 Found 104 hits with Last Name = 'stier' and Initial = 'm'
Found 104 hits with Last Name = 'stier' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50205450
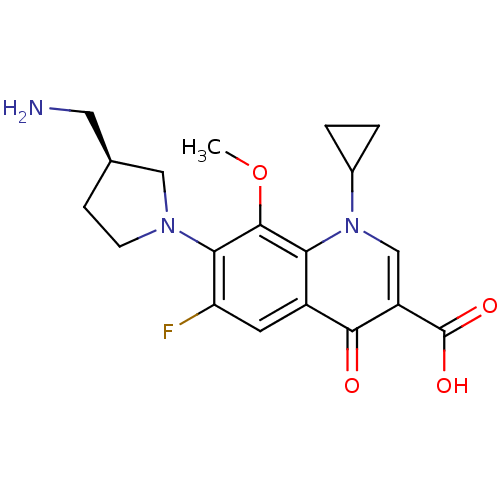
((S)-7-(3-(aminomethyl)pyrrolidin-1-yl)-1-cycloprop...)Show SMILES COc1c(N2CC[C@@H](CN)C2)c(F)cc2c1n(cc(C(O)=O)c2=O)C1CC1 Show InChI InChI=1S/C19H22FN3O4/c1-27-18-15-12(6-14(20)16(18)22-5-4-10(7-21)8-22)17(24)13(19(25)26)9-23(15)11-2-3-11/h6,9-11H,2-5,7-8,21H2,1H3,(H,25,26)/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG by fliter binding assay |
Bioorg Med Chem Lett 17: 2150-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.090
BindingDB Entry DOI: 10.7270/Q2GM86Z7 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50205452
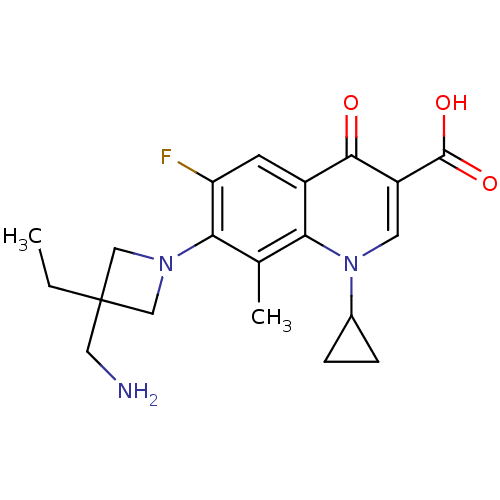
(7-(3-(aminomethyl)-3-ethylazetidin-1-yl)-1-cyclopr...)Show SMILES CCC1(CN)CN(C1)c1c(F)cc2c(c1C)n(cc(C(O)=O)c2=O)C1CC1 Show InChI InChI=1S/C20H24FN3O3/c1-3-20(8-22)9-23(10-20)17-11(2)16-13(6-15(17)21)18(25)14(19(26)27)7-24(16)12-4-5-12/h6-7,12H,3-5,8-10,22H2,1-2H3,(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG by fliter binding assay |
Bioorg Med Chem Lett 17: 2150-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.090
BindingDB Entry DOI: 10.7270/Q2GM86Z7 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50205465
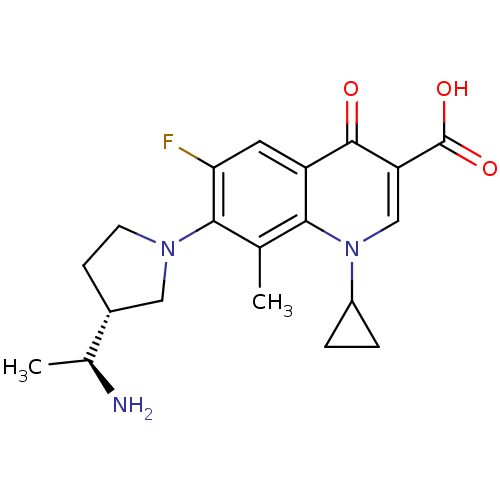
(7-((R)-3-((S)-1-aminoethyl)pyrrolidin-1-yl)-1-cycl...)Show SMILES C[C@H](N)[C@@H]1CCN(C1)c1c(F)cc2c(c1C)n(cc(C(O)=O)c2=O)C1CC1 Show InChI InChI=1S/C20H24FN3O3/c1-10-17-14(19(25)15(20(26)27)9-24(17)13-3-4-13)7-16(21)18(10)23-6-5-12(8-23)11(2)22/h7,9,11-13H,3-6,8,22H2,1-2H3,(H,26,27)/t11-,12+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG by fliter binding assay |
Bioorg Med Chem Lett 17: 2150-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.090
BindingDB Entry DOI: 10.7270/Q2GM86Z7 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50205451
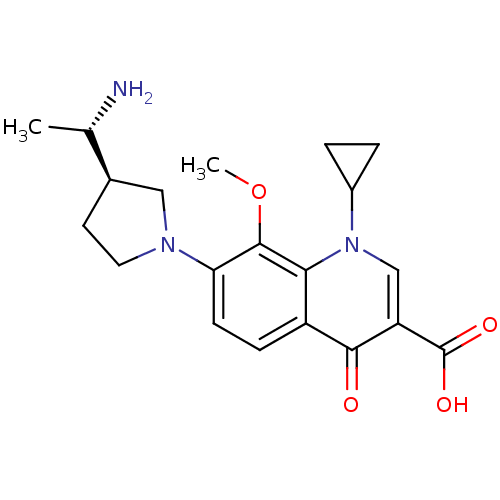
(7-((R)-3-((S)-1-aminoethyl)pyrrolidin-1-yl)-1-cycl...)Show SMILES COc1c(ccc2c1n(cc(C(O)=O)c2=O)C1CC1)N1CC[C@H](C1)[C@H](C)N Show InChI InChI=1S/C20H25N3O4/c1-11(21)12-7-8-22(9-12)16-6-5-14-17(19(16)27-2)23(13-3-4-13)10-15(18(14)24)20(25)26/h5-6,10-13H,3-4,7-9,21H2,1-2H3,(H,25,26)/t11-,12+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG by fliter binding assay |
Bioorg Med Chem Lett 17: 2150-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.090
BindingDB Entry DOI: 10.7270/Q2GM86Z7 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50205466
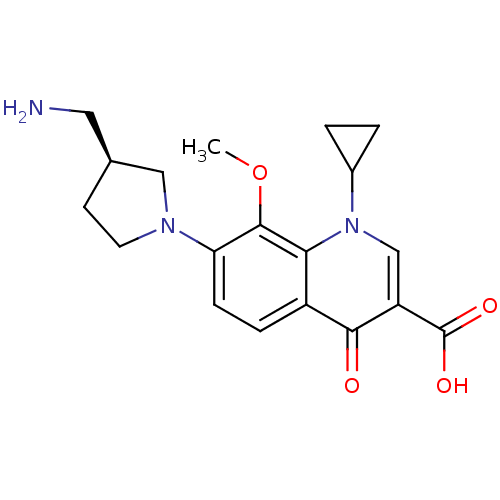
((S)-7-(3-(aminomethyl)pyrrolidin-1-yl)-1-cycloprop...)Show SMILES COc1c(ccc2c1n(cc(C(O)=O)c2=O)C1CC1)N1CC[C@@H](CN)C1 Show InChI InChI=1S/C19H23N3O4/c1-26-18-15(21-7-6-11(8-20)9-21)5-4-13-16(18)22(12-2-3-12)10-14(17(13)23)19(24)25/h4-5,10-12H,2-3,6-9,20H2,1H3,(H,24,25)/t11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG by fliter binding assay |
Bioorg Med Chem Lett 17: 2150-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.090
BindingDB Entry DOI: 10.7270/Q2GM86Z7 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50205457

(7-((R)-3-((S)-1-(2-cyanoethylamino)ethyl)pyrrolidi...)Show SMILES C[C@H](NCCC#N)[C@@H]1CCN(C1)c1c(F)cc2c(c1C)n(cc(C(O)=O)c2=O)C1CC1 Show InChI InChI=1S/C23H27FN4O3/c1-13-20-17(22(29)18(23(30)31)12-28(20)16-4-5-16)10-19(24)21(13)27-9-6-15(11-27)14(2)26-8-3-7-25/h10,12,14-16,26H,3-6,8-9,11H2,1-2H3,(H,30,31)/t14-,15+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG by fliter binding assay |
Bioorg Med Chem Lett 17: 2150-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.090
BindingDB Entry DOI: 10.7270/Q2GM86Z7 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50205453
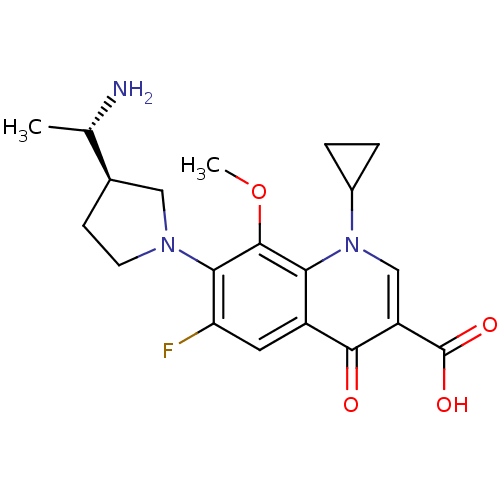
(7-((R)-3-((S)-1-aminoethyl)pyrrolidin-1-yl)-1-cycl...)Show SMILES COc1c(N2CC[C@H](C2)[C@H](C)N)c(F)cc2c1n(cc(C(O)=O)c2=O)C1CC1 Show InChI InChI=1S/C20H24FN3O4/c1-10(22)11-5-6-23(8-11)17-15(21)7-13-16(19(17)28-2)24(12-3-4-12)9-14(18(13)25)20(26)27/h7,9-12H,3-6,8,22H2,1-2H3,(H,26,27)/t10-,11+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG by fliter binding assay |
Bioorg Med Chem Lett 17: 2150-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.090
BindingDB Entry DOI: 10.7270/Q2GM86Z7 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50205446
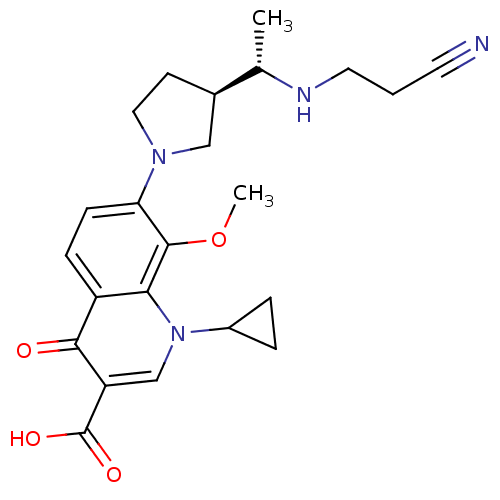
(7-((R)-3-((S)-1-(2-cyanoethylamino)ethyl)pyrrolidi...)Show SMILES COc1c(ccc2c1n(cc(C(O)=O)c2=O)C1CC1)N1CC[C@H](C1)[C@H](C)NCCC#N Show InChI InChI=1S/C23H28N4O4/c1-14(25-10-3-9-24)15-8-11-26(12-15)19-7-6-17-20(22(19)31-2)27(16-4-5-16)13-18(21(17)28)23(29)30/h6-7,13-16,25H,3-5,8,10-12H2,1-2H3,(H,29,30)/t14-,15+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG by fliter binding assay |
Bioorg Med Chem Lett 17: 2150-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.090
BindingDB Entry DOI: 10.7270/Q2GM86Z7 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50205447

(10-((R)-3-((S)-1-aminoethyl)pyrrolidin-1-yl)-9-flu...)Show SMILES C[C@H](N)[C@@H]1CCN(C1)c1c(F)cc2c3c1OCC(C)n3cc(C(O)=O)c2=O |w:17.19| Show InChI InChI=1S/C19H22FN3O4/c1-9-8-27-18-15-12(17(24)13(19(25)26)7-23(9)15)5-14(20)16(18)22-4-3-11(6-22)10(2)21/h5,7,9-11H,3-4,6,8,21H2,1-2H3,(H,25,26)/t9?,10-,11+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG by fliter binding assay |
Bioorg Med Chem Lett 17: 2150-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.090
BindingDB Entry DOI: 10.7270/Q2GM86Z7 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM21690

(1-cyclopropyl-6-fluoro-4-oxo-7-(piperazin-1-yl)-1,...)Show InChI InChI=1S/C17H18FN3O3/c18-13-7-11-14(8-15(13)20-5-3-19-4-6-20)21(10-1-2-10)9-12(16(11)22)17(23)24/h7-10,19H,1-6H2,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG by fliter binding assay |
Bioorg Med Chem Lett 17: 2150-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.090
BindingDB Entry DOI: 10.7270/Q2GM86Z7 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50205461
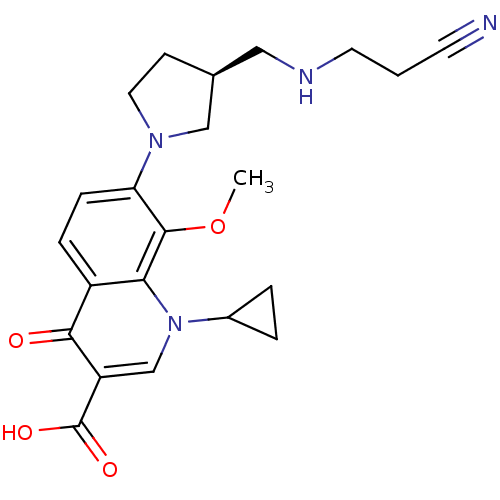
((S)-7-(3-((2-cyanoethylamino)methyl)pyrrolidin-1-y...)Show SMILES COc1c(ccc2c1n(cc(C(O)=O)c2=O)C1CC1)N1CC[C@@H](CNCCC#N)C1 Show InChI InChI=1S/C22H26N4O4/c1-30-21-18(25-10-7-14(12-25)11-24-9-2-8-23)6-5-16-19(21)26(15-3-4-15)13-17(20(16)27)22(28)29/h5-6,13-15,24H,2-4,7,9-12H2,1H3,(H,28,29)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG by fliter binding assay |
Bioorg Med Chem Lett 17: 2150-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.090
BindingDB Entry DOI: 10.7270/Q2GM86Z7 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50205459
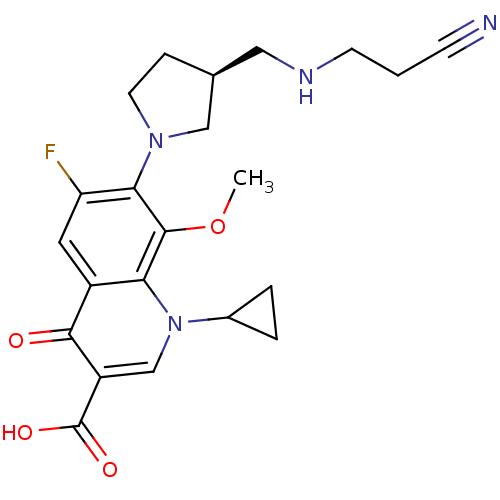
((S)-7-(3-(aminomethyl)pyrrolidin-1-yl)-1-cycloprop...)Show SMILES COc1c(N2CC[C@@H](CNCCC#N)C2)c(F)cc2c1n(cc(C(O)=O)c2=O)C1CC1 Show InChI InChI=1S/C22H25FN4O4/c1-31-21-18-15(20(28)16(22(29)30)12-27(18)14-3-4-14)9-17(23)19(21)26-8-5-13(11-26)10-25-7-2-6-24/h9,12-14,25H,2-5,7-8,10-11H2,1H3,(H,29,30)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.06E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG by fliter binding assay |
Bioorg Med Chem Lett 17: 2150-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.090
BindingDB Entry DOI: 10.7270/Q2GM86Z7 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50205455
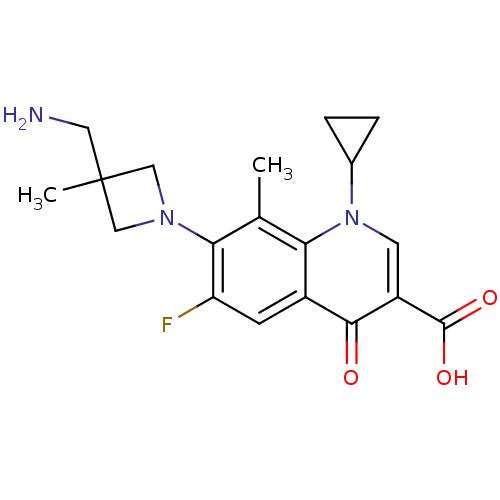
(7-(3-(aminomethyl)-3-methylazetidin-1-yl)-1-cyclop...)Show SMILES Cc1c(N2CC(C)(CN)C2)c(F)cc2c1n(cc(C(O)=O)c2=O)C1CC1 Show InChI InChI=1S/C19H22FN3O3/c1-10-15-12(5-14(20)16(10)22-8-19(2,7-21)9-22)17(24)13(18(25)26)6-23(15)11-3-4-11/h5-6,11H,3-4,7-9,21H2,1-2H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.12E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG by fliter binding assay |
Bioorg Med Chem Lett 17: 2150-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.090
BindingDB Entry DOI: 10.7270/Q2GM86Z7 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50205449
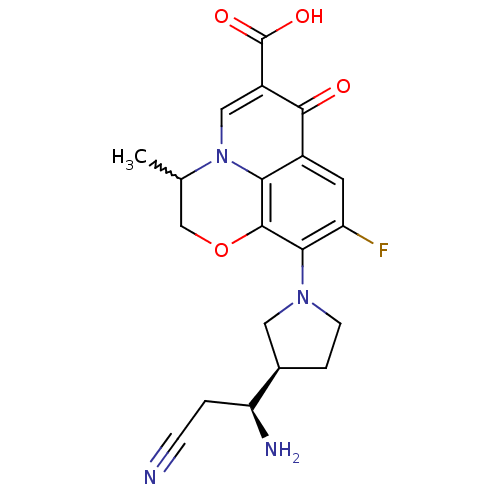
(10-((R)-3-((S)-1-amino-2-cyanoethyl)pyrrolidin-1-y...)Show SMILES CC1COc2c(N3CC[C@H](C3)[C@@H](N)CC#N)c(F)cc3c2n1cc(C(O)=O)c3=O |w:1.0| Show InChI InChI=1S/C20H21FN4O4/c1-10-9-29-19-16-12(18(26)13(20(27)28)8-25(10)16)6-14(21)17(19)24-5-3-11(7-24)15(23)2-4-22/h6,8,10-11,15H,2-3,5,7,9,23H2,1H3,(H,27,28)/t10?,11-,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG by fliter binding assay |
Bioorg Med Chem Lett 17: 2150-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.090
BindingDB Entry DOI: 10.7270/Q2GM86Z7 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50131445
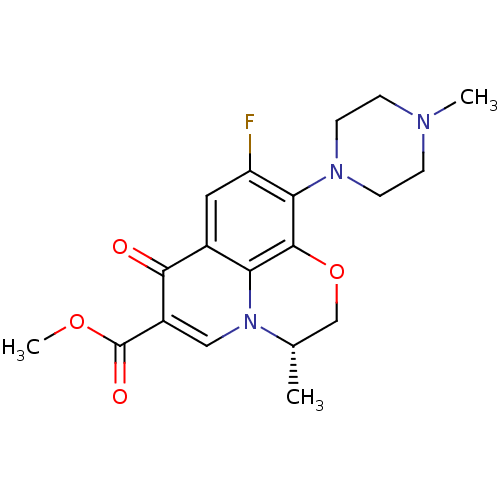
((3S)-9-fluoro-3-methyl-10-(4-methylpiperazin-1-yl)...)Show SMILES COC(=O)c1cn2[C@@H](C)COc3c(N4CCN(C)CC4)c(F)cc(c23)c1=O Show InChI InChI=1S/C19H22FN3O4/c1-11-10-27-18-15-12(17(24)13(9-23(11)15)19(25)26-3)8-14(20)16(18)22-6-4-21(2)5-7-22/h8-9,11H,4-7,10H2,1-3H3/t11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG by fliter binding assay |
Bioorg Med Chem Lett 17: 2150-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.090
BindingDB Entry DOI: 10.7270/Q2GM86Z7 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50205448
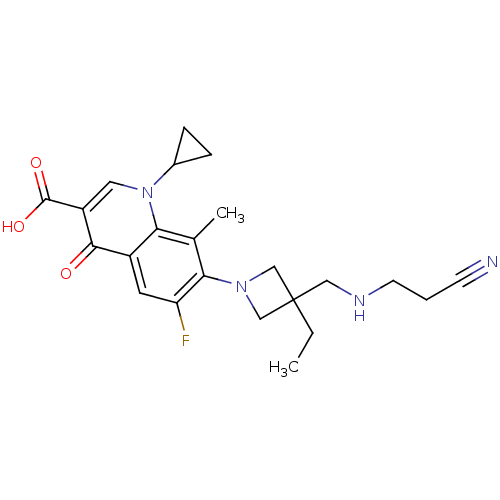
(7-(3-((2-cyanoethylamino)methyl)-3-ethylazetidin-1...)Show SMILES CCC1(CNCCC#N)CN(C1)c1c(F)cc2c(c1C)n(cc(C(O)=O)c2=O)C1CC1 Show InChI InChI=1S/C23H27FN4O3/c1-3-23(11-26-8-4-7-25)12-27(13-23)20-14(2)19-16(9-18(20)24)21(29)17(22(30)31)10-28(19)15-5-6-15/h9-10,15,26H,3-6,8,11-13H2,1-2H3,(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG by fliter binding assay |
Bioorg Med Chem Lett 17: 2150-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.090
BindingDB Entry DOI: 10.7270/Q2GM86Z7 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50205454
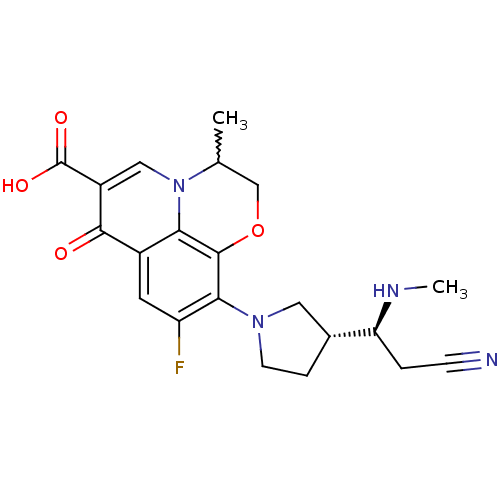
(10-((R)-3-((S)-2-cyano-1-(methylamino)ethyl)pyrrol...)Show SMILES CN[C@@H](CC#N)[C@@H]1CCN(C1)c1c(F)cc2c3c1OCC(C)n3cc(C(O)=O)c2=O |w:20.22| Show InChI InChI=1S/C21H23FN4O4/c1-11-10-30-20-17-13(19(27)14(21(28)29)9-26(11)17)7-15(22)18(20)25-6-4-12(8-25)16(24-2)3-5-23/h7,9,11-12,16,24H,3-4,6,8,10H2,1-2H3,(H,28,29)/t11?,12-,16+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG by fliter binding assay |
Bioorg Med Chem Lett 17: 2150-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.090
BindingDB Entry DOI: 10.7270/Q2GM86Z7 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50205467
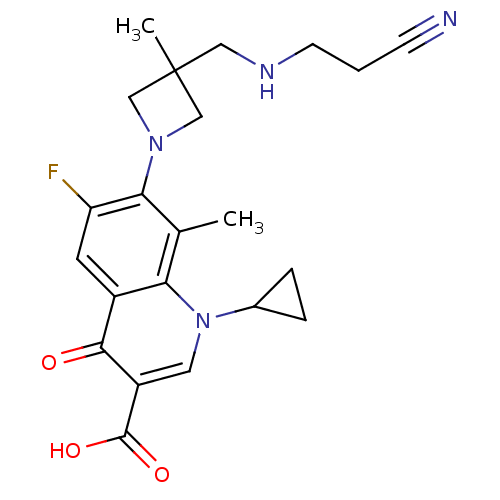
(7-(3-((2-cyanoethylamino)methyl)-3-methylazetidin-...)Show SMILES Cc1c(N2CC(C)(CNCCC#N)C2)c(F)cc2c1n(cc(C(O)=O)c2=O)C1CC1 Show InChI InChI=1S/C22H25FN4O3/c1-13-18-15(20(28)16(21(29)30)9-27(18)14-4-5-14)8-17(23)19(13)26-11-22(2,12-26)10-25-7-3-6-24/h8-9,14,25H,3-5,7,10-12H2,1-2H3,(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG by fliter binding assay |
Bioorg Med Chem Lett 17: 2150-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.090
BindingDB Entry DOI: 10.7270/Q2GM86Z7 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50205464
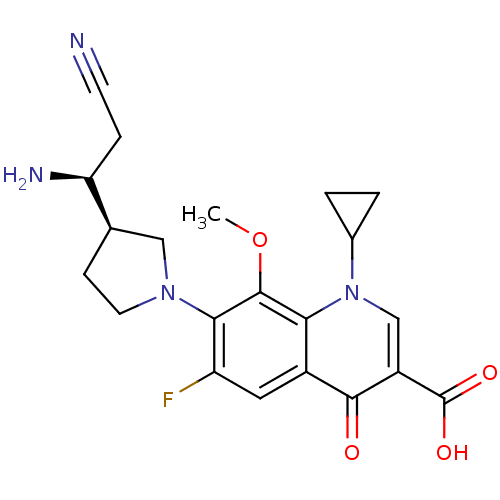
(7-((R)-3-((S)-1-amino-2-cyanoethyl)pyrrolidin-1-yl...)Show SMILES COc1c(N2CC[C@H](C2)[C@@H](N)CC#N)c(F)cc2c1n(cc(C(O)=O)c2=O)C1CC1 Show InChI InChI=1S/C21H23FN4O4/c1-30-20-17-13(19(27)14(21(28)29)10-26(17)12-2-3-12)8-15(22)18(20)25-7-5-11(9-25)16(24)4-6-23/h8,10-12,16H,2-5,7,9,24H2,1H3,(H,28,29)/t11-,16+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG by fliter binding assay |
Bioorg Med Chem Lett 17: 2150-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.090
BindingDB Entry DOI: 10.7270/Q2GM86Z7 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50205460
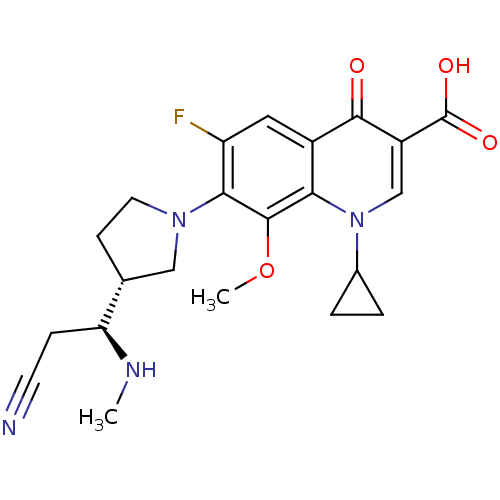
(7-((R)-3-((S)-2-cyano-1-(methylamino)ethyl)pyrroli...)Show SMILES CN[C@@H](CC#N)[C@@H]1CCN(C1)c1c(F)cc2c(c1OC)n(cc(C(O)=O)c2=O)C1CC1 Show InChI InChI=1S/C22H25FN4O4/c1-25-17(5-7-24)12-6-8-26(10-12)19-16(23)9-14-18(21(19)31-2)27(13-3-4-13)11-15(20(14)28)22(29)30/h9,11-13,17,25H,3-6,8,10H2,1-2H3,(H,29,30)/t12-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG by fliter binding assay |
Bioorg Med Chem Lett 17: 2150-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.090
BindingDB Entry DOI: 10.7270/Q2GM86Z7 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50205458
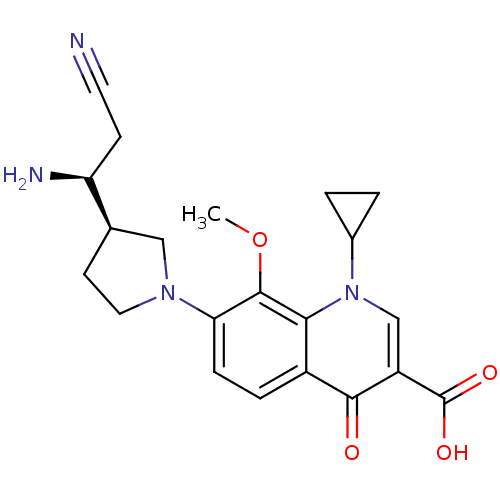
(7-((R)-3-((S)-1-amino-2-cyanoethyl)pyrrolidin-1-yl...)Show SMILES COc1c(ccc2c1n(cc(C(O)=O)c2=O)C1CC1)N1CC[C@H](C1)[C@@H](N)CC#N Show InChI InChI=1S/C21H24N4O4/c1-29-20-17(24-9-7-12(10-24)16(23)6-8-22)5-4-14-18(20)25(13-2-3-13)11-15(19(14)26)21(27)28/h4-5,11-13,16H,2-3,6-7,9-10,23H2,1H3,(H,27,28)/t12-,16+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG by fliter binding assay |
Bioorg Med Chem Lett 17: 2150-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.090
BindingDB Entry DOI: 10.7270/Q2GM86Z7 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50205463
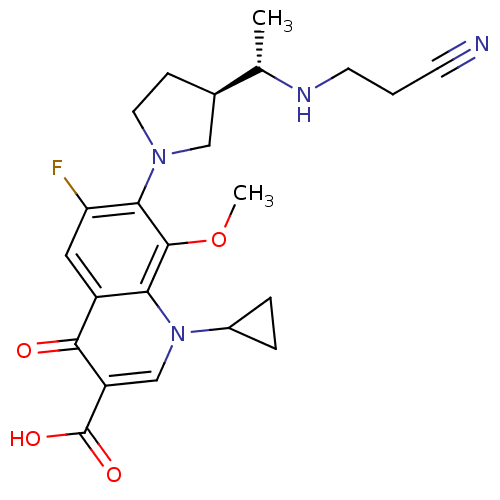
(7-((R)-3-((S)-1-(2-cyanoethylamino)ethyl)pyrrolidi...)Show SMILES COc1c(N2CC[C@H](C2)[C@H](C)NCCC#N)c(F)cc2c1n(cc(C(O)=O)c2=O)C1CC1 Show InChI InChI=1S/C23H27FN4O4/c1-13(26-8-3-7-25)14-6-9-27(11-14)20-18(24)10-16-19(22(20)32-2)28(15-4-5-15)12-17(21(16)29)23(30)31/h10,12-15,26H,3-6,8-9,11H2,1-2H3,(H,30,31)/t13-,14+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG by fliter binding assay |
Bioorg Med Chem Lett 17: 2150-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.090
BindingDB Entry DOI: 10.7270/Q2GM86Z7 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50205462
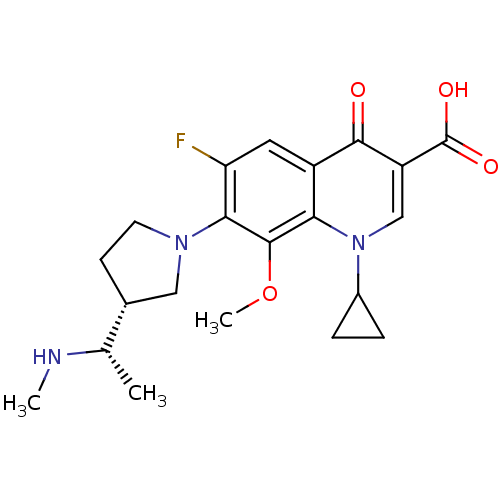
(1-cyclopropyl-6-fluoro-8-methoxy-7-((R)-3-((S)-1-(...)Show SMILES CN[C@@H](C)[C@@H]1CCN(C1)c1c(F)cc2c(c1OC)n(cc(C(O)=O)c2=O)C1CC1 Show InChI InChI=1S/C21H26FN3O4/c1-11(23-2)12-6-7-24(9-12)18-16(22)8-14-17(20(18)29-3)25(13-4-5-13)10-15(19(14)26)21(27)28/h8,10-13,23H,4-7,9H2,1-3H3,(H,27,28)/t11-,12+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG by fliter binding assay |
Bioorg Med Chem Lett 17: 2150-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.090
BindingDB Entry DOI: 10.7270/Q2GM86Z7 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50205456
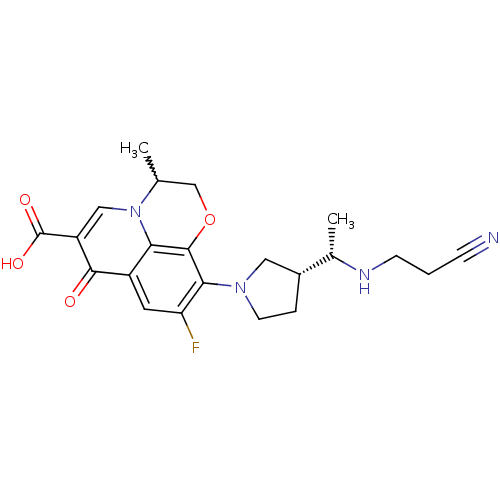
(10-((R)-3-((S)-1-(2-cyanoethylamino)ethyl)pyrrolid...)Show SMILES C[C@H](NCCC#N)[C@@H]1CCN(C1)c1c(F)cc2c3c1OCC(C)n3cc(C(O)=O)c2=O |w:21.23| Show InChI InChI=1S/C22H25FN4O4/c1-12-11-31-21-18-15(20(28)16(22(29)30)10-27(12)18)8-17(23)19(21)26-7-4-14(9-26)13(2)25-6-3-5-24/h8,10,12-14,25H,3-4,6-7,9,11H2,1-2H3,(H,29,30)/t12?,13-,14+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG by fliter binding assay |
Bioorg Med Chem Lett 17: 2150-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.090
BindingDB Entry DOI: 10.7270/Q2GM86Z7 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM18012
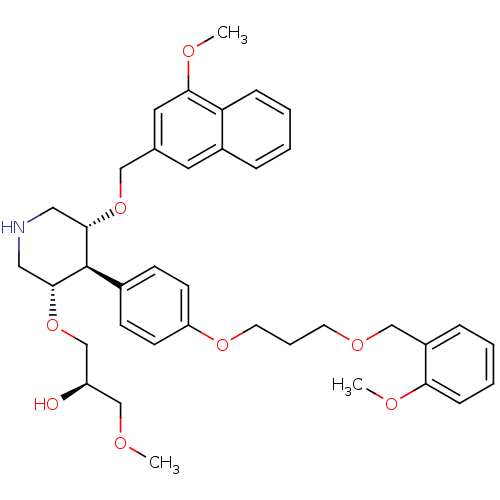
(trans,trans-4-arylpiperidine-based compound, 1)Show SMILES COC[C@@H](O)CO[C@@H]1CNC[C@H](OCc2cc(OC)c3ccccc3c2)[C@H]1c1ccc(OCCCOCc2ccccc2OC)cc1 |r| Show InChI InChI=1S/C38H47NO8/c1-41-25-31(40)26-47-37-22-39-21-36(46-23-27-19-29-9-4-6-11-33(29)35(20-27)43-3)38(37)28-13-15-32(16-14-28)45-18-8-17-44-24-30-10-5-7-12-34(30)42-2/h4-7,9-16,19-20,31,36-40H,8,17-18,21-26H2,1-3H3/t31-,36+,37-,38-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0670 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Pfizer
| Assay Description
The renin assay utilized a tandem Green Flourescent Protein (tGFP) substrate that was hydrolyzed by human rennin. The tGFP substrate contained a nine... |
Bioorg Med Chem Lett 17: 3575-80 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.052
BindingDB Entry DOI: 10.7270/Q2B56H0X |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM18033
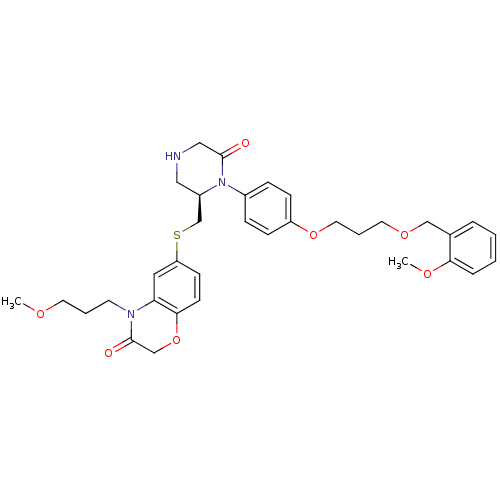
(Ketopiperazine-based inhibitor, 13)Show SMILES COCCCN1C(=O)COc2ccc(SC[C@H]3CNCC(=O)N3c3ccc(OCCCOCc4ccccc4OC)cc3)cc12 |r| Show InChI InChI=1S/C34H41N3O7S/c1-40-16-5-15-36-30-19-29(13-14-32(30)44-23-34(36)39)45-24-27-20-35-21-33(38)37(27)26-9-11-28(12-10-26)43-18-6-17-42-22-25-7-3-4-8-31(25)41-2/h3-4,7-14,19,27,35H,5-6,15-18,20-24H2,1-2H3/t27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Pfizer
| Assay Description
The renin assay utilized a tandem Green Flourescent Protein (tGFP) substrate that was hydrolyzed by human rennin. The tGFP substrate contained a nine... |
Bioorg Med Chem Lett 16: 2500-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.084
BindingDB Entry DOI: 10.7270/Q26D5R7R |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM18025
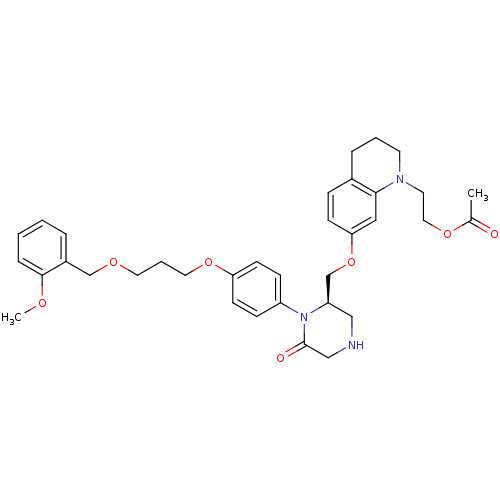
(2-(7-{[(2R)-1-(4-{3-[(2-methoxyphenyl)methoxy]prop...)Show SMILES COc1ccccc1COCCCOc1ccc(cc1)N1[C@@H](COc2ccc3CCCN(CCOC(C)=O)c3c2)CNCC1=O |r| Show InChI InChI=1S/C35H43N3O7/c1-26(39)43-20-17-37-16-5-8-27-10-13-32(21-33(27)37)45-25-30-22-36-23-35(40)38(30)29-11-14-31(15-12-29)44-19-6-18-42-24-28-7-3-4-9-34(28)41-2/h3-4,7,9-15,21,30,36H,5-6,8,16-20,22-25H2,1-2H3/t30-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Pfizer
| Assay Description
The renin assay utilized a tandem Green Flourescent Protein (tGFP) substrate that was hydrolyzed by human rennin. The tGFP substrate contained a nine... |
Bioorg Med Chem Lett 16: 2500-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.084
BindingDB Entry DOI: 10.7270/Q26D5R7R |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Renin
(Homo sapiens (Human)) | BDBM18032

(Ketopiperazine-based inhibitor, 12)Show SMILES COCCCN1C(=O)COc2ccc(OC[C@H]3CNCC(=O)N3c3ccc(OCCCOCc4ccccc4OC)cc3)cc12 |r| Show InChI InChI=1S/C34H41N3O8/c1-40-16-5-15-36-30-19-29(13-14-32(30)45-24-34(36)39)44-23-27-20-35-21-33(38)37(27)26-9-11-28(12-10-26)43-18-6-17-42-22-25-7-3-4-8-31(25)41-2/h3-4,7-14,19,27,35H,5-6,15-18,20-24H2,1-2H3/t27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.680 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Pfizer
| Assay Description
The renin assay utilized a tandem Green Flourescent Protein (tGFP) substrate that was hydrolyzed by human rennin. The tGFP substrate contained a nine... |
Bioorg Med Chem Lett 16: 2500-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.084
BindingDB Entry DOI: 10.7270/Q26D5R7R |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM18028
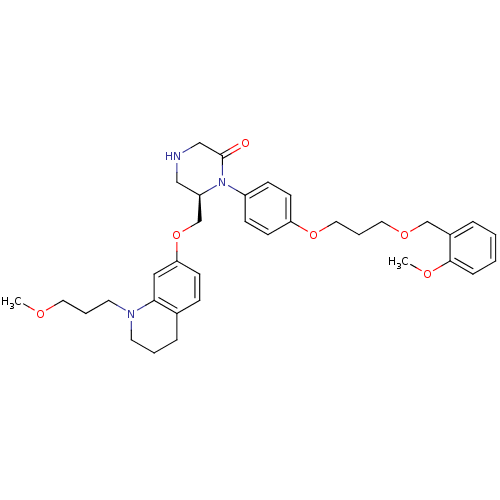
((6R)-1-(4-{3-[(2-methoxyphenyl)methoxy]propoxy}phe...)Show SMILES COCCCN1CCCc2ccc(OC[C@H]3CNCC(=O)N3c3ccc(OCCCOCc4ccccc4OC)cc3)cc12 |r| Show InChI InChI=1S/C35H45N3O6/c1-40-19-6-18-37-17-5-9-27-11-14-32(22-33(27)37)44-26-30-23-36-24-35(39)38(30)29-12-15-31(16-13-29)43-21-7-20-42-25-28-8-3-4-10-34(28)41-2/h3-4,8,10-16,22,30,36H,5-7,9,17-21,23-26H2,1-2H3/t30-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Pfizer
| Assay Description
The renin assay utilized a tandem Green Flourescent Protein (tGFP) substrate that was hydrolyzed by human rennin. The tGFP substrate contained a nine... |
Bioorg Med Chem Lett 16: 2500-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.084
BindingDB Entry DOI: 10.7270/Q26D5R7R |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM18022
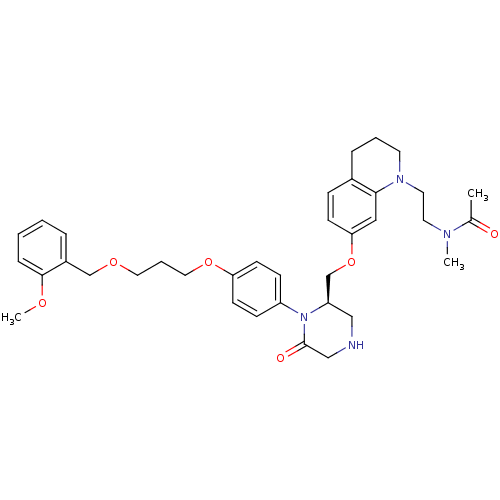
(Ketopiperazine-based inhibitor, 2)Show SMILES COc1ccccc1COCCCOc1ccc(cc1)N1[C@@H](COc2ccc3CCCN(CCN(C)C(C)=O)c3c2)CNCC1=O |r| Show InChI InChI=1S/C36H46N4O6/c1-27(41)38(2)18-19-39-17-6-9-28-11-14-33(22-34(28)39)46-26-31-23-37-24-36(42)40(31)30-12-15-32(16-13-30)45-21-7-20-44-25-29-8-4-5-10-35(29)43-3/h4-5,8,10-16,22,31,37H,6-7,9,17-21,23-26H2,1-3H3/t31-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Pfizer
| Assay Description
The renin assay utilized a tandem Green Flourescent Protein (tGFP) substrate that was hydrolyzed by human rennin. The tGFP substrate contained a nine... |
Bioorg Med Chem Lett 16: 2500-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.084
BindingDB Entry DOI: 10.7270/Q26D5R7R |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Renin
(Homo sapiens (Human)) | BDBM50016033

(CHEMBL3085574 | {1-[1-{1-[2-(1-Benzylcarbamoyl-3-m...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)[C@@H](O)CC(=O)N[C@@H](CC(C)C)C(=O)NCc1ccccc1 Show InChI InChI=1S/C41H59N7O7/c1-26(2)18-31(35(49)22-36(50)45-32(19-27(3)4)37(51)43-23-29-16-12-9-13-17-29)46-39(53)34(21-30-24-42-25-44-30)47-38(52)33(20-28-14-10-8-11-15-28)48-40(54)55-41(5,6)7/h8-17,24-27,31-35,49H,18-23H2,1-7H3,(H,42,44)(H,43,51)(H,45,50)(H,46,53)(H,47,52)(H,48,54)/t31-,32-,33-,34-,35-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against human renin |
J Med Chem 33: 838-45 (1990)
BindingDB Entry DOI: 10.7270/Q2T152M6 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM18031
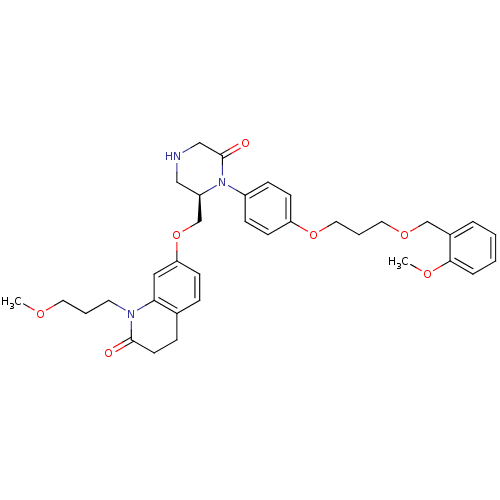
(CHEMBL193816 | Ketopiperazine-based inhibitor, 11)Show SMILES COCCCN1C(=O)CCc2ccc(OC[C@H]3CNCC(=O)N3c3ccc(OCCCOCc4ccccc4OC)cc3)cc12 |r| Show InChI InChI=1S/C35H43N3O7/c1-41-18-5-17-37-32-21-31(13-9-26(32)10-16-34(37)39)45-25-29-22-36-23-35(40)38(29)28-11-14-30(15-12-28)44-20-6-19-43-24-27-7-3-4-8-33(27)42-2/h3-4,7-9,11-15,21,29,36H,5-6,10,16-20,22-25H2,1-2H3/t29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Pfizer
| Assay Description
The renin assay utilized a tandem Green Flourescent Protein (tGFP) substrate that was hydrolyzed by human rennin. The tGFP substrate contained a nine... |
Bioorg Med Chem Lett 16: 2500-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.084
BindingDB Entry DOI: 10.7270/Q26D5R7R |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM18026
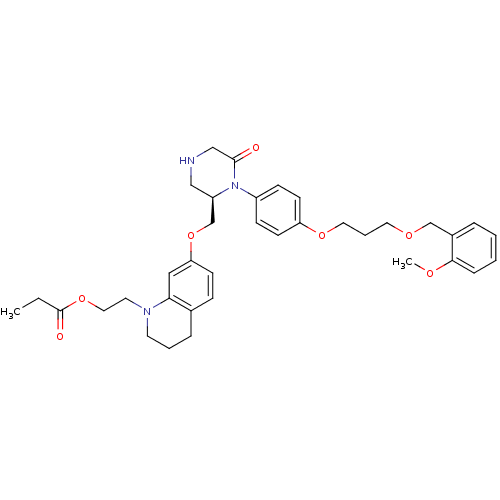
(2-(7-{[(2R)-1-(4-{3-[(2-methoxyphenyl)methoxy]prop...)Show SMILES CCC(=O)OCCN1CCCc2ccc(OC[C@H]3CNCC(=O)N3c3ccc(OCCCOCc4ccccc4OC)cc3)cc12 |r| Show InChI InChI=1S/C36H45N3O7/c1-3-36(41)45-21-18-38-17-6-9-27-11-14-32(22-33(27)38)46-26-30-23-37-24-35(40)39(30)29-12-15-31(16-13-29)44-20-7-19-43-25-28-8-4-5-10-34(28)42-2/h4-5,8,10-16,22,30,37H,3,6-7,9,17-21,23-26H2,1-2H3/t30-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Pfizer
| Assay Description
The renin assay utilized a tandem Green Flourescent Protein (tGFP) substrate that was hydrolyzed by human rennin. The tGFP substrate contained a nine... |
Bioorg Med Chem Lett 16: 2500-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.084
BindingDB Entry DOI: 10.7270/Q26D5R7R |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Androgen receptor
(Homo sapiens (Human)) | BDBM25437

((trifluoromethyl)benzonitrile, 14 | 4-{[(1R,2S)-2-...)Show SMILES FC(F)(F)c1cc(O[C@@H]2CCCC[C@H]2C#N)ccc1C#N |r| Show InChI InChI=1S/C15H13F3N2O/c16-15(17,18)13-7-12(6-5-10(13)8-19)21-14-4-2-1-3-11(14)9-20/h5-7,11,14H,1-4H2/t11-,14+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at androgen receptor expressed in MDA-MB-453 cells assessed as inhibition of dihydrotestosterone-induced response in reporter gen... |
Bioorg Med Chem Lett 17: 5983-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.075
BindingDB Entry DOI: 10.7270/Q27W6BWT |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50221436

(4-(2-cyano-cyclohexyl-oxy)-2-trifluoromethyl-benzo...)Show SMILES FC(F)(F)c1cc(OC2CCCCC2C#N)ccc1C#N |w:13.14,8.7| Show InChI InChI=1S/C15H13F3N2O/c16-15(17,18)13-7-12(6-5-10(13)8-19)21-14-4-2-1-3-11(14)9-20/h5-7,11,14H,1-4H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at androgen receptor expressed in MDA-MB-453 cells assessed as inhibition of dihydrotestosterone-induced response in reporter gen... |
Bioorg Med Chem Lett 17: 5983-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.075
BindingDB Entry DOI: 10.7270/Q27W6BWT |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50016034

((4-{3-[1-(4-Aminomethyl-benzylcarbamoyl)-3-methyl-...)Show SMILES CC(C)C[C@H](NC(=O)C[C@@H](O)C(CC1CCCCC1)NC(=O)CCC(O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)C(=O)NCc1ccc(CN)cc1 Show InChI InChI=1S/C42H65N5O7/c1-28(2)22-35(40(52)44-27-32-18-16-31(26-43)17-19-32)46-39(51)25-37(49)34(24-30-14-10-7-11-15-30)45-38(50)21-20-36(48)33(23-29-12-8-6-9-13-29)47-41(53)54-42(3,4)5/h6,8-9,12-13,16-19,28,30,33-37,48-49H,7,10-11,14-15,20-27,43H2,1-5H3,(H,44,52)(H,45,50)(H,46,51)(H,47,53)/t33-,34?,35-,36?,37+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against human renin |
J Med Chem 33: 838-45 (1990)
BindingDB Entry DOI: 10.7270/Q2T152M6 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM18029
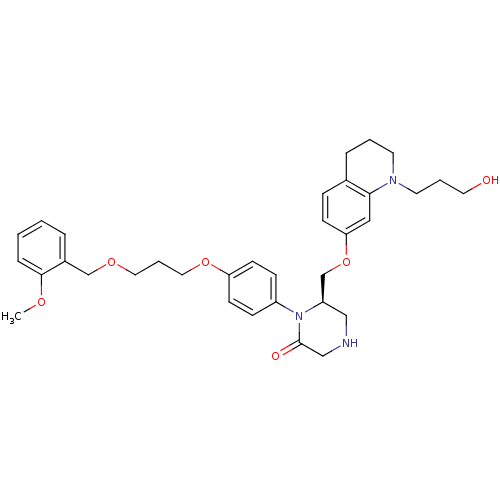
((6R)-6-({[1-(3-hydroxypropyl)-1,2,3,4-tetrahydroqu...)Show SMILES COc1ccccc1COCCCOc1ccc(cc1)N1[C@@H](COc2ccc3CCCN(CCCO)c3c2)CNCC1=O |r| Show InChI InChI=1S/C34H43N3O6/c1-40-33-9-3-2-7-27(33)24-41-19-6-20-42-30-14-11-28(12-15-30)37-29(22-35-23-34(37)39)25-43-31-13-10-26-8-4-16-36(17-5-18-38)32(26)21-31/h2-3,7,9-15,21,29,35,38H,4-6,8,16-20,22-25H2,1H3/t29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Pfizer
| Assay Description
The renin assay utilized a tandem Green Flourescent Protein (tGFP) substrate that was hydrolyzed by human rennin. The tGFP substrate contained a nine... |
Bioorg Med Chem Lett 16: 2500-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.084
BindingDB Entry DOI: 10.7270/Q26D5R7R |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Androgen receptor
(Homo sapiens (Human)) | BDBM50221435

(4-((trans)-2-cyanocyclohexyloxy)-2-(trifluoromethy...)Show InChI InChI=1S/C15H13F3N2O/c16-15(17,18)13-7-12(6-5-10(13)8-19)21-14-4-2-1-3-11(14)9-20/h5-7,11,14H,1-4H2/t11-,14+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from androgen receptor expressed in Sf9 cells |
Bioorg Med Chem Lett 17: 5983-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.075
BindingDB Entry DOI: 10.7270/Q27W6BWT |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50221436

(4-(2-cyano-cyclohexyl-oxy)-2-trifluoromethyl-benzo...)Show SMILES FC(F)(F)c1cc(OC2CCCCC2C#N)ccc1C#N |w:13.14,8.7| Show InChI InChI=1S/C15H13F3N2O/c16-15(17,18)13-7-12(6-5-10(13)8-19)21-14-4-2-1-3-11(14)9-20/h5-7,11,14H,1-4H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from androgen receptor expressed in Sf9 cells |
Bioorg Med Chem Lett 17: 5983-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.075
BindingDB Entry DOI: 10.7270/Q27W6BWT |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM25437

((trifluoromethyl)benzonitrile, 14 | 4-{[(1R,2S)-2-...)Show SMILES FC(F)(F)c1cc(O[C@@H]2CCCC[C@H]2C#N)ccc1C#N |r| Show InChI InChI=1S/C15H13F3N2O/c16-15(17,18)13-7-12(6-5-10(13)8-19)21-14-4-2-1-3-11(14)9-20/h5-7,11,14H,1-4H2/t11-,14+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from androgen receptor expressed in Sf9 cells |
Bioorg Med Chem Lett 17: 5983-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.075
BindingDB Entry DOI: 10.7270/Q27W6BWT |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50016025

(CHEMBL354821 | {1-Benzyl-4-[3-(1-benzylcarbamoyl-3...)Show SMILES CC(C)C[C@H](NC(=O)C[C@@H](O)C(CC1CCCCC1)NC(=O)CCC(O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)C(=O)NCc1ccccc1 Show InChI InChI=1S/C41H62N4O7/c1-28(2)23-34(39(50)42-27-31-19-13-8-14-20-31)44-38(49)26-36(47)33(25-30-17-11-7-12-18-30)43-37(48)22-21-35(46)32(24-29-15-9-6-10-16-29)45-40(51)52-41(3,4)5/h6,8-10,13-16,19-20,28,30,32-36,46-47H,7,11-12,17-18,21-27H2,1-5H3,(H,42,50)(H,43,48)(H,44,49)(H,45,51)/t32-,33?,34-,35?,36+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against human renin |
J Med Chem 33: 838-45 (1990)
BindingDB Entry DOI: 10.7270/Q2T152M6 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50016046
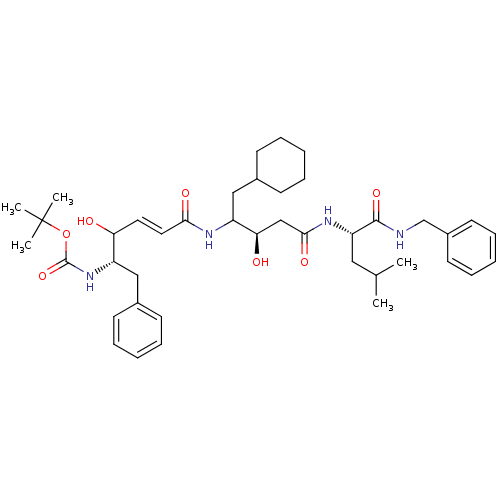
(CHEMBL167426 | {1-Benzyl-4-[3-(1-benzylcarbamoyl-3...)Show SMILES CC(C)C[C@H](NC(=O)C[C@@H](O)C(CC1CCCCC1)NC(=O)\C=C\C(O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)C(=O)NCc1ccccc1 Show InChI InChI=1S/C41H60N4O7/c1-28(2)23-34(39(50)42-27-31-19-13-8-14-20-31)44-38(49)26-36(47)33(25-30-17-11-7-12-18-30)43-37(48)22-21-35(46)32(24-29-15-9-6-10-16-29)45-40(51)52-41(3,4)5/h6,8-10,13-16,19-22,28,30,32-36,46-47H,7,11-12,17-18,23-27H2,1-5H3,(H,42,50)(H,43,48)(H,44,49)(H,45,51)/b22-21+/t32-,33?,34-,35?,36+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against human renin |
J Med Chem 33: 838-45 (1990)
BindingDB Entry DOI: 10.7270/Q2T152M6 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM18019
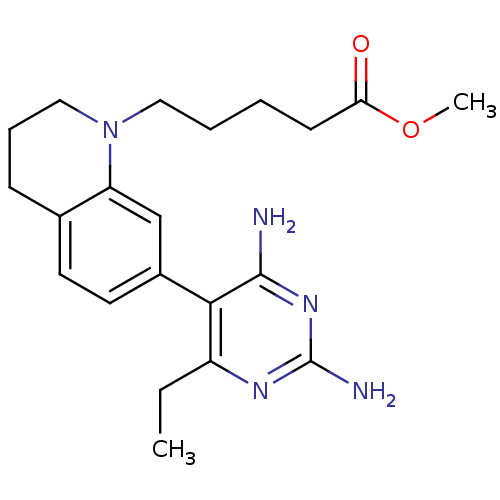
(6-ethyl-2,4-diaminopyrimidine-based compound, 14 |...)Show SMILES CCc1nc(N)nc(N)c1-c1ccc2CCCN(CCCCC(=O)OC)c2c1 Show InChI InChI=1S/C21H29N5O2/c1-3-16-19(20(22)25-21(23)24-16)15-10-9-14-7-6-12-26(17(14)13-15)11-5-4-8-18(27)28-2/h9-10,13H,3-8,11-12H2,1-2H3,(H4,22,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 91 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Pfizer
| Assay Description
The renin assay utilized a tandem Green Flourescent Protein (tGFP) substrate that was hydrolyzed by human rennin. The tGFP substrate contained a nine... |
Bioorg Med Chem Lett 17: 3575-80 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.052
BindingDB Entry DOI: 10.7270/Q2B56H0X |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Renin
(Homo sapiens (Human)) | BDBM18018
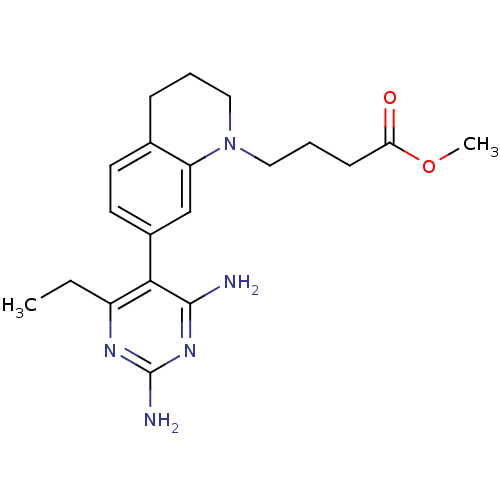
(6-ethyl-2,4-diaminopyrimidine-based compound, 13 |...)Show SMILES CCc1nc(N)nc(N)c1-c1ccc2CCCN(CCCC(=O)OC)c2c1 Show InChI InChI=1S/C20H27N5O2/c1-3-15-18(19(21)24-20(22)23-15)14-9-8-13-6-4-10-25(16(13)12-14)11-5-7-17(26)27-2/h8-9,12H,3-7,10-11H2,1-2H3,(H4,21,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Pfizer
| Assay Description
The renin assay utilized a tandem Green Flourescent Protein (tGFP) substrate that was hydrolyzed by human rennin. The tGFP substrate contained a nine... |
Bioorg Med Chem Lett 17: 3575-80 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.052
BindingDB Entry DOI: 10.7270/Q2B56H0X |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Renin
(Homo sapiens (Human)) | BDBM50016026

(CHEMBL424543 | [1-(1-{1-[2-(1-Benzylcarbamoyl-3-me...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)C(O)CC(=O)N[C@@H](CC(C)C)C(=O)NCc1ccccc1 Show InChI InChI=1S/C44H61N5O7/c1-29(2)23-34(38(50)27-39(51)46-35(24-30(3)4)40(52)45-28-33-21-15-10-16-22-33)47-41(53)36(25-31-17-11-8-12-18-31)48-42(54)37(26-32-19-13-9-14-20-32)49-43(55)56-44(5,6)7/h8-22,29-30,34-38,50H,23-28H2,1-7H3,(H,45,52)(H,46,51)(H,47,53)(H,48,54)(H,49,55)/t34-,35-,36-,37-,38?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against human renin |
J Med Chem 33: 838-45 (1990)
BindingDB Entry DOI: 10.7270/Q2T152M6 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM18020
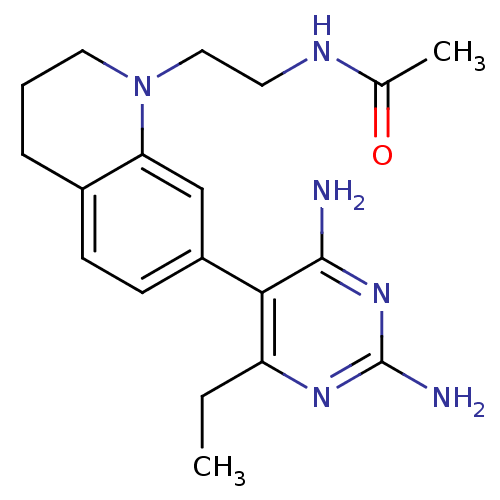
(6-ethyl-2,4-diaminopyrimidine-based compound, 15 |...)Show InChI InChI=1S/C19H26N6O/c1-3-15-17(18(20)24-19(21)23-15)14-7-6-13-5-4-9-25(16(13)11-14)10-8-22-12(2)26/h6-7,11H,3-5,8-10H2,1-2H3,(H,22,26)(H4,20,21,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 178 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Pfizer
| Assay Description
The renin assay utilized a tandem Green Flourescent Protein (tGFP) substrate that was hydrolyzed by human rennin. The tGFP substrate contained a nine... |
Bioorg Med Chem Lett 17: 3575-80 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.052
BindingDB Entry DOI: 10.7270/Q2B56H0X |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Androgen receptor
(Homo sapiens (Human)) | BDBM50221435

(4-((trans)-2-cyanocyclohexyloxy)-2-(trifluoromethy...)Show InChI InChI=1S/C15H13F3N2O/c16-15(17,18)13-7-12(6-5-10(13)8-19)21-14-4-2-1-3-11(14)9-20/h5-7,11,14H,1-4H2/t11-,14+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 196 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at androgen receptor expressed in MDA-MB-453 cells assessed as inhibition of dihydrotestosterone-induced response in reporter gen... |
Bioorg Med Chem Lett 17: 5983-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.075
BindingDB Entry DOI: 10.7270/Q27W6BWT |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM18017
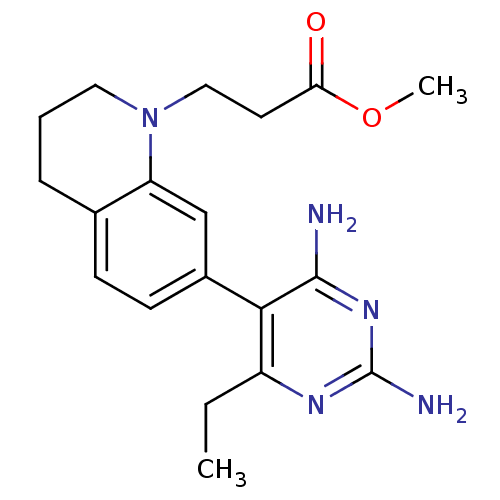
(6-ethyl-2,4-diaminopyrimidine-based compound, 12 |...)Show InChI InChI=1S/C19H25N5O2/c1-3-14-17(18(20)23-19(21)22-14)13-7-6-12-5-4-9-24(15(12)11-13)10-8-16(25)26-2/h6-7,11H,3-5,8-10H2,1-2H3,(H4,20,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 198 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Pfizer
| Assay Description
The renin assay utilized a tandem Green Flourescent Protein (tGFP) substrate that was hydrolyzed by human rennin. The tGFP substrate contained a nine... |
Bioorg Med Chem Lett 17: 3575-80 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.052
BindingDB Entry DOI: 10.7270/Q2B56H0X |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
DNA gyrase subunit A/B
(Escherichia coli (strain K12)) | BDBM21690

(1-cyclopropyl-6-fluoro-4-oxo-7-(piperazin-1-yl)-1,...)Show InChI InChI=1S/C17H18FN3O3/c18-13-7-11-14(8-15(13)20-5-3-19-4-6-20)21(10-1-2-10)9-12(16(11)22)17(23)24/h7-10,19H,1-6H2,(H,23,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli gyrase |
J Med Chem 49: 6435-8 (2006)
Article DOI: 10.1021/jm060505l
BindingDB Entry DOI: 10.7270/Q21N80RC |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Escherichia coli (strain K12)) | BDBM50197073

(3-amino-7-((R)-3-((S)-1-aminoethyl)pyrrolidin-1-yl...)Show SMILES C[C@H](N)[C@@H]1CCN(C1)c1c(F)cc2c(c1C)n(C1CC1)c(=O)n(N)c2=O |r| Show InChI InChI=1S/C18H24FN5O2/c1-9-15-13(17(25)24(21)18(26)23(15)12-3-4-12)7-14(19)16(9)22-6-5-11(8-22)10(2)20/h7,10-12H,3-6,8,20-21H2,1-2H3/t10-,11+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli gyrase |
J Med Chem 49: 6435-8 (2006)
Article DOI: 10.1021/jm060505l
BindingDB Entry DOI: 10.7270/Q21N80RC |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data