 Found 562 hits with Last Name = 'spear' and Initial = 'kl'
Found 562 hits with Last Name = 'spear' and Initial = 'kl' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Atrial natriuretic peptide receptor 3
(Homo sapiens (Human)) | BDBM50016816
(CHEMBL412913 | S-S-C-F-G-G-R-I-D-R-I-G-A-Q-S-G-L-G...)Show SMILES CC[C@H](C)[C@H]1NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)CNC(=O)CNC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@@H](CSSC[C@@H](NC(=O)CNC(=O)[C@@H](CC(C)C)NC(=O)CNC(=O)[C@@H](CO)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](C)NC(=O)CNC1=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](N)CO)[C@@H](C)CC Show InChI InChI=1S/C107H165N35O34S2/c1-8-53(5)84-102(173)124-43-79(151)125-55(7)86(157)129-64(30-31-76(109)148)92(163)138-71(47-144)90(161)123-44-81(153)127-65(35-52(3)4)88(159)122-45-82(154)128-74(100(171)134-68(39-77(110)149)96(167)139-73(49-146)98(169)133-67(37-57-21-14-11-15-22-57)95(166)130-62(24-17-33-118-106(113)114)91(162)136-70(104(175)176)38-58-26-28-59(147)29-27-58)50-177-178-51-75(140-99(170)72(48-145)137-87(158)60(108)46-143)101(172)132-66(36-56-19-12-10-13-20-56)89(160)121-41-78(150)120-42-80(152)126-61(23-16-32-117-105(111)112)93(164)142-85(54(6)9-2)103(174)135-69(40-83(155)156)97(168)131-63(94(165)141-84)25-18-34-119-107(115)116/h10-15,19-22,26-29,52-55,60-75,84-85,143-147H,8-9,16-18,23-25,30-51,108H2,1-7H3,(H2,109,148)(H2,110,149)(H,120,150)(H,121,160)(H,122,159)(H,123,161)(H,124,173)(H,125,151)(H,126,152)(H,127,153)(H,128,154)(H,129,157)(H,130,166)(H,131,168)(H,132,172)(H,133,169)(H,134,171)(H,135,174)(H,136,162)(H,137,158)(H,138,163)(H,139,167)(H,140,170)(H,141,165)(H,142,164)(H,155,156)(H,175,176)(H4,111,112,117)(H4,113,114,118)(H4,115,116,119)/t53-,54-,55+,60-,61-,62-,63-,64-,65+,66+,67-,68-,69-,70-,71+,72-,73-,74+,75?,84+,85+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
G. D. Searle and Company
Curated by ChEMBL
| Assay Description
Apparent binding affinity for non-vasorelaxant receptor |
J Med Chem 32: 67-72 (1989)
BindingDB Entry DOI: 10.7270/Q2FN156B |
More data for this
Ligand-Target Pair | |
Atrial natriuretic peptide receptor 2
(Homo sapiens (Human)) | BDBM50228215
(CHEMBL3349651)Show SMILES [H][C@]1([#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)[C@@]([H])([#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6@H](-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6]-[#7]-[#6]1=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-[#8])[#6@@H](-[#6])-[#6]-[#6])[#6@@H](-[#6])-[#6]-[#6] Show InChI InChI=1S/C107H165N35O34S2/c1-8-53(5)84-102(173)124-43-79(151)125-55(7)86(157)129-64(30-31-76(109)148)92(163)138-71(47-144)90(161)123-44-81(153)127-65(35-52(3)4)88(159)122-45-82(154)128-74(100(171)134-68(39-77(110)149)96(167)139-73(49-146)98(169)133-67(37-57-21-14-11-15-22-57)95(166)130-62(24-17-33-118-106(113)114)91(162)136-70(104(175)176)38-58-26-28-59(147)29-27-58)50-177-178-51-75(140-99(170)72(48-145)137-87(158)60(108)46-143)101(172)132-66(36-56-19-12-10-13-20-56)89(160)121-41-78(150)120-42-80(152)126-61(23-16-32-117-105(111)112)93(164)142-85(54(6)9-2)103(174)135-69(40-83(155)156)97(168)131-63(94(165)141-84)25-18-34-119-107(115)116/h10-15,19-22,26-29,52-55,60-75,84-85,143-147H,8-9,16-18,23-25,30-51,108H2,1-7H3,(H2,109,148)(H2,110,149)(H,120,150)(H,121,160)(H,122,159)(H,123,161)(H,124,173)(H,125,151)(H,126,152)(H,127,153)(H,128,154)(H,129,157)(H,130,166)(H,131,168)(H,132,172)(H,133,169)(H,134,171)(H,135,174)(H,136,162)(H,137,158)(H,138,163)(H,139,167)(H,140,170)(H,141,165)(H,142,164)(H,155,156)(H,175,176)(H4,111,112,117)(H4,113,114,118)(H4,115,116,119)/t53-,54-,55-,60-,61-,62-,63-,64-,65-,66-,67-,68-,69-,70-,71-,72-,73-,74-,75-,84-,85-/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
G. D. Searle and Company
Curated by ChEMBL
| Assay Description
Apparent binding constant against multiple binding sites |
J Med Chem 32: 1094-8 (1989)
BindingDB Entry DOI: 10.7270/Q26112J6 |
More data for this
Ligand-Target Pair | |
D-amino-acid oxidase
(Homo sapiens (Human)) | BDBM50433372
(CHEMBL2375520)Show InChI InChI=1S/C7H3Cl2NO2/c8-4-1-3-6(2-5(4)9)12-10-7(3)11/h1-2H,(H,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunovion Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant DAAO by Michaelis-Menten plot analysis in presence of D-serine |
J Med Chem 56: 3710-24 (2013)
Article DOI: 10.1021/jm4002583
BindingDB Entry DOI: 10.7270/Q2X92CPC |
More data for this
Ligand-Target Pair | |
D-amino-acid oxidase
(Homo sapiens (Human)) | BDBM50260725
(4H-thieno[3,2-b]pyrrole-5-carboxylic acid | CHEMBL...)Show InChI InChI=1S/C7H5NO2S/c9-7(10)5-3-6-4(8-5)1-2-11-6/h1-3,8H,(H,9,10) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunovion Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant DAAO by Michaelis-Menten plot analysis in presence of D-serine |
J Med Chem 56: 3710-24 (2013)
Article DOI: 10.1021/jm4002583
BindingDB Entry DOI: 10.7270/Q2X92CPC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
D-amino-acid oxidase
(Homo sapiens (Human)) | BDBM50260722
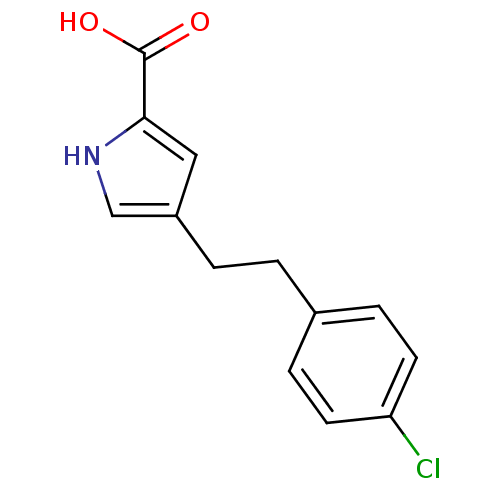
(4-(4-chlorophenethyl)-1H-pyrrole-2-carboxylic acid...)Show InChI InChI=1S/C13H12ClNO2/c14-11-5-3-9(4-6-11)1-2-10-7-12(13(16)17)15-8-10/h3-8,15H,1-2H2,(H,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunovion Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant DAAO by Michaelis-Menten plot analysis in presence of D-serine |
J Med Chem 56: 3710-24 (2013)
Article DOI: 10.1021/jm4002583
BindingDB Entry DOI: 10.7270/Q2X92CPC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
D-amino-acid oxidase
(Homo sapiens (Human)) | BDBM50206007
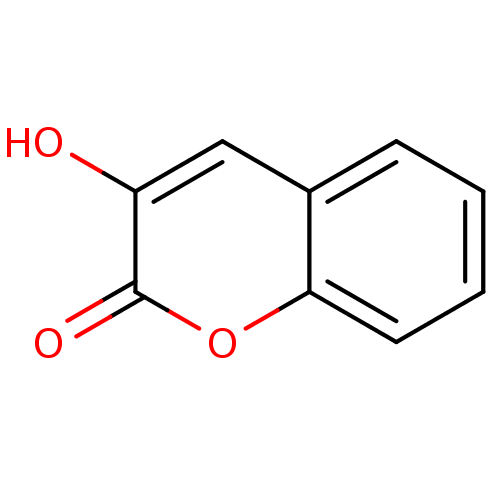
(3-Hydroxy-chromen-2-one | 3-hydroxy-2H-chromen-2-o...)Show InChI InChI=1S/C9H6O3/c10-7-5-6-3-1-2-4-8(6)12-9(7)11/h1-5,10H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunovion Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant DAAO by Michaelis-Menten plot analysis in presence of D-serine |
J Med Chem 56: 3710-24 (2013)
Article DOI: 10.1021/jm4002583
BindingDB Entry DOI: 10.7270/Q2X92CPC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
D-amino-acid oxidase
(Homo sapiens (Human)) | BDBM50433371
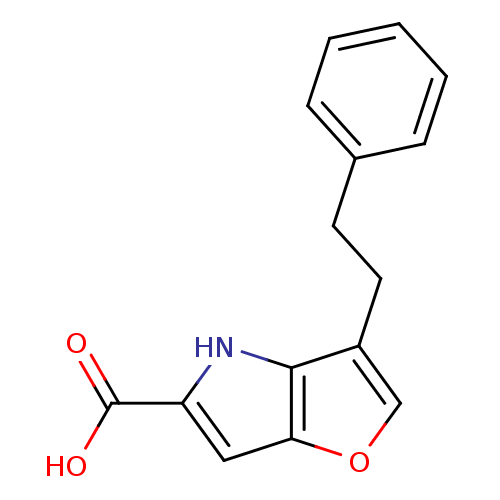
(CHEMBL2375519)Show InChI InChI=1S/C15H13NO3/c17-15(18)12-8-13-14(16-12)11(9-19-13)7-6-10-4-2-1-3-5-10/h1-5,8-9,16H,6-7H2,(H,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
| Article
PubMed
| 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunovion Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant DAAO by Michaelis-Menten plot analysis in presence of D-serine |
J Med Chem 56: 3710-24 (2013)
Article DOI: 10.1021/jm4002583
BindingDB Entry DOI: 10.7270/Q2X92CPC |
More data for this
Ligand-Target Pair | |
D-amino-acid oxidase
(Homo sapiens (Human)) | BDBM36181
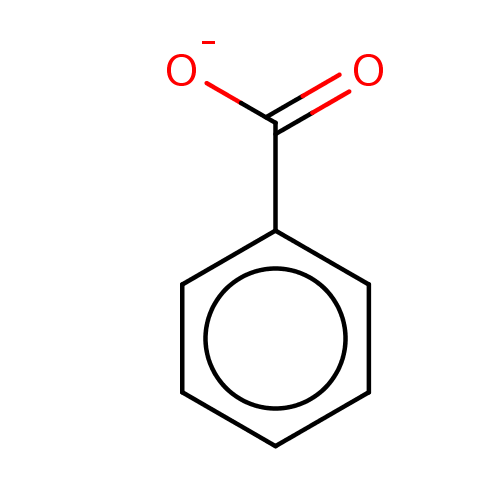
(SODIUM BENZOATE | benzoate | benzoic acid | benzoi...)Show InChI InChI=1S/C7H6O2/c8-7(9)6-4-2-1-3-5-6/h1-5H,(H,8,9)/p-1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunovion Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant DAAO by Michaelis-Menten plot analysis in presence of D-serine |
J Med Chem 56: 3710-24 (2013)
Article DOI: 10.1021/jm4002583
BindingDB Entry DOI: 10.7270/Q2X92CPC |
More data for this
Ligand-Target Pair | |
D-amino-acid oxidase
(Sus scrofa (pig)) | BDBM50004955
(1H-Indole-2-carboxylic acid | CHEMBL278390 | Indol...)Show InChI InChI=1S/C9H7NO2/c11-9(12)8-5-6-3-1-2-4-7(6)10-8/h1-5,10H,(H,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunovion Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Competitive inhibition of pig kidney DAAO in presence of D-alanine |
J Med Chem 56: 3710-24 (2013)
Article DOI: 10.1021/jm4002583
BindingDB Entry DOI: 10.7270/Q2X92CPC |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50452851
(CHEMBL2373017)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@@H]1CCSSCC[C@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)CNC)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N1)C(O)=O |wU:8.7,4.4,15.16,53.55,2.1,wD:32.34,25.26,36.38,(-13.57,-1.73,;-12.05,-1.44,;-11.05,-2.61,;-11.56,-4.06,;-9.54,-2.32,;-8.53,-3.49,;-7.02,-3.2,;-6.51,-1.75,;-6.01,-4.37,;-6.36,-5.86,;-5.05,-6.66,;-3.88,-5.66,;-4.48,-4.24,;-3.68,-2.92,;-4.42,-1.57,;-2.14,-2.95,;-1.02,-4.01,;-1.37,-5.51,;-.36,-6.68,;-1.16,-7.99,;-2.66,-7.64,;-2.79,-6.1,;-1.34,-1.64,;.2,-1.67,;.94,-3.02,;1,-.35,;.23,.98,;1,2.31,;.23,3.65,;1,4.98,;2.54,4.98,;3.31,3.65,;4.85,3.65,;5.62,4.98,;7.16,4.98,;7.93,3.65,;7.93,6.31,;7.16,7.65,;7.93,8.98,;7.16,10.31,;7.93,11.65,;7.16,12.98,;7.93,14.32,;5.62,12.98,;9.47,6.31,;10.24,7.65,;9.47,8.98,;11.78,7.65,;12.55,8.98,;14.09,8.98,;5.62,2.31,;7.16,2.35,;4.84,.82,;5.16,-.69,;6.57,-1.31,;6.73,-2.85,;8.14,-3.47,;8.3,-5,;7.05,-5.91,;7.21,-7.44,;5.64,-5.28,;5.48,-3.75,;3.83,-1.46,;3.67,-2.99,;2.68,-.43,;-9.03,-.87,;-7.51,-.58,;-10.03,.3,)| Show InChI InChI=1S/C43H65N13O10S2/c1-4-24(2)35(42(65)66)55-40(63)33-8-6-16-56(33)41(64)32(20-26-21-47-23-49-26)54-38(61)30-14-18-68-67-17-13-29(37(60)53-31(39(62)52-30)19-25-9-11-27(57)12-10-25)51-36(59)28(50-34(58)22-46-3)7-5-15-48-43(44)45/h9-12,21,23-24,28-33,35,46,57H,4-8,13-20,22H2,1-3H3,(H,47,49)(H,50,58)(H,51,59)(H,52,62)(H,53,60)(H,54,61)(H,55,63)(H,65,66)(H4,44,45,48)/t24-,28-,29-,30-,31-,32-,33-,35-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
G. D. Searle and Company
Curated by ChEMBL
| Assay Description
Concentration required to 50% inhibition in specific binding of [125- I]A-II to Angiotensin II receptor in rat uterine membrane |
J Med Chem 33: 1935-40 (1990)
BindingDB Entry DOI: 10.7270/Q2R78D70 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50334773
(CHEMBL1642904 | trans-((2R,4S)-4-(3,4-dichlorophen...)Show SMILES NC[C@@H]1C[C@@H](c2ccc(Cl)c(Cl)c2)c2ccccc2C1 |r| Show InChI InChI=1S/C17H17Cl2N/c18-16-6-5-13(9-17(16)19)15-8-11(10-20)7-12-3-1-2-4-14(12)15/h1-6,9,11,15H,7-8,10,20H2/t11-,15-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Sepracor Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]serotonin reuptake at human SERT expressed in HEK293 cells by scintillation counting |
Bioorg Med Chem 19: 663-76 (2011)
Article DOI: 10.1016/j.bmc.2010.10.034
BindingDB Entry DOI: 10.7270/Q2639Q0M |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50334781
(CHEMBL1642912 | Cis-2-((2S,4S)-4-(3,4-dichlorophen...)Show SMILES NCC[C@H]1C[C@@H](c2ccc(Cl)c(Cl)c2)c2ccccc2C1 |r| Show InChI InChI=1S/C18H19Cl2N/c19-17-6-5-14(11-18(17)20)16-10-12(7-8-21)9-13-3-1-2-4-15(13)16/h1-6,11-12,16H,7-10,21H2/t12-,16+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Sepracor Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]serotonin reuptake at human SERT expressed in HEK293 cells by scintillation counting |
Bioorg Med Chem 19: 663-76 (2011)
Article DOI: 10.1016/j.bmc.2010.10.034
BindingDB Entry DOI: 10.7270/Q2639Q0M |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM50337829
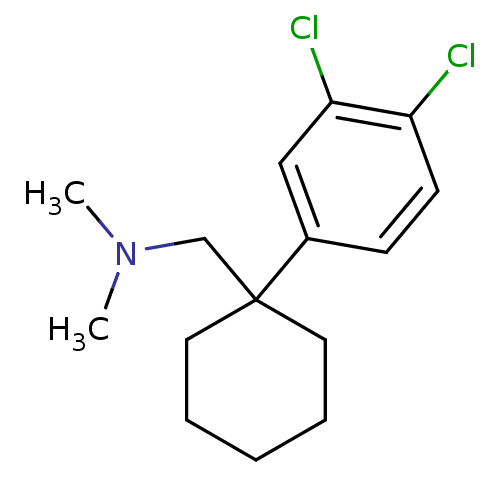
(1-(1-(3,4-dichlorophenyl)cyclohexyl)-N,N-dimethylm...)Show InChI InChI=1S/C15H21Cl2N/c1-18(2)11-15(8-4-3-5-9-15)12-6-7-13(16)14(17)10-12/h6-7,10H,3-5,8-9,11H2,1-2H3 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunovion Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant dopamine transporter |
Bioorg Med Chem Lett 21: 1438-41 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.016
BindingDB Entry DOI: 10.7270/Q2S46S72 |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM50337830
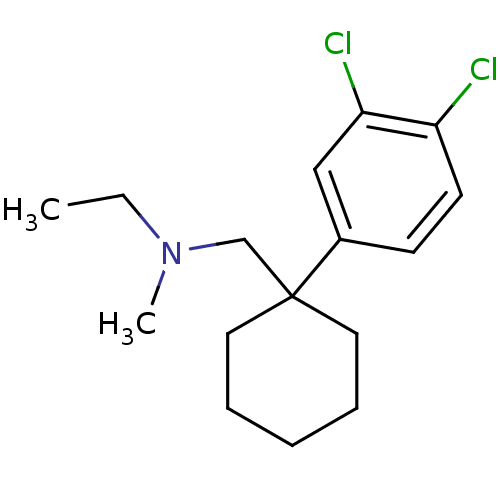
(CHEMBL1683875 | N-((1-(3,4-dichlorophenyl)cyclohex...)Show InChI InChI=1S/C16H23Cl2N/c1-3-19(2)12-16(9-5-4-6-10-16)13-7-8-14(17)15(18)11-13/h7-8,11H,3-6,9-10,12H2,1-2H3 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunovion Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant dopamine transporter |
Bioorg Med Chem Lett 21: 1438-41 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.016
BindingDB Entry DOI: 10.7270/Q2S46S72 |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM50337831

(CHEMBL1683899 | N,N-dimethyl-1-(1-(naphthalen-1-yl...)Show InChI InChI=1S/C19H25N/c1-20(2)15-19(13-6-3-7-14-19)18-12-8-10-16-9-4-5-11-17(16)18/h4-5,8-12H,3,6-7,13-15H2,1-2H3 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunovion Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant dopamine transporter |
Bioorg Med Chem Lett 21: 1438-41 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.016
BindingDB Entry DOI: 10.7270/Q2S46S72 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50228335
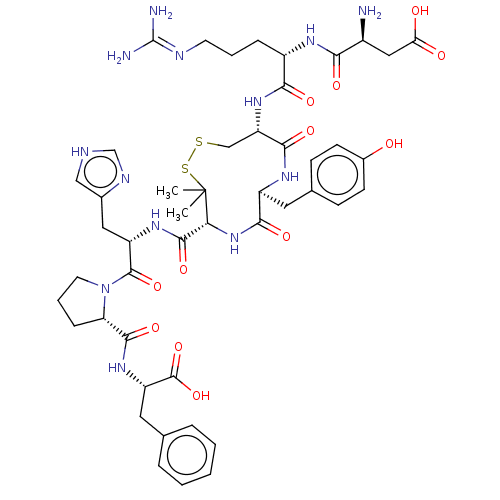
(CHEMBL263034)Show SMILES CC1(C)SSC[C@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](N)CC(O)=O)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@@H]1C(=O)N[C@@H](Cc1c[nH]cn1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O |wU:42.44,59.63,46.47,6.6,wD:63.66,30.30,21.21,10.10,(2.55,2.37,;2.49,1.14,;3.45,.38,;1.48,2.3,;,2.73,;-1.48,2.3,;-2.49,1.14,;-3.89,1.78,;-4.04,3.32,;-3.03,4.03,;-5.44,3.96,;-6.69,3.07,;-8.09,3.72,;-9.35,2.83,;-10.75,3.47,;-12.01,2.58,;-13.13,3.1,;-11.9,1.36,;-5.58,5.49,;-6.98,6.13,;-7.98,5.42,;-7.12,7.67,;-6.12,8.38,;-8.53,8.31,;-8.67,9.84,;-7.67,10.56,;-9.79,10.36,;-2.71,-.39,;-3.93,-.56,;-2.07,-1.79,;-.77,-2.62,;-1.21,-4.1,;-2.71,-4.47,;-3.77,-3.35,;-5.27,-3.7,;-5.71,-5.18,;-6.9,-5.47,;-4.65,-6.3,;-3.15,-5.94,;.77,-2.62,;1.12,-3.8,;2.06,-1.79,;2.7,-.39,;4.23,-.6,;4.7,-1.75,;5.18,.61,;6.71,.39,;7.66,1.6,;7.09,3.03,;7.91,4.32,;6.93,5.5,;5.5,4.92,;5.6,3.39,;7.29,-1.04,;6.53,-2.01,;8.82,-1.25,;9.86,-.14,;11.25,-.81,;11.04,-2.34,;9.52,-2.61,;8.83,-3.98,;9.51,-5.01,;7.3,-4.08,;6.61,-5.46,;5.07,-5.56,;4.38,-6.93,;2.85,-7.03,;2.16,-8.41,;3.02,-9.7,;4.56,-9.6,;5.24,-8.22,;7.46,-6.75,;8.69,-6.67,;6.91,-7.85,)| Show InChI InChI=1S/C47H63N13O12S2/c1-47(2)37(43(69)56-32(20-27-22-51-24-53-27)44(70)60-17-7-11-35(60)42(68)57-33(45(71)72)19-25-8-4-3-5-9-25)59-40(66)31(18-26-12-14-28(61)15-13-26)55-41(67)34(23-73-74-47)58-39(65)30(10-6-16-52-46(49)50)54-38(64)29(48)21-36(62)63/h3-5,8-9,12-15,22,24,29-35,37,61H,6-7,10-11,16-21,23,48H2,1-2H3,(H,51,53)(H,54,64)(H,55,67)(H,56,69)(H,57,68)(H,58,65)(H,59,66)(H,62,63)(H,71,72)(H4,49,50,52)/t29-,30-,31-,32-,33-,34-,35-,37+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
G. D. Searle and Company
Curated by ChEMBL
| Assay Description
Concentration required to 50% inhibition in specific binding of [125- I]A-II to Angiotensin II receptor in rat uterine membrane |
J Med Chem 33: 1935-40 (1990)
BindingDB Entry DOI: 10.7270/Q2R78D70 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50030815
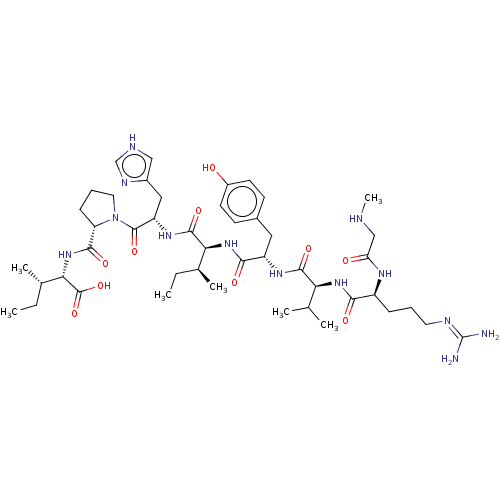
(CHEMBL404594)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)CNC)C(C)C)[C@@H](C)CC)C(O)=O |wU:8.7,25.27,wD:41.44,4.4,15.16,29.31,45.48,2.2,62.65,(8.44,-18.06,;9.36,-17.24,;9.05,-15.73,;7.88,-15.34,;10.19,-14.7,;9.88,-13.19,;11.03,-12.16,;12.2,-12.55,;10.71,-10.66,;11.74,-9.51,;10.97,-8.18,;9.46,-8.5,;9.33,-10.02,;7.99,-10.79,;7.99,-12.02,;6.66,-10.02,;5.32,-10.79,;5.32,-12.33,;4.07,-13.22,;4.55,-14.68,;6.09,-14.68,;6.56,-13.21,;6.66,-8.48,;5.33,-7.71,;4.43,-8.22,;5.33,-6.17,;3.99,-5.39,;4,-3.85,;5.06,-3.24,;2.66,-3.08,;1.33,-3.85,;-.01,-3.08,;-1.34,-3.84,;-2.67,-3.07,;-2.67,-1.53,;-3.73,-.91,;-1.33,-.76,;,-1.54,;2.67,-1.54,;4,-.77,;5.07,-1.39,;4.01,.77,;5.35,1.53,;5.36,3.07,;4.29,3.69,;6.69,3.84,;6.7,5.37,;8.04,6.14,;8.04,7.68,;9.38,8.44,;9.39,9.99,;10.45,10.6,;8.32,10.61,;8.03,3.06,;8.02,1.52,;6.94,.91,;9.35,.74,;9.34,-.8,;10.22,-1.32,;2.68,1.54,;1.61,.93,;2.69,2.78,;6.66,-5.4,;7.73,-6.01,;6.66,-3.86,;7.73,-3.24,;11.66,-15.17,;11.91,-16.38,;12.58,-14.35,)| Show InChI InChI=1S/C46H73N13O10/c1-8-26(5)37(43(66)55-33(21-29-22-50-24-52-29)44(67)59-19-11-13-34(59)41(64)58-38(45(68)69)27(6)9-2)57-40(63)32(20-28-14-16-30(60)17-15-28)54-42(65)36(25(3)4)56-39(62)31(53-35(61)23-49-7)12-10-18-51-46(47)48/h14-17,22,24-27,31-34,36-38,49,60H,8-13,18-21,23H2,1-7H3,(H,50,52)(H,53,61)(H,54,65)(H,55,66)(H,56,62)(H,57,63)(H,58,64)(H,68,69)(H4,47,48,51)/t26-,27-,31-,32-,33-,34-,36-,37-,38-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
G. D. Searle and Company
Curated by ChEMBL
| Assay Description
Concentration required to 50% inhibition in specific binding of [125- I]A-II to Angiotensin II receptor from rat uterine membrane |
J Med Chem 33: 1935-40 (1990)
BindingDB Entry DOI: 10.7270/Q2R78D70 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50334787
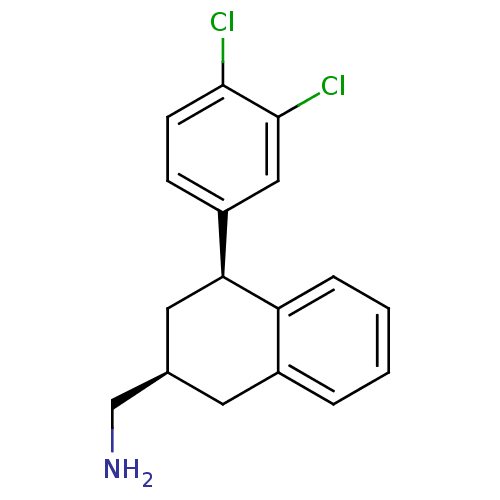
(CHEMBL1642902 | cis-((2S,4S)-4-(3,4-dichlorophenyl...)Show SMILES NC[C@H]1C[C@@H](c2ccc(Cl)c(Cl)c2)c2ccccc2C1 |r| Show InChI InChI=1S/C17H17Cl2N/c18-16-6-5-13(9-17(16)19)15-8-11(10-20)7-12-3-1-2-4-14(12)15/h1-6,9,11,15H,7-8,10,20H2/t11-,15+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Sepracor Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]serotonin reuptake at human SERT expressed in HEK293 cells by scintillation counting |
Bioorg Med Chem 19: 663-76 (2011)
Article DOI: 10.1016/j.bmc.2010.10.034
BindingDB Entry DOI: 10.7270/Q2639Q0M |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM50337832
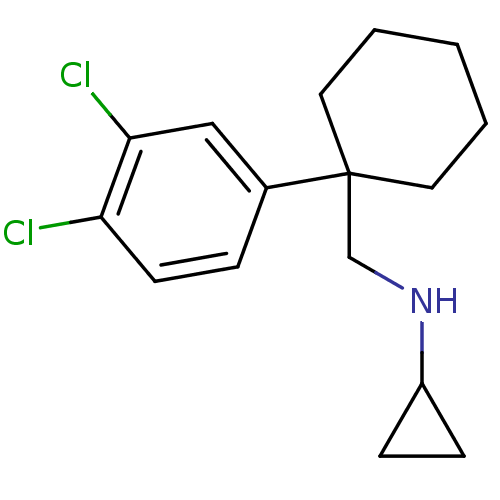
(CHEMBL1683873 | N-((1-(3,4-dichlorophenyl)cyclohex...)Show InChI InChI=1S/C16H21Cl2N/c17-14-7-4-12(10-15(14)18)16(8-2-1-3-9-16)11-19-13-5-6-13/h4,7,10,13,19H,1-3,5-6,8-9,11H2 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunovion Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant dopamine transporter |
Bioorg Med Chem Lett 21: 1438-41 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.016
BindingDB Entry DOI: 10.7270/Q2S46S72 |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM50337823
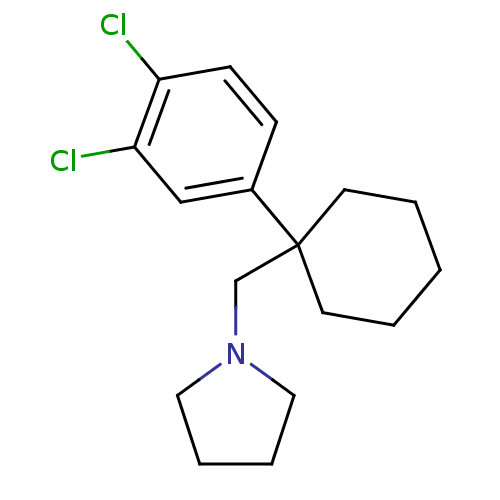
(1-((1-(3,4-dichlorophenyl)cyclohexyl)methyl)pyrrol...)Show InChI InChI=1S/C17H23Cl2N/c18-15-7-6-14(12-16(15)19)17(8-2-1-3-9-17)13-20-10-4-5-11-20/h6-7,12H,1-5,8-11,13H2 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunovion Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant dopamine transporter |
Bioorg Med Chem Lett 21: 1438-41 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.016
BindingDB Entry DOI: 10.7270/Q2S46S72 |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM50337822
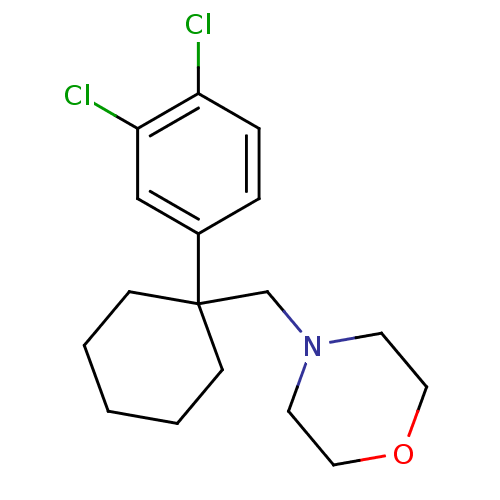
(4-((1-(3,4-dichlorophenyl)cyclohexyl)methyl)morpho...)Show InChI InChI=1S/C17H23Cl2NO/c18-15-5-4-14(12-16(15)19)17(6-2-1-3-7-17)13-20-8-10-21-11-9-20/h4-5,12H,1-3,6-11,13H2 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunovion Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant dopamine transporter |
Bioorg Med Chem Lett 21: 1438-41 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.016
BindingDB Entry DOI: 10.7270/Q2S46S72 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50228272
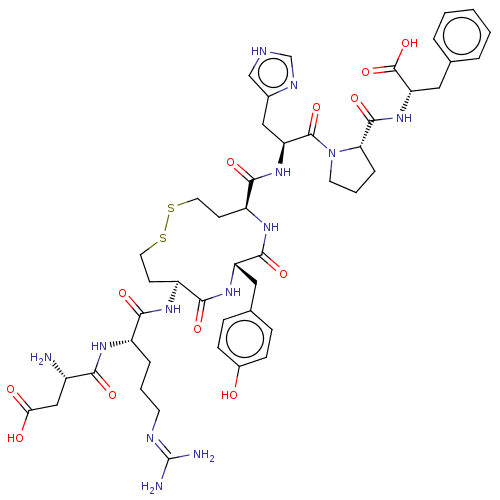
(CHEMBL411997)Show SMILES N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@H]1CCSSCC[C@H](NC(=O)[C@H](Cc2ccc(O)cc2)NC1=O)C(=O)N[C@@H](Cc1c[nH]cn1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O |wU:59.63,46.47,9.8,31.31,1.0,wD:20.19,27.44,63.66,(4.96,9.59,;6.07,9.07,;7.34,9.95,;7.21,11.48,;6.1,12.01,;8.22,12.19,;6.2,7.53,;7.31,7.01,;4.93,6.66,;5.06,5.12,;6.45,4.47,;7.72,5.35,;9.11,4.69,;10.38,5.57,;11.77,4.91,;12.78,5.62,;11.87,3.69,;3.79,4.24,;2.68,4.77,;3.92,2.71,;2.65,1.83,;1.49,2.85,;,3.22,;-1.5,2.85,;-2.65,1.83,;-3.19,.39,;-3.01,-1.14,;-2.13,-2.4,;-.77,-3.12,;.77,-3.12,;1.06,-4.32,;2.13,-2.4,;3.16,-3.57,;4.67,-3.26,;5.69,-4.41,;7.19,-4.11,;7.68,-2.65,;8.89,-2.4,;6.66,-1.5,;5.15,-1.8,;3.01,-1.14,;3.19,.39,;4.41,.54,;-3.16,-3.57,;-4.37,-3.32,;-2.67,-5.03,;-3.69,-6.18,;-3.2,-7.64,;-1.69,-7.95,;-1.07,-9.35,;.46,-9.17,;.76,-7.66,;-.58,-6.9,;-5.2,-5.88,;-5.59,-4.71,;-6.21,-7.04,;-5.86,-8.52,;-7.18,-9.31,;-8.34,-8.29,;-7.73,-6.88,;-8.51,-5.55,;-9.74,-5.56,;-7.74,-4.21,;-8.52,-2.88,;-7.75,-1.54,;-8.52,-.21,;-7.76,1.12,;-8.54,2.46,;-10.08,2.45,;-10.84,1.11,;-10.06,-.22,;-10.06,-2.88,;-10.67,-3.95,;-10.68,-1.82,)| Show InChI InChI=1S/C47H63N13O12S2/c48-30(23-38(62)63)39(64)54-31(8-4-16-52-47(49)50)40(65)55-32-14-18-73-74-19-15-33(56-43(68)34(57-41(32)66)20-27-10-12-29(61)13-11-27)42(67)58-35(22-28-24-51-25-53-28)45(70)60-17-5-9-37(60)44(69)59-36(46(71)72)21-26-6-2-1-3-7-26/h1-3,6-7,10-13,24-25,30-37,61H,4-5,8-9,14-23,48H2,(H,51,53)(H,54,64)(H,55,65)(H,56,68)(H,57,66)(H,58,67)(H,59,69)(H,62,63)(H,71,72)(H4,49,50,52)/t30-,31-,32-,33-,34-,35-,36-,37-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
G. D. Searle and Company
Curated by ChEMBL
| Assay Description
pA2 value for Angiotensin II receptor |
J Med Chem 33: 1935-40 (1990)
BindingDB Entry DOI: 10.7270/Q2R78D70 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50228195

(Angiotensin Ii | CHEBI:2719)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](N)CC(O)=O)C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C50H71N13O12/c1-5-28(4)41(47(72)59-36(23-31-25-54-26-56-31)48(73)63-20-10-14-38(63)45(70)60-37(49(74)75)22-29-11-7-6-8-12-29)62-44(69)35(21-30-15-17-32(64)18-16-30)58-46(71)40(27(2)3)61-43(68)34(13-9-19-55-50(52)53)57-42(67)33(51)24-39(65)66/h6-8,11-12,15-18,25-28,33-38,40-41,64H,5,9-10,13-14,19-24,51H2,1-4H3,(H,54,56)(H,57,67)(H,58,71)(H,59,72)(H,60,70)(H,61,68)(H,62,69)(H,65,66)(H,74,75)(H4,52,53,55)/t28-,33-,34-,35-,36-,37-,38-,40-,41-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
G. D. Searle and Company
Curated by ChEMBL
| Assay Description
Concentration required to 50% inhibition in specific binding of [125- I]A-II to Angiotensin II receptor in rat uterine membrane |
J Med Chem 33: 1935-40 (1990)
BindingDB Entry DOI: 10.7270/Q2R78D70 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50228308
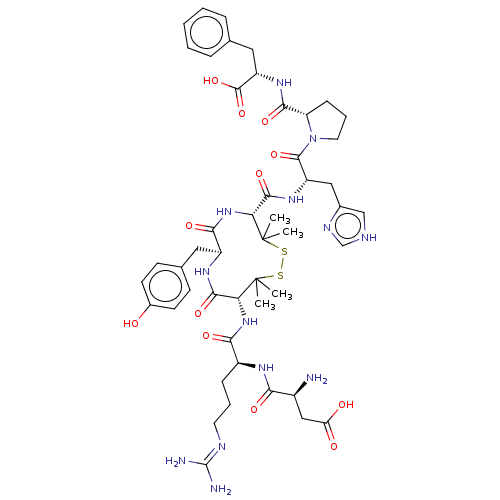
(CHEMBL413740)Show SMILES CC1(C)SSC(C)(C)[C@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](N)CC(O)=O)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@@H]1C(=O)N[C@@H](Cc1c[nH]cn1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O |wU:44.46,8.8,61.65,48.49,wD:65.68,32.32,23.23,12.12,(2.55,2.37,;2.49,1.14,;3.45,.38,;1.48,2.3,;,2.73,;-1.48,2.3,;-2.71,2.19,;-.86,3.37,;-2.49,1.14,;-3.89,1.78,;-4.04,3.32,;-3.03,4.03,;-5.44,3.96,;-6.7,3.07,;-8.09,3.72,;-9.35,2.83,;-10.75,3.47,;-12.01,2.58,;-13.13,3.1,;-11.9,1.36,;-5.58,5.49,;-6.98,6.14,;-7.98,5.42,;-7.12,7.67,;-6.12,8.38,;-8.53,8.31,;-8.67,9.84,;-7.67,10.56,;-9.79,10.36,;-2.71,-.39,;-3.93,-.56,;-2.07,-1.79,;-.77,-2.62,;-1.21,-4.1,;-2.71,-4.47,;-3.77,-3.35,;-5.27,-3.7,;-5.71,-5.18,;-6.9,-5.47,;-4.65,-6.3,;-3.15,-5.94,;.77,-2.62,;1.12,-3.8,;2.06,-1.79,;2.7,-.39,;4.24,-.6,;4.7,-1.75,;5.19,.61,;6.71,.39,;7.66,1.6,;7.09,3.03,;7.91,4.32,;6.93,5.5,;5.5,4.92,;5.6,3.39,;7.29,-1.04,;6.53,-2.01,;8.82,-1.25,;9.86,-.14,;11.25,-.81,;11.04,-2.34,;9.52,-2.61,;8.83,-3.98,;9.52,-5.01,;7.3,-4.08,;6.61,-5.46,;5.07,-5.56,;4.39,-6.94,;2.85,-7.03,;2.16,-8.42,;3.02,-9.7,;4.56,-9.6,;5.24,-8.22,;7.46,-6.75,;8.69,-6.67,;6.91,-7.85,)| Show InChI InChI=1S/C49H67N13O12S2/c1-48(2)37(60-40(67)31(12-8-18-54-47(51)52)56-39(66)30(50)23-36(64)65)43(70)57-32(20-27-14-16-29(63)17-15-27)41(68)61-38(49(3,4)76-75-48)44(71)58-33(22-28-24-53-25-55-28)45(72)62-19-9-13-35(62)42(69)59-34(46(73)74)21-26-10-6-5-7-11-26/h5-7,10-11,14-17,24-25,30-35,37-38,63H,8-9,12-13,18-23,50H2,1-4H3,(H,53,55)(H,56,66)(H,57,70)(H,58,71)(H,59,69)(H,60,67)(H,61,68)(H,64,65)(H,73,74)(H4,51,52,54)/t30-,31-,32-,33-,34-,35-,37+,38+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
G. D. Searle and Company
Curated by ChEMBL
| Assay Description
Concentration required to 50% inhibition in specific binding of [125- I]A-II to Angiotensin II receptor in rat uterine membrane |
J Med Chem 33: 1935-40 (1990)
BindingDB Entry DOI: 10.7270/Q2R78D70 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50028094
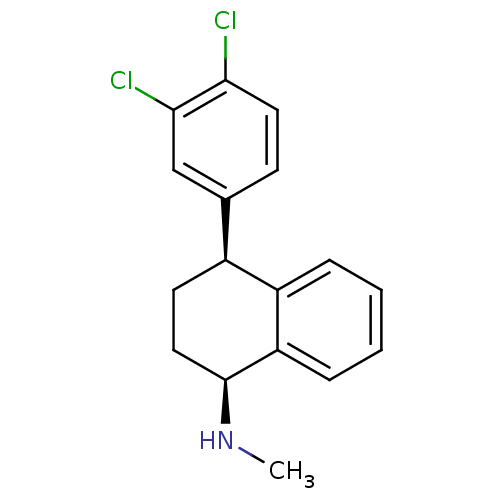
((1S,4S)-4-(3,4-dichlorophenyl)-N-methyl-1,2,3,4-te...)Show SMILES CN[C@H]1CC[C@@H](c2ccc(Cl)c(Cl)c2)c2ccccc12 |r| Show InChI InChI=1S/C17H17Cl2N/c1-20-17-9-7-12(13-4-2-3-5-14(13)17)11-6-8-15(18)16(19)10-11/h2-6,8,10,12,17,20H,7,9H2,1H3/t12-,17-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sepracor Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]serotonin reuptake at human SERT expressed in HEK293 cells by scintillation counting |
Bioorg Med Chem 19: 663-76 (2011)
Article DOI: 10.1016/j.bmc.2010.10.034
BindingDB Entry DOI: 10.7270/Q2639Q0M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50334770
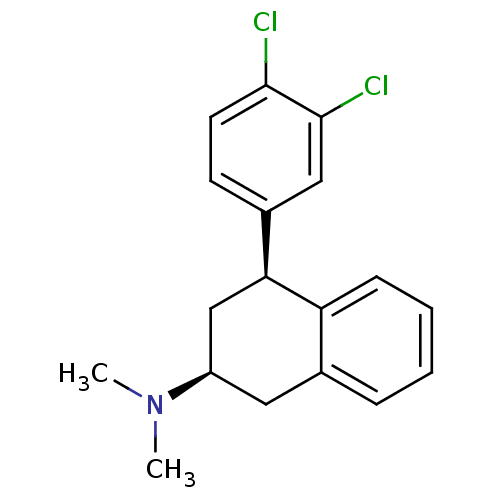
(CHEMBL1642900 | cis-(2S,4S)-4-(3,4-dichlorophenyl)...)Show SMILES CN(C)[C@H]1C[C@@H](c2ccc(Cl)c(Cl)c2)c2ccccc2C1 |r| Show InChI InChI=1S/C18H19Cl2N/c1-21(2)14-9-12-5-3-4-6-15(12)16(11-14)13-7-8-17(19)18(20)10-13/h3-8,10,14,16H,9,11H2,1-2H3/t14-,16+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sepracor Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]serotonin reuptake at human SERT expressed in HEK293 cells by scintillation counting |
Bioorg Med Chem 19: 663-76 (2011)
Article DOI: 10.1016/j.bmc.2010.10.034
BindingDB Entry DOI: 10.7270/Q2639Q0M |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50334916

(1-(3,4-dichlorophenyl)-N,N-dimethyl-1,2,3,4-tetrah...)Show InChI InChI=1S/C17H18Cl2N2/c1-20(2)16-9-10-21(17-6-4-3-5-13(16)17)12-7-8-14(18)15(19)11-12/h3-8,11,16H,9-10H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunovion Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant 5HT transporter |
Bioorg Med Chem Lett 21: 520-3 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.086
BindingDB Entry DOI: 10.7270/Q2DJ5FWS |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50337833
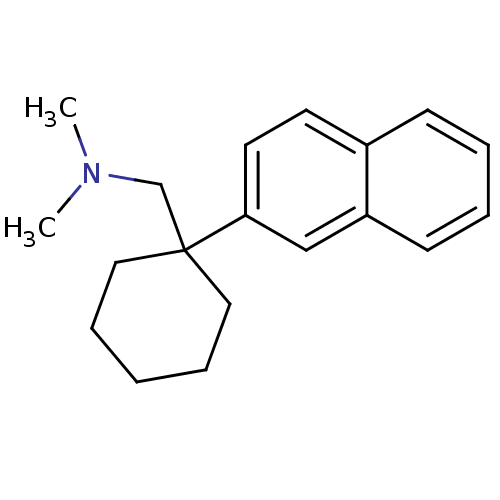
(CHEMBL1683902 | N,N-dimethyl-1-(1-(naphthalen-2-yl...)Show InChI InChI=1S/C19H25N/c1-20(2)15-19(12-6-3-7-13-19)18-11-10-16-8-4-5-9-17(16)14-18/h4-5,8-11,14H,3,6-7,12-13,15H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunovion Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant serotonin transporter |
Bioorg Med Chem Lett 21: 1438-41 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.016
BindingDB Entry DOI: 10.7270/Q2S46S72 |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM50337833

(CHEMBL1683902 | N,N-dimethyl-1-(1-(naphthalen-2-yl...)Show InChI InChI=1S/C19H25N/c1-20(2)15-19(12-6-3-7-13-19)18-11-10-16-8-4-5-9-17(16)14-18/h4-5,8-11,14H,3,6-7,12-13,15H2,1-2H3 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunovion Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant dopamine transporter |
Bioorg Med Chem Lett 21: 1438-41 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.016
BindingDB Entry DOI: 10.7270/Q2S46S72 |
More data for this
Ligand-Target Pair | |
D-amino-acid oxidase
(Homo sapiens (Human)) | BDBM50433372

(CHEMBL2375520)Show InChI InChI=1S/C7H3Cl2NO2/c8-4-1-3-6(2-5(4)9)12-10-7(3)11/h1-2H,(H,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunovion Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant DAAO after 1 hr by coupled enzyme assay in presence of D-serine |
J Med Chem 56: 3710-24 (2013)
Article DOI: 10.1021/jm4002583
BindingDB Entry DOI: 10.7270/Q2X92CPC |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM50337834
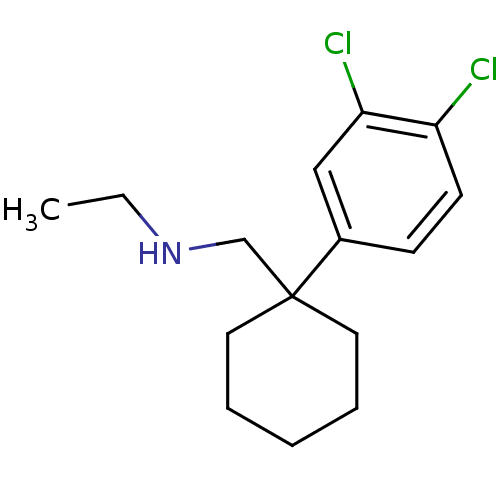
(CHEMBL1683872 | N-((1-(3,4-dichlorophenyl)cyclohex...)Show InChI InChI=1S/C15H21Cl2N/c1-2-18-11-15(8-4-3-5-9-15)12-6-7-13(16)14(17)10-12/h6-7,10,18H,2-5,8-9,11H2,1H3 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunovion Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant dopamine transporter |
Bioorg Med Chem Lett 21: 1438-41 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.016
BindingDB Entry DOI: 10.7270/Q2S46S72 |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50337829

(1-(1-(3,4-dichlorophenyl)cyclohexyl)-N,N-dimethylm...)Show InChI InChI=1S/C15H21Cl2N/c1-18(2)11-15(8-4-3-5-9-15)12-6-7-13(16)14(17)10-12/h6-7,10H,3-5,8-9,11H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunovion Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Norepinephrine transporter |
Bioorg Med Chem Lett 21: 1438-41 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.016
BindingDB Entry DOI: 10.7270/Q2S46S72 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM30130

(CHEMBL1201082 | CHEMBL41 | Fluoxetin | Fluoxetine ...)Show InChI InChI=1S/C17H18F3NO/c1-21-12-11-16(13-5-3-2-4-6-13)22-15-9-7-14(8-10-15)17(18,19)20/h2-10,16,21H,11-12H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Sepracor Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]serotonin reuptake at human SERT expressed in HEK293 cells by scintillation counting |
Bioorg Med Chem 19: 663-76 (2011)
Article DOI: 10.1016/j.bmc.2010.10.034
BindingDB Entry DOI: 10.7270/Q2639Q0M |
More data for this
Ligand-Target Pair | |
D-amino-acid oxidase
(Homo sapiens (Human)) | BDBM50260725

(4H-thieno[3,2-b]pyrrole-5-carboxylic acid | CHEMBL...)Show InChI InChI=1S/C7H5NO2S/c9-7(10)5-3-6-4(8-5)1-2-11-6/h1-3,8H,(H,9,10) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunovion Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant DAAO after 1 hr by coupled enzyme assay in presence of D-serine |
J Med Chem 56: 3710-24 (2013)
Article DOI: 10.1021/jm4002583
BindingDB Entry DOI: 10.7270/Q2X92CPC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
D-amino-acid oxidase
(Homo sapiens (Human)) | BDBM50260725

(4H-thieno[3,2-b]pyrrole-5-carboxylic acid | CHEMBL...)Show InChI InChI=1S/C7H5NO2S/c9-7(10)5-3-6-4(8-5)1-2-11-6/h1-3,8H,(H,9,10) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunovion Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of DAAO (unknown origin) |
J Med Chem 56: 3710-24 (2013)
Article DOI: 10.1021/jm4002583
BindingDB Entry DOI: 10.7270/Q2X92CPC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50334764
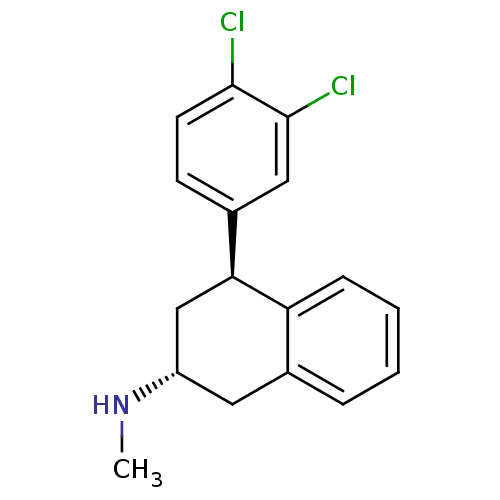
(CHEMBL1642894 | trans-(2R,4S)-4-(3,4-dichloropheny...)Show SMILES CN[C@@H]1C[C@@H](c2ccc(Cl)c(Cl)c2)c2ccccc2C1 |r| Show InChI InChI=1S/C17H17Cl2N/c1-20-13-8-11-4-2-3-5-14(11)15(10-13)12-6-7-16(18)17(19)9-12/h2-7,9,13,15,20H,8,10H2,1H3/t13-,15-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Sepracor Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]serotonin reuptake at human SERT expressed in HEK293 cells by scintillation counting |
Bioorg Med Chem 19: 663-76 (2011)
Article DOI: 10.1016/j.bmc.2010.10.034
BindingDB Entry DOI: 10.7270/Q2639Q0M |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50337912

(CHEMBL1684056 | N-methyl-1-(1-(naphthalen-2-yl)cyc...)Show InChI InChI=1S/C19H25N/c1-15(20-2)19(12-6-3-7-13-19)18-11-10-16-8-4-5-9-17(16)14-18/h4-5,8-11,14-15,20H,3,6-7,12-13H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunovion Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]serotonin reuptake at human recombinant SERT expressed in LLC-PK1 cells by liquid scintillation counting |
Bioorg Med Chem Lett 21: 1434-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.019
BindingDB Entry DOI: 10.7270/Q2CV4J2P |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50337912

(CHEMBL1684056 | N-methyl-1-(1-(naphthalen-2-yl)cyc...)Show InChI InChI=1S/C19H25N/c1-15(20-2)19(12-6-3-7-13-19)18-11-10-16-8-4-5-9-17(16)14-18/h4-5,8-11,14-15,20H,3,6-7,12-13H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunovion Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]serotonin reuptake at human recombinant SERT expressed in LLC-PK1 cells by liquid scintillation counting |
Bioorg Med Chem Lett 21: 1434-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.019
BindingDB Entry DOI: 10.7270/Q2CV4J2P |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50337818

(CHEMBL1683876 | N-((1-(3,4-dichlorophenyl)cyclohex...)Show InChI InChI=1S/C17H25Cl2N/c1-3-20(4-2)13-17(10-6-5-7-11-17)14-8-9-15(18)16(19)12-14/h8-9,12H,3-7,10-11,13H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunovion Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Norepinephrine transporter |
Bioorg Med Chem Lett 21: 1438-41 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.016
BindingDB Entry DOI: 10.7270/Q2S46S72 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50334768

(CHEMBL1642898 | trans-(2R,4S)-4-(3,4-dichloropheny...)Show SMILES CN(C)[C@@H]1C[C@@H](c2ccc(Cl)c(Cl)c2)c2ccccc2C1 |r| Show InChI InChI=1S/C18H19Cl2N/c1-21(2)14-9-12-5-3-4-6-15(12)16(11-14)13-7-8-17(19)18(20)10-13/h3-8,10,14,16H,9,11H2,1-2H3/t14-,16-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Sepracor Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]serotonin reuptake at human SERT expressed in HEK293 cells by scintillation counting |
Bioorg Med Chem 19: 663-76 (2011)
Article DOI: 10.1016/j.bmc.2010.10.034
BindingDB Entry DOI: 10.7270/Q2639Q0M |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50334920
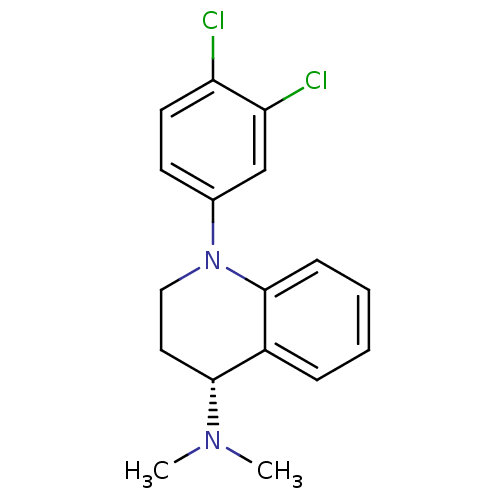
((R)-1-(3,4-dichlorophenyl)-N,N-dimethyl-1,2,3,4-te...)Show SMILES CN(C)[C@@H]1CCN(c2ccc(Cl)c(Cl)c2)c2ccccc12 |r| Show InChI InChI=1S/C17H18Cl2N2/c1-20(2)16-9-10-21(17-6-4-3-5-13(16)17)12-7-8-14(18)15(19)11-12/h3-8,11,16H,9-10H2,1-2H3/t16-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunovion Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant 5HT transporter |
Bioorg Med Chem Lett 21: 520-3 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.086
BindingDB Entry DOI: 10.7270/Q2DJ5FWS |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50337911
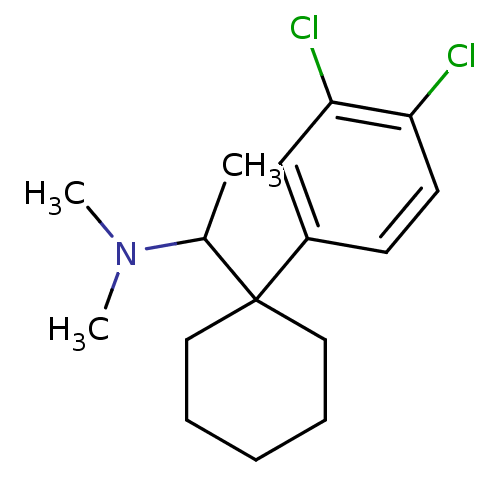
((+/-)-1-(1-(3,4-dichlorophenyl)cyclohexyl)-N,N-dim...)Show InChI InChI=1S/C16H23Cl2N/c1-12(19(2)3)16(9-5-4-6-10-16)13-7-8-14(17)15(18)11-13/h7-8,11-12H,4-6,9-10H2,1-3H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunovion Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]norepinephrine reuptake at human human recombinant NET expressed in HEK293 cells by scintillation counting |
Bioorg Med Chem Lett 21: 1434-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.019
BindingDB Entry DOI: 10.7270/Q2CV4J2P |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50337911

((+/-)-1-(1-(3,4-dichlorophenyl)cyclohexyl)-N,N-dim...)Show InChI InChI=1S/C16H23Cl2N/c1-12(19(2)3)16(9-5-4-6-10-16)13-7-8-14(17)15(18)11-13/h7-8,11-12H,4-6,9-10H2,1-3H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunovion Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]norepinephrine reuptake at human human recombinant NET expressed in HEK293 cells by scintillation counting |
Bioorg Med Chem Lett 21: 1434-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.019
BindingDB Entry DOI: 10.7270/Q2CV4J2P |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50337833

(CHEMBL1683902 | N,N-dimethyl-1-(1-(naphthalen-2-yl...)Show InChI InChI=1S/C19H25N/c1-20(2)15-19(12-6-3-7-13-19)18-11-10-16-8-4-5-9-17(16)14-18/h4-5,8-11,14H,3,6-7,12-13,15H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunovion Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Norepinephrine transporter |
Bioorg Med Chem Lett 21: 1438-41 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.016
BindingDB Entry DOI: 10.7270/Q2S46S72 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50334766
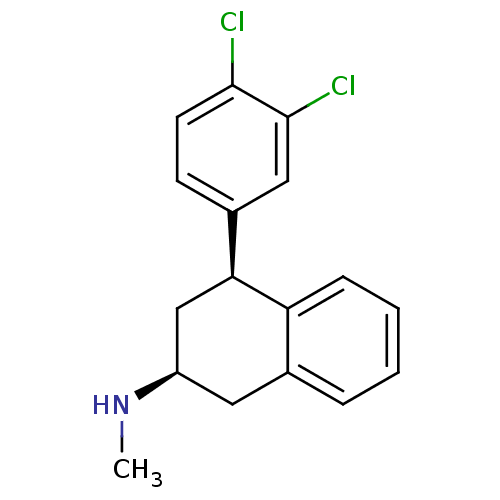
(CHEMBL1642896 | cis-(2S,4S)-4-(3,4-dichlorophenyl)...)Show SMILES CN[C@H]1C[C@@H](c2ccc(Cl)c(Cl)c2)c2ccccc2C1 |r| Show InChI InChI=1S/C17H17Cl2N/c1-20-13-8-11-4-2-3-5-14(11)15(10-13)12-6-7-16(18)17(19)9-12/h2-7,9,13,15,20H,8,10H2,1H3/t13-,15+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Sepracor Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]serotonin reuptake at human SERT expressed in HEK293 cells by scintillation counting |
Bioorg Med Chem 19: 663-76 (2011)
Article DOI: 10.1016/j.bmc.2010.10.034
BindingDB Entry DOI: 10.7270/Q2639Q0M |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
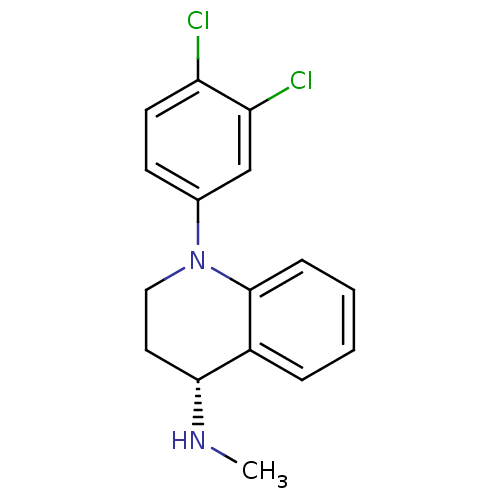
(Homo sapiens (Human)) | BDBM50334919
((R)-1-(3,4-dichlorophenyl)-N-methyl-1,2,3,4-tetrah...)Show SMILES CN[C@@H]1CCN(c2ccc(Cl)c(Cl)c2)c2ccccc12 |r| Show InChI InChI=1S/C16H16Cl2N2/c1-19-15-8-9-20(16-5-3-2-4-12(15)16)11-6-7-13(17)14(18)10-11/h2-7,10,15,19H,8-9H2,1H3/t15-/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunovion Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant dopamine transporter |
Bioorg Med Chem Lett 21: 520-3 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.086
BindingDB Entry DOI: 10.7270/Q2DJ5FWS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50334783
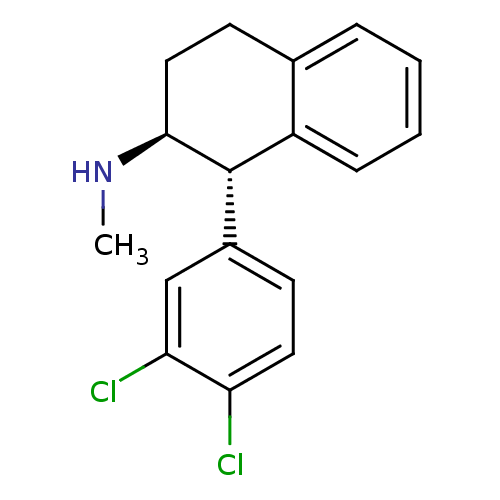
(CHEMBL1642914 | trans-(1S,2S)-1-(3,4-dichloropheny...)Show SMILES CN[C@H]1CCc2ccccc2[C@@H]1c1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C17H17Cl2N/c1-20-16-9-7-11-4-2-3-5-13(11)17(16)12-6-8-14(18)15(19)10-12/h2-6,8,10,16-17,20H,7,9H2,1H3/t16-,17-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Sepracor Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem 19: 663-76 (2011)
Article DOI: 10.1016/j.bmc.2010.10.034
BindingDB Entry DOI: 10.7270/Q2639Q0M |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM22417
(3-(2-methoxyphenoxy)-N-methyl-3-phenylpropan-1-ami...)Show InChI InChI=1S/C17H21NO2/c1-18-13-12-15(14-8-4-3-5-9-14)20-17-11-7-6-10-16(17)19-2/h3-11,15,18H,12-13H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Sepracor Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]norepinephrine reuptake at human NET expressed in MDCK cells by scintillation counting |
Bioorg Med Chem 19: 663-76 (2011)
Article DOI: 10.1016/j.bmc.2010.10.034
BindingDB Entry DOI: 10.7270/Q2639Q0M |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50334775
(CHEMBL1642906 | trans-1-((2R,4S)-4-(3,4-dichloroph...)Show SMILES CNC[C@@H]1C[C@@H](c2ccc(Cl)c(Cl)c2)c2ccccc2C1 |r| Show InChI InChI=1S/C18H19Cl2N/c1-21-11-12-8-13-4-2-3-5-15(13)16(9-12)14-6-7-17(19)18(20)10-14/h2-7,10,12,16,21H,8-9,11H2,1H3/t12-,16-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Sepracor Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]serotonin reuptake at human SERT expressed in HEK293 cells by scintillation counting |
Bioorg Med Chem 19: 663-76 (2011)
Article DOI: 10.1016/j.bmc.2010.10.034
BindingDB Entry DOI: 10.7270/Q2639Q0M |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM50337835

(1-(1-(3,4-dichlorophenyl)cyclopentyl)-N,N-dimethyl...)Show InChI InChI=1S/C14H19Cl2N/c1-17(2)10-14(7-3-4-8-14)11-5-6-12(15)13(16)9-11/h5-6,9H,3-4,7-8,10H2,1-2H3 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunovion Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant dopamine transporter |
Bioorg Med Chem Lett 21: 1438-41 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.016
BindingDB Entry DOI: 10.7270/Q2S46S72 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data