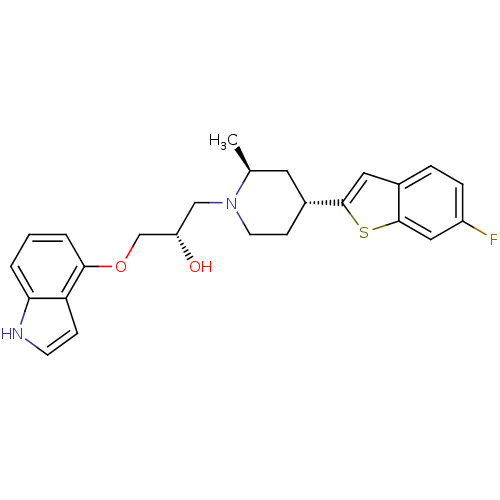 ((S)-1-(1H-indol-4-yloxy)-3-((2S,4R)-4-(6-fluoroben...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro affinity of the compound at the 5-HT reuptake site using [3H]-paroxetine as radioligand in rat frontal cortex membranes | Bioorg Med Chem Lett 13: 2393-7 (2003) BindingDB Entry DOI: 10.7270/Q2Z31Z1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50130152 ((S)-1-(1H-indol-4-yloxy)-3-((2S,4R)-4-(6-fluoroben...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]paroxetine from 5HT reuptake site | Bioorg Med Chem Lett 16: 2347-51 (2006) Article DOI: 10.1016/j.bmcl.2005.11.007 BindingDB Entry DOI: 10.7270/Q2W958RV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398598 (CHEMBL2152922) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from human 5HT4E receptor expressed in CHO cells after 30 mins by liquid scintillation counting | J Med Chem 55: 9240-54 (2012) Article DOI: 10.1021/jm300953p BindingDB Entry DOI: 10.7270/Q2FQ9XRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50452111 (CHEMBL2112353) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity at 5-HT reuptake site labeled with [3H]-paroxetine | Bioorg Med Chem Lett 14: 2653-6 (2004) Article DOI: 10.1016/j.bmcl.2004.02.088 BindingDB Entry DOI: 10.7270/Q2GF0SZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398598 (CHEMBL2152922) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from human 5HT4A receptor expressed in HEK293 cells after 30 mins by liquid scintillation counting | J Med Chem 55: 9240-54 (2012) Article DOI: 10.1021/jm300953p BindingDB Entry DOI: 10.7270/Q2FQ9XRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398596 (CHEMBL2179589) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from human 5HT4D receptor expressed in HEK293 cells after 30 mins by liquid scintillation counting | J Med Chem 55: 9240-54 (2012) Article DOI: 10.1021/jm300953p BindingDB Entry DOI: 10.7270/Q2FQ9XRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 2 (Homo sapiens (Human)) | BDBM50034503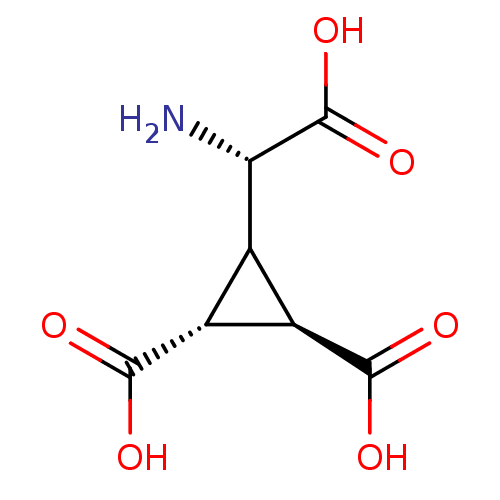 ((1R,2R)-3-((S)-Amino-carboxy-methyl)-cyclopropane-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 0.368 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by PDSP Ki Database | J Neurochem 71: 2558-64 (1998) Article DOI: 10.1046/j.1471-4159.1998.71062558.x BindingDB Entry DOI: 10.7270/Q25D8QCZ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50135248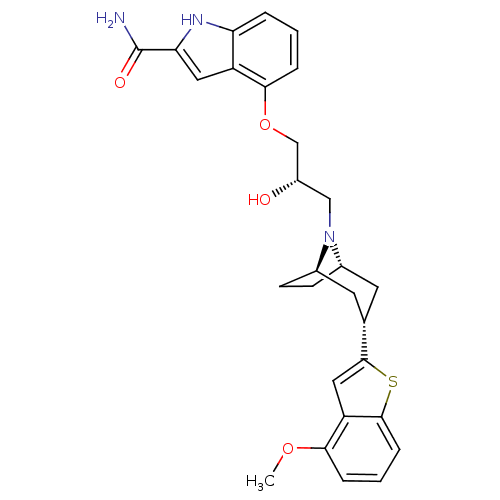 (4-{(S)-2-Hydroxy-3-[(1R,3S,5S)-3-(4-methoxy-benzo[...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Affinity at the 5-HT reuptake site labeled with [3H]-paroxetine using rat frontal cortex membranes | Bioorg Med Chem Lett 13: 3939-42 (2003) BindingDB Entry DOI: 10.7270/Q24T6HSX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 2 (Homo sapiens (Human)) | BDBM50004899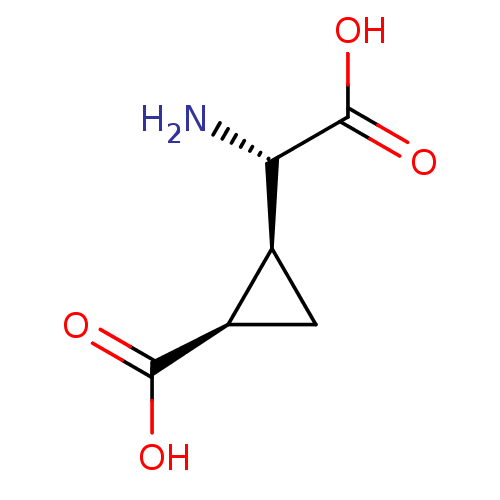 ((1R,2S)-2-((S)-Amino-carboxy-methyl)-cyclopropanec...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.378 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by PDSP Ki Database | J Neurochem 71: 2558-64 (1998) Article DOI: 10.1046/j.1471-4159.1998.71062558.x BindingDB Entry DOI: 10.7270/Q25D8QCZ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50130173 ((S)-1-[(4S,6R)-4-(5-Fluoro-benzo[b]thiophen-2-yl)-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro affinity of the compound at the 5-HT reuptake site using [3H]-paroxetine as radioligand in rat frontal cortex membranes | Bioorg Med Chem Lett 13: 2393-7 (2003) BindingDB Entry DOI: 10.7270/Q2Z31Z1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50135244 ((S)-1-[(2S,4R)-4-(6-Fluoro-benzo[b]thiophen-2-yl)-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Affinity at the 5-HT reuptake site labeled with [3H]-paroxetine using rat frontal cortex membranes | Bioorg Med Chem Lett 13: 3939-42 (2003) BindingDB Entry DOI: 10.7270/Q24T6HSX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50452114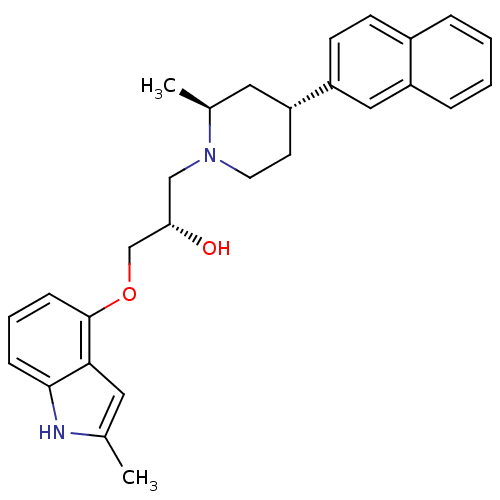 (CHEMBL2112350) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity at 5-HT reuptake site labeled with [3H]-paroxetine | Bioorg Med Chem Lett 14: 2653-6 (2004) Article DOI: 10.1016/j.bmcl.2004.02.088 BindingDB Entry DOI: 10.7270/Q2GF0SZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50130168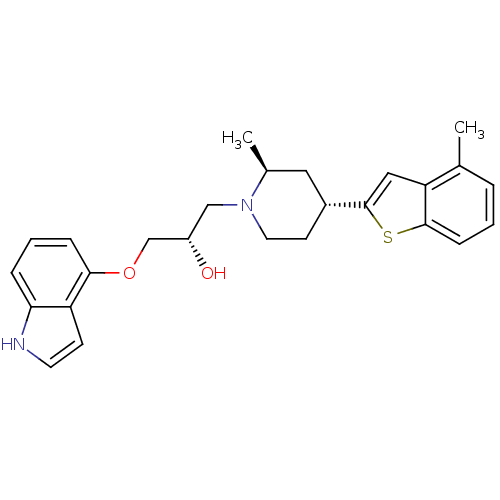 ((S)-1-(1H-Indol-4-yloxy)-3-[(4S,6R)-2-methyl-4-(4-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro affinity of the compound at the 5-HT reuptake site using [3H]-paroxetine as radioligand in rat frontal cortex membranes | Bioorg Med Chem Lett 13: 2393-7 (2003) BindingDB Entry DOI: 10.7270/Q2Z31Z1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50130168 ((S)-1-(1H-Indol-4-yloxy)-3-[(4S,6R)-2-methyl-4-(4-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]paroxetine from 5HT reuptake site | Bioorg Med Chem Lett 16: 2347-51 (2006) Article DOI: 10.1016/j.bmcl.2005.11.007 BindingDB Entry DOI: 10.7270/Q2W958RV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50135253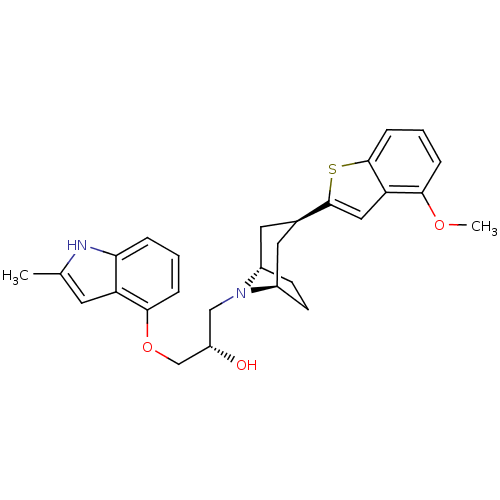 ((S)-1-[(1S,3R,5R)-3-(4-Methoxy-benzo[b]thiophen-2-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Affinity at the 5-HT reuptake site labeled with [3H]-paroxetine using rat frontal cortex membranes | Bioorg Med Chem Lett 13: 3939-42 (2003) BindingDB Entry DOI: 10.7270/Q24T6HSX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398598 (CHEMBL2152922) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from human 5HT4B receptor expressed in HEK293 cells after 30 mins by liquid scintillation counting | J Med Chem 55: 9240-54 (2012) Article DOI: 10.1021/jm300953p BindingDB Entry DOI: 10.7270/Q2FQ9XRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398599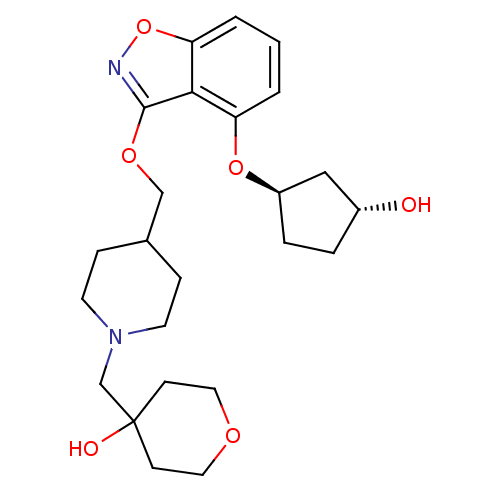 (CHEMBL2179580) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from human 5HT4D receptor expressed in HEK293 cells after 30 mins by liquid scintillation counting | J Med Chem 55: 9240-54 (2012) Article DOI: 10.1021/jm300953p BindingDB Entry DOI: 10.7270/Q2FQ9XRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50350987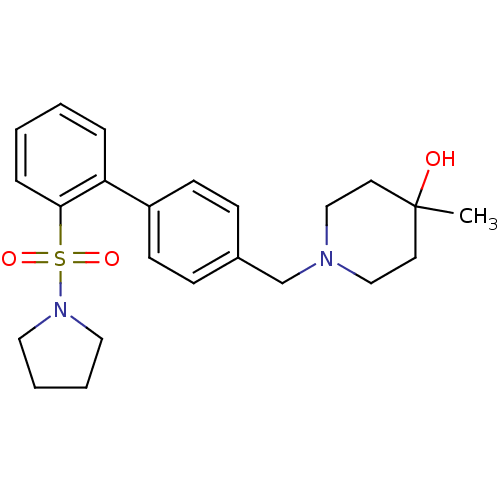 (CHEMBL1818233) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO cells after 1 hr by liquid scintillation counting | J Med Chem 54: 5868-77 (2011) Article DOI: 10.1021/jm2006035 BindingDB Entry DOI: 10.7270/Q2X63NBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50130157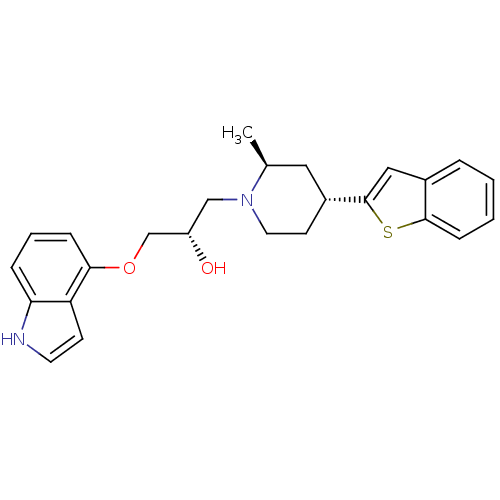 ((S)-1-((4S,6R)-4-Benzo[b]thiophen-2-yl-2-methyl-pi...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]paroxetine from 5HT reuptake site | Bioorg Med Chem Lett 16: 2347-51 (2006) Article DOI: 10.1016/j.bmcl.2005.11.007 BindingDB Entry DOI: 10.7270/Q2W958RV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50130157 ((S)-1-((4S,6R)-4-Benzo[b]thiophen-2-yl-2-methyl-pi...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro affinity of the compound at the 5-HT reuptake site using [3H]-paroxetine as radioligand in rat frontal cortex membranes | Bioorg Med Chem Lett 13: 2393-7 (2003) BindingDB Entry DOI: 10.7270/Q2Z31Z1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50135246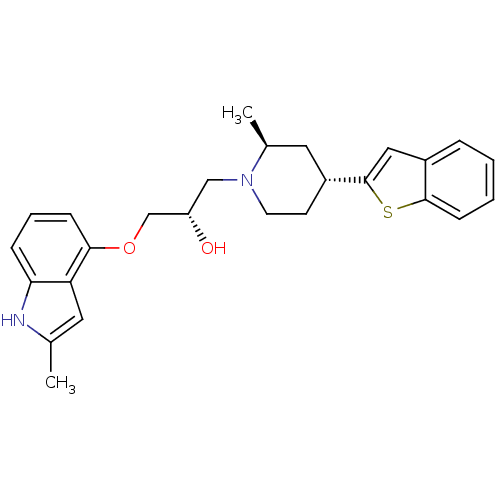 ((S)-1-((2S,4R)-4-(benzo[b]thiophen-2-yl)-2-methylp...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]paroxetine from 5HT reuptake site | Bioorg Med Chem Lett 16: 2347-51 (2006) Article DOI: 10.1016/j.bmcl.2005.11.007 BindingDB Entry DOI: 10.7270/Q2W958RV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50135246 ((S)-1-((2S,4R)-4-(benzo[b]thiophen-2-yl)-2-methylp...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Affinity at the 5-HT reuptake site labeled with [3H]-paroxetine using rat frontal cortex membranes | Bioorg Med Chem Lett 13: 3939-42 (2003) BindingDB Entry DOI: 10.7270/Q24T6HSX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 3007 total ) | Next | Last >> |