 Found 266 hits with Last Name = 'severin' and Initial = 'a'
Found 266 hits with Last Name = 'severin' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Free fatty acid receptor 1
(Homo sapiens (Human)) | BDBM50344068

(CHEMBL1777861 | rac-3-(4-(2-(difluoromethoxy)-4-(t...)Show SMILES CC#CC(CC(O)=O)c1ccc(Oc2ccc(cc2OC(F)F)C(F)(F)F)cc1 Show InChI InChI=1S/C20H15F5O4/c1-2-3-13(10-18(26)27)12-4-7-15(8-5-12)28-16-9-6-14(20(23,24)25)11-17(16)29-19(21)22/h4-9,11,13,19H,10H2,1H3,(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human GPR40 expressed in CHO cells by SPA based binding assay |
Bioorg Med Chem Lett 21: 3390-4 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.114
BindingDB Entry DOI: 10.7270/Q2KP82GX |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Homo sapiens (Human)) | BDBM50344071

(CHEMBL1777864 | rac-3-(4-(4-cyanonaphthalen-1-ylox...)Show SMILES CC#CC(CC(O)=O)c1ccc(Oc2ccc(C#N)c3ccccc23)cc1 Show InChI InChI=1S/C23H17NO3/c1-2-5-17(14-23(25)26)16-8-11-19(12-9-16)27-22-13-10-18(15-24)20-6-3-4-7-21(20)22/h3-4,6-13,17H,14H2,1H3,(H,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human GPR40 expressed in CHO cells by SPA based binding assay |
Bioorg Med Chem Lett 21: 3390-4 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.114
BindingDB Entry DOI: 10.7270/Q2KP82GX |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Homo sapiens (Human)) | BDBM50344084
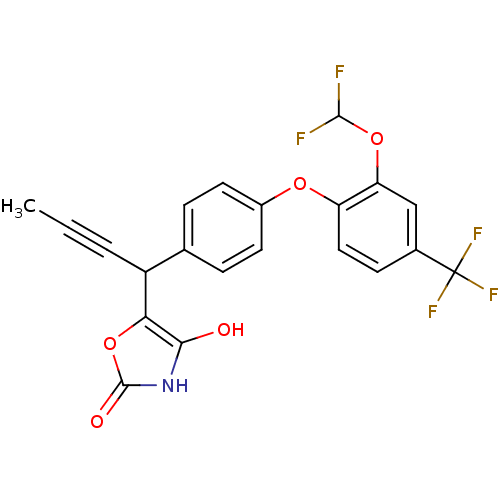
(CHEMBL1777876 | rac-5-(1-(4-(2-(difluoromethoxy)-4...)Show SMILES CC#CC(c1oc(=O)[nH]c1O)c1ccc(Oc2ccc(cc2OC(F)F)C(F)(F)F)cc1 Show InChI InChI=1S/C21H14F5NO5/c1-2-3-14(17-18(28)27-20(29)32-17)11-4-7-13(8-5-11)30-15-9-6-12(21(24,25)26)10-16(15)31-19(22)23/h4-10,14,19,28H,1H3,(H,27,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human GPR40 expressed in CHO cells by SPA based binding assay |
Bioorg Med Chem Lett 21: 3390-4 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.114
BindingDB Entry DOI: 10.7270/Q2KP82GX |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Homo sapiens (Human)) | BDBM50344069
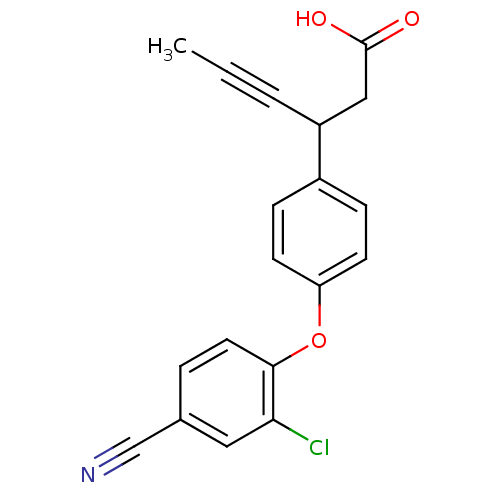
(CHEMBL1777862 | rac-3-(4-(2-chloro-4-cyanophenoxy)...)Show InChI InChI=1S/C19H14ClNO3/c1-2-3-15(11-19(22)23)14-5-7-16(8-6-14)24-18-9-4-13(12-21)10-17(18)20/h4-10,15H,11H2,1H3,(H,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human GPR40 expressed in CHO cells by SPA based binding assay |
Bioorg Med Chem Lett 21: 3390-4 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.114
BindingDB Entry DOI: 10.7270/Q2KP82GX |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Homo sapiens (Human)) | BDBM50344078
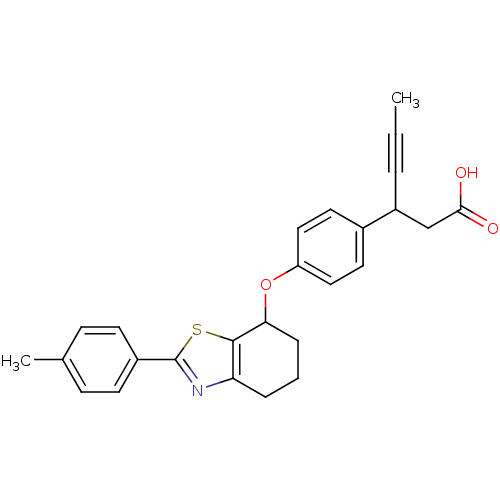
(CHEMBL1777872 | rac-3-(4-(2-p-tolyl-4,5,6,7-tetrah...)Show SMILES CC#CC(CC(O)=O)c1ccc(OC2CCCc3nc(sc23)-c2ccc(C)cc2)cc1 Show InChI InChI=1S/C26H25NO3S/c1-3-5-20(16-24(28)29)18-12-14-21(15-13-18)30-23-7-4-6-22-25(23)31-26(27-22)19-10-8-17(2)9-11-19/h8-15,20,23H,4,6-7,16H2,1-2H3,(H,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human GPR40 expressed in CHO cells by SPA based binding assay |
Bioorg Med Chem Lett 21: 3390-4 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.114
BindingDB Entry DOI: 10.7270/Q2KP82GX |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Homo sapiens (Human)) | BDBM50344073

(CHEMBL1777866 | rac-3-(4-(3-chloro-5-(trifluoromet...)Show SMILES CC#CC(CC(O)=O)c1ccc(Oc2ncc(cc2Cl)C(F)(F)F)cc1 Show InChI InChI=1S/C18H13ClF3NO3/c1-2-3-12(8-16(24)25)11-4-6-14(7-5-11)26-17-15(19)9-13(10-23-17)18(20,21)22/h4-7,9-10,12H,8H2,1H3,(H,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human GPR40 expressed in CHO cells by SPA based binding assay |
Bioorg Med Chem Lett 21: 3390-4 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.114
BindingDB Entry DOI: 10.7270/Q2KP82GX |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Homo sapiens (Human)) | BDBM50344070
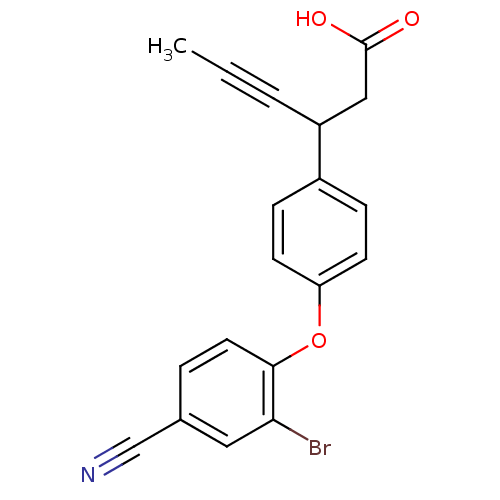
(CHEMBL1777863 | rac-3-(4-(2-bromo-4-cyanophenoxy)p...)Show InChI InChI=1S/C19H14BrNO3/c1-2-3-15(11-19(22)23)14-5-7-16(8-6-14)24-18-9-4-13(12-21)10-17(18)20/h4-10,15H,11H2,1H3,(H,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human GPR40 expressed in CHO cells by SPA based binding assay |
Bioorg Med Chem Lett 21: 3390-4 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.114
BindingDB Entry DOI: 10.7270/Q2KP82GX |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Homo sapiens (Human)) | BDBM50344067

(CHEMBL1777860 | rac-3-(4-(2-methyl-4-(trifluoromet...)Show SMILES CC#CC(CC(O)=O)c1ccc(Oc2ccc(cc2C)C(F)(F)F)cc1 Show InChI InChI=1S/C20H17F3O3/c1-3-4-15(12-19(24)25)14-5-8-17(9-6-14)26-18-10-7-16(11-13(18)2)20(21,22)23/h5-11,15H,12H2,1-2H3,(H,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human GPR40 expressed in CHO cells by SPA based binding assay |
Bioorg Med Chem Lett 21: 3390-4 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.114
BindingDB Entry DOI: 10.7270/Q2KP82GX |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Homo sapiens (Human)) | BDBM50344077

(3-(4-((R)-2,3-dihydro-1H-inden-1-yloxy)phenyl)hex-...)Show SMILES CC#CC(CC(O)=O)c1ccc(O[C@@H]2CCc3ccccc23)cc1 |r| Show InChI InChI=1S/C21H20O3/c1-2-5-17(14-21(22)23)15-8-11-18(12-9-15)24-20-13-10-16-6-3-4-7-19(16)20/h3-4,6-9,11-12,17,20H,10,13-14H2,1H3,(H,22,23)/t17?,20-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human GPR40 expressed in CHO cells by SPA based binding assay |
Bioorg Med Chem Lett 21: 3390-4 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.114
BindingDB Entry DOI: 10.7270/Q2KP82GX |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Homo sapiens (Human)) | BDBM50344080

(CHEMBL1777855 | rac-3-(4-(2-(difluoromethoxy)-4-(t...)Show SMILES CC#CC(CC(O)=O)c1ccc(Oc2ccc(cc2OC(F)F)C(F)(F)F)cc1F Show InChI InChI=1S/C20H14F6O4/c1-2-3-11(8-18(27)28)14-6-5-13(10-15(14)21)29-16-7-4-12(20(24,25)26)9-17(16)30-19(22)23/h4-7,9-11,19H,8H2,1H3,(H,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human GPR40 expressed in CHO cells by SPA based binding assay |
Bioorg Med Chem Lett 21: 3390-4 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.114
BindingDB Entry DOI: 10.7270/Q2KP82GX |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Homo sapiens (Human)) | BDBM50344086

(CHEMBL1777878 | cis-rac-5-(1-(4-(2-(difluoromethox...)Show SMILES CC#C[C@H](c1oc(=O)[nH]c1O)c1ccc(Oc2ccc(cc2OC(F)F)C(F)(F)F)cc1 |r| Show InChI InChI=1S/C21H14F5NO5/c1-2-3-14(17-18(28)27-20(29)32-17)11-4-7-13(8-5-11)30-15-9-6-12(21(24,25)26)10-16(15)31-19(22)23/h4-10,14,19,28H,1H3,(H,27,29)/t14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 151 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human GPR40 expressed in CHO cells by SPA based binding assay |
Bioorg Med Chem Lett 21: 3390-4 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.114
BindingDB Entry DOI: 10.7270/Q2KP82GX |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Homo sapiens (Human)) | BDBM50344072

(CHEMBL1777865 | rac-3-(4-(4-(trifluoromethyl)napht...)Show SMILES CC#CC(CC(O)=O)c1ccc(Oc2ccc(c3ccccc23)C(F)(F)F)cc1 Show InChI InChI=1S/C23H17F3O3/c1-2-5-16(14-22(27)28)15-8-10-17(11-9-15)29-21-13-12-20(23(24,25)26)18-6-3-4-7-19(18)21/h3-4,6-13,16H,14H2,1H3,(H,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 211 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human GPR40 expressed in CHO cells by SPA based binding assay |
Bioorg Med Chem Lett 21: 3390-4 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.114
BindingDB Entry DOI: 10.7270/Q2KP82GX |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Homo sapiens (Human)) | BDBM50344079

(CHEMBL1777873 | rac-3-(4-(cyclopentyloxy)phenyl)he...)Show InChI InChI=1S/C17H20O3/c1-2-5-14(12-17(18)19)13-8-10-16(11-9-13)20-15-6-3-4-7-15/h8-11,14-15H,3-4,6-7,12H2,1H3,(H,18,19) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 312 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human GPR40 expressed in CHO cells by SPA based binding assay |
Bioorg Med Chem Lett 21: 3390-4 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.114
BindingDB Entry DOI: 10.7270/Q2KP82GX |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Homo sapiens (Human)) | BDBM50344076

(CHEMBL1777869 | rac-3-(4-(isoquinolin-1-yloxy)phen...)Show InChI InChI=1S/C21H17NO3/c1-2-5-17(14-20(23)24)15-8-10-18(11-9-15)25-21-19-7-4-3-6-16(19)12-13-22-21/h3-4,6-13,17H,14H2,1H3,(H,23,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 342 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human GPR40 expressed in CHO cells by SPA based binding assay |
Bioorg Med Chem Lett 21: 3390-4 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.114
BindingDB Entry DOI: 10.7270/Q2KP82GX |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Homo sapiens (Human)) | BDBM50344074

(CHEMBL1777867 | rac-3-(4-(5-bromopyrimidin-2-yloxy...)Show InChI InChI=1S/C16H13BrN2O3/c1-2-3-12(8-15(20)21)11-4-6-14(7-5-11)22-16-18-9-13(17)10-19-16/h4-7,9-10,12H,8H2,1H3,(H,20,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 433 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human GPR40 expressed in CHO cells by SPA based binding assay |
Bioorg Med Chem Lett 21: 3390-4 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.114
BindingDB Entry DOI: 10.7270/Q2KP82GX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50344086

(CHEMBL1777878 | cis-rac-5-(1-(4-(2-(difluoromethox...)Show SMILES CC#C[C@H](c1oc(=O)[nH]c1O)c1ccc(Oc2ccc(cc2OC(F)F)C(F)(F)F)cc1 |r| Show InChI InChI=1S/C21H14F5NO5/c1-2-3-14(17-18(28)27-20(29)32-17)11-4-7-13(8-5-11)30-15-9-6-12(21(24,25)26)10-16(15)31-19(22)23/h4-10,14,19,28H,1H3,(H,27,29)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
Bioorg Med Chem Lett 21: 3390-4 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.114
BindingDB Entry DOI: 10.7270/Q2KP82GX |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Homo sapiens (Human)) | BDBM50344085
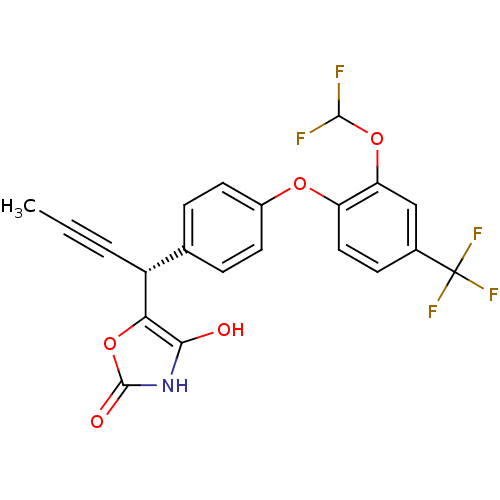
(CHEMBL1777877 | trans-rac-5-(1-(4-(2-(difluorometh...)Show SMILES CC#C[C@@H](c1oc(=O)[nH]c1O)c1ccc(Oc2ccc(cc2OC(F)F)C(F)(F)F)cc1 |r| Show InChI InChI=1S/C21H14F5NO5/c1-2-3-14(17-18(28)27-20(29)32-17)11-4-7-13(8-5-11)30-15-9-6-12(21(24,25)26)10-16(15)31-19(22)23/h4-10,14,19,28H,1H3,(H,27,29)/t14-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human GPR40 expressed in CHO cells by SPA based binding assay |
Bioorg Med Chem Lett 21: 3390-4 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.114
BindingDB Entry DOI: 10.7270/Q2KP82GX |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Homo sapiens (Human)) | BDBM50344075
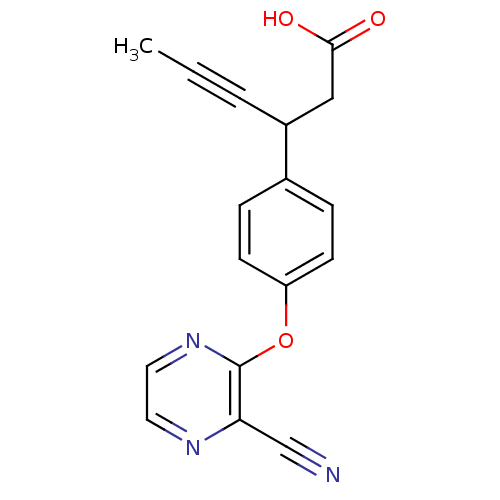
(CHEMBL1777868 | rac-3-(4-(3-cyanopyrazin-2-yloxy)p...)Show InChI InChI=1S/C17H13N3O3/c1-2-3-13(10-16(21)22)12-4-6-14(7-5-12)23-17-15(11-18)19-8-9-20-17/h4-9,13H,10H2,1H3,(H,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.49E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human GPR40 expressed in CHO cells by SPA based binding assay |
Bioorg Med Chem Lett 21: 3390-4 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.114
BindingDB Entry DOI: 10.7270/Q2KP82GX |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50344086

(CHEMBL1777878 | cis-rac-5-(1-(4-(2-(difluoromethox...)Show SMILES CC#C[C@H](c1oc(=O)[nH]c1O)c1ccc(Oc2ccc(cc2OC(F)F)C(F)(F)F)cc1 |r| Show InChI InChI=1S/C21H14F5NO5/c1-2-3-14(17-18(28)27-20(29)32-17)11-4-7-13(8-5-11)30-15-9-6-12(21(24,25)26)10-16(15)31-19(22)23/h4-10,14,19,28H,1H3,(H,27,29)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 21: 3390-4 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.114
BindingDB Entry DOI: 10.7270/Q2KP82GX |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Homo sapiens (Human)) | BDBM50344081

(CHEMBL1777874 | cis-rac-2-(4-(2-chloro-4-(trifluor...)Show SMILES CC#C[C@@]1(C[C@@H]1C(O)=O)c1ccc(Oc2ccc(cc2Cl)C(F)(F)F)cc1 |r| Show InChI InChI=1S/C20H14ClF3O3/c1-2-9-19(11-15(19)18(25)26)12-3-6-14(7-4-12)27-17-8-5-13(10-16(17)21)20(22,23)24/h3-8,10,15H,11H2,1H3,(H,25,26)/t15-,19-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.44E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human GPR40 expressed in CHO cells by SPA based binding assay |
Bioorg Med Chem Lett 21: 3390-4 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.114
BindingDB Entry DOI: 10.7270/Q2KP82GX |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Homo sapiens (Human)) | BDBM50344083
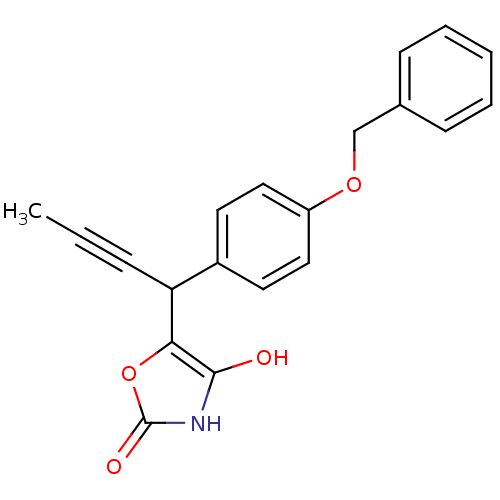
(CHEMBL1777871 | rac-5-(1-(4-(benzyloxy)phenyl)but-...)Show InChI InChI=1S/C20H17NO4/c1-2-6-17(18-19(22)21-20(23)25-18)15-9-11-16(12-10-15)24-13-14-7-4-3-5-8-14/h3-5,7-12,17,22H,13H2,1H3,(H,21,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human GPR40 expressed in CHO cells by SPA based binding assay |
Bioorg Med Chem Lett 21: 3390-4 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.114
BindingDB Entry DOI: 10.7270/Q2KP82GX |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50195560

(4-(4-butoxybenzoyl)-1,2-bis(4-chlorophenyl)-5-hydr...)Show SMILES CCCCOc1ccc(cc1)C(=O)c1c(O)n(-c2ccc(Cl)cc2)n(-c2ccc(Cl)cc2)c1=O Show InChI InChI=1S/C26H22Cl2N2O4/c1-2-3-16-34-22-14-4-17(5-15-22)24(31)23-25(32)29(20-10-6-18(27)7-11-20)30(26(23)33)21-12-8-19(28)9-13-21/h4-15,32H,2-3,16H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth
Curated by ChEMBL
| Assay Description
Inhibition of MurB activity |
J Med Chem 49: 6027-36 (2006)
Article DOI: 10.1021/jm060499t
BindingDB Entry DOI: 10.7270/Q22V2FRV |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50195576

(1,2-bis(3,4-dichlorophenyl)-5-hydroxy-3-oxo-2,3-di...)Show SMILES Oc1c(C(=O)Nc2ccc(Cl)c(Cl)c2)c(=O)n(-c2ccc(Cl)c(Cl)c2)n1-c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C22H11Cl6N3O3/c23-13-4-1-10(7-16(13)26)29-20(32)19-21(33)30(11-2-5-14(24)17(27)8-11)31(22(19)34)12-3-6-15(25)18(28)9-12/h1-9,33H,(H,29,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth
Curated by ChEMBL
| Assay Description
Inhibition of MurB activity |
J Med Chem 49: 6027-36 (2006)
Article DOI: 10.1021/jm060499t
BindingDB Entry DOI: 10.7270/Q22V2FRV |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50195550
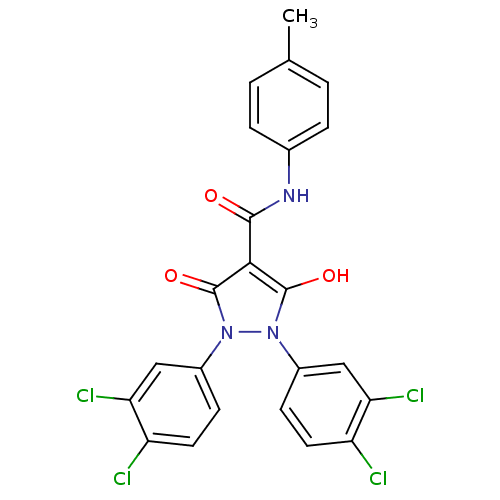
(1,2-bis(3,4-dichlorophenyl)-5-hydroxy-3-oxo-2,3-di...)Show SMILES Cc1ccc(NC(=O)c2c(O)n(-c3ccc(Cl)c(Cl)c3)n(-c3ccc(Cl)c(Cl)c3)c2=O)cc1 Show InChI InChI=1S/C23H15Cl4N3O3/c1-12-2-4-13(5-3-12)28-21(31)20-22(32)29(14-6-8-16(24)18(26)10-14)30(23(20)33)15-7-9-17(25)19(27)11-15/h2-11,32H,1H3,(H,28,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth
Curated by ChEMBL
| Assay Description
Inhibition of MurB activity |
J Med Chem 49: 6027-36 (2006)
Article DOI: 10.1021/jm060499t
BindingDB Entry DOI: 10.7270/Q22V2FRV |
More data for this
Ligand-Target Pair | |
Voltage-dependent L-type calcium channel subunit alpha-1C
(Homo sapiens (Human)) | BDBM50344086

(CHEMBL1777878 | cis-rac-5-(1-(4-(2-(difluoromethox...)Show SMILES CC#C[C@H](c1oc(=O)[nH]c1O)c1ccc(Oc2ccc(cc2OC(F)F)C(F)(F)F)cc1 |r| Show InChI InChI=1S/C21H14F5NO5/c1-2-3-14(17-18(28)27-20(29)32-17)11-4-7-13(8-5-11)30-15-9-6-12(21(24,25)26)10-16(15)31-19(22)23/h4-10,14,19,28H,1H3,(H,27,29)/t14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Cav1.2 calcium channel |
Bioorg Med Chem Lett 21: 3390-4 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.114
BindingDB Entry DOI: 10.7270/Q2KP82GX |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Staphylococcus aureus (Firmicutes)) | BDBM50166493

(4-(2-Benzylsulfanyl-ethyl)-1,2-bis-(4-chloro-pheny...)Show SMILES Oc1c(CCSCc2ccccc2)c(=O)n(-c2ccc(Cl)cc2)n1-c1ccc(Cl)cc1 Show InChI InChI=1S/C24H20Cl2N2O2S/c25-18-6-10-20(11-7-18)27-23(29)22(14-15-31-16-17-4-2-1-3-5-17)24(30)28(27)21-12-8-19(26)9-13-21/h1-13,29H,14-16H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MurB enzyme in Staphylococcus aureus |
Bioorg Med Chem Lett 15: 2527-31 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.058
BindingDB Entry DOI: 10.7270/Q2BG2NHW |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Staphylococcus aureus (Firmicutes)) | BDBM50166485
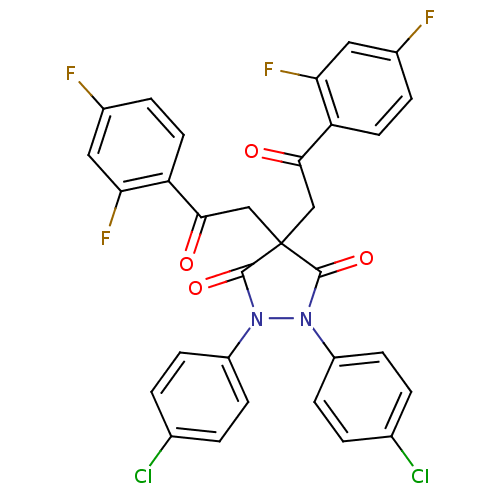
(1,2-Bis-(4-chloro-phenyl)-4,4-bis-[2-(2,4-difluoro...)Show SMILES Fc1ccc(C(=O)CC2(CC(=O)c3ccc(F)cc3F)C(=O)N(N(C2=O)c2ccc(Cl)cc2)c2ccc(Cl)cc2)c(F)c1 Show InChI InChI=1S/C31H18Cl2F4N2O4/c32-17-1-7-21(8-2-17)38-29(42)31(15-27(40)23-11-5-19(34)13-25(23)36,16-28(41)24-12-6-20(35)14-26(24)37)30(43)39(38)22-9-3-18(33)4-10-22/h1-14H,15-16H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MurB enzyme in Staphylococcus aureus |
Bioorg Med Chem Lett 15: 2527-31 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.058
BindingDB Entry DOI: 10.7270/Q2BG2NHW |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Staphylococcus aureus (Firmicutes)) | BDBM50166480
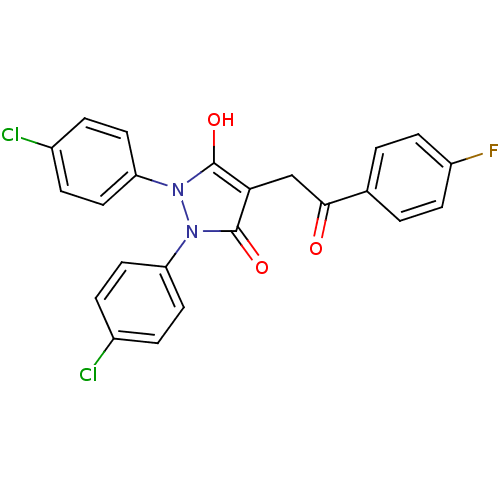
(1,2-Bis-(4-chloro-phenyl)-4-[2-(4-fluoro-phenyl)-2...)Show SMILES Oc1c(CC(=O)c2ccc(F)cc2)c(=O)n(-c2ccc(Cl)cc2)n1-c1ccc(Cl)cc1 Show InChI InChI=1S/C23H15Cl2FN2O3/c24-15-3-9-18(10-4-15)27-22(30)20(13-21(29)14-1-7-17(26)8-2-14)23(31)28(27)19-11-5-16(25)6-12-19/h1-12,30H,13H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MurB enzyme in Staphylococcus aureus |
Bioorg Med Chem Lett 15: 2527-31 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.058
BindingDB Entry DOI: 10.7270/Q2BG2NHW |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50166493

(4-(2-Benzylsulfanyl-ethyl)-1,2-bis-(4-chloro-pheny...)Show SMILES Oc1c(CCSCc2ccccc2)c(=O)n(-c2ccc(Cl)cc2)n1-c1ccc(Cl)cc1 Show InChI InChI=1S/C24H20Cl2N2O2S/c25-18-6-10-20(11-7-18)27-23(29)22(14-15-31-16-17-4-2-1-3-5-17)24(30)28(27)21-12-8-19(26)9-13-21/h1-13,29H,14-16H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MurB enzyme in Escherichia coli |
Bioorg Med Chem Lett 15: 2527-31 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.058
BindingDB Entry DOI: 10.7270/Q2BG2NHW |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50195551

(1,2-bis(4-chlorophenyl)-5-hydroxy-4-(4-trifluorome...)Show SMILES Oc1c(C(=O)c2ccc(OC(F)(F)F)cc2)c(=O)n(-c2ccc(Cl)cc2)n1-c1ccc(Cl)cc1 Show InChI InChI=1S/C23H13Cl2F3N2O4/c24-14-3-7-16(8-4-14)29-21(32)19(22(33)30(29)17-9-5-15(25)6-10-17)20(31)13-1-11-18(12-2-13)34-23(26,27)28/h1-12,32H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth
Curated by ChEMBL
| Assay Description
Inhibition of MurB activity |
J Med Chem 49: 6027-36 (2006)
Article DOI: 10.1021/jm060499t
BindingDB Entry DOI: 10.7270/Q22V2FRV |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50166480

(1,2-Bis-(4-chloro-phenyl)-4-[2-(4-fluoro-phenyl)-2...)Show SMILES Oc1c(CC(=O)c2ccc(F)cc2)c(=O)n(-c2ccc(Cl)cc2)n1-c1ccc(Cl)cc1 Show InChI InChI=1S/C23H15Cl2FN2O3/c24-15-3-9-18(10-4-15)27-22(30)20(13-21(29)14-1-7-17(26)8-2-14)23(31)28(27)19-11-5-16(25)6-12-19/h1-12,30H,13H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MurB enzyme in Escherichia coli |
Bioorg Med Chem Lett 15: 2527-31 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.058
BindingDB Entry DOI: 10.7270/Q2BG2NHW |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50195567
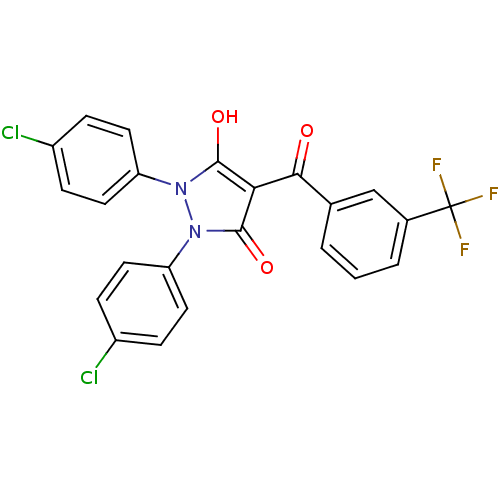
(1,2-bis(4-chlorophenyl)-5-hydroxy-3-oxo-2,3-dihydr...)Show SMILES Oc1c(C(=O)c2cccc(c2)C(F)(F)F)c(=O)n(-c2ccc(Cl)cc2)n1-c1ccc(Cl)cc1 Show InChI InChI=1S/C23H13Cl2F3N2O3/c24-15-4-8-17(9-5-15)29-21(32)19(22(33)30(29)18-10-6-16(25)7-11-18)20(31)13-2-1-3-14(12-13)23(26,27)28/h1-12,32H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth
Curated by ChEMBL
| Assay Description
Inhibition of MurB activity |
J Med Chem 49: 6027-36 (2006)
Article DOI: 10.1021/jm060499t
BindingDB Entry DOI: 10.7270/Q22V2FRV |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50166500
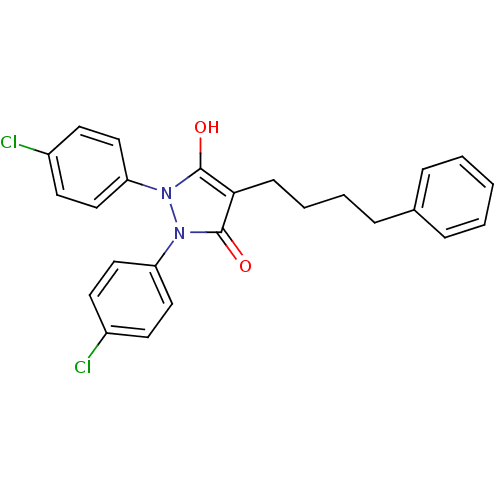
(1,2-Bis-(4-chloro-phenyl)-4-(4-phenyl-butyl)-pyraz...)Show SMILES Oc1c(CCCCc2ccccc2)c(=O)n(-c2ccc(Cl)cc2)n1-c1ccc(Cl)cc1 Show InChI InChI=1S/C25H22Cl2N2O2/c26-19-10-14-21(15-11-19)28-24(30)23(9-5-4-8-18-6-2-1-3-7-18)25(31)29(28)22-16-12-20(27)13-17-22/h1-3,6-7,10-17,30H,4-5,8-9H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MurB enzyme in Escherichia coli |
Bioorg Med Chem Lett 15: 2527-31 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.058
BindingDB Entry DOI: 10.7270/Q2BG2NHW |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50195557
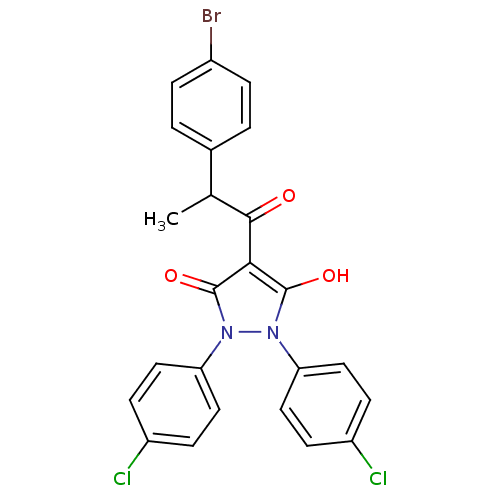
(1,2-bis(4-chlorophenyl)-5-hydroxy-3-oxo-2,3-dihydr...)Show SMILES CC(C(=O)c1c(O)n(-c2ccc(Cl)cc2)n(-c2ccc(Cl)cc2)c1=O)c1ccc(Br)cc1 Show InChI InChI=1S/C24H17BrCl2N2O3/c1-14(15-2-4-16(25)5-3-15)22(30)21-23(31)28(19-10-6-17(26)7-11-19)29(24(21)32)20-12-8-18(27)9-13-20/h2-14,31H,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth
Curated by ChEMBL
| Assay Description
Inhibition of MurB activity |
J Med Chem 49: 6027-36 (2006)
Article DOI: 10.1021/jm060499t
BindingDB Entry DOI: 10.7270/Q22V2FRV |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50195555
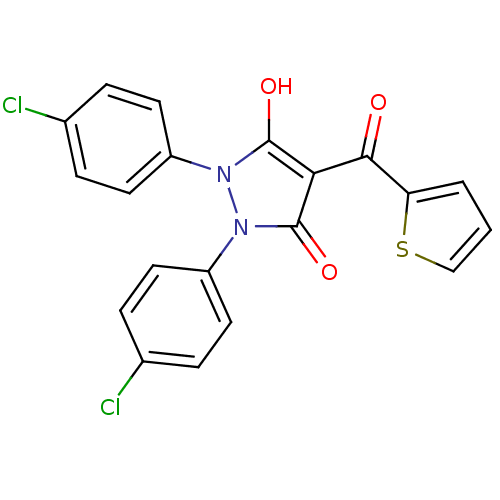
(1,2-bis(4-chlorophenyl)-5-hydroxy-4-(thiophene-2-c...)Show SMILES Oc1c(C(=O)c2cccs2)c(=O)n(-c2ccc(Cl)cc2)n1-c1ccc(Cl)cc1 Show InChI InChI=1S/C20H12Cl2N2O3S/c21-12-3-7-14(8-4-12)23-19(26)17(18(25)16-2-1-11-28-16)20(27)24(23)15-9-5-13(22)6-10-15/h1-11,26H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth
Curated by ChEMBL
| Assay Description
Inhibition of MurB activity |
J Med Chem 49: 6027-36 (2006)
Article DOI: 10.1021/jm060499t
BindingDB Entry DOI: 10.7270/Q22V2FRV |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50166485

(1,2-Bis-(4-chloro-phenyl)-4,4-bis-[2-(2,4-difluoro...)Show SMILES Fc1ccc(C(=O)CC2(CC(=O)c3ccc(F)cc3F)C(=O)N(N(C2=O)c2ccc(Cl)cc2)c2ccc(Cl)cc2)c(F)c1 Show InChI InChI=1S/C31H18Cl2F4N2O4/c32-17-1-7-21(8-2-17)38-29(42)31(15-27(40)23-11-5-19(34)13-25(23)36,16-28(41)24-12-6-20(35)14-26(24)37)30(43)39(38)22-9-3-18(33)4-10-22/h1-14H,15-16H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MurB enzyme in Escherichia coli |
Bioorg Med Chem Lett 15: 2527-31 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.058
BindingDB Entry DOI: 10.7270/Q2BG2NHW |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50195564
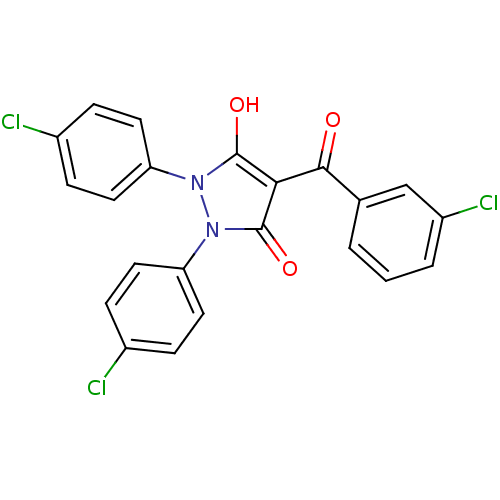
(1,2-bis(4-chlorophenyl)-5-hydroxy-3-oxo-2,3-dihydr...)Show SMILES Oc1c(C(=O)c2cccc(Cl)c2)c(=O)n(-c2ccc(Cl)cc2)n1-c1ccc(Cl)cc1 Show InChI InChI=1S/C22H13Cl3N2O3/c23-14-4-8-17(9-5-14)26-21(29)19(20(28)13-2-1-3-16(25)12-13)22(30)27(26)18-10-6-15(24)7-11-18/h1-12,29H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth
Curated by ChEMBL
| Assay Description
Inhibition of MurB activity |
J Med Chem 49: 6027-36 (2006)
Article DOI: 10.1021/jm060499t
BindingDB Entry DOI: 10.7270/Q22V2FRV |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50195577
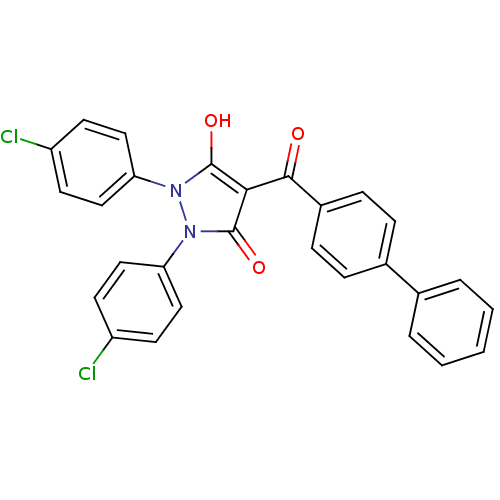
(4-(biphenyl-4-carbonyl)-1,2-bis(4-chlorophenyl)-5-...)Show SMILES Oc1c(C(=O)c2ccc(cc2)-c2ccccc2)c(=O)n(-c2ccc(Cl)cc2)n1-c1ccc(Cl)cc1 Show InChI InChI=1S/C28H18Cl2N2O3/c29-21-10-14-23(15-11-21)31-27(34)25(28(35)32(31)24-16-12-22(30)13-17-24)26(33)20-8-6-19(7-9-20)18-4-2-1-3-5-18/h1-17,34H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth
Curated by ChEMBL
| Assay Description
Inhibition of MurB activity |
J Med Chem 49: 6027-36 (2006)
Article DOI: 10.1021/jm060499t
BindingDB Entry DOI: 10.7270/Q22V2FRV |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Staphylococcus aureus (Firmicutes)) | BDBM50166500

(1,2-Bis-(4-chloro-phenyl)-4-(4-phenyl-butyl)-pyraz...)Show SMILES Oc1c(CCCCc2ccccc2)c(=O)n(-c2ccc(Cl)cc2)n1-c1ccc(Cl)cc1 Show InChI InChI=1S/C25H22Cl2N2O2/c26-19-10-14-21(15-11-19)28-24(30)23(9-5-4-8-18-6-2-1-3-7-18)25(31)29(28)22-16-12-20(27)13-17-22/h1-3,6-7,10-17,30H,4-5,8-9H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MurB enzyme in Staphylococcus aureus |
Bioorg Med Chem Lett 15: 2527-31 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.058
BindingDB Entry DOI: 10.7270/Q2BG2NHW |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50166475

(1,2-Bis-(4-chloro-phenyl)-4,4-bis-(6-phenyl-hexyl)...)Show SMILES Clc1ccc(cc1)N1N(C(=O)C(CCCCCCc2ccccc2)(CCCCCCc2ccccc2)C1=O)c1ccc(Cl)cc1 Show InChI InChI=1S/C39H42Cl2N2O2/c40-33-21-25-35(26-22-33)42-37(44)39(38(45)43(42)36-27-23-34(41)24-28-36,29-13-3-1-7-15-31-17-9-5-10-18-31)30-14-4-2-8-16-32-19-11-6-12-20-32/h5-6,9-12,17-28H,1-4,7-8,13-16,29-30H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MurB enzyme in Escherichia coli |
Bioorg Med Chem Lett 15: 2527-31 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.058
BindingDB Entry DOI: 10.7270/Q2BG2NHW |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50166499

(4,4-Bis-(2-benzyloxy-ethyl)-1,2-bis-(4-chloro-phen...)Show SMILES Clc1ccc(cc1)N1N(C(=O)C(CCOCc2ccccc2)(CCOCc2ccccc2)C1=O)c1ccc(Cl)cc1 Show InChI InChI=1S/C33H30Cl2N2O4/c34-27-11-15-29(16-12-27)36-31(38)33(19-21-40-23-25-7-3-1-4-8-25,20-22-41-24-26-9-5-2-6-10-26)32(39)37(36)30-17-13-28(35)14-18-30/h1-18H,19-24H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MurB enzyme in Escherichia coli |
Bioorg Med Chem Lett 15: 2527-31 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.058
BindingDB Entry DOI: 10.7270/Q2BG2NHW |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Staphylococcus aureus (Firmicutes)) | BDBM50166499

(4,4-Bis-(2-benzyloxy-ethyl)-1,2-bis-(4-chloro-phen...)Show SMILES Clc1ccc(cc1)N1N(C(=O)C(CCOCc2ccccc2)(CCOCc2ccccc2)C1=O)c1ccc(Cl)cc1 Show InChI InChI=1S/C33H30Cl2N2O4/c34-27-11-15-29(16-12-27)36-31(38)33(19-21-40-23-25-7-3-1-4-8-25,20-22-41-24-26-9-5-2-6-10-26)32(39)37(36)30-17-13-28(35)14-18-30/h1-18H,19-24H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MurB enzyme in Staphylococcus aureus |
Bioorg Med Chem Lett 15: 2527-31 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.058
BindingDB Entry DOI: 10.7270/Q2BG2NHW |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Staphylococcus aureus (Firmicutes)) | BDBM50166475

(1,2-Bis-(4-chloro-phenyl)-4,4-bis-(6-phenyl-hexyl)...)Show SMILES Clc1ccc(cc1)N1N(C(=O)C(CCCCCCc2ccccc2)(CCCCCCc2ccccc2)C1=O)c1ccc(Cl)cc1 Show InChI InChI=1S/C39H42Cl2N2O2/c40-33-21-25-35(26-22-33)42-37(44)39(38(45)43(42)36-27-23-34(41)24-28-36,29-13-3-1-7-15-31-17-9-5-10-18-31)30-14-4-2-8-16-32-19-11-6-12-20-32/h5-6,9-12,17-28H,1-4,7-8,13-16,29-30H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MurB enzyme in Staphylococcus aureus |
Bioorg Med Chem Lett 15: 2527-31 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.058
BindingDB Entry DOI: 10.7270/Q2BG2NHW |
More data for this
Ligand-Target Pair | |
UDP-N-acetylglucosamine 1-carboxyvinyltransferase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50166480

(1,2-Bis-(4-chloro-phenyl)-4-[2-(4-fluoro-phenyl)-2...)Show SMILES Oc1c(CC(=O)c2ccc(F)cc2)c(=O)n(-c2ccc(Cl)cc2)n1-c1ccc(Cl)cc1 Show InChI InChI=1S/C23H15Cl2FN2O3/c24-15-3-9-18(10-4-15)27-22(30)20(13-21(29)14-1-7-17(26)8-2-14)23(31)28(27)19-11-5-16(25)6-12-19/h1-12,30H,13H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MurA enzyme in Escherichia coli |
Bioorg Med Chem Lett 15: 2527-31 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.058
BindingDB Entry DOI: 10.7270/Q2BG2NHW |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50195549

(1,2-bis(3-chlorophenyl)-3,5-dioxopyrazolidine-4-ca...)Show SMILES Oc1c(C(=O)Nc2ccc(Cl)cc2)c(=O)n(-c2cccc(Cl)c2)n1-c1cccc(Cl)c1 Show InChI InChI=1S/C22H14Cl3N3O3/c23-13-7-9-16(10-8-13)26-20(29)19-21(30)27(17-5-1-3-14(24)11-17)28(22(19)31)18-6-2-4-15(25)12-18/h1-12,30H,(H,26,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth
Curated by ChEMBL
| Assay Description
Inhibition of MurB activity |
J Med Chem 49: 6027-36 (2006)
Article DOI: 10.1021/jm060499t
BindingDB Entry DOI: 10.7270/Q22V2FRV |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50195561
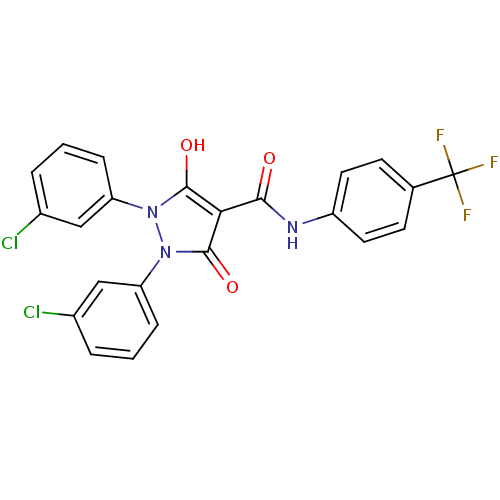
(1,2-bis(3-chlorophenyl)-5-hydroxy-3-oxo-2,3-dihydr...)Show SMILES Oc1c(C(=O)Nc2ccc(cc2)C(F)(F)F)c(=O)n(-c2cccc(Cl)c2)n1-c1cccc(Cl)c1 Show InChI InChI=1S/C23H14Cl2F3N3O3/c24-14-3-1-5-17(11-14)30-21(33)19(22(34)31(30)18-6-2-4-15(25)12-18)20(32)29-16-9-7-13(8-10-16)23(26,27)28/h1-12,33H,(H,29,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth
Curated by ChEMBL
| Assay Description
Inhibition of MurB activity |
J Med Chem 49: 6027-36 (2006)
Article DOI: 10.1021/jm060499t
BindingDB Entry DOI: 10.7270/Q22V2FRV |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50166478

(1,2-Bis-(4-chloro-phenyl)-4-(6-phenyl-hexyl)-pyraz...)Show SMILES Oc1c(CCCCCCc2ccccc2)c(=O)n(-c2ccc(Cl)cc2)n1-c1ccc(Cl)cc1 Show InChI InChI=1S/C27H26Cl2N2O2/c28-21-12-16-23(17-13-21)30-26(32)25(27(33)31(30)24-18-14-22(29)15-19-24)11-7-2-1-4-8-20-9-5-3-6-10-20/h3,5-6,9-10,12-19,32H,1-2,4,7-8,11H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MurB enzyme in Escherichia coli |
Bioorg Med Chem Lett 15: 2527-31 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.058
BindingDB Entry DOI: 10.7270/Q2BG2NHW |
More data for this
Ligand-Target Pair | |
UDP-N-acetylglucosamine 1-carboxyvinyltransferase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50166485

(1,2-Bis-(4-chloro-phenyl)-4,4-bis-[2-(2,4-difluoro...)Show SMILES Fc1ccc(C(=O)CC2(CC(=O)c3ccc(F)cc3F)C(=O)N(N(C2=O)c2ccc(Cl)cc2)c2ccc(Cl)cc2)c(F)c1 Show InChI InChI=1S/C31H18Cl2F4N2O4/c32-17-1-7-21(8-2-17)38-29(42)31(15-27(40)23-11-5-19(34)13-25(23)36,16-28(41)24-12-6-20(35)14-26(24)37)30(43)39(38)22-9-3-18(33)4-10-22/h1-14H,15-16H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MurA enzyme in Escherichia coli |
Bioorg Med Chem Lett 15: 2527-31 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.058
BindingDB Entry DOI: 10.7270/Q2BG2NHW |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50195572
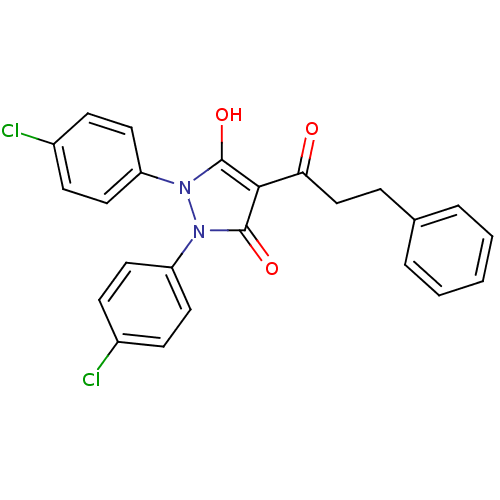
(1,2-bis(4-chlorophenyl)-5-hydroxy-3-oxo-2,3-dihydr...)Show SMILES Oc1c(C(=O)CCc2ccccc2)c(=O)n(-c2ccc(Cl)cc2)n1-c1ccc(Cl)cc1 Show InChI InChI=1S/C24H18Cl2N2O3/c25-17-7-11-19(12-8-17)27-23(30)22(21(29)15-6-16-4-2-1-3-5-16)24(31)28(27)20-13-9-18(26)10-14-20/h1-5,7-14,30H,6,15H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth
Curated by ChEMBL
| Assay Description
Inhibition of MurB activity |
J Med Chem 49: 6027-36 (2006)
Article DOI: 10.1021/jm060499t
BindingDB Entry DOI: 10.7270/Q22V2FRV |
More data for this
Ligand-Target Pair | |
UDP-N-acetylglucosamine 1-carboxyvinyltransferase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50166493

(4-(2-Benzylsulfanyl-ethyl)-1,2-bis-(4-chloro-pheny...)Show SMILES Oc1c(CCSCc2ccccc2)c(=O)n(-c2ccc(Cl)cc2)n1-c1ccc(Cl)cc1 Show InChI InChI=1S/C24H20Cl2N2O2S/c25-18-6-10-20(11-7-18)27-23(29)22(14-15-31-16-17-4-2-1-3-5-17)24(30)28(27)21-12-8-19(26)9-13-21/h1-13,29H,14-16H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MurA enzyme in Escherichia coli |
Bioorg Med Chem Lett 15: 2527-31 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.058
BindingDB Entry DOI: 10.7270/Q2BG2NHW |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data