

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D-amino-acid oxidase (Homo sapiens (Human)) | BDBM31147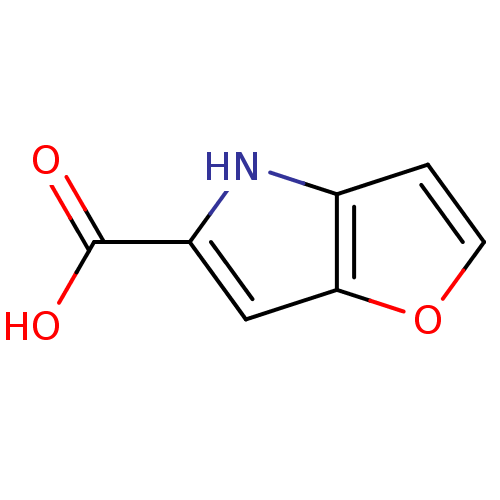 (4H-furo[3,2-b]pyrrole-5-carboxylic acid | 5-carbox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents | MMDB PDB Article PubMed | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal His-tagged DAO expressed in Escherichia coli BL21(DE3) using D-serine as substrate by Lineweaver-Burk plot... | J Med Chem 56: 1894-907 (2013) Article DOI: 10.1021/jm3017865 BindingDB Entry DOI: 10.7270/Q2H70H4P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D-amino-acid oxidase (Homo sapiens (Human)) | BDBM50206007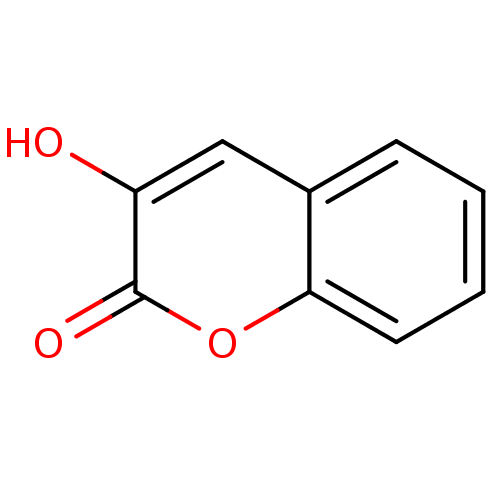 (3-Hydroxy-chromen-2-one | 3-hydroxy-2H-chromen-2-o...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 156 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal His-tagged DAO expressed in Escherichia coli BL21(DE3) using D-serine as substrate by Lineweaver-Burk plot... | J Med Chem 56: 1894-907 (2013) Article DOI: 10.1021/jm3017865 BindingDB Entry DOI: 10.7270/Q2H70H4P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Solute carrier organic anion transporter family member 1A4 (Rattus norvegicus) | BDBM18957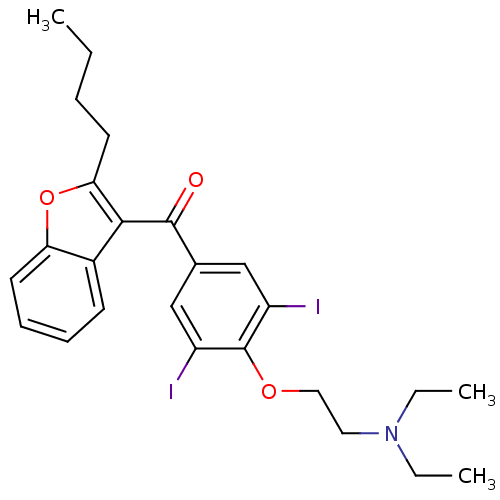 ((2-{4-[(2-butyl-1-benzofuran-3-yl)carbonyl]-2,6-di...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku University School of Medicine Curated by ChEMBL | Assay Description TP_TRANSPORTER: inhibition of Digoxin uptake in Xenopus laevis oocytes | Endocrinology 142: 2005-12 (2001) Article DOI: 10.1210/endo.142.5.8115 BindingDB Entry DOI: 10.7270/Q2GF0XB3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1,4-galactosyltransferase 1 (Homo sapiens (Human)) | BDBM50324492 (CHEMBL1214871) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.86E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Inhibition of human Beta-1,4-galactosyltransferase 1 | J Med Chem 53: 5607-19 (2010) Article DOI: 10.1021/jm100612r BindingDB Entry DOI: 10.7270/Q2WS8TFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-aspartate oxidase (Homo sapiens (Human)) | BDBM50427221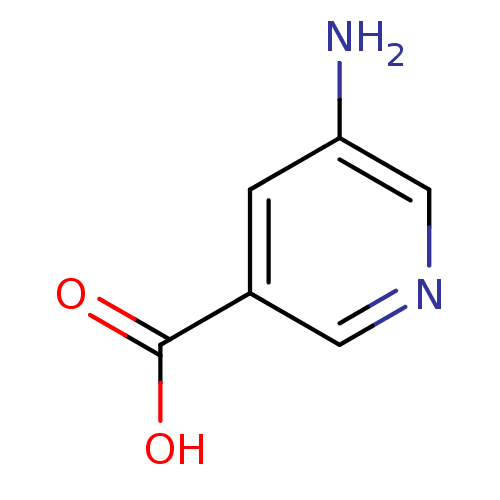 (5-Aminonicotinic Acid | CHEMBL1491941) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University Curated by ChEMBL | Assay Description Binding affinity to human recombinant DDO | J Med Chem 58: 7328-40 (2015) Article DOI: 10.1021/acs.jmedchem.5b00871 BindingDB Entry DOI: 10.7270/Q2WM1G6R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RCG38204, isoform CRA_d (Rattus norvegicus) | BDBM50427221 (5-Aminonicotinic Acid | CHEMBL1491941) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | 5.89E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University Curated by ChEMBL | Assay Description Inhibition of rat recombinant DDO using D-Asp | J Med Chem 58: 7328-40 (2015) Article DOI: 10.1021/acs.jmedchem.5b00871 BindingDB Entry DOI: 10.7270/Q2WM1G6R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-amino-acid oxidase (Homo sapiens (Human)) | BDBM36181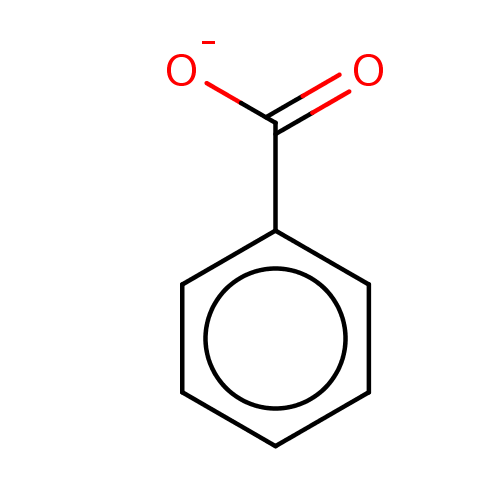 (SODIUM BENZOATE | benzoate | benzoic acid | benzoi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University Curated by ChEMBL | Assay Description Inhibition of human recombinant DAO expressed in Escherichia coli BL21(DE3) by oxygraphic assay | J Med Chem 56: 1894-907 (2013) Article DOI: 10.1021/jm3017865 BindingDB Entry DOI: 10.7270/Q2H70H4P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-amino-acid oxidase (Homo sapiens (Human)) | BDBM50074328 (CHEMBL164585 | Thiophene-3-carboxylic acid) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents | Article PubMed | 8.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal His-tagged DAO expressed in Escherichia coli BL21(DE3) using D-serine as substrate by Lineweaver-Burk plot... | J Med Chem 56: 1894-907 (2013) Article DOI: 10.1021/jm3017865 BindingDB Entry DOI: 10.7270/Q2H70H4P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-aspartate oxidase (Mus musculus) | BDBM50427221 (5-Aminonicotinic Acid | CHEMBL1491941) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | 8.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University Curated by ChEMBL | Assay Description Inhibition of mouse recombinant DDO using D-Asp | J Med Chem 58: 7328-40 (2015) Article DOI: 10.1021/acs.jmedchem.5b00871 BindingDB Entry DOI: 10.7270/Q2WM1G6R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-amino-acid oxidase (Homo sapiens (Human)) | BDBM50427206 (CHEMBL2324852) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 9.92E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal His-tagged DAO expressed in Escherichia coli BL21(DE3) using D-serine as substrate by Lineweaver-Burk plot... | J Med Chem 56: 1894-907 (2013) Article DOI: 10.1021/jm3017865 BindingDB Entry DOI: 10.7270/Q2H70H4P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-amino-acid oxidase (Homo sapiens (Human)) | BDBM36181 (SODIUM BENZOATE | benzoate | benzoic acid | benzoi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 1.09E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal His-tagged DAO expressed in Escherichia coli BL21(DE3) using D-serine as substrate by Lineweaver-Burk plot... | J Med Chem 56: 1894-907 (2013) Article DOI: 10.1021/jm3017865 BindingDB Entry DOI: 10.7270/Q2H70H4P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-aspartate oxidase (Homo sapiens (Human)) | BDBM50121995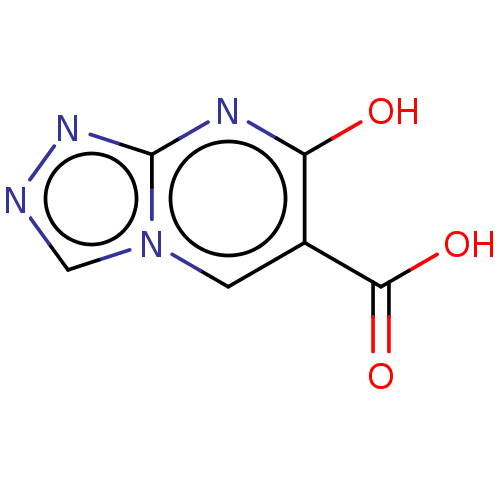 (CHEMBL3617316) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University Curated by ChEMBL | Assay Description Binding affinity to human recombinant DDO | J Med Chem 58: 7328-40 (2015) Article DOI: 10.1021/acs.jmedchem.5b00871 BindingDB Entry DOI: 10.7270/Q2WM1G6R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-aspartate oxidase (Mus musculus) | BDBM50121995 (CHEMBL3617316) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University Curated by ChEMBL | Assay Description Inhibition of mouse recombinant DDO using D-Asp | J Med Chem 58: 7328-40 (2015) Article DOI: 10.1021/acs.jmedchem.5b00871 BindingDB Entry DOI: 10.7270/Q2WM1G6R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RCG38204, isoform CRA_d (Rattus norvegicus) | BDBM50121995 (CHEMBL3617316) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.59E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University Curated by ChEMBL | Assay Description Inhibition of rat recombinant DDO using D-Asp | J Med Chem 58: 7328-40 (2015) Article DOI: 10.1021/acs.jmedchem.5b00871 BindingDB Entry DOI: 10.7270/Q2WM1G6R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-aspartate oxidase (Homo sapiens (Human)) | BDBM14673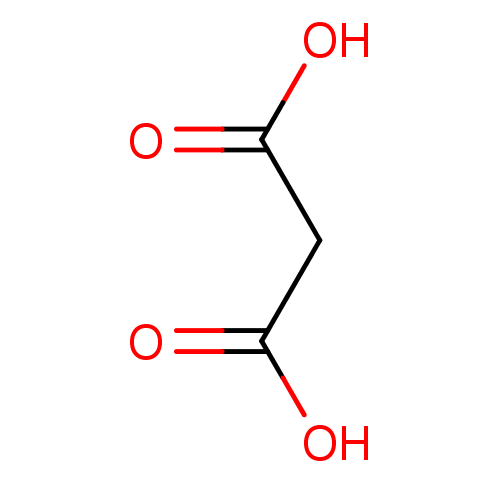 (Fragment 3 | Malonic Acid | propanedioic acid) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.53E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University Curated by ChEMBL | Assay Description Binding affinity to human recombinant DDO | J Med Chem 58: 7328-40 (2015) Article DOI: 10.1021/acs.jmedchem.5b00871 BindingDB Entry DOI: 10.7270/Q2WM1G6R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-aspartate oxidase (Mus musculus) | BDBM14673 (Fragment 3 | Malonic Acid | propanedioic acid) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.22E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University Curated by ChEMBL | Assay Description Inhibition of mouse recombinant DDO using D-Asp | J Med Chem 58: 7328-40 (2015) Article DOI: 10.1021/acs.jmedchem.5b00871 BindingDB Entry DOI: 10.7270/Q2WM1G6R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RCG38204, isoform CRA_d (Rattus norvegicus) | BDBM14673 (Fragment 3 | Malonic Acid | propanedioic acid) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.56E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University Curated by ChEMBL | Assay Description Inhibition of rat recombinant DDO using D-Asp | J Med Chem 58: 7328-40 (2015) Article DOI: 10.1021/acs.jmedchem.5b00871 BindingDB Entry DOI: 10.7270/Q2WM1G6R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 [Y641F] (Homo sapiens (Human)) | BDBM172085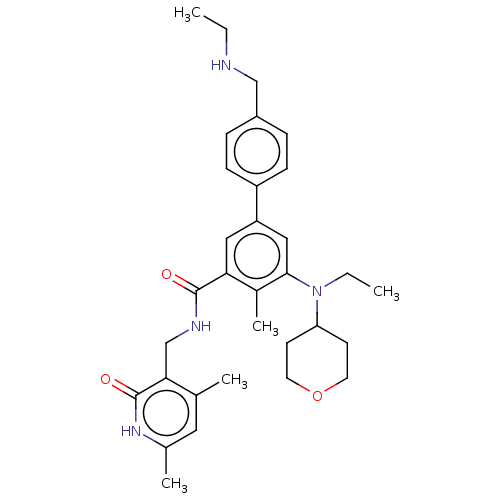 (US10155002, Compound 91 | US11052093, Compound 91 ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme, Inc. US Patent | Assay Description The assays were all performed in a buffer consisting of 20 mM bicine (pH=7.6), 0.5 mM DTT, 0.005% BSG and 0.002% Tween20, prepared on the day of use.... | US Patent US10155002 (2018) BindingDB Entry DOI: 10.7270/Q2KS6TM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 [Y641F] (Homo sapiens (Human)) | BDBM172085 (US10155002, Compound 91 | US11052093, Compound 91 ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | 25 |
Epizyme, Inc. US Patent | Assay Description The assays were all performed in a buffer consisting of 20 mM bicine (pH=7.6), 0.5 mM DTT, 0.005% BSG and 0.002% Tween20, prepared on the day of use.... | US Patent US9090562 (2015) BindingDB Entry DOI: 10.7270/Q2057DPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 [Y641F] (Homo sapiens (Human)) | BDBM172085 (US10155002, Compound 91 | US11052093, Compound 91 ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The assays were all performed in a buffer consisting of 20 mM bicine (pH=7.6), 0.5 mM DTT, 0.005% BSG and 0.002% Tween20, prepared on the day of use.... | Citation and Details BindingDB Entry DOI: 10.7270/Q2SB48WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 [Y641F] (Homo sapiens (Human)) | BDBM172163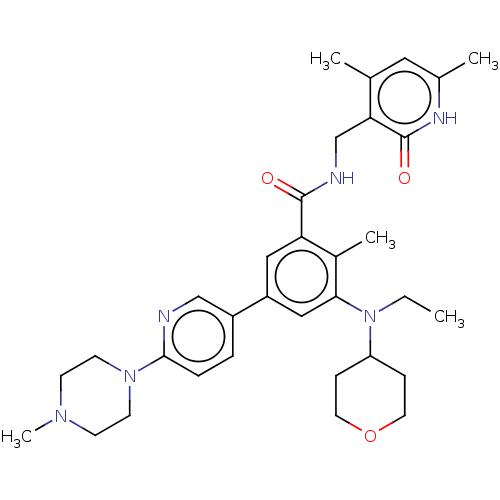 (US10155002, Compound 171 | US11052093, Compound 17...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The assays were all performed in a buffer consisting of 20 mM bicine (pH=7.6), 0.5 mM DTT, 0.005% BSG and 0.002% Tween20, prepared on the day of use.... | Citation and Details BindingDB Entry DOI: 10.7270/Q2SB48WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 [Y641F] (Homo sapiens (Human)) | BDBM172163 (US10155002, Compound 171 | US11052093, Compound 17...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme, Inc. US Patent | Assay Description The assays were all performed in a buffer consisting of 20 mM bicine (pH=7.6), 0.5 mM DTT, 0.005% BSG and 0.002% Tween20, prepared on the day of use.... | US Patent US10155002 (2018) BindingDB Entry DOI: 10.7270/Q2KS6TM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 [Y641F] (Homo sapiens (Human)) | BDBM172163 (US10155002, Compound 171 | US11052093, Compound 17...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.760 | n/a | n/a | n/a | n/a | n/a | 25 |
Epizyme, Inc. US Patent | Assay Description The assays were all performed in a buffer consisting of 20 mM bicine (pH=7.6), 0.5 mM DTT, 0.005% BSG and 0.002% Tween20, prepared on the day of use.... | US Patent US9090562 (2015) BindingDB Entry DOI: 10.7270/Q2057DPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 [Y641F] (Homo sapiens (Human)) | BDBM172087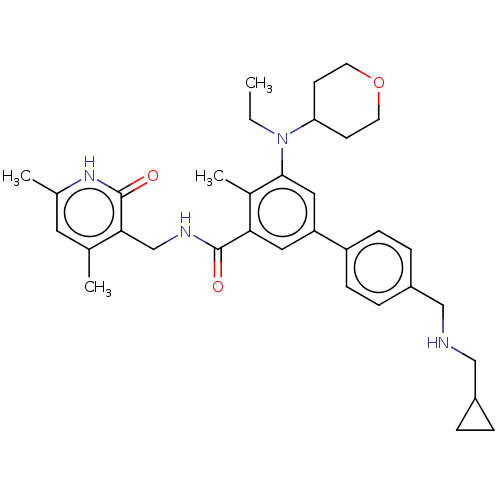 (US10155002, Compound 93 | US11052093, Compound 93 ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme, Inc. US Patent | Assay Description The assays were all performed in a buffer consisting of 20 mM bicine (pH=7.6), 0.5 mM DTT, 0.005% BSG and 0.002% Tween20, prepared on the day of use.... | US Patent US10155002 (2018) BindingDB Entry DOI: 10.7270/Q2KS6TM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 [Y641F] (Homo sapiens (Human)) | BDBM172087 (US10155002, Compound 93 | US11052093, Compound 93 ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.870 | n/a | n/a | n/a | n/a | n/a | 25 |
Epizyme, Inc. US Patent | Assay Description The assays were all performed in a buffer consisting of 20 mM bicine (pH=7.6), 0.5 mM DTT, 0.005% BSG and 0.002% Tween20, prepared on the day of use.... | US Patent US9090562 (2015) BindingDB Entry DOI: 10.7270/Q2057DPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 [Y641F] (Homo sapiens (Human)) | BDBM172087 (US10155002, Compound 93 | US11052093, Compound 93 ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The assays were all performed in a buffer consisting of 20 mM bicine (pH=7.6), 0.5 mM DTT, 0.005% BSG and 0.002% Tween20, prepared on the day of use.... | Citation and Details BindingDB Entry DOI: 10.7270/Q2SB48WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM172176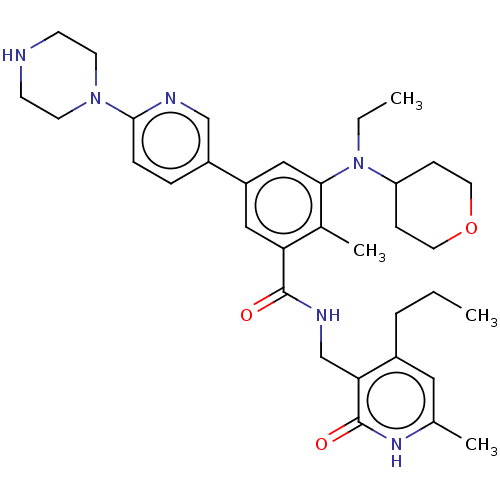 (EPZ009161 | US10155002, Compound 184 | US11052093,...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme, Inc. US Patent | Assay Description The assays were all performed in a buffer consisting of 20 mM bicine (pH=7.6), 0.5 mM DTT, 0.005% BSG and 0.002% Tween20, prepared on the day of use.... | US Patent US10155002 (2018) BindingDB Entry DOI: 10.7270/Q2KS6TM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM172176 (EPZ009161 | US10155002, Compound 184 | US11052093,...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The assays were all performed in a buffer consisting of 20 mM bicine (pH=7.6), 0.5 mM DTT, 0.005% BSG and 0.002% Tween20, prepared on the day of use.... | Citation and Details BindingDB Entry DOI: 10.7270/Q2SB48WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM172176 (EPZ009161 | US10155002, Compound 184 | US11052093,...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.920 | n/a | n/a | n/a | n/a | n/a | 25 |
Epizyme, Inc. US Patent | Assay Description The assays were all performed in a buffer consisting of 20 mM bicine (pH=7.6), 0.5 mM DTT, 0.005% BSG and 0.002% Tween20, prepared on the day of use.... | US Patent US9090562 (2015) BindingDB Entry DOI: 10.7270/Q2057DPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 [Y641F] (Homo sapiens (Human)) | BDBM172080 (US10155002, Compound 86 | US11052093, Compound 86 ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The assays were all performed in a buffer consisting of 20 mM bicine (pH=7.6), 0.5 mM DTT, 0.005% BSG and 0.002% Tween20, prepared on the day of use.... | Citation and Details BindingDB Entry DOI: 10.7270/Q2SB48WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 [Y641F] (Homo sapiens (Human)) | BDBM172080 (US10155002, Compound 86 | US11052093, Compound 86 ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.12 | n/a | n/a | n/a | n/a | n/a | 25 |
Epizyme, Inc. US Patent | Assay Description The assays were all performed in a buffer consisting of 20 mM bicine (pH=7.6), 0.5 mM DTT, 0.005% BSG and 0.002% Tween20, prepared on the day of use.... | US Patent US9090562 (2015) BindingDB Entry DOI: 10.7270/Q2057DPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 [Y641F] (Homo sapiens (Human)) | BDBM172080 (US10155002, Compound 86 | US11052093, Compound 86 ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.12 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme, Inc. US Patent | Assay Description The assays were all performed in a buffer consisting of 20 mM bicine (pH=7.6), 0.5 mM DTT, 0.005% BSG and 0.002% Tween20, prepared on the day of use.... | US Patent US10155002 (2018) BindingDB Entry DOI: 10.7270/Q2KS6TM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM172138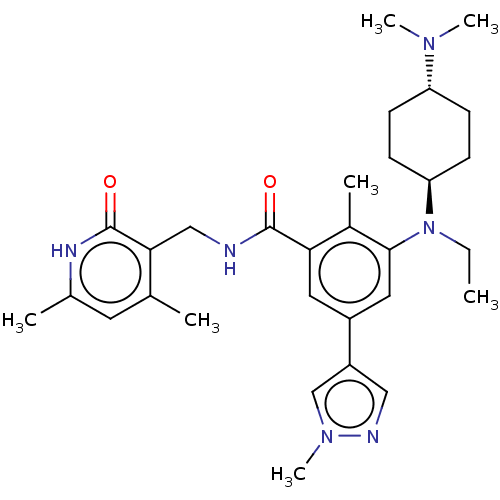 (EPZ007428 | US10155002, Compound 145 | US11052093,...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.39 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The assays were all performed in a buffer consisting of 20 mM bicine (pH=7.6), 0.5 mM DTT, 0.005% BSG and 0.002% Tween20, prepared on the day of use.... | Citation and Details BindingDB Entry DOI: 10.7270/Q2SB48WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM172138 (EPZ007428 | US10155002, Compound 145 | US11052093,...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.39 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme, Inc. US Patent | Assay Description The assays were all performed in a buffer consisting of 20 mM bicine (pH=7.6), 0.5 mM DTT, 0.005% BSG and 0.002% Tween20, prepared on the day of use.... | US Patent US10155002 (2018) BindingDB Entry DOI: 10.7270/Q2KS6TM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM172138 (EPZ007428 | US10155002, Compound 145 | US11052093,...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.39 | n/a | n/a | n/a | n/a | n/a | 25 |
Epizyme, Inc. US Patent | Assay Description The assays were all performed in a buffer consisting of 20 mM bicine (pH=7.6), 0.5 mM DTT, 0.005% BSG and 0.002% Tween20, prepared on the day of use.... | US Patent US9090562 (2015) BindingDB Entry DOI: 10.7270/Q2057DPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM172135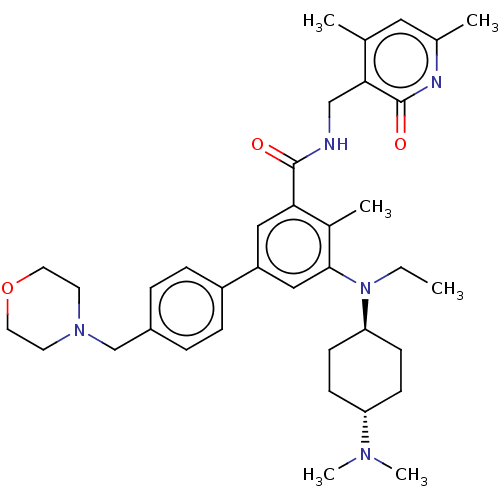 (EPZ007210 | US10155002, Compound 141 | US11052093,...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.51 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The assays were all performed in a buffer consisting of 20 mM bicine (pH=7.6), 0.5 mM DTT, 0.005% BSG and 0.002% Tween20, prepared on the day of use.... | Citation and Details BindingDB Entry DOI: 10.7270/Q2SB48WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM172135 (EPZ007210 | US10155002, Compound 141 | US11052093,...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.51 | n/a | n/a | n/a | n/a | n/a | 25 |
Epizyme, Inc. US Patent | Assay Description The assays were all performed in a buffer consisting of 20 mM bicine (pH=7.6), 0.5 mM DTT, 0.005% BSG and 0.002% Tween20, prepared on the day of use.... | US Patent US9090562 (2015) BindingDB Entry DOI: 10.7270/Q2057DPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM172135 (EPZ007210 | US10155002, Compound 141 | US11052093,...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.51 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme, Inc. US Patent | Assay Description The assays were all performed in a buffer consisting of 20 mM bicine (pH=7.6), 0.5 mM DTT, 0.005% BSG and 0.002% Tween20, prepared on the day of use.... | US Patent US10155002 (2018) BindingDB Entry DOI: 10.7270/Q2KS6TM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 [Y641F] (Homo sapiens (Human)) | BDBM172089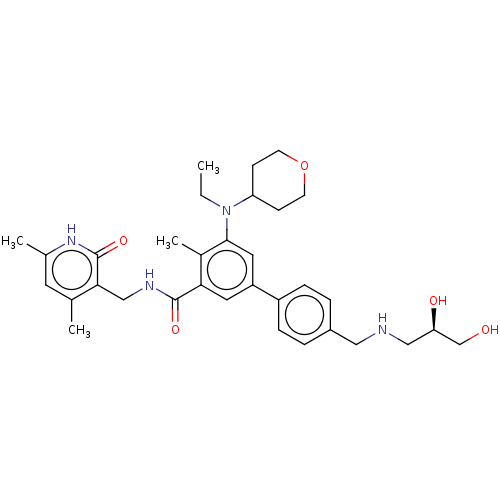 (US10155002, Compound 95 | US11052093, Compound 95 ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.55 | n/a | n/a | n/a | n/a | n/a | 25 |
Epizyme, Inc. US Patent | Assay Description The assays were all performed in a buffer consisting of 20 mM bicine (pH=7.6), 0.5 mM DTT, 0.005% BSG and 0.002% Tween20, prepared on the day of use.... | US Patent US9090562 (2015) BindingDB Entry DOI: 10.7270/Q2057DPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 [Y641F] (Homo sapiens (Human)) | BDBM172089 (US10155002, Compound 95 | US11052093, Compound 95 ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.55 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme, Inc. US Patent | Assay Description The assays were all performed in a buffer consisting of 20 mM bicine (pH=7.6), 0.5 mM DTT, 0.005% BSG and 0.002% Tween20, prepared on the day of use.... | US Patent US10155002 (2018) BindingDB Entry DOI: 10.7270/Q2KS6TM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 [Y641F] (Homo sapiens (Human)) | BDBM172089 (US10155002, Compound 95 | US11052093, Compound 95 ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.55 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The assays were all performed in a buffer consisting of 20 mM bicine (pH=7.6), 0.5 mM DTT, 0.005% BSG and 0.002% Tween20, prepared on the day of use.... | Citation and Details BindingDB Entry DOI: 10.7270/Q2SB48WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 [Y641F] (Homo sapiens (Human)) | BDBM172162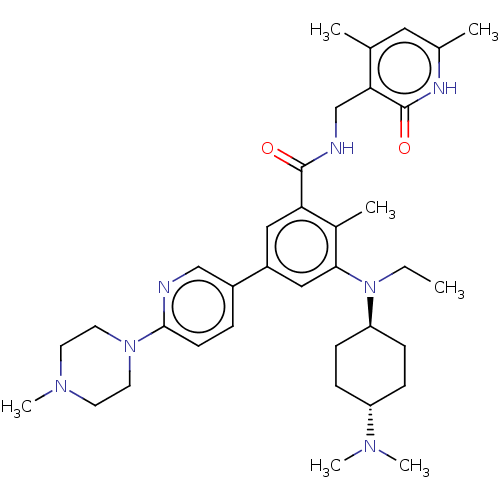 (US10155002, Compound 170 | US11052093, Compound 17...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.63 | n/a | n/a | n/a | n/a | n/a | 25 |
Epizyme, Inc. US Patent | Assay Description The assays were all performed in a buffer consisting of 20 mM bicine (pH=7.6), 0.5 mM DTT, 0.005% BSG and 0.002% Tween20, prepared on the day of use.... | US Patent US9090562 (2015) BindingDB Entry DOI: 10.7270/Q2057DPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 [Y641F] (Homo sapiens (Human)) | BDBM172162 (US10155002, Compound 170 | US11052093, Compound 17...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.63 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The assays were all performed in a buffer consisting of 20 mM bicine (pH=7.6), 0.5 mM DTT, 0.005% BSG and 0.002% Tween20, prepared on the day of use.... | Citation and Details BindingDB Entry DOI: 10.7270/Q2SB48WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 [Y641F] (Homo sapiens (Human)) | BDBM172162 (US10155002, Compound 170 | US11052093, Compound 17...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.63 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme, Inc. US Patent | Assay Description The assays were all performed in a buffer consisting of 20 mM bicine (pH=7.6), 0.5 mM DTT, 0.005% BSG and 0.002% Tween20, prepared on the day of use.... | US Patent US10155002 (2018) BindingDB Entry DOI: 10.7270/Q2KS6TM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM172181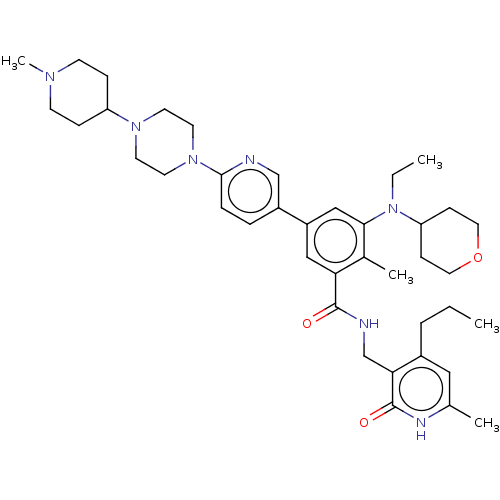 (US10155002, Compound 189 | US11052093, Compound 18...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.64 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme, Inc. US Patent | Assay Description The assays were all performed in a buffer consisting of 20 mM bicine (pH=7.6), 0.5 mM DTT, 0.005% BSG and 0.002% Tween20, prepared on the day of use.... | US Patent US10155002 (2018) BindingDB Entry DOI: 10.7270/Q2KS6TM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM172181 (US10155002, Compound 189 | US11052093, Compound 18...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.64 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The assays were all performed in a buffer consisting of 20 mM bicine (pH=7.6), 0.5 mM DTT, 0.005% BSG and 0.002% Tween20, prepared on the day of use.... | Citation and Details BindingDB Entry DOI: 10.7270/Q2SB48WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM172181 (US10155002, Compound 189 | US11052093, Compound 18...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.64 | n/a | n/a | n/a | n/a | n/a | 25 |
Epizyme, Inc. US Patent | Assay Description The assays were all performed in a buffer consisting of 20 mM bicine (pH=7.6), 0.5 mM DTT, 0.005% BSG and 0.002% Tween20, prepared on the day of use.... | US Patent US9090562 (2015) BindingDB Entry DOI: 10.7270/Q2057DPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 [Y641F] (Homo sapiens (Human)) | BDBM172181 (US10155002, Compound 189 | US11052093, Compound 18...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.82 | n/a | n/a | n/a | n/a | n/a | 25 |
Epizyme, Inc. US Patent | Assay Description The assays were all performed in a buffer consisting of 20 mM bicine (pH=7.6), 0.5 mM DTT, 0.005% BSG and 0.002% Tween20, prepared on the day of use.... | US Patent US9090562 (2015) BindingDB Entry DOI: 10.7270/Q2057DPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 [Y641F] (Homo sapiens (Human)) | BDBM172181 (US10155002, Compound 189 | US11052093, Compound 18...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.82 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The assays were all performed in a buffer consisting of 20 mM bicine (pH=7.6), 0.5 mM DTT, 0.005% BSG and 0.002% Tween20, prepared on the day of use.... | Citation and Details BindingDB Entry DOI: 10.7270/Q2SB48WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 [Y641F] (Homo sapiens (Human)) | BDBM172181 (US10155002, Compound 189 | US11052093, Compound 18...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.82 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme, Inc. US Patent | Assay Description The assays were all performed in a buffer consisting of 20 mM bicine (pH=7.6), 0.5 mM DTT, 0.005% BSG and 0.002% Tween20, prepared on the day of use.... | US Patent US10155002 (2018) BindingDB Entry DOI: 10.7270/Q2KS6TM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1244 total ) | Next | Last >> |