 Found 1105 hits with Last Name = 'shima' and Initial = 'a'
Found 1105 hits with Last Name = 'shima' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50370683

(CHEMBL1169543)Show SMILES O=C(O[C@H]1CN2CCC1CC2)N1CCc2ccccc2[C@H]1c1ccccc1 |r,wD:20.24,3.2,THB:2:3:7.6:9.10,(-1.33,3.85,;-1.33,2.31,;-2.67,1.54,;-4,2.31,;-4.18,3.72,;-5.76,3.08,;-7.14,3.75,;-6.88,2.32,;-5.51,1.68,;-5.61,0,;-6.05,1.14,;,1.54,;;1.33,-.77,;2.67,,;4,-.77,;5.33,,;5.33,1.54,;4,2.31,;2.67,1.54,;1.33,2.31,;1.33,3.85,;2.67,4.62,;2.67,6.16,;1.33,6.93,;,6.16,;,4.62,)| Show InChI InChI=1S/C23H26N2O2/c26-23(27-21-16-24-13-10-18(21)11-14-24)25-15-12-17-6-4-5-9-20(17)22(25)19-7-2-1-3-8-19/h1-9,18,21-22H,10-16H2/t21-,22+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for rat cortex muscarinic acetylcholine receptor M1 using [3H]pirenzepine |
J Med Chem 48: 6597-606 (2005)
Article DOI: 10.1021/jm050099q
BindingDB Entry DOI: 10.7270/Q2NP257C |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(RAT) | BDBM50370683

(CHEMBL1169543)Show SMILES O=C(O[C@H]1CN2CCC1CC2)N1CCc2ccccc2[C@H]1c1ccccc1 |r,wD:20.24,3.2,THB:2:3:7.6:9.10,(-1.33,3.85,;-1.33,2.31,;-2.67,1.54,;-4,2.31,;-4.18,3.72,;-5.76,3.08,;-7.14,3.75,;-6.88,2.32,;-5.51,1.68,;-5.61,0,;-6.05,1.14,;,1.54,;;1.33,-.77,;2.67,,;4,-.77,;5.33,,;5.33,1.54,;4,2.31,;2.67,1.54,;1.33,2.31,;1.33,3.85,;2.67,4.62,;2.67,6.16,;1.33,6.93,;,6.16,;,4.62,)| Show InChI InChI=1S/C23H26N2O2/c26-23(27-21-16-24-13-10-18(21)11-14-24)25-15-12-17-6-4-5-9-20(17)22(25)19-7-2-1-3-8-19/h1-9,18,21-22H,10-16H2/t21-,22+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for rat salivary gland muscarinic acetylcholine receptor M3 using [3H]N-methylscopolamine |
J Med Chem 48: 6597-606 (2005)
Article DOI: 10.1021/jm050099q
BindingDB Entry DOI: 10.7270/Q2NP257C |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50175574
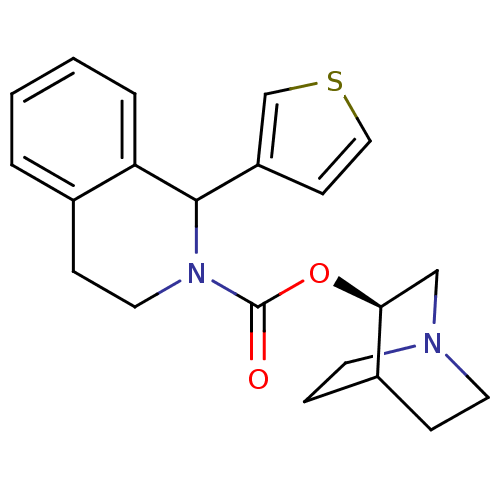
((S)-1-Thiophen-3-yl-3,4-dihydro-1H-isoquinoline-2-...)Show SMILES O=C(O[C@H]1CN2CCC1CC2)N1CCc2ccccc2C1c1ccsc1 |wD:3.2,(.51,-2.2,;.49,-.66,;1.8,.13,;3.16,-.61,;3.17,-2.17,;4.51,-2.92,;5.18,-1.77,;3.76,-1.12,;4.46,.18,;5.82,-.58,;5.84,-2.12,;-.76,.07,;-.81,1.61,;-2.16,2.35,;-3.47,1.52,;-4.76,2.23,;-6.03,1.47,;-6.01,-.01,;-4.7,-.73,;-3.41,-.02,;-2.05,-.73,;-2.01,-2.27,;-3.24,-3.22,;-2.7,-4.67,;-1.16,-4.6,;-.74,-3.13,)| Show InChI InChI=1S/C21H24N2O2S/c24-21(25-19-13-22-9-5-16(19)6-10-22)23-11-7-15-3-1-2-4-18(15)20(23)17-8-12-26-14-17/h1-4,8,12,14,16,19-20H,5-7,9-11,13H2/t19-,20?/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for rat cortex muscarinic acetylcholine receptor M1 using [3H]pirenzepine |
J Med Chem 48: 6597-606 (2005)
Article DOI: 10.1021/jm050099q
BindingDB Entry DOI: 10.7270/Q2NP257C |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50260712

((S)-N-(1-amino-3,3-dimethyl-1-oxobutan-2-yl)-3-iso...)Show SMILES CC(C)CCn1c2ccccc2n(C(=O)N[C@H](C(N)=O)C(C)(C)C)c1=O |r| Show InChI InChI=1S/C19H28N4O3/c1-12(2)10-11-22-13-8-6-7-9-14(13)23(18(22)26)17(25)21-15(16(20)24)19(3,4)5/h6-9,12,15H,10-11H2,1-5H3,(H2,20,24)(H,21,25)/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human CB2 receptor |
Bioorg Med Chem Lett 18: 3310-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.032
BindingDB Entry DOI: 10.7270/Q2VD70C3 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50175570
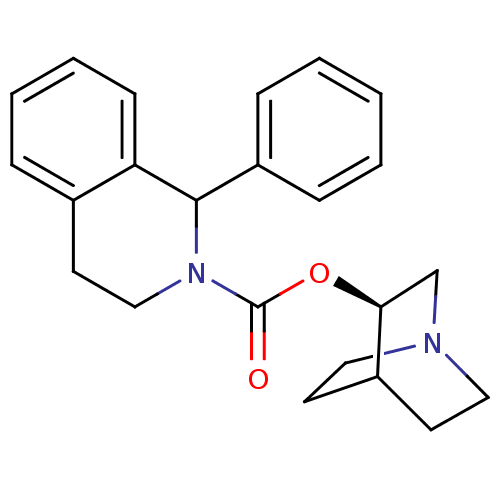
((S)-1-Phenyl-3,4-dihydro-1H-isoquinoline-2-carboxy...)Show SMILES O=C(O[C@H]1CN2CCC1CC2)N1CCc2ccccc2C1c1ccccc1 |wD:3.2,(1.1,-.87,;1.08,.67,;2.4,1.47,;3.75,.72,;3.75,-.83,;5.11,-1.58,;5.78,-.43,;4.36,.23,;5.06,1.51,;6.42,.76,;6.44,-.78,;-.18,1.42,;-.23,2.96,;-1.58,3.69,;-2.89,2.87,;-4.19,3.58,;-5.47,2.82,;-5.45,1.33,;-4.13,.61,;-2.83,1.33,;-1.47,.6,;-1.54,-.89,;-.18,-1.62,;-.16,-3.17,;-1.47,-3.98,;-2.83,-3.21,;-2.86,-1.69,)| Show InChI InChI=1S/C23H26N2O2/c26-23(27-21-16-24-13-10-18(21)11-14-24)25-15-12-17-6-4-5-9-20(17)22(25)19-7-2-1-3-8-19/h1-9,18,21-22H,10-16H2/t21-,22?/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for rat cortex muscarinic acetylcholine receptor M1 using [3H]pirenzepine |
J Med Chem 48: 6597-606 (2005)
Article DOI: 10.1021/jm050099q
BindingDB Entry DOI: 10.7270/Q2NP257C |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50175581
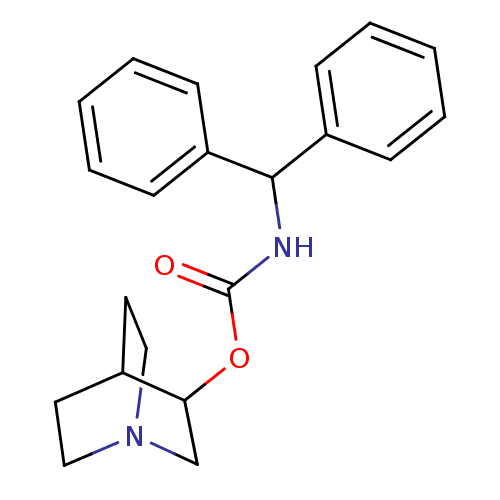
(Benzhydryl-carbamic acid 1-aza-bicyclo[2.2.2]oct-3...)Show SMILES O=C(NC(c1ccccc1)c1ccccc1)OC1CN2CCC1CC2 |(1.67,-1.57,;1.69,-.03,;.37,.76,;-.93,.04,;-2.2,.81,;-2.19,2.29,;-3.45,3.05,;-4.76,2.33,;-4.78,.84,;-3.5,.09,;-.95,-1.5,;-2.31,-2.25,;-2.33,-3.79,;-1,-4.59,;.35,-3.83,;.36,-2.29,;3.05,.72,;4.37,-.08,;4.33,-1.62,;5.66,-2.41,;6.36,-1.29,;4.96,-.59,;5.71,.67,;7.03,-.12,;6.99,-1.67,)| Show InChI InChI=1S/C21H24N2O2/c24-21(25-19-15-23-13-11-16(19)12-14-23)22-20(17-7-3-1-4-8-17)18-9-5-2-6-10-18/h1-10,16,19-20H,11-15H2,(H,22,24) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for rat cortex muscarinic acetylcholine receptor M1 using [3H]pirenzepine |
J Med Chem 48: 6597-606 (2005)
Article DOI: 10.1021/jm050099q
BindingDB Entry DOI: 10.7270/Q2NP257C |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50175575
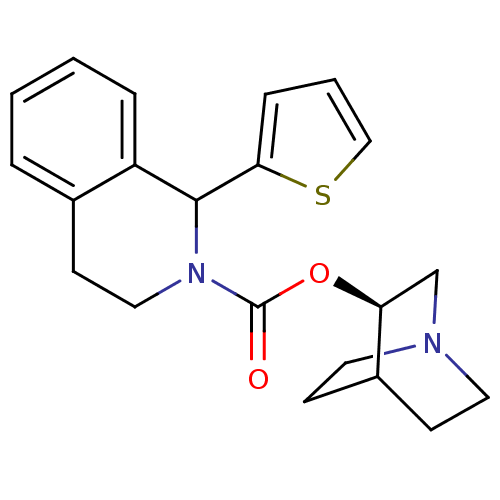
(1-Thiophen-2-yl-3,4-dihydro-1H-isoquinoline-2-carb...)Show SMILES O=C(O[C@H]1CN2CCC1CC2)N1CCc2ccccc2C1c1cccs1 |wD:3.2,(-1.66,-4.44,;-1.68,-2.9,;-.37,-2.1,;.99,-2.85,;1,-4.4,;2.34,-5.15,;3.01,-4,;1.6,-3.35,;2.3,-2.06,;3.65,-2.81,;3.68,-4.35,;-2.94,-2.16,;-2.98,-.62,;-4.34,.12,;-5.64,-.71,;-6.94,.02,;-8.21,-.76,;-8.19,-2.24,;-6.88,-2.95,;-5.58,-2.25,;-4.23,-2.97,;-4.18,-4.49,;-5.41,-5.45,;-4.87,-6.9,;-3.33,-6.84,;-2.91,-5.36,)| Show InChI InChI=1S/C21H24N2O2S/c24-21(25-18-14-22-10-7-16(18)8-11-22)23-12-9-15-4-1-2-5-17(15)20(23)19-6-3-13-26-19/h1-6,13,16,18,20H,7-12,14H2/t18-,20?/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for rat cortex muscarinic acetylcholine receptor M1 using [3H]pirenzepine |
J Med Chem 48: 6597-606 (2005)
Article DOI: 10.1021/jm050099q
BindingDB Entry DOI: 10.7270/Q2NP257C |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50175575

(1-Thiophen-2-yl-3,4-dihydro-1H-isoquinoline-2-carb...)Show SMILES O=C(O[C@H]1CN2CCC1CC2)N1CCc2ccccc2C1c1cccs1 |wD:3.2,(-1.66,-4.44,;-1.68,-2.9,;-.37,-2.1,;.99,-2.85,;1,-4.4,;2.34,-5.15,;3.01,-4,;1.6,-3.35,;2.3,-2.06,;3.65,-2.81,;3.68,-4.35,;-2.94,-2.16,;-2.98,-.62,;-4.34,.12,;-5.64,-.71,;-6.94,.02,;-8.21,-.76,;-8.19,-2.24,;-6.88,-2.95,;-5.58,-2.25,;-4.23,-2.97,;-4.18,-4.49,;-5.41,-5.45,;-4.87,-6.9,;-3.33,-6.84,;-2.91,-5.36,)| Show InChI InChI=1S/C21H24N2O2S/c24-21(25-18-14-22-10-7-16(18)8-11-22)23-12-9-15-4-1-2-5-17(15)20(23)19-6-3-13-26-19/h1-6,13,16,18,20H,7-12,14H2/t18-,20?/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for rat cortex muscarinic acetylcholine receptor M1 using [3H]pirenzepine |
J Med Chem 48: 6597-606 (2005)
Article DOI: 10.1021/jm050099q
BindingDB Entry DOI: 10.7270/Q2NP257C |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(RAT) | BDBM50175581

(Benzhydryl-carbamic acid 1-aza-bicyclo[2.2.2]oct-3...)Show SMILES O=C(NC(c1ccccc1)c1ccccc1)OC1CN2CCC1CC2 |(1.67,-1.57,;1.69,-.03,;.37,.76,;-.93,.04,;-2.2,.81,;-2.19,2.29,;-3.45,3.05,;-4.76,2.33,;-4.78,.84,;-3.5,.09,;-.95,-1.5,;-2.31,-2.25,;-2.33,-3.79,;-1,-4.59,;.35,-3.83,;.36,-2.29,;3.05,.72,;4.37,-.08,;4.33,-1.62,;5.66,-2.41,;6.36,-1.29,;4.96,-.59,;5.71,.67,;7.03,-.12,;6.99,-1.67,)| Show InChI InChI=1S/C21H24N2O2/c24-21(25-19-15-23-13-11-16(19)12-14-23)22-20(17-7-3-1-4-8-17)18-9-5-2-6-10-18/h1-10,16,19-20H,11-15H2,(H,22,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for rat salivary gland muscarinic acetylcholine receptor M3 using [3H]N-methylscopolamine |
J Med Chem 48: 6597-606 (2005)
Article DOI: 10.1021/jm050099q
BindingDB Entry DOI: 10.7270/Q2NP257C |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor
(Mus musculus) | BDBM50350352
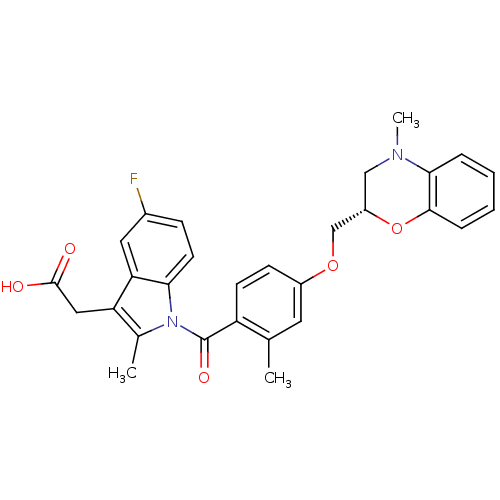
(CHEMBL1813275)Show SMILES CN1C[C@@H](COc2ccc(C(=O)n3c(C)c(CC(O)=O)c4cc(F)ccc34)c(C)c2)Oc2ccccc12 |r| Show InChI InChI=1S/C29H27FN2O5/c1-17-12-20(36-16-21-15-31(3)26-6-4-5-7-27(26)37-21)9-10-22(17)29(35)32-18(2)23(14-28(33)34)24-13-19(30)8-11-25(24)32/h4-13,21H,14-16H2,1-3H3,(H,33,34)/t21-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]-PGD2 from mouse DP receptor expressed in CHO cells after 20 mins by liquid scintillation counting |
Bioorg Med Chem 19: 4574-88 (2011)
Article DOI: 10.1016/j.bmc.2011.06.014
BindingDB Entry DOI: 10.7270/Q2FB5398 |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor
(Mus musculus) | BDBM50350350
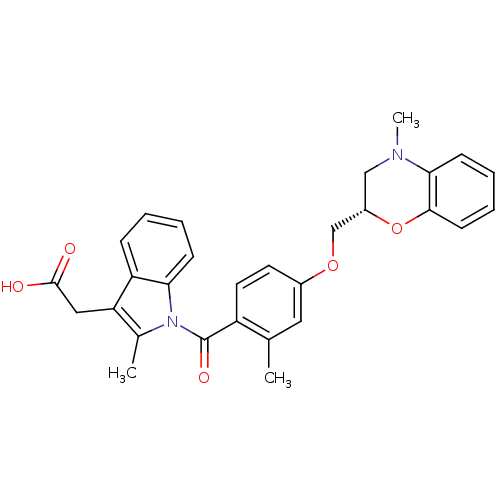
(CHEMBL1813120)Show SMILES CN1C[C@@H](COc2ccc(C(=O)n3c(C)c(CC(O)=O)c4ccccc34)c(C)c2)Oc2ccccc12 |r| Show InChI InChI=1S/C29H28N2O5/c1-18-14-20(35-17-21-16-30(3)26-10-6-7-11-27(26)36-21)12-13-22(18)29(34)31-19(2)24(15-28(32)33)23-8-4-5-9-25(23)31/h4-14,21H,15-17H2,1-3H3,(H,32,33)/t21-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]-PGD2 from mouse DP receptor expressed in CHO cells after 20 mins by liquid scintillation counting |
Bioorg Med Chem 19: 4574-88 (2011)
Article DOI: 10.1016/j.bmc.2011.06.014
BindingDB Entry DOI: 10.7270/Q2FB5398 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(RAT) | BDBM50175574

((S)-1-Thiophen-3-yl-3,4-dihydro-1H-isoquinoline-2-...)Show SMILES O=C(O[C@H]1CN2CCC1CC2)N1CCc2ccccc2C1c1ccsc1 |wD:3.2,(.51,-2.2,;.49,-.66,;1.8,.13,;3.16,-.61,;3.17,-2.17,;4.51,-2.92,;5.18,-1.77,;3.76,-1.12,;4.46,.18,;5.82,-.58,;5.84,-2.12,;-.76,.07,;-.81,1.61,;-2.16,2.35,;-3.47,1.52,;-4.76,2.23,;-6.03,1.47,;-6.01,-.01,;-4.7,-.73,;-3.41,-.02,;-2.05,-.73,;-2.01,-2.27,;-3.24,-3.22,;-2.7,-4.67,;-1.16,-4.6,;-.74,-3.13,)| Show InChI InChI=1S/C21H24N2O2S/c24-21(25-19-13-22-9-5-16(19)6-10-22)23-11-7-15-3-1-2-4-18(15)20(23)17-8-12-26-14-17/h1-4,8,12,14,16,19-20H,5-7,9-11,13H2/t19-,20?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for rat salivary gland muscarinic acetylcholine receptor M3 using [3H]N-methylscopolamine |
J Med Chem 48: 6597-606 (2005)
Article DOI: 10.1021/jm050099q
BindingDB Entry DOI: 10.7270/Q2NP257C |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(RAT) | BDBM50175575

(1-Thiophen-2-yl-3,4-dihydro-1H-isoquinoline-2-carb...)Show SMILES O=C(O[C@H]1CN2CCC1CC2)N1CCc2ccccc2C1c1cccs1 |wD:3.2,(-1.66,-4.44,;-1.68,-2.9,;-.37,-2.1,;.99,-2.85,;1,-4.4,;2.34,-5.15,;3.01,-4,;1.6,-3.35,;2.3,-2.06,;3.65,-2.81,;3.68,-4.35,;-2.94,-2.16,;-2.98,-.62,;-4.34,.12,;-5.64,-.71,;-6.94,.02,;-8.21,-.76,;-8.19,-2.24,;-6.88,-2.95,;-5.58,-2.25,;-4.23,-2.97,;-4.18,-4.49,;-5.41,-5.45,;-4.87,-6.9,;-3.33,-6.84,;-2.91,-5.36,)| Show InChI InChI=1S/C21H24N2O2S/c24-21(25-18-14-22-10-7-16(18)8-11-22)23-12-9-15-4-1-2-5-17(15)20(23)19-6-3-13-26-19/h1-6,13,16,18,20H,7-12,14H2/t18-,20?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for rat salivary gland muscarinic acetylcholine receptor M3 using [3H]N-methylscopolamine |
J Med Chem 48: 6597-606 (2005)
Article DOI: 10.1021/jm050099q
BindingDB Entry DOI: 10.7270/Q2NP257C |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(RAT) | BDBM50175575

(1-Thiophen-2-yl-3,4-dihydro-1H-isoquinoline-2-carb...)Show SMILES O=C(O[C@H]1CN2CCC1CC2)N1CCc2ccccc2C1c1cccs1 |wD:3.2,(-1.66,-4.44,;-1.68,-2.9,;-.37,-2.1,;.99,-2.85,;1,-4.4,;2.34,-5.15,;3.01,-4,;1.6,-3.35,;2.3,-2.06,;3.65,-2.81,;3.68,-4.35,;-2.94,-2.16,;-2.98,-.62,;-4.34,.12,;-5.64,-.71,;-6.94,.02,;-8.21,-.76,;-8.19,-2.24,;-6.88,-2.95,;-5.58,-2.25,;-4.23,-2.97,;-4.18,-4.49,;-5.41,-5.45,;-4.87,-6.9,;-3.33,-6.84,;-2.91,-5.36,)| Show InChI InChI=1S/C21H24N2O2S/c24-21(25-18-14-22-10-7-16(18)8-11-22)23-12-9-15-4-1-2-5-17(15)20(23)19-6-3-13-26-19/h1-6,13,16,18,20H,7-12,14H2/t18-,20?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for rat salivary gland muscarinic acetylcholine receptor M3 using [3H]N-methylscopolamine |
J Med Chem 48: 6597-606 (2005)
Article DOI: 10.1021/jm050099q
BindingDB Entry DOI: 10.7270/Q2NP257C |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor
(Mus musculus) | BDBM50350354
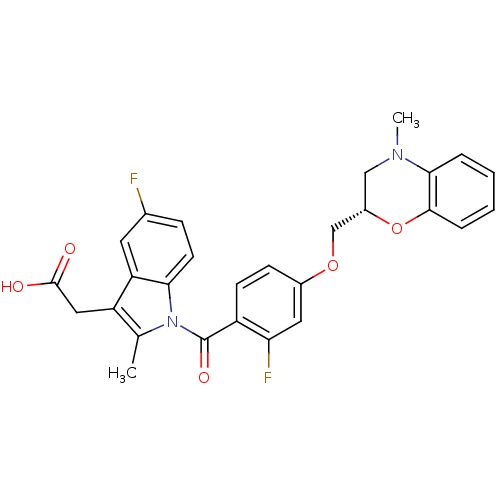
(CHEMBL1813277)Show SMILES CN1C[C@@H](COc2ccc(C(=O)n3c(C)c(CC(O)=O)c4cc(F)ccc34)c(F)c2)Oc2ccccc12 |r| Show InChI InChI=1S/C28H24F2N2O5/c1-16-21(13-27(33)34)22-11-17(29)7-10-24(22)32(16)28(35)20-9-8-18(12-23(20)30)36-15-19-14-31(2)25-5-3-4-6-26(25)37-19/h3-12,19H,13-15H2,1-2H3,(H,33,34)/t19-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]-PGD2 from mouse DP receptor expressed in CHO cells after 20 mins by liquid scintillation counting |
Bioorg Med Chem 19: 4574-88 (2011)
Article DOI: 10.1016/j.bmc.2011.06.014
BindingDB Entry DOI: 10.7270/Q2FB5398 |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor
(Mus musculus) | BDBM50350371
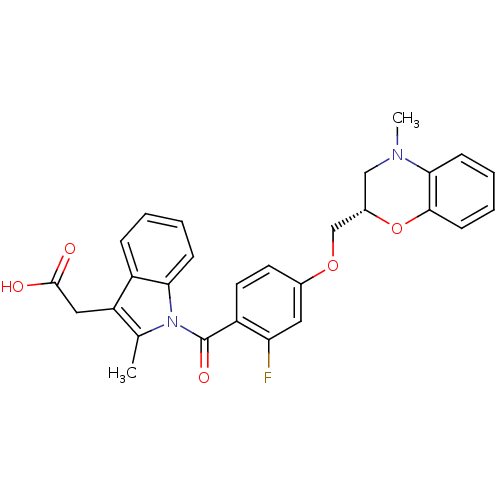
(CHEMBL1813122)Show SMILES CN1C[C@@H](COc2ccc(C(=O)n3c(C)c(CC(O)=O)c4ccccc34)c(F)c2)Oc2ccccc12 |r| Show InChI InChI=1S/C28H25FN2O5/c1-17-22(14-27(32)33)20-7-3-4-8-24(20)31(17)28(34)21-12-11-18(13-23(21)29)35-16-19-15-30(2)25-9-5-6-10-26(25)36-19/h3-13,19H,14-16H2,1-2H3,(H,32,33)/t19-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]-PGD2 from mouse DP receptor expressed in CHO cells after 20 mins by liquid scintillation counting |
Bioorg Med Chem 19: 4574-88 (2011)
Article DOI: 10.1016/j.bmc.2011.06.014
BindingDB Entry DOI: 10.7270/Q2FB5398 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50175578
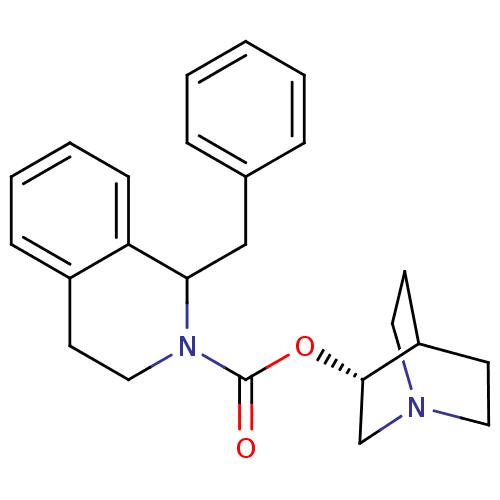
(1-Benzyl-3,4-dihydro-1H-isoquinoline-2-carboxylic ...)Show SMILES O=C(O[C@H]1CN2CCC1CC2)N1CCc2ccccc2C1Cc1ccccc1 |wD:3.2,(-1.07,-1.94,;-1.1,-.4,;.22,.4,;1.57,-.35,;1.59,-1.9,;2.92,-2.64,;3.59,-1.5,;2.18,-.85,;2.88,.44,;4.23,-.31,;4.25,-1.85,;-2.35,.34,;-2.39,1.88,;-3.75,2.61,;-5.05,1.79,;-6.34,2.51,;-7.62,1.74,;-7.6,.26,;-6.29,-.45,;-4.99,.25,;-3.64,-.47,;-3.59,-2.01,;-4.91,-2.82,;-4.85,-4.35,;-6.16,-5.16,;-7.53,-4.44,;-7.57,-2.9,;-6.27,-2.08,)| Show InChI InChI=1S/C24H28N2O2/c27-24(28-23-17-25-13-10-20(23)11-14-25)26-15-12-19-8-4-5-9-21(19)22(26)16-18-6-2-1-3-7-18/h1-9,20,22-23H,10-17H2/t22?,23-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for rat cortex muscarinic acetylcholine receptor M1 using [3H]pirenzepine |
J Med Chem 48: 6597-606 (2005)
Article DOI: 10.1021/jm050099q
BindingDB Entry DOI: 10.7270/Q2NP257C |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50175578

(1-Benzyl-3,4-dihydro-1H-isoquinoline-2-carboxylic ...)Show SMILES O=C(O[C@H]1CN2CCC1CC2)N1CCc2ccccc2C1Cc1ccccc1 |wD:3.2,(-1.07,-1.94,;-1.1,-.4,;.22,.4,;1.57,-.35,;1.59,-1.9,;2.92,-2.64,;3.59,-1.5,;2.18,-.85,;2.88,.44,;4.23,-.31,;4.25,-1.85,;-2.35,.34,;-2.39,1.88,;-3.75,2.61,;-5.05,1.79,;-6.34,2.51,;-7.62,1.74,;-7.6,.26,;-6.29,-.45,;-4.99,.25,;-3.64,-.47,;-3.59,-2.01,;-4.91,-2.82,;-4.85,-4.35,;-6.16,-5.16,;-7.53,-4.44,;-7.57,-2.9,;-6.27,-2.08,)| Show InChI InChI=1S/C24H28N2O2/c27-24(28-23-17-25-13-10-20(23)11-14-25)26-15-12-19-8-4-5-9-21(19)22(26)16-18-6-2-1-3-7-18/h1-9,20,22-23H,10-17H2/t22?,23-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for rat cortex muscarinic acetylcholine receptor M1 using [3H]pirenzepine |
J Med Chem 48: 6597-606 (2005)
Article DOI: 10.1021/jm050099q
BindingDB Entry DOI: 10.7270/Q2NP257C |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50377918

(CHEMBL1162994)Show SMILES CC(C)(C)C(NC(=O)n1c2ccccc2n(CCC2CCOCC2)c1=O)C(N)=O Show InChI InChI=1S/C21H30N4O4/c1-21(2,3)17(18(22)26)23-19(27)25-16-7-5-4-6-15(16)24(20(25)28)11-8-14-9-12-29-13-10-14/h4-7,14,17H,8-13H2,1-3H3,(H2,22,26)(H,23,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human CB2 receptor |
Bioorg Med Chem Lett 18: 3310-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.032
BindingDB Entry DOI: 10.7270/Q2VD70C3 |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor
(Mus musculus) | BDBM50350351
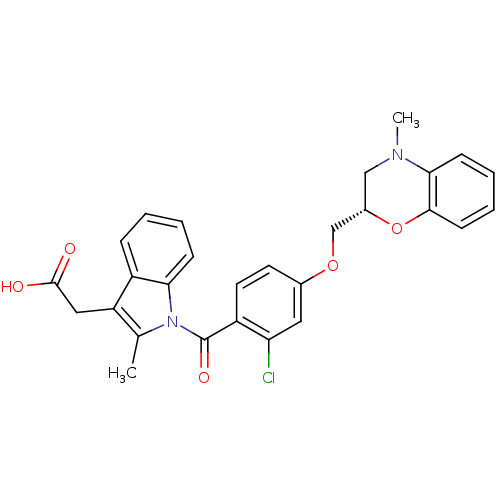
(CHEMBL1813121)Show SMILES CN1C[C@@H](COc2ccc(C(=O)n3c(C)c(CC(O)=O)c4ccccc34)c(Cl)c2)Oc2ccccc12 |r| Show InChI InChI=1S/C28H25ClN2O5/c1-17-22(14-27(32)33)20-7-3-4-8-24(20)31(17)28(34)21-12-11-18(13-23(21)29)35-16-19-15-30(2)25-9-5-6-10-26(25)36-19/h3-13,19H,14-16H2,1-2H3,(H,32,33)/t19-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]-PGD2 from mouse DP receptor expressed in CHO cells after 20 mins by liquid scintillation counting |
Bioorg Med Chem 19: 4574-88 (2011)
Article DOI: 10.1016/j.bmc.2011.06.014
BindingDB Entry DOI: 10.7270/Q2FB5398 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM18656

((2R,5S)-4-[4-cyano-3-(trifluoromethyl)phenyl]-2,5-...)Show SMILES C[C@@H]1CN([C@@H](C)CN1C(=O)Nc1ccc(nc1)C(F)(F)F)c1ccc(C#N)c(c1)C(F)(F)F |r| Show InChI InChI=1S/C21H19F6N5O/c1-12-11-32(19(33)30-15-4-6-18(29-9-15)21(25,26)27)13(2)10-31(12)16-5-3-14(8-28)17(7-16)20(22,23)24/h3-7,9,12-13H,10-11H2,1-2H3,(H,30,33)/t12-,13+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
| Assay Description
The Ki values were determined by the application of the Cheng-Prusoff equation: Ki=IC50/(1+[L]/Kd] where [L] is the concentration of labeled ligand a... |
J Med Chem 49: 716-26 (2006)
Article DOI: 10.1021/jm050293c
BindingDB Entry DOI: 10.7270/Q2P84952 |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor
(Mus musculus) | BDBM50350366
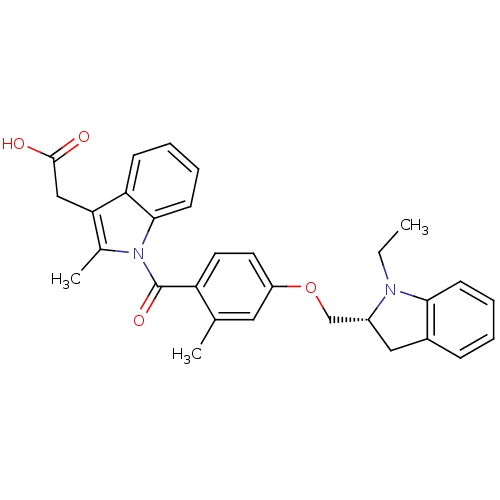
(CHEMBL1813287)Show SMILES CCN1[C@@H](COc2ccc(C(=O)n3c(C)c(CC(O)=O)c4ccccc34)c(C)c2)Cc2ccccc12 |r| Show InChI InChI=1S/C30H30N2O4/c1-4-31-22(16-21-9-5-7-11-27(21)31)18-36-23-13-14-24(19(2)15-23)30(35)32-20(3)26(17-29(33)34)25-10-6-8-12-28(25)32/h5-15,22H,4,16-18H2,1-3H3,(H,33,34)/t22-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]-PGD2 from mouse DP receptor expressed in CHO cells after 20 mins by liquid scintillation counting |
Bioorg Med Chem 19: 4574-88 (2011)
Article DOI: 10.1016/j.bmc.2011.06.014
BindingDB Entry DOI: 10.7270/Q2FB5398 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50260627

((S)-N-(1-amino-3,3-dimethyl-1-oxobutan-2-yl)-3-(2-...)Show SMILES CC(C)(C)[C@H](NC(=O)n1c2ccccc2n(CCN2CCOCC2)c1=O)C(N)=O |r| Show InChI InChI=1S/C20H29N5O4/c1-20(2,3)16(17(21)26)22-18(27)25-15-7-5-4-6-14(15)24(19(25)28)9-8-23-10-12-29-13-11-23/h4-7,16H,8-13H2,1-3H3,(H2,21,26)(H,22,27)/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human CB2 receptor |
Bioorg Med Chem Lett 18: 3310-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.032
BindingDB Entry DOI: 10.7270/Q2VD70C3 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50260672
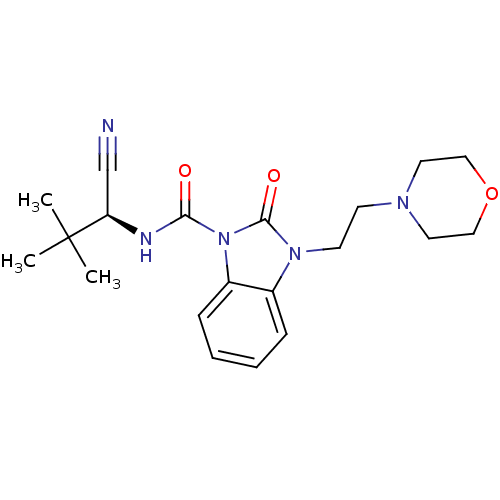
((S)-N-(1-cyano-2,2-dimethylpropyl)-3-(2-morpholino...)Show SMILES CC(C)(C)[C@H](NC(=O)n1c2ccccc2n(CCN2CCOCC2)c1=O)C#N |r| Show InChI InChI=1S/C20H27N5O3/c1-20(2,3)17(14-21)22-18(26)25-16-7-5-4-6-15(16)24(19(25)27)9-8-23-10-12-28-13-11-23/h4-7,17H,8-13H2,1-3H3,(H,22,26)/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human CB2 receptor |
Bioorg Med Chem Lett 18: 3310-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.032
BindingDB Entry DOI: 10.7270/Q2VD70C3 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50260673

((S)-N-(2,2-dimethyl-1-(2-methyl-2H-tetrazol-5-yl)p...)Show SMILES Cn1nnc(n1)[C@@H](NC(=O)n1c2ccccc2n(CCN2CCOCC2)c1=O)C(C)(C)C |r| Show InChI InChI=1S/C21H30N8O3/c1-21(2,3)17(18-23-25-26(4)24-18)22-19(30)29-16-8-6-5-7-15(16)28(20(29)31)10-9-27-11-13-32-14-12-27/h5-8,17H,9-14H2,1-4H3,(H,22,30)/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human CB2 receptor |
Bioorg Med Chem Lett 18: 3310-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.032
BindingDB Entry DOI: 10.7270/Q2VD70C3 |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor
(Mus musculus) | BDBM50350353
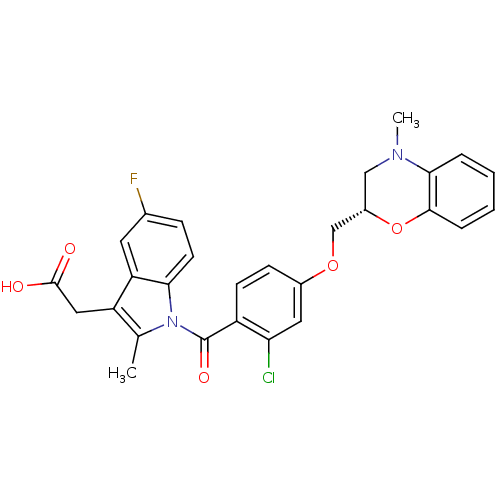
(CHEMBL1813276)Show SMILES CN1C[C@@H](COc2ccc(C(=O)n3c(C)c(CC(O)=O)c4cc(F)ccc34)c(Cl)c2)Oc2ccccc12 |r| Show InChI InChI=1S/C28H24ClFN2O5/c1-16-21(13-27(33)34)22-11-17(30)7-10-24(22)32(16)28(35)20-9-8-18(12-23(20)29)36-15-19-14-31(2)25-5-3-4-6-26(25)37-19/h3-12,19H,13-15H2,1-2H3,(H,33,34)/t19-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]-PGD2 from mouse DP receptor expressed in CHO cells after 20 mins by liquid scintillation counting |
Bioorg Med Chem 19: 4574-88 (2011)
Article DOI: 10.1016/j.bmc.2011.06.014
BindingDB Entry DOI: 10.7270/Q2FB5398 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(RAT) | BDBM50165019
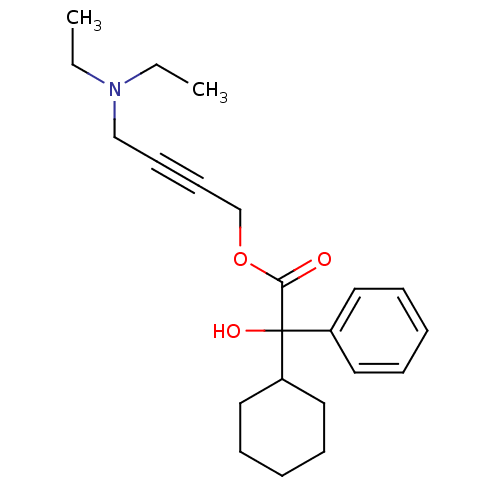
(4-(Diethylamino)-2-butynyl alpha-phenylcyclohexane...)Show InChI InChI=1S/C22H31NO3/c1-3-23(4-2)17-11-12-18-26-21(24)22(25,19-13-7-5-8-14-19)20-15-9-6-10-16-20/h5,7-8,13-14,20,25H,3-4,6,9-10,15-18H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for rat salivary gland muscarinic acetylcholine receptor M3 using [3H]N-methylscopolamine |
J Med Chem 48: 6597-606 (2005)
Article DOI: 10.1021/jm050099q
BindingDB Entry DOI: 10.7270/Q2NP257C |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor
(Mus musculus) | BDBM50350361
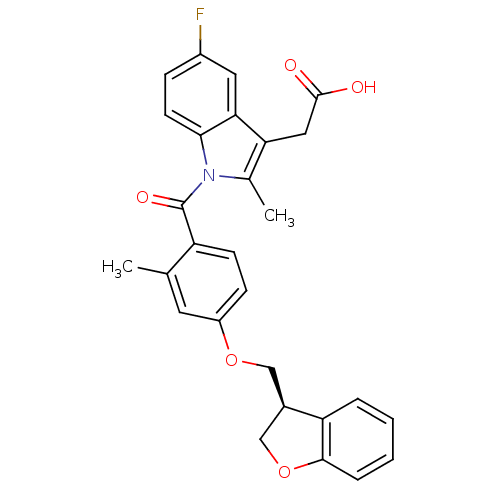
(CHEMBL1813289)Show SMILES Cc1c(CC(O)=O)c2cc(F)ccc2n1C(=O)c1ccc(OC[C@@H]2COc3ccccc23)cc1C |r| Show InChI InChI=1S/C28H24FNO5/c1-16-11-20(34-14-18-15-35-26-6-4-3-5-22(18)26)8-9-21(16)28(33)30-17(2)23(13-27(31)32)24-12-19(29)7-10-25(24)30/h3-12,18H,13-15H2,1-2H3,(H,31,32)/t18-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]-PGD2 from mouse DP receptor expressed in CHO cells after 20 mins by liquid scintillation counting |
Bioorg Med Chem 19: 4574-88 (2011)
Article DOI: 10.1016/j.bmc.2011.06.014
BindingDB Entry DOI: 10.7270/Q2FB5398 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(RAT) | BDBM50175570

((S)-1-Phenyl-3,4-dihydro-1H-isoquinoline-2-carboxy...)Show SMILES O=C(O[C@H]1CN2CCC1CC2)N1CCc2ccccc2C1c1ccccc1 |wD:3.2,(1.1,-.87,;1.08,.67,;2.4,1.47,;3.75,.72,;3.75,-.83,;5.11,-1.58,;5.78,-.43,;4.36,.23,;5.06,1.51,;6.42,.76,;6.44,-.78,;-.18,1.42,;-.23,2.96,;-1.58,3.69,;-2.89,2.87,;-4.19,3.58,;-5.47,2.82,;-5.45,1.33,;-4.13,.61,;-2.83,1.33,;-1.47,.6,;-1.54,-.89,;-.18,-1.62,;-.16,-3.17,;-1.47,-3.98,;-2.83,-3.21,;-2.86,-1.69,)| Show InChI InChI=1S/C23H26N2O2/c26-23(27-21-16-24-13-10-18(21)11-14-24)25-15-12-17-6-4-5-9-20(17)22(25)19-7-2-1-3-8-19/h1-9,18,21-22H,10-16H2/t21-,22?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for rat salivary gland muscarinic acetylcholine receptor M3 using [3H]N-methylscopolamine |
J Med Chem 48: 6597-606 (2005)
Article DOI: 10.1021/jm050099q
BindingDB Entry DOI: 10.7270/Q2NP257C |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor
(Mus musculus) | BDBM50350369
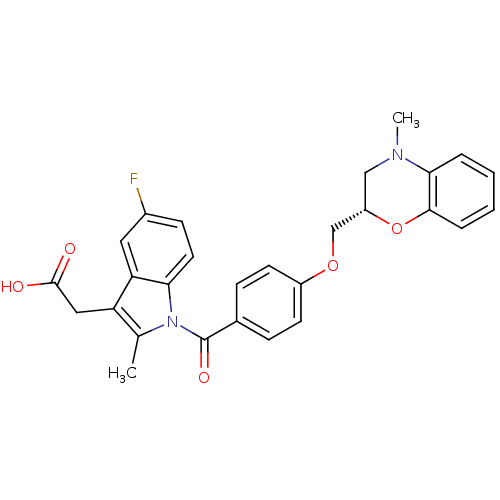
(CHEMBL1813118)Show SMILES CN1C[C@@H](COc2ccc(cc2)C(=O)n2c(C)c(CC(O)=O)c3cc(F)ccc23)Oc2ccccc12 |r| Show InChI InChI=1S/C28H25FN2O5/c1-17-22(14-27(32)33)23-13-19(29)9-12-24(23)31(17)28(34)18-7-10-20(11-8-18)35-16-21-15-30(2)25-5-3-4-6-26(25)36-21/h3-13,21H,14-16H2,1-2H3,(H,32,33)/t21-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]-PGD2 from mouse DP receptor expressed in CHO cells after 20 mins by liquid scintillation counting |
Bioorg Med Chem 19: 4574-88 (2011)
Article DOI: 10.1016/j.bmc.2011.06.014
BindingDB Entry DOI: 10.7270/Q2FB5398 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50165019

(4-(Diethylamino)-2-butynyl alpha-phenylcyclohexane...)Show InChI InChI=1S/C22H31NO3/c1-3-23(4-2)17-11-12-18-26-21(24)22(25,19-13-7-5-8-14-19)20-15-9-6-10-16-20/h5,7-8,13-14,20,25H,3-4,6,9-10,15-18H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for rat cortex muscarinic acetylcholine receptor M1 using [3H]pirenzepine |
J Med Chem 48: 6597-606 (2005)
Article DOI: 10.1021/jm050099q
BindingDB Entry DOI: 10.7270/Q2NP257C |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50260709
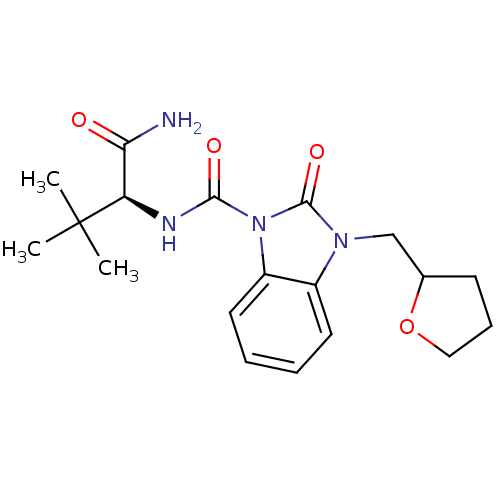
(CHEMBL496497 | N-((S)-1-amino-3,3-dimethyl-1-oxobu...)Show SMILES CC(C)(C)[C@H](NC(=O)n1c2ccccc2n(CC2CCCO2)c1=O)C(N)=O |r| Show InChI InChI=1S/C19H26N4O4/c1-19(2,3)15(16(20)24)21-17(25)23-14-9-5-4-8-13(14)22(18(23)26)11-12-7-6-10-27-12/h4-5,8-9,12,15H,6-7,10-11H2,1-3H3,(H2,20,24)(H,21,25)/t12?,15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human CB2 receptor |
Bioorg Med Chem Lett 18: 3310-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.032
BindingDB Entry DOI: 10.7270/Q2VD70C3 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Rattus norvegicus (Rat)) | BDBM18656

((2R,5S)-4-[4-cyano-3-(trifluoromethyl)phenyl]-2,5-...)Show SMILES C[C@@H]1CN([C@@H](C)CN1C(=O)Nc1ccc(nc1)C(F)(F)F)c1ccc(C#N)c(c1)C(F)(F)F |r| Show InChI InChI=1S/C21H19F6N5O/c1-12-11-32(19(33)30-15-4-6-18(29-9-15)21(25,26)27)13(2)10-31(12)16-5-3-14(8-28)17(7-16)20(22,23)24/h3-7,9,12-13H,10-11H2,1-2H3,(H,30,33)/t12-,13+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
| Assay Description
The Ki values were determined by the application of the Cheng-Prusoff equation: Ki=IC50/(1+[L]/Kd] where [L] is the concentration of labeled ligand a... |
J Med Chem 49: 716-26 (2006)
Article DOI: 10.1021/jm050293c
BindingDB Entry DOI: 10.7270/Q2P84952 |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor
(Mus musculus) | BDBM50357626
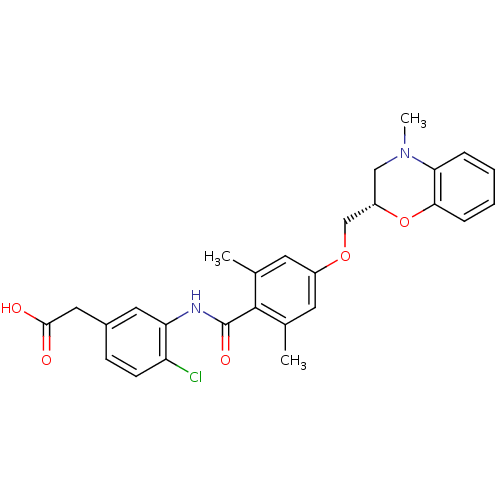
(CHEMBL1915856)Show SMILES CN1C[C@@H](COc2cc(C)c(C(=O)Nc3cc(CC(O)=O)ccc3Cl)c(C)c2)Oc2ccccc12 |r| Show InChI InChI=1S/C27H27ClN2O5/c1-16-10-19(34-15-20-14-30(3)23-6-4-5-7-24(23)35-20)11-17(2)26(16)27(33)29-22-12-18(13-25(31)32)8-9-21(22)28/h4-12,20H,13-15H2,1-3H3,(H,29,33)(H,31,32)/t20-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-PGD2 from mouse DP receptor expressed in CHO cells after 20 mins by liquid scintillation counting |
Bioorg Med Chem 19: 6935-48 (2011)
Article DOI: 10.1016/j.bmc.2011.08.065
BindingDB Entry DOI: 10.7270/Q2K35V22 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50175566
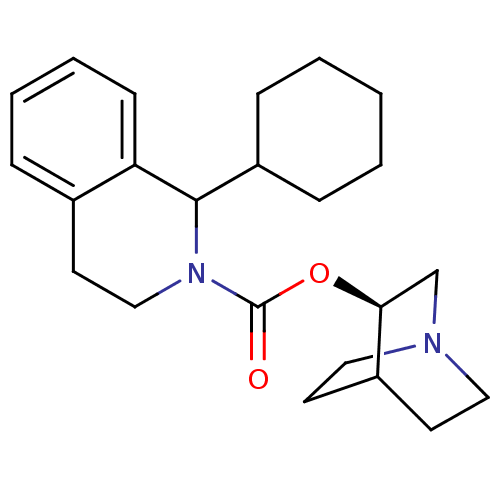
(1-Cyclohexyl-3,4-dihydro-1H-isoquinoline-2-carboxy...)Show SMILES O=C(O[C@H]1CN2CCC1CC2)N1CCc2ccccc2C1C1CCCCC1 |wD:3.2,(-1.26,-.59,;-1.28,.95,;.05,1.75,;1.39,1,;1.41,-.55,;2.76,-1.3,;3.43,-.15,;2,.51,;2.7,1.8,;4.06,1.05,;4.08,-.5,;-2.53,1.7,;-2.59,3.25,;-3.95,3.97,;-5.25,3.15,;-6.55,3.86,;-7.82,3.11,;-7.8,1.61,;-6.49,.89,;-5.19,1.61,;-3.83,.88,;-3.79,-.66,;-2.43,-1.39,;-2.38,-2.93,;-3.69,-3.75,;-5.06,-3.02,;-5.1,-1.48,)| Show InChI InChI=1S/C23H32N2O2/c26-23(27-21-16-24-13-10-18(21)11-14-24)25-15-12-17-6-4-5-9-20(17)22(25)19-7-2-1-3-8-19/h4-6,9,18-19,21-22H,1-3,7-8,10-16H2/t21-,22?/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for rat cortex muscarinic acetylcholine receptor M1 using [3H]pirenzepine |
J Med Chem 48: 6597-606 (2005)
Article DOI: 10.1021/jm050099q
BindingDB Entry DOI: 10.7270/Q2NP257C |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50175566

(1-Cyclohexyl-3,4-dihydro-1H-isoquinoline-2-carboxy...)Show SMILES O=C(O[C@H]1CN2CCC1CC2)N1CCc2ccccc2C1C1CCCCC1 |wD:3.2,(-1.26,-.59,;-1.28,.95,;.05,1.75,;1.39,1,;1.41,-.55,;2.76,-1.3,;3.43,-.15,;2,.51,;2.7,1.8,;4.06,1.05,;4.08,-.5,;-2.53,1.7,;-2.59,3.25,;-3.95,3.97,;-5.25,3.15,;-6.55,3.86,;-7.82,3.11,;-7.8,1.61,;-6.49,.89,;-5.19,1.61,;-3.83,.88,;-3.79,-.66,;-2.43,-1.39,;-2.38,-2.93,;-3.69,-3.75,;-5.06,-3.02,;-5.1,-1.48,)| Show InChI InChI=1S/C23H32N2O2/c26-23(27-21-16-24-13-10-18(21)11-14-24)25-15-12-17-6-4-5-9-20(17)22(25)19-7-2-1-3-8-19/h4-6,9,18-19,21-22H,1-3,7-8,10-16H2/t21-,22?/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for rat cortex muscarinic acetylcholine receptor M1 using [3H]pirenzepine |
J Med Chem 48: 6597-606 (2005)
Article DOI: 10.1021/jm050099q
BindingDB Entry DOI: 10.7270/Q2NP257C |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(RAT) | BDBM50175566

(1-Cyclohexyl-3,4-dihydro-1H-isoquinoline-2-carboxy...)Show SMILES O=C(O[C@H]1CN2CCC1CC2)N1CCc2ccccc2C1C1CCCCC1 |wD:3.2,(-1.26,-.59,;-1.28,.95,;.05,1.75,;1.39,1,;1.41,-.55,;2.76,-1.3,;3.43,-.15,;2,.51,;2.7,1.8,;4.06,1.05,;4.08,-.5,;-2.53,1.7,;-2.59,3.25,;-3.95,3.97,;-5.25,3.15,;-6.55,3.86,;-7.82,3.11,;-7.8,1.61,;-6.49,.89,;-5.19,1.61,;-3.83,.88,;-3.79,-.66,;-2.43,-1.39,;-2.38,-2.93,;-3.69,-3.75,;-5.06,-3.02,;-5.1,-1.48,)| Show InChI InChI=1S/C23H32N2O2/c26-23(27-21-16-24-13-10-18(21)11-14-24)25-15-12-17-6-4-5-9-20(17)22(25)19-7-2-1-3-8-19/h4-6,9,18-19,21-22H,1-3,7-8,10-16H2/t21-,22?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for rat salivary gland muscarinic acetylcholine receptor M3 using [3H]N-methylscopolamine |
J Med Chem 48: 6597-606 (2005)
Article DOI: 10.1021/jm050099q
BindingDB Entry DOI: 10.7270/Q2NP257C |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(RAT) | BDBM50175566

(1-Cyclohexyl-3,4-dihydro-1H-isoquinoline-2-carboxy...)Show SMILES O=C(O[C@H]1CN2CCC1CC2)N1CCc2ccccc2C1C1CCCCC1 |wD:3.2,(-1.26,-.59,;-1.28,.95,;.05,1.75,;1.39,1,;1.41,-.55,;2.76,-1.3,;3.43,-.15,;2,.51,;2.7,1.8,;4.06,1.05,;4.08,-.5,;-2.53,1.7,;-2.59,3.25,;-3.95,3.97,;-5.25,3.15,;-6.55,3.86,;-7.82,3.11,;-7.8,1.61,;-6.49,.89,;-5.19,1.61,;-3.83,.88,;-3.79,-.66,;-2.43,-1.39,;-2.38,-2.93,;-3.69,-3.75,;-5.06,-3.02,;-5.1,-1.48,)| Show InChI InChI=1S/C23H32N2O2/c26-23(27-21-16-24-13-10-18(21)11-14-24)25-15-12-17-6-4-5-9-20(17)22(25)19-7-2-1-3-8-19/h4-6,9,18-19,21-22H,1-3,7-8,10-16H2/t21-,22?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for rat salivary gland muscarinic acetylcholine receptor M3 using [3H]N-methylscopolamine |
J Med Chem 48: 6597-606 (2005)
Article DOI: 10.1021/jm050099q
BindingDB Entry DOI: 10.7270/Q2NP257C |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50175581

(Benzhydryl-carbamic acid 1-aza-bicyclo[2.2.2]oct-3...)Show SMILES O=C(NC(c1ccccc1)c1ccccc1)OC1CN2CCC1CC2 |(1.67,-1.57,;1.69,-.03,;.37,.76,;-.93,.04,;-2.2,.81,;-2.19,2.29,;-3.45,3.05,;-4.76,2.33,;-4.78,.84,;-3.5,.09,;-.95,-1.5,;-2.31,-2.25,;-2.33,-3.79,;-1,-4.59,;.35,-3.83,;.36,-2.29,;3.05,.72,;4.37,-.08,;4.33,-1.62,;5.66,-2.41,;6.36,-1.29,;4.96,-.59,;5.71,.67,;7.03,-.12,;6.99,-1.67,)| Show InChI InChI=1S/C21H24N2O2/c24-21(25-19-15-23-13-11-16(19)12-14-23)22-20(17-7-3-1-4-8-17)18-9-5-2-6-10-18/h1-10,16,19-20H,11-15H2,(H,22,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for rat heart muscarinic acetylcholine receptor M2 using [3H]quinuclidinyl benzilate |
J Med Chem 48: 6597-606 (2005)
Article DOI: 10.1021/jm050099q
BindingDB Entry DOI: 10.7270/Q2NP257C |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50261052
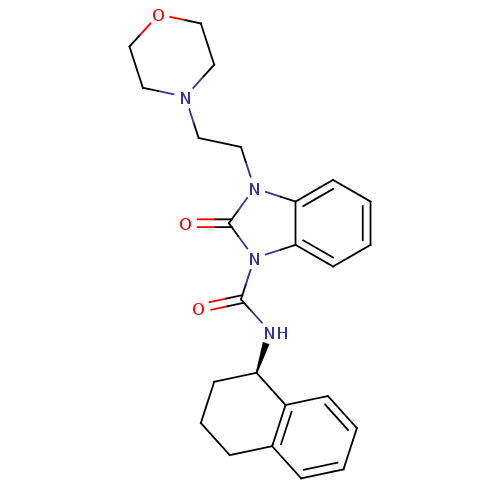
((R)-3-(2-morpholinoethyl)-2-oxo-N-(1,2,3,4-tetrahy...)Show SMILES O=C(N[C@@H]1CCCc2ccccc12)n1c2ccccc2n(CCN2CCOCC2)c1=O |r| Show InChI InChI=1S/C24H28N4O3/c29-23(25-20-9-5-7-18-6-1-2-8-19(18)20)28-22-11-4-3-10-21(22)27(24(28)30)13-12-26-14-16-31-17-15-26/h1-4,6,8,10-11,20H,5,7,9,12-17H2,(H,25,29)/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human CB2 receptor |
Bioorg Med Chem Lett 18: 3310-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.032
BindingDB Entry DOI: 10.7270/Q2VD70C3 |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor
(Mus musculus) | BDBM50350362
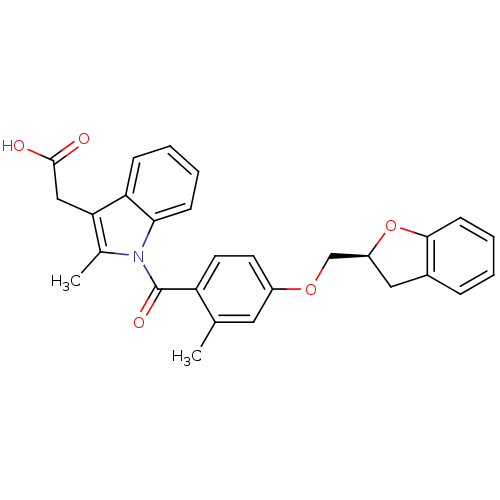
(CHEMBL1813284)Show SMILES Cc1c(CC(O)=O)c2ccccc2n1C(=O)c1ccc(OC[C@@H]2Cc3ccccc3O2)cc1C |r| Show InChI InChI=1S/C28H25NO5/c1-17-13-20(33-16-21-14-19-7-3-6-10-26(19)34-21)11-12-22(17)28(32)29-18(2)24(15-27(30)31)23-8-4-5-9-25(23)29/h3-13,21H,14-16H2,1-2H3,(H,30,31)/t21-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]-PGD2 from mouse DP receptor expressed in CHO cells after 20 mins by liquid scintillation counting |
Bioorg Med Chem 19: 4574-88 (2011)
Article DOI: 10.1016/j.bmc.2011.06.014
BindingDB Entry DOI: 10.7270/Q2FB5398 |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor
(Mus musculus) | BDBM50351489

(CHEMBL1819614)Show SMILES CN1C[C@@H](COc2ccc(C(=O)Nc3cc(CC(O)=O)ccc3Cl)c(C)c2)Oc2ccccc12 |r| Show InChI InChI=1S/C26H25ClN2O5/c1-16-11-18(33-15-19-14-29(2)23-5-3-4-6-24(23)34-19)8-9-20(16)26(32)28-22-12-17(13-25(30)31)7-10-21(22)27/h3-12,19H,13-15H2,1-2H3,(H,28,32)(H,30,31)/t19-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-PGD2 from mouse DP receptor expressed in CHO cells after 20 mins by liquid scintillation counting |
Bioorg Med Chem 19: 6935-48 (2011)
Article DOI: 10.1016/j.bmc.2011.08.065
BindingDB Entry DOI: 10.7270/Q2K35V22 |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor
(Mus musculus) | BDBM50350349
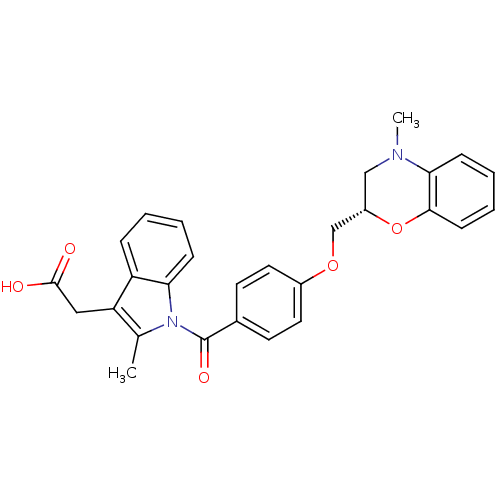
(CHEMBL1813115)Show SMILES CN1C[C@@H](COc2ccc(cc2)C(=O)n2c(C)c(CC(O)=O)c3ccccc23)Oc2ccccc12 |r| Show InChI InChI=1S/C28H26N2O5/c1-18-23(15-27(31)32)22-7-3-4-8-24(22)30(18)28(33)19-11-13-20(14-12-19)34-17-21-16-29(2)25-9-5-6-10-26(25)35-21/h3-14,21H,15-17H2,1-2H3,(H,31,32)/t21-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]-PGD2 from mouse DP receptor expressed in CHO cells after 20 mins by liquid scintillation counting |
Bioorg Med Chem 19: 4574-88 (2011)
Article DOI: 10.1016/j.bmc.2011.06.014
BindingDB Entry DOI: 10.7270/Q2FB5398 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50261051

(3-(2-morpholinoethyl)-N-(naphthalen-1-yl)-2-oxo-2,...)Show SMILES O=C(Nc1cccc2ccccc12)n1c2ccccc2n(CCN2CCOCC2)c1=O Show InChI InChI=1S/C24H24N4O3/c29-23(25-20-9-5-7-18-6-1-2-8-19(18)20)28-22-11-4-3-10-21(22)27(24(28)30)13-12-26-14-16-31-17-15-26/h1-11H,12-17H2,(H,25,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human CB2 receptor |
Bioorg Med Chem Lett 18: 3310-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.032
BindingDB Entry DOI: 10.7270/Q2VD70C3 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50260753
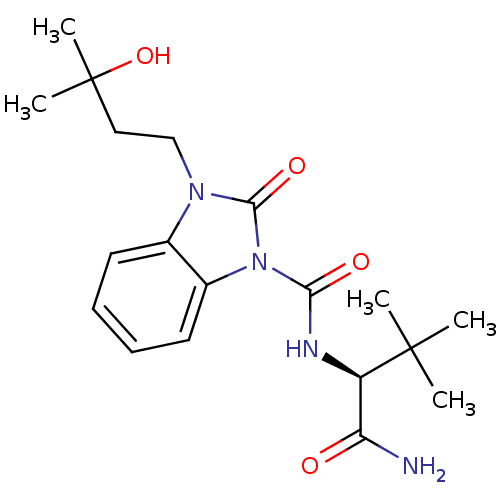
((S)-N-(1-amino-3,3-dimethyl-1-oxobutan-2-yl)-3-(3-...)Show SMILES CC(C)(O)CCn1c2ccccc2n(C(=O)N[C@H](C(N)=O)C(C)(C)C)c1=O |r| Show InChI InChI=1S/C19H28N4O4/c1-18(2,3)14(15(20)24)21-16(25)23-13-9-7-6-8-12(13)22(17(23)26)11-10-19(4,5)27/h6-9,14,27H,10-11H2,1-5H3,(H2,20,24)(H,21,25)/t14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human CB2 receptor |
Bioorg Med Chem Lett 18: 3310-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.032
BindingDB Entry DOI: 10.7270/Q2VD70C3 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50175574

((S)-1-Thiophen-3-yl-3,4-dihydro-1H-isoquinoline-2-...)Show SMILES O=C(O[C@H]1CN2CCC1CC2)N1CCc2ccccc2C1c1ccsc1 |wD:3.2,(.51,-2.2,;.49,-.66,;1.8,.13,;3.16,-.61,;3.17,-2.17,;4.51,-2.92,;5.18,-1.77,;3.76,-1.12,;4.46,.18,;5.82,-.58,;5.84,-2.12,;-.76,.07,;-.81,1.61,;-2.16,2.35,;-3.47,1.52,;-4.76,2.23,;-6.03,1.47,;-6.01,-.01,;-4.7,-.73,;-3.41,-.02,;-2.05,-.73,;-2.01,-2.27,;-3.24,-3.22,;-2.7,-4.67,;-1.16,-4.6,;-.74,-3.13,)| Show InChI InChI=1S/C21H24N2O2S/c24-21(25-19-13-22-9-5-16(19)6-10-22)23-11-7-15-3-1-2-4-18(15)20(23)17-8-12-26-14-17/h1-4,8,12,14,16,19-20H,5-7,9-11,13H2/t19-,20?/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for rat cortex muscarinic acetylcholine receptor M1 using [3H]pirenzepine |
J Med Chem 48: 6597-606 (2005)
Article DOI: 10.1021/jm050099q
BindingDB Entry DOI: 10.7270/Q2NP257C |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50175567
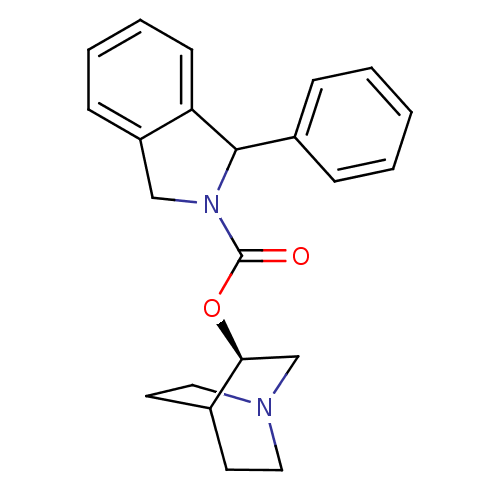
(1-Phenyl-1,3-dihydro-isoindole-2-carboxylic acid (...)Show SMILES O=C(O[C@H]1CN2CCC1CC2)N1Cc2ccccc2C1c1ccccc1 |wD:3.2,TLB:2:3:7.6:9.10,(17.05,5.78,;16.28,4.44,;17.05,3.1,;18.6,3.1,;19.37,1.77,;20.91,1.77,;20.93,3.09,;19.37,2.96,;19.36,4.43,;20.9,4.43,;21.68,3.1,;14.74,4.43,;13.83,5.67,;12.37,5.21,;11.04,5.98,;9.69,5.21,;9.69,3.66,;11.04,2.89,;12.37,3.66,;13.83,3.18,;14.15,1.68,;15.63,1.21,;15.95,-.29,;14.81,-1.34,;13.35,-.85,;13.02,.65,)| Show InChI InChI=1S/C22H24N2O2/c25-22(26-20-15-23-12-10-16(20)11-13-23)24-14-18-8-4-5-9-19(18)21(24)17-6-2-1-3-7-17/h1-9,16,20-21H,10-15H2/t20-,21?/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 8.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for rat cortex muscarinic acetylcholine receptor M1 using [3H]pirenzepine |
J Med Chem 48: 6597-606 (2005)
Article DOI: 10.1021/jm050099q
BindingDB Entry DOI: 10.7270/Q2NP257C |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50370683

(CHEMBL1169543)Show SMILES O=C(O[C@H]1CN2CCC1CC2)N1CCc2ccccc2[C@H]1c1ccccc1 |r,wD:20.24,3.2,THB:2:3:7.6:9.10,(-1.33,3.85,;-1.33,2.31,;-2.67,1.54,;-4,2.31,;-4.18,3.72,;-5.76,3.08,;-7.14,3.75,;-6.88,2.32,;-5.51,1.68,;-5.61,0,;-6.05,1.14,;,1.54,;;1.33,-.77,;2.67,,;4,-.77,;5.33,,;5.33,1.54,;4,2.31,;2.67,1.54,;1.33,2.31,;1.33,3.85,;2.67,4.62,;2.67,6.16,;1.33,6.93,;,6.16,;,4.62,)| Show InChI InChI=1S/C23H26N2O2/c26-23(27-21-16-24-13-10-18(21)11-14-24)25-15-12-17-6-4-5-9-20(17)22(25)19-7-2-1-3-8-19/h1-9,18,21-22H,10-16H2/t21-,22+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for rat heart muscarinic acetylcholine receptor M2 using [3H]quinuclidinyl benzilate |
J Med Chem 48: 6597-606 (2005)
Article DOI: 10.1021/jm050099q
BindingDB Entry DOI: 10.7270/Q2NP257C |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50261099

((R)-1-(2-morpholinoethyl)-2-oxo-N-(1,2,3,4-tetrahy...)Show SMILES O=C(N[C@@H]1CCCc2ccccc12)N1Cc2ccccc2N(CCN2CCOCC2)C1=O |r| Show InChI InChI=1S/C25H30N4O3/c30-24(26-22-10-5-8-19-6-1-3-9-21(19)22)29-18-20-7-2-4-11-23(20)28(25(29)31)13-12-27-14-16-32-17-15-27/h1-4,6-7,9,11,22H,5,8,10,12-18H2,(H,26,30)/t22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human CB2 receptor |
Bioorg Med Chem Lett 18: 3310-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.032
BindingDB Entry DOI: 10.7270/Q2VD70C3 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50175569
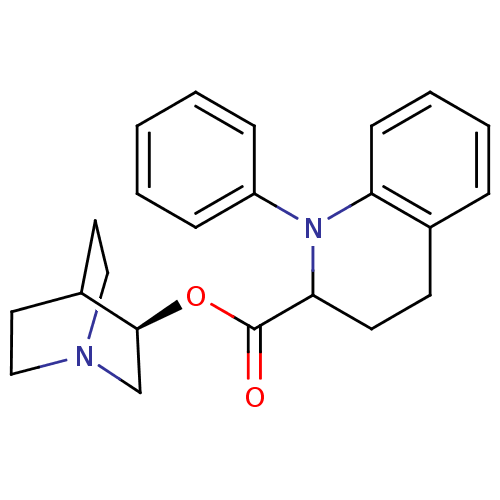
(1-Phenyl-1,2,3,4-tetrahydro-quinoline-2-carboxylic...)Show SMILES O=C(O[C@H]1CN2CCC1CC2)C1CCc2ccccc2N1c1ccccc1 |wD:3.2,(13.54,-1.45,;13.56,.09,;14.91,.84,;16.24,.05,;16.21,-1.51,;17.53,-2.3,;18.24,-1.18,;16.84,-.48,;17.59,.8,;18.91,-.01,;18.89,-1.56,;12.23,.88,;12.25,2.38,;10.97,3.16,;9.67,2.43,;8.4,3.18,;7.09,2.45,;7.06,.98,;8.35,.2,;9.65,.93,;10.93,.16,;10.9,-1.38,;12.23,-2.17,;12.2,-3.71,;10.84,-4.48,;9.52,-3.68,;9.54,-2.13,)| Show InChI InChI=1S/C23H26N2O2/c26-23(27-22-16-24-14-12-18(22)13-15-24)21-11-10-17-6-4-5-9-20(17)25(21)19-7-2-1-3-8-19/h1-9,18,21-22H,10-16H2/t21?,22-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 9.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for rat cortex muscarinic acetylcholine receptor M1 using [3H]pirenzepine |
J Med Chem 48: 6597-606 (2005)
Article DOI: 10.1021/jm050099q
BindingDB Entry DOI: 10.7270/Q2NP257C |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data