

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mu-type opioid receptor (CALF) | BDBM85393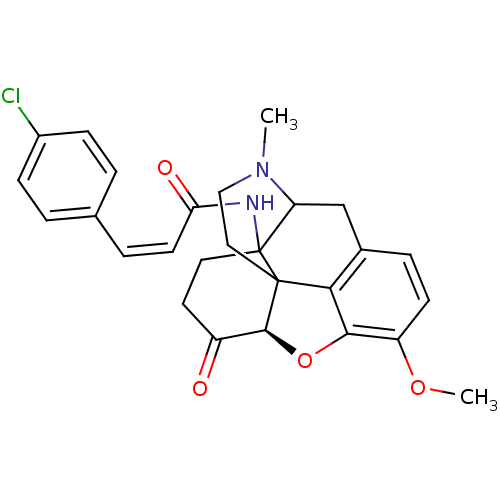 (14β-(p-chlorocinnamoylamino)-7,8-dihydrocodei...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rochester Curated by PDSP Ki Database | J Pharmacol Exp Ther 289: 304-11 (1999) BindingDB Entry DOI: 10.7270/Q2MC8XJC | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (CALF) | BDBM85396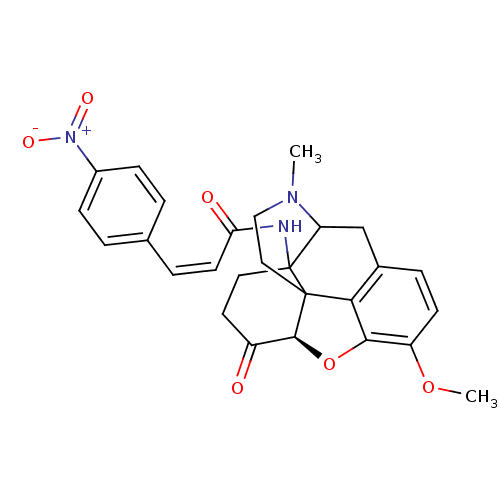 (CACO) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rochester Curated by PDSP Ki Database | J Pharmacol Exp Ther 289: 304-11 (1999) BindingDB Entry DOI: 10.7270/Q2MC8XJC | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Opioid receptor delta 1 (Bos taurus) | BDBM85393 (14β-(p-chlorocinnamoylamino)-7,8-dihydrocodei...) | PDB Reactome pathway UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rochester Curated by PDSP Ki Database | J Pharmacol Exp Ther 289: 304-11 (1999) BindingDB Entry DOI: 10.7270/Q2MC8XJC | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (CALF) | BDBM50000092 ((-)-(etorphine) | (-)-morphine | (1S,5R,13R,14S)-1...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Activity of the compound was evaluated by inhibition of the binding of 0.8 nM [3H]- DAMGO at Opioid receptor mu 1 binding site | J Med Chem 39: 1956-66 (1996) Article DOI: 10.1021/jm950817g BindingDB Entry DOI: 10.7270/Q2CC0ZR8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Kappa-type opioid receptor (CALF) | BDBM85393 (14β-(p-chlorocinnamoylamino)-7,8-dihydrocodei...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rochester Curated by PDSP Ki Database | J Pharmacol Exp Ther 289: 304-11 (1999) BindingDB Entry DOI: 10.7270/Q2MC8XJC | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (CALF) | BDBM85394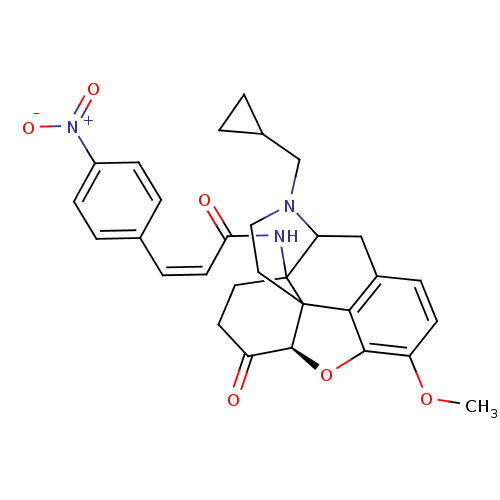 (N-CPM-CACO) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rochester Curated by PDSP Ki Database | J Pharmacol Exp Ther 289: 304-11 (1999) BindingDB Entry DOI: 10.7270/Q2MC8XJC | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (CALF) | BDBM85395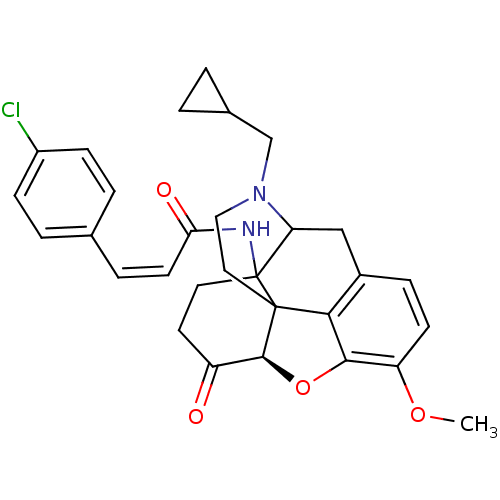 (MC-CAM) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rochester Curated by PDSP Ki Database | J Pharmacol Exp Ther 289: 304-11 (1999) BindingDB Entry DOI: 10.7270/Q2MC8XJC | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (CALF) | BDBM50050482 (10-Amino-9-methoxymethyl-1-methyl-tricyclo[7.3.1.0...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Activity of the compound was evaluated by inhibition of the binding of 0.8 nM [3H]- DAMGO at Opioid receptor mu 1 binding site | J Med Chem 39: 1956-66 (1996) Article DOI: 10.1021/jm950817g BindingDB Entry DOI: 10.7270/Q2CC0ZR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (CALF) | BDBM50050482 (10-Amino-9-methoxymethyl-1-methyl-tricyclo[7.3.1.0...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Activity of the compound was evaluated by inhibition of the binding of 0.8 nM [3H]- DAMGO at Opioid receptor mu 1 binding site | J Med Chem 39: 1956-66 (1996) Article DOI: 10.1021/jm950817g BindingDB Entry DOI: 10.7270/Q2CC0ZR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50050482 (10-Amino-9-methoxymethyl-1-methyl-tricyclo[7.3.1.0...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Activity of the compound was evaluated by inhibition of the binding of 1 nM [3H]U69,593 at Opioid receptor kappa 1 binding site | J Med Chem 39: 1956-66 (1996) Article DOI: 10.1021/jm950817g BindingDB Entry DOI: 10.7270/Q2CC0ZR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (CALF) | BDBM85394 (N-CPM-CACO) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rochester Curated by PDSP Ki Database | J Pharmacol Exp Ther 289: 304-11 (1999) BindingDB Entry DOI: 10.7270/Q2MC8XJC | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Opioid receptor delta 1 (Bos taurus) | BDBM85395 (MC-CAM) | PDB Reactome pathway UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rochester Curated by PDSP Ki Database | J Pharmacol Exp Ther 289: 304-11 (1999) BindingDB Entry DOI: 10.7270/Q2MC8XJC | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (CALF) | BDBM85395 (MC-CAM) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rochester Curated by PDSP Ki Database | J Pharmacol Exp Ther 289: 304-11 (1999) BindingDB Entry DOI: 10.7270/Q2MC8XJC | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Opioid receptor delta 1 (Bos taurus) | BDBM85394 (N-CPM-CACO) | PDB Reactome pathway UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rochester Curated by PDSP Ki Database | J Pharmacol Exp Ther 289: 304-11 (1999) BindingDB Entry DOI: 10.7270/Q2MC8XJC | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50050479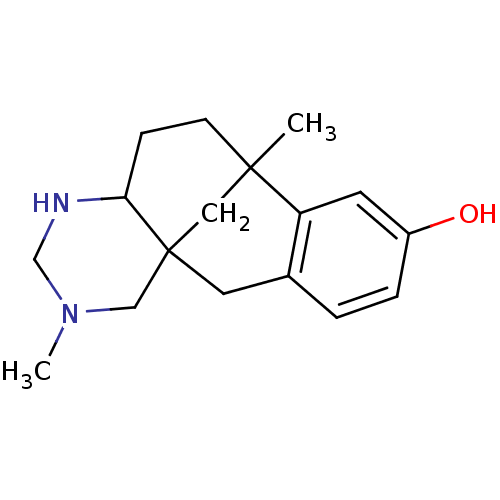 (3,9-dimethyl-3,5-diazatetracyclo[7.7.1.01,6.010,15...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Activity of the compound was evaluated by inhibition of the binding of 1 nM [3H]U69,593 at Opioid receptor kappa 1 binding site | J Med Chem 39: 1956-66 (1996) Article DOI: 10.1021/jm950817g BindingDB Entry DOI: 10.7270/Q2CC0ZR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (CALF) | BDBM85396 (CACO) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rochester Curated by PDSP Ki Database | J Pharmacol Exp Ther 289: 304-11 (1999) BindingDB Entry DOI: 10.7270/Q2MC8XJC | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (CALF) | BDBM50050487 (10-Dimethylamino-9-methoxymethyl-1-methyl-tricyclo...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 25.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Activity of the compound was evaluated by inhibition of the binding of 0.8 nM [3H]- DAMGO at Opioid receptor mu 1 binding site | J Med Chem 39: 1956-66 (1996) Article DOI: 10.1021/jm950817g BindingDB Entry DOI: 10.7270/Q2CC0ZR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (CALF) | BDBM50050487 (10-Dimethylamino-9-methoxymethyl-1-methyl-tricyclo...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 25.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Activity of the compound was evaluated by inhibition of the binding of 0.8 nM [3H]- DAMGO at Opioid receptor mu 1 binding site | J Med Chem 39: 1956-66 (1996) Article DOI: 10.1021/jm950817g BindingDB Entry DOI: 10.7270/Q2CC0ZR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (CALF) | BDBM50050479 (3,9-dimethyl-3,5-diazatetracyclo[7.7.1.01,6.010,15...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 27.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Activity of the compound was evaluated by inhibition of the binding of 0.8 nM [3H]- DAMGO at Opioid receptor mu 1 binding site | J Med Chem 39: 1956-66 (1996) Article DOI: 10.1021/jm950817g BindingDB Entry DOI: 10.7270/Q2CC0ZR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (CALF) | BDBM50050484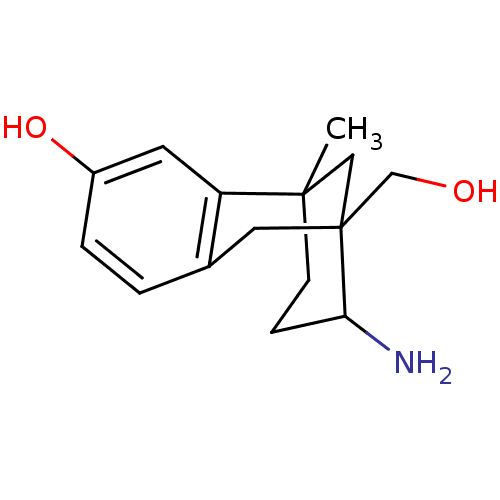 (10-Amino-9-hydroxymethyl-1-methyl-tricyclo[7.3.1.0...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 40.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Activity of the compound was evaluated by inhibition of the binding of 0.8 nM [3H]- DAMGO at Opioid receptor mu 1 binding site | J Med Chem 39: 1956-66 (1996) Article DOI: 10.1021/jm950817g BindingDB Entry DOI: 10.7270/Q2CC0ZR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (CALF) | BDBM50050484 (10-Amino-9-hydroxymethyl-1-methyl-tricyclo[7.3.1.0...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Activity of the compound was evaluated by inhibition of the binding of 0.8 nM [3H]- DAMGO at Opioid receptor mu 1 binding site | J Med Chem 39: 1956-66 (1996) Article DOI: 10.1021/jm950817g BindingDB Entry DOI: 10.7270/Q2CC0ZR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50050481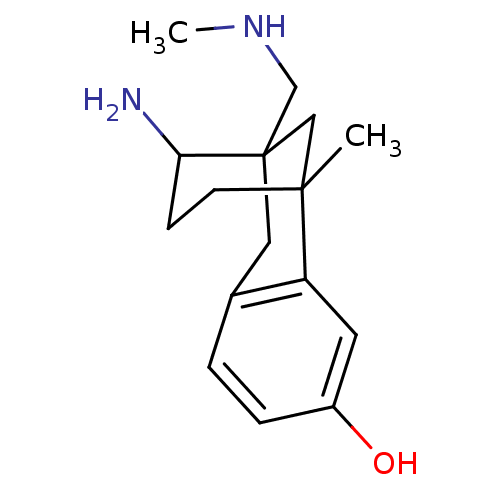 (10-Amino-1-methyl-9-methylaminomethyl-tricyclo[7.3...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Activity of the compound was evaluated by inhibition of the binding of 1 nM [3H]U69,593 at Opioid receptor kappa 1 binding site | J Med Chem 39: 1956-66 (1996) Article DOI: 10.1021/jm950817g BindingDB Entry DOI: 10.7270/Q2CC0ZR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (CALF) | BDBM50050481 (10-Amino-1-methyl-9-methylaminomethyl-tricyclo[7.3...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 91.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Activity of the compound was evaluated by inhibition of the binding of 0.8 nM [3H]- DAMGO at Opioid receptor mu 1 binding site | J Med Chem 39: 1956-66 (1996) Article DOI: 10.1021/jm950817g BindingDB Entry DOI: 10.7270/Q2CC0ZR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (CALF) | BDBM50050485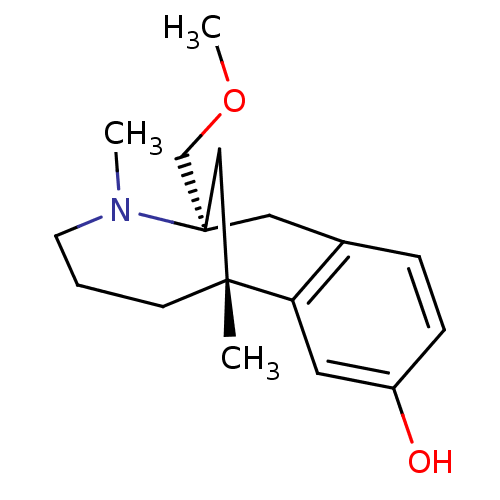 ((1R,9R)-9-Methoxymethyl-1,10-dimethyl-10-aza-tricy...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 102 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Activity of the compound was evaluated by inhibition of the binding of 0.8 nM [3H]- DAMGO at Opioid receptor mu 1 binding site | J Med Chem 39: 1956-66 (1996) Article DOI: 10.1021/jm950817g BindingDB Entry DOI: 10.7270/Q2CC0ZR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50050484 (10-Amino-9-hydroxymethyl-1-methyl-tricyclo[7.3.1.0...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 109 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Activity of the compound was evaluated by inhibition of the binding of 1 nM [3H]U69,593 at Opioid receptor kappa 1 binding site | J Med Chem 39: 1956-66 (1996) Article DOI: 10.1021/jm950817g BindingDB Entry DOI: 10.7270/Q2CC0ZR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50050487 (10-Dimethylamino-9-methoxymethyl-1-methyl-tricyclo...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 123 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Activity of the compound was evaluated by inhibition of the binding of 1 nM [3H]U69,593 at Opioid receptor kappa 1 binding site | J Med Chem 39: 1956-66 (1996) Article DOI: 10.1021/jm950817g BindingDB Entry DOI: 10.7270/Q2CC0ZR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50000092 ((-)-(etorphine) | (-)-morphine | (1S,5R,13R,14S)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 132 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Activity of the compound was evaluated by inhibition of the binding of 1 nM [3H]-DPDPE at Opioid receptor delta 1 binding site | J Med Chem 39: 1956-66 (1996) Article DOI: 10.1021/jm950817g BindingDB Entry DOI: 10.7270/Q2CC0ZR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (CALF) | BDBM50050483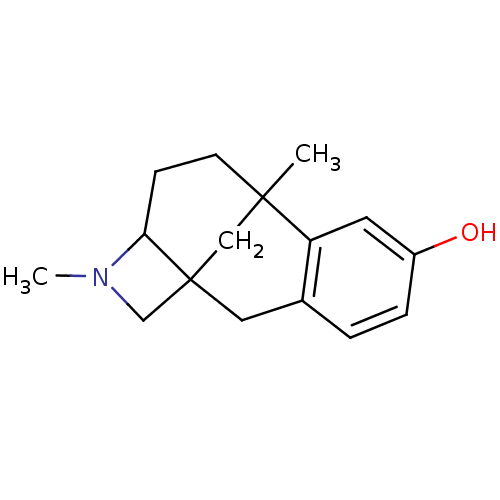 (9,13-dimethyl-13-azatetracyclo[7.5.1.01,12.03,8]pe...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 179 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Activity of the compound was evaluated by inhibition of the binding of 0.8 nM [3H]- DAMGO at Opioid receptor mu 1 binding site | J Med Chem 39: 1956-66 (1996) Article DOI: 10.1021/jm950817g BindingDB Entry DOI: 10.7270/Q2CC0ZR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50050483 (9,13-dimethyl-13-azatetracyclo[7.5.1.01,12.03,8]pe...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 215 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Activity of the compound was evaluated by inhibition of the binding of 1 nM [3H]U69,593 at Opioid receptor kappa 1 binding site | J Med Chem 39: 1956-66 (1996) Article DOI: 10.1021/jm950817g BindingDB Entry DOI: 10.7270/Q2CC0ZR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (CALF) | BDBM50050478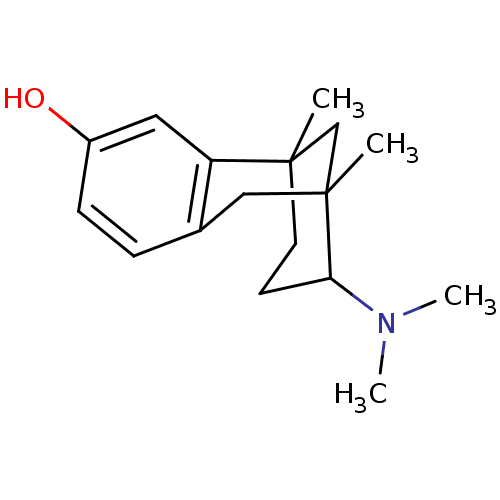 (10-Dimethylamino-1,9-dimethyl-tricyclo[7.3.1.0*2,7...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 297 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Activity of the compound was evaluated by inhibition of the binding of 0.8 nM [3H]- DAMGO at Opioid receptor mu 1 binding site | J Med Chem 39: 1956-66 (1996) Article DOI: 10.1021/jm950817g BindingDB Entry DOI: 10.7270/Q2CC0ZR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (CALF) | BDBM50050478 (10-Dimethylamino-1,9-dimethyl-tricyclo[7.3.1.0*2,7...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 297 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Activity of the compound was evaluated by inhibition of the binding of 0.8 nM [3H]- DAMGO at Opioid receptor mu 1 binding site | J Med Chem 39: 1956-66 (1996) Article DOI: 10.1021/jm950817g BindingDB Entry DOI: 10.7270/Q2CC0ZR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50000092 ((-)-(etorphine) | (-)-morphine | (1S,5R,13R,14S)-1...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Activity of the compound was evaluated by inhibition of the binding of 1 nM [3H]U69,593 at Opioid receptor kappa 1 binding site | J Med Chem 39: 1956-66 (1996) Article DOI: 10.1021/jm950817g BindingDB Entry DOI: 10.7270/Q2CC0ZR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (CALF) | BDBM50050480 (9-Hydroxymethyl-1-methyl-10-methylamino-tricyclo[7...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Activity of the compound was evaluated by inhibition of the binding of 0.8 nM [3H]- DAMGO at Opioid receptor mu 1 binding site | J Med Chem 39: 1956-66 (1996) Article DOI: 10.1021/jm950817g BindingDB Entry DOI: 10.7270/Q2CC0ZR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (CALF) | BDBM50050480 (9-Hydroxymethyl-1-methyl-10-methylamino-tricyclo[7...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Activity of the compound was evaluated by inhibition of the binding of 0.8 nM [3H]- DAMGO at Opioid receptor mu 1 binding site | J Med Chem 39: 1956-66 (1996) Article DOI: 10.1021/jm950817g BindingDB Entry DOI: 10.7270/Q2CC0ZR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50050478 (10-Dimethylamino-1,9-dimethyl-tricyclo[7.3.1.0*2,7...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 409 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Activity of the compound was evaluated by inhibition of the binding of 1 nM [3H]U69,593 at Opioid receptor kappa 1 binding site | J Med Chem 39: 1956-66 (1996) Article DOI: 10.1021/jm950817g BindingDB Entry DOI: 10.7270/Q2CC0ZR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50050481 (10-Amino-1-methyl-9-methylaminomethyl-tricyclo[7.3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 459 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Activity of the compound was evaluated by inhibition of the binding of 1 nM [3H]-DPDPE at Opioid receptor delta 1 binding site | J Med Chem 39: 1956-66 (1996) Article DOI: 10.1021/jm950817g BindingDB Entry DOI: 10.7270/Q2CC0ZR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50050482 (10-Amino-9-methoxymethyl-1-methyl-tricyclo[7.3.1.0...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 467 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Activity of the compound was evaluated by inhibition of the binding of 1 nM [3H]-DPDPE at Opioid receptor delta 1 binding site | J Med Chem 39: 1956-66 (1996) Article DOI: 10.1021/jm950817g BindingDB Entry DOI: 10.7270/Q2CC0ZR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50050480 (9-Hydroxymethyl-1-methyl-10-methylamino-tricyclo[7...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Activity of the compound was evaluated by inhibition of the binding of 1 nM [3H]U69,593 at Opioid receptor kappa 1 binding site | J Med Chem 39: 1956-66 (1996) Article DOI: 10.1021/jm950817g BindingDB Entry DOI: 10.7270/Q2CC0ZR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50050485 ((1R,9R)-9-Methoxymethyl-1,10-dimethyl-10-aza-tricy...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 795 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Activity of the compound was evaluated by inhibition of the binding of 1 nM [3H]U69,593 at Opioid receptor kappa 1 binding site | J Med Chem 39: 1956-66 (1996) Article DOI: 10.1021/jm950817g BindingDB Entry DOI: 10.7270/Q2CC0ZR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (CALF) | BDBM50050477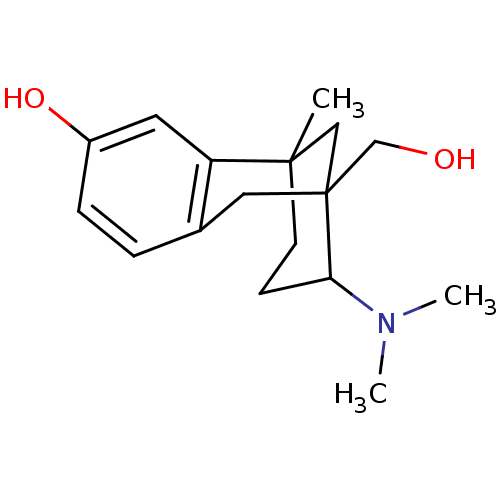 (10-Dimethylamino-9-hydroxymethyl-1-methyl-tricyclo...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Activity of the compound was evaluated by inhibition of the binding of 0.8 nM [3H]- DAMGO at Opioid receptor mu 1 binding site | J Med Chem 39: 1956-66 (1996) Article DOI: 10.1021/jm950817g BindingDB Entry DOI: 10.7270/Q2CC0ZR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (CALF) | BDBM50050477 (10-Dimethylamino-9-hydroxymethyl-1-methyl-tricyclo...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Activity of the compound was evaluated by inhibition of the binding of 0.8 nM [3H]- DAMGO at Opioid receptor mu 1 binding site | J Med Chem 39: 1956-66 (1996) Article DOI: 10.1021/jm950817g BindingDB Entry DOI: 10.7270/Q2CC0ZR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50050479 (3,9-dimethyl-3,5-diazatetracyclo[7.7.1.01,6.010,15...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 863 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Activity of the compound was evaluated by inhibition of the binding of 1 nM [3H]-DPDPE at Opioid receptor delta 1 binding site | J Med Chem 39: 1956-66 (1996) Article DOI: 10.1021/jm950817g BindingDB Entry DOI: 10.7270/Q2CC0ZR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50050487 (10-Dimethylamino-9-methoxymethyl-1-methyl-tricyclo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Activity of the compound was evaluated by inhibition of the binding of 1 nM [3H]-DPDPE at Opioid receptor delta 1 binding site | J Med Chem 39: 1956-66 (1996) Article DOI: 10.1021/jm950817g BindingDB Entry DOI: 10.7270/Q2CC0ZR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50050477 (10-Dimethylamino-9-hydroxymethyl-1-methyl-tricyclo...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Activity of the compound was evaluated by inhibition of the binding of 1 nM [3H]U69,593 at Opioid receptor kappa 1 binding site | J Med Chem 39: 1956-66 (1996) Article DOI: 10.1021/jm950817g BindingDB Entry DOI: 10.7270/Q2CC0ZR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (CALF) | BDBM50050486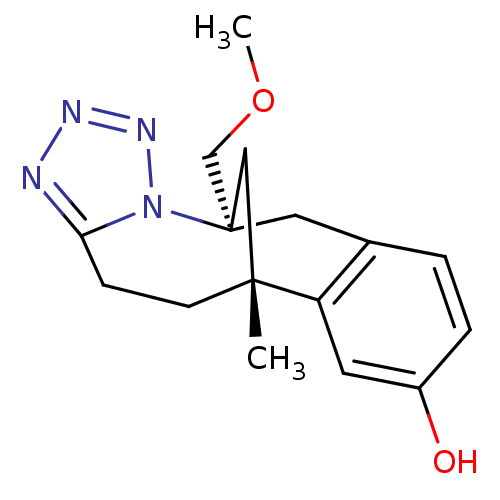 (1-methoxymethyl-9-methyl-(1S,9R)-2,3,4,5-tetraazat...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Activity of the compound was evaluated by inhibition of the binding of 0.8 nM [3H]- DAMGO at Opioid receptor mu 1 binding site | J Med Chem 39: 1956-66 (1996) Article DOI: 10.1021/jm950817g BindingDB Entry DOI: 10.7270/Q2CC0ZR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50050485 ((1R,9R)-9-Methoxymethyl-1,10-dimethyl-10-aza-tricy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Activity of the compound was evaluated by inhibition of the binding of 1 nM [3H]-DPDPE at Opioid receptor delta 1 binding site | J Med Chem 39: 1956-66 (1996) Article DOI: 10.1021/jm950817g BindingDB Entry DOI: 10.7270/Q2CC0ZR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50050483 (9,13-dimethyl-13-azatetracyclo[7.5.1.01,12.03,8]pe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Activity of the compound was evaluated by inhibition of the binding of 1 nM [3H]-DPDPE at Opioid receptor delta 1 binding site | J Med Chem 39: 1956-66 (1996) Article DOI: 10.1021/jm950817g BindingDB Entry DOI: 10.7270/Q2CC0ZR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50050478 (10-Dimethylamino-1,9-dimethyl-tricyclo[7.3.1.0*2,7...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Activity of the compound was evaluated by inhibition of the binding of 1 nM [3H]-DPDPE at Opioid receptor delta 1 binding site | J Med Chem 39: 1956-66 (1996) Article DOI: 10.1021/jm950817g BindingDB Entry DOI: 10.7270/Q2CC0ZR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50050486 (1-methoxymethyl-9-methyl-(1S,9R)-2,3,4,5-tetraazat...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Activity of the compound was evaluated by inhibition of the binding of 1 nM [3H]U69,593 at Opioid receptor kappa 1 binding site | J Med Chem 39: 1956-66 (1996) Article DOI: 10.1021/jm950817g BindingDB Entry DOI: 10.7270/Q2CC0ZR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50050486 (1-methoxymethyl-9-methyl-(1S,9R)-2,3,4,5-tetraazat...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Activity of the compound was evaluated by inhibition of the binding of 1 nM [3H]-DPDPE at Opioid receptor delta 1 binding site | J Med Chem 39: 1956-66 (1996) Article DOI: 10.1021/jm950817g BindingDB Entry DOI: 10.7270/Q2CC0ZR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 300 total ) | Next | Last >> |