 Found 137 hits with Last Name = 'stocker' and Initial = 'a'
Found 137 hits with Last Name = 'stocker' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Thymidylate synthase
(Mus musculus) | BDBM50008294

(2-(4-(((2-amino-4-oxo-1,4-dihydroquinazolin-6-yl)m...)Show SMILES Nc1nc2ccc(CN(CC#C)c3ccc(cc3)C(=O)NC(CCC(O)=O)C(O)=O)cc2c(=O)[nH]1 Show InChI InChI=1S/C24H23N5O6/c1-2-11-29(13-14-3-8-18-17(12-14)22(33)28-24(25)27-18)16-6-4-15(5-7-16)21(32)26-19(23(34)35)9-10-20(30)31/h1,3-8,12,19H,9-11,13H2,(H,26,32)(H,30,31)(H,34,35)(H3,25,27,28,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity was evaluated against thymidylate synthase |
J Med Chem 28: 1468-76 (1985)
BindingDB Entry DOI: 10.7270/Q2348JDZ |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Mus musculus (Mouse)) | BDBM50008294

(2-(4-(((2-amino-4-oxo-1,4-dihydroquinazolin-6-yl)m...)Show SMILES Nc1nc2ccc(CN(CC#C)c3ccc(cc3)C(=O)NC(CCC(O)=O)C(O)=O)cc2c(=O)[nH]1 Show InChI InChI=1S/C24H23N5O6/c1-2-11-29(13-14-3-8-18-17(12-14)22(33)28-24(25)27-18)16-6-4-15(5-7-16)21(32)26-19(23(34)35)9-10-20(30)31/h1,3-8,12,19H,9-11,13H2,(H,26,32)(H,30,31)(H,34,35)(H3,25,27,28,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity was evaluated against dihydrofolate reductase |
J Med Chem 28: 1468-76 (1985)
BindingDB Entry DOI: 10.7270/Q2348JDZ |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50392216
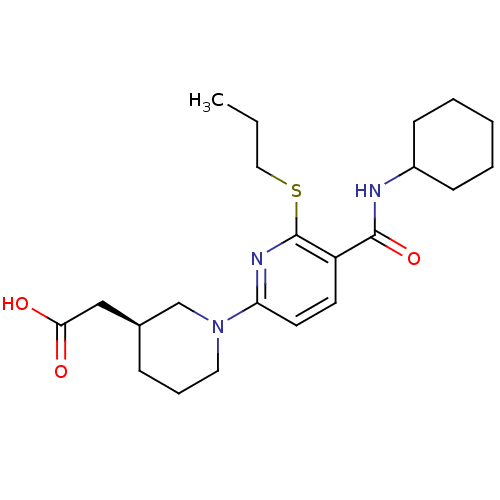
(CHEMBL2153191 | US8673938, 7)Show SMILES CCCSc1nc(ccc1C(=O)NC1CCCCC1)N1CCC[C@@H](CC(O)=O)C1 |r| Show InChI InChI=1S/C22H33N3O3S/c1-2-13-29-22-18(21(28)23-17-8-4-3-5-9-17)10-11-19(24-22)25-12-6-7-16(15-25)14-20(26)27/h10-11,16-17H,2-9,12-15H2,1H3,(H,23,28)(H,26,27)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of 11betaHSD1 in human adipocytes assessed as cortisone to cortisol conversion by scintillation counting method |
J Med Chem 55: 10136-47 (2012)
Article DOI: 10.1021/jm301252n
BindingDB Entry DOI: 10.7270/Q2WM1FJT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50399335

(CHEMBL2177609)Show SMILES CC(C)(C)c1c(cnn1-c1ccc(cc1)C(O)=O)C(=O)NC1C2CC3CC(C2)CC1C3 |TLB:20:21:23.30.24:28.26.27,30:29:27:23.24.25,THB:20:21:27:23.24.25,30:24:21.29.28:27,25:24:21:28.26.27,25:26:21:23.30.24,(26.01,-25.71,;26.64,-24.31,;28.17,-24.15,;27.4,-25.64,;25.74,-23.06,;26.22,-21.6,;24.97,-20.69,;23.72,-21.6,;24.2,-23.06,;23.29,-24.31,;21.76,-24.13,;20.85,-25.38,;21.48,-26.79,;23.02,-26.95,;23.91,-25.7,;20.57,-28.04,;19.04,-27.88,;21.2,-29.44,;27.68,-21.12,;28,-19.62,;28.82,-22.16,;30.29,-21.68,;31.49,-20.41,;32.81,-20.9,;34.21,-20.55,;34.22,-19.02,;32.82,-18.44,;31.48,-18.92,;31.79,-19.67,;31.79,-21.26,;33.2,-21.83,)| Show InChI InChI=1S/C25H31N3O3/c1-25(2,3)22-20(13-26-28(22)19-6-4-16(5-7-19)24(30)31)23(29)27-21-17-9-14-8-15(11-17)12-18(21)10-14/h4-7,13-15,17-18,21H,8-12H2,1-3H3,(H,27,29)(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of 11betaHSD1 in human adipocytes assessed as cortisone to cortisol conversion by scintillation counting method |
J Med Chem 55: 10136-47 (2012)
Article DOI: 10.1021/jm301252n
BindingDB Entry DOI: 10.7270/Q2WM1FJT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50394015
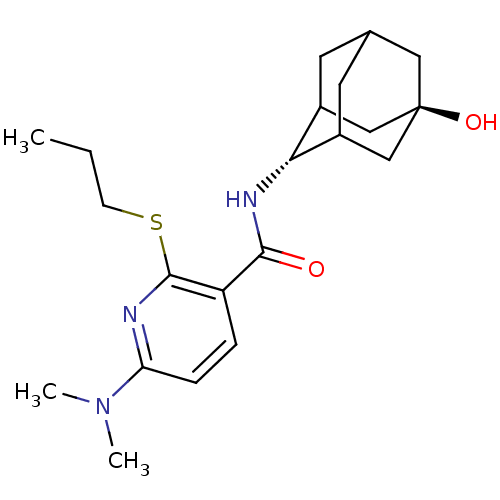
(CHEMBL2158468)Show SMILES CCCSc1nc(ccc1C(=O)N[C@H]1C2CC3CC1C[C@](O)(C3)C2)N(C)C |r,wU:13.13,wD:20.22,TLB:17:18:23:15.16.22,17:16:13.18.19:23,21:20:13:15.17.16,THB:12:13:23:15.16.22,22:16:13:19.20.23,22:20:13:15.17.16,(1.9,-44.89,;.56,-45.66,;-.77,-44.89,;-.77,-43.35,;-2.11,-42.59,;-3.45,-43.36,;-4.78,-42.59,;-4.78,-41.04,;-3.45,-40.27,;-2.11,-41.04,;-.78,-40.26,;-.79,-38.72,;.55,-41.03,;1.88,-40.25,;3.05,-38.95,;4.39,-39.41,;5.78,-39.04,;4.79,-40.33,;3.37,-39.8,;3.34,-38.2,;4.35,-36.96,;4.34,-35.41,;5.76,-37.51,;3.01,-37.46,;-6.11,-43.36,;-7.45,-42.59,;-6.12,-44.9,)| Show InChI InChI=1S/C21H31N3O2S/c1-4-7-27-20-16(5-6-17(22-20)24(2)3)19(25)23-18-14-8-13-9-15(18)12-21(26,10-13)11-14/h5-6,13-15,18,26H,4,7-12H2,1-3H3,(H,23,25)/t13?,14?,15?,18-,21- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human 11betaHSD1 by HTRF assay |
Bioorg Med Chem Lett 22: 6756-61 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.070
BindingDB Entry DOI: 10.7270/Q23R0V0G |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50394013
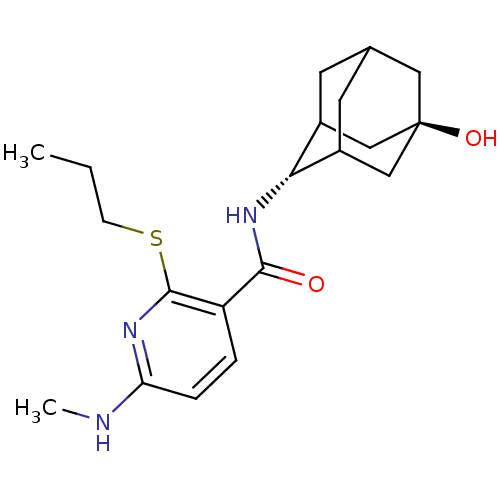
(CHEMBL2158466)Show SMILES CCCSc1nc(NC)ccc1C(=O)N[C@H]1C2CC3CC1C[C@](O)(C3)C2 |r,wU:15.15,wD:22.24,TLB:19:20:25:17.18.24,19:18:15.20.21:25,23:22:15:17.19.18,THB:14:15:25:17.18.24,24:18:15:21.22.25,24:22:15:17.19.18,(1.31,-29.92,;-.02,-30.69,;-1.35,-29.92,;-1.36,-28.38,;-2.69,-27.61,;-4.03,-28.39,;-5.36,-27.62,;-6.7,-28.38,;-8.03,-27.61,;-5.36,-26.07,;-4.03,-25.3,;-2.7,-26.06,;-1.37,-25.29,;-1.37,-23.75,;-.03,-26.05,;1.3,-25.28,;2.47,-23.97,;3.81,-24.44,;5.19,-24.06,;4.2,-25.36,;2.79,-24.82,;2.75,-23.23,;3.77,-21.98,;3.76,-20.44,;5.18,-22.54,;2.43,-22.49,)| Show InChI InChI=1S/C20H29N3O2S/c1-3-6-26-19-15(4-5-16(21-2)22-19)18(24)23-17-13-7-12-8-14(17)11-20(25,9-12)10-13/h4-5,12-14,17,25H,3,6-11H2,1-2H3,(H,21,22)(H,23,24)/t12?,13?,14?,17-,20- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human 11betaHSD1 by HTRF assay |
Bioorg Med Chem Lett 22: 6756-61 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.070
BindingDB Entry DOI: 10.7270/Q23R0V0G |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50399353
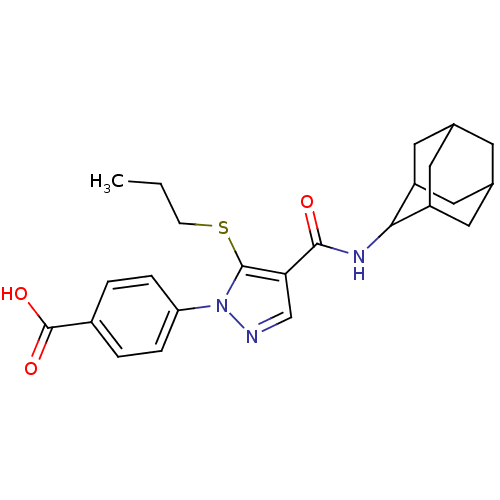
(CHEMBL2177615)Show SMILES CCCSc1c(cnn1-c1ccc(cc1)C(O)=O)C(=O)NC1C2CC3CC(C2)CC1C3 |TLB:30:29:27:23.24.25,20:21:23.30.24:28.26.27,THB:30:24:21.29.28:27,20:21:27:23.24.25,25:24:21:28.26.27,25:26:21:23.30.24,(42.49,-16.8,;40.96,-16.96,;40.05,-15.71,;40.68,-14.31,;39.78,-13.06,;40.26,-11.6,;39.01,-10.69,;37.76,-11.6,;38.24,-13.06,;37.33,-14.3,;35.8,-14.13,;34.89,-15.38,;35.51,-16.79,;37.05,-16.94,;37.95,-15.7,;34.61,-18.03,;33.08,-17.87,;35.24,-19.44,;41.72,-11.12,;42.04,-9.62,;42.86,-12.15,;44.33,-11.68,;45.53,-10.4,;46.85,-10.89,;48.25,-10.55,;48.26,-9.02,;46.86,-8.44,;45.52,-8.92,;45.83,-9.67,;45.83,-11.26,;47.24,-11.82,)| Show InChI InChI=1S/C24H29N3O3S/c1-2-7-31-23-20(13-25-27(23)19-5-3-16(4-6-19)24(29)30)22(28)26-21-17-9-14-8-15(11-17)12-18(21)10-14/h3-6,13-15,17-18,21H,2,7-12H2,1H3,(H,26,28)(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal 6-His tagged full length human 11betaHSD1 incubated for 25 mins by HTRF assay |
J Med Chem 55: 10136-47 (2012)
Article DOI: 10.1021/jm301252n
BindingDB Entry DOI: 10.7270/Q2WM1FJT |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50399348
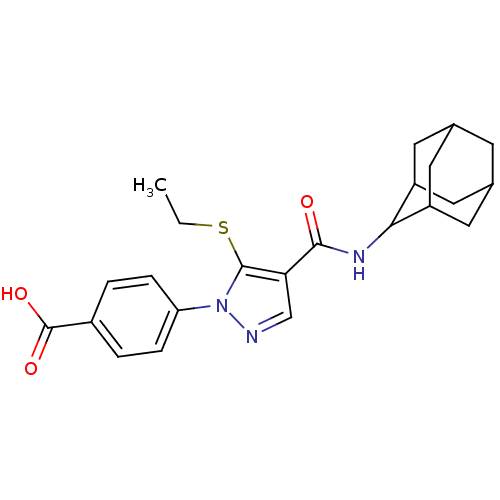
(CHEMBL2177620)Show SMILES CCSc1c(cnn1-c1ccc(cc1)C(O)=O)C(=O)NC1C2CC3CC(C2)CC1C3 |TLB:19:20:22.29.23:27.25.26,29:28:26:22.23.24,THB:19:20:26:22.23.24,29:23:20.28.27:26,24:23:20:27.25.26,24:25:20:22.29.23,(-1.15,-44.09,;-2.05,-42.84,;-1.42,-41.44,;-2.34,-40.19,;-1.85,-38.73,;-3.11,-37.83,;-4.34,-38.73,;-3.88,-40.19,;-4.77,-41.44,;-6.3,-41.27,;-7.22,-42.51,;-6.58,-43.92,;-5.04,-44.07,;-4.15,-42.83,;-7.49,-45.16,;-9.03,-45,;-6.86,-46.57,;-.38,-38.26,;-.07,-36.76,;.76,-39.29,;2.22,-38.82,;3.42,-37.54,;4.75,-38.03,;6.14,-37.69,;6.15,-36.16,;4.76,-35.58,;3.41,-36.06,;3.72,-36.81,;3.72,-38.4,;5.13,-38.96,)| Show InChI InChI=1S/C23H27N3O3S/c1-2-30-22-19(12-24-26(22)18-5-3-15(4-6-18)23(28)29)21(27)25-20-16-8-13-7-14(10-16)11-17(20)9-13/h3-6,12-14,16-17,20H,2,7-11H2,1H3,(H,25,27)(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal 6-His tagged full length human 11betaHSD1 incubated for 25 mins by HTRF assay |
J Med Chem 55: 10136-47 (2012)
Article DOI: 10.1021/jm301252n
BindingDB Entry DOI: 10.7270/Q2WM1FJT |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50394016
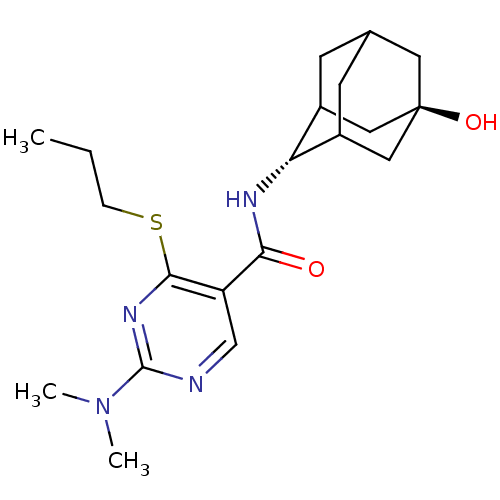
(CHEMBL2158469)Show SMILES CCCSc1nc(ncc1C(=O)N[C@H]1C2CC3CC1C[C@](O)(C3)C2)N(C)C |r,wU:13.13,wD:20.22,TLB:17:18:23:15.16.22,17:16:13.18.19:23,21:20:13:15.17.16,THB:12:13:23:15.16.22,22:16:13:19.20.23,22:20:13:15.17.16,(23.18,-44.42,;21.85,-45.2,;20.52,-44.43,;20.51,-42.89,;19.18,-42.12,;17.84,-42.89,;16.51,-42.12,;16.51,-40.58,;17.84,-39.81,;19.17,-40.57,;20.5,-39.79,;20.5,-38.25,;21.84,-40.56,;23.17,-39.79,;24.34,-38.48,;25.68,-38.94,;27.07,-38.57,;26.08,-39.87,;24.66,-39.33,;24.62,-37.74,;25.64,-36.49,;25.63,-34.95,;27.05,-37.04,;24.3,-36.99,;15.17,-42.89,;13.84,-42.12,;15.17,-44.43,)| Show InChI InChI=1S/C20H30N4O2S/c1-4-5-27-18-15(11-21-19(23-18)24(2)3)17(25)22-16-13-6-12-7-14(16)10-20(26,8-12)9-13/h11-14,16,26H,4-10H2,1-3H3,(H,22,25)/t12?,13?,14?,16-,20- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human 11betaHSD1 by HTRF assay |
Bioorg Med Chem Lett 22: 6756-61 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.070
BindingDB Entry DOI: 10.7270/Q23R0V0G |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50394017
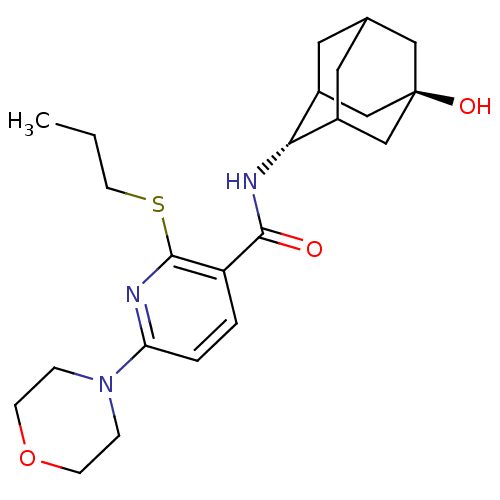
(CHEMBL2158470)Show SMILES CCCSc1nc(ccc1C(=O)N[C@H]1C2CC3CC1C[C@](O)(C3)C2)N1CCOCC1 |r,wU:13.13,wD:20.22,TLB:17:18:23:15.16.22,17:16:13.18.19:23,21:20:13:15.17.16,THB:12:13:23:15.16.22,22:16:13:19.20.23,22:20:13:15.17.16,(2.84,-3.33,;1.5,-4.1,;.17,-3.33,;.17,-1.79,;-1.17,-1.02,;-2.5,-1.79,;-3.84,-1.02,;-3.84,.52,;-2.51,1.29,;-1.17,.53,;.16,1.3,;.15,2.84,;1.49,.54,;2.82,1.31,;3.99,2.62,;5.33,2.15,;6.72,2.53,;5.73,1.23,;4.31,1.77,;4.28,3.36,;5.29,4.61,;5.28,6.15,;6.7,4.06,;3.96,4.1,;-5.17,-1.8,;-6.5,-1.02,;-7.83,-1.78,;-7.84,-3.32,;-6.51,-4.1,;-5.17,-3.33,)| Show InChI InChI=1S/C23H33N3O3S/c1-2-9-30-22-18(3-4-19(24-22)26-5-7-29-8-6-26)21(27)25-20-16-10-15-11-17(20)14-23(28,12-15)13-16/h3-4,15-17,20,28H,2,5-14H2,1H3,(H,25,27)/t15?,16?,17?,20-,23- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human 11betaHSD1 by HTRF assay |
Bioorg Med Chem Lett 22: 6756-61 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.070
BindingDB Entry DOI: 10.7270/Q23R0V0G |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50394014
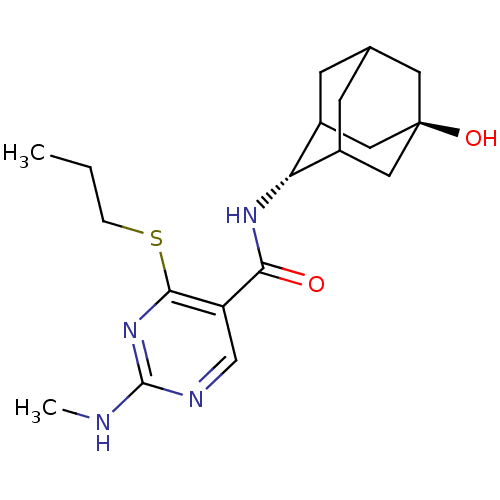
(CHEMBL2158467)Show SMILES CCCSc1nc(NC)ncc1C(=O)N[C@H]1C2CC3CC1C[C@](O)(C3)C2 |r,wU:15.15,wD:22.24,TLB:19:20:25:17.18.24,19:18:15.20.21:25,23:22:15:17.19.18,THB:14:15:25:17.18.24,24:18:15:21.22.25,24:22:15:17.19.18,(21.02,-29.8,;19.69,-30.57,;18.35,-29.81,;18.35,-28.27,;17.02,-27.5,;15.68,-28.27,;14.35,-27.5,;13.01,-28.27,;11.68,-27.5,;14.35,-25.95,;15.68,-25.18,;17.01,-25.95,;18.34,-25.17,;18.34,-23.63,;19.68,-25.94,;21.01,-25.16,;22.18,-23.86,;23.51,-24.32,;24.9,-23.95,;23.91,-25.24,;22.5,-24.71,;22.46,-23.12,;23.48,-21.87,;23.47,-20.32,;24.88,-22.42,;22.14,-22.37,)| Show InChI InChI=1S/C19H28N4O2S/c1-3-4-26-17-14(10-21-18(20-2)23-17)16(24)22-15-12-5-11-6-13(15)9-19(25,7-11)8-12/h10-13,15,25H,3-9H2,1-2H3,(H,22,24)(H,20,21,23)/t11?,12?,13?,15-,19- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human 11betaHSD1 by HTRF assay |
Bioorg Med Chem Lett 22: 6756-61 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.070
BindingDB Entry DOI: 10.7270/Q23R0V0G |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50399346

(CHEMBL2180883)Show SMILES OC(=O)c1ccc(cc1)-n1ncc(C(=O)NC2C3CC4CC(C3)CC2C4)c1SC1CCCC1 |TLB:15:16:18.25.19:23.21.22,25:24:22:18.19.20,THB:15:16:22:18.19.20,25:19:16.24.23:22,20:19:16:23.21.22,20:21:16:18.25.19,(27.7,-42.8,;29.23,-42.96,;29.85,-44.37,;30.13,-41.71,;29.51,-40.3,;30.41,-39.06,;31.95,-39.23,;32.57,-40.63,;31.67,-41.87,;32.85,-37.99,;32.38,-36.52,;33.62,-35.61,;34.87,-36.52,;36.34,-36.05,;36.66,-34.54,;37.48,-37.08,;38.95,-36.61,;40.14,-35.33,;41.47,-35.82,;42.87,-35.47,;42.88,-33.95,;41.48,-33.37,;40.13,-33.85,;40.44,-34.6,;40.45,-36.19,;41.85,-36.75,;34.39,-37.99,;35.3,-39.23,;34.67,-40.64,;33.17,-40.96,;33,-42.49,;34.41,-43.12,;35.44,-41.97,)| Show InChI InChI=1S/C26H31N3O3S/c30-24(28-23-18-10-15-9-16(12-18)13-19(23)11-15)22-14-27-29(25(22)33-21-3-1-2-4-21)20-7-5-17(6-8-20)26(31)32/h5-8,14-16,18-19,21,23H,1-4,9-13H2,(H,28,30)(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal 6-His tagged full length human 11betaHSD1 incubated for 25 mins by HTRF assay |
J Med Chem 55: 10136-47 (2012)
Article DOI: 10.1021/jm301252n
BindingDB Entry DOI: 10.7270/Q2WM1FJT |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50194411
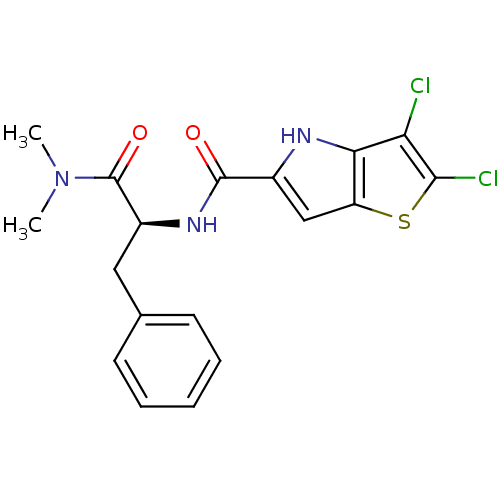
((S)-2,3-dichloro-N-(1-(dimethylamino)-1-oxo-3-phen...)Show SMILES CN(C)C(=O)[C@H](Cc1ccccc1)NC(=O)c1cc2sc(Cl)c(Cl)c2[nH]1 Show InChI InChI=1S/C18H17Cl2N3O2S/c1-23(2)18(25)12(8-10-6-4-3-5-7-10)22-17(24)11-9-13-15(21-11)14(19)16(20)26-13/h3-7,9,12,21H,8H2,1-2H3,(H,22,24)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human liver GPa by multienzyme coupled assay |
Bioorg Med Chem Lett 16: 5567-71 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.047
BindingDB Entry DOI: 10.7270/Q2KW5FP3 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50399351
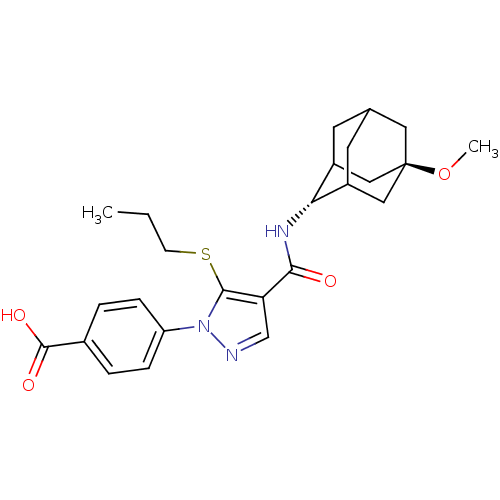
(CHEMBL2177617)Show SMILES CCCSc1c(cnn1-c1ccc(cc1)C(O)=O)C(=O)N[C@H]1C2CC3CC1C[C@](C3)(C2)OC |r,wU:21.22,wD:28.35,TLB:20:21:30.27.28:25.24.23,20:21:23:30.28.29,31:28:21.26.25:23,THB:27:26:23:30.28.29,27:28:21.26.25:23,29:28:21:25.24.23,29:24:21:30.27.28,31:28:21:25.24.23,(6.35,-29.86,;4.82,-30.02,;3.92,-28.78,;4.54,-27.37,;3.64,-26.12,;4.12,-24.66,;2.87,-23.75,;1.63,-24.66,;2.1,-26.12,;1.19,-27.37,;-.35,-27.2,;-1.26,-28.44,;-.62,-29.85,;.92,-30.01,;1.82,-28.76,;-1.54,-31.1,;-3.07,-30.94,;-.9,-32.51,;5.58,-24.19,;5.91,-22.68,;6.73,-25.22,;8.19,-24.75,;9.39,-23.47,;9.38,-21.98,;10.73,-21.51,;9.69,-22.73,;9.69,-24.32,;11.1,-24.89,;12.11,-23.61,;12.12,-22.08,;10.72,-23.96,;13.65,-23.55,;14.47,-24.85,)| Show InChI InChI=1S/C25H31N3O4S/c1-3-8-33-23-20(14-26-28(23)19-6-4-16(5-7-19)24(30)31)22(29)27-21-17-9-15-10-18(21)13-25(11-15,12-17)32-2/h4-7,14-15,17-18,21H,3,8-13H2,1-2H3,(H,27,29)(H,30,31)/t15?,17?,18?,21-,25- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal 6-His tagged full length human 11betaHSD1 incubated for 25 mins by HTRF assay |
J Med Chem 55: 10136-47 (2012)
Article DOI: 10.1021/jm301252n
BindingDB Entry DOI: 10.7270/Q2WM1FJT |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50394000
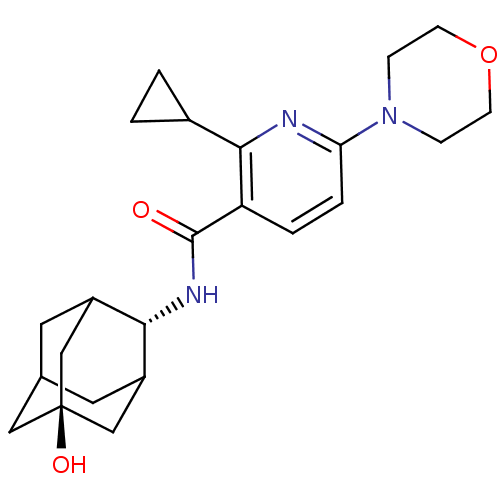
(CHEMBL2158481)Show SMILES O[C@@]12CC3CC(C1)[C@H](NC(=O)c1ccc(nc1C1CC1)N1CCOCC1)C(C3)C2 |r,wU:7.8,wD:1.0,TLB:0:1:7:4.27.3,27:26:6:4.3.2,THB:8:7:6:4.3.2,27:3:7.26.28:6,2:3:7:28.1.6,2:1:7:4.27.3,(5.69,-7.49,;5.71,-9.03,;7.11,-9.59,;7.13,-11.11,;5.74,-11.49,;4.4,-11.02,;4.37,-9.54,;3.23,-12.33,;1.91,-13.1,;.57,-12.34,;.56,-10.8,;-.76,-13.11,;-2.1,-12.35,;-3.42,-13.12,;-3.43,-14.67,;-2.09,-15.44,;-.76,-14.66,;.58,-15.43,;1.35,-16.76,;2.12,-15.43,;-4.76,-15.44,;-6.09,-14.66,;-7.42,-15.42,;-7.43,-16.97,;-6.1,-17.74,;-4.76,-16.98,;4.73,-11.87,;6.14,-12.41,;4.69,-10.28,)| Show InChI InChI=1S/C23H31N3O3/c27-22(25-20-16-9-14-10-17(20)13-23(28,11-14)12-16)18-3-4-19(24-21(18)15-1-2-15)26-5-7-29-8-6-26/h3-4,14-17,20,28H,1-2,5-13H2,(H,25,27)/t14?,16?,17?,20-,23- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11betaHSD1 by HTRF assay |
Bioorg Med Chem Lett 22: 6756-61 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.070
BindingDB Entry DOI: 10.7270/Q23R0V0G |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50399336

(CHEMBL2177608)Show SMILES CC1(CC1)c1c(cnn1-c1ccc(cc1)C(O)=O)C(=O)NC1C2CC3CC(C2)CC1C3 |TLB:20:21:23.30.24:28.26.27,30:29:27:23.24.25,THB:20:21:27:23.24.25,30:24:21.29.28:27,25:24:21:28.26.27,25:26:21:23.30.24,(10.13,-23.34,;8.59,-23.5,;8.59,-25.04,;7.26,-24.26,;7.69,-22.25,;8.17,-20.79,;6.92,-19.88,;5.68,-20.79,;6.15,-22.25,;5.25,-23.5,;3.71,-23.33,;2.81,-24.57,;3.43,-25.98,;4.97,-26.14,;5.87,-24.89,;2.53,-27.23,;.99,-27.07,;3.15,-28.63,;9.64,-20.31,;9.96,-18.81,;10.78,-21.35,;12.24,-20.87,;13.44,-19.6,;14.77,-20.09,;16.17,-19.74,;16.18,-18.21,;14.78,-17.63,;13.43,-18.11,;13.74,-18.86,;13.75,-20.45,;15.15,-21.02,)| Show InChI InChI=1S/C25H29N3O3/c1-25(6-7-25)22-20(13-26-28(22)19-4-2-16(3-5-19)24(30)31)23(29)27-21-17-9-14-8-15(11-17)12-18(21)10-14/h2-5,13-15,17-18,21H,6-12H2,1H3,(H,27,29)(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal 6-His tagged full length human 11betaHSD1 incubated for 25 mins by HTRF assay |
J Med Chem 55: 10136-47 (2012)
Article DOI: 10.1021/jm301252n
BindingDB Entry DOI: 10.7270/Q2WM1FJT |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50399352

(CHEMBL2177616)Show SMILES CCCSc1c(cnn1-c1ccc(cc1)C(O)=O)C(=O)N[C@H]1C2CC3CC1C[C@](O)(C3)C2 |r,wU:21.22,wD:28.31,TLB:20:21:31.27.28:25.24.23,20:21:23:31.28.30,29:28:21.26.25:23,THB:27:26:23:31.28.30,27:28:21.26.25:23,30:28:21:25.24.23,30:24:21:31.27.28,29:28:21:25.24.23,(58.75,-15.24,;57.22,-15.4,;56.32,-14.15,;56.94,-12.74,;56.04,-11.5,;56.52,-10.03,;55.27,-9.12,;54.03,-10.03,;54.5,-11.5,;53.59,-12.74,;52.06,-12.57,;51.15,-13.81,;51.78,-15.22,;53.32,-15.38,;54.22,-14.14,;50.87,-16.47,;49.34,-16.31,;51.5,-17.88,;57.98,-9.56,;58.31,-8.05,;59.13,-10.59,;60.59,-10.12,;61.79,-8.84,;61.78,-7.36,;63.13,-6.88,;62.09,-8.11,;62.09,-9.7,;63.5,-10.26,;64.51,-8.98,;66.05,-8.92,;64.52,-7.46,;63.12,-9.33,)| Show InChI InChI=1S/C24H29N3O4S/c1-2-7-32-22-19(13-25-27(22)18-5-3-15(4-6-18)23(29)30)21(28)26-20-16-8-14-9-17(20)12-24(31,10-14)11-16/h3-6,13-14,16-17,20,31H,2,7-12H2,1H3,(H,26,28)(H,29,30)/t14?,16?,17?,20-,24- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal 6-His tagged full length human 11betaHSD1 incubated for 25 mins by HTRF assay |
J Med Chem 55: 10136-47 (2012)
Article DOI: 10.1021/jm301252n
BindingDB Entry DOI: 10.7270/Q2WM1FJT |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50392216

(CHEMBL2153191 | US8673938, 7)Show SMILES CCCSc1nc(ccc1C(=O)NC1CCCCC1)N1CCC[C@@H](CC(O)=O)C1 |r| Show InChI InChI=1S/C22H33N3O3S/c1-2-13-29-22-18(21(28)23-17-8-4-3-5-9-17)10-11-19(24-22)25-12-6-7-16(15-25)14-20(26)27/h10-11,16-17H,2-9,12-15H2,1H3,(H,23,28)(H,26,27)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal 6-His tagged full length human 11betaHSD1 incubated for 25 mins by HTRF assay |
J Med Chem 55: 10136-47 (2012)
Article DOI: 10.1021/jm301252n
BindingDB Entry DOI: 10.7270/Q2WM1FJT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50394011
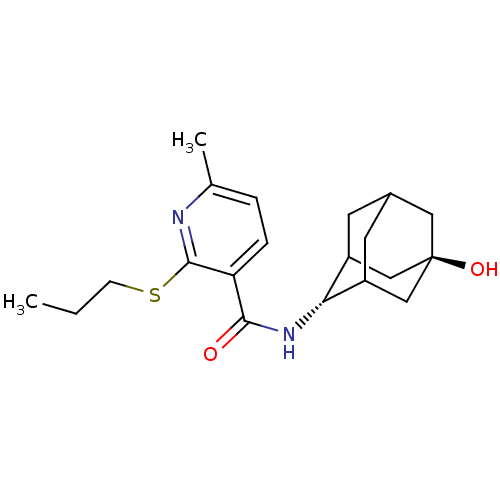
(CHEMBL2158464)Show SMILES CCCSc1nc(C)ccc1C(=O)N[C@H]1C2CC3CC1C[C@](O)(C3)C2 |r,wU:14.14,wD:21.23,TLB:18:19:24:16.17.23,18:17:14.19.20:24,22:21:14:16.18.17,THB:13:14:24:16.17.23,23:17:14:20.21.24,23:21:14:16.18.17,(1.43,-17.15,;.1,-17.92,;-1.24,-17.15,;-1.24,-15.61,;-2.58,-14.84,;-3.91,-15.62,;-5.25,-14.84,;-6.58,-15.61,;-5.24,-13.3,;-3.92,-12.53,;-2.58,-13.29,;-1.25,-12.52,;-1.26,-10.98,;.09,-13.28,;1.41,-12.51,;2.58,-11.2,;3.92,-11.67,;5.31,-11.29,;4.32,-12.59,;2.91,-12.05,;2.87,-10.46,;3.89,-9.21,;3.87,-7.67,;5.29,-9.77,;2.55,-9.72,)| Show InChI InChI=1S/C20H28N2O2S/c1-3-6-25-19-16(5-4-12(2)21-19)18(23)22-17-14-7-13-8-15(17)11-20(24,9-13)10-14/h4-5,13-15,17,24H,3,6-11H2,1-2H3,(H,22,23)/t13?,14?,15?,17-,20- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human 11betaHSD1 by HTRF assay |
Bioorg Med Chem Lett 22: 6756-61 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.070
BindingDB Entry DOI: 10.7270/Q23R0V0G |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50399335

(CHEMBL2177609)Show SMILES CC(C)(C)c1c(cnn1-c1ccc(cc1)C(O)=O)C(=O)NC1C2CC3CC(C2)CC1C3 |TLB:20:21:23.30.24:28.26.27,30:29:27:23.24.25,THB:20:21:27:23.24.25,30:24:21.29.28:27,25:24:21:28.26.27,25:26:21:23.30.24,(26.01,-25.71,;26.64,-24.31,;28.17,-24.15,;27.4,-25.64,;25.74,-23.06,;26.22,-21.6,;24.97,-20.69,;23.72,-21.6,;24.2,-23.06,;23.29,-24.31,;21.76,-24.13,;20.85,-25.38,;21.48,-26.79,;23.02,-26.95,;23.91,-25.7,;20.57,-28.04,;19.04,-27.88,;21.2,-29.44,;27.68,-21.12,;28,-19.62,;28.82,-22.16,;30.29,-21.68,;31.49,-20.41,;32.81,-20.9,;34.21,-20.55,;34.22,-19.02,;32.82,-18.44,;31.48,-18.92,;31.79,-19.67,;31.79,-21.26,;33.2,-21.83,)| Show InChI InChI=1S/C25H31N3O3/c1-25(2,3)22-20(13-26-28(22)19-6-4-16(5-7-19)24(30)31)23(29)27-21-17-9-14-8-15(11-17)12-18(21)10-14/h4-7,13-15,17-18,21H,8-12H2,1-3H3,(H,27,29)(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal 6-His tagged full length human 11betaHSD1 incubated for 25 mins by HTRF assay |
J Med Chem 55: 10136-47 (2012)
Article DOI: 10.1021/jm301252n
BindingDB Entry DOI: 10.7270/Q2WM1FJT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50399338
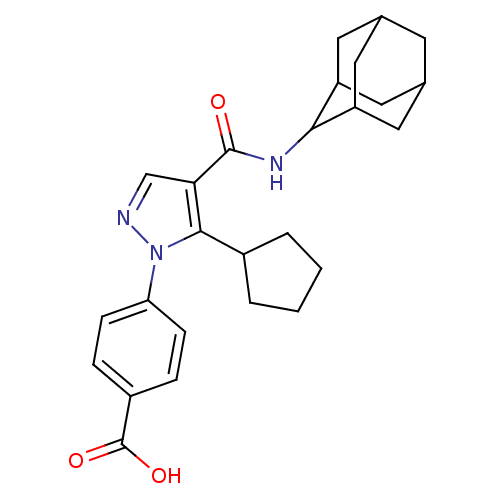
(CHEMBL2177606)Show SMILES OC(=O)c1ccc(cc1)-n1ncc(C(=O)NC2C3CC4CC(C3)CC2C4)c1C1CCCC1 |TLB:15:16:18.25.19:23.21.22,25:24:22:18.19.20,THB:15:16:22:18.19.20,25:19:16.24.23:22,20:19:16:23.21.22,20:21:16:18.25.19,(33.58,-8.41,;35.11,-8.57,;35.73,-9.98,;36.01,-7.32,;35.39,-5.91,;36.3,-4.67,;37.83,-4.84,;38.45,-6.23,;37.55,-7.48,;38.73,-3.59,;38.26,-2.13,;39.5,-1.22,;40.75,-2.13,;42.22,-1.66,;42.54,-.15,;43.36,-2.69,;44.83,-2.21,;46.02,-.94,;47.35,-1.43,;48.75,-1.08,;48.76,.46,;47.36,1.03,;46.02,.56,;46.32,-.2,;46.33,-1.79,;47.73,-2.36,;40.27,-3.59,;41.18,-4.84,;40.7,-6.3,;41.94,-7.21,;43.19,-6.3,;42.71,-4.84,)| Show InChI InChI=1S/C26H31N3O3/c30-25(28-23-19-10-15-9-16(12-19)13-20(23)11-15)22-14-27-29(24(22)17-3-1-2-4-17)21-7-5-18(6-8-21)26(31)32/h5-8,14-17,19-20,23H,1-4,9-13H2,(H,28,30)(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal 6-His tagged full length human 11betaHSD1 incubated for 25 mins by HTRF assay |
J Med Chem 55: 10136-47 (2012)
Article DOI: 10.1021/jm301252n
BindingDB Entry DOI: 10.7270/Q2WM1FJT |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50194415
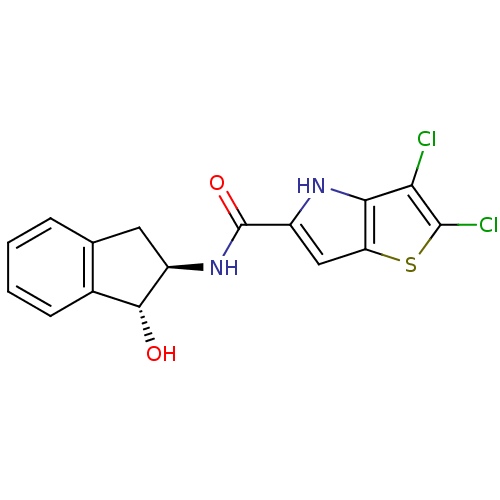
(2,3-dichloro-N-((1R,2R)-1-hydroxy-2,3-dihydro-1H-i...)Show SMILES O[C@H]1[C@@H](Cc2ccccc12)NC(=O)c1cc2sc(Cl)c(Cl)c2[nH]1 Show InChI InChI=1S/C16H12Cl2N2O2S/c17-12-13-11(23-15(12)18)6-10(19-13)16(22)20-9-5-7-3-1-2-4-8(7)14(9)21/h1-4,6,9,14,19,21H,5H2,(H,20,22)/t9-,14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human liver GPa by multienzyme coupled assay |
Bioorg Med Chem Lett 16: 5567-71 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.047
BindingDB Entry DOI: 10.7270/Q2KW5FP3 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50394010
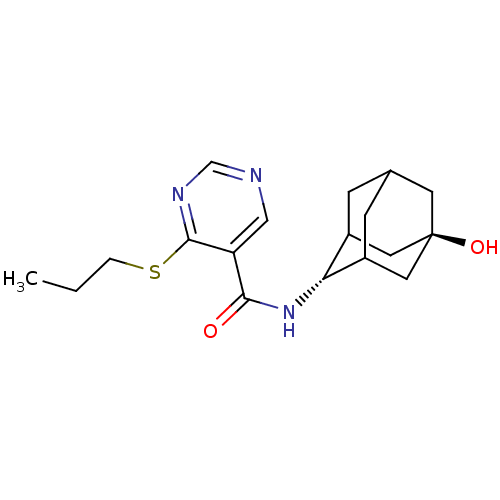
(CHEMBL2158007)Show SMILES CCCSc1ncncc1C(=O)N[C@H]1C2CC3CC1C[C@](O)(C3)C2 |r,wU:13.13,wD:20.22,TLB:17:18:23:15.16.22,17:16:13.18.19:23,21:20:13:15.17.16,THB:12:13:23:15.16.22,22:16:13:19.20.23,22:20:13:15.17.16,(16.53,-3.98,;15.2,-4.75,;13.87,-3.98,;13.86,-2.44,;12.53,-1.68,;11.19,-2.45,;9.86,-1.68,;9.86,-.13,;11.19,.64,;12.52,-.13,;13.85,.65,;13.85,2.19,;15.19,-.12,;16.52,.66,;17.69,1.97,;19.03,1.5,;20.42,1.87,;19.43,.58,;18.01,1.12,;17.97,2.71,;18.99,3.95,;18.98,5.5,;20.4,3.4,;17.65,3.45,)| Show InChI InChI=1S/C18H25N3O2S/c1-2-3-24-17-14(9-19-10-20-17)16(22)21-15-12-4-11-5-13(15)8-18(23,6-11)7-12/h9-13,15,23H,2-8H2,1H3,(H,21,22)/t11?,12?,13?,15-,18- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human 11betaHSD1 by HTRF assay |
Bioorg Med Chem Lett 22: 6756-61 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.070
BindingDB Entry DOI: 10.7270/Q23R0V0G |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50399339
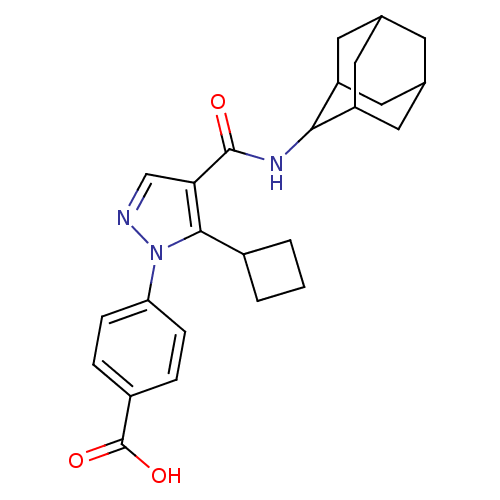
(CHEMBL2177605)Show SMILES OC(=O)c1ccc(cc1)-n1ncc(C(=O)NC2C3CC4CC(C3)CC2C4)c1C1CCC1 |TLB:15:16:18.25.19:23.21.22,25:24:22:18.19.20,THB:15:16:22:18.19.20,25:19:16.24.23:22,20:19:16:23.21.22,20:21:16:18.25.19,(16.81,-9.09,;18.35,-9.25,;18.97,-10.66,;19.25,-8,;18.63,-6.59,;19.53,-5.35,;21.06,-5.52,;21.69,-6.92,;20.79,-8.16,;21.97,-4.28,;21.5,-2.81,;22.74,-1.91,;23.99,-2.81,;25.46,-2.34,;25.78,-.83,;26.6,-3.37,;28.06,-2.9,;29.26,-1.62,;30.59,-2.11,;31.98,-1.77,;32,-.24,;30.6,.34,;29.25,-.14,;29.56,-.89,;29.57,-2.48,;30.97,-3.04,;23.51,-4.28,;24.42,-5.52,;24.18,-7.05,;25.7,-7.29,;25.94,-5.77,)| Show InChI InChI=1S/C25H29N3O3/c29-24(27-22-18-9-14-8-15(11-18)12-19(22)10-14)21-13-26-28(23(21)16-2-1-3-16)20-6-4-17(5-7-20)25(30)31/h4-7,13-16,18-19,22H,1-3,8-12H2,(H,27,29)(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal 6-His tagged full length human 11betaHSD1 incubated for 25 mins by HTRF assay |
J Med Chem 55: 10136-47 (2012)
Article DOI: 10.1021/jm301252n
BindingDB Entry DOI: 10.7270/Q2WM1FJT |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50399347

(CHEMBL2177621)Show SMILES CSc1c(cnn1-c1ccc(cc1)C(O)=O)C(=O)NC1C2CC3CC(C2)CC1C3 |TLB:18:19:21.28.22:26.24.25,28:27:25:21.22.23,THB:18:19:25:21.22.23,28:22:19.27.26:25,23:22:19:26.24.25,23:24:19:21.28.22,(16.98,-40.7,;17.61,-39.29,;16.71,-38.05,;17.19,-36.58,;15.94,-35.68,;14.69,-36.58,;15.17,-38.05,;14.26,-39.29,;12.73,-39.12,;11.82,-40.36,;12.45,-41.77,;13.99,-41.93,;14.88,-40.69,;11.54,-43.02,;10.01,-42.86,;12.17,-44.43,;18.65,-36.11,;18.97,-34.6,;19.79,-37.14,;21.26,-36.67,;22.46,-35.39,;23.78,-35.88,;25.18,-35.54,;25.19,-34.01,;23.79,-33.43,;22.45,-33.91,;22.76,-34.66,;22.76,-36.25,;24.17,-36.81,)| Show InChI InChI=1S/C22H25N3O3S/c1-29-21-18(11-23-25(21)17-4-2-14(3-5-17)22(27)28)20(26)24-19-15-7-12-6-13(9-15)10-16(19)8-12/h2-5,11-13,15-16,19H,6-10H2,1H3,(H,24,26)(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal 6-His tagged full length human 11betaHSD1 incubated for 25 mins by HTRF assay |
J Med Chem 55: 10136-47 (2012)
Article DOI: 10.1021/jm301252n
BindingDB Entry DOI: 10.7270/Q2WM1FJT |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50394012
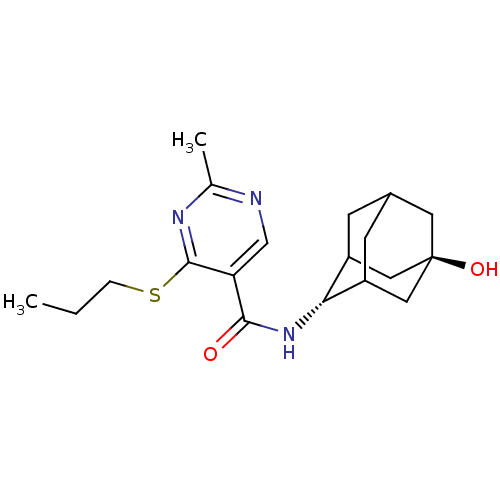
(CHEMBL2158465)Show SMILES CCCSc1nc(C)ncc1C(=O)N[C@H]1C2CC3CC1C[C@](O)(C3)C2 |r,wU:14.14,wD:21.23,TLB:18:19:24:16.17.23,18:17:14.19.20:24,22:21:14:16.18.17,THB:13:14:24:16.17.23,23:17:14:20.21.24,23:21:14:16.18.17,(18.17,-16.91,;16.83,-17.69,;15.5,-16.92,;15.5,-15.38,;14.16,-14.61,;12.83,-15.38,;11.49,-14.61,;10.16,-15.38,;11.49,-13.07,;12.82,-12.3,;14.16,-13.06,;15.49,-12.28,;15.48,-10.74,;16.82,-13.05,;18.15,-12.28,;19.32,-10.97,;20.66,-11.43,;22.05,-11.06,;21.06,-12.36,;19.64,-11.82,;19.61,-10.23,;20.62,-8.98,;20.61,-7.44,;22.03,-9.53,;19.29,-9.48,)| Show InChI InChI=1S/C19H27N3O2S/c1-3-4-25-18-15(10-20-11(2)21-18)17(23)22-16-13-5-12-6-14(16)9-19(24,7-12)8-13/h10,12-14,16,24H,3-9H2,1-2H3,(H,22,23)/t12?,13?,14?,16-,19- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human 11betaHSD1 by HTRF assay |
Bioorg Med Chem Lett 22: 6756-61 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.070
BindingDB Entry DOI: 10.7270/Q23R0V0G |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50399345
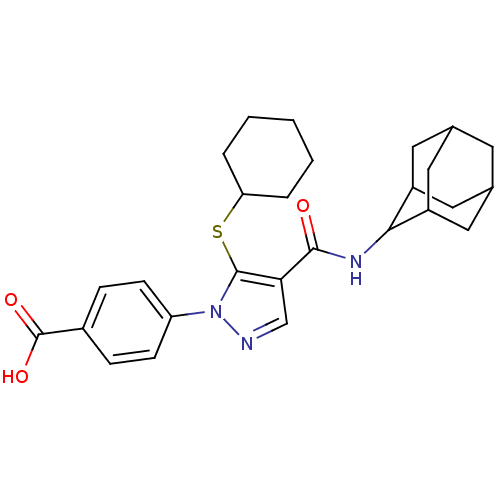
(CHEMBL2180884)Show SMILES OC(=O)c1ccc(cc1)-n1ncc(C(=O)NC2C3CC4CC(C3)CC2C4)c1SC1CCCCC1 |TLB:15:16:18.25.19:23.21.22,25:24:22:18.19.20,THB:15:16:22:18.19.20,25:19:16.24.23:22,20:19:16:23.21.22,20:21:16:18.25.19,(43.67,-41.61,;45.2,-41.77,;45.83,-43.18,;46.1,-40.52,;45.48,-39.11,;46.39,-37.87,;47.92,-38.04,;48.54,-39.43,;47.64,-40.68,;48.83,-36.79,;48.35,-35.33,;49.6,-34.42,;50.84,-35.33,;52.31,-34.86,;52.63,-33.35,;53.45,-35.89,;54.92,-35.41,;56.11,-34.14,;57.44,-34.63,;58.84,-34.28,;58.85,-32.75,;57.45,-32.18,;56.11,-32.65,;56.41,-33.4,;56.42,-34.99,;57.82,-35.56,;50.37,-36.79,;51.27,-38.04,;50.64,-39.45,;49.11,-39.6,;48.48,-41,;49.38,-42.25,;50.91,-42.09,;51.55,-40.69,)| Show InChI InChI=1S/C27H33N3O3S/c31-25(29-24-19-11-16-10-17(13-19)14-20(24)12-16)23-15-28-30(21-8-6-18(7-9-21)27(32)33)26(23)34-22-4-2-1-3-5-22/h6-9,15-17,19-20,22,24H,1-5,10-14H2,(H,29,31)(H,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal 6-His tagged full length human 11betaHSD1 incubated for 25 mins by HTRF assay |
J Med Chem 55: 10136-47 (2012)
Article DOI: 10.1021/jm301252n
BindingDB Entry DOI: 10.7270/Q2WM1FJT |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50394018
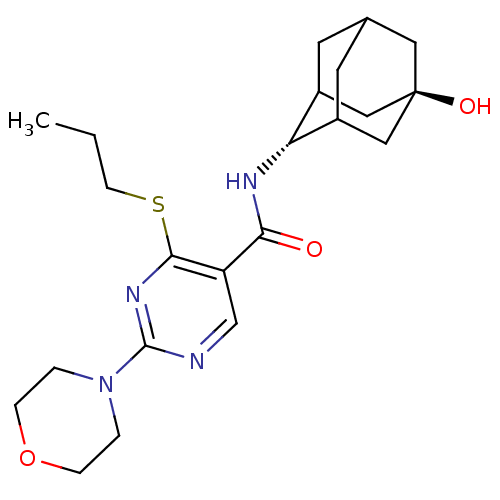
(CHEMBL2158471)Show SMILES CCCSc1nc(ncc1C(=O)N[C@H]1C2CC3CC1C[C@](O)(C3)C2)N1CCOCC1 |r,wU:13.13,wD:20.22,TLB:17:18:23:15.16.22,17:16:13.18.19:23,21:20:13:15.17.16,THB:12:13:23:15.16.22,22:16:13:19.20.23,22:20:13:15.17.16,(23.49,-4.45,;22.16,-5.23,;20.83,-4.46,;20.83,-2.92,;19.49,-2.15,;18.15,-2.92,;16.82,-2.15,;16.82,-.61,;18.15,.16,;19.48,-.6,;20.82,.18,;20.81,1.72,;22.15,-.59,;23.48,.18,;24.65,1.49,;25.99,1.03,;27.38,1.4,;26.39,.1,;24.97,.64,;24.94,2.23,;25.95,3.48,;25.94,5.02,;27.36,2.93,;24.61,2.98,;15.49,-2.92,;14.16,-2.14,;12.83,-2.9,;12.82,-4.44,;14.15,-5.22,;15.49,-4.45,)| Show InChI InChI=1S/C22H32N4O3S/c1-2-7-30-20-17(13-23-21(25-20)26-3-5-29-6-4-26)19(27)24-18-15-8-14-9-16(18)12-22(28,10-14)11-15/h13-16,18,28H,2-12H2,1H3,(H,24,27)/t14?,15?,16?,18-,22- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human 11betaHSD1 by HTRF assay |
Bioorg Med Chem Lett 22: 6756-61 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.070
BindingDB Entry DOI: 10.7270/Q23R0V0G |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50399350

(CHEMBL2177618)Show SMILES CCCSc1c(cnn1-c1ccc(cc1)C(O)=O)C(=O)N[C@H]1C2CC3CC1C[C@](C3)(C2)OC(F)F |r,wU:21.22,wD:28.35,TLB:20:21:30.27.28:25.24.23,20:21:23:30.28.29,31:28:21.26.25:23,THB:27:26:23:30.28.29,27:28:21.26.25:23,29:28:21:25.24.23,29:24:21:30.27.28,31:28:21:25.24.23,(27.09,-29.55,;25.56,-29.71,;24.65,-28.46,;25.28,-27.05,;24.38,-25.81,;24.85,-24.34,;23.61,-23.43,;22.36,-24.34,;22.84,-25.81,;21.93,-27.05,;20.4,-26.88,;19.49,-28.12,;20.11,-29.53,;21.65,-29.69,;22.55,-28.45,;19.21,-30.78,;17.68,-30.62,;19.84,-32.19,;26.32,-23.87,;26.64,-22.36,;27.46,-24.9,;28.93,-24.43,;30.13,-23.15,;30.12,-21.67,;31.46,-21.19,;30.43,-22.42,;30.43,-24.01,;31.84,-24.57,;32.85,-23.3,;32.86,-21.77,;31.45,-23.64,;34.39,-23.23,;35.1,-21.87,;36.64,-21.81,;34.28,-20.57,)| Show InChI InChI=1S/C25H29F2N3O4S/c1-2-7-35-22-19(13-28-30(22)18-5-3-15(4-6-18)23(32)33)21(31)29-20-16-8-14-9-17(20)12-25(10-14,11-16)34-24(26)27/h3-6,13-14,16-17,20,24H,2,7-12H2,1H3,(H,29,31)(H,32,33)/t14?,16?,17?,20-,25- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal 6-His tagged full length human 11betaHSD1 incubated for 25 mins by HTRF assay |
J Med Chem 55: 10136-47 (2012)
Article DOI: 10.1021/jm301252n
BindingDB Entry DOI: 10.7270/Q2WM1FJT |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50394001
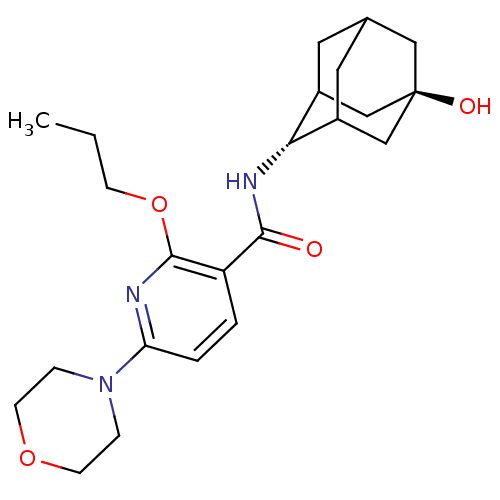
(CHEMBL2158482)Show SMILES CCCOc1nc(ccc1C(=O)N[C@H]1C2CC3CC1C[C@](O)(C3)C2)N1CCOCC1 |r,wU:13.13,wD:20.22,TLB:21:20:13:15.17.16,17:18:23:15.16.22,17:16:13.18.19:23,THB:12:13:23:15.16.22,22:16:13:19.20.23,22:20:13:15.17.16,(18.84,-18.34,;17.51,-19.11,;16.18,-18.34,;16.17,-16.8,;14.84,-16.03,;13.5,-16.81,;12.17,-16.03,;12.17,-14.49,;13.5,-13.72,;14.83,-14.48,;16.16,-13.71,;16.16,-12.17,;17.5,-14.47,;18.83,-13.7,;20,-12.39,;21.34,-12.86,;22.73,-12.48,;21.74,-13.78,;20.32,-13.24,;20.28,-11.65,;21.3,-10.4,;21.29,-8.86,;22.71,-10.96,;19.96,-10.91,;10.84,-16.81,;9.5,-16.03,;8.17,-16.79,;8.16,-18.33,;9.5,-19.11,;10.84,-18.34,)| Show InChI InChI=1S/C23H33N3O4/c1-2-7-30-22-18(3-4-19(24-22)26-5-8-29-9-6-26)21(27)25-20-16-10-15-11-17(20)14-23(28,12-15)13-16/h3-4,15-17,20,28H,2,5-14H2,1H3,(H,25,27)/t15?,16?,17?,20-,23- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human 11betaHSD1 by HTRF assay |
Bioorg Med Chem Lett 22: 6756-61 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.070
BindingDB Entry DOI: 10.7270/Q23R0V0G |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50320331
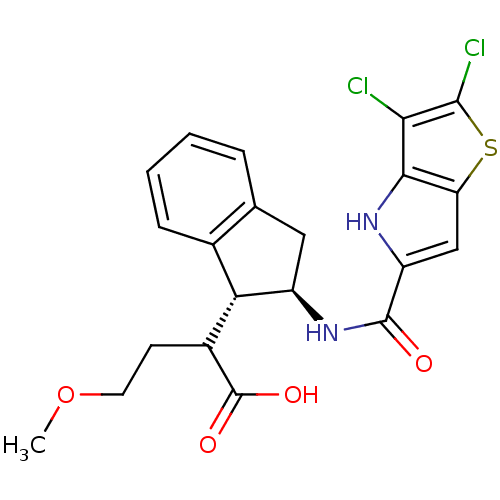
((S)-2-((1R,2R)-2-(2,3-DICHLORO-4H-THIENO[3,2-B]PYR...)Show SMILES COCCC([C@H]1[C@@H](Cc2ccccc12)NC(=O)c1cc2sc(Cl)c(Cl)c2[nH]1)C(O)=O |r| Show InChI InChI=1S/C21H20Cl2N2O4S/c1-29-7-6-12(21(27)28)16-11-5-3-2-4-10(11)8-13(16)25-20(26)14-9-15-18(24-14)17(22)19(23)30-15/h2-5,9,12-13,16,24H,6-8H2,1H3,(H,25,26)(H,27,28)/t12?,13-,16+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant liver glycogen phosphorylase by multienzyme-coupled reaction |
Bioorg Med Chem Lett 20: 3511-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.147
BindingDB Entry DOI: 10.7270/Q2571C66 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50399341
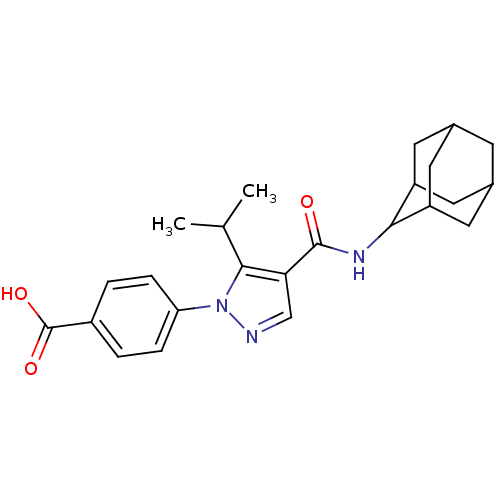
(CHEMBL2177603)Show SMILES CC(C)c1c(cnn1-c1ccc(cc1)C(O)=O)C(=O)NC1C2CC3CC(C2)CC1C3 |TLB:19:20:22.29.23:27.25.26,29:28:26:22.23.24,THB:19:20:26:22.23.24,29:23:20.28.27:26,24:23:20:27.25.26,24:25:20:22.29.23,(53.92,-53.92,;54.55,-52.52,;56.08,-52.36,;53.64,-51.27,;54.12,-49.81,;52.87,-48.9,;51.63,-49.81,;52.1,-51.27,;51.2,-52.52,;49.66,-52.34,;48.76,-53.59,;49.38,-55,;50.92,-55.16,;51.82,-53.91,;48.48,-56.25,;46.95,-56.09,;49.1,-57.65,;55.59,-49.33,;55.91,-47.83,;56.73,-50.37,;58.2,-49.89,;59.39,-48.62,;60.72,-49.11,;62.12,-48.76,;62.13,-47.23,;60.73,-46.65,;59.38,-47.13,;59.69,-47.88,;59.7,-49.47,;61.1,-50.04,)| Show InChI InChI=1S/C24H29N3O3/c1-13(2)22-20(12-25-27(22)19-5-3-16(4-6-19)24(29)30)23(28)26-21-17-8-14-7-15(10-17)11-18(21)9-14/h3-6,12-15,17-18,21H,7-11H2,1-2H3,(H,26,28)(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal 6-His tagged full length human 11betaHSD1 incubated for 25 mins by HTRF assay |
J Med Chem 55: 10136-47 (2012)
Article DOI: 10.1021/jm301252n
BindingDB Entry DOI: 10.7270/Q2WM1FJT |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50194414
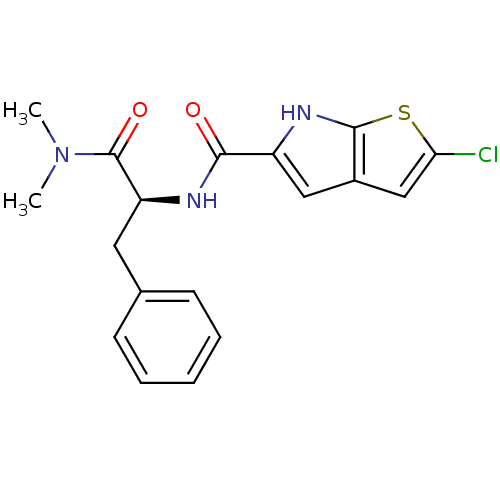
((S)-2-chloro-N-(1-(dimethylamino)-1-oxo-3-phenylpr...)Show SMILES CN(C)C(=O)[C@H](Cc1ccccc1)NC(=O)c1cc2cc(Cl)sc2[nH]1 Show InChI InChI=1S/C18H18ClN3O2S/c1-22(2)18(24)14(8-11-6-4-3-5-7-11)20-16(23)13-9-12-10-15(19)25-17(12)21-13/h3-7,9-10,14,21H,8H2,1-2H3,(H,20,23)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human liver GPa by multienzyme coupled assay |
Bioorg Med Chem Lett 16: 5567-71 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.047
BindingDB Entry DOI: 10.7270/Q2KW5FP3 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50320321
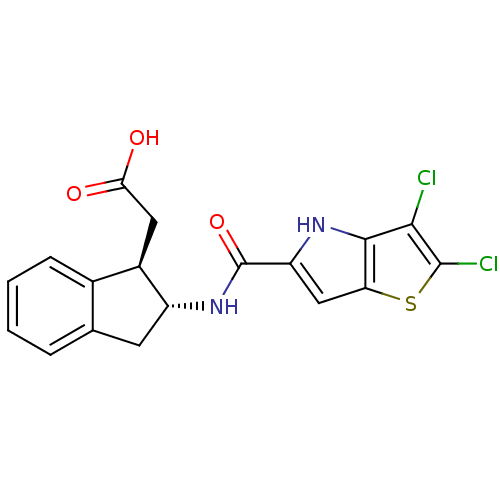
(2-((1R,2R)-2-(2,3-dichloro-4H-thieno[3,2-b]pyrrole...)Show SMILES OC(=O)C[C@H]1[C@@H](Cc2ccccc12)NC(=O)c1cc2sc(Cl)c(Cl)c2[nH]1 |r| Show InChI InChI=1S/C18H14Cl2N2O3S/c19-15-16-13(26-17(15)20)7-12(21-16)18(25)22-11-5-8-3-1-2-4-9(8)10(11)6-14(23)24/h1-4,7,10-11,21H,5-6H2,(H,22,25)(H,23,24)/t10-,11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant liver glycogen phosphorylase by multienzyme-coupled reaction |
Bioorg Med Chem Lett 20: 3511-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.147
BindingDB Entry DOI: 10.7270/Q2571C66 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50399359

(CHEMBL2177610)Show InChI InChI=1S/C14H23N3OS/c1-3-9-19-14-12(10-15-17(14)2)13(18)16-11-7-5-4-6-8-11/h10-11H,3-9H2,1-2H3,(H,16,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal 6-His tagged full length human 11betaHSD1 incubated for 25 mins by HTRF assay |
J Med Chem 55: 10136-47 (2012)
Article DOI: 10.1021/jm301252n
BindingDB Entry DOI: 10.7270/Q2WM1FJT |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50394001

(CHEMBL2158482)Show SMILES CCCOc1nc(ccc1C(=O)N[C@H]1C2CC3CC1C[C@](O)(C3)C2)N1CCOCC1 |r,wU:13.13,wD:20.22,TLB:21:20:13:15.17.16,17:18:23:15.16.22,17:16:13.18.19:23,THB:12:13:23:15.16.22,22:16:13:19.20.23,22:20:13:15.17.16,(18.84,-18.34,;17.51,-19.11,;16.18,-18.34,;16.17,-16.8,;14.84,-16.03,;13.5,-16.81,;12.17,-16.03,;12.17,-14.49,;13.5,-13.72,;14.83,-14.48,;16.16,-13.71,;16.16,-12.17,;17.5,-14.47,;18.83,-13.7,;20,-12.39,;21.34,-12.86,;22.73,-12.48,;21.74,-13.78,;20.32,-13.24,;20.28,-11.65,;21.3,-10.4,;21.29,-8.86,;22.71,-10.96,;19.96,-10.91,;10.84,-16.81,;9.5,-16.03,;8.17,-16.79,;8.16,-18.33,;9.5,-19.11,;10.84,-18.34,)| Show InChI InChI=1S/C23H33N3O4/c1-2-7-30-22-18(3-4-19(24-22)26-5-8-29-9-6-26)21(27)25-20-16-10-15-11-17(20)14-23(28,12-15)13-16/h3-4,15-17,20,28H,2,5-14H2,1H3,(H,25,27)/t15?,16?,17?,20-,23- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11betaHSD1 by HTRF assay |
Bioorg Med Chem Lett 22: 6756-61 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.070
BindingDB Entry DOI: 10.7270/Q23R0V0G |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50394013

(CHEMBL2158466)Show SMILES CCCSc1nc(NC)ccc1C(=O)N[C@H]1C2CC3CC1C[C@](O)(C3)C2 |r,wU:15.15,wD:22.24,TLB:19:20:25:17.18.24,19:18:15.20.21:25,23:22:15:17.19.18,THB:14:15:25:17.18.24,24:18:15:21.22.25,24:22:15:17.19.18,(1.31,-29.92,;-.02,-30.69,;-1.35,-29.92,;-1.36,-28.38,;-2.69,-27.61,;-4.03,-28.39,;-5.36,-27.62,;-6.7,-28.38,;-8.03,-27.61,;-5.36,-26.07,;-4.03,-25.3,;-2.7,-26.06,;-1.37,-25.29,;-1.37,-23.75,;-.03,-26.05,;1.3,-25.28,;2.47,-23.97,;3.81,-24.44,;5.19,-24.06,;4.2,-25.36,;2.79,-24.82,;2.75,-23.23,;3.77,-21.98,;3.76,-20.44,;5.18,-22.54,;2.43,-22.49,)| Show InChI InChI=1S/C20H29N3O2S/c1-3-6-26-19-15(4-5-16(21-2)22-19)18(24)23-17-13-7-12-8-14(17)11-20(25,9-12)10-13/h4-5,12-14,17,25H,3,6-11H2,1-2H3,(H,21,22)(H,23,24)/t12?,13?,14?,17-,20- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11betaHSD1 by HTRF assay |
Bioorg Med Chem Lett 22: 6756-61 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.070
BindingDB Entry DOI: 10.7270/Q23R0V0G |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Mus musculus) | BDBM50008294

(2-(4-(((2-amino-4-oxo-1,4-dihydroquinazolin-6-yl)m...)Show SMILES Nc1nc2ccc(CN(CC#C)c3ccc(cc3)C(=O)NC(CCC(O)=O)C(O)=O)cc2c(=O)[nH]1 Show InChI InChI=1S/C24H23N5O6/c1-2-11-29(13-14-3-8-18-17(12-14)22(33)28-24(25)27-18)16-6-4-15(5-7-16)21(32)26-19(23(34)35)9-10-20(30)31/h1,3-8,12,19H,9-11,13H2,(H,26,32)(H,30,31)(H,34,35)(H3,25,27,28,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against thymidylate synthase |
J Med Chem 28: 1468-76 (1985)
BindingDB Entry DOI: 10.7270/Q2348JDZ |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50399340
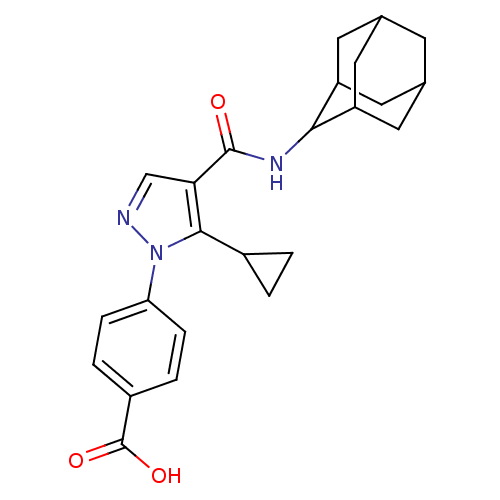
(CHEMBL2177604)Show SMILES OC(=O)c1ccc(cc1)-n1ncc(C(=O)NC2C3CC4CC(C3)CC2C4)c1C1CC1 |TLB:15:16:18.25.19:23.21.22,25:24:22:18.19.20,THB:15:16:22:18.19.20,25:19:16.24.23:22,20:19:16:23.21.22,20:21:16:18.25.19,(-2.53,-10.16,;-1,-10.32,;-.37,-11.73,;-.09,-9.07,;-.72,-7.66,;.2,-6.42,;1.73,-6.59,;2.35,-7.98,;1.46,-9.23,;2.64,-5.34,;2.17,-3.88,;3.41,-2.97,;4.66,-3.88,;6.12,-3.41,;6.44,-1.9,;7.26,-4.44,;8.73,-3.96,;9.93,-2.69,;11.25,-3.18,;12.65,-2.83,;12.66,-1.3,;11.26,-.72,;9.92,-1.2,;10.23,-1.95,;10.23,-3.54,;11.64,-4.11,;4.18,-5.34,;5.08,-6.59,;5.23,-8.13,;6.48,-7.23,)| Show InChI InChI=1S/C24H27N3O3/c28-23(26-21-17-8-13-7-14(10-17)11-18(21)9-13)20-12-25-27(22(20)15-1-2-15)19-5-3-16(4-6-19)24(29)30/h3-6,12-15,17-18,21H,1-2,7-11H2,(H,26,28)(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal 6-His tagged full length human 11betaHSD1 incubated for 25 mins by HTRF assay |
J Med Chem 55: 10136-47 (2012)
Article DOI: 10.1021/jm301252n
BindingDB Entry DOI: 10.7270/Q2WM1FJT |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50394000

(CHEMBL2158481)Show SMILES O[C@@]12CC3CC(C1)[C@H](NC(=O)c1ccc(nc1C1CC1)N1CCOCC1)C(C3)C2 |r,wU:7.8,wD:1.0,TLB:0:1:7:4.27.3,27:26:6:4.3.2,THB:8:7:6:4.3.2,27:3:7.26.28:6,2:3:7:28.1.6,2:1:7:4.27.3,(5.69,-7.49,;5.71,-9.03,;7.11,-9.59,;7.13,-11.11,;5.74,-11.49,;4.4,-11.02,;4.37,-9.54,;3.23,-12.33,;1.91,-13.1,;.57,-12.34,;.56,-10.8,;-.76,-13.11,;-2.1,-12.35,;-3.42,-13.12,;-3.43,-14.67,;-2.09,-15.44,;-.76,-14.66,;.58,-15.43,;1.35,-16.76,;2.12,-15.43,;-4.76,-15.44,;-6.09,-14.66,;-7.42,-15.42,;-7.43,-16.97,;-6.1,-17.74,;-4.76,-16.98,;4.73,-11.87,;6.14,-12.41,;4.69,-10.28,)| Show InChI InChI=1S/C23H31N3O3/c27-22(25-20-16-9-14-10-17(20)13-23(28,11-14)12-16)18-3-4-19(24-21(18)15-1-2-15)26-5-7-29-8-6-26/h3-4,14-17,20,28H,1-2,5-13H2,(H,25,27)/t14?,16?,17?,20-,23- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human 11betaHSD1 by HTRF assay |
Bioorg Med Chem Lett 22: 6756-61 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.070
BindingDB Entry DOI: 10.7270/Q23R0V0G |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50394006
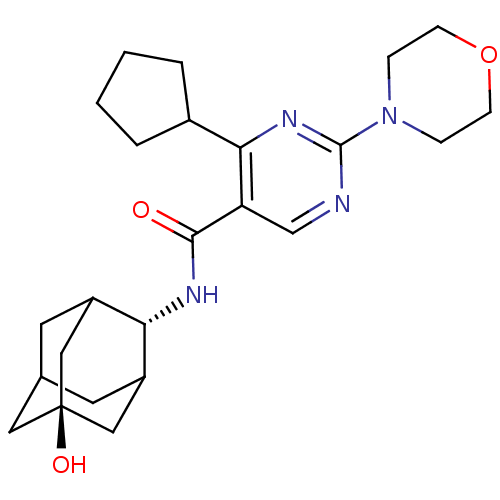
(CHEMBL2158532)Show SMILES O[C@@]12CC3CC(C1)[C@H](NC(=O)c1cnc(nc1C1CCCC1)N1CCOCC1)C(C3)C2 |r,wU:7.8,wD:1.0,TLB:0:1:7:4.29.3,29:28:6:4.3.2,THB:8:7:6:4.3.2,29:3:7.28.30:6,2:3:7:30.1.6,2:1:7:4.29.3,(6.13,-38.89,;6.14,-40.43,;7.55,-40.99,;7.57,-42.51,;6.18,-42.89,;4.84,-42.42,;4.8,-40.94,;3.67,-43.73,;2.34,-44.5,;1.01,-43.74,;1,-42.2,;-.33,-44.51,;-1.66,-43.75,;-2.99,-44.52,;-2.99,-46.06,;-1.66,-46.84,;-.32,-46.06,;.92,-46.98,;.91,-48.51,;2.38,-49,;3.29,-47.75,;2.39,-46.5,;-4.32,-46.84,;-5.66,-46.06,;-6.99,-46.82,;-7,-48.36,;-5.66,-49.14,;-4.32,-48.37,;5.16,-43.27,;6.58,-43.81,;5.13,-41.68,)| Show InChI InChI=1S/C24H34N4O3/c29-22(26-20-17-9-15-10-18(20)13-24(30,11-15)12-17)19-14-25-23(28-5-7-31-8-6-28)27-21(19)16-3-1-2-4-16/h14-18,20,30H,1-13H2,(H,26,29)/t15?,17?,18?,20-,24- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human 11betaHSD1 by HTRF assay |
Bioorg Med Chem Lett 22: 6756-61 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.070
BindingDB Entry DOI: 10.7270/Q23R0V0G |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50194420
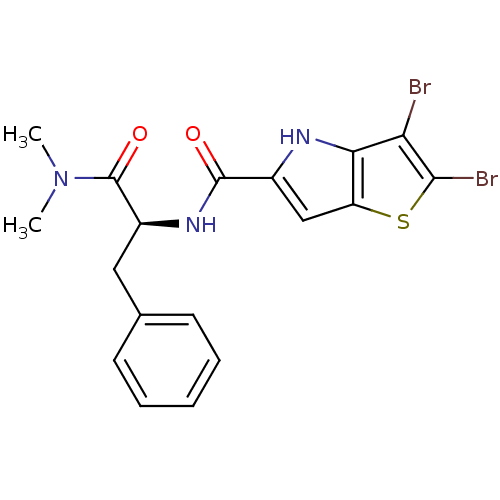
((S)-2,3-dibromo-N-(1-(dimethylamino)-1-oxo-3-pheny...)Show SMILES CN(C)C(=O)[C@H](Cc1ccccc1)NC(=O)c1cc2sc(Br)c(Br)c2[nH]1 Show InChI InChI=1S/C18H17Br2N3O2S/c1-23(2)18(25)12(8-10-6-4-3-5-7-10)22-17(24)11-9-13-15(21-11)14(19)16(20)26-13/h3-7,9,12,21H,8H2,1-2H3,(H,22,24)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human liver GPa by multienzyme coupled assay |
Bioorg Med Chem Lett 16: 5567-71 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.047
BindingDB Entry DOI: 10.7270/Q2KW5FP3 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50394017

(CHEMBL2158470)Show SMILES CCCSc1nc(ccc1C(=O)N[C@H]1C2CC3CC1C[C@](O)(C3)C2)N1CCOCC1 |r,wU:13.13,wD:20.22,TLB:17:18:23:15.16.22,17:16:13.18.19:23,21:20:13:15.17.16,THB:12:13:23:15.16.22,22:16:13:19.20.23,22:20:13:15.17.16,(2.84,-3.33,;1.5,-4.1,;.17,-3.33,;.17,-1.79,;-1.17,-1.02,;-2.5,-1.79,;-3.84,-1.02,;-3.84,.52,;-2.51,1.29,;-1.17,.53,;.16,1.3,;.15,2.84,;1.49,.54,;2.82,1.31,;3.99,2.62,;5.33,2.15,;6.72,2.53,;5.73,1.23,;4.31,1.77,;4.28,3.36,;5.29,4.61,;5.28,6.15,;6.7,4.06,;3.96,4.1,;-5.17,-1.8,;-6.5,-1.02,;-7.83,-1.78,;-7.84,-3.32,;-6.51,-4.1,;-5.17,-3.33,)| Show InChI InChI=1S/C23H33N3O3S/c1-2-9-30-22-18(3-4-19(24-22)26-5-7-29-8-6-26)21(27)25-20-16-10-15-11-17(20)14-23(28,12-15)13-16/h3-4,15-17,20,28H,2,5-14H2,1H3,(H,25,27)/t15?,16?,17?,20-,23- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11betaHSD1 by HTRF assay |
Bioorg Med Chem Lett 22: 6756-61 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.070
BindingDB Entry DOI: 10.7270/Q23R0V0G |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50394015

(CHEMBL2158468)Show SMILES CCCSc1nc(ccc1C(=O)N[C@H]1C2CC3CC1C[C@](O)(C3)C2)N(C)C |r,wU:13.13,wD:20.22,TLB:17:18:23:15.16.22,17:16:13.18.19:23,21:20:13:15.17.16,THB:12:13:23:15.16.22,22:16:13:19.20.23,22:20:13:15.17.16,(1.9,-44.89,;.56,-45.66,;-.77,-44.89,;-.77,-43.35,;-2.11,-42.59,;-3.45,-43.36,;-4.78,-42.59,;-4.78,-41.04,;-3.45,-40.27,;-2.11,-41.04,;-.78,-40.26,;-.79,-38.72,;.55,-41.03,;1.88,-40.25,;3.05,-38.95,;4.39,-39.41,;5.78,-39.04,;4.79,-40.33,;3.37,-39.8,;3.34,-38.2,;4.35,-36.96,;4.34,-35.41,;5.76,-37.51,;3.01,-37.46,;-6.11,-43.36,;-7.45,-42.59,;-6.12,-44.9,)| Show InChI InChI=1S/C21H31N3O2S/c1-4-7-27-20-16(5-6-17(22-20)24(2)3)19(25)23-18-14-8-13-9-15(18)12-21(26,10-13)11-14/h5-6,13-15,18,26H,4,7-12H2,1-3H3,(H,23,25)/t13?,14?,15?,18-,21- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11betaHSD1 by HTRF assay |
Bioorg Med Chem Lett 22: 6756-61 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.070
BindingDB Entry DOI: 10.7270/Q23R0V0G |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50394009
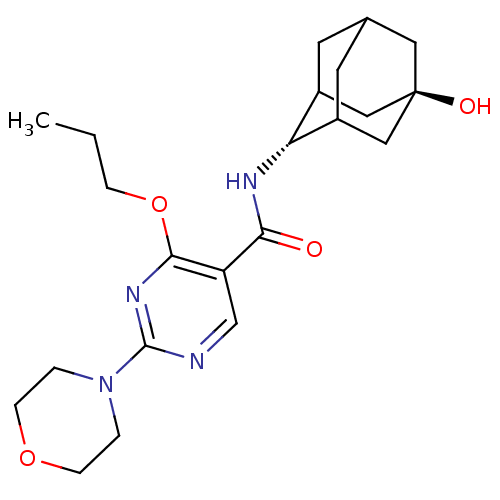
(CHEMBL2158535)Show SMILES CCCOc1nc(ncc1C(=O)N[C@H]1C2CC3CC1C[C@](O)(C3)C2)N1CCOCC1 |r,wU:13.13,wD:20.22,TLB:21:20:13:15.17.16,17:18:23:15.16.22,17:16:13.18.19:23,THB:12:13:23:15.16.22,22:16:13:19.20.23,22:20:13:15.17.16,(26.63,-7.07,;25.3,-7.84,;23.96,-7.07,;23.96,-5.53,;22.62,-4.76,;21.29,-5.54,;19.95,-4.76,;19.96,-3.22,;21.28,-2.45,;22.62,-3.21,;23.95,-2.44,;23.94,-.9,;25.29,-3.2,;26.61,-2.43,;27.78,-1.12,;29.12,-1.59,;30.51,-1.21,;29.52,-2.51,;28.11,-1.97,;28.07,-.38,;29.09,.87,;29.07,2.41,;30.49,.31,;27.75,.36,;18.62,-5.54,;17.29,-4.76,;15.96,-5.52,;15.95,-7.06,;17.28,-7.84,;18.62,-7.07,)| Show InChI InChI=1S/C22H32N4O4/c1-2-5-30-20-17(13-23-21(25-20)26-3-6-29-7-4-26)19(27)24-18-15-8-14-9-16(18)12-22(28,10-14)11-15/h13-16,18,28H,2-12H2,1H3,(H,24,27)/t14?,15?,16?,18-,22- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human 11betaHSD1 by HTRF assay |
Bioorg Med Chem Lett 22: 6756-61 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.070
BindingDB Entry DOI: 10.7270/Q23R0V0G |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50394014

(CHEMBL2158467)Show SMILES CCCSc1nc(NC)ncc1C(=O)N[C@H]1C2CC3CC1C[C@](O)(C3)C2 |r,wU:15.15,wD:22.24,TLB:19:20:25:17.18.24,19:18:15.20.21:25,23:22:15:17.19.18,THB:14:15:25:17.18.24,24:18:15:21.22.25,24:22:15:17.19.18,(21.02,-29.8,;19.69,-30.57,;18.35,-29.81,;18.35,-28.27,;17.02,-27.5,;15.68,-28.27,;14.35,-27.5,;13.01,-28.27,;11.68,-27.5,;14.35,-25.95,;15.68,-25.18,;17.01,-25.95,;18.34,-25.17,;18.34,-23.63,;19.68,-25.94,;21.01,-25.16,;22.18,-23.86,;23.51,-24.32,;24.9,-23.95,;23.91,-25.24,;22.5,-24.71,;22.46,-23.12,;23.48,-21.87,;23.47,-20.32,;24.88,-22.42,;22.14,-22.37,)| Show InChI InChI=1S/C19H28N4O2S/c1-3-4-26-17-14(10-21-18(20-2)23-17)16(24)22-15-12-5-11-6-13(15)9-19(25,7-11)8-12/h10-13,15,25H,3-9H2,1-2H3,(H,22,24)(H,20,21,23)/t11?,12?,13?,15-,19- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11betaHSD1 by HTRF assay |
Bioorg Med Chem Lett 22: 6756-61 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.070
BindingDB Entry DOI: 10.7270/Q23R0V0G |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50399356

(CHEMBL2177613)Show SMILES CCCSc1c(cnn1-c1ccc(cc1)C(O)=O)C(=O)NC1CCCCC1 Show InChI InChI=1S/C20H25N3O3S/c1-2-12-27-19-17(18(24)22-15-6-4-3-5-7-15)13-21-23(19)16-10-8-14(9-11-16)20(25)26/h8-11,13,15H,2-7,12H2,1H3,(H,22,24)(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal 6-His tagged full length human 11betaHSD1 incubated for 25 mins by HTRF assay |
J Med Chem 55: 10136-47 (2012)
Article DOI: 10.1021/jm301252n
BindingDB Entry DOI: 10.7270/Q2WM1FJT |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50320326
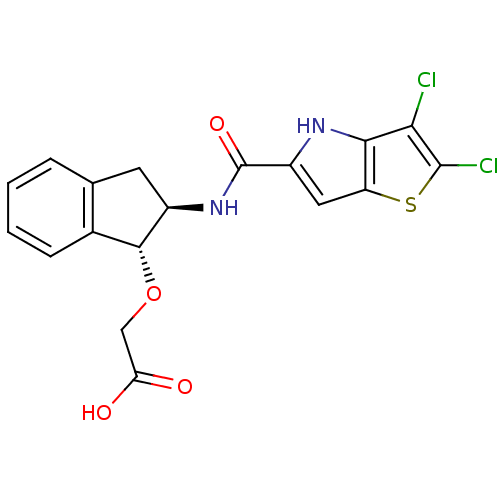
(2-((1R,2R)-2-(2,3-dichloro-4H-thieno[3,2-b]pyrrole...)Show SMILES OC(=O)CO[C@H]1[C@@H](Cc2ccccc12)NC(=O)c1cc2sc(Cl)c(Cl)c2[nH]1 |r| Show InChI InChI=1S/C18H14Cl2N2O4S/c19-14-15-12(27-17(14)20)6-11(21-15)18(25)22-10-5-8-3-1-2-4-9(8)16(10)26-7-13(23)24/h1-4,6,10,16,21H,5,7H2,(H,22,25)(H,23,24)/t10-,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant liver glycogen phosphorylase by multienzyme-coupled reaction |
Bioorg Med Chem Lett 20: 3511-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.147
BindingDB Entry DOI: 10.7270/Q2571C66 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50194417
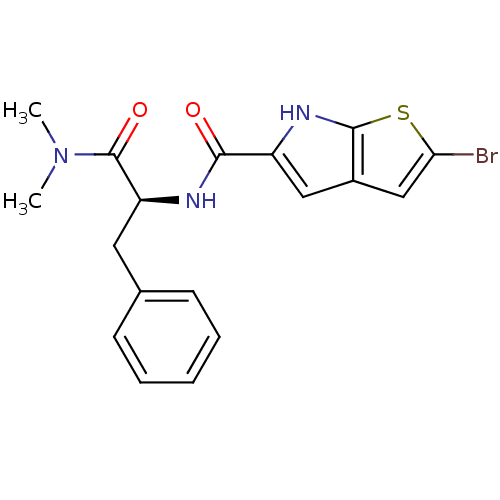
((S)-2-bromo-N-(1-(dimethylamino)-1-oxo-3-phenylpro...)Show SMILES CN(C)C(=O)[C@H](Cc1ccccc1)NC(=O)c1cc2cc(Br)sc2[nH]1 Show InChI InChI=1S/C18H18BrN3O2S/c1-22(2)18(24)14(8-11-6-4-3-5-7-11)20-16(23)13-9-12-10-15(19)25-17(12)21-13/h3-7,9-10,14,21H,8H2,1-2H3,(H,20,23)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human liver GPa by multienzyme coupled assay |
Bioorg Med Chem Lett 16: 5567-71 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.047
BindingDB Entry DOI: 10.7270/Q2KW5FP3 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50320328
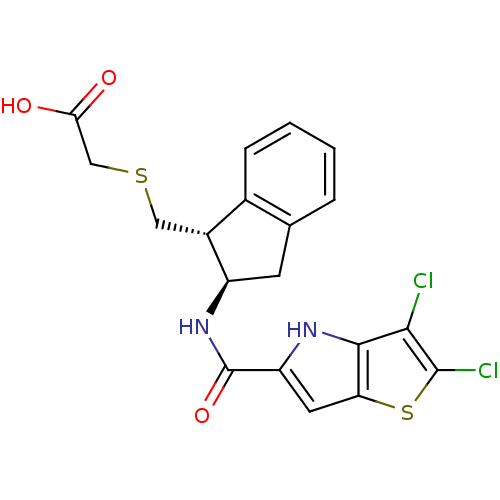
(2-(((1R,2R)-2-(2,3-dichloro-4H-thieno[3,2-b]pyrrol...)Show SMILES OC(=O)CSC[C@H]1[C@@H](Cc2ccccc12)NC(=O)c1cc2sc(Cl)c(Cl)c2[nH]1 |r| Show InChI InChI=1S/C19H16Cl2N2O3S2/c20-16-17-14(28-18(16)21)6-13(22-17)19(26)23-12-5-9-3-1-2-4-10(9)11(12)7-27-8-15(24)25/h1-4,6,11-12,22H,5,7-8H2,(H,23,26)(H,24,25)/t11-,12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant liver glycogen phosphorylase by multienzyme-coupled reaction |
Bioorg Med Chem Lett 20: 3511-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.147
BindingDB Entry DOI: 10.7270/Q2571C66 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data