 Found 54212 hits with Last Name = 'son' and Initial = 'e'
Found 54212 hits with Last Name = 'son' and Initial = 'e' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50042235

(2-butyl-3-{[2'-(1H-tetrazol-5-yl)[1,1'-biphenyl]-4...)Show SMILES CCCCC1=NC2(CCCC2)C(=O)N1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 |t:4| Show InChI InChI=1S/C25H28N6O/c1-2-3-10-22-26-25(15-6-7-16-25)24(32)31(22)17-18-11-13-19(14-12-18)20-8-4-5-9-21(20)23-27-29-30-28-23/h4-5,8-9,11-14H,2-3,6-7,10,15-17H2,1H3,(H,27,28,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >0.000100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity towards human Endothelin A receptor |
J Med Chem 45: 3829-35 (2002)
BindingDB Entry DOI: 10.7270/Q2Z60NDM |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50091105

(4''-Oxazol-2-yl-biphenyl-2-sulfonic acid (3,4-dime...)Show SMILES Cc1noc(NS(=O)(=O)c2ccccc2-c2ccc(cc2)-c2ncco2)c1C Show InChI InChI=1S/C20H17N3O4S/c1-13-14(2)22-27-19(13)23-28(24,25)18-6-4-3-5-17(18)15-7-9-16(10-8-15)20-21-11-12-26-20/h3-12,23H,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >0.000100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity towards rat Angiotensin II receptor, type 1 |
J Med Chem 45: 3829-35 (2002)
BindingDB Entry DOI: 10.7270/Q2Z60NDM |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50091105

(4''-Oxazol-2-yl-biphenyl-2-sulfonic acid (3,4-dime...)Show SMILES Cc1noc(NS(=O)(=O)c2ccccc2-c2ccc(cc2)-c2ncco2)c1C Show InChI InChI=1S/C20H17N3O4S/c1-13-14(2)22-27-19(13)23-28(24,25)18-6-4-3-5-17(18)15-7-9-16(10-8-15)20-21-11-12-26-20/h3-12,23H,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >0.000100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity towards human Angiotensin II receptor, type 1 |
J Med Chem 45: 3829-35 (2002)
BindingDB Entry DOI: 10.7270/Q2Z60NDM |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50091105

(4''-Oxazol-2-yl-biphenyl-2-sulfonic acid (3,4-dime...)Show SMILES Cc1noc(NS(=O)(=O)c2ccccc2-c2ccc(cc2)-c2ncco2)c1C Show InChI InChI=1S/C20H17N3O4S/c1-13-14(2)22-27-19(13)23-28(24,25)18-6-4-3-5-17(18)15-7-9-16(10-8-15)20-21-11-12-26-20/h3-12,23H,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >0.000100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity towards human Angiotensin II receptor, type 1 |
J Med Chem 45: 3829-35 (2002)
BindingDB Entry DOI: 10.7270/Q2Z60NDM |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50091105

(4''-Oxazol-2-yl-biphenyl-2-sulfonic acid (3,4-dime...)Show SMILES Cc1noc(NS(=O)(=O)c2ccccc2-c2ccc(cc2)-c2ncco2)c1C Show InChI InChI=1S/C20H17N3O4S/c1-13-14(2)22-27-19(13)23-28(24,25)18-6-4-3-5-17(18)15-7-9-16(10-8-15)20-21-11-12-26-20/h3-12,23H,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >0.000100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity towards rat Angiotensin II receptor, type 1 |
J Med Chem 45: 3829-35 (2002)
BindingDB Entry DOI: 10.7270/Q2Z60NDM |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50042235

(2-butyl-3-{[2'-(1H-tetrazol-5-yl)[1,1'-biphenyl]-4...)Show SMILES CCCCC1=NC2(CCCC2)C(=O)N1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 |t:4| Show InChI InChI=1S/C25H28N6O/c1-2-3-10-22-26-25(15-6-7-16-25)24(32)31(22)17-18-11-13-19(14-12-18)20-8-4-5-9-21(20)23-27-29-30-28-23/h4-5,8-9,11-14H,2-3,6-7,10,15-17H2,1H3,(H,27,28,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >0.000100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity towards human Endothelin A receptor |
J Med Chem 45: 3829-35 (2002)
BindingDB Entry DOI: 10.7270/Q2Z60NDM |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Rattus norvegicus) | BDBM50470113
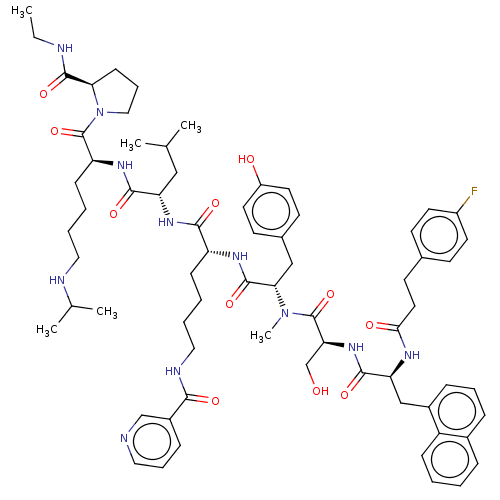
(CHEMBL439134)Show SMILES CCNC(=O)[C@H]1CCCN1C(=O)[C@H](CCCCNC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](CCCCNC(=O)c1cccnc1)NC(=O)[C@H](Cc1ccc(O)cc1)N(C)C(=O)[C@H](CO)NC(=O)[C@H](Cc1cccc2ccccc12)NC(=O)CCc1ccc(F)cc1 Show InChI InChI=1S/C69H92FN11O11/c1-7-72-66(89)59-24-16-38-81(59)69(92)55(23-11-12-36-73-45(4)5)77-64(87)56(39-44(2)3)78-63(86)54(22-10-13-37-74-62(85)50-20-15-35-71-42-50)76-67(90)60(40-47-27-32-52(83)33-28-47)80(6)68(91)58(43-82)79-65(88)57(41-49-19-14-18-48-17-8-9-21-53(48)49)75-61(84)34-29-46-25-30-51(70)31-26-46/h8-9,14-15,17-21,25-28,30-33,35,42,44-45,54-60,73,82-83H,7,10-13,16,22-24,29,34,36-41,43H2,1-6H3,(H,72,89)(H,74,85)(H,75,84)(H,76,90)(H,77,87)(H,78,86)(H,79,88)/t54-,55+,56+,57+,58+,59-,60+/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TAP Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of the in vitro binding of [125I]-labeled leuprolide to the rat pituitary luteinizing hormone releasing hormone (LHRH) receptor. |
J Med Chem 37: 701-5 (1994)
Article DOI: 10.1021/jm00031a021
BindingDB Entry DOI: 10.7270/Q2MG7S7B |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50039635
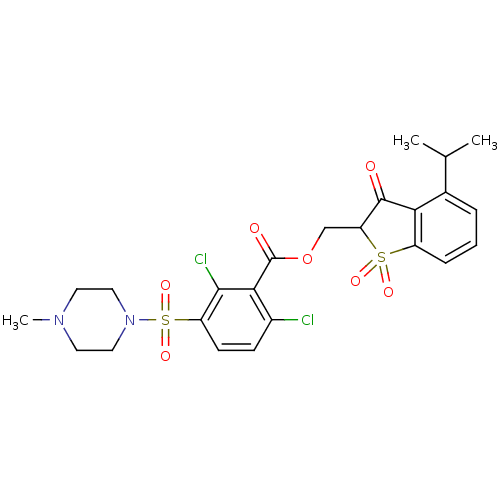
(2,6-Dichloro-3-(4-methyl-piperazine-1-sulfonyl)-be...)Show SMILES CC(C)c1cccc2c1C(=O)C(COC(=O)c1c(Cl)ccc(c1Cl)S(=O)(=O)N1CCN(C)CC1)S2(=O)=O Show InChI InChI=1S/C24H26Cl2N2O7S2/c1-14(2)15-5-4-6-17-20(15)23(29)19(36(17,31)32)13-35-24(30)21-16(25)7-8-18(22(21)26)37(33,34)28-11-9-27(3)10-12-28/h4-8,14,19H,9-13H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceutical Research Division
Curated by ChEMBL
| Assay Description
In vitro inhibition constant of human leukocyte elastase. |
J Med Chem 37: 2623-6 (1994)
BindingDB Entry DOI: 10.7270/Q2ST7QGN |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50036480

(2,6-Dichloro-3-(2-morpholin-4-yl-ethoxy)-benzoic a...)Show SMILES CC(C)c1cccc2c1C(=O)N(COC(=O)c1c(Cl)ccc(OCCN3CCOCC3)c1Cl)S2(=O)=O Show InChI InChI=1S/C24H26Cl2N2O7S/c1-15(2)16-4-3-5-19-20(16)23(29)28(36(19,31)32)14-35-24(30)21-17(25)6-7-18(22(21)26)34-13-10-27-8-11-33-12-9-27/h3-7,15H,8-14H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Inc.
Curated by ChEMBL
| Assay Description
Potency of inhibition against human leukocyte elastase (HLE) expressed as an apparent binding constant |
J Med Chem 38: 739-44 (1995)
BindingDB Entry DOI: 10.7270/Q2W66JTD |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50039646
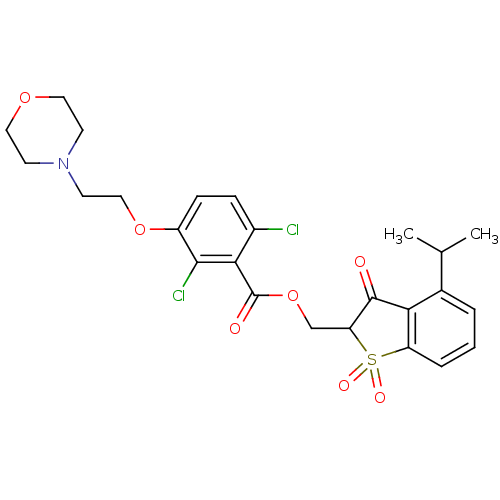
(2,6-Dichloro-3-(2-morpholin-4-yl-ethoxy)-benzoic a...)Show SMILES CC(C)c1cccc2c1C(=O)C(COC(=O)c1c(Cl)ccc(OCCN3CCOCC3)c1Cl)S2(=O)=O Show InChI InChI=1S/C25H27Cl2NO7S/c1-15(2)16-4-3-5-19-21(16)24(29)20(36(19,31)32)14-35-25(30)22-17(26)6-7-18(23(22)27)34-13-10-28-8-11-33-12-9-28/h3-7,15,20H,8-14H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceutical Research Division
Curated by ChEMBL
| Assay Description
In vitro inhibition constant of human leukocyte elastase. |
J Med Chem 37: 2623-6 (1994)
BindingDB Entry DOI: 10.7270/Q2ST7QGN |
More data for this
Ligand-Target Pair | |
Progonadoliberin-1
(RAT) | BDBM84726
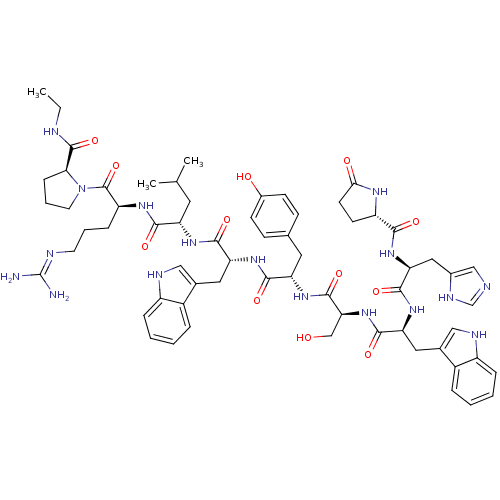
(deslorelin)Show SMILES CCNC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H]1CCC(=O)N1 |r,wU:31.44,23.28,12.20,57.63,63.79,5.4,77.90,87.93,wD:45.57,(-8.25,5.5,;-8.21,4.01,;-6.91,3.28,;-6.91,1.76,;-5.56,.97,;-8.21,.86,;-9.55,1.3,;-10.59,.25,;-9.77,-1.09,;-8.21,-.6,;-6.97,-1.49,;-5.62,-.85,;-7.01,-3.06,;-8.43,-3.76,;-9.77,-2.88,;-11.13,-3.55,;-12.37,-2.75,;-13.77,-3.43,;-15.09,-2.58,;-13.89,-4.95,;-5.75,-3.91,;-4.4,-3.25,;-4.38,-1.66,;-3.12,-4.06,;-3.52,-5.56,;-2.46,-6.71,;-2.79,-8.25,;-.84,-7.09,;-1.7,-3.39,;-.45,-4.25,;-.33,-5.83,;.61,-3.28,;.76,-1.8,;-.23,-.66,;.18,.7,;-.73,1.73,;-2.18,1.46,;-3.12,2.52,;-4.51,2.21,;-4.98,.86,;-4.03,-.18,;-2.61,.1,;2.04,-3.86,;3.22,-2.91,;4.71,-3.45,;2.98,-1.4,;1.57,-.7,;1.7,.88,;.39,1.92,;.68,3.61,;2.13,4.32,;2.37,5.95,;3.43,3.26,;3.26,1.51,;4.09,-.39,;5.66,-.45,;6.38,.82,;6.32,-1.82,;5.59,-3.1,;6.26,-4.46,;7.87,-1.86,;8.66,-.54,;7.93,.8,;10.17,-.6,;10.89,-1.99,;10.45,-3.49,;9,-4.01,;9.07,-5.58,;10.52,-6,;11.2,-7.39,;12.75,-7.49,;13.62,-6.2,;12.93,-4.81,;11.38,-4.71,;10.95,.6,;10.29,1.94,;8.75,1.98,;11.11,3.26,;12.59,3.16,;13.35,1.86,;12.66,.45,;13.72,-.54,;15.09,.1,;14.84,1.64,;10.35,4.51,;8.83,4.51,;8.02,3.22,;8.02,5.91,;8.59,7.19,;7.54,8.25,;6.16,7.59,;4.77,8.19,;6.53,5.95,)| Show InChI InChI=1S/C64H83N17O12/c1-4-68-62(92)53-16-10-24-81(53)63(93)46(15-9-23-69-64(65)66)74-56(86)47(25-35(2)3)75-58(88)49(27-37-30-70-43-13-7-5-11-41(37)43)77-57(87)48(26-36-17-19-40(83)20-18-36)76-61(91)52(33-82)80-59(89)50(28-38-31-71-44-14-8-6-12-42(38)44)78-60(90)51(29-39-32-67-34-72-39)79-55(85)45-21-22-54(84)73-45/h5-8,11-14,17-20,30-32,34-35,45-53,70-71,82-83H,4,9-10,15-16,21-29,33H2,1-3H3,(H,67,72)(H,68,92)(H,73,84)(H,74,86)(H,75,88)(H,76,91)(H,77,87)(H,78,90)(H,79,85)(H,80,89)(H4,65,66,69)/t45-,46-,47-,48-,49+,50-,51-,52-,53-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Med Chem 36: 363-9 (1993)
Article DOI: 10.1021/jm00055a007
BindingDB Entry DOI: 10.7270/Q2GQ6W8T |
More data for this
Ligand-Target Pair | |
Progonadoliberin-1
(RAT) | BDBM84707

(nafarelin)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](Cc1ccc2ccccc2c1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H]1CCC(=O)N1)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N1CCC[C@H]1C(=O)NCC(N)=O |r,wU:41.56,65.70,23.34,74.80,88.96,wD:35.40,55.67,8.21,4.4,(4.31,-3.28,;5.62,-2.47,;6.98,-3.2,;5.58,-.92,;7.27,.05,;7.24,1.6,;5.89,2.38,;4.25,1.42,;5.89,3.78,;7.3,4.67,;8.68,4.11,;8.64,2.57,;9.96,1.77,;11.33,2.51,;12.66,1.72,;14.02,2.47,;14.04,4.01,;12.72,4.83,;11.36,4.07,;10.03,4.87,;4.65,4.7,;3.3,3.93,;3.3,2.31,;1.92,4.76,;1.92,6.36,;3.25,7.09,;4.54,6.36,;5.85,7.07,;5.85,8.62,;7.01,9.29,;4.55,9.38,;3.25,8.62,;.5,3.94,;-.84,4.7,;-.87,6.2,;-2.13,3.93,;-2.1,2.42,;-.79,1.7,;-3.45,4.64,;-4.69,3.8,;-4.62,2.07,;-6.14,4.56,;-6.14,6.66,;-7.38,7.5,;-7.54,9.01,;-9,9.32,;-9.76,8.03,;-11.24,7.72,;-11.7,6.27,;-10.72,5.17,;-9.23,5.47,;-8.74,6.92,;-7.5,3.63,;-8.71,4.41,;-8.64,5.92,;-9.94,3.67,;-9.94,2.17,;-8.64,1.41,;-7.14,1.48,;-6.6,.06,;-7.77,-.88,;-9.04,-.05,;-11.26,4.41,;-12.55,3.67,;-12.53,2.1,;-13.85,4.43,;-14.01,5.87,;-15.44,6.27,;-16.37,5.01,;-17.87,4.96,;-15.32,3.81,;8.64,-.67,;9.96,.12,;8.68,-2.21,;10,-2.98,;11.35,-2.2,;12.39,-3.01,;13.81,-2.21,;15.17,-3.2,;16.48,-2.17,;17.87,-2.85,;16.38,-.62,;10,-4.51,;8.68,-5.3,;11.36,-5.3,;12.78,-4.67,;13.81,-5.86,;13.05,-7.16,;11.52,-6.83,;10.37,-7.86,;10.7,-9.38,;8.9,-7.38,;7.75,-8.42,;6.28,-7.95,;5.95,-6.42,;5.11,-8.96,)| Show InChI InChI=1S/C66H83N17O13/c1-36(2)25-48(58(89)76-47(13-7-23-71-66(68)69)65(96)83-24-8-14-54(83)64(95)73-33-55(67)86)77-60(91)50(28-38-15-18-39-9-3-4-10-40(39)26-38)78-59(90)49(27-37-16-19-43(85)20-17-37)79-63(94)53(34-84)82-61(92)51(29-41-31-72-45-12-6-5-11-44(41)45)80-62(93)52(30-42-32-70-35-74-42)81-57(88)46-21-22-56(87)75-46/h3-6,9-12,15-20,26,31-32,35-36,46-54,72,84-85H,7-8,13-14,21-25,27-30,33-34H2,1-2H3,(H2,67,86)(H,70,74)(H,73,95)(H,75,87)(H,76,89)(H,77,91)(H,78,90)(H,79,94)(H,80,93)(H,81,88)(H,82,92)(H4,68,69,71)/t46-,47-,48-,49-,50+,51-,52-,53-,54-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Med Chem 36: 363-9 (1993)
Article DOI: 10.1021/jm00055a007
BindingDB Entry DOI: 10.7270/Q2GQ6W8T |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50039637
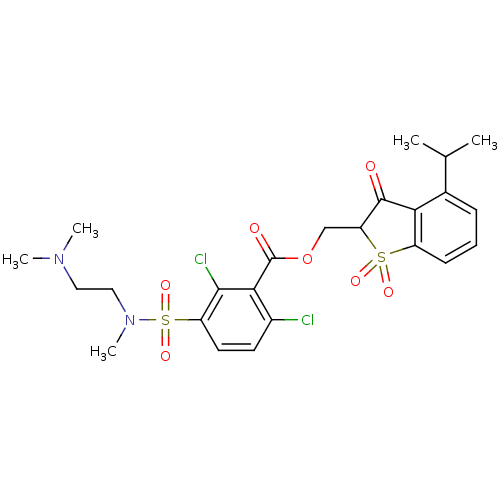
(2,6-Dichloro-3-[(2-dimethylamino-ethyl)-methyl-sul...)Show SMILES CC(C)c1cccc2c1C(=O)C(COC(=O)c1c(Cl)ccc(c1Cl)S(=O)(=O)N(C)CCN(C)C)S2(=O)=O Show InChI InChI=1S/C24H28Cl2N2O7S2/c1-14(2)15-7-6-8-17-20(15)23(29)19(36(17,31)32)13-35-24(30)21-16(25)9-10-18(22(21)26)37(33,34)28(5)12-11-27(3)4/h6-10,14,19H,11-13H2,1-5H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceutical Research Division
Curated by ChEMBL
| Assay Description
In vitro inhibition constant of human leukocyte elastase. |
J Med Chem 37: 2623-6 (1994)
BindingDB Entry DOI: 10.7270/Q2ST7QGN |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM23334

(3-(pyridin-3-yl)propyl (2S)-1-(3,3-dimethyl-2-oxop...)Show SMILES CCC(C)(C)C(=O)C(=O)N1CCC[C@H]1C(=O)OCCCc1cccnc1 |r| Show InChI InChI=1S/C20H28N2O4/c1-4-20(2,3)17(23)18(24)22-12-6-10-16(22)19(25)26-13-7-9-15-8-5-11-21-14-15/h5,8,11,14,16H,4,6-7,9-10,12-13H2,1-3H3/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Ability to inhibit peptidyl-prolyl isomerase (PPIase, or rotamase) activity of FK506 binding protein 12 |
Bioorg Med Chem Lett 12: 1429-33 (2002)
BindingDB Entry DOI: 10.7270/Q23J3C9S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Gonadotropin-releasing hormone receptor
(Rattus norvegicus) | BDBM50470098
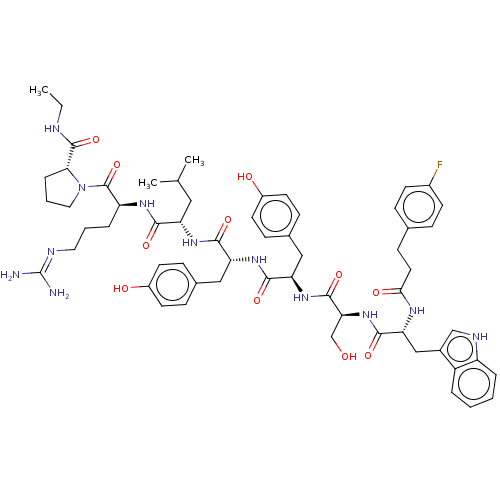
(CHEMBL2371296)Show SMILES CCNC(=O)[C@H]1CCCN1C(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](Cc1ccc(O)cc1)NC(=O)[C@@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(=O)CCc1ccc(F)cc1 |wU:55.60,43.54,12.20,wD:31.41,23.28,5.4,61.64,(-4.86,4.47,;-3.76,3.4,;-4.14,1.91,;-3.04,.83,;-1.56,1.25,;-3.42,-.66,;-4.85,-1.23,;-4.76,-2.77,;-3.26,-3.15,;-2.44,-1.85,;-.9,-1.75,;-.22,-.37,;-.05,-3.03,;-.73,-4.41,;-2.27,-4.51,;-2.95,-5.89,;-4.49,-5.99,;-5.17,-7.37,;-6.71,-7.47,;-4.32,-8.65,;1.49,-2.93,;2.34,-4.21,;1.66,-5.59,;3.88,-4.12,;4.56,-2.74,;3.71,-1.45,;2.17,-1.55,;4.39,-.07,;4.73,-5.4,;6.27,-5.3,;6.95,-3.92,;7.12,-6.58,;6.44,-7.96,;4.9,-8.06,;4.05,-6.78,;2.51,-6.88,;1.83,-8.26,;.29,-8.35,;2.68,-9.54,;4.22,-9.44,;8.66,-6.48,;9.51,-7.76,;8.83,-9.14,;11.05,-7.66,;11.73,-6.28,;10.88,-5,;11.56,-3.62,;10.71,-2.34,;9.17,-2.44,;8.32,-1.16,;8.49,-3.82,;9.34,-5.1,;11.9,-8.95,;13.44,-8.85,;14.12,-7.47,;14.29,-10.13,;13.61,-11.51,;12.07,-11.61,;15.83,-10.03,;16.69,-11.31,;16,-12.69,;18.22,-11.21,;19.08,-12.5,;20.61,-12.4,;21.6,-13.58,;23.03,-13.01,;22.93,-11.48,;24,-10.37,;23.59,-8.89,;22.1,-8.51,;21.02,-9.61,;21.44,-11.1,;18.91,-9.83,;18.05,-8.55,;16.51,-8.65,;18.73,-7.17,;17.88,-5.89,;18.56,-4.51,;17.71,-3.23,;18.39,-1.85,;19.93,-1.75,;20.61,-.37,;20.78,-3.03,;20.1,-4.41,)| Show InChI InChI=1S/C60H77FN12O11/c1-4-64-58(83)51-12-8-28-73(51)59(84)45(11-7-27-65-60(62)63)68-53(78)46(29-35(2)3)69-54(79)47(30-37-15-22-41(75)23-16-37)70-55(80)48(31-38-17-24-42(76)25-18-38)71-57(82)50(34-74)72-56(81)49(32-39-33-66-44-10-6-5-9-43(39)44)67-52(77)26-19-36-13-20-40(61)21-14-36/h5-6,9-10,13-18,20-25,33,35,45-51,66,74-76H,4,7-8,11-12,19,26-32,34H2,1-3H3,(H,64,83)(H,67,77)(H,68,78)(H,69,79)(H,70,80)(H,71,82)(H,72,81)(H4,62,63,65)/t45-,46-,47+,48+,49+,50-,51+/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TAP Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of the in vitro binding of [125I]-labeled leuprolide to the rat pituitary luteinizing hormone releasing hormone (LHRH) receptor. |
J Med Chem 37: 701-5 (1994)
Article DOI: 10.1021/jm00031a021
BindingDB Entry DOI: 10.7270/Q2MG7S7B |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50036478

(3-Carboxymethoxy-2,6-dichloro-benzoic acid 4-isopr...)Show SMILES COc1cc2c(C(=O)N(COC(=O)c3c(Cl)ccc(OCC(O)=O)c3Cl)S2(=O)=O)c(c1)C(C)C Show InChI InChI=1S/C21H19Cl2NO9S/c1-10(2)12-6-11(31-3)7-15-17(12)20(27)24(34(15,29)30)9-33-21(28)18-13(22)4-5-14(19(18)23)32-8-16(25)26/h4-7,10H,8-9H2,1-3H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Inc.
Curated by ChEMBL
| Assay Description
Potency of inhibition against human leukocyte elastase (HLE) expressed as an apparent binding constant |
J Med Chem 38: 739-44 (1995)
BindingDB Entry DOI: 10.7270/Q2W66JTD |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50036476

(2,6-Dichloro-3-(4-methyl-piperazine-1-sulfonyl)-be...)Show SMILES COc1cc2c(C(=O)N(COC(=O)c3c(Cl)ccc(c3Cl)S(=O)(=O)N3CCN(C)CC3)S2(=O)=O)c(c1)C(C)C Show InChI InChI=1S/C24H27Cl2N3O8S2/c1-14(2)16-11-15(36-4)12-19-20(16)23(30)29(39(19,34)35)13-37-24(31)21-17(25)5-6-18(22(21)26)38(32,33)28-9-7-27(3)8-10-28/h5-6,11-12,14H,7-10,13H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Inc.
Curated by ChEMBL
| Assay Description
Potency of inhibition against human leukocyte elastase (HLE) expressed as an apparent binding constant |
J Med Chem 38: 739-44 (1995)
BindingDB Entry DOI: 10.7270/Q2W66JTD |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Rattus norvegicus) | BDBM50470117
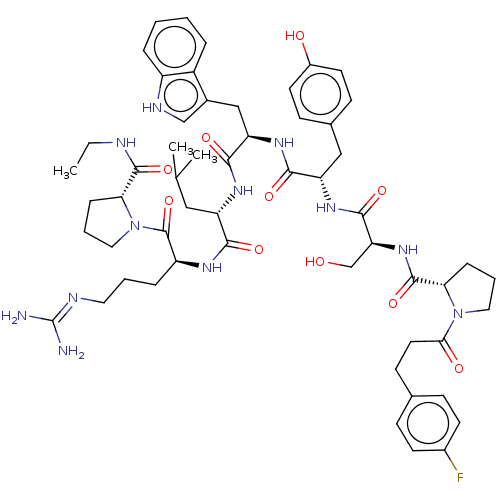
(CHEMBL264989)Show SMILES CCNC(=O)[C@H]1CCCN1C(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(=O)[C@@H]1CCCN1C(=O)CCc1ccc(F)cc1 |wU:57.63,12.20,wD:63.66,45.57,31.44,23.28,5.4,(20.41,-5.17,;20.43,-6.7,;19.11,-7.49,;17.76,-6.71,;16.46,-7.49,;17.73,-5.22,;18.94,-4.33,;18.43,-2.88,;16.89,-2.9,;16.47,-4.33,;15.14,-5.09,;15.14,-6.64,;13.8,-4.33,;13.82,-2.78,;15.15,-2.01,;15.18,-.48,;16.51,.28,;16.53,1.83,;17.86,2.58,;15.19,2.6,;12.48,-5.07,;11.15,-4.3,;11.16,-2.75,;9.81,-5.06,;9.79,-6.6,;11.13,-7.39,;12.45,-6.62,;11.09,-8.92,;8.47,-4.29,;7.13,-5.04,;7.13,-6.57,;5.81,-4.26,;5.84,-2.72,;5.36,-1.27,;3.9,-.78,;3.9,.76,;5.36,1.25,;6,2.63,;7.52,2.79,;8.44,1.55,;7.8,.13,;6.26,-.01,;4.49,-5.03,;3.14,-4.25,;3.14,-2.71,;1.8,-5,;1.8,-6.54,;3.13,-7.32,;4.46,-6.55,;5.8,-7.35,;5.78,-8.89,;7.1,-9.64,;4.45,-9.64,;3.1,-8.87,;.48,-4.22,;-.86,-4.97,;-.86,-6.52,;-2.18,-4.2,;-2.16,-2.67,;-.84,-1.91,;-3.51,-4.96,;-4.85,-4.19,;-4.83,-2.65,;-6.15,-4.93,;-6.43,-6.44,;-7.96,-6.64,;-8.62,-5.23,;-7.53,-4.17,;-8.3,-2.65,;-7.24,-1.36,;-9.62,-1.85,;-11.1,-2.72,;-12.62,-1.87,;-14.1,-2.74,;-15.57,-1.9,;-15.57,-.17,;-17.05,.76,;-14.1,.7,;-12.62,-.14,)| Show InChI InChI=1S/C56H75FN12O10/c1-4-60-53(77)46-13-9-27-69(46)55(79)41(12-7-25-61-56(58)59)63-49(73)42(28-33(2)3)64-51(75)44(30-36-31-62-40-11-6-5-10-39(36)40)66-50(74)43(29-35-17-22-38(71)23-18-35)65-52(76)45(32-70)67-54(78)47-14-8-26-68(47)48(72)24-19-34-15-20-37(57)21-16-34/h5-6,10-11,15-18,20-23,31,33,41-47,62,70-71H,4,7-9,12-14,19,24-30,32H2,1-3H3,(H,60,77)(H,63,73)(H,64,75)(H,65,76)(H,66,74)(H,67,78)(H4,58,59,61)/t41-,42-,43-,44+,45-,46+,47-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TAP Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of the in vitro binding of [125I]-labeled leuprolide to the rat pituitary luteinizing hormone releasing hormone (LHRH) receptor. |
J Med Chem 37: 701-5 (1994)
Article DOI: 10.1021/jm00031a021
BindingDB Entry DOI: 10.7270/Q2MG7S7B |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50036477

(2,6-Dichloro-3-(2-morpholin-4-yl-ethoxy)-benzoic a...)Show SMILES COc1cc2c(C(=O)N(COC(=O)c3c(Cl)ccc(OCCN4CCOCC4)c3Cl)S2(=O)=O)c(c1)C(C)C Show InChI InChI=1S/C25H28Cl2N2O8S/c1-15(2)17-12-16(34-3)13-20-21(17)24(30)29(38(20,32)33)14-37-25(31)22-18(26)4-5-19(23(22)27)36-11-8-28-6-9-35-10-7-28/h4-5,12-13,15H,6-11,14H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Inc.
Curated by ChEMBL
| Assay Description
Potency of inhibition against human leukocyte elastase (HLE) expressed as an apparent binding constant |
J Med Chem 38: 739-44 (1995)
BindingDB Entry DOI: 10.7270/Q2W66JTD |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50036481

(2,6-Dichloro-3-[(2-dimethylamino-ethyl)-methyl-sul...)Show SMILES COc1cc2c(C(=O)N(COC(=O)c3c(Cl)ccc(c3Cl)S(=O)(=O)N(C)CCN(C)C)S2(=O)=O)c(c1)C(C)C Show InChI InChI=1S/C24H29Cl2N3O8S2/c1-14(2)16-11-15(36-6)12-19-20(16)23(30)29(39(19,34)35)13-37-24(31)21-17(25)7-8-18(22(21)26)38(32,33)28(5)10-9-27(3)4/h7-8,11-12,14H,9-10,13H2,1-6H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Inc.
Curated by ChEMBL
| Assay Description
Potency of inhibition against human leukocyte elastase (HLE) expressed as an apparent binding constant |
J Med Chem 38: 739-44 (1995)
BindingDB Entry DOI: 10.7270/Q2W66JTD |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Rattus norvegicus) | BDBM50469862
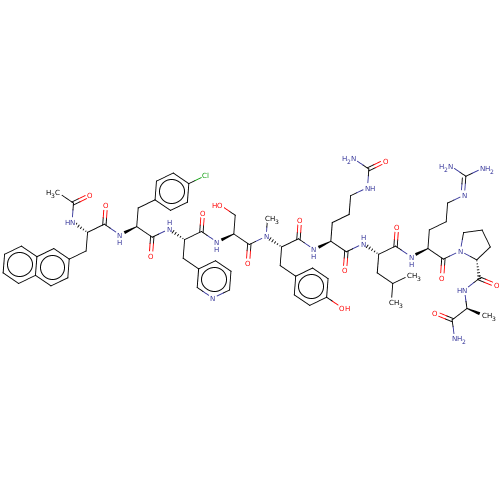
(CHEMBL409219)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#7]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7](-[#6])-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-c1cccnc1)-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(Cl)cc1)-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc2ccccc2c1)-[#7]-[#6](-[#6])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](-[#7])=O Show InChI InChI=1S/C71H94ClN17O14/c1-40(2)32-53(62(95)83-52(16-10-29-78-70(74)75)69(102)89-31-11-17-58(89)66(99)80-41(3)60(73)93)84-61(94)51(15-9-30-79-71(76)103)82-67(100)59(37-44-21-26-50(92)27-22-44)88(5)68(101)57(39-90)87-65(98)56(36-46-12-8-28-77-38-46)86-64(97)55(34-43-19-24-49(72)25-20-43)85-63(96)54(81-42(4)91)35-45-18-23-47-13-6-7-14-48(47)33-45/h6-8,12-14,18-28,33,38,40-41,51-59,90,92H,9-11,15-17,29-32,34-37,39H2,1-5H3,(H2,73,93)(H,80,99)(H,81,91)(H,82,100)(H,83,95)(H,84,94)(H,85,96)(H,86,97)(H,87,98)(H4,74,75,78)(H3,76,79,103)/t41-,51-,52-,53-,54-,55-,56-,57-,58+,59-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0135 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
The negative logarithm of the concentration of antagonist that inhibits 50% of the binding of 125 I-labeled leuprolide to the rat pituitary LHRH rece... |
J Med Chem 36: 928-33 (1993)
Article DOI: 10.1021/jm00059a020
BindingDB Entry DOI: 10.7270/Q2Z03BW7 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50036475

(2,6-Dichloro-3-(2-pyrrolidin-1-yl-ethoxy)-benzoic ...)Show SMILES COc1cc2c(C(=O)N(COC(=O)c3c(Cl)ccc(OCCN4CCCC4)c3Cl)S2(=O)=O)c(c1)C(C)C Show InChI InChI=1S/C25H28Cl2N2O7S/c1-15(2)17-12-16(34-3)13-20-21(17)24(30)29(37(20,32)33)14-36-25(31)22-18(26)6-7-19(23(22)27)35-11-10-28-8-4-5-9-28/h6-7,12-13,15H,4-5,8-11,14H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Inc.
Curated by ChEMBL
| Assay Description
Potency of inhibition against human leukocyte elastase (HLE) expressed as an apparent binding constant |
J Med Chem 38: 739-44 (1995)
BindingDB Entry DOI: 10.7270/Q2W66JTD |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Rattus norvegicus) | BDBM50470118
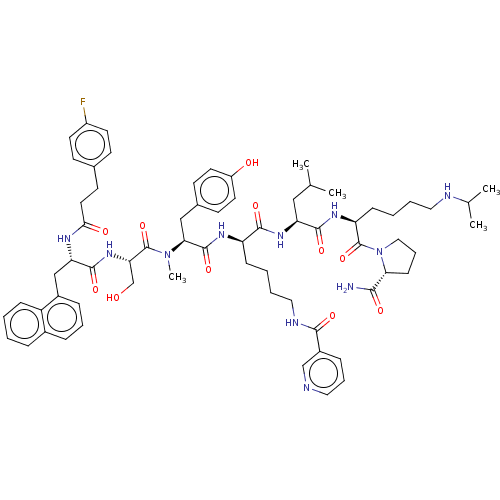
(CHEMBL412751)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](CCCCNC(=O)c1cccnc1)NC(=O)[C@H](Cc1ccc(O)cc1)N(C)C(=O)[C@H](CO)NC(=O)[C@H](Cc1cccc2ccccc12)NC(=O)CCc1ccc(F)cc1)C(=O)N[C@@H](CCCCNC(C)C)C(=O)N1CCC[C@@H]1C(N)=O Show InChI InChI=1S/C67H88FN11O11/c1-42(2)37-54(63(86)75-53(21-9-10-34-71-43(3)4)67(90)79-36-14-22-57(79)60(69)83)76-62(85)52(20-8-11-35-72-61(84)48-18-13-33-70-40-48)74-65(88)58(38-45-25-30-50(81)31-26-45)78(5)66(89)56(41-80)77-64(87)55(39-47-17-12-16-46-15-6-7-19-51(46)47)73-59(82)32-27-44-23-28-49(68)29-24-44/h6-7,12-13,15-19,23-26,28-31,33,40,42-43,52-58,71,80-81H,8-11,14,20-22,27,32,34-39,41H2,1-5H3,(H2,69,83)(H,72,84)(H,73,82)(H,74,88)(H,75,86)(H,76,85)(H,77,87)/t52-,53+,54+,55+,56+,57-,58+/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TAP Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of the in vitro binding of [125I]-labeled leuprolide to the rat pituitary luteinizing hormone releasing hormone (LHRH) receptor. |
J Med Chem 37: 701-5 (1994)
Article DOI: 10.1021/jm00031a021
BindingDB Entry DOI: 10.7270/Q2MG7S7B |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM570555
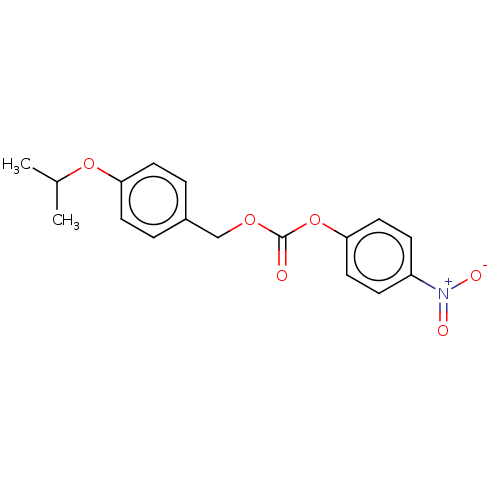
(US11440884, Example 18 | [4-(propan-2-yloxy)phenyl...)Show SMILES CC(C)Oc1ccc(COC(=O)Oc2ccc(cc2)[N+]([O-])=O)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
R-SAT's were generally performed with 50 ng/well of receptor and 20 ng/well of β-galactosidase plasmid DNA. All receptor constructs used wer... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZC864D |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Rattus norvegicus) | BDBM50470082

(CHEMBL408861)Show SMILES CCNC(=O)[C@H]1CCCN1C(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(=O)C(Cc1cccc2ccccc12)NC(=O)CCc1ccc(F)cc1 |wU:57.63,12.20,wD:45.57,31.44,23.28,5.4,(25.92,-4.76,;25.93,-6.28,;24.61,-7.08,;23.26,-6.31,;21.98,-7.08,;23.23,-4.8,;24.45,-3.92,;23.94,-2.49,;22.42,-2.49,;21.98,-3.93,;20.66,-4.67,;20.65,-6.22,;19.33,-3.92,;19.34,-2.38,;20.68,-1.62,;20.69,-.08,;22.03,.68,;22.04,2.21,;23.36,2.98,;20.71,2.99,;17.99,-4.67,;16.67,-3.9,;16.67,-2.36,;15.32,-4.64,;15.32,-6.19,;16.64,-6.98,;16.63,-8.5,;17.98,-6.21,;14,-3.87,;12.67,-4.63,;12.65,-6.15,;11.35,-3.86,;11.36,-2.33,;10.88,-.87,;9.43,-.39,;9.43,1.16,;10.9,1.63,;11.52,3.02,;13.06,3.18,;13.96,1.95,;13.33,.54,;11.78,.38,;10.01,-4.61,;8.68,-3.84,;8.68,-2.29,;7.34,-4.6,;7.34,-6.12,;8.66,-6.91,;8.65,-8.46,;9.98,-9.23,;11.32,-8.47,;12.64,-9.24,;11.33,-6.93,;10,-6.15,;6.02,-3.81,;4.7,-4.57,;4.67,-6.12,;3.36,-3.79,;3.38,-2.26,;4.71,-1.51,;2.03,-4.55,;.69,-3.77,;.72,-2.25,;-.64,-4.53,;-.64,-6.08,;.68,-6.88,;2.04,-6.14,;3.36,-6.93,;3.29,-8.47,;1.96,-9.21,;1.94,-10.75,;.59,-11.49,;-.73,-10.71,;-.71,-9.17,;.64,-8.43,;-1.96,-3.77,;-3.3,-4.51,;-3.28,-6.05,;-4.65,-3.77,;-6.1,-4.73,;-7.62,-3.96,;-7.72,-2.23,;-9.25,-1.46,;-10.7,-2.39,;-12.19,-1.55,;-10.58,-4.13,;-9.06,-4.9,)| Show InChI InChI=1S/C64H79FN12O10/c1-4-68-62(86)55-19-11-31-77(55)63(87)49(18-10-30-69-64(66)67)72-57(81)50(32-38(2)3)73-60(84)53(35-43-36-70-48-17-8-7-16-47(43)48)75-58(82)51(33-40-22-27-45(79)28-23-40)74-61(85)54(37-78)76-59(83)52(34-42-14-9-13-41-12-5-6-15-46(41)42)71-56(80)29-24-39-20-25-44(65)26-21-39/h5-9,12-17,20-23,25-28,36,38,49-55,70,78-79H,4,10-11,18-19,24,29-35,37H2,1-3H3,(H,68,86)(H,71,80)(H,72,81)(H,73,84)(H,74,85)(H,75,82)(H,76,83)(H4,66,67,69)/t49-,50-,51-,52?,53+,54-,55+/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TAP Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of the in vitro binding of [125I]-labeled leuprolide to the rat pituitary luteinizing hormone releasing hormone (LHRH) receptor. |
J Med Chem 37: 701-5 (1994)
Article DOI: 10.1021/jm00031a021
BindingDB Entry DOI: 10.7270/Q2MG7S7B |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Rattus norvegicus) | BDBM50470097

(CHEMBL262235)Show SMILES CCNC(=O)[C@H]1CCCN1C(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1cccc2ccccc12)NC(=O)CCc1ccc(F)cc1 |wU:57.63,12.20,wD:45.57,31.44,23.28,5.4,63.80,(30.87,-6.95,;30.88,-8.47,;29.57,-9.26,;28.22,-8.5,;26.93,-9.26,;28.18,-6.99,;29.41,-6.11,;28.89,-4.67,;27.37,-4.67,;26.93,-6.12,;25.61,-6.86,;25.6,-8.4,;24.28,-6.11,;24.29,-4.57,;25.63,-3.81,;25.64,-2.26,;26.98,-1.51,;26.99,.02,;28.31,.79,;25.66,.8,;22.94,-6.86,;21.62,-6.09,;21.62,-4.55,;20.27,-6.83,;20.27,-8.37,;21.59,-9.17,;21.58,-10.69,;22.93,-8.39,;18.95,-6.06,;17.62,-6.82,;17.6,-8.34,;16.31,-6.05,;16.32,-4.51,;15.83,-3.06,;14.39,-2.57,;14.39,-1.03,;15.85,-.56,;16.48,.84,;18.01,1,;18.91,-.24,;18.28,-1.65,;16.73,-1.81,;14.97,-6.8,;13.64,-6.02,;13.64,-4.48,;12.3,-6.79,;12.29,-8.31,;13.6,-9.1,;13.6,-10.62,;14.93,-11.42,;16.27,-10.66,;17.59,-11.43,;16.28,-9.11,;14.95,-8.34,;10.98,-5.99,;9.65,-6.75,;9.63,-8.31,;8.31,-5.98,;8.33,-4.45,;9.66,-3.7,;6.98,-6.73,;5.64,-5.96,;5.67,-4.44,;4.31,-6.72,;4.31,-8.27,;5.63,-9.07,;6.99,-8.33,;8.31,-9.11,;8.24,-10.66,;6.91,-11.4,;6.89,-12.94,;5.54,-13.68,;4.22,-12.9,;4.25,-11.35,;5.6,-10.61,;2.99,-5.96,;1.64,-6.7,;1.72,-8.21,;.26,-6.02,;-1.15,-7.02,;-2.7,-6.31,;-2.88,-4.61,;-4.43,-3.9,;-5.84,-4.9,;-7.36,-4.12,;-5.65,-6.6,;-4.11,-7.31,)| Show InChI InChI=1S/C64H79FN12O10/c1-4-68-62(86)55-19-11-31-77(55)63(87)49(18-10-30-69-64(66)67)72-57(81)50(32-38(2)3)73-60(84)53(35-43-36-70-48-17-8-7-16-47(43)48)75-58(82)51(33-40-22-27-45(79)28-23-40)74-61(85)54(37-78)76-59(83)52(34-42-14-9-13-41-12-5-6-15-46(41)42)71-56(80)29-24-39-20-25-44(65)26-21-39/h5-9,12-17,20-23,25-28,36,38,49-55,70,78-79H,4,10-11,18-19,24,29-35,37H2,1-3H3,(H,68,86)(H,71,80)(H,72,81)(H,73,84)(H,74,85)(H,75,82)(H,76,83)(H4,66,67,69)/t49-,50-,51-,52-,53+,54-,55+/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TAP Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of the in vitro binding of [125I]-labeled leuprolide to the rat pituitary luteinizing hormone releasing hormone (LHRH) receptor. |
J Med Chem 37: 701-5 (1994)
Article DOI: 10.1021/jm00031a021
BindingDB Entry DOI: 10.7270/Q2MG7S7B |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Rattus norvegicus) | BDBM50470080

(CHEMBL266475)Show SMILES CCNC(=O)[C@H]1CCCN1C(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](CCCCNC(=O)c1cccnc1)NC(=O)[C@H](Cc1ccc(O)cc1)N(C)C(=O)[C@H](CO)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)CCc1ccc(F)cc1 |wU:61.66,12.20,wD:23.28,31.46,5.4,67.70,48.50,(24.58,-7.94,;24.59,-9.48,;23.27,-10.25,;21.92,-9.5,;20.64,-10.25,;21.89,-8,;23.11,-7.1,;22.6,-5.68,;21.08,-5.68,;20.64,-7.12,;19.32,-7.87,;19.31,-9.4,;18,-7.1,;18.01,-5.56,;19.35,-4.81,;19.36,-3.28,;20.69,-2.51,;20.7,-.99,;22.02,-.24,;19.38,-.2,;16.66,-7.86,;15.35,-7.09,;15.35,-5.55,;14,-7.84,;14,-9.37,;15.31,-10.15,;16.65,-9.38,;15.3,-11.69,;12.68,-7.07,;11.36,-7.81,;11.34,-9.35,;10.04,-7.04,;10.12,-5.53,;8.92,-4.56,;9.41,-3.11,;8.08,-2.09,;8.74,-.71,;7.87,.6,;8.54,1.98,;6.32,.45,;5.46,1.73,;3.91,1.59,;3.25,.25,;4.15,-1.04,;5.66,-.91,;8.71,-7.81,;7.38,-7.03,;7.38,-5.5,;6.04,-7.77,;6.03,-9.31,;7.35,-10.08,;7.35,-11.62,;8.67,-12.39,;10.01,-11.66,;11.33,-12.42,;10.02,-10.12,;8.69,-9.34,;4.73,-7,;4.73,-5.46,;3.4,-7.74,;3.38,-9.3,;2.06,-6.97,;2.09,-5.46,;3.41,-4.69,;.74,-7.74,;-.6,-6.97,;-.57,-5.43,;-1.92,-7.71,;-1.95,-9.25,;-.61,-10.04,;.8,-9.41,;1.81,-10.57,;1.04,-11.88,;1.49,-13.36,;.46,-14.48,;-1.03,-14.16,;-1.5,-12.68,;-.47,-11.56,;-3.24,-6.96,;-4.58,-7.71,;-4.59,-9.22,;-5.92,-6.96,;-7.37,-7.87,;-8.88,-7.07,;-10.32,-7.99,;-11.84,-7.2,;-11.92,-5.46,;-13.4,-4.6,;-10.48,-4.57,;-8.95,-5.36,)| Show InChI InChI=1S/C64H85FN14O11/c1-5-69-60(87)53-18-12-32-79(53)63(90)49(17-11-31-71-64(66)67)75-58(85)50(33-39(2)3)76-57(84)48(16-8-9-30-70-56(83)42-13-10-29-68-36-42)74-61(88)54(34-41-21-26-45(81)27-22-41)78(4)62(89)52(38-80)77-59(86)51(35-43-37-72-47-15-7-6-14-46(43)47)73-55(82)28-23-40-19-24-44(65)25-20-40/h6-7,10,13-15,19-22,24-27,29,36-37,39,48-54,72,80-81H,5,8-9,11-12,16-18,23,28,30-35,38H2,1-4H3,(H,69,87)(H,70,83)(H,73,82)(H,74,88)(H,75,85)(H,76,84)(H,77,86)(H4,66,67,71)/t48-,49+,50+,51+,52+,53-,54+/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TAP Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of the in vitro binding of [125I]-labeled leuprolide to the rat pituitary luteinizing hormone releasing hormone (LHRH) receptor. |
J Med Chem 37: 701-5 (1994)
Article DOI: 10.1021/jm00031a021
BindingDB Entry DOI: 10.7270/Q2MG7S7B |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Rattus norvegicus) | BDBM50470086
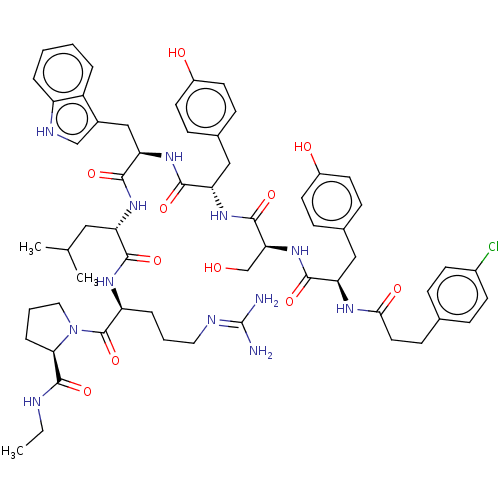
(CHEMBL2369140)Show SMILES CCNC(=O)[C@H]1CCCN1C(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(=O)[C@@H](Cc1ccc(O)cc1)NC(=O)CCc1ccc(Cl)cc1 |wU:57.63,12.20,63.76,wD:45.57,31.44,23.28,5.4,(27.37,-7.76,;27.37,-9.28,;26.05,-10.07,;24.72,-9.31,;23.41,-10.07,;24.68,-7.8,;25.89,-6.92,;25.37,-5.48,;23.86,-5.48,;23.43,-6.93,;22.09,-7.67,;22.09,-9.22,;20.77,-6.92,;20.77,-5.37,;22.12,-4.61,;22.12,-3.07,;23.47,-2.32,;23.47,-.78,;24.81,-.01,;22.15,,;19.44,-7.67,;18.1,-6.9,;18.12,-5.35,;16.77,-7.64,;16.75,-9.18,;18.09,-9.98,;18.07,-11.5,;19.42,-9.2,;15.43,-6.87,;14.11,-7.63,;14.1,-9.15,;12.79,-6.86,;12.79,-5.32,;12.33,-3.86,;10.86,-3.38,;10.86,-1.83,;12.34,-1.36,;12.95,.03,;14.49,.19,;15.39,-1.04,;14.75,-2.46,;13.23,-2.62,;11.46,-7.61,;10.12,-6.83,;10.12,-5.29,;8.76,-7.6,;8.76,-9.12,;10.08,-9.91,;11.44,-9.15,;12.76,-9.92,;12.76,-11.47,;14.08,-12.24,;11.4,-12.23,;10.08,-11.44,;7.45,-6.8,;6.13,-7.56,;6.09,-9.12,;4.8,-6.79,;4.81,-5.26,;6.13,-4.51,;3.45,-7.54,;2.12,-6.77,;2.13,-5.25,;.78,-7.53,;.78,-9.08,;2.1,-9.88,;3.45,-9.14,;4.77,-9.92,;4.73,-11.47,;6.06,-12.27,;3.38,-12.21,;2.07,-11.43,;-.54,-6.77,;-1.89,-7.51,;-1.82,-9.04,;-3.25,-6.82,;-4.67,-7.8,;-6.23,-7.06,;-7.63,-8.05,;-9.17,-7.32,;-9.33,-5.6,;-10.87,-4.8,;-7.92,-4.61,;-6.36,-5.35,)| Show InChI InChI=1S/C60H77ClN12O11/c1-4-64-58(83)51-12-8-28-73(51)59(84)45(11-7-27-65-60(62)63)68-53(78)46(29-35(2)3)69-56(81)49(32-39-33-66-44-10-6-5-9-43(39)44)71-55(80)48(31-38-17-24-42(76)25-18-38)70-57(82)50(34-74)72-54(79)47(30-37-15-22-41(75)23-16-37)67-52(77)26-19-36-13-20-40(61)21-14-36/h5-6,9-10,13-18,20-25,33,35,45-51,66,74-76H,4,7-8,11-12,19,26-32,34H2,1-3H3,(H,64,83)(H,67,77)(H,68,78)(H,69,81)(H,70,82)(H,71,80)(H,72,79)(H4,62,63,65)/t45-,46-,47+,48-,49+,50-,51+/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TAP Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of the in vitro binding of [125I]-labeled leuprolide to the rat pituitary luteinizing hormone releasing hormone (LHRH) receptor. |
J Med Chem 37: 701-5 (1994)
Article DOI: 10.1021/jm00031a021
BindingDB Entry DOI: 10.7270/Q2MG7S7B |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Rattus norvegicus) | BDBM50470099
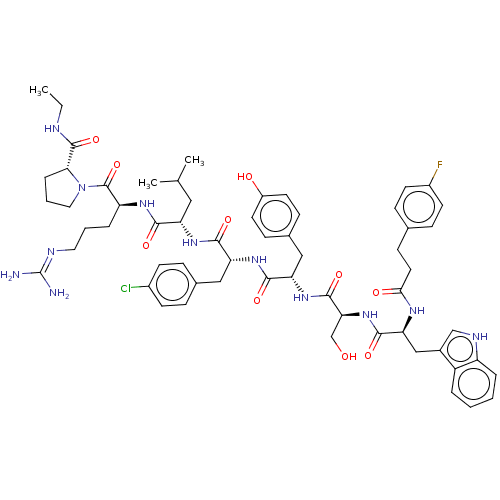
(CHEMBL2371295)Show SMILES CCNC(=O)[C@H]1CCCN1C(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](Cc1ccc(Cl)cc1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)CCc1ccc(F)cc1 |wU:55.60,12.20,wD:43.54,31.41,23.28,5.4,61.64,(27.36,-6,;27.38,-7.53,;26.06,-8.31,;24.72,-7.54,;23.42,-8.31,;24.69,-6.05,;25.9,-5.16,;25.38,-3.71,;23.85,-3.73,;23.43,-5.16,;22.1,-5.92,;22.08,-7.44,;20.76,-5.16,;20.78,-3.61,;22.11,-2.84,;22.14,-1.32,;23.47,-.56,;23.49,.99,;24.82,1.74,;22.15,1.76,;19.44,-5.9,;18.11,-5.13,;18.12,-3.58,;16.77,-5.89,;16.76,-7.43,;18.09,-8.22,;19.41,-7.44,;18.06,-9.75,;15.44,-5.12,;14.1,-5.87,;14.1,-7.4,;12.78,-5.09,;12.88,-3.57,;13.64,-2.23,;12.85,-.9,;13.59,.45,;15.15,.47,;15.9,1.82,;15.94,-.87,;15.19,-2.2,;11.46,-5.86,;10.11,-5.06,;10.11,-3.54,;8.78,-5.83,;8.78,-7.37,;10.1,-8.15,;10.08,-9.7,;11.42,-10.47,;12.75,-9.72,;14.07,-10.47,;12.77,-8.17,;11.43,-7.38,;7.46,-5.05,;6.12,-5.8,;6.11,-7.35,;4.8,-5.03,;4.8,-3.51,;6.12,-2.75,;3.45,-5.79,;2.13,-5.02,;2.16,-3.49,;.78,-5.77,;.78,-7.31,;2.1,-8.09,;3.52,-7.47,;4.55,-8.62,;3.76,-9.95,;4.23,-11.42,;3.2,-12.55,;1.68,-12.23,;1.22,-10.76,;2.26,-9.63,;-.54,-5,;-1.87,-5.76,;-1.79,-7.27,;-3.25,-5.08,;-4.65,-6.09,;-6.21,-5.38,;-7.61,-6.4,;-9.17,-5.71,;-9.35,-4,;-10.9,-3.22,;-7.97,-2.97,;-6.39,-3.68,)| Show InChI InChI=1S/C60H76ClFN12O10/c1-4-65-58(83)51-12-8-28-74(51)59(84)45(11-7-27-66-60(63)64)69-53(78)46(29-35(2)3)70-54(79)47(30-37-13-20-40(61)21-14-37)71-55(80)48(31-38-17-24-42(76)25-18-38)72-57(82)50(34-75)73-56(81)49(32-39-33-67-44-10-6-5-9-43(39)44)68-52(77)26-19-36-15-22-41(62)23-16-36/h5-6,9-10,13-18,20-25,33,35,45-51,67,75-76H,4,7-8,11-12,19,26-32,34H2,1-3H3,(H,65,83)(H,68,77)(H,69,78)(H,70,79)(H,71,80)(H,72,82)(H,73,81)(H4,63,64,66)/t45-,46-,47+,48-,49-,50-,51+/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TAP Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of the in vitro binding of [125I]-labeled leuprolide to the rat pituitary luteinizing hormone releasing hormone (LHRH) receptor. |
J Med Chem 37: 701-5 (1994)
Article DOI: 10.1021/jm00031a021
BindingDB Entry DOI: 10.7270/Q2MG7S7B |
More data for this
Ligand-Target Pair | |
Lutropin-choriogonadotropic hormone receptor
(Rattus norvegicus) | BDBM50405789

(CHEMBL412984)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(=O)[C@@H](Cc1ccc(Cl)cc1)NC(=O)[C@@H](Cc1ccc(Cl)cc1)NC(C)=O)C(=O)N[C@H](CCCNC(N)=N)C(=O)N1CCC[C@@H]1C(=O)N[C@H](C)C(N)=O Show InChI InChI=1S/C69H92Cl2N18O13/c1-37(2)30-51(60(95)83-50(13-8-28-78-69(75)76)67(102)89-29-9-14-57(89)66(101)80-38(3)58(72)93)84-59(94)49(12-7-27-77-68(73)74)82-62(97)53(33-42-19-25-46(92)26-20-42)86-65(100)56(36-90)88-64(99)55(34-43-35-79-48-11-6-5-10-47(43)48)87-63(98)54(32-41-17-23-45(71)24-18-41)85-61(96)52(81-39(4)91)31-40-15-21-44(70)22-16-40/h5-6,10-11,15-26,35,37-38,49-57,79,90,92H,7-9,12-14,27-34,36H2,1-4H3,(H2,72,93)(H,80,101)(H,81,91)(H,82,97)(H,83,95)(H,84,94)(H,85,96)(H,86,100)(H,87,98)(H,88,99)(H4,73,74,77)(H4,75,76,78)/t38-,49-,50-,51+,52-,53+,54-,55-,56+,57-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0182 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory
Curated by ChEMBL
| Assay Description
In vitro binding affinity for Luteinizing hormone releasing hormone receptor from rat pituitary cells, expressed as negative logarithm of the equilib... |
J Med Chem 32: 2340-4 (1989)
BindingDB Entry DOI: 10.7270/Q2H70H1B |
More data for this
Ligand-Target Pair | |
Progonadoliberin-1
(RAT) | BDBM84712
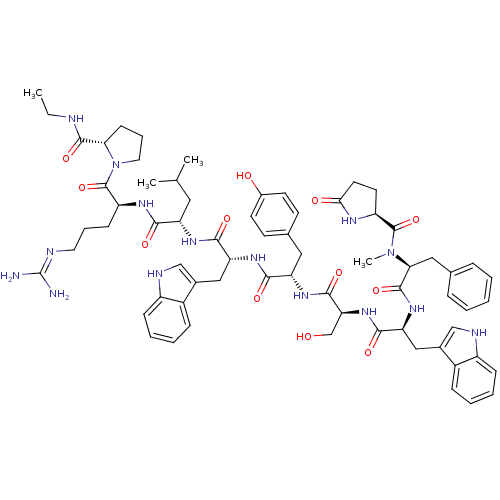
(deslorelin 2NMePhe | deslorelin 2Phe)Show SMILES CCNC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1ccccc1)N(C)C(=O)[C@@H]1CCC(=O)N1 |r,wU:31.44,23.28,12.20,57.63,63.79,5.4,77.91,89.95,wD:45.57,(-8.25,5.5,;-8.21,4.01,;-6.91,3.28,;-6.91,1.76,;-5.56,.97,;-8.21,.86,;-9.55,1.3,;-10.59,.25,;-9.77,-1.09,;-8.21,-.6,;-6.97,-1.49,;-5.62,-.85,;-7.01,-3.06,;-8.43,-3.76,;-9.77,-2.88,;-11.13,-3.55,;-12.37,-2.75,;-13.77,-3.43,;-15.09,-2.58,;-13.89,-4.95,;-5.75,-3.91,;-4.4,-3.25,;-4.38,-1.66,;-3.12,-4.06,;-3.52,-5.56,;-2.46,-6.71,;-2.79,-8.25,;-.84,-7.09,;-1.7,-3.39,;-.45,-4.25,;-.33,-5.83,;.61,-3.28,;.76,-1.8,;-.23,-.66,;.18,.7,;-.73,1.73,;-2.18,1.46,;-3.12,2.52,;-4.51,2.21,;-4.98,.86,;-4.03,-.18,;-2.61,.1,;2.04,-3.86,;3.22,-2.91,;4.71,-3.45,;2.98,-1.4,;1.57,-.7,;1.7,.88,;.39,1.92,;.68,3.61,;2.13,4.32,;2.37,5.95,;3.43,3.26,;3.26,1.51,;4.09,-.39,;5.66,-.45,;6.38,.82,;6.32,-1.82,;5.59,-3.1,;6.26,-4.46,;7.87,-1.86,;8.66,-.54,;7.93,.8,;10.17,-.6,;10.89,-1.99,;10.45,-3.49,;9,-4.01,;9.07,-5.58,;10.52,-6,;11.2,-7.39,;12.75,-7.49,;13.62,-6.2,;12.93,-4.81,;11.38,-4.71,;10.95,.6,;10.29,1.94,;8.75,1.98,;11.11,3.26,;12.59,3.16,;13.53,2.14,;13.13,.66,;14.22,-.43,;15.71,-.03,;16.11,1.45,;15.02,2.54,;10.35,4.51,;11.12,5.85,;8.83,4.51,;8.02,3.22,;8.02,5.91,;8.59,7.19,;7.54,8.25,;6.16,7.59,;4.77,8.19,;6.53,5.95,)| Show InChI InChI=1S/C68H87N15O12/c1-5-71-64(92)56-22-14-30-83(56)67(95)49(21-13-29-72-68(69)70)76-59(87)51(31-39(2)3)77-61(89)53(34-42-36-73-47-19-11-9-17-45(42)47)79-60(88)52(32-41-23-25-44(85)26-24-41)78-63(91)55(38-84)81-62(90)54(35-43-37-74-48-20-12-10-18-46(43)48)80-65(93)57(33-40-15-7-6-8-16-40)82(4)66(94)50-27-28-58(86)75-50/h6-12,15-20,23-26,36-37,39,49-57,73-74,84-85H,5,13-14,21-22,27-35,38H2,1-4H3,(H,71,92)(H,75,86)(H,76,87)(H,77,89)(H,78,91)(H,79,88)(H,80,93)(H,81,90)(H4,69,70,72)/t49-,50-,51-,52-,53+,54-,55-,56-,57-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Med Chem 36: 363-9 (1993)
Article DOI: 10.1021/jm00055a007
BindingDB Entry DOI: 10.7270/Q2GQ6W8T |
More data for this
Ligand-Target Pair | |
Progonadoliberin-1
(RAT) | BDBM84708
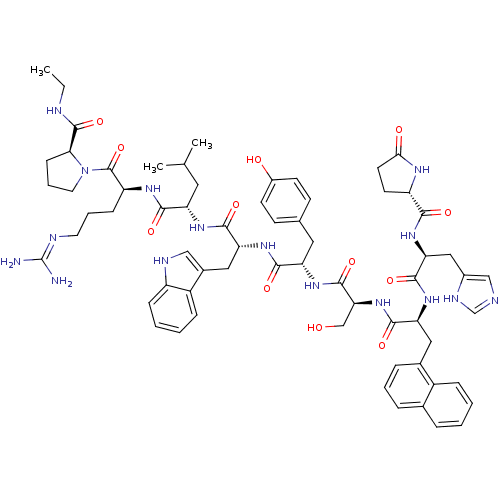
(deslorelin 31Nal)Show SMILES CCNC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1cccc2ccccc12)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H]1CCC(=O)N1 |r,wU:31.44,23.28,12.20,57.63,63.80,5.4,78.91,88.94,wD:45.57,(-8.25,5.5,;-8.21,4.01,;-6.91,3.28,;-6.91,1.76,;-5.56,.97,;-8.21,.86,;-9.55,1.3,;-10.59,.25,;-9.77,-1.09,;-8.21,-.6,;-6.97,-1.49,;-5.62,-.85,;-7.01,-3.06,;-8.43,-3.76,;-9.77,-2.88,;-11.13,-3.55,;-12.37,-2.75,;-13.77,-3.43,;-15.09,-2.58,;-13.89,-4.95,;-5.75,-3.91,;-4.4,-3.25,;-4.38,-1.66,;-3.12,-4.06,;-3.52,-5.56,;-2.46,-6.71,;-2.79,-8.25,;-.84,-7.09,;-1.7,-3.39,;-.45,-4.25,;-.33,-5.83,;.61,-3.28,;.76,-1.8,;-.23,-.66,;.18,.7,;-.73,1.73,;-2.18,1.46,;-3.12,2.52,;-4.51,2.21,;-4.98,.86,;-4.03,-.18,;-2.61,.1,;2.04,-3.86,;3.22,-2.91,;4.71,-3.45,;2.98,-1.4,;1.57,-.7,;1.7,.88,;.39,1.92,;.68,3.61,;2.13,4.32,;2.37,5.95,;3.43,3.26,;3.26,1.51,;4.09,-.39,;5.66,-.45,;6.38,.82,;6.32,-1.82,;5.59,-3.1,;6.26,-4.46,;7.87,-1.86,;8.66,-.54,;7.93,.8,;10.17,-.6,;10.89,-1.99,;10.27,-3.14,;8.73,-3.13,;7.96,-4.47,;8.73,-5.8,;10.27,-5.8,;11.04,-7.14,;12.58,-7.14,;13.35,-5.8,;12.58,-4.47,;11.04,-4.47,;10.95,.6,;10.29,1.94,;8.75,1.98,;11.11,3.26,;12.59,3.16,;13.35,1.86,;12.66,.45,;13.72,-.54,;15.09,.1,;14.84,1.64,;10.35,4.51,;8.83,4.51,;8.02,3.22,;8.02,5.91,;8.59,7.19,;7.54,8.25,;6.16,7.59,;4.77,8.19,;6.53,5.95,)| Show InChI InChI=1S/C66H84N16O12/c1-4-70-64(93)55-19-11-27-82(55)65(94)48(18-10-26-71-66(67)68)75-58(87)49(28-37(2)3)76-60(89)52(31-41-33-72-46-17-8-7-16-45(41)46)79-59(88)50(29-38-20-22-43(84)23-21-38)77-63(92)54(35-83)81-61(90)51(30-40-14-9-13-39-12-5-6-15-44(39)40)78-62(91)53(32-42-34-69-36-73-42)80-57(86)47-24-25-56(85)74-47/h5-9,12-17,20-23,33-34,36-37,47-55,72,83-84H,4,10-11,18-19,24-32,35H2,1-3H3,(H,69,73)(H,70,93)(H,74,85)(H,75,87)(H,76,89)(H,77,92)(H,78,91)(H,79,88)(H,80,86)(H,81,90)(H4,67,68,71)/t47-,48-,49-,50-,51-,52+,53-,54-,55-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Med Chem 36: 363-9 (1993)
Article DOI: 10.1021/jm00055a007
BindingDB Entry DOI: 10.7270/Q2GQ6W8T |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Rattus norvegicus) | BDBM50470085

(CHEMBL438195)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](CCCCNC(=O)c1cccnc1)NC(=O)[C@H](Cc1ccc(O)cc1)N(C)C(=O)[C@H](CO)NC(=O)[C@H](Cc1cccc2ccccc12)NC(=O)CCc1ccc(F)cc1)C(=O)N[C@@H](CCCCNC(C)C)C(=O)N1CCC[C@@H]1C(=O)N[C@H](C)C(N)=O Show InChI InChI=1S/C70H93FN12O12/c1-43(2)38-56(65(90)79-55(22-10-11-35-74-44(3)4)70(95)83-37-15-23-59(83)67(92)76-45(5)62(72)87)80-64(89)54(21-9-12-36-75-63(88)50-19-14-34-73-41-50)78-68(93)60(39-47-26-31-52(85)32-27-47)82(6)69(94)58(42-84)81-66(91)57(40-49-18-13-17-48-16-7-8-20-53(48)49)77-61(86)33-28-46-24-29-51(71)30-25-46/h7-8,13-14,16-20,24-27,29-32,34,41,43-45,54-60,74,84-85H,9-12,15,21-23,28,33,35-40,42H2,1-6H3,(H2,72,87)(H,75,88)(H,76,92)(H,77,86)(H,78,93)(H,79,90)(H,80,89)(H,81,91)/t45-,54-,55+,56+,57+,58+,59-,60+/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TAP Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of the in vitro binding of [125I]-labeled leuprolide to the rat pituitary luteinizing hormone releasing hormone (LHRH) receptor. |
J Med Chem 37: 701-5 (1994)
Article DOI: 10.1021/jm00031a021
BindingDB Entry DOI: 10.7270/Q2MG7S7B |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Rattus norvegicus) | BDBM50470084

(CHEMBL428299)Show SMILES CCNC(=O)[C@H]1CCCN1C(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](CCCCNC(=O)c1cccnc1)NC(=O)[C@H](Cc1ccc(O)cc1)N(C)C(=O)[C@H](CO)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)CCc1ccc(Cl)cc1 |wU:61.66,12.20,wD:23.28,31.46,5.4,67.70,48.50,(21.23,-6.64,;21.25,-8.17,;19.93,-8.96,;18.58,-8.2,;17.28,-8.96,;18.55,-6.69,;19.77,-5.8,;19.25,-4.36,;17.71,-4.36,;17.29,-5.81,;15.96,-6.56,;15.94,-8.1,;14.62,-5.8,;14.64,-4.26,;15.97,-3.49,;15.99,-1.95,;17.33,-1.19,;17.35,.34,;18.68,1.12,;16,1.13,;13.3,-6.56,;11.96,-5.78,;11.97,-4.23,;10.62,-6.52,;10.61,-8.07,;11.94,-8.87,;13.26,-8.09,;11.91,-10.39,;9.29,-5.75,;7.94,-6.51,;7.94,-8.04,;6.62,-5.74,;6.72,-4.2,;5.49,-3.24,;6.01,-1.78,;4.65,-.75,;5.32,.63,;4.46,1.93,;5.13,3.32,;2.88,1.79,;2.04,3.08,;.46,2.96,;-.18,1.58,;.71,.29,;2.24,.42,;5.29,-6.49,;3.94,-5.72,;3.94,-4.17,;2.61,-6.48,;2.59,-8.01,;3.91,-8.8,;3.91,-10.33,;5.26,-11.12,;6.59,-10.36,;7.91,-11.14,;6.61,-8.81,;5.27,-8.04,;1.29,-5.69,;1.3,-4.14,;-.05,-6.45,;-.06,-8.01,;-1.38,-5.68,;-1.38,-4.14,;-.03,-3.39,;-2.73,-6.43,;-4.05,-5.65,;-4.03,-4.13,;-5.41,-6.42,;-5.41,-7.96,;-4.08,-8.75,;-2.67,-8.13,;-1.63,-9.27,;-2.42,-10.61,;-1.96,-12.07,;-2.99,-13.2,;-4.5,-12.88,;-4.97,-11.41,;-3.92,-10.29,;-6.73,-5.65,;-8.05,-6.42,;-8.08,-7.93,;-9.38,-5.62,;-10.86,-6.52,;-12.37,-5.69,;-13.83,-6.59,;-15.34,-5.77,;-15.4,-4.04,;-17.04,-3.56,;-13.92,-3.14,;-12.4,-3.98,)| Show InChI InChI=1S/C64H85ClN14O11/c1-5-69-60(87)53-18-12-32-79(53)63(90)49(17-11-31-71-64(66)67)75-58(85)50(33-39(2)3)76-57(84)48(16-8-9-30-70-56(83)42-13-10-29-68-36-42)74-61(88)54(34-41-21-26-45(81)27-22-41)78(4)62(89)52(38-80)77-59(86)51(35-43-37-72-47-15-7-6-14-46(43)47)73-55(82)28-23-40-19-24-44(65)25-20-40/h6-7,10,13-15,19-22,24-27,29,36-37,39,48-54,72,80-81H,5,8-9,11-12,16-18,23,28,30-35,38H2,1-4H3,(H,69,87)(H,70,83)(H,73,82)(H,74,88)(H,75,85)(H,76,84)(H,77,86)(H4,66,67,71)/t48-,49+,50+,51+,52+,53-,54+/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TAP Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of the in vitro binding of [125I]-labeled leuprolide to the rat pituitary luteinizing hormone releasing hormone (LHRH) receptor. |
J Med Chem 37: 701-5 (1994)
Article DOI: 10.1021/jm00031a021
BindingDB Entry DOI: 10.7270/Q2MG7S7B |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Rattus norvegicus) | BDBM50470083

(CHEMBL267709)Show SMILES CCNC(=O)[C@H]1CCCN1C(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](CCCCNC(=O)c1cccnc1)NC(=O)[C@H](Cc1ccc(O)cc1)N(C)C(=O)[C@H](CO)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)CCc1ccc(F)cc1F |wU:61.66,12.20,wD:23.28,31.46,5.4,67.70,48.50,(22.09,-4.06,;22.09,-5.58,;20.77,-6.37,;19.44,-5.61,;18.13,-6.37,;19.41,-4.1,;20.61,-3.22,;20.09,-1.78,;18.58,-1.78,;18.15,-3.23,;16.81,-3.97,;16.81,-5.51,;15.49,-3.22,;15.49,-1.67,;16.84,-.91,;16.84,.63,;18.2,1.38,;18.2,2.92,;19.54,3.69,;16.88,3.7,;14.16,-3.97,;12.82,-3.2,;12.84,-1.65,;11.49,-3.94,;11.47,-5.48,;12.81,-6.28,;14.14,-5.5,;12.79,-7.8,;10.15,-3.17,;8.83,-3.93,;8.82,-5.45,;7.51,-3.15,;7.6,-1.62,;6.38,-.65,;6.89,.79,;5.55,1.82,;6.19,3.21,;5.32,4.51,;6,5.89,;3.78,4.37,;2.94,5.65,;1.36,5.53,;.71,4.15,;1.59,2.86,;3.12,2.99,;6.18,-3.91,;4.84,-3.13,;4.84,-1.59,;3.49,-3.89,;3.49,-5.42,;4.81,-6.21,;4.81,-7.76,;6.13,-8.53,;7.48,-7.77,;8.8,-8.54,;7.48,-6.22,;6.16,-5.45,;2.17,-3.1,;2.19,-1.56,;.85,-3.86,;.82,-5.42,;-.48,-3.09,;-.47,-1.56,;.85,-.8,;-1.82,-3.84,;-3.15,-3.07,;-3.14,-1.54,;-4.5,-3.83,;-4.51,-5.38,;-3.18,-6.16,;-1.76,-5.54,;-.74,-6.67,;-1.53,-8.01,;-1.06,-9.47,;-2.09,-10.6,;-3.59,-10.28,;-4.08,-8.82,;-3.03,-7.69,;-5.81,-3.07,;-7.17,-3.81,;-7.13,-5.34,;-8.52,-3.1,;-9.95,-4.06,;-11.51,-3.29,;-11.61,-1.58,;-13.15,-.82,;-14.58,-1.78,;-16.07,-.94,;-14.45,-3.51,;-12.93,-4.26,;-12.9,-5.99,)| Show InChI InChI=1S/C64H84F2N14O11/c1-5-70-60(88)53-18-12-30-80(53)63(91)49(17-11-29-72-64(67)68)76-58(86)50(31-38(2)3)77-57(85)48(16-8-9-28-71-56(84)41-13-10-27-69-35-41)75-61(89)54(32-39-19-24-44(82)25-20-39)79(4)62(90)52(37-81)78-59(87)51(33-42-36-73-47-15-7-6-14-45(42)47)74-55(83)26-22-40-21-23-43(65)34-46(40)66/h6-7,10,13-15,19-21,23-25,27,34-36,38,48-54,73,81-82H,5,8-9,11-12,16-18,22,26,28-33,37H2,1-4H3,(H,70,88)(H,71,84)(H,74,83)(H,75,89)(H,76,86)(H,77,85)(H,78,87)(H4,67,68,72)/t48-,49+,50+,51+,52+,53-,54+/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TAP Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of the in vitro binding of [125I]-labeled leuprolide to the rat pituitary luteinizing hormone releasing hormone (LHRH) receptor. |
J Med Chem 37: 701-5 (1994)
Article DOI: 10.1021/jm00031a021
BindingDB Entry DOI: 10.7270/Q2MG7S7B |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(RAT) | BDBM50089269
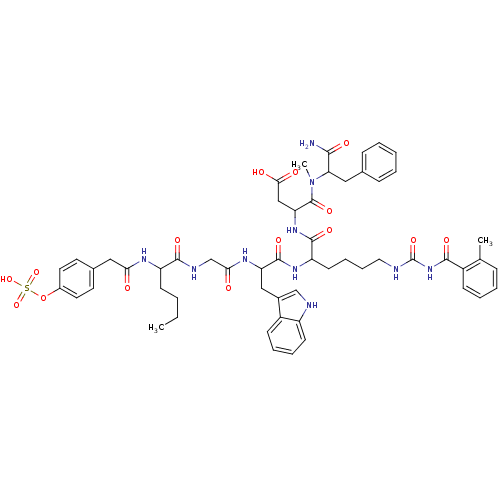
(CHEMBL276192 | N-(1-Carbamoyl-2-phenyl-ethyl)-3-{2...)Show SMILES CCCCC(NC(=O)Cc1ccc(OS(O)(=O)=O)cc1)C(=O)NCC(=O)NC(Cc1c[nH]c2ccccc12)C(=O)NC(CCCCNC(=O)NC(=O)c1ccccc1C)C(=O)NC(CC(O)=O)C(=O)N(C)C(Cc1ccccc1)C(N)=O Show InChI InChI=1S/C56H68N10O15S/c1-4-5-20-42(61-47(67)29-36-23-25-38(26-24-36)81-82(78,79)80)52(73)60-33-48(68)62-44(30-37-32-59-41-21-12-11-19-40(37)41)54(75)63-43(22-13-14-27-58-56(77)65-51(72)39-18-10-9-15-34(39)2)53(74)64-45(31-49(69)70)55(76)66(3)46(50(57)71)28-35-16-7-6-8-17-35/h6-12,15-19,21,23-26,32,42-46,59H,4-5,13-14,20,22,27-31,33H2,1-3H3,(H2,57,71)(H,60,73)(H,61,67)(H,62,68)(H,63,75)(H,64,74)(H,69,70)(H,78,79,80)(H2,58,65,72,77) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
Inhibition of [125I]BH-CCK-8 binding to Cholecystokinin type A receptor of rat pancreatic tissue |
J Med Chem 43: 2350-5 (2000)
BindingDB Entry DOI: 10.7270/Q2FX7B4J |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50285289

(2,6-Dichloro-3-(2-morpholin-4-yl-ethoxy)-benzoic a...)Show SMILES CCOc1cc(OC)cc2c1C(=O)N(COC(=O)c1c(Cl)ccc(OCCN3CCOCC3)c1Cl)S2(=O)=O Show InChI InChI=1S/C24H26Cl2N2O9S/c1-3-35-18-12-15(33-2)13-19-21(18)23(29)28(38(19,31)32)14-37-24(30)20-16(25)4-5-17(22(20)26)36-11-8-27-6-9-34-10-7-27/h4-5,12-13H,3,6-11,14H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Human Leukocyte Elastase (HLE) as apparent binding constant (kreact/kinact) |
Bioorg Med Chem Lett 5: 105-109 (1995)
Article DOI: 10.1016/0960-894X(94)00466-S
BindingDB Entry DOI: 10.7270/Q2V69JJN |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Rattus norvegicus) | BDBM50470106

(CHEMBL413660)Show SMILES CCNC(=O)[C@H]1CCCN1C(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)CCc1ccc(F)cc1 |wU:57.63,12.20,wD:45.57,31.44,23.28,5.4,63.76,(28.79,-5.89,;28.8,-7.42,;27.48,-8.2,;26.14,-7.43,;24.85,-8.2,;26.1,-5.94,;27.32,-5.06,;26.81,-3.61,;25.29,-3.63,;24.85,-5.06,;23.53,-5.82,;23.52,-7.34,;22.2,-5.06,;22.21,-3.51,;23.55,-2.74,;23.56,-1.22,;24.9,-.46,;24.91,1.08,;26.23,1.83,;23.59,1.85,;20.87,-5.79,;19.55,-5.02,;19.55,-3.48,;18.2,-5.78,;18.2,-7.33,;19.52,-8.11,;19.5,-9.64,;20.85,-7.34,;16.88,-5.01,;15.55,-5.76,;15.53,-7.29,;14.24,-4.99,;14.25,-3.45,;13.76,-2,;12.32,-1.52,;12.32,.02,;13.79,.5,;14.41,1.89,;15.94,2.05,;16.84,.8,;16.21,-.61,;14.66,-.75,;12.9,-5.75,;11.57,-4.96,;11.57,-3.44,;10.23,-5.73,;10.23,-7.26,;11.55,-8.04,;12.89,-7.27,;14.21,-8.07,;14.2,-9.61,;15.52,-10.36,;12.86,-10.36,;11.54,-9.59,;8.91,-4.95,;7.58,-5.7,;7.56,-7.24,;6.24,-4.93,;6.27,-3.41,;7.59,-2.65,;4.92,-5.69,;3.58,-4.92,;3.61,-3.38,;2.25,-5.67,;2.25,-7.21,;3.57,-8.01,;4.93,-7.27,;6.24,-8.07,;6.18,-9.61,;7.52,-10.41,;4.83,-10.35,;3.53,-9.55,;.93,-4.9,;-.41,-5.63,;-.41,-7.17,;-1.76,-4.9,;-3.2,-5.83,;-4.74,-5.04,;-6.16,-5.98,;-7.7,-5.18,;-7.78,-3.47,;-9.28,-2.61,;-6.33,-2.54,;-4.81,-3.32,)| Show InChI InChI=1S/C60H77FN12O11/c1-4-64-58(83)51-12-8-28-73(51)59(84)45(11-7-27-65-60(62)63)68-53(78)46(29-35(2)3)69-56(81)49(32-39-33-66-44-10-6-5-9-43(39)44)71-55(80)48(31-38-17-24-42(76)25-18-38)70-57(82)50(34-74)72-54(79)47(30-37-15-22-41(75)23-16-37)67-52(77)26-19-36-13-20-40(61)21-14-36/h5-6,9-10,13-18,20-25,33,35,45-51,66,74-76H,4,7-8,11-12,19,26-32,34H2,1-3H3,(H,64,83)(H,67,77)(H,68,78)(H,69,81)(H,70,82)(H,71,80)(H,72,79)(H4,62,63,65)/t45-,46-,47-,48-,49+,50-,51+/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TAP Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of the in vitro binding of [125I]-labeled leuprolide to the rat pituitary luteinizing hormone releasing hormone (LHRH) receptor. |
J Med Chem 37: 701-5 (1994)
Article DOI: 10.1021/jm00031a021
BindingDB Entry DOI: 10.7270/Q2MG7S7B |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50561628
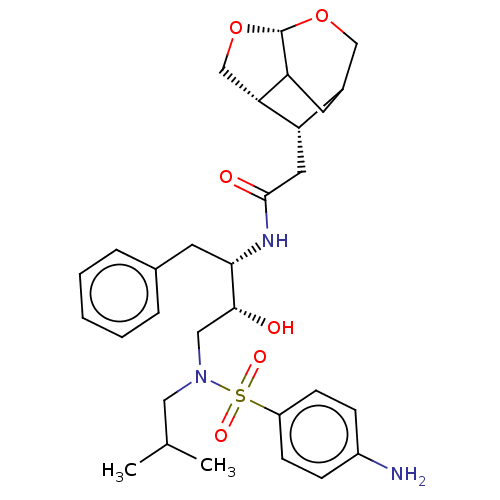
(CHEMBL4800615)Show SMILES [H][C@@]12CO[C@]3([H])OCC(CC13)[C@@H]2CC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(CC(C)C)S(=O)(=O)c1ccc(N)cc1 |r,TLB:2:1:7.6.4:9,THB:12:11:7.6.4:9,3:4:11.1:9| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HIV1 protease by fluorescence based assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.9b00670
BindingDB Entry DOI: 10.7270/Q2GF0Z75 |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Rattus norvegicus) | BDBM50470105

(CHEMBL405993)Show SMILES CCNC(=O)[C@H]1CCCN1C(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](CCCCNC(=O)c1cccnc1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)CCc1ccc(F)cc1 |wU:60.65,12.20,wD:48.59,23.28,31.46,5.4,66.69,(29.35,-7.64,;29.38,-9.17,;28.05,-9.96,;26.71,-9.2,;25.41,-9.96,;26.68,-7.69,;27.89,-6.8,;27.38,-5.36,;25.84,-5.36,;25.42,-6.81,;24.09,-7.56,;24.07,-9.1,;22.74,-6.8,;22.77,-5.26,;24.1,-4.49,;24.12,-2.95,;25.45,-2.19,;25.47,-.66,;26.81,.12,;24.13,.13,;21.42,-7.56,;20.09,-6.78,;20.1,-5.23,;18.75,-7.52,;18.73,-9.07,;20.07,-9.87,;20.04,-11.39,;21.39,-9.09,;17.41,-6.75,;16.07,-7.51,;16.07,-9.04,;14.75,-6.74,;14.84,-5.2,;13.62,-4.24,;14.13,-2.78,;12.78,-1.75,;13.45,-.37,;12.59,.94,;13.25,2.32,;11.01,.8,;10.17,2.09,;8.59,1.96,;7.94,.58,;8.84,-.71,;10.36,-.56,;13.43,-7.49,;12.07,-6.72,;12.07,-5.17,;10.74,-7.48,;10.74,-9.01,;12.06,-9.8,;13.39,-9.04,;14.74,-9.81,;14.71,-11.36,;16.04,-12.14,;13.38,-12.12,;12.04,-11.35,;9.42,-6.69,;8.07,-7.45,;8.06,-9.01,;6.75,-6.67,;6.75,-5.14,;8.09,-4.39,;5.4,-7.43,;4.07,-6.65,;4.1,-5.13,;2.72,-7.42,;2.72,-8.96,;4.04,-9.75,;5.46,-9.13,;6.49,-10.26,;5.71,-11.61,;6.17,-13.07,;5.14,-14.2,;3.62,-13.88,;3.16,-12.41,;4.2,-11.29,;1.4,-6.65,;.06,-7.4,;.06,-8.93,;-1.28,-6.65,;-2.74,-7.58,;-4.28,-6.78,;-4.34,-5.06,;-5.87,-4.27,;-7.32,-5.19,;-8.82,-4.32,;-7.24,-6.91,;-5.71,-7.71,)| Show InChI InChI=1S/C63H83FN14O11/c1-4-68-61(88)53-17-11-31-78(53)62(89)48(16-10-30-70-63(65)66)74-57(84)49(32-38(2)3)75-56(83)47(15-7-8-29-69-55(82)41-12-9-28-67-35-41)73-58(85)50(33-40-20-25-44(80)26-21-40)76-60(87)52(37-79)77-59(86)51(34-42-36-71-46-14-6-5-13-45(42)46)72-54(81)27-22-39-18-23-43(64)24-19-39/h5-6,9,12-14,18-21,23-26,28,35-36,38,47-53,71,79-80H,4,7-8,10-11,15-17,22,27,29-34,37H2,1-3H3,(H,68,88)(H,69,82)(H,72,81)(H,73,85)(H,74,84)(H,75,83)(H,76,87)(H,77,86)(H4,65,66,70)/t47-,48+,49+,50+,51+,52+,53-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TAP Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of the in vitro binding of [125I]-labeled leuprolide to the rat pituitary luteinizing hormone releasing hormone (LHRH) receptor. |
J Med Chem 37: 701-5 (1994)
Article DOI: 10.1021/jm00031a021
BindingDB Entry DOI: 10.7270/Q2MG7S7B |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50029699
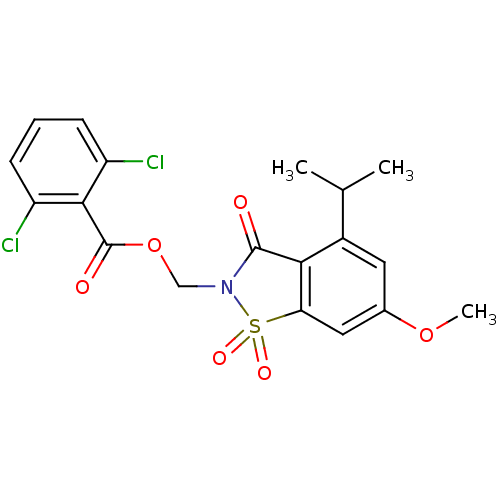
(2,6-Dichloro-benzoic acid 4-isopropyl-6-methoxy-1,...)Show SMILES COc1cc2c(C(=O)N(COC(=O)c3c(Cl)cccc3Cl)S2(=O)=O)c(c1)C(C)C Show InChI InChI=1S/C19H17Cl2NO6S/c1-10(2)12-7-11(27-3)8-15-16(12)18(23)22(29(15,25)26)9-28-19(24)17-13(20)5-4-6-14(17)21/h4-8,10H,9H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Evaluated for inhibitory activity against Human leukocyte elastase (HLE) |
Bioorg Med Chem Lett 5: 331-336 (1995)
Article DOI: 10.1016/0960-894X(95)00030-W
BindingDB Entry DOI: 10.7270/Q2SX6D60 |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Rattus norvegicus) | BDBM50469858
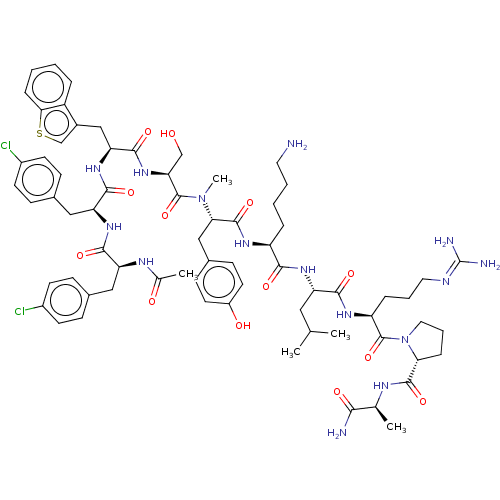
(CHEMBL268397)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7](-[#6])-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-c1csc2ccccc12)-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(Cl)cc1)-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(Cl)cc1)-[#7]-[#6](-[#6])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](-[#7])=O Show InChI InChI=1S/C70H93Cl2N15O13S/c1-39(2)32-52(62(93)81-51(14-10-30-77-70(75)76)69(100)87-31-11-15-57(87)66(97)78-40(3)60(74)91)82-61(92)50(13-8-9-29-73)80-67(98)58(35-44-21-27-48(90)28-22-44)86(5)68(99)56(37-88)85-65(96)55(36-45-38-101-59-16-7-6-12-49(45)59)84-64(95)54(34-43-19-25-47(72)26-20-43)83-63(94)53(79-41(4)89)33-42-17-23-46(71)24-18-42/h6-7,12,16-28,38-40,50-58,88,90H,8-11,13-15,29-37,73H2,1-5H3,(H2,74,91)(H,78,97)(H,79,89)(H,80,98)(H,81,93)(H,82,92)(H,83,94)(H,84,95)(H,85,96)(H4,75,76,77)/t40-,50-,51-,52-,53-,54-,55-,56-,57+,58-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0234 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
The negative logarithm of the concentration of antagonist that inhibits 50% of the binding of 125 I-labeled leuprolide to the rat pituitary LHRH rece... |
J Med Chem 36: 928-33 (1993)
Article DOI: 10.1021/jm00059a020
BindingDB Entry DOI: 10.7270/Q2Z03BW7 |
More data for this
Ligand-Target Pair | |
Lutropin-choriogonadotropic hormone receptor
(Rattus norvegicus) | BDBM50230127
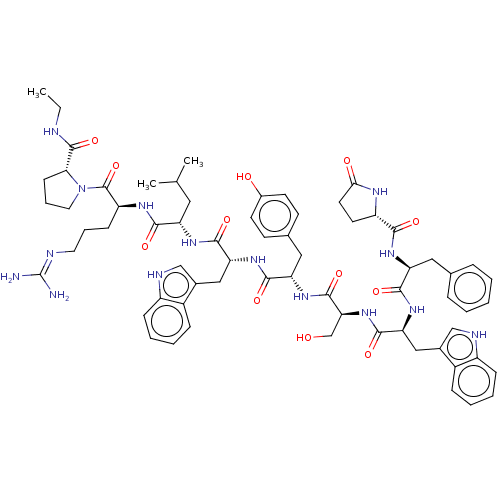
(CHEMBL407606)Show SMILES CCNC(=O)[C@H]1CCCN1C(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCC(=O)N1 |wU:45.57,31.44,63.79,23.28,5.4,88.94,wD:57.63,77.91,12.20,(27.09,-8.95,;27.47,-10.44,;28.96,-10.86,;30.04,-11.96,;31.53,-11.6,;29.61,-13.45,;30.56,-14.67,;29.69,-15.95,;28.21,-15.53,;28.16,-13.97,;26.88,-13.11,;26.98,-11.58,;25.49,-13.78,;25.38,-15.33,;26.66,-16.2,;26.55,-17.73,;27.83,-18.59,;27.72,-20.13,;29,-21,;26.33,-20.8,;24.22,-12.92,;22.82,-13.6,;22.72,-15.13,;21.54,-12.73,;21.65,-11.2,;23.05,-10.52,;24.32,-11.38,;23.15,-8.98,;20.15,-13.41,;18.89,-12.55,;18.99,-11.01,;17.49,-13.23,;17.39,-14.76,;18.14,-16.08,;19.64,-16.43,;19.78,-17.94,;18.38,-18.56,;17.91,-20.01,;16.43,-20.33,;15.39,-19.17,;15.88,-17.73,;17.36,-17.42,;16.22,-12.36,;14.83,-13.03,;14.71,-14.57,;13.55,-12.17,;13.66,-10.62,;15.04,-9.95,;15.16,-8.42,;16.54,-7.74,;17.82,-8.6,;19.2,-7.93,;17.71,-10.14,;16.32,-10.81,;12.16,-12.85,;10.88,-11.97,;10.99,-10.44,;9.49,-12.65,;9.39,-14.19,;10.67,-15.06,;8.21,-11.79,;6.83,-12.46,;6.71,-14.01,;5.54,-11.6,;5.65,-10.07,;7.05,-9.39,;8.58,-9.18,;8.85,-7.67,;7.49,-6.93,;7.12,-5.44,;5.63,-5,;4.53,-6.07,;4.89,-7.56,;6.37,-8,;4.17,-12.27,;2.88,-11.41,;3,-9.87,;1.49,-12.09,;1.38,-13.62,;2.15,-14.95,;3.66,-14.95,;4.41,-16.27,;3.65,-17.59,;2.12,-17.59,;1.37,-16.26,;.22,-11.23,;-1.18,-11.9,;-1.28,-13.44,;-2.34,-11.11,;-2.34,-9.69,;-3.87,-9.46,;-4.55,-10.83,;-6.06,-11.09,;-3.45,-11.92,)| Show InChI InChI=1S/C67H85N15O12/c1-4-70-65(93)56-21-13-29-82(56)66(94)49(20-12-28-71-67(68)69)75-59(87)50(30-38(2)3)76-62(90)53(33-41-35-72-46-18-10-8-16-44(41)46)79-61(89)52(32-40-22-24-43(84)25-23-40)78-64(92)55(37-83)81-63(91)54(34-42-36-73-47-19-11-9-17-45(42)47)80-60(88)51(31-39-14-6-5-7-15-39)77-58(86)48-26-27-57(85)74-48/h5-11,14-19,22-25,35-36,38,48-56,72-73,83-84H,4,12-13,20-21,26-34,37H2,1-3H3,(H,70,93)(H,74,85)(H,75,87)(H,76,90)(H,77,86)(H,78,92)(H,79,89)(H,80,88)(H,81,91)(H4,68,69,71)/t48-,49-,50-,51-,52-,53+,54-,55-,56+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0245 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Negative logarithm of equilibrium dissociation constant in the rat pituitary luteinizing releasing hormone receptor binding assay |
J Med Chem 35: 3890-4 (1992)
BindingDB Entry DOI: 10.7270/Q24Q7X6M |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Rattus norvegicus) | BDBM50470132

(CHEMBL2370208)Show SMILES CCNC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCC(=O)N1 |wU:88.94,63.67,31.32,45.48,23.24,wD:5.4,77.83,57.61,12.13,(36.44,8.14,;34.94,7.83,;34.46,6.38,;32.95,6.07,;31.92,7.22,;32.47,4.61,;33.37,3.36,;32.44,2.11,;30.99,2.61,;30.99,4.14,;29.68,4.91,;29.62,6.47,;28.37,4.08,;28.43,2.53,;29.81,1.83,;29.87,.29,;31.25,-.44,;31.32,-1.99,;32.69,-2.69,;30.02,-2.82,;26.99,4.81,;25.69,3.97,;25.76,2.41,;24.33,4.68,;24.25,6.23,;25.54,7.06,;26.92,6.35,;25.48,8.6,;23.01,3.84,;21.63,4.56,;21.57,6.12,;20.32,3.73,;20.41,2.18,;21.76,1.47,;23.3,1.54,;23.85,.09,;22.66,-.87,;22.56,-2.4,;21.21,-3.08,;19.9,-2.25,;20,-.71,;21.38,-.02,;18.96,4.45,;17.66,3.62,;17.72,2.06,;16.28,4.33,;16.22,5.86,;17.52,6.71,;18.9,5.99,;20.19,6.83,;20.13,8.36,;21.44,9.2,;18.75,9.08,;17.43,8.25,;14.97,3.49,;13.59,4.21,;13.52,5.75,;12.3,3.37,;12.37,1.83,;13.74,1.12,;10.92,4.1,;9.61,3.27,;9.68,1.71,;8.23,3.97,;8.17,5.51,;7.08,6.61,;5.53,6.54,;4.97,7.98,;6.17,8.95,;6.25,10.48,;7.62,11.19,;8.91,10.35,;8.83,8.81,;7.47,8.11,;6.94,3.14,;5.56,3.84,;5.48,5.38,;4.26,3.01,;4.32,1.47,;5.7,.77,;6.98,1.6,;8.35,.9,;8.43,-.64,;7.13,-1.48,;5.77,-.77,;2.88,3.73,;1.57,2.89,;1.63,1.34,;.22,3.62,;.21,5.16,;-1.26,5.62,;-2.16,4.37,;-3.7,4.36,;-1.25,3.14,)| Show InChI InChI=1S/C67H85N15O12/c1-4-70-65(93)56-21-13-29-82(56)66(94)49(20-12-28-71-67(68)69)75-59(87)50(30-38(2)3)76-62(90)53(33-41-35-72-46-18-10-8-16-44(41)46)79-61(89)52(32-40-22-24-43(84)25-23-40)78-64(92)55(37-83)81-63(91)54(34-42-36-73-47-19-11-9-17-45(42)47)80-60(88)51(31-39-14-6-5-7-15-39)77-58(86)48-26-27-57(85)74-48/h5-11,14-19,22-25,35-36,38,48-56,72-73,83-84H,4,12-13,20-21,26-34,37H2,1-3H3,(H,70,93)(H,74,85)(H,75,87)(H,76,90)(H,77,86)(H,78,92)(H,79,89)(H,80,88)(H,81,91)(H4,68,69,71)/t48-,49-,50-,51-,52-,53+,54-,55-,56-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TAP Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of the in vitro binding of [125I]-labeled leuprolide to the rat pituitary luteinizing hormone releasing hormone (LHRH) receptor. |
J Med Chem 37: 701-5 (1994)
Article DOI: 10.1021/jm00031a021
BindingDB Entry DOI: 10.7270/Q2MG7S7B |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Rattus norvegicus) | BDBM50470101

(CHEMBL406887)Show SMILES CCNC(=O)[C@H]1CCCN1C(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](CCCCNC(=O)c1cccnc1)NC(=O)[C@H](Cc1ccc(O)cc1)N(C)C(=O)[C@H](CO)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)CCc1cccc(F)c1 |wU:61.66,12.20,wD:23.28,31.46,5.4,67.70,48.50,(28.94,-13.15,;28.96,-14.67,;27.64,-15.47,;26.3,-14.7,;25,-15.47,;26.27,-13.19,;27.48,-12.31,;26.95,-10.87,;25.43,-10.87,;25.01,-12.32,;23.68,-13.06,;23.66,-14.61,;22.34,-12.31,;22.36,-10.76,;23.69,-10,;23.71,-8.45,;25.04,-7.7,;25.07,-6.16,;26.4,-5.39,;23.72,-5.38,;21.02,-13.06,;19.69,-12.29,;19.7,-10.74,;18.34,-13.03,;18.33,-14.57,;19.66,-15.37,;20.99,-14.6,;19.63,-16.89,;17.01,-12.25,;15.67,-13.02,;15.67,-14.54,;14.35,-12.24,;14.44,-10.71,;13.22,-9.74,;13.74,-8.29,;12.38,-7.26,;13.05,-5.87,;12.19,-4.58,;12.86,-3.19,;10.61,-4.71,;9.77,-3.43,;8.19,-3.55,;7.55,-4.93,;8.44,-6.22,;9.97,-6.09,;13.02,-13,;11.67,-12.22,;11.67,-10.68,;10.34,-12.98,;10.32,-14.51,;11.64,-15.3,;11.64,-16.85,;12.98,-17.62,;14.32,-16.86,;15.64,-17.63,;14.34,-15.31,;13,-14.54,;9.02,-12.19,;9.03,-10.64,;7.68,-12.95,;7.67,-14.51,;6.36,-12.18,;6.36,-10.64,;7.7,-9.89,;5,-12.93,;3.68,-12.16,;3.71,-10.63,;2.33,-12.92,;2.33,-14.47,;3.65,-15.25,;5.07,-14.63,;6.1,-15.77,;5.32,-17.11,;5.78,-18.57,;4.75,-19.7,;3.23,-19.37,;2.77,-17.91,;3.81,-16.79,;1.01,-12.16,;-.32,-12.9,;-.29,-14.43,;-1.7,-12.19,;-3.11,-13.15,;-4.66,-12.38,;-4.79,-10.64,;-6.31,-9.9,;-7.75,-10.87,;-7.63,-12.6,;-9.12,-13.5,;-6.08,-13.35,)| Show InChI InChI=1S/C64H85FN14O11/c1-5-69-60(87)53-21-13-31-79(53)63(90)49(20-12-30-71-64(66)67)75-58(85)50(32-39(2)3)76-57(84)48(19-8-9-29-70-56(83)42-15-11-28-68-36-42)74-61(88)54(34-41-22-25-45(81)26-23-41)78(4)62(89)52(38-80)77-59(86)51(35-43-37-72-47-18-7-6-17-46(43)47)73-55(82)27-24-40-14-10-16-44(65)33-40/h6-7,10-11,14-18,22-23,25-26,28,33,36-37,39,48-54,72,80-81H,5,8-9,12-13,19-21,24,27,29-32,34-35,38H2,1-4H3,(H,69,87)(H,70,83)(H,73,82)(H,74,88)(H,75,85)(H,76,84)(H,77,86)(H4,66,67,71)/t48-,49+,50+,51+,52+,53-,54+/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TAP Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of the in vitro binding of [125I]-labeled leuprolide to the rat pituitary luteinizing hormone releasing hormone (LHRH) receptor. |
J Med Chem 37: 701-5 (1994)
Article DOI: 10.1021/jm00031a021
BindingDB Entry DOI: 10.7270/Q2MG7S7B |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM81768

(BENZYLIC ACID | CAS_6581-06-2 | NSC_5311391 | Quin...)Show InChI InChI=1S/C14H12O3/c15-13(16)14(17,11-7-3-1-4-8-11)12-9-5-2-6-10-12/h1-10,17H,(H,15,16) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 0.0270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic Jacksonville
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 260: 576-80 (1992)
BindingDB Entry DOI: 10.7270/Q28P5Z0G |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Rattus norvegicus) | BDBM50470127

(CHEMBL2369141)Show SMILES CCNC(=O)[C@H]1CCCN1C(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(=O)CCc1ccc(F)cc1 |wU:57.63,12.20,63.67,wD:45.57,31.44,23.28,5.4,(37.09,-9.27,;37.09,-10.82,;35.77,-11.59,;34.44,-10.84,;33.14,-11.59,;34.41,-9.34,;35.61,-8.44,;35.1,-7.01,;33.59,-7.01,;33.16,-8.46,;31.82,-9.21,;31.82,-10.74,;30.5,-8.44,;30.5,-6.89,;31.85,-6.14,;31.85,-4.61,;33.2,-3.84,;33.2,-2.32,;34.54,-1.55,;31.88,-1.52,;29.17,-9.2,;27.83,-8.43,;27.85,-6.88,;26.5,-9.18,;26.48,-10.71,;27.82,-11.49,;27.8,-13.04,;29.15,-10.72,;25.16,-8.4,;23.84,-9.14,;23.83,-10.69,;22.52,-8.37,;22.52,-6.85,;22.06,-5.38,;20.59,-4.9,;20.59,-3.36,;22.07,-2.89,;22.68,-1.49,;24.23,-1.33,;25.13,-2.58,;24.48,-4,;22.96,-4.15,;21.19,-9.14,;19.85,-8.36,;19.85,-6.83,;18.5,-9.11,;18.5,-10.66,;19.82,-11.43,;19.82,-12.97,;21.14,-13.74,;22.49,-13,;23.81,-13.76,;22.49,-11.46,;21.17,-10.68,;17.18,-8.34,;15.87,-9.08,;15.83,-10.62,;14.54,-8.31,;14.55,-6.8,;15.87,-6.02,;13.2,-9.08,;11.87,-8.31,;11.88,-6.76,;10.53,-9.05,;10.52,-10.59,;11.85,-11.38,;13.26,-10.75,;14.28,-11.91,;13.49,-13.23,;13.96,-14.71,;12.93,-15.83,;11.43,-15.51,;10.95,-14.03,;12,-12.91,;9.21,-8.3,;7.86,-9.05,;7.89,-10.56,;6.51,-8.31,;5.08,-9.27,;3.54,-8.52,;3.42,-6.8,;1.88,-6.05,;.45,-7.01,;-1.05,-6.17,;.58,-8.73,;2.12,-9.47,)| Show InChI InChI=1S/C62H78FN13O10/c1-4-66-60(85)53-16-10-28-76(53)61(86)47(15-9-27-67-62(64)65)71-55(80)48(29-36(2)3)72-58(83)51(32-40-34-69-46-14-8-6-12-44(40)46)74-56(81)49(30-38-19-24-42(78)25-20-38)73-59(84)52(35-77)75-57(82)50(31-39-33-68-45-13-7-5-11-43(39)45)70-54(79)26-21-37-17-22-41(63)23-18-37/h5-8,11-14,17-20,22-25,33-34,36,47-53,68-69,77-78H,4,9-10,15-16,21,26-32,35H2,1-3H3,(H,66,85)(H,70,79)(H,71,80)(H,72,83)(H,73,84)(H,74,81)(H,75,82)(H4,64,65,67)/t47-,48-,49-,50+,51+,52-,53+/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TAP Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of the in vitro binding of [125I]-labeled leuprolide to the rat pituitary luteinizing hormone releasing hormone (LHRH) receptor. |
J Med Chem 37: 701-5 (1994)
Article DOI: 10.1021/jm00031a021
BindingDB Entry DOI: 10.7270/Q2MG7S7B |
More data for this
Ligand-Target Pair | |
Progonadoliberin-1
(RAT) | BDBM84711

(nafarelin 31Nal)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](Cc1ccc2ccccc2c1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1cccc2ccccc12)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H]1CCC(=O)N1)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N1CCC[C@H]1C(=O)NCC(N)=O |r,wU:41.57,66.71,23.34,75.81,89.97,wD:35.40,56.68,8.21,4.4,(4.31,-3.28,;5.62,-2.47,;6.98,-3.2,;5.58,-.92,;7.27,.05,;7.24,1.59,;5.89,2.38,;4.25,1.42,;5.89,3.78,;7.3,4.67,;8.68,4.11,;8.64,2.57,;9.96,1.77,;11.33,2.51,;12.66,1.72,;14.02,2.47,;14.03,4.01,;12.72,4.83,;11.36,4.07,;10.03,4.87,;4.65,4.7,;3.3,3.93,;3.3,2.31,;1.92,4.76,;1.92,6.36,;3.25,7.09,;4.53,6.36,;5.85,7.07,;5.85,8.62,;7.01,9.29,;4.55,9.38,;3.25,8.62,;.5,3.94,;-.84,4.7,;-.87,6.2,;-2.13,3.93,;-2.1,2.42,;-.79,1.7,;-3.45,4.64,;-4.69,3.8,;-4.62,2.07,;-6.14,4.56,;-6.14,6.1,;-6.59,7.15,;-5.5,8.24,;-5.9,9.73,;-7.38,10.12,;-8.47,9.04,;-9.96,9.43,;-11.05,8.35,;-10.65,6.86,;-9.16,6.46,;-8.07,7.55,;-7.5,3.63,;-8.71,4.41,;-8.64,5.92,;-9.94,3.67,;-9.94,2.17,;-8.64,1.41,;-7.14,1.48,;-6.59,.06,;-7.77,-.88,;-9.04,-.05,;-11.26,4.41,;-12.55,3.67,;-12.53,2.1,;-13.85,4.43,;-14.01,5.87,;-15.44,6.27,;-16.37,5.01,;-17.87,4.96,;-15.32,3.81,;8.64,-.67,;9.96,.12,;8.68,-2.21,;10,-2.98,;11.34,-2.2,;12.39,-3.01,;13.81,-2.21,;15.16,-3.2,;16.48,-2.17,;17.87,-2.85,;16.38,-.62,;10,-4.51,;8.68,-5.3,;11.36,-5.3,;12.78,-4.67,;13.81,-5.86,;13.05,-7.16,;11.52,-6.83,;10.37,-7.86,;10.7,-9.38,;8.9,-7.37,;7.75,-8.42,;6.28,-7.95,;5.95,-6.42,;5.11,-8.96,)| Show InChI InChI=1S/C68H84N16O13/c1-38(2)28-50(60(90)77-49(16-8-26-73-68(70)71)67(97)84-27-9-17-56(84)66(96)74-35-57(69)87)78-62(92)52(31-40-18-21-41-10-3-4-12-43(41)29-40)79-61(91)51(30-39-19-22-46(86)23-20-39)80-65(95)55(36-85)83-63(93)53(32-44-14-7-13-42-11-5-6-15-47(42)44)81-64(94)54(33-45-34-72-37-75-45)82-59(89)48-24-25-58(88)76-48/h3-7,10-15,18-23,29,34,37-38,48-56,85-86H,8-9,16-17,24-28,30-33,35-36H2,1-2H3,(H2,69,87)(H,72,75)(H,74,96)(H,76,88)(H,77,90)(H,78,92)(H,79,91)(H,80,95)(H,81,94)(H,82,89)(H,83,93)(H4,70,71,73)/t48-,49-,50-,51-,52+,53-,54-,55-,56-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Med Chem 36: 363-9 (1993)
Article DOI: 10.1021/jm00055a007
BindingDB Entry DOI: 10.7270/Q2GQ6W8T |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50561630
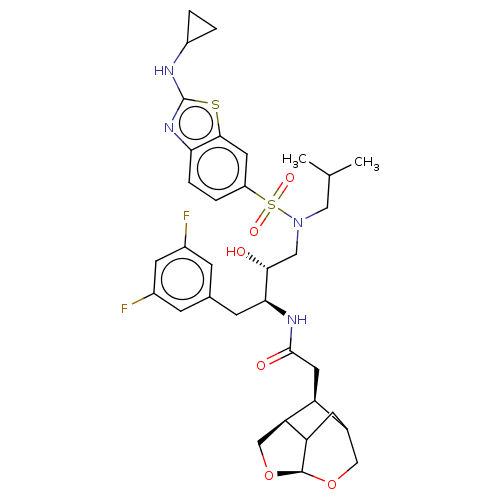
(CHEMBL4743665)Show SMILES [H][C@]12OC[C@@]3([H])[C@@H](CC(=O)N[C@@H](Cc4cc(F)cc(F)c4)[C@H](O)CN(CC(C)C)S(=O)(=O)c4ccc5nc(NC6CC6)sc5c4)C(CC13)CO2 |r,TLB:7:6:48.49.1:46,2:1:6.4:46,THB:3:4:48.49.1:46| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HIV1 protease by fluorescence based assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.9b00670
BindingDB Entry DOI: 10.7270/Q2GF0Z75 |
More data for this
Ligand-Target Pair | |
Progonadoliberin-1
(RAT) | BDBM84717
(nafarelin 7NMeLeu)Show SMILES CC(C)C[C@H](N(C)C(=O)[C@@H](Cc1ccc2ccccc2c1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H]1CCC(=O)N1)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N1CCC[C@H]1C(=O)NCC(N)=O |r,wU:42.57,66.71,24.35,75.81,89.97,wD:36.41,56.68,9.22,4.4,(4.31,-3.28,;5.62,-2.47,;6.98,-3.2,;5.58,-.92,;7.27,.05,;7.24,1.6,;8.78,1.6,;5.89,2.38,;4.25,1.42,;5.89,3.78,;7.3,4.67,;8.68,4.11,;8.64,2.57,;9.96,1.77,;11.33,2.51,;12.66,1.72,;14.02,2.47,;14.04,4.01,;12.72,4.83,;11.36,4.07,;10.03,4.87,;4.65,4.7,;3.3,3.93,;3.3,2.31,;1.92,4.76,;1.92,6.36,;3.25,7.09,;4.54,6.36,;5.85,7.07,;5.85,8.62,;7.01,9.29,;4.55,9.38,;3.25,8.62,;.5,3.94,;-.84,4.7,;-.87,6.2,;-2.13,3.93,;-2.1,2.42,;-.79,1.7,;-3.45,4.64,;-4.69,3.8,;-4.62,2.07,;-6.14,4.56,;-6.14,6.66,;-7.38,7.5,;-7.54,9.01,;-9,9.32,;-9.76,8.03,;-11.24,7.72,;-11.7,6.27,;-10.72,5.17,;-9.23,5.47,;-8.74,6.92,;-7.5,3.63,;-8.71,4.41,;-8.64,5.92,;-9.94,3.67,;-9.94,2.17,;-8.64,1.41,;-7.14,1.48,;-6.6,.06,;-7.77,-.88,;-9.04,-.05,;-11.26,4.41,;-12.55,3.67,;-12.53,2.1,;-13.85,4.43,;-14.01,5.87,;-15.44,6.27,;-16.37,5.01,;-17.87,4.96,;-15.32,3.81,;8.64,-.67,;9.96,.12,;8.68,-2.21,;10,-2.98,;11.35,-2.2,;12.39,-3.01,;13.81,-2.21,;15.17,-3.2,;16.48,-2.17,;17.87,-2.85,;16.38,-.62,;10,-4.51,;8.68,-5.3,;11.36,-5.3,;12.78,-4.67,;13.81,-5.86,;13.05,-7.16,;11.52,-6.83,;10.37,-7.86,;10.7,-9.38,;8.9,-7.38,;7.75,-8.42,;6.28,-7.95,;5.95,-6.42,;5.11,-8.96,)| Show InChI InChI=1S/C67H85N17O13/c1-37(2)26-55(64(95)77-48(14-8-24-72-67(69)70)66(97)84-25-9-15-54(84)63(94)74-34-56(68)87)83(3)65(96)52(29-39-16-19-40-10-4-5-11-41(40)27-39)81-59(90)49(28-38-17-20-44(86)21-18-38)78-62(93)53(35-85)82-60(91)50(30-42-32-73-46-13-7-6-12-45(42)46)79-61(92)51(31-43-33-71-36-75-43)80-58(89)47-22-23-57(88)76-47/h4-7,10-13,16-21,27,32-33,36-37,47-55,73,85-86H,8-9,14-15,22-26,28-31,34-35H2,1-3H3,(H2,68,87)(H,71,75)(H,74,94)(H,76,88)(H,77,95)(H,78,93)(H,79,92)(H,80,89)(H,81,90)(H,82,91)(H4,69,70,72)/t47-,48-,49-,50-,51-,52+,53-,54-,55-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Med Chem 36: 363-9 (1993)
Article DOI: 10.1021/jm00055a007
BindingDB Entry DOI: 10.7270/Q2GQ6W8T |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data


















































