

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020840 (CHEMBL113276 | [Cyclopentyl-(2-mercapto-3-methoxy-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin I converting enzyme | J Med Chem 28: 328-32 (1985) BindingDB Entry DOI: 10.7270/Q2DN45MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020833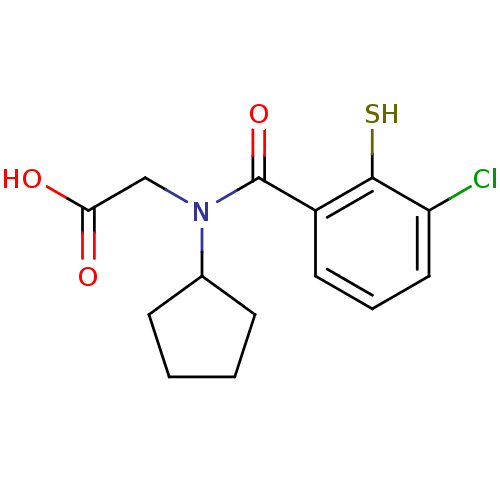 (CHEMBL326137 | [(3-Chloro-2-mercapto-benzoyl)-cycl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin I converting enzyme | J Med Chem 28: 328-32 (1985) BindingDB Entry DOI: 10.7270/Q2DN45MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM21642 ((2S)-1-[(2S)-2-methyl-3-sulfanylpropanoyl]pyrrolid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin I converting enzyme | J Med Chem 28: 328-32 (1985) BindingDB Entry DOI: 10.7270/Q2DN45MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020830 (CHEMBL263056 | [Cyclopentyl-(2-mercapto-3-trifluor...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin I converting enzyme | J Med Chem 28: 328-32 (1985) BindingDB Entry DOI: 10.7270/Q2DN45MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020829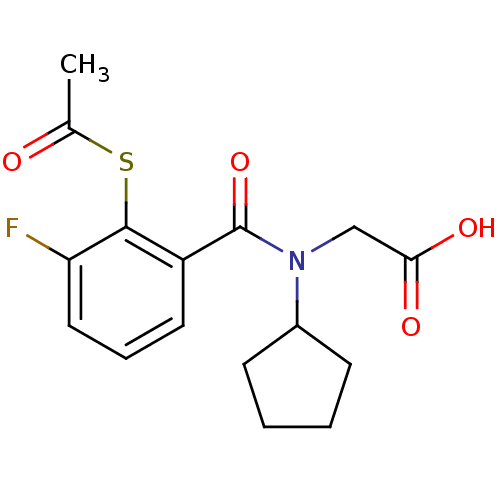 (CHEMBL114242 | [(2-Acetylsulfanyl-3-fluoro-benzoyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin I converting enzyme | J Med Chem 28: 328-32 (1985) BindingDB Entry DOI: 10.7270/Q2DN45MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020824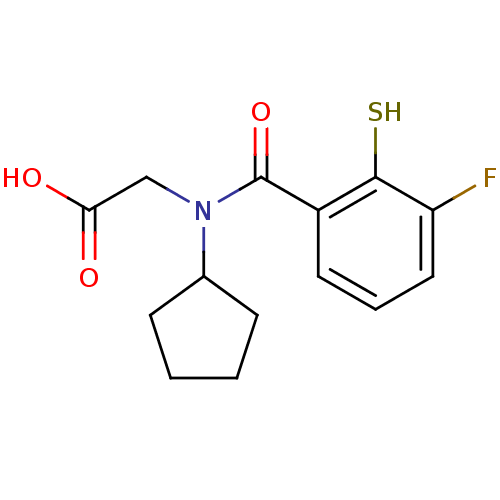 (CHEMBL325659 | [Cyclopentyl-(3-fluoro-2-mercapto-b...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin I converting enzyme | J Med Chem 28: 328-32 (1985) BindingDB Entry DOI: 10.7270/Q2DN45MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020831 (CHEMBL113315 | [(2-Acetylsulfanyl-3-methyl-benzoyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin I converting enzyme | J Med Chem 28: 328-32 (1985) BindingDB Entry DOI: 10.7270/Q2DN45MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020842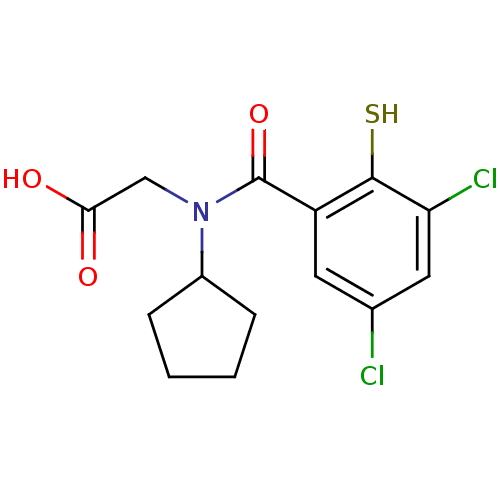 (CHEMBL114294 | [Cyclopentyl-(3,5-dichloro-2-mercap...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin I converting enzyme | J Med Chem 28: 328-32 (1985) BindingDB Entry DOI: 10.7270/Q2DN45MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020841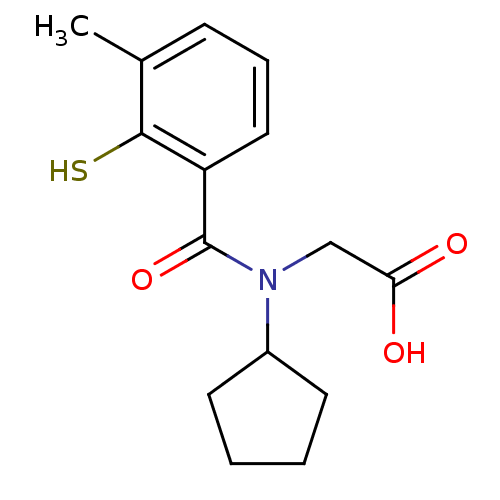 (CHEMBL324242 | [Cyclopentyl-(2-mercapto-3-methyl-b...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin I converting enzyme | J Med Chem 28: 328-32 (1985) BindingDB Entry DOI: 10.7270/Q2DN45MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020825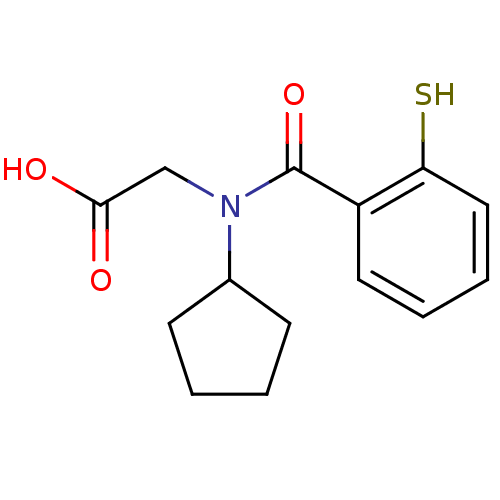 (CHEMBL112168 | [Cyclopentyl-(2-mercapto-benzoyl)-a...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin I converting enzyme | J Med Chem 28: 328-32 (1985) BindingDB Entry DOI: 10.7270/Q2DN45MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020823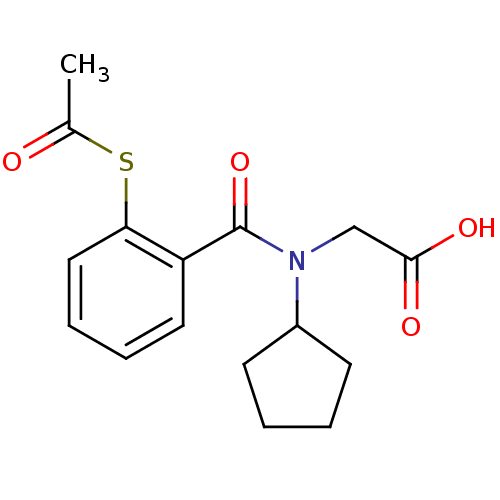 (CHEMBL112477 | [(2-Acetylsulfanyl-benzoyl)-cyclope...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin I converting enzyme | J Med Chem 28: 328-32 (1985) BindingDB Entry DOI: 10.7270/Q2DN45MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020821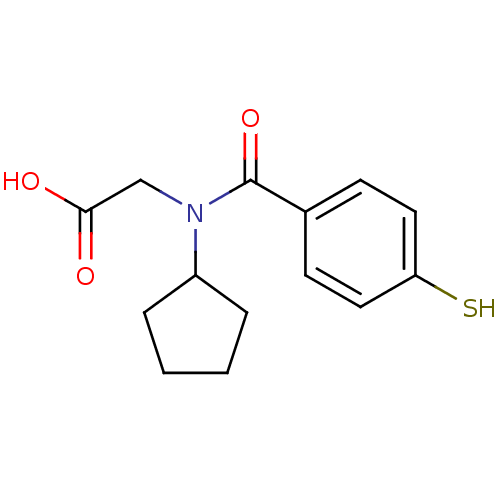 (CHEMBL113612 | [Cyclopentyl-(4-mercapto-benzoyl)-a...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin I converting enzyme | J Med Chem 28: 328-32 (1985) BindingDB Entry DOI: 10.7270/Q2DN45MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020832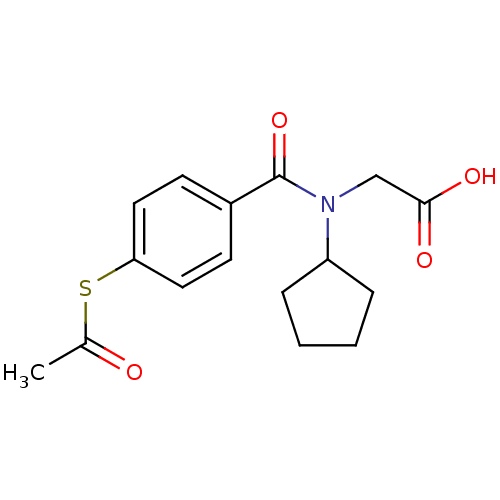 (CHEMBL112589 | [(4-Acetylsulfanyl-benzoyl)-cyclope...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin I converting enzyme | J Med Chem 28: 328-32 (1985) BindingDB Entry DOI: 10.7270/Q2DN45MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020827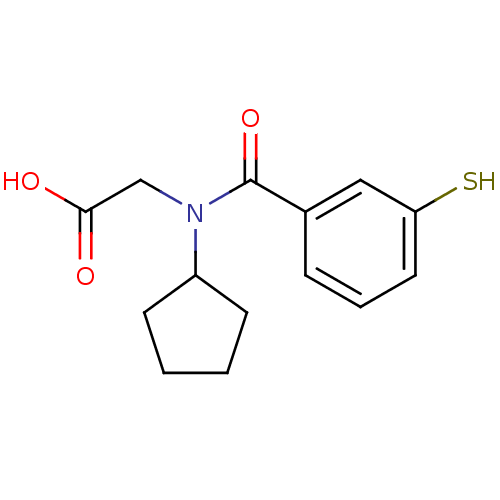 (CHEMBL324898 | [Cyclopentyl-(3-mercapto-benzoyl)-a...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin I converting enzyme | J Med Chem 28: 328-32 (1985) BindingDB Entry DOI: 10.7270/Q2DN45MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020838 (CHEMBL323879 | [(3-Acetylsulfanyl-benzoyl)-cyclope...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0280 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin I converting enzyme | J Med Chem 28: 328-32 (1985) BindingDB Entry DOI: 10.7270/Q2DN45MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020820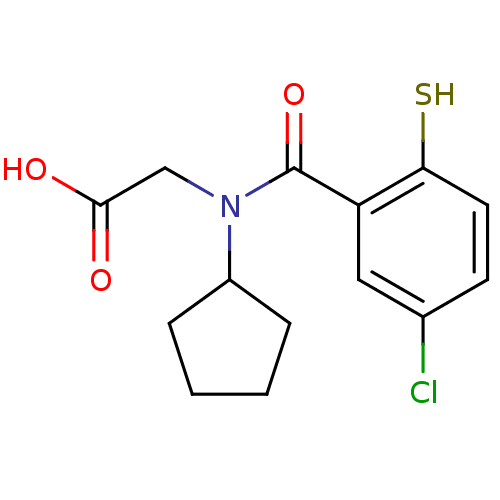 (CHEMBL112922 | [(5-Chloro-2-mercapto-benzoyl)-cycl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin I converting enzyme | J Med Chem 28: 328-32 (1985) BindingDB Entry DOI: 10.7270/Q2DN45MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020839 (CHEMBL324703 | [Cyclopentyl-(2-nitro-benzoyl)-amin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin I converting enzyme | J Med Chem 28: 328-32 (1985) BindingDB Entry DOI: 10.7270/Q2DN45MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020836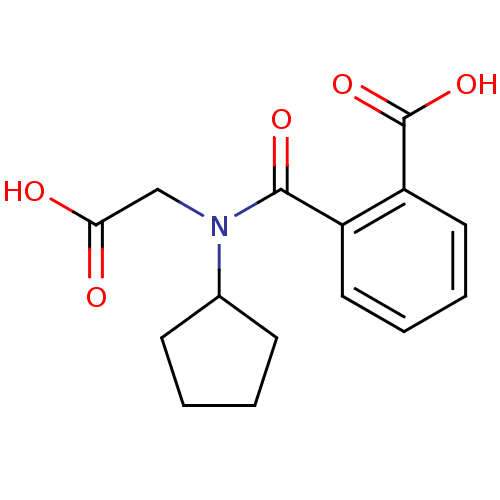 (CHEMBL115153 | N-Carboxymethyl-N-cyclopentyl-phtha...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin I converting enzyme | J Med Chem 28: 328-32 (1985) BindingDB Entry DOI: 10.7270/Q2DN45MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020837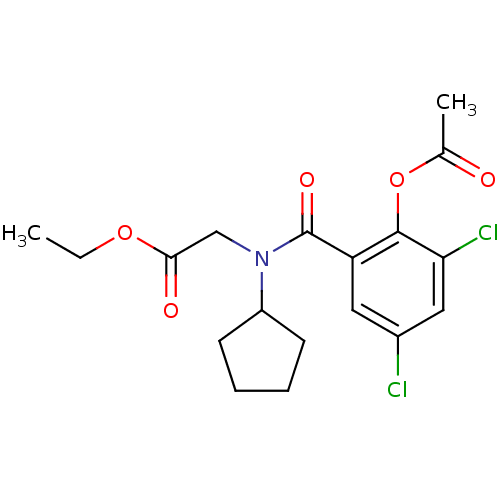 (CHEMBL114018 | [(2-Acetoxy-3,5-dichloro-benzoyl)-c...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin I converting enzyme | J Med Chem 28: 328-32 (1985) BindingDB Entry DOI: 10.7270/Q2DN45MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020828 (CHEMBL324676 | [Cyclopentyl-(3,5-dichloro-2-hydrox...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin I converting enzyme | J Med Chem 28: 328-32 (1985) BindingDB Entry DOI: 10.7270/Q2DN45MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020834 (CHEMBL325305 | [Cyclopentyl-(3-isopropyl-2-mercapt...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin I converting enzyme | J Med Chem 28: 328-32 (1985) BindingDB Entry DOI: 10.7270/Q2DN45MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme 2 (Homo sapiens (Human)) | BDBM50367254 (ENALAPRILAT) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | PDB PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Rorer Central Research Curated by ChEMBL | Assay Description In vitro inhibition of Angiotensin I converting enzyme in Hog plasma | J Med Chem 33: 1606-15 (1990) BindingDB Entry DOI: 10.7270/Q2CF9QPK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020771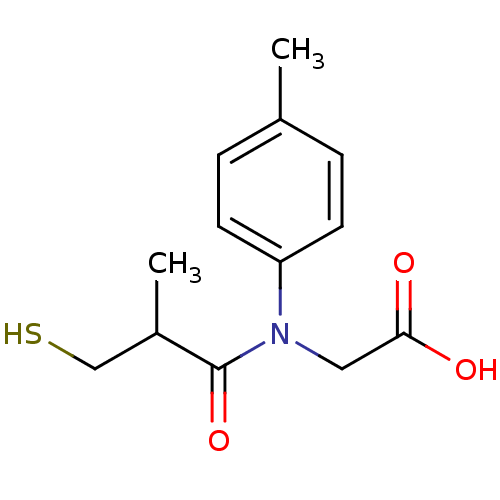 (CHEMBL160361 | [(3-Mercapto-2-methyl-propionyl)-p-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against rabbit lung Angiotensin I converting enzyme at pH 8.3 | J Med Chem 28: 57-66 (1985) BindingDB Entry DOI: 10.7270/Q2M90975 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50367254 (ENALAPRILAT) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Rorer Central Research Curated by ChEMBL | Assay Description In vitro inhibition of Angiotensin I converting enzyme in rabbit lung | J Med Chem 33: 1606-15 (1990) BindingDB Entry DOI: 10.7270/Q2CF9QPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50013611 (4-(4-Amino-2-chloro-5-sulfamoyl-benzenesulfonylami...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Rorer Central Research Curated by ChEMBL | Assay Description In vitro inhibition of angiotensin I converting enzyme (ACE) in rabbit lung | J Med Chem 33: 1606-15 (1990) BindingDB Entry DOI: 10.7270/Q2CF9QPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50013613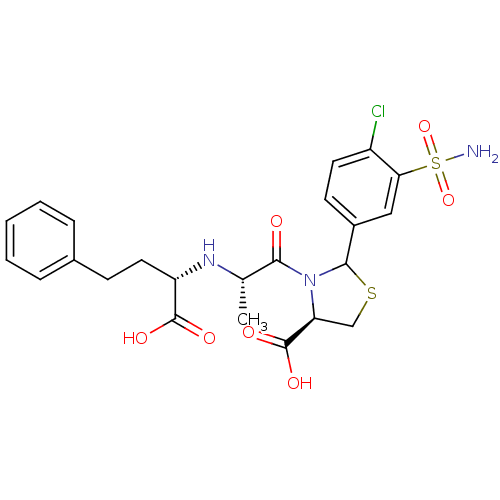 (3-[2-(1-Carboxy-3-phenyl-propylamino)-propionyl]-2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Rorer Central Research Curated by ChEMBL | Assay Description In vitro inhibition of angiotensin I converting enzyme (ACE) in rabbit lung | J Med Chem 33: 1606-15 (1990) BindingDB Entry DOI: 10.7270/Q2CF9QPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50367217 (CHEMBL1907938) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against rabbit lung Angiotensin I converting enzyme at pH 8.3 | J Med Chem 28: 57-66 (1985) BindingDB Entry DOI: 10.7270/Q2M90975 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM21642 ((2S)-1-[(2S)-2-methyl-3-sulfanylpropanoyl]pyrrolid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against rabbit lung Angiotensin I converting enzyme at pH 8.3 | J Med Chem 28: 57-66 (1985) BindingDB Entry DOI: 10.7270/Q2M90975 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50013616 (1-[2-(1-Carboxy-3-phenyl-propylamino)-propionyl]-4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Rorer Central Research Curated by ChEMBL | Assay Description In vitro inhibition of angiotensin I converting enzyme (ACE) in rabbit lung | J Med Chem 33: 1606-15 (1990) BindingDB Entry DOI: 10.7270/Q2CF9QPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020794 (CHEMBL348040 | [Cyclobutyl-(3-mercapto-2-methyl-pr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against rabbit lung Angiotensin I converting enzyme at pH 8.3 | J Med Chem 28: 57-66 (1985) BindingDB Entry DOI: 10.7270/Q2M90975 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020766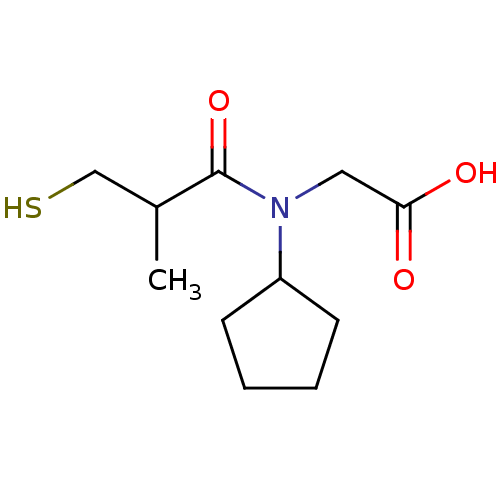 ((R+S)-[Cyclopentyl-(3-mercapto-2-methyl-propionyl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against rabbit lung Angiotensin I converting enzyme at pH 8.3 | J Med Chem 28: 57-66 (1985) BindingDB Entry DOI: 10.7270/Q2M90975 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM21642 ((2S)-1-[(2S)-2-methyl-3-sulfanylpropanoyl]pyrrolid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Rorer Central Research Curated by ChEMBL | Assay Description In vitro inhibition of Angiotensin I converting enzyme in rabbit lung | J Med Chem 33: 1606-15 (1990) BindingDB Entry DOI: 10.7270/Q2CF9QPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020761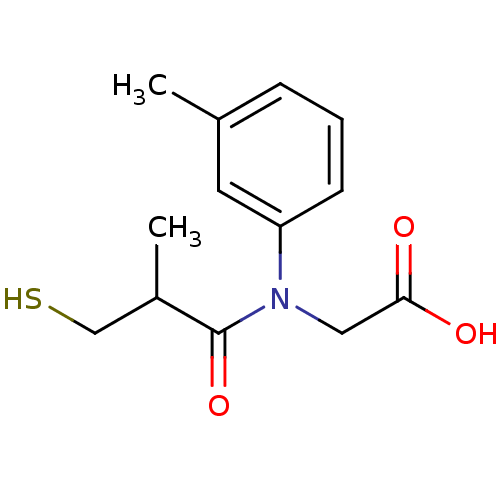 (CHEMBL346255 | [(3-Mercapto-2-methyl-propionyl)-m-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against rabbit lung Angiotensin I converting enzyme at pH 8.3 | J Med Chem 28: 57-66 (1985) BindingDB Entry DOI: 10.7270/Q2M90975 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020791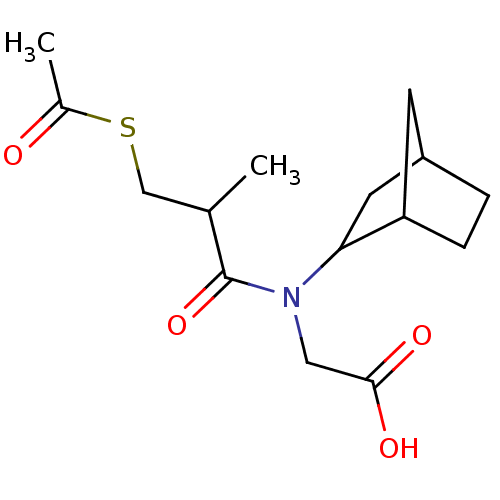 (CHEMBL346561 | [(3-Acetylsulfanyl-2-methyl-propion...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against rabbit lung Angiotensin I converting enzyme at pH 8.3 | J Med Chem 28: 57-66 (1985) BindingDB Entry DOI: 10.7270/Q2M90975 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020791 (CHEMBL346561 | [(3-Acetylsulfanyl-2-methyl-propion...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | 8.3 | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against rabbit lung Angiotensin I converting enzyme at pH 8.3 | J Med Chem 28: 57-66 (1985) BindingDB Entry DOI: 10.7270/Q2M90975 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50013609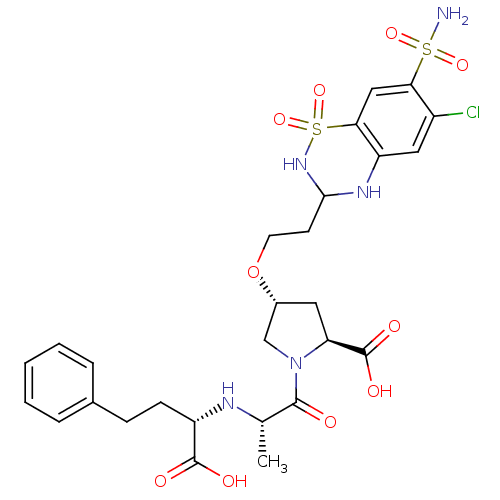 (1-[2-(1-Carboxy-3-phenyl-propylamino)-propionyl]-4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Rorer Central Research Curated by ChEMBL | Assay Description In vitro inhibition of angiotensin I converting enzyme(ACE) in rabbit lung | J Med Chem 33: 1606-15 (1990) BindingDB Entry DOI: 10.7270/Q2CF9QPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM21642 ((2S)-1-[(2S)-2-methyl-3-sulfanylpropanoyl]pyrrolid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against rabbit lung Angiotensin I converting enzyme | J Med Chem 28: 57-66 (1985) BindingDB Entry DOI: 10.7270/Q2M90975 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020793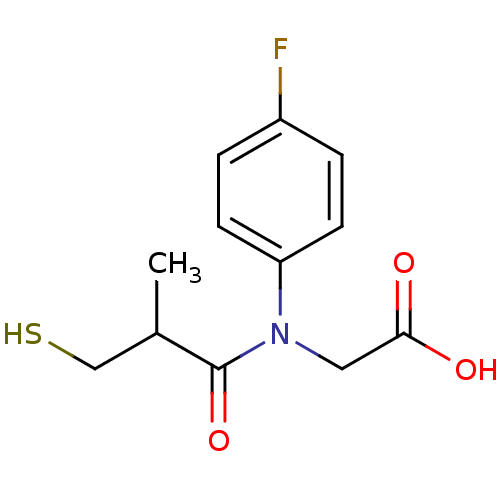 (CHEMBL23727 | [(4-Fluoro-phenyl)-(3-mercapto-2-met...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against rabbit lung Angiotensin I converting enzyme at pH 8.3 | J Med Chem 28: 57-66 (1985) BindingDB Entry DOI: 10.7270/Q2M90975 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM21642 ((2S)-1-[(2S)-2-methyl-3-sulfanylpropanoyl]pyrrolid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against rabbit lung Angiotensin I converting enzyme | J Med Chem 28: 57-66 (1985) BindingDB Entry DOI: 10.7270/Q2M90975 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50013608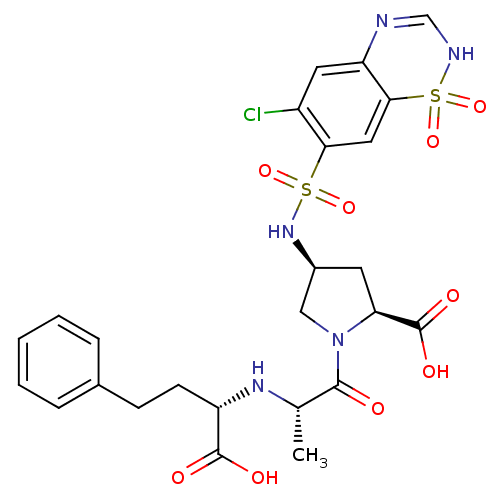 (1-[2-(1-Carboxy-3-phenyl-propylamino)-propionyl]-4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Rorer Central Research Curated by ChEMBL | Assay Description In vitro inhibition of angiotensin I converting enzyme (ACE) in rabbit lung | J Med Chem 33: 1606-15 (1990) BindingDB Entry DOI: 10.7270/Q2CF9QPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme 2 (Homo sapiens (Human)) | BDBM21642 ((2S)-1-[(2S)-2-methyl-3-sulfanylpropanoyl]pyrrolid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Rorer Central Research Curated by ChEMBL | Assay Description In vitro inhibition of Angiotensin I converting enzyme in Hog plasma | J Med Chem 33: 1606-15 (1990) BindingDB Entry DOI: 10.7270/Q2CF9QPK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50021340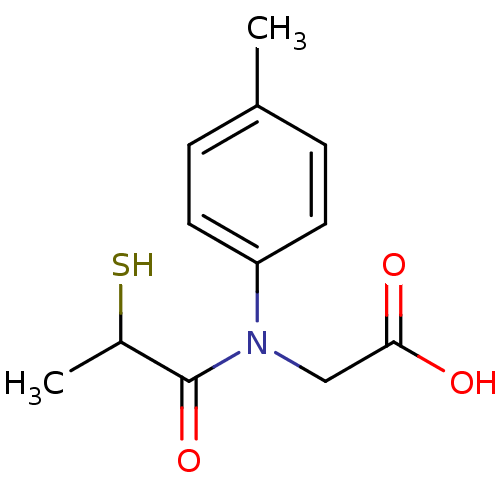 (CHEMBL166743 | [(2-Mercapto-propionyl)-p-tolyl-ami...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of the activity of rabbit lung Angiotensin I converting enzyme | J Med Chem 29: 784-96 (1986) BindingDB Entry DOI: 10.7270/Q2F47N4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50013615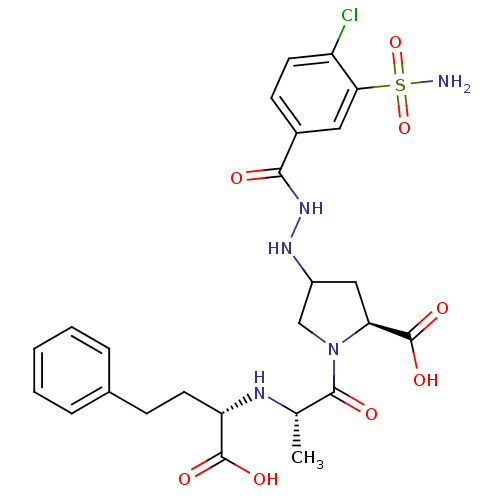 (1-[2-(1-Carboxy-3-phenyl-propylamino)-propionyl]-4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Rorer Central Research Curated by ChEMBL | Assay Description In vitro inhibition of Angiotensin I converting enzyme in rabbit lung | J Med Chem 33: 1606-15 (1990) BindingDB Entry DOI: 10.7270/Q2CF9QPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020779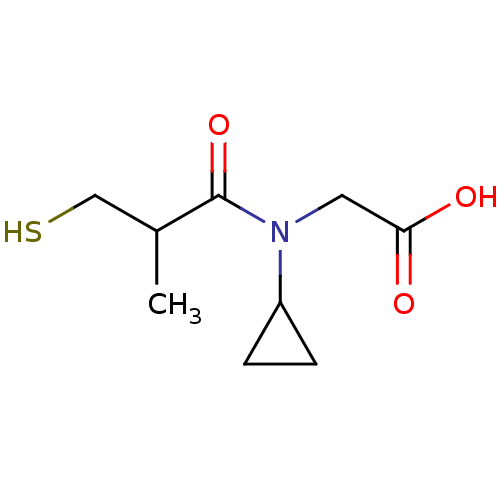 (CHEMBL158962 | [Cyclopropyl-(3-mercapto-2-methyl-p...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against rabbit lung Angiotensin I converting enzyme at pH 8.3 | J Med Chem 28: 57-66 (1985) BindingDB Entry DOI: 10.7270/Q2M90975 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020808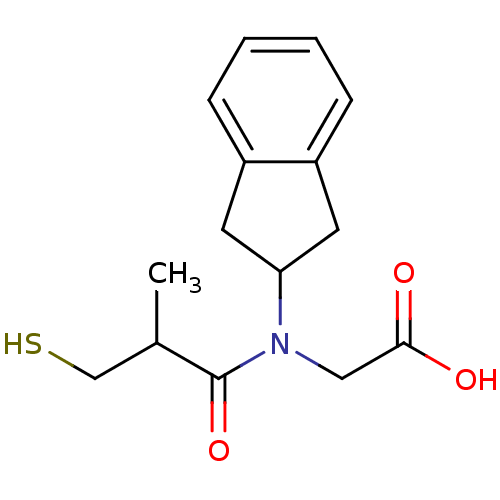 (CHEMBL1788147 | CHEMBL278348 | [Indan-2-yl-(3-merc...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against rabbit lung Angiotensin I converting enzyme at pH 8.3 | J Med Chem 28: 57-66 (1985) BindingDB Entry DOI: 10.7270/Q2M90975 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020798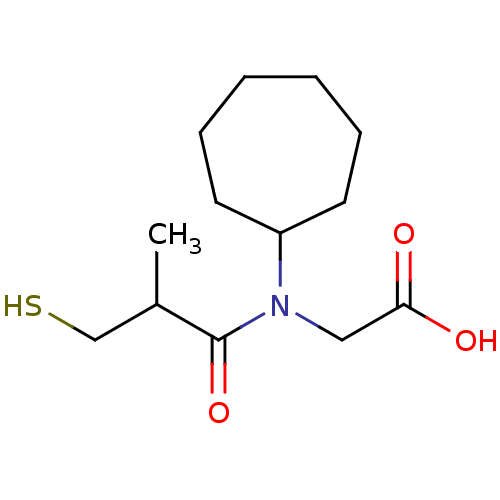 (CHEMBL1788148 | CHEMBL23841 | [Cycloheptyl-(3-merc...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against rabbit lung Angiotensin I converting enzyme at pH 8.3 | J Med Chem 28: 57-66 (1985) BindingDB Entry DOI: 10.7270/Q2M90975 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020769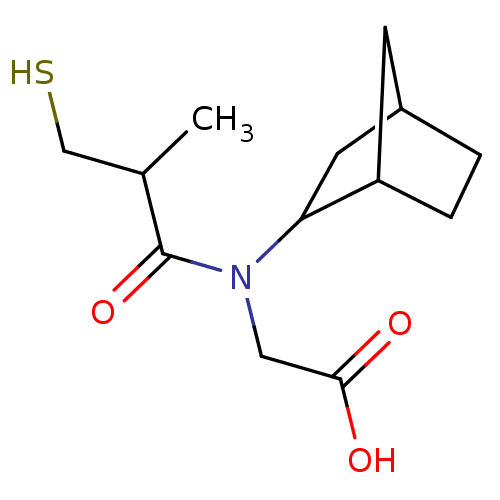 (CHEMBL345858 | [Bicyclo[2.2.1]hept-2-yl-(3-mercapt...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against rabbit lung Angiotensin I converting enzyme at pH 8.3 | J Med Chem 28: 57-66 (1985) BindingDB Entry DOI: 10.7270/Q2M90975 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020801 (CHEMBL23641 | [Indan-5-yl-(3-mercapto-2-methyl-pro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against rabbit lung Angiotensin I converting enzyme at pH 8.3 | J Med Chem 28: 57-66 (1985) BindingDB Entry DOI: 10.7270/Q2M90975 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020768 (CHEMBL1788152 | CHEMBL23518 | [Cyclohexyl-(3-merca...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against rabbit lung Angiotensin I converting enzyme at pH 8.3 | J Med Chem 28: 57-66 (1985) BindingDB Entry DOI: 10.7270/Q2M90975 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020763 (CHEMBL422013 | [(3-Acetylsulfanyl-2-methyl-propion...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against rabbit lung Angiotensin I converting enzyme at pH 8.3 | J Med Chem 28: 57-66 (1985) BindingDB Entry DOI: 10.7270/Q2M90975 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 123 total ) | Next | Last >> |