

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50422828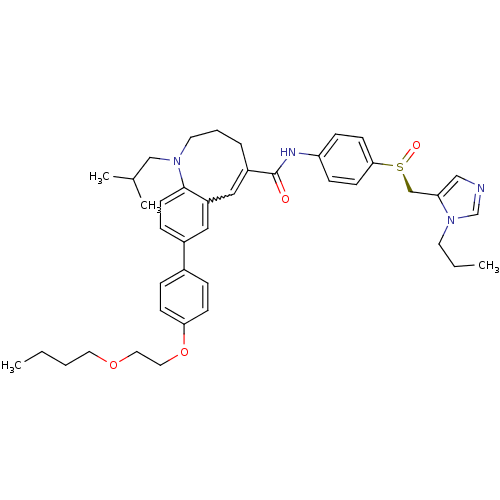 (CENICRIVIROC | CENICRIVIROC MESYLATE | TAK-652 | T...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of membrane fusion between HIV1 JR-FL Env-expressing COS7 cells and MOLT4/CCR5 | J Med Chem 49: 2037-48 (2006) Article DOI: 10.1021/jm0509703 BindingDB Entry DOI: 10.7270/Q23N2303 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitin synthase 1 (Candida albicans) | BDBM50089536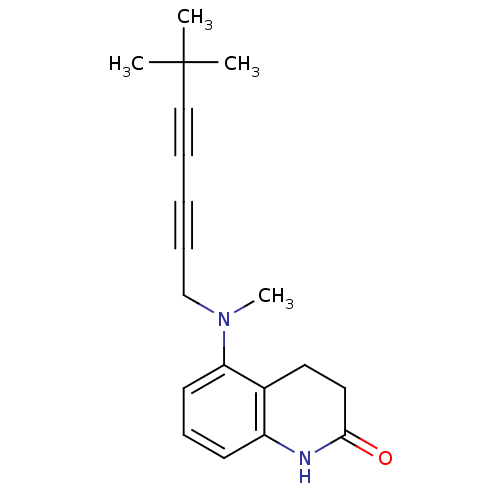 (5-[(6,6-Dimethyl-hepta-2,4-diynyl)-methyl-amino]-3...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center Curated by ChEMBL | Assay Description Inhibitory of Candida albicans chitin synthase 1 | Bioorg Med Chem Lett 10: 1459-62 (2000) BindingDB Entry DOI: 10.7270/Q2R210MK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50184396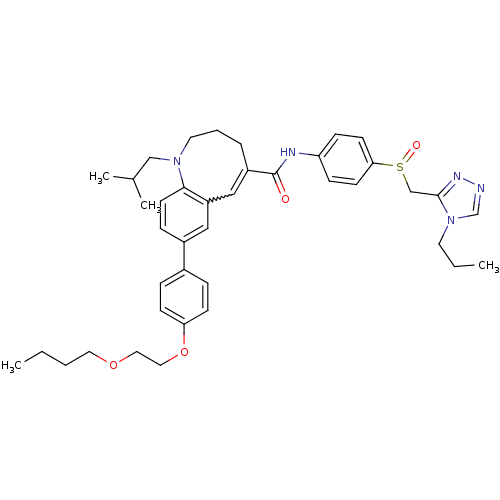 ((S)-(-)-8-{4-[2-(butoxy)ethoxy]phenyl}-1-isobutyl-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of membrane fusion between HIV1 JR-FL Env-expressing COS7 cells and MOLT4/CCR5 | J Med Chem 49: 2037-48 (2006) Article DOI: 10.1021/jm0509703 BindingDB Entry DOI: 10.7270/Q23N2303 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50422828 (CENICRIVIROC | CENICRIVIROC MESYLATE | TAK-652 | T...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of replication of R5 HIV1 Ba-L in MOLT4/CCR5 cells | J Med Chem 49: 2037-48 (2006) Article DOI: 10.1021/jm0509703 BindingDB Entry DOI: 10.7270/Q23N2303 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50184399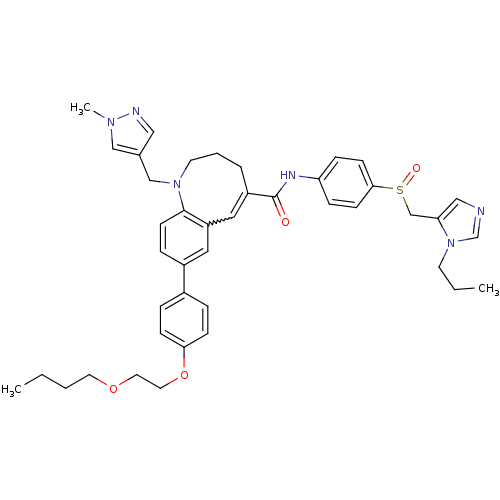 ((S)-(-)-8-{4-[2-(butoxy)ethoxy]phenyl}-1-[(1-methy...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of membrane fusion between HIV1 JR-FL Env-expressing COS7 cells and MOLT4/CCR5 | J Med Chem 49: 2037-48 (2006) Article DOI: 10.1021/jm0509703 BindingDB Entry DOI: 10.7270/Q23N2303 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50184398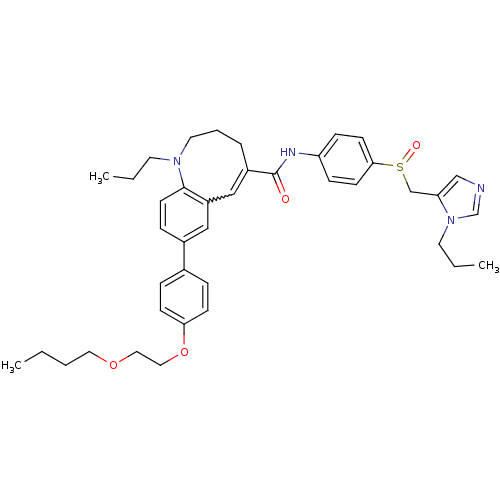 ((S)-(-)-8-{4-[2-(butoxy)ethoxy]phenyl}-1-propyl-N-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of membrane fusion between HIV1 JR-FL Env-expressing COS7 cells and MOLT4/CCR5 | J Med Chem 49: 2037-48 (2006) Article DOI: 10.1021/jm0509703 BindingDB Entry DOI: 10.7270/Q23N2303 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitin synthase 1 (Candida albicans) | BDBM50089557 (5-[Butyl-((E)-6,6-dimethyl-hept-2-en-4-ynyl)-amino...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center Curated by ChEMBL | Assay Description Inhibitory of Candida albicans chitin synthase 1 | Bioorg Med Chem Lett 10: 1459-62 (2000) BindingDB Entry DOI: 10.7270/Q2R210MK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50184400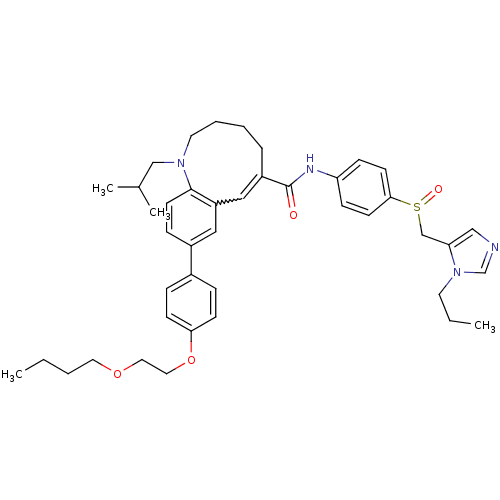 ((S)-(-)-9-{4-[2-(butoxy)ethoxy]phenyl}-1-isobutyl-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of membrane fusion between HIV1 JR-FL Env-expressing COS7 cells and MOLT4/CCR5 | J Med Chem 49: 2037-48 (2006) Article DOI: 10.1021/jm0509703 BindingDB Entry DOI: 10.7270/Q23N2303 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitin synthase 1 (Candida albicans) | BDBM50089551 (5-[((E)-6,6-Dimethyl-hept-2-en-4-ynyl)-propyl-amin...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center Curated by ChEMBL | Assay Description Inhibitory of Candida albicans chitin synthase 1 | Bioorg Med Chem Lett 10: 1459-62 (2000) BindingDB Entry DOI: 10.7270/Q2R210MK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitin synthase 1 (Candida albicans) | BDBM50089540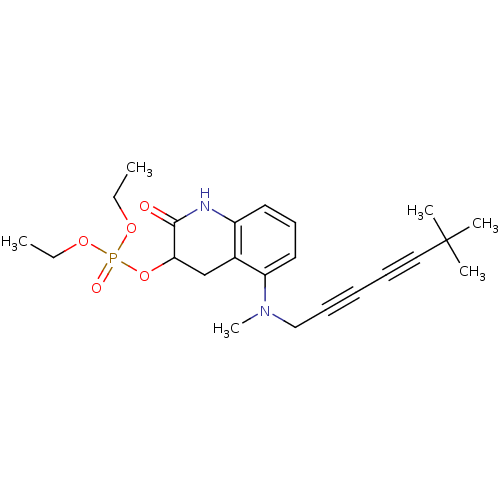 (CHEMBL32125 | Phosphoric acid 5-[(6,6-dimethyl-hep...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center Curated by ChEMBL | Assay Description Inhibitory of Candida albicans chitin synthase 1 | Bioorg Med Chem Lett 10: 1459-62 (2000) BindingDB Entry DOI: 10.7270/Q2R210MK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50068091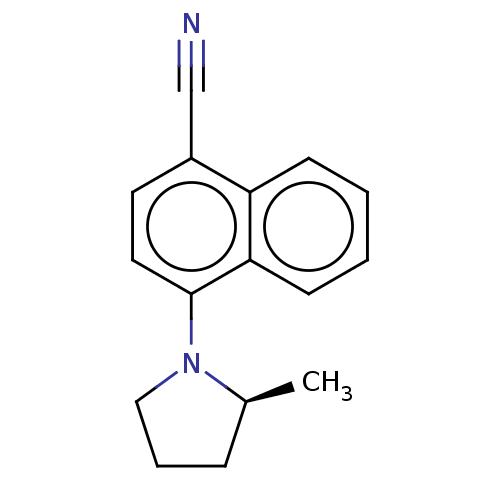 (CHEMBL3402221) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Displacement of [3H]mibolerone from androgen receptor (unknown origin) after 3 hrs | Bioorg Med Chem 23: 2568-78 (2015) Article DOI: 10.1016/j.bmc.2015.03.032 BindingDB Entry DOI: 10.7270/Q2JM2C94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitin synthase 1 (Candida albicans) | BDBM50089563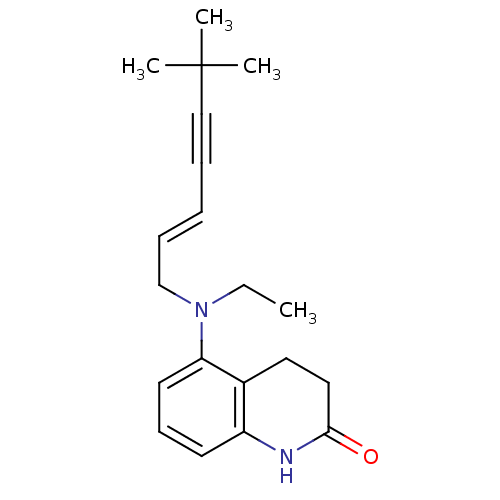 (5-[((E)-6,6-Dimethyl-hept-2-en-4-ynyl)-ethyl-amino...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center Curated by ChEMBL | Assay Description Inhibitory of Candida albicans chitin synthase 1 | Bioorg Med Chem Lett 10: 1459-62 (2000) BindingDB Entry DOI: 10.7270/Q2R210MK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50184402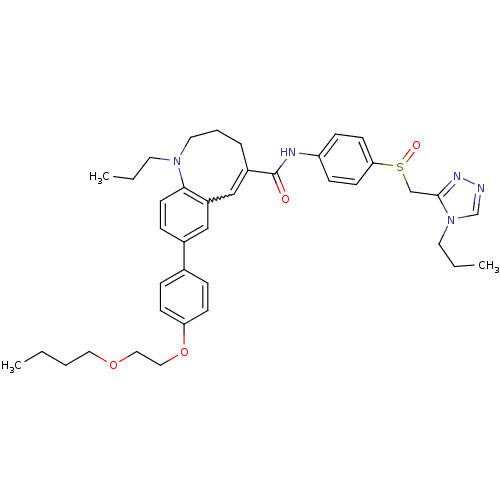 ((S)-(-)-8-{4-[2-(butoxy)ethoxy]phenyl}-1-propyl-N-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of membrane fusion between HIV1 JR-FL Env-expressing COS7 cells and MOLT4/CCR5 | J Med Chem 49: 2037-48 (2006) Article DOI: 10.1021/jm0509703 BindingDB Entry DOI: 10.7270/Q23N2303 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50184403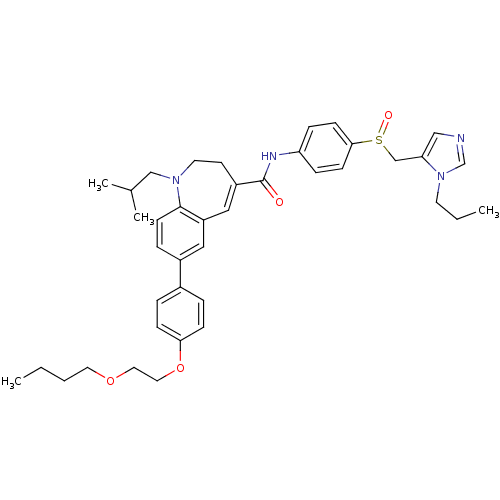 (7-(4-(2-butoxyethoxy)phenyl)-1-isobutyl-N-(4-((1-p...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of membrane fusion between HIV1 JR-FL Env-expressing COS7 cells and MOLT4/CCR5 | J Med Chem 49: 2037-48 (2006) Article DOI: 10.1021/jm0509703 BindingDB Entry DOI: 10.7270/Q23N2303 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50068096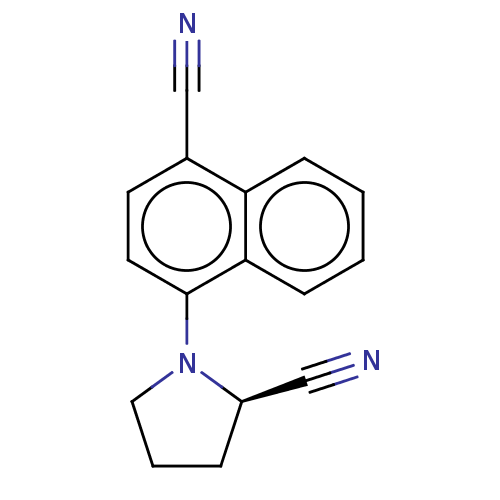 (CHEMBL3402227) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Displacement of [3H]mibolerone from androgen receptor (unknown origin) after 3 hrs | Bioorg Med Chem 23: 2568-78 (2015) Article DOI: 10.1016/j.bmc.2015.03.032 BindingDB Entry DOI: 10.7270/Q2JM2C94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitin synthase 1 (Candida albicans) | BDBM50089538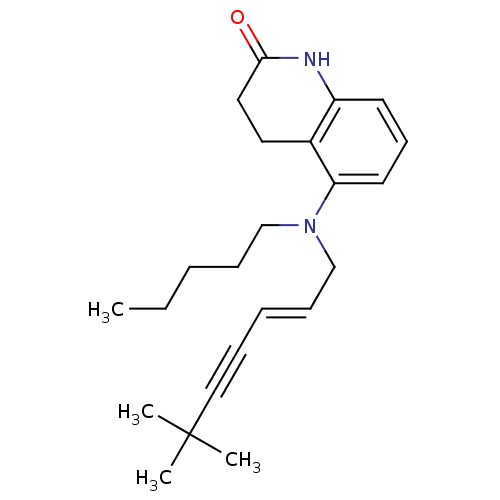 (5-[((E)-6,6-Dimethyl-hept-2-en-4-ynyl)-pentyl-amin...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center Curated by ChEMBL | Assay Description Inhibitory of Candida albicans chitin synthase 1 | Bioorg Med Chem Lett 10: 1459-62 (2000) BindingDB Entry DOI: 10.7270/Q2R210MK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50068093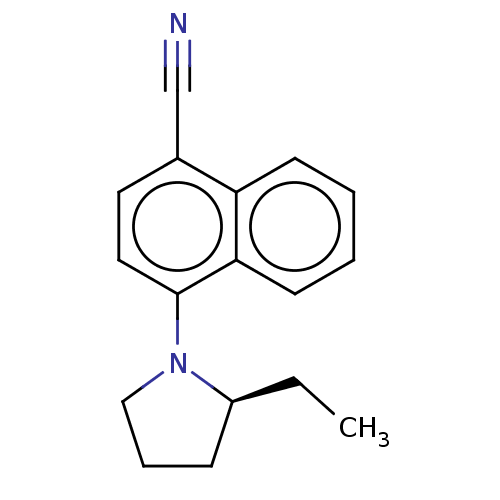 (CHEMBL3402224) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Displacement of [3H]mibolerone from androgen receptor (unknown origin) after 3 hrs | Bioorg Med Chem 23: 2568-78 (2015) Article DOI: 10.1016/j.bmc.2015.03.032 BindingDB Entry DOI: 10.7270/Q2JM2C94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitin synthase 1 (Candida albicans) | BDBM50089559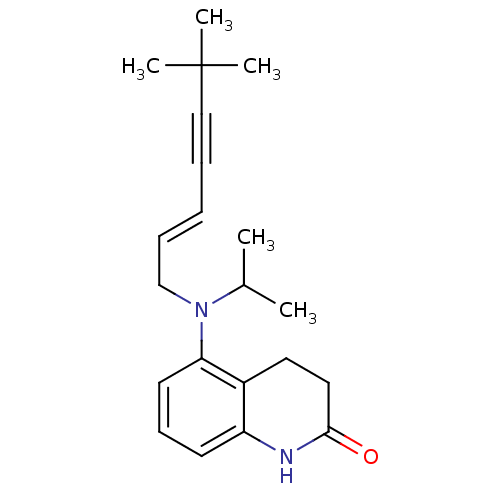 (5-[((E)-6,6-Dimethyl-hept-2-en-4-ynyl)-isopropyl-a...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center Curated by ChEMBL | Assay Description Inhibitory of Candida albicans chitin synthase 1 | Bioorg Med Chem Lett 10: 1459-62 (2000) BindingDB Entry DOI: 10.7270/Q2R210MK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50068101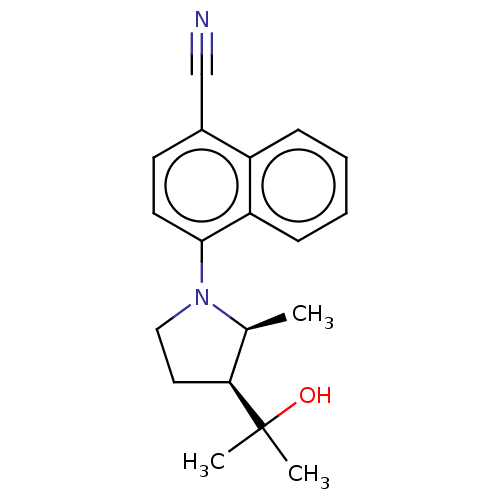 (CHEMBL3402232) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Displacement of [3H]mibolerone from androgen receptor (unknown origin) after 3 hrs | Bioorg Med Chem 23: 2568-78 (2015) Article DOI: 10.1016/j.bmc.2015.03.032 BindingDB Entry DOI: 10.7270/Q2JM2C94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50088301 ((E)-N,N-dimethyl-N-(4-(2-p-tolyl-6,7-dihydro-5H-be...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]RANTES from CCR5 expressed in CHO cells | J Med Chem 49: 2037-48 (2006) Article DOI: 10.1021/jm0509703 BindingDB Entry DOI: 10.7270/Q23N2303 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50068094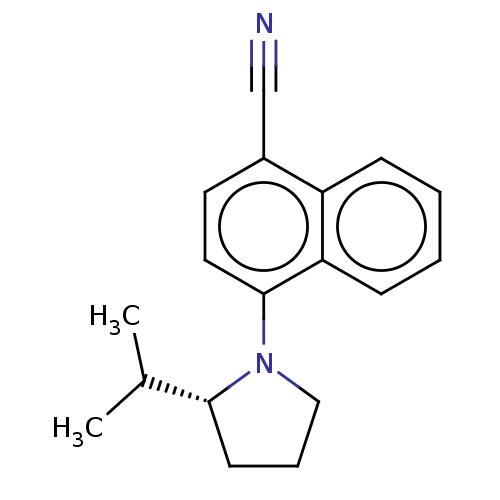 (CHEMBL3402225) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Displacement of [3H]mibolerone from androgen receptor (unknown origin) after 3 hrs | Bioorg Med Chem 23: 2568-78 (2015) Article DOI: 10.1016/j.bmc.2015.03.032 BindingDB Entry DOI: 10.7270/Q2JM2C94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50088321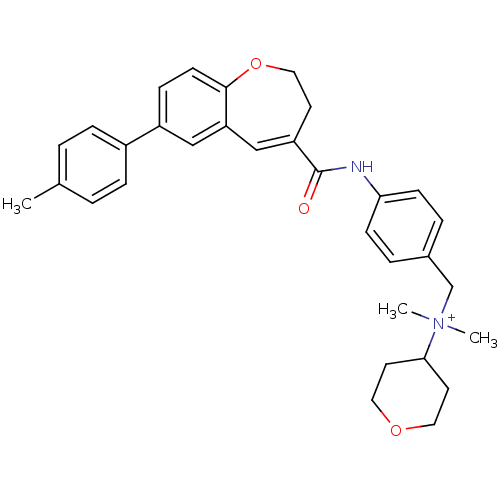 (CHEMBL292548 | Dimethyl-(tetrahydro-pyran-4-yl)-{4...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibitory effect on Chemokine binding to C-C chemokine receptor type 5 using [125I]-RANTES | J Med Chem 43: 2049-63 (2000) BindingDB Entry DOI: 10.7270/Q26D5S75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50088301 ((E)-N,N-dimethyl-N-(4-(2-p-tolyl-6,7-dihydro-5H-be...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibitory effect on Chemokine binding to C-C chemokine receptor type 5 using [125I]-RANTES | J Med Chem 43: 2049-63 (2000) BindingDB Entry DOI: 10.7270/Q26D5S75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50184398 ((S)-(-)-8-{4-[2-(butoxy)ethoxy]phenyl}-1-propyl-N-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of replication of R5 HIV1 Ba-L in MOLT4/CCR5 cells | J Med Chem 49: 2037-48 (2006) Article DOI: 10.1021/jm0509703 BindingDB Entry DOI: 10.7270/Q23N2303 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50088301 ((E)-N,N-dimethyl-N-(4-(2-p-tolyl-6,7-dihydro-5H-be...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of membrane fusion between HIV1 JR-FL Env-expressing COS7 cells and MOLT4/CCR5 | J Med Chem 49: 2037-48 (2006) Article DOI: 10.1021/jm0509703 BindingDB Entry DOI: 10.7270/Q23N2303 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50088302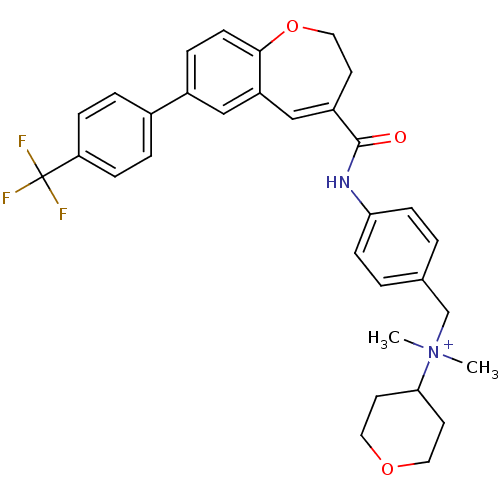 (CHEMBL56565 | Dimethyl-(tetrahydro-pyran-4-yl)-(4-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibitory effect on Chemokine binding to C-C chemokine receptor type 5 using [125I]-RANTES | J Med Chem 43: 2049-63 (2000) BindingDB Entry DOI: 10.7270/Q26D5S75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50184399 ((S)-(-)-8-{4-[2-(butoxy)ethoxy]phenyl}-1-[(1-methy...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]RANTES from CCR5 expressed in CHO cells | J Med Chem 49: 2037-48 (2006) Article DOI: 10.1021/jm0509703 BindingDB Entry DOI: 10.7270/Q23N2303 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50184398 ((S)-(-)-8-{4-[2-(butoxy)ethoxy]phenyl}-1-propyl-N-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]RANTES from CCR5 expressed in CHO cells | J Med Chem 49: 2037-48 (2006) Article DOI: 10.1021/jm0509703 BindingDB Entry DOI: 10.7270/Q23N2303 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50088322 ((4-{[7-(4-Ethoxy-phenyl)-2,3-dihydro-benzo[b]oxepi...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibitory effect on Chemokine binding to C-C chemokine receptor type 5 using [125I]-RANTES | J Med Chem 43: 2049-63 (2000) BindingDB Entry DOI: 10.7270/Q26D5S75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50088301 ((E)-N,N-dimethyl-N-(4-(2-p-tolyl-6,7-dihydro-5H-be...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibitory effect on Chemokine binding to C-C chemokine receptor type 5 using [125I]-RANTES | J Med Chem 43: 2049-63 (2000) BindingDB Entry DOI: 10.7270/Q26D5S75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50184403 (7-(4-(2-butoxyethoxy)phenyl)-1-isobutyl-N-(4-((1-p...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]RANTES from CCR5 expressed in CHO cells | J Med Chem 49: 2037-48 (2006) Article DOI: 10.1021/jm0509703 BindingDB Entry DOI: 10.7270/Q23N2303 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50184400 ((S)-(-)-9-{4-[2-(butoxy)ethoxy]phenyl}-1-isobutyl-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of replication of R5 HIV1 Ba-L in MOLT4/CCR5 cells | J Med Chem 49: 2037-48 (2006) Article DOI: 10.1021/jm0509703 BindingDB Entry DOI: 10.7270/Q23N2303 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane A2 receptor (Homo sapiens (Human)) | BDBM50228336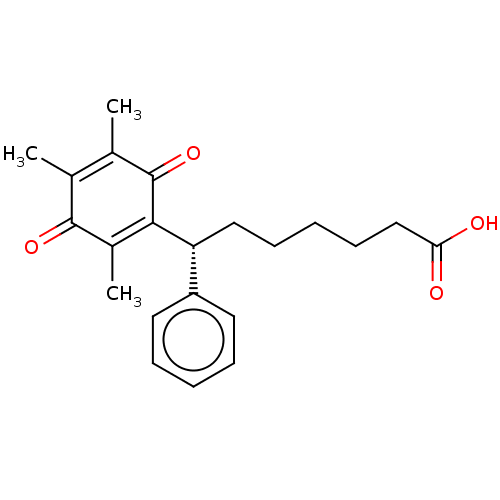 (CHEMBL71879) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of Escherichia coli dihydrofolate reductase. | J Med Chem 32: 2214-21 (1989) BindingDB Entry DOI: 10.7270/Q2WH2P0W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50068097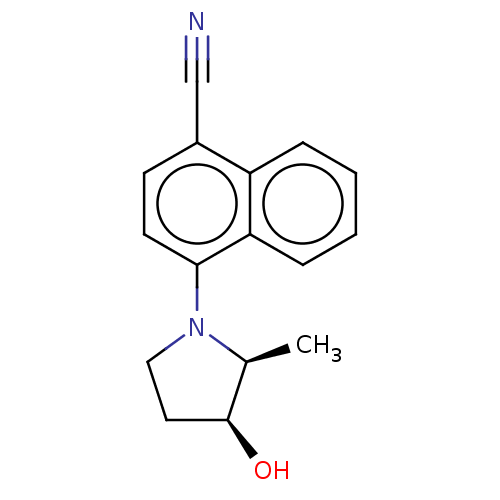 (CHEMBL3402228) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Displacement of [3H]mibolerone from androgen receptor (unknown origin) after 3 hrs | Bioorg Med Chem 23: 2568-78 (2015) Article DOI: 10.1016/j.bmc.2015.03.032 BindingDB Entry DOI: 10.7270/Q2JM2C94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50184402 ((S)-(-)-8-{4-[2-(butoxy)ethoxy]phenyl}-1-propyl-N-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]RANTES from CCR5 expressed in CHO cells | J Med Chem 49: 2037-48 (2006) Article DOI: 10.1021/jm0509703 BindingDB Entry DOI: 10.7270/Q23N2303 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50088319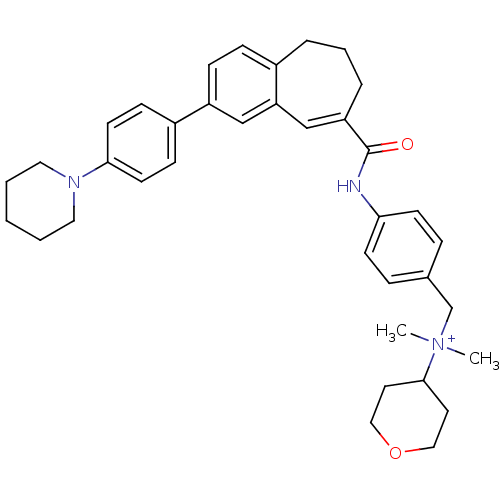 (CHEMBL62339 | Dimethyl-(4-{[3-(4-piperidin-1-yl-ph...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibitory effect on Chemokine binding to C-C chemokine receptor type 5 using [125I]-RANTES | J Med Chem 43: 2049-63 (2000) BindingDB Entry DOI: 10.7270/Q26D5S75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50184399 ((S)-(-)-8-{4-[2-(butoxy)ethoxy]phenyl}-1-[(1-methy...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of replication of R5 HIV1 Ba-L in MOLT4/CCR5 cells | J Med Chem 49: 2037-48 (2006) Article DOI: 10.1021/jm0509703 BindingDB Entry DOI: 10.7270/Q23N2303 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50184396 ((S)-(-)-8-{4-[2-(butoxy)ethoxy]phenyl}-1-isobutyl-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]RANTES from CCR5 expressed in CHO cells | J Med Chem 49: 2037-48 (2006) Article DOI: 10.1021/jm0509703 BindingDB Entry DOI: 10.7270/Q23N2303 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50068098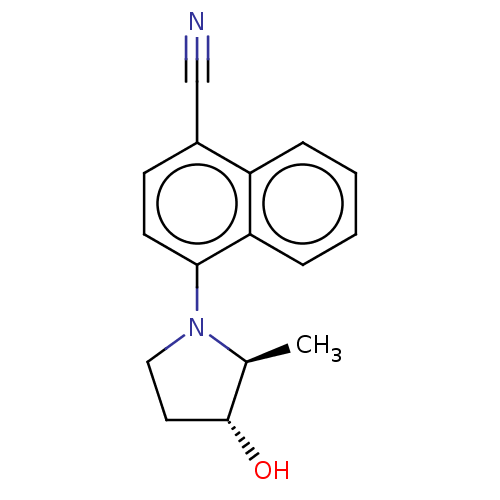 (CHEMBL3402229) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Displacement of [3H]mibolerone from androgen receptor (unknown origin) after 3 hrs | Bioorg Med Chem 23: 2568-78 (2015) Article DOI: 10.1016/j.bmc.2015.03.032 BindingDB Entry DOI: 10.7270/Q2JM2C94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50415098 (CHEMBL570898) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Displacement of [3H]mibolerone from androgen receptor (unknown origin) after 3 hrs | Bioorg Med Chem 23: 2568-78 (2015) Article DOI: 10.1016/j.bmc.2015.03.032 BindingDB Entry DOI: 10.7270/Q2JM2C94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50415097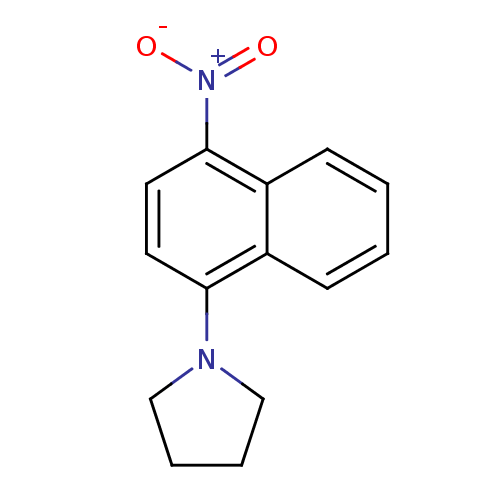 (CHEMBL570897) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Displacement of [3H]mibolerone from androgen receptor (unknown origin) after 3 hrs | Bioorg Med Chem 23: 2568-78 (2015) Article DOI: 10.1016/j.bmc.2015.03.032 BindingDB Entry DOI: 10.7270/Q2JM2C94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitin synthase 1 (Candida albicans) | BDBM50089543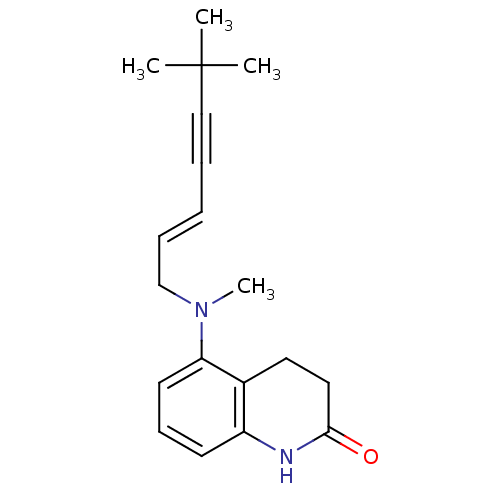 (5-[((E)-6,6-Dimethyl-hept-2-en-4-ynyl)-methyl-amin...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center Curated by ChEMBL | Assay Description Inhibitory of Candida albicans chitin synthase 1 | Bioorg Med Chem Lett 10: 1459-62 (2000) BindingDB Entry DOI: 10.7270/Q2R210MK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitin synthase 1 (Candida albicans) | BDBM50089547 (5-[(6,6-Dimethyl-hepta-2,4-diynyl)-methyl-amino]-3...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center Curated by ChEMBL | Assay Description Inhibitory of Candida albicans chitin synthase 1 | Bioorg Med Chem Lett 10: 1459-62 (2000) BindingDB Entry DOI: 10.7270/Q2R210MK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50422828 (CENICRIVIROC | CENICRIVIROC MESYLATE | TAK-652 | T...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]RANTES from CCR5 expressed in CHO cells | J Med Chem 49: 2037-48 (2006) Article DOI: 10.1021/jm0509703 BindingDB Entry DOI: 10.7270/Q23N2303 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50088311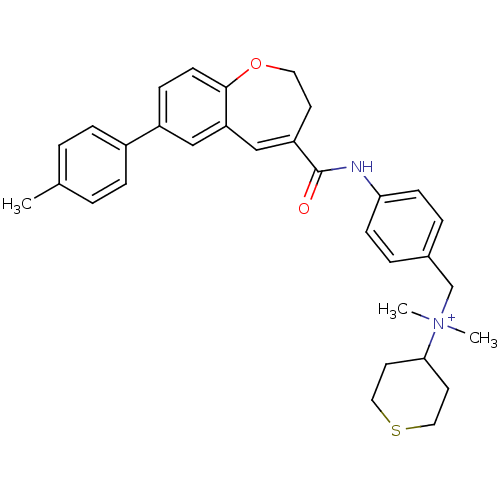 (CHEMBL61208 | Dimethyl-(tetrahydro-thiopyran-4-yl)...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibitory effect on Chemokine binding to C-C chemokine receptor type 5 using [125I]-RANTES | J Med Chem 43: 2049-63 (2000) BindingDB Entry DOI: 10.7270/Q26D5S75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50088306 ((1-Ethyl-propyl)-dimethyl-{4-[(7-p-tolyl-2,3-dihyd...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibitory effect on Chemokine binding to C-C chemokine receptor type 5 using [125I]-RANTES | J Med Chem 43: 2049-63 (2000) BindingDB Entry DOI: 10.7270/Q26D5S75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50088312 (CHEMBL62180 | Dimethyl-(4-{[3-(4-pyrrolidin-1-yl-p...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibitory effect on Chemokine binding to C-C chemokine receptor type 5 using [125I]-RANTES | J Med Chem 43: 2049-63 (2000) BindingDB Entry DOI: 10.7270/Q26D5S75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50068100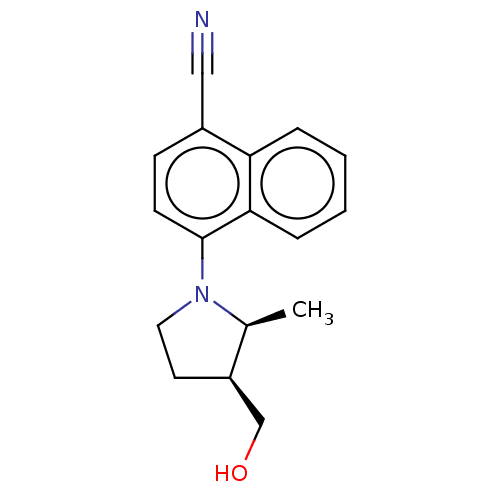 (CHEMBL3402231) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Displacement of [3H]mibolerone from androgen receptor (unknown origin) after 3 hrs | Bioorg Med Chem 23: 2568-78 (2015) Article DOI: 10.1016/j.bmc.2015.03.032 BindingDB Entry DOI: 10.7270/Q2JM2C94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane A2 receptor (Homo sapiens (Human)) | BDBM50005097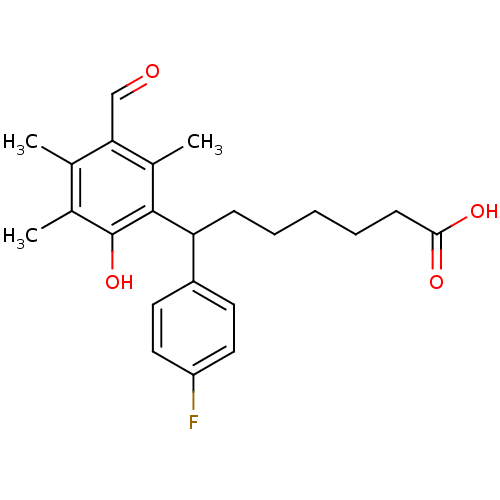 (7-(4-Fluoro-phenyl)-7-(3-formyl-6-hydroxy-2,4,5-tr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Compound was evaluated for its ability to inhibit specific binding of [3H]-U-46,619 to Thromboxane A2/ Prostaglandin H2 receptor in guinea pig platel... | J Med Chem 35: 2202-9 (1992) BindingDB Entry DOI: 10.7270/Q2JQ0ZZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50088325 (CHEMBL62598 | Dimethyl-(4-oxo-cyclohexyl)-{4-[(7-p...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibitory effect on Chemokine binding to C-C chemokine receptor type 5 using [125I]-RANTES | J Med Chem 43: 2049-63 (2000) BindingDB Entry DOI: 10.7270/Q26D5S75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 184 total ) | Next | Last >> |