 Found 548 hits with Last Name = 'shapiro' and Initial = 'r'
Found 548 hits with Last Name = 'shapiro' and Initial = 'r' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Hrh3 protein
(RAT) | BDBM27213

(4-[3-(4-cyclopropanecarbonylphenoxy)propyl]-1H-imi...)Show InChI InChI=1S/C16H18N2O2/c19-16(12-3-4-12)13-5-7-15(8-6-13)20-9-1-2-14-10-17-11-18-14/h5-8,10-12H,1-4,9H2,(H,17,18) | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.427 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
Eur J Pharmacol 487: 183-97 (2004)
Article DOI: 10.1016/j.ejphar.2004.01.015
BindingDB Entry DOI: 10.7270/Q2NZ866C |
More data for this
Ligand-Target Pair | |
Hrh3 protein
(RAT) | BDBM27213

(4-[3-(4-cyclopropanecarbonylphenoxy)propyl]-1H-imi...)Show InChI InChI=1S/C16H18N2O2/c19-16(12-3-4-12)13-5-7-15(8-6-13)20-9-1-2-14-10-17-11-18-14/h5-8,10-12H,1-4,9H2,(H,17,18) | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.654 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
Eur J Pharmacol 487: 183-97 (2004)
Article DOI: 10.1016/j.ejphar.2004.01.015
BindingDB Entry DOI: 10.7270/Q2NZ866C |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM7966

(2-(1H-imidazol-4-yl)ethan-1-amine | CHEMBL544208 |...)Show InChI InChI=1S/C5H9N3/c6-2-1-5-3-7-4-8-5/h3-4H,1-2,6H2,(H,7,8) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 2.32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
Eur J Pharmacol 487: 183-97 (2004)
Article DOI: 10.1016/j.ejphar.2004.01.015
BindingDB Entry DOI: 10.7270/Q2NZ866C |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM7966

(2-(1H-imidazol-4-yl)ethan-1-amine | CHEMBL544208 |...)Show InChI InChI=1S/C5H9N3/c6-2-1-5-3-7-4-8-5/h3-4H,1-2,6H2,(H,7,8) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 2.61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
Eur J Pharmacol 487: 183-97 (2004)
Article DOI: 10.1016/j.ejphar.2004.01.015
BindingDB Entry DOI: 10.7270/Q2NZ866C |
More data for this
Ligand-Target Pair | |
Hrh3 protein
(RAT) | BDBM7966

(2-(1H-imidazol-4-yl)ethan-1-amine | CHEMBL544208 |...)Show InChI InChI=1S/C5H9N3/c6-2-1-5-3-7-4-8-5/h3-4H,1-2,6H2,(H,7,8) | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
Eur J Pharmacol 487: 183-97 (2004)
Article DOI: 10.1016/j.ejphar.2004.01.015
BindingDB Entry DOI: 10.7270/Q2NZ866C |
More data for this
Ligand-Target Pair | |
Hrh3 protein
(RAT) | BDBM22914

(CHEMBL260374 | N-cyclohexyl-4-(1H-imidazol-5-yl)pi...)Show InChI InChI=1S/C15H24N4S/c20-15(18-13-4-2-1-3-5-13)19-8-6-12(7-9-19)14-10-16-11-17-14/h10-13H,1-9H2,(H,16,17)(H,18,20) | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
Eur J Pharmacol 487: 183-97 (2004)
Article DOI: 10.1016/j.ejphar.2004.01.015
BindingDB Entry DOI: 10.7270/Q2NZ866C |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50240709
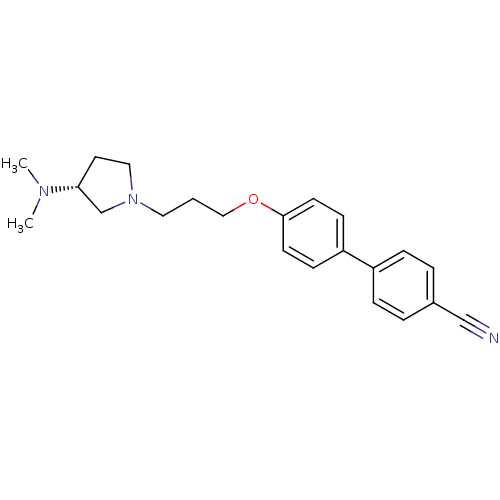
((R)-4'-(3-(3-(dimethylamino)pyrrolidin-1-yl)propox...)Show SMILES CN(C)[C@@H]1CCN(CCCOc2ccc(cc2)-c2ccc(cc2)C#N)C1 |r| Show InChI InChI=1S/C22H27N3O/c1-24(2)21-12-14-25(17-21)13-3-15-26-22-10-8-20(9-11-22)19-6-4-18(16-23)5-7-19/h4-11,21H,3,12-15,17H2,1-2H3/t21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
Eur J Pharmacol 487: 183-97 (2004)
Article DOI: 10.1016/j.ejphar.2004.01.015
BindingDB Entry DOI: 10.7270/Q2NZ866C |
More data for this
Ligand-Target Pair | |
Hrh3 protein
(RAT) | BDBM7966

(2-(1H-imidazol-4-yl)ethan-1-amine | CHEMBL544208 |...)Show InChI InChI=1S/C5H9N3/c6-2-1-5-3-7-4-8-5/h3-4H,1-2,6H2,(H,7,8) | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
Eur J Pharmacol 487: 183-97 (2004)
Article DOI: 10.1016/j.ejphar.2004.01.015
BindingDB Entry DOI: 10.7270/Q2NZ866C |
More data for this
Ligand-Target Pair | |
Hrh3 protein
(RAT) | BDBM50240709

((R)-4'-(3-(3-(dimethylamino)pyrrolidin-1-yl)propox...)Show SMILES CN(C)[C@@H]1CCN(CCCOc2ccc(cc2)-c2ccc(cc2)C#N)C1 |r| Show InChI InChI=1S/C22H27N3O/c1-24(2)21-12-14-25(17-21)13-3-15-26-22-10-8-20(9-11-22)19-6-4-18(16-23)5-7-19/h4-11,21H,3,12-15,17H2,1-2H3/t21-/m1/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
Eur J Pharmacol 487: 183-97 (2004)
Article DOI: 10.1016/j.ejphar.2004.01.015
BindingDB Entry DOI: 10.7270/Q2NZ866C |
More data for this
Ligand-Target Pair | |
Hrh3 protein
(RAT) | BDBM22914

(CHEMBL260374 | N-cyclohexyl-4-(1H-imidazol-5-yl)pi...)Show InChI InChI=1S/C15H24N4S/c20-15(18-13-4-2-1-3-5-13)19-8-6-12(7-9-19)14-10-16-11-17-14/h10-13H,1-9H2,(H,16,17)(H,18,20) | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 7.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
Eur J Pharmacol 487: 183-97 (2004)
Article DOI: 10.1016/j.ejphar.2004.01.015
BindingDB Entry DOI: 10.7270/Q2NZ866C |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50193788
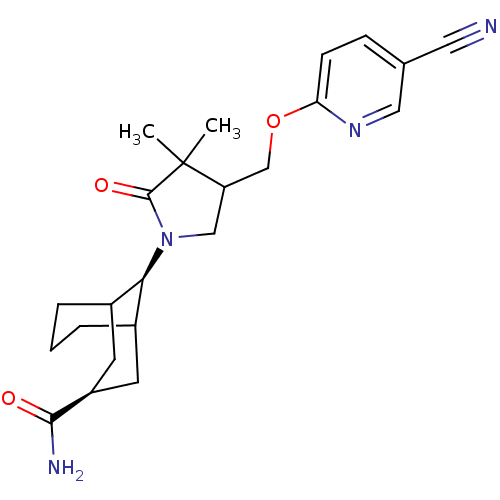
((3s,9s)-9-(4-((5-cyanopyridin-2-yloxy)methyl)-3,3-...)Show SMILES CC1(C)C(COc2ccc(cn2)C#N)CN([C@@H]2C3CCCC2C[C@H](C3)C(N)=O)C1=O |wU:16.16,wD:23.27,THB:15:16:20.19.18:24.23.22,25:23:20.19.18:16,(12.85,-27.96,;11.71,-28.98,;10.46,-28.1,;12.95,-29.89,;14.41,-29.43,;14.86,-27.95,;16.36,-27.6,;17.41,-28.73,;18.91,-28.38,;19.35,-26.91,;18.29,-25.79,;16.8,-26.14,;20.85,-26.55,;22.35,-26.2,;12.47,-31.35,;10.93,-31.34,;10.56,-32.84,;9.51,-33.59,;9.55,-35.33,;8.74,-36.36,;10.25,-36.1,;10.25,-34.29,;8.38,-34.27,;8.46,-31.93,;7.83,-33.51,;6.98,-32.34,;5.88,-31.26,;6.59,-33.83,;10.46,-29.89,;9,-29.41,)| Show InChI InChI=1S/C23H30N4O3/c1-23(2)18(13-30-19-7-6-14(10-24)11-26-19)12-27(22(23)29)20-15-4-3-5-16(20)9-17(8-15)21(25)28/h6-7,11,15-18,20H,3-5,8-9,12-13H2,1-2H3,(H2,25,28)/t15?,16?,17-,18?,20+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11beta-HSD1 |
Bioorg Med Chem Lett 16: 5555-60 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.034
BindingDB Entry DOI: 10.7270/Q25X28K5 |
More data for this
Ligand-Target Pair | |
Hrh3 protein
(RAT) | BDBM50240709

((R)-4'-(3-(3-(dimethylamino)pyrrolidin-1-yl)propox...)Show SMILES CN(C)[C@@H]1CCN(CCCOc2ccc(cc2)-c2ccc(cc2)C#N)C1 |r| Show InChI InChI=1S/C22H27N3O/c1-24(2)21-12-14-25(17-21)13-3-15-26-22-10-8-20(9-11-22)19-6-4-18(16-23)5-7-19/h4-11,21H,3,12-15,17H2,1-2H3/t21-/m1/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 21.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
Eur J Pharmacol 487: 183-97 (2004)
Article DOI: 10.1016/j.ejphar.2004.01.015
BindingDB Entry DOI: 10.7270/Q2NZ866C |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50240709

((R)-4'-(3-(3-(dimethylamino)pyrrolidin-1-yl)propox...)Show SMILES CN(C)[C@@H]1CCN(CCCOc2ccc(cc2)-c2ccc(cc2)C#N)C1 |r| Show InChI InChI=1S/C22H27N3O/c1-24(2)21-12-14-25(17-21)13-3-15-26-22-10-8-20(9-11-22)19-6-4-18(16-23)5-7-19/h4-11,21H,3,12-15,17H2,1-2H3/t21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 22.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
Eur J Pharmacol 487: 183-97 (2004)
Article DOI: 10.1016/j.ejphar.2004.01.015
BindingDB Entry DOI: 10.7270/Q2NZ866C |
More data for this
Ligand-Target Pair | |
Hrh3 protein
(RAT) | BDBM86490
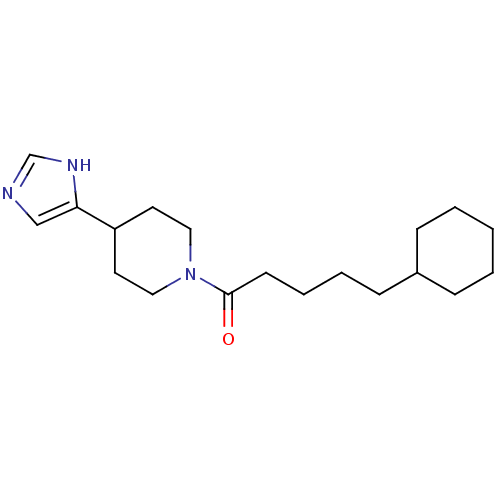
(CAS_0 | CHEMBL14812 | GT 2016 | NSC_0)Show InChI InChI=1S/C19H31N3O/c23-19(9-5-4-8-16-6-2-1-3-7-16)22-12-10-17(11-13-22)18-14-20-15-21-18/h14-17H,1-13H2,(H,20,21) | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 25.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
Eur J Pharmacol 487: 183-97 (2004)
Article DOI: 10.1016/j.ejphar.2004.01.015
BindingDB Entry DOI: 10.7270/Q2NZ866C |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50193788

((3s,9s)-9-(4-((5-cyanopyridin-2-yloxy)methyl)-3,3-...)Show SMILES CC1(C)C(COc2ccc(cn2)C#N)CN([C@@H]2C3CCCC2C[C@H](C3)C(N)=O)C1=O |wU:16.16,wD:23.27,THB:15:16:20.19.18:24.23.22,25:23:20.19.18:16,(12.85,-27.96,;11.71,-28.98,;10.46,-28.1,;12.95,-29.89,;14.41,-29.43,;14.86,-27.95,;16.36,-27.6,;17.41,-28.73,;18.91,-28.38,;19.35,-26.91,;18.29,-25.79,;16.8,-26.14,;20.85,-26.55,;22.35,-26.2,;12.47,-31.35,;10.93,-31.34,;10.56,-32.84,;9.51,-33.59,;9.55,-35.33,;8.74,-36.36,;10.25,-36.1,;10.25,-34.29,;8.38,-34.27,;8.46,-31.93,;7.83,-33.51,;6.98,-32.34,;5.88,-31.26,;6.59,-33.83,;10.46,-29.89,;9,-29.41,)| Show InChI InChI=1S/C23H30N4O3/c1-23(2)18(13-30-19-7-6-14(10-24)11-26-19)12-27(22(23)29)20-15-4-3-5-16(20)9-17(8-15)21(25)28/h6-7,11,15-18,20H,3-5,8-9,12-13H2,1-2H3,(H2,25,28)/t15?,16?,17-,18?,20+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 |
Bioorg Med Chem Lett 16: 5555-60 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.034
BindingDB Entry DOI: 10.7270/Q25X28K5 |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM7966

(2-(1H-imidazol-4-yl)ethan-1-amine | CHEMBL544208 |...)Show InChI InChI=1S/C5H9N3/c6-2-1-5-3-7-4-8-5/h3-4H,1-2,6H2,(H,7,8) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 39.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
Eur J Pharmacol 487: 183-97 (2004)
Article DOI: 10.1016/j.ejphar.2004.01.015
BindingDB Entry DOI: 10.7270/Q2NZ866C |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM22914

(CHEMBL260374 | N-cyclohexyl-4-(1H-imidazol-5-yl)pi...)Show InChI InChI=1S/C15H24N4S/c20-15(18-13-4-2-1-3-5-13)19-8-6-12(7-9-19)14-10-16-11-17-14/h10-13H,1-9H2,(H,16,17)(H,18,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 47.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
Eur J Pharmacol 487: 183-97 (2004)
Article DOI: 10.1016/j.ejphar.2004.01.015
BindingDB Entry DOI: 10.7270/Q2NZ866C |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50193783

((1R,7S,8r)-ethyl 4-(4-((5-cyanopyridin-2-yloxy)met...)Show SMILES CCOC(=O)[C@@H]1[C@H]2CCC(CC[C@@H]12)N1CC(COc2ccc(cn2)C#N)C(C)(C)C1=O Show InChI InChI=1S/C24H31N3O4/c1-4-30-22(28)21-18-8-6-17(7-9-19(18)21)27-13-16(24(2,3)23(27)29)14-31-20-10-5-15(11-25)12-26-20/h5,10,12,16-19,21H,4,6-9,13-14H2,1-3H3/t16?,17?,18-,19+,21+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 |
Bioorg Med Chem Lett 16: 5555-60 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.034
BindingDB Entry DOI: 10.7270/Q25X28K5 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM22914

(CHEMBL260374 | N-cyclohexyl-4-(1H-imidazol-5-yl)pi...)Show InChI InChI=1S/C15H24N4S/c20-15(18-13-4-2-1-3-5-13)19-8-6-12(7-9-19)14-10-16-11-17-14/h10-13H,1-9H2,(H,16,17)(H,18,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 61.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
Eur J Pharmacol 487: 183-97 (2004)
Article DOI: 10.1016/j.ejphar.2004.01.015
BindingDB Entry DOI: 10.7270/Q2NZ866C |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM27213

(4-[3-(4-cyclopropanecarbonylphenoxy)propyl]-1H-imi...)Show InChI InChI=1S/C16H18N2O2/c19-16(12-3-4-12)13-5-7-15(8-6-13)20-9-1-2-14-10-17-11-18-14/h5-8,10-12H,1-4,9H2,(H,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 62.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
Eur J Pharmacol 487: 183-97 (2004)
Article DOI: 10.1016/j.ejphar.2004.01.015
BindingDB Entry DOI: 10.7270/Q2NZ866C |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM22914

(CHEMBL260374 | N-cyclohexyl-4-(1H-imidazol-5-yl)pi...)Show InChI InChI=1S/C15H24N4S/c20-15(18-13-4-2-1-3-5-13)19-8-6-12(7-9-19)14-10-16-11-17-14/h10-13H,1-9H2,(H,16,17)(H,18,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 72.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
Eur J Pharmacol 487: 183-97 (2004)
Article DOI: 10.1016/j.ejphar.2004.01.015
BindingDB Entry DOI: 10.7270/Q2NZ866C |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM27213

(4-[3-(4-cyclopropanecarbonylphenoxy)propyl]-1H-imi...)Show InChI InChI=1S/C16H18N2O2/c19-16(12-3-4-12)13-5-7-15(8-6-13)20-9-1-2-14-10-17-11-18-14/h5-8,10-12H,1-4,9H2,(H,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 89.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
Eur J Pharmacol 487: 183-97 (2004)
Article DOI: 10.1016/j.ejphar.2004.01.015
BindingDB Entry DOI: 10.7270/Q2NZ866C |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM86490

(CAS_0 | CHEMBL14812 | GT 2016 | NSC_0)Show InChI InChI=1S/C19H31N3O/c23-19(9-5-4-8-16-6-2-1-3-7-16)22-12-10-17(11-13-22)18-14-20-15-21-18/h14-17H,1-13H2,(H,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 136 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
Eur J Pharmacol 487: 183-97 (2004)
Article DOI: 10.1016/j.ejphar.2004.01.015
BindingDB Entry DOI: 10.7270/Q2NZ866C |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50193800
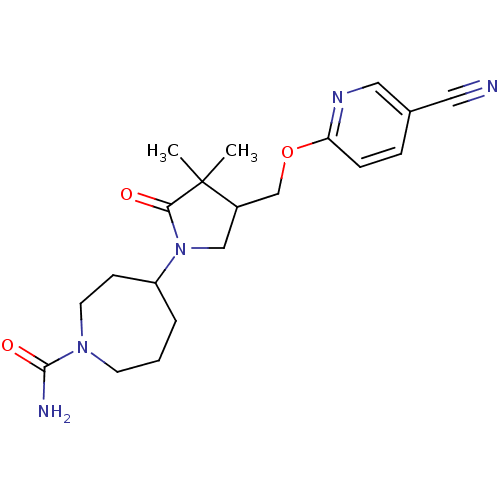
(4-(4-((5-cyanopyridin-2-yloxy)methyl)-3,3-dimethyl...)Show SMILES CC1(C)C(COc2ccc(cn2)C#N)CN(C2CCCN(CC2)C(N)=O)C1=O Show InChI InChI=1S/C20H27N5O3/c1-20(2)15(13-28-17-6-5-14(10-21)11-23-17)12-25(18(20)26)16-4-3-8-24(9-7-16)19(22)27/h5-6,11,15-16H,3-4,7-9,12-13H2,1-2H3,(H2,22,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 |
Bioorg Med Chem Lett 16: 5555-60 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.034
BindingDB Entry DOI: 10.7270/Q25X28K5 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50193800

(4-(4-((5-cyanopyridin-2-yloxy)methyl)-3,3-dimethyl...)Show SMILES CC1(C)C(COc2ccc(cn2)C#N)CN(C2CCCN(CC2)C(N)=O)C1=O Show InChI InChI=1S/C20H27N5O3/c1-20(2)15(13-28-17-6-5-14(10-21)11-23-17)12-25(18(20)26)16-4-3-8-24(9-7-16)19(22)27/h5-6,11,15-16H,3-4,7-9,12-13H2,1-2H3,(H2,22,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11beta-HSD1 |
Bioorg Med Chem Lett 16: 5555-60 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.034
BindingDB Entry DOI: 10.7270/Q25X28K5 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50193783

((1R,7S,8r)-ethyl 4-(4-((5-cyanopyridin-2-yloxy)met...)Show SMILES CCOC(=O)[C@@H]1[C@H]2CCC(CC[C@@H]12)N1CC(COc2ccc(cn2)C#N)C(C)(C)C1=O Show InChI InChI=1S/C24H31N3O4/c1-4-30-22(28)21-18-8-6-17(7-9-19(18)21)27-13-16(24(2,3)23(27)29)14-31-20-10-5-15(11-25)12-26-20/h5,10,12,16-19,21H,4,6-9,13-14H2,1-3H3/t16?,17?,18-,19+,21+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11beta-HSD1 |
Bioorg Med Chem Lett 16: 5555-60 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.034
BindingDB Entry DOI: 10.7270/Q25X28K5 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM86490

(CAS_0 | CHEMBL14812 | GT 2016 | NSC_0)Show InChI InChI=1S/C19H31N3O/c23-19(9-5-4-8-16-6-2-1-3-7-16)22-12-10-17(11-13-22)18-14-20-15-21-18/h14-17H,1-13H2,(H,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 499 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
Eur J Pharmacol 487: 183-97 (2004)
Article DOI: 10.1016/j.ejphar.2004.01.015
BindingDB Entry DOI: 10.7270/Q2NZ866C |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM86490

(CAS_0 | CHEMBL14812 | GT 2016 | NSC_0)Show InChI InChI=1S/C19H31N3O/c23-19(9-5-4-8-16-6-2-1-3-7-16)22-12-10-17(11-13-22)18-14-20-15-21-18/h14-17H,1-13H2,(H,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 734 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
Eur J Pharmacol 487: 183-97 (2004)
Article DOI: 10.1016/j.ejphar.2004.01.015
BindingDB Entry DOI: 10.7270/Q2NZ866C |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM27213

(4-[3-(4-cyclopropanecarbonylphenoxy)propyl]-1H-imi...)Show InChI InChI=1S/C16H18N2O2/c19-16(12-3-4-12)13-5-7-15(8-6-13)20-9-1-2-14-10-17-11-18-14/h5-8,10-12H,1-4,9H2,(H,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.88E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
Eur J Pharmacol 487: 183-97 (2004)
Article DOI: 10.1016/j.ejphar.2004.01.015
BindingDB Entry DOI: 10.7270/Q2NZ866C |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM86490

(CAS_0 | CHEMBL14812 | GT 2016 | NSC_0)Show InChI InChI=1S/C19H31N3O/c23-19(9-5-4-8-16-6-2-1-3-7-16)22-12-10-17(11-13-22)18-14-20-15-21-18/h14-17H,1-13H2,(H,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
Eur J Pharmacol 487: 183-97 (2004)
Article DOI: 10.1016/j.ejphar.2004.01.015
BindingDB Entry DOI: 10.7270/Q2NZ866C |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM7966

(2-(1H-imidazol-4-yl)ethan-1-amine | CHEMBL544208 |...)Show InChI InChI=1S/C5H9N3/c6-2-1-5-3-7-4-8-5/h3-4H,1-2,6H2,(H,7,8) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 7.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
Eur J Pharmacol 487: 183-97 (2004)
Article DOI: 10.1016/j.ejphar.2004.01.015
BindingDB Entry DOI: 10.7270/Q2NZ866C |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM86490

(CAS_0 | CHEMBL14812 | GT 2016 | NSC_0)Show InChI InChI=1S/C19H31N3O/c23-19(9-5-4-8-16-6-2-1-3-7-16)22-12-10-17(11-13-22)18-14-20-15-21-18/h14-17H,1-13H2,(H,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
Eur J Pharmacol 487: 183-97 (2004)
Article DOI: 10.1016/j.ejphar.2004.01.015
BindingDB Entry DOI: 10.7270/Q2NZ866C |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50240709

((R)-4'-(3-(3-(dimethylamino)pyrrolidin-1-yl)propox...)Show SMILES CN(C)[C@@H]1CCN(CCCOc2ccc(cc2)-c2ccc(cc2)C#N)C1 |r| Show InChI InChI=1S/C22H27N3O/c1-24(2)21-12-14-25(17-21)13-3-15-26-22-10-8-20(9-11-22)19-6-4-18(16-23)5-7-19/h4-11,21H,3,12-15,17H2,1-2H3/t21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
Eur J Pharmacol 487: 183-97 (2004)
Article DOI: 10.1016/j.ejphar.2004.01.015
BindingDB Entry DOI: 10.7270/Q2NZ866C |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM27213

(4-[3-(4-cyclopropanecarbonylphenoxy)propyl]-1H-imi...)Show InChI InChI=1S/C16H18N2O2/c19-16(12-3-4-12)13-5-7-15(8-6-13)20-9-1-2-14-10-17-11-18-14/h5-8,10-12H,1-4,9H2,(H,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
Eur J Pharmacol 487: 183-97 (2004)
Article DOI: 10.1016/j.ejphar.2004.01.015
BindingDB Entry DOI: 10.7270/Q2NZ866C |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM22914

(CHEMBL260374 | N-cyclohexyl-4-(1H-imidazol-5-yl)pi...)Show InChI InChI=1S/C15H24N4S/c20-15(18-13-4-2-1-3-5-13)19-8-6-12(7-9-19)14-10-16-11-17-14/h10-13H,1-9H2,(H,16,17)(H,18,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
Eur J Pharmacol 487: 183-97 (2004)
Article DOI: 10.1016/j.ejphar.2004.01.015
BindingDB Entry DOI: 10.7270/Q2NZ866C |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM50240709

((R)-4'-(3-(3-(dimethylamino)pyrrolidin-1-yl)propox...)Show SMILES CN(C)[C@@H]1CCN(CCCOc2ccc(cc2)-c2ccc(cc2)C#N)C1 |r| Show InChI InChI=1S/C22H27N3O/c1-24(2)21-12-14-25(17-21)13-3-15-26-22-10-8-20(9-11-22)19-6-4-18(16-23)5-7-19/h4-11,21H,3,12-15,17H2,1-2H3/t21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
Eur J Pharmacol 487: 183-97 (2004)
Article DOI: 10.1016/j.ejphar.2004.01.015
BindingDB Entry DOI: 10.7270/Q2NZ866C |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM27213

(4-[3-(4-cyclopropanecarbonylphenoxy)propyl]-1H-imi...)Show InChI InChI=1S/C16H18N2O2/c19-16(12-3-4-12)13-5-7-15(8-6-13)20-9-1-2-14-10-17-11-18-14/h5-8,10-12H,1-4,9H2,(H,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
Eur J Pharmacol 487: 183-97 (2004)
Article DOI: 10.1016/j.ejphar.2004.01.015
BindingDB Entry DOI: 10.7270/Q2NZ866C |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM22914

(CHEMBL260374 | N-cyclohexyl-4-(1H-imidazol-5-yl)pi...)Show InChI InChI=1S/C15H24N4S/c20-15(18-13-4-2-1-3-5-13)19-8-6-12(7-9-19)14-10-16-11-17-14/h10-13H,1-9H2,(H,16,17)(H,18,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
Eur J Pharmacol 487: 183-97 (2004)
Article DOI: 10.1016/j.ejphar.2004.01.015
BindingDB Entry DOI: 10.7270/Q2NZ866C |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM7966

(2-(1H-imidazol-4-yl)ethan-1-amine | CHEMBL544208 |...)Show InChI InChI=1S/C5H9N3/c6-2-1-5-3-7-4-8-5/h3-4H,1-2,6H2,(H,7,8) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
Eur J Pharmacol 487: 183-97 (2004)
Article DOI: 10.1016/j.ejphar.2004.01.015
BindingDB Entry DOI: 10.7270/Q2NZ866C |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM19386
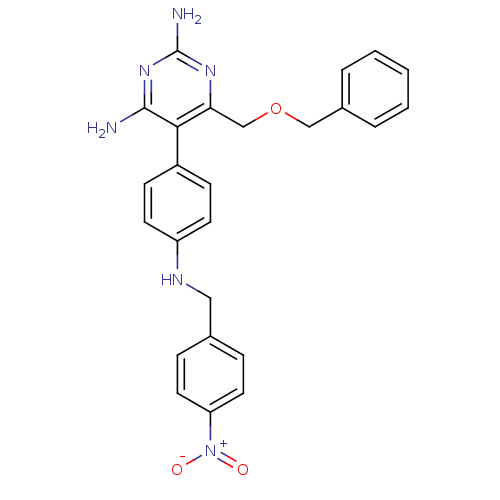
(2,4-diaminopyrimidine derivative, 8b | 6-[(benzylo...)Show SMILES Nc1nc(N)c(c(COCc2ccccc2)n1)-c1ccc(NCc2ccc(cc2)[N+]([O-])=O)cc1 Show InChI InChI=1S/C25H24N6O3/c26-24-23(22(29-25(27)30-24)16-34-15-18-4-2-1-3-5-18)19-8-10-20(11-9-19)28-14-17-6-12-21(13-7-17)31(32)33/h1-13,28H,14-16H2,(H4,26,27,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | 5.80 | n/a | n/a | 7.4 | 22 |
Abbott Laboratories
| Assay Description
Specific binding was determined by incubation of membranes from GHS-R1a transfected CHO-K cells with 125I-His9-ghrelin in the presence of increasing ... |
J Med Chem 49: 2568-78 (2006)
Article DOI: 10.1021/jm0510934
BindingDB Entry DOI: 10.7270/Q27W69G1 |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM19387
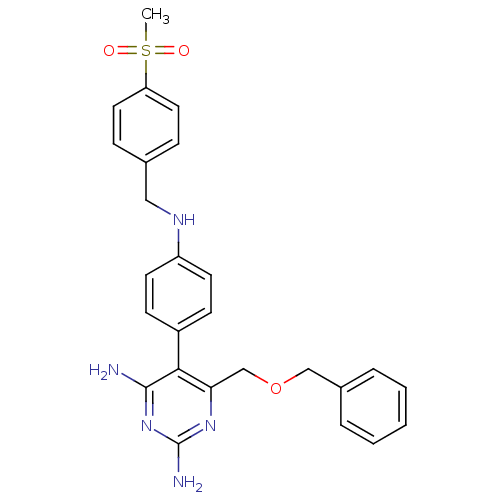
(2,4-diaminopyrimidine derivative, 8c | 6-[(benzylo...)Show SMILES CS(=O)(=O)c1ccc(CNc2ccc(cc2)-c2c(N)nc(N)nc2COCc2ccccc2)cc1 Show InChI InChI=1S/C26H27N5O3S/c1-35(32,33)22-13-7-18(8-14-22)15-29-21-11-9-20(10-12-21)24-23(30-26(28)31-25(24)27)17-34-16-19-5-3-2-4-6-19/h2-14,29H,15-17H2,1H3,(H4,27,28,30,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | 7.20 | n/a | n/a | 7.4 | 22 |
Abbott Laboratories
| Assay Description
Specific binding was determined by incubation of membranes from GHS-R1a transfected CHO-K cells with 125I-His9-ghrelin in the presence of increasing ... |
J Med Chem 49: 2568-78 (2006)
Article DOI: 10.1021/jm0510934
BindingDB Entry DOI: 10.7270/Q27W69G1 |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM19403
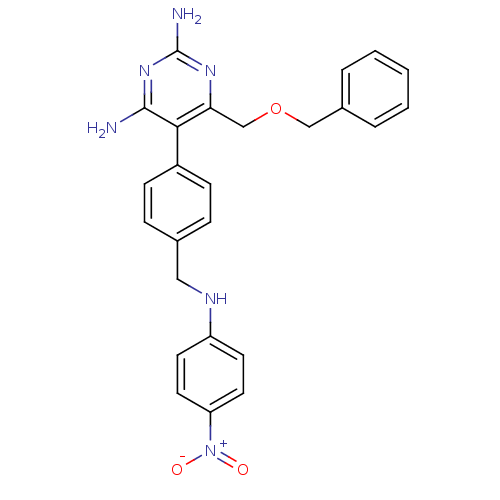
(2,4-diaminopyrimidine derivative, 11a | 6-[(benzyl...)Show SMILES Nc1nc(N)c(c(COCc2ccccc2)n1)-c1ccc(CNc2ccc(cc2)[N+]([O-])=O)cc1 Show InChI InChI=1S/C25H24N6O3/c26-24-23(22(29-25(27)30-24)16-34-15-18-4-2-1-3-5-18)19-8-6-17(7-9-19)14-28-20-10-12-21(13-11-20)31(32)33/h1-13,28H,14-16H2,(H4,26,27,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | 16 | n/a | n/a | n/a | n/a |
Abbott Laboratories
| Assay Description
Specific binding was determined by incubation of membranes from GHS-R1a transfected CHO-K cells with 125I-His9-ghrelin in the presence of increasing ... |
J Med Chem 49: 2568-78 (2006)
Article DOI: 10.1021/jm0510934
BindingDB Entry DOI: 10.7270/Q27W69G1 |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM19388
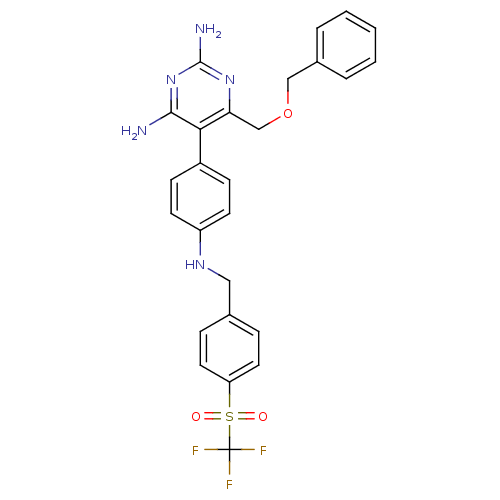
(2,4-diaminopyrimidine derivative, 8d | 6-[(benzylo...)Show SMILES Nc1nc(N)c(c(COCc2ccccc2)n1)-c1ccc(NCc2ccc(cc2)S(=O)(=O)C(F)(F)F)cc1 Show InChI InChI=1S/C26H24F3N5O3S/c27-26(28,29)38(35,36)21-12-6-17(7-13-21)14-32-20-10-8-19(9-11-20)23-22(33-25(31)34-24(23)30)16-37-15-18-4-2-1-3-5-18/h1-13,32H,14-16H2,(H4,30,31,33,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | 85 | n/a | n/a | 7.4 | 22 |
Abbott Laboratories
| Assay Description
Specific binding was determined by incubation of membranes from GHS-R1a transfected CHO-K cells with 125I-His9-ghrelin in the presence of increasing ... |
J Med Chem 49: 2568-78 (2006)
Article DOI: 10.1021/jm0510934
BindingDB Entry DOI: 10.7270/Q27W69G1 |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM19389

(1-(4-{[(4-{2,4-diamino-6-[(benzyloxy)methyl]pyrimi...)Show SMILES CC(=O)c1ccc(CNc2ccc(cc2)-c2c(N)nc(N)nc2COCc2ccccc2)cc1 Show InChI InChI=1S/C27H27N5O2/c1-18(33)21-9-7-19(8-10-21)15-30-23-13-11-22(12-14-23)25-24(31-27(29)32-26(25)28)17-34-16-20-5-3-2-4-6-20/h2-14,30H,15-17H2,1H3,(H4,28,29,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | 10 | n/a | n/a | 7.4 | 22 |
Abbott Laboratories
| Assay Description
Specific binding was determined by incubation of membranes from GHS-R1a transfected CHO-K cells with 125I-His9-ghrelin in the presence of increasing ... |
J Med Chem 49: 2568-78 (2006)
Article DOI: 10.1021/jm0510934
BindingDB Entry DOI: 10.7270/Q27W69G1 |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50162116
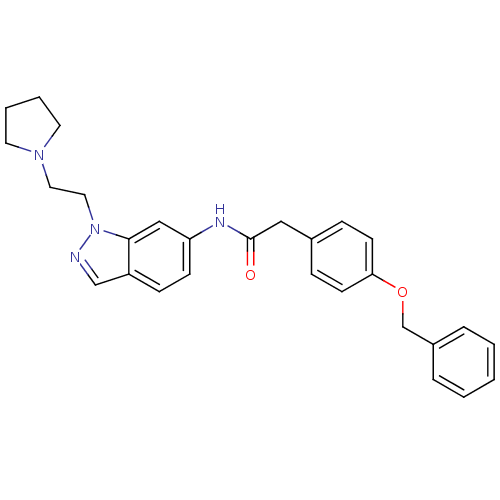
(2-(4-(benzyloxy)phenyl)-N-(1-(2-(pyrrolidin-1-yl)e...)Show SMILES O=C(Cc1ccc(OCc2ccccc2)cc1)Nc1ccc2cnn(CCN3CCCC3)c2c1 Show InChI InChI=1S/C28H30N4O2/c33-28(18-22-8-12-26(13-9-22)34-21-23-6-2-1-3-7-23)30-25-11-10-24-20-29-32(27(24)19-25)17-16-31-14-4-5-15-31/h1-3,6-13,19-20H,4-5,14-18,21H2,(H,30,33) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inihibition of melanin concentrating hormone receptor 1 from human neuronal IMR-32 cells |
J Med Chem 48: 1318-21 (2005)
Article DOI: 10.1021/jm0490890
BindingDB Entry DOI: 10.7270/Q2794459 |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Mus musculus) | BDBM50162116

(2-(4-(benzyloxy)phenyl)-N-(1-(2-(pyrrolidin-1-yl)e...)Show SMILES O=C(Cc1ccc(OCc2ccccc2)cc1)Nc1ccc2cnn(CCN3CCCC3)c2c1 Show InChI InChI=1S/C28H30N4O2/c33-28(18-22-8-12-26(13-9-22)34-21-23-6-2-1-3-7-23)30-25-11-10-24-20-29-32(27(24)19-25)17-16-31-14-4-5-15-31/h1-3,6-13,19-20H,4-5,14-18,21H2,(H,30,33) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Melanin-concentrating hormone receptor 1 in diet-induced obese mice |
Bioorg Med Chem Lett 15: 2752-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.114
BindingDB Entry DOI: 10.7270/Q2QJ7GSD |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50185040
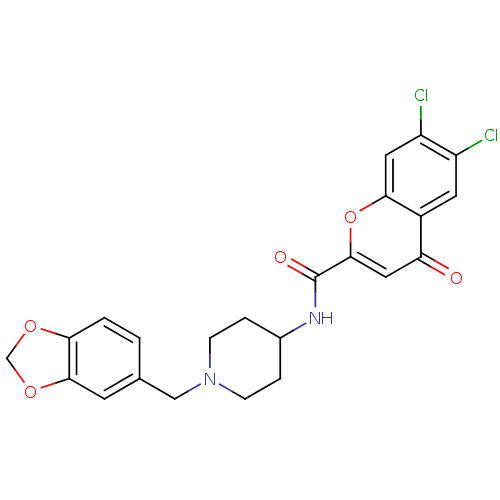
(CHEMBL204828 | N-[1-(1,3-benzodioxol-5-ylmethyl)pi...)Show SMILES Clc1cc2oc(cc(=O)c2cc1Cl)C(=O)NC1CCN(Cc2ccc3OCOc3c2)CC1 Show InChI InChI=1S/C23H20Cl2N2O5/c24-16-8-15-18(28)10-22(32-20(15)9-17(16)25)23(29)26-14-3-5-27(6-4-14)11-13-1-2-19-21(7-13)31-12-30-19/h1-2,7-10,14H,3-6,11-12H2,(H,26,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from hERG |
J Med Chem 49: 6569-84 (2006)
Article DOI: 10.1021/jm060683e
BindingDB Entry DOI: 10.7270/Q2NG4Q7P |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM19390

(2,4-diaminopyrimidine derivative, 8f | 6-[(benzylo...)Show SMILES Nc1nc(N)c(c(COCc2ccccc2)n1)-c1ccc(NCc2ccnc(Cl)c2)cc1 Show InChI InChI=1S/C24H23ClN6O/c25-21-12-17(10-11-28-21)13-29-19-8-6-18(7-9-19)22-20(30-24(27)31-23(22)26)15-32-14-16-4-2-1-3-5-16/h1-12,29H,13-15H2,(H4,26,27,30,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | 3.60 | n/a | n/a | 7.4 | 22 |
Abbott Laboratories
| Assay Description
Specific binding was determined by incubation of membranes from GHS-R1a transfected CHO-K cells with 125I-His9-ghrelin in the presence of increasing ... |
J Med Chem 49: 2568-78 (2006)
Article DOI: 10.1021/jm0510934
BindingDB Entry DOI: 10.7270/Q27W69G1 |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Mus musculus) | BDBM50167565
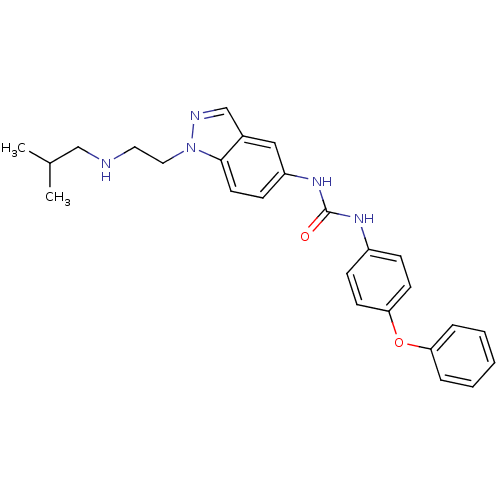
(1-[1-(2-Isobutylamino-ethyl)-1H-indazol-5-yl]-3-(4...)Show SMILES CC(C)CNCCn1ncc2cc(NC(=O)Nc3ccc(Oc4ccccc4)cc3)ccc12 Show InChI InChI=1S/C26H29N5O2/c1-19(2)17-27-14-15-31-25-13-10-22(16-20(25)18-28-31)30-26(32)29-21-8-11-24(12-9-21)33-23-6-4-3-5-7-23/h3-13,16,18-19,27H,14-15,17H2,1-2H3,(H2,29,30,32) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against mouse Melanin-concentrating hormone receptor 1 expressed in IMR-32 cells using [125I]-MCH |
Bioorg Med Chem Lett 15: 2752-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.114
BindingDB Entry DOI: 10.7270/Q2QJ7GSD |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50197135
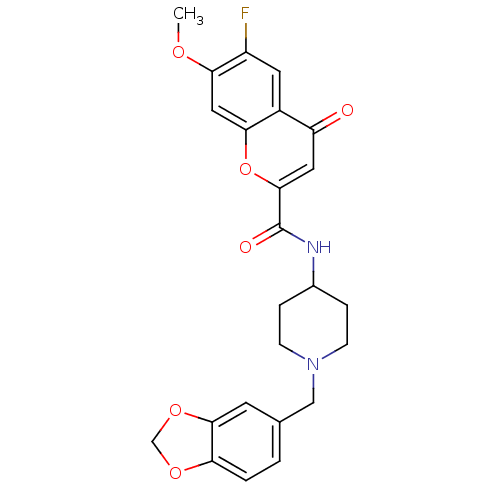
(CHEMBL217442 | N-[1-(1,3-benzodioxol-5-ylmethyl)pi...)Show SMILES COc1cc2oc(cc(=O)c2cc1F)C(=O)NC1CCN(Cc2ccc3OCOc3c2)CC1 Show InChI InChI=1S/C24H23FN2O6/c1-30-21-11-20-16(9-17(21)25)18(28)10-23(33-20)24(29)26-15-4-6-27(7-5-15)12-14-2-3-19-22(8-14)32-13-31-19/h2-3,8-11,15H,4-7,12-13H2,1H3,(H,26,29) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]MCH from MCHr1 expressed in IMR32 cells |
J Med Chem 49: 6569-84 (2006)
Article DOI: 10.1021/jm060683e
BindingDB Entry DOI: 10.7270/Q2NG4Q7P |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data