 Found 132 hits with Last Name = 'strick' and Initial = 'ca'
Found 132 hits with Last Name = 'strick' and Initial = 'ca' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50426340
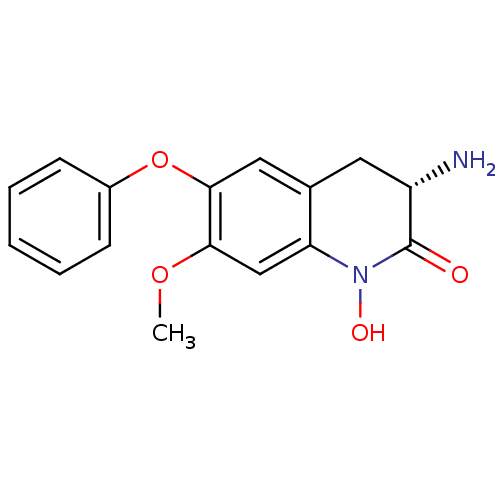
(CHEMBL2321943)Show SMILES COc1cc2N(O)C(=O)[C@@H](N)Cc2cc1Oc1ccccc1 |r| Show InChI InChI=1S/C16H16N2O4/c1-21-14-9-13-10(7-12(17)16(19)18(13)20)8-15(14)22-11-5-3-2-4-6-11/h2-6,8-9,12,20H,7,17H2,1H3/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Irreversible inhibition of human KAT2 using L-kynurenine as substrate measured every 5 mins over 16 hrs by SpectraMax plate reader analysis |
ACS Med Chem Lett 4: 37-40 (2013)
Article DOI: 10.1021/ml300237v
BindingDB Entry DOI: 10.7270/Q2KH0PNV |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50426341
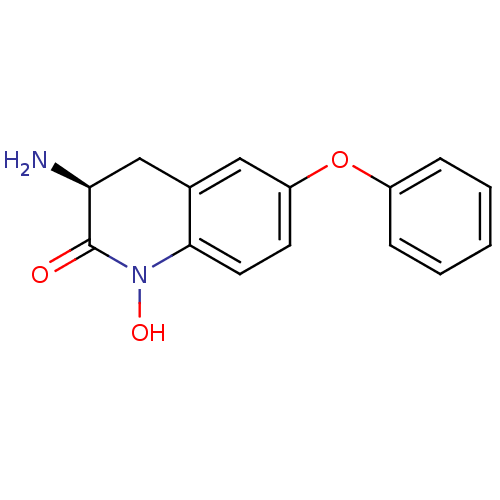
(CHEMBL2321944)Show InChI InChI=1S/C15H14N2O3/c16-13-9-10-8-12(20-11-4-2-1-3-5-11)6-7-14(10)17(19)15(13)18/h1-8,13,19H,9,16H2/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Irreversible inhibition of human KAT2 using L-kynurenine as substrate measured every 5 mins over 16 hrs by SpectraMax plate reader analysis |
ACS Med Chem Lett 4: 37-40 (2013)
Article DOI: 10.1021/ml300237v
BindingDB Entry DOI: 10.7270/Q2KH0PNV |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50386310
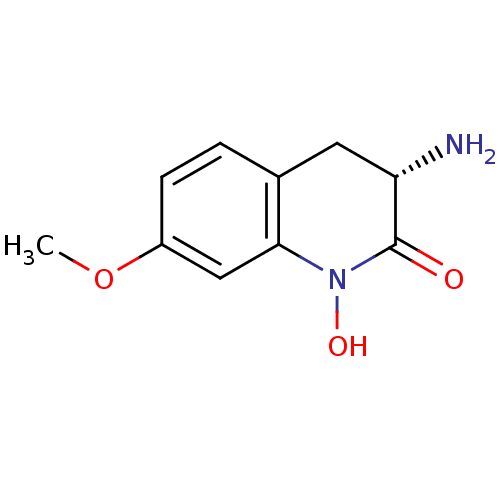
(CHEMBL2049092)Show InChI InChI=1S/C10H12N2O3/c1-15-7-3-2-6-4-8(11)10(13)12(14)9(6)5-7/h2-3,5,8,14H,4,11H2,1H3/t8-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Irreversible inhibition of human KAT2 using L-kynurenine as substrate measured every 5 mins over 16 hrs by SpectraMax plate reader analysis |
ACS Med Chem Lett 4: 37-40 (2013)
Article DOI: 10.1021/ml300237v
BindingDB Entry DOI: 10.7270/Q2KH0PNV |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50386292
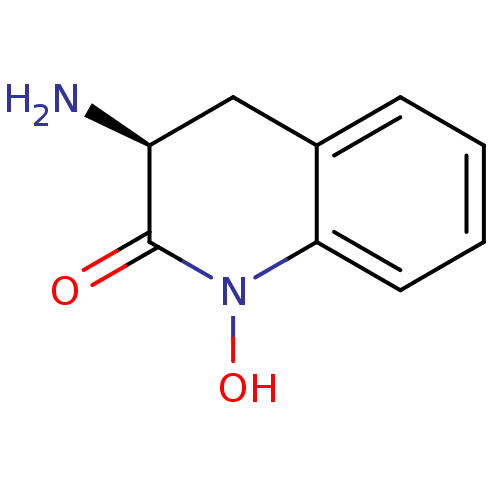
(CHEMBL2047851)Show InChI InChI=1S/C9H10N2O2/c10-7-5-6-3-1-2-4-8(6)11(13)9(7)12/h1-4,7,13H,5,10H2/t7-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Irreversible inhibition of human KAT2 using L-kynurenine as substrate measured every 5 mins over 16 hrs by SpectraMax plate reader analysis |
ACS Med Chem Lett 4: 37-40 (2013)
Article DOI: 10.1021/ml300237v
BindingDB Entry DOI: 10.7270/Q2KH0PNV |
More data for this
Ligand-Target Pair | |
D-amino-acid oxidase
(Homo sapiens (Human)) | BDBM31149
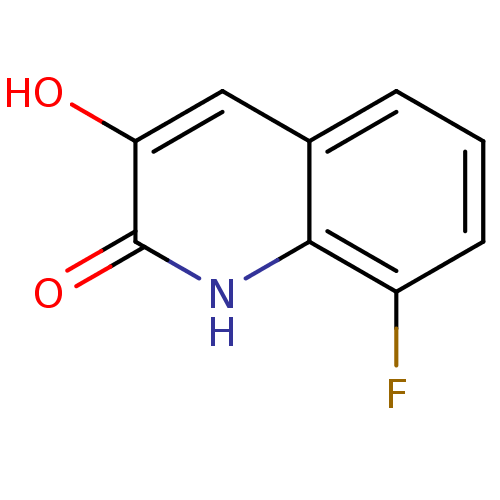
(3-hydroxyquinolin-2(1H)-one, 3)Show InChI InChI=1S/C9H6FNO2/c10-6-3-1-2-5-4-7(12)9(13)11-8(5)6/h1-4,12H,(H,11,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | 8.5 | 22 |
Pfizer
| Assay Description
Inhibitory effect of compounds was determined in a cell free fluorescence assay. The H2O2 generated from the degradation of D-serine was linked to ox... |
J Med Chem 52: 3576-85 (2009)
Article DOI: 10.1021/jm900128w
BindingDB Entry DOI: 10.7270/Q2CZ35HM |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
D-amino-acid oxidase
(Homo sapiens (Human)) | BDBM31173
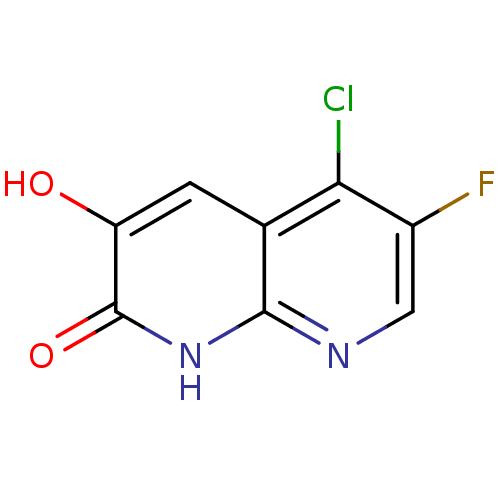
(naphthyridinone analog., 27)Show InChI InChI=1S/C8H4ClFN2O2/c9-6-3-1-5(13)8(14)12-7(3)11-2-4(6)10/h1-2,13H,(H,11,12,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | 8.5 | 22 |
Pfizer
| Assay Description
Inhibitory effect of compounds was determined in a cell free fluorescence assay. The H2O2 generated from the degradation of D-serine was linked to ox... |
J Med Chem 52: 3576-85 (2009)
Article DOI: 10.1021/jm900128w
BindingDB Entry DOI: 10.7270/Q2CZ35HM |
More data for this
Ligand-Target Pair | |
D-amino-acid oxidase
(Homo sapiens (Human)) | BDBM31156

(3-hydroxyquinolin-2(1H)-one, 10)Show InChI InChI=1S/C9H6ClNO2/c10-6-2-1-3-7-5(6)4-8(12)9(13)11-7/h1-4,12H,(H,11,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | 8.5 | 22 |
Pfizer
| Assay Description
Inhibitory effect of compounds was determined in a cell free fluorescence assay. The H2O2 generated from the degradation of D-serine was linked to ox... |
J Med Chem 52: 3576-85 (2009)
Article DOI: 10.1021/jm900128w
BindingDB Entry DOI: 10.7270/Q2CZ35HM |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
D-amino-acid oxidase
(Homo sapiens (Human)) | BDBM31148
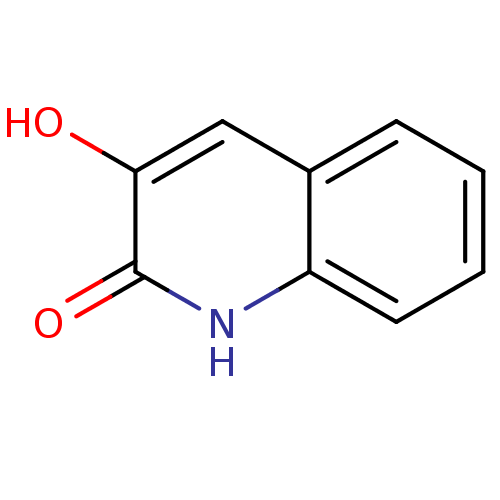
(3-hydroxyquinolin-2(1H)-one, 2 | US9701638, 1)Show InChI InChI=1S/C9H7NO2/c11-8-5-6-3-1-2-4-7(6)10-9(8)12/h1-5,11H,(H,10,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | 8.5 | 22 |
Pfizer
| Assay Description
Inhibitory effect of compounds was determined in a cell free fluorescence assay. The H2O2 generated from the degradation of D-serine was linked to ox... |
J Med Chem 52: 3576-85 (2009)
Article DOI: 10.1021/jm900128w
BindingDB Entry DOI: 10.7270/Q2CZ35HM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
D-amino-acid oxidase
(Rattus norvegicus (rat)) | BDBM31173

(naphthyridinone analog., 27)Show InChI InChI=1S/C8H4ClFN2O2/c9-6-3-1-5(13)8(14)12-7(3)11-2-4(6)10/h1-2,13H,(H,11,12,14) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer
| Assay Description
Inhibitory effect of compounds was determined in a cell free fluorescence assay. The H2O2 generated from the degradation of D-serine was linked to ox... |
J Med Chem 52: 3576-85 (2009)
Article DOI: 10.1021/jm900128w
BindingDB Entry DOI: 10.7270/Q2CZ35HM |
More data for this
Ligand-Target Pair | |
D-amino-acid oxidase
(Homo sapiens (Human)) | BDBM31172
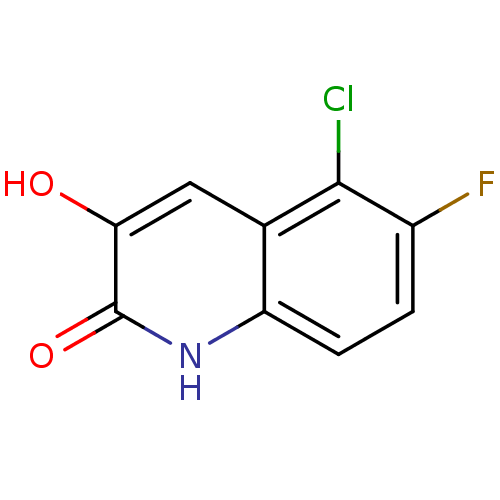
(3-hydroxyquinolin-2(1H)-one, 26)Show InChI InChI=1S/C9H5ClFNO2/c10-8-4-3-7(13)9(14)12-6(4)2-1-5(8)11/h1-3,13H,(H,12,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | 8.5 | 22 |
Pfizer
| Assay Description
Inhibitory effect of compounds was determined in a cell free fluorescence assay. The H2O2 generated from the degradation of D-serine was linked to ox... |
J Med Chem 52: 3576-85 (2009)
Article DOI: 10.1021/jm900128w
BindingDB Entry DOI: 10.7270/Q2CZ35HM |
More data for this
Ligand-Target Pair | |
D-amino-acid oxidase
(Homo sapiens (Human)) | BDBM31161
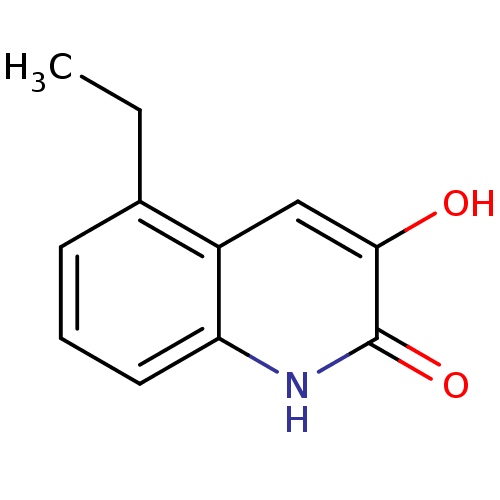
(3-hydroxyquinolin-2(1H)-one, 15)Show InChI InChI=1S/C11H11NO2/c1-2-7-4-3-5-9-8(7)6-10(13)11(14)12-9/h3-6,13H,2H2,1H3,(H,12,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | 8.5 | 22 |
Pfizer
| Assay Description
Inhibitory effect of compounds was determined in a cell free fluorescence assay. The H2O2 generated from the degradation of D-serine was linked to ox... |
J Med Chem 52: 3576-85 (2009)
Article DOI: 10.1021/jm900128w
BindingDB Entry DOI: 10.7270/Q2CZ35HM |
More data for this
Ligand-Target Pair | |
D-amino-acid oxidase
(Homo sapiens (Human)) | BDBM31152
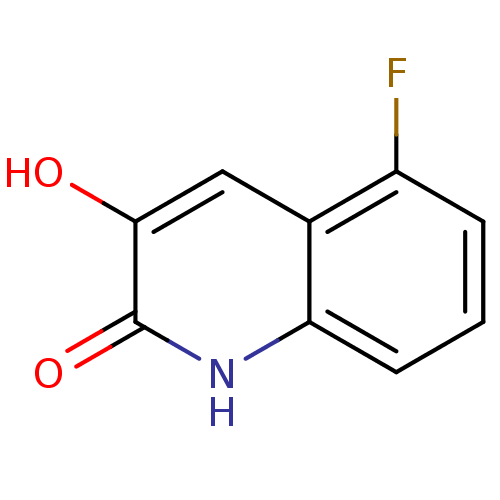
(3-hydroxyquinolin-2(1H)-one, 6)Show InChI InChI=1S/C9H6FNO2/c10-6-2-1-3-7-5(6)4-8(12)9(13)11-7/h1-4,12H,(H,11,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | 8.5 | 22 |
Pfizer
| Assay Description
Inhibitory effect of compounds was determined in a cell free fluorescence assay. The H2O2 generated from the degradation of D-serine was linked to ox... |
J Med Chem 52: 3576-85 (2009)
Article DOI: 10.1021/jm900128w
BindingDB Entry DOI: 10.7270/Q2CZ35HM |
More data for this
Ligand-Target Pair | |
D-amino-acid oxidase
(Homo sapiens (Human)) | BDBM31164
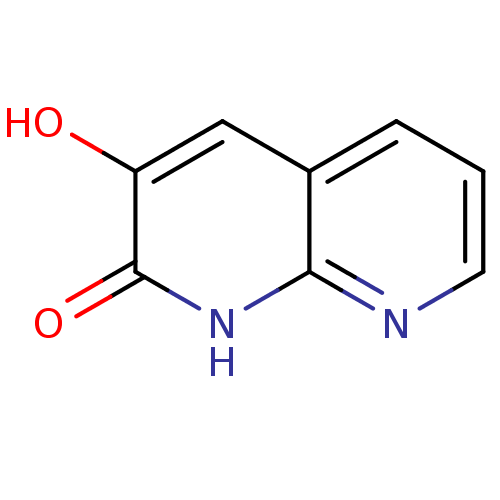
(naphthyridinone analog.,18)Show InChI InChI=1S/C8H6N2O2/c11-6-4-5-2-1-3-9-7(5)10-8(6)12/h1-4,11H,(H,9,10,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | 8.5 | 22 |
Pfizer
| Assay Description
Inhibitory effect of compounds was determined in a cell free fluorescence assay. The H2O2 generated from the degradation of D-serine was linked to ox... |
J Med Chem 52: 3576-85 (2009)
Article DOI: 10.1021/jm900128w
BindingDB Entry DOI: 10.7270/Q2CZ35HM |
More data for this
Ligand-Target Pair | |
D-amino-acid oxidase
(Homo sapiens (Human)) | BDBM31147
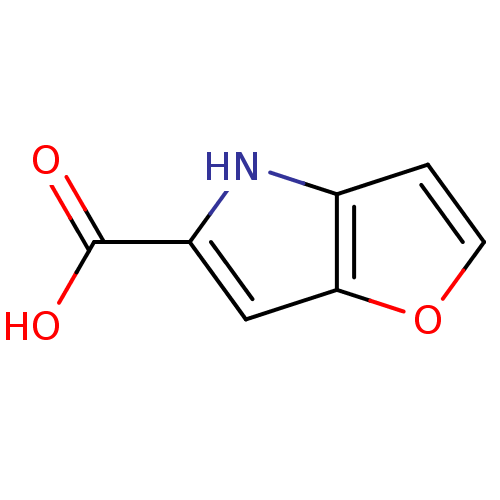
(4H-furo[3,2-b]pyrrole-5-carboxylic acid | 5-carbox...)Show InChI InChI=1S/C7H5NO3/c9-7(10)5-3-6-4(8-5)1-2-11-6/h1-3,8H,(H,9,10) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| MMDB
PDB
Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | 8.5 | 22 |
Pfizer
| Assay Description
Inhibitory effect of compounds was determined in a cell free fluorescence assay. The H2O2 generated from the degradation of D-serine was linked to ox... |
J Med Chem 52: 3576-85 (2009)
Article DOI: 10.1021/jm900128w
BindingDB Entry DOI: 10.7270/Q2CZ35HM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
D-amino-acid oxidase
(Homo sapiens (Human)) | BDBM31151
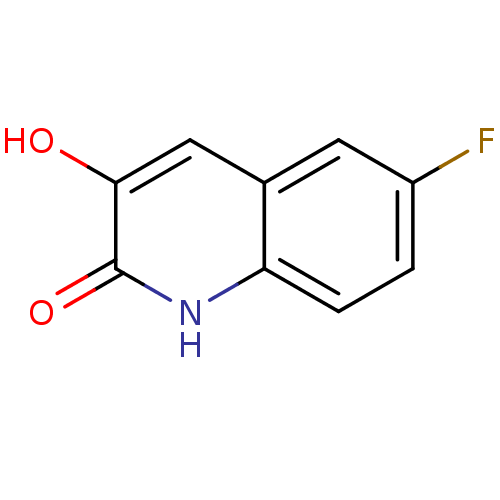
(3-hydroxyquinolin-2(1H)-one, 5)Show InChI InChI=1S/C9H6FNO2/c10-6-1-2-7-5(3-6)4-8(12)9(13)11-7/h1-4,12H,(H,11,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | 8.5 | 22 |
Pfizer
| Assay Description
Inhibitory effect of compounds was determined in a cell free fluorescence assay. The H2O2 generated from the degradation of D-serine was linked to ox... |
J Med Chem 52: 3576-85 (2009)
Article DOI: 10.1021/jm900128w
BindingDB Entry DOI: 10.7270/Q2CZ35HM |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
D-amino-acid oxidase
(Homo sapiens (Human)) | BDBM31150
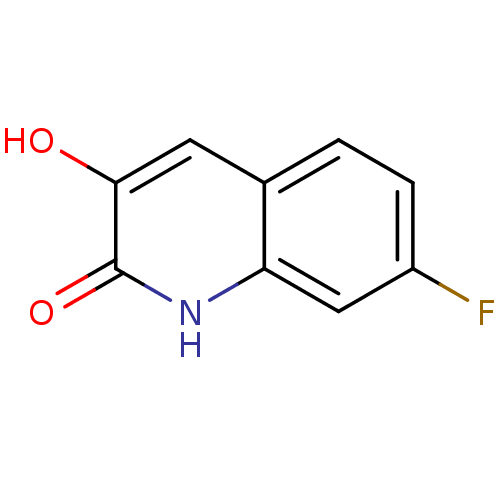
(3-hydroxyquinolin-2(1H)-one, 4)Show InChI InChI=1S/C9H6FNO2/c10-6-2-1-5-3-8(12)9(13)11-7(5)4-6/h1-4,12H,(H,11,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | 8.5 | 22 |
Pfizer
| Assay Description
Inhibitory effect of compounds was determined in a cell free fluorescence assay. The H2O2 generated from the degradation of D-serine was linked to ox... |
J Med Chem 52: 3576-85 (2009)
Article DOI: 10.1021/jm900128w
BindingDB Entry DOI: 10.7270/Q2CZ35HM |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
D-amino-acid oxidase
(Homo sapiens (Human)) | BDBM31160
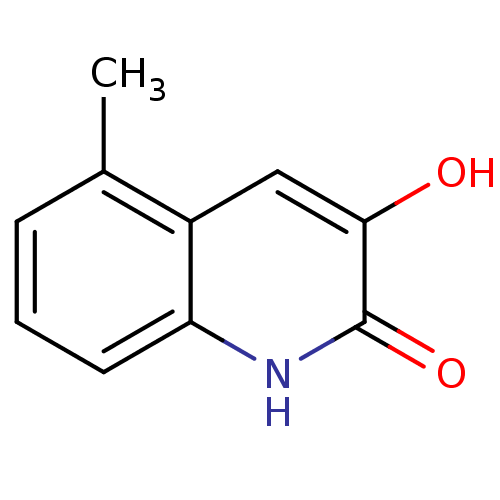
(3-hydroxyquinolin-2(1H)-one, 14)Show InChI InChI=1S/C10H9NO2/c1-6-3-2-4-8-7(6)5-9(12)10(13)11-8/h2-5,12H,1H3,(H,11,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | 8.5 | 22 |
Pfizer
| Assay Description
Inhibitory effect of compounds was determined in a cell free fluorescence assay. The H2O2 generated from the degradation of D-serine was linked to ox... |
J Med Chem 52: 3576-85 (2009)
Article DOI: 10.1021/jm900128w
BindingDB Entry DOI: 10.7270/Q2CZ35HM |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50386310

(CHEMBL2049092)Show InChI InChI=1S/C10H12N2O3/c1-15-7-3-2-6-4-8(11)10(13)12(14)9(6)5-7/h2-3,5,8,14H,4,11H2,1H3/t8-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human KAT2 using L-kynurenine as substrate measured after 15-20 hrs by SpectraMax plate reader analysis |
ACS Med Chem Lett 4: 37-40 (2013)
Article DOI: 10.1021/ml300237v
BindingDB Entry DOI: 10.7270/Q2KH0PNV |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50386310

(CHEMBL2049092)Show InChI InChI=1S/C10H12N2O3/c1-15-7-3-2-6-4-8(11)10(13)12(14)9(6)5-7/h2-3,5,8,14H,4,11H2,1H3/t8-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant KAT2 assessed as conversion of L-kynurenine into kynurenic acid after 15 to 20 hrs |
ACS Med Chem Lett 3: 187-192 (2012)
Article DOI: 10.1021/ml200204m
BindingDB Entry DOI: 10.7270/Q2MC9135 |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50386292

(CHEMBL2047851)Show InChI InChI=1S/C9H10N2O2/c10-7-5-6-3-1-2-4-8(6)11(13)9(7)12/h1-4,7,13H,5,10H2/t7-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant KAT2 assessed as conversion of L-kynurenine into kynurenic acid after 15 to 20 hrs |
ACS Med Chem Lett 3: 187-192 (2012)
Article DOI: 10.1021/ml200204m
BindingDB Entry DOI: 10.7270/Q2MC9135 |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50426341

(CHEMBL2321944)Show InChI InChI=1S/C15H14N2O3/c16-13-9-10-8-12(20-11-4-2-1-3-5-11)6-7-14(10)17(19)15(13)18/h1-8,13,19H,9,16H2/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human KAT2 using L-kynurenine as substrate measured after 15-20 hrs by SpectraMax plate reader analysis |
ACS Med Chem Lett 4: 37-40 (2013)
Article DOI: 10.1021/ml300237v
BindingDB Entry DOI: 10.7270/Q2KH0PNV |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50386292

(CHEMBL2047851)Show InChI InChI=1S/C9H10N2O2/c10-7-5-6-3-1-2-4-8(6)11(13)9(7)12/h1-4,7,13H,5,10H2/t7-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human KAT2 using L-kynurenine as substrate measured after 15-20 hrs by SpectraMax plate reader analysis |
ACS Med Chem Lett 4: 37-40 (2013)
Article DOI: 10.1021/ml300237v
BindingDB Entry DOI: 10.7270/Q2KH0PNV |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50386308
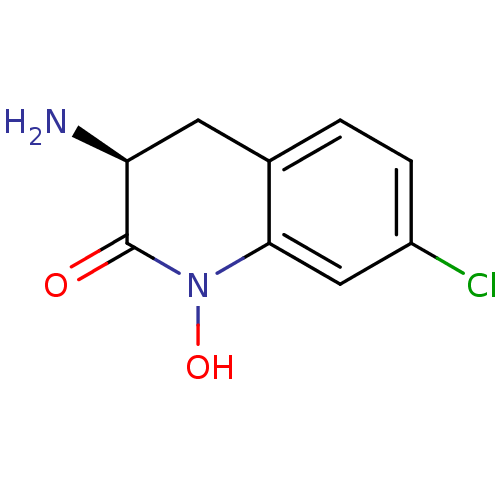
(CHEMBL2047861)Show InChI InChI=1S/C9H9ClN2O2/c10-6-2-1-5-3-7(11)9(13)12(14)8(5)4-6/h1-2,4,7,14H,3,11H2/t7-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant KAT2 assessed as conversion of L-kynurenine into kynurenic acid after 15 to 20 hrs |
ACS Med Chem Lett 3: 187-192 (2012)
Article DOI: 10.1021/ml200204m
BindingDB Entry DOI: 10.7270/Q2MC9135 |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50386294
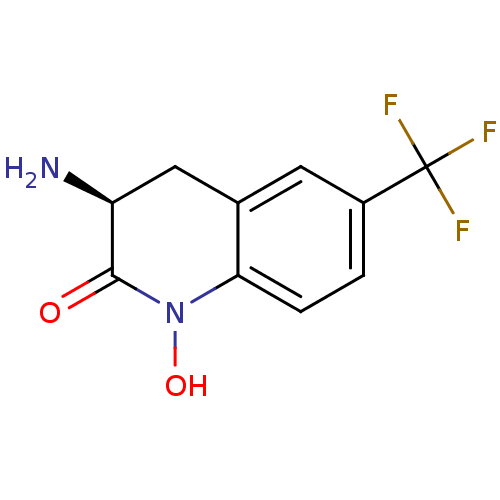
(CHEMBL2049095)Show InChI InChI=1S/C10H9F3N2O2/c11-10(12,13)6-1-2-8-5(3-6)4-7(14)9(16)15(8)17/h1-3,7,17H,4,14H2/t7-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant KAT2 assessed as conversion of L-kynurenine into kynurenic acid after 15 to 20 hrs |
ACS Med Chem Lett 3: 187-192 (2012)
Article DOI: 10.1021/ml200204m
BindingDB Entry DOI: 10.7270/Q2MC9135 |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50386312
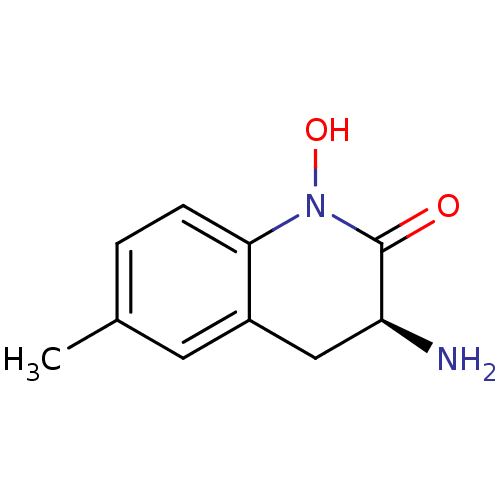
(CHEMBL2049094)Show InChI InChI=1S/C10H12N2O2/c1-6-2-3-9-7(4-6)5-8(11)10(13)12(9)14/h2-4,8,14H,5,11H2,1H3/t8-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant KAT2 assessed as conversion of L-kynurenine into kynurenic acid after 15 to 20 hrs |
ACS Med Chem Lett 3: 187-192 (2012)
Article DOI: 10.1021/ml200204m
BindingDB Entry DOI: 10.7270/Q2MC9135 |
More data for this
Ligand-Target Pair | |
D-amino-acid oxidase
(Homo sapiens (Human)) | BDBM31165
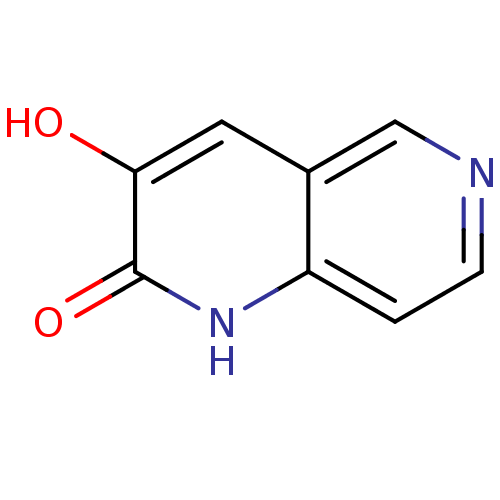
(naphthyridinone analog.,19)Show InChI InChI=1S/C8H6N2O2/c11-7-3-5-4-9-2-1-6(5)10-8(7)12/h1-4,11H,(H,10,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | 8.5 | 22 |
Pfizer
| Assay Description
Inhibitory effect of compounds was determined in a cell free fluorescence assay. The H2O2 generated from the degradation of D-serine was linked to ox... |
J Med Chem 52: 3576-85 (2009)
Article DOI: 10.1021/jm900128w
BindingDB Entry DOI: 10.7270/Q2CZ35HM |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
D-amino-acid oxidase
(Homo sapiens (Human)) | BDBM31153
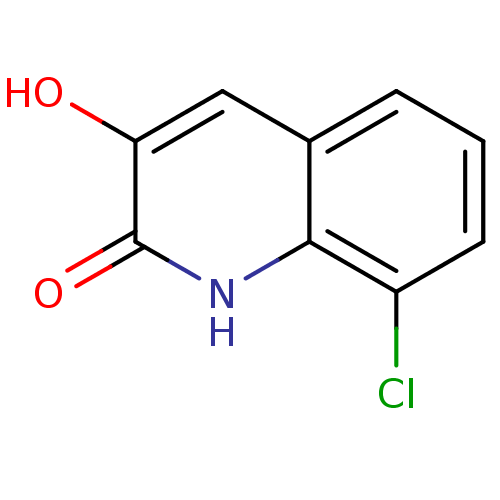
(3-hydroxyquinolin-2(1H)-one, 7)Show InChI InChI=1S/C9H6ClNO2/c10-6-3-1-2-5-4-7(12)9(13)11-8(5)6/h1-4,12H,(H,11,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | 8.5 | 22 |
Pfizer
| Assay Description
Inhibitory effect of compounds was determined in a cell free fluorescence assay. The H2O2 generated from the degradation of D-serine was linked to ox... |
J Med Chem 52: 3576-85 (2009)
Article DOI: 10.1021/jm900128w
BindingDB Entry DOI: 10.7270/Q2CZ35HM |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50386311
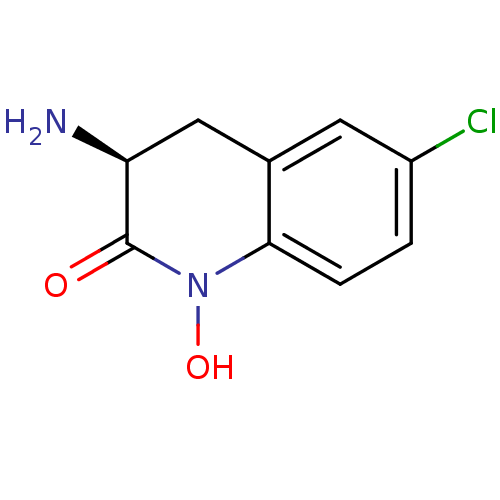
(CHEMBL2049093)Show InChI InChI=1S/C9H9ClN2O2/c10-6-1-2-8-5(3-6)4-7(11)9(13)12(8)14/h1-3,7,14H,4,11H2/t7-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant KAT2 assessed as conversion of L-kynurenine into kynurenic acid after 15 to 20 hrs |
ACS Med Chem Lett 3: 187-192 (2012)
Article DOI: 10.1021/ml200204m
BindingDB Entry DOI: 10.7270/Q2MC9135 |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50426340

(CHEMBL2321943)Show SMILES COc1cc2N(O)C(=O)[C@@H](N)Cc2cc1Oc1ccccc1 |r| Show InChI InChI=1S/C16H16N2O4/c1-21-14-9-13-10(7-12(17)16(19)18(13)20)8-15(14)22-11-5-3-2-4-6-11/h2-6,8-9,12,20H,7,17H2,1H3/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human KAT2 using L-kynurenine as substrate measured after 15-20 hrs by SpectraMax plate reader analysis |
ACS Med Chem Lett 4: 37-40 (2013)
Article DOI: 10.1021/ml300237v
BindingDB Entry DOI: 10.7270/Q2KH0PNV |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50386309
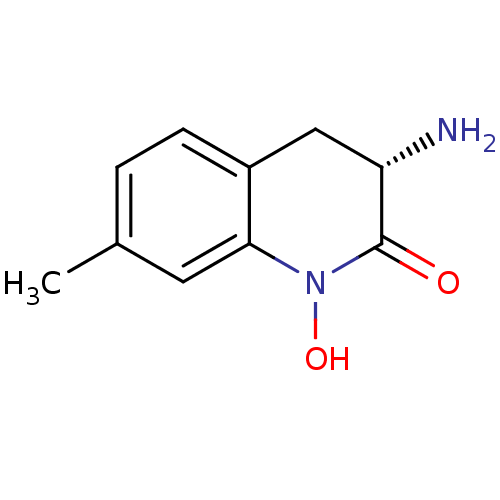
(CHEMBL2047862)Show InChI InChI=1S/C10H12N2O2/c1-6-2-3-7-5-8(11)10(13)12(14)9(7)4-6/h2-4,8,14H,5,11H2,1H3/t8-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant KAT2 assessed as conversion of L-kynurenine into kynurenic acid after 15 to 20 hrs |
ACS Med Chem Lett 3: 187-192 (2012)
Article DOI: 10.1021/ml200204m
BindingDB Entry DOI: 10.7270/Q2MC9135 |
More data for this
Ligand-Target Pair | |
D-amino-acid oxidase
(Homo sapiens (Human)) | BDBM31157
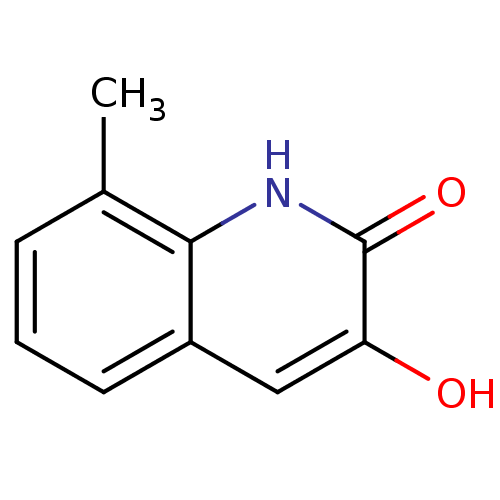
(3-hydroxyquinolin-2(1H)-one, 11)Show InChI InChI=1S/C10H9NO2/c1-6-3-2-4-7-5-8(12)10(13)11-9(6)7/h2-5,12H,1H3,(H,11,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | 8.5 | 22 |
Pfizer
| Assay Description
Inhibitory effect of compounds was determined in a cell free fluorescence assay. The H2O2 generated from the degradation of D-serine was linked to ox... |
J Med Chem 52: 3576-85 (2009)
Article DOI: 10.1021/jm900128w
BindingDB Entry DOI: 10.7270/Q2CZ35HM |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50386303
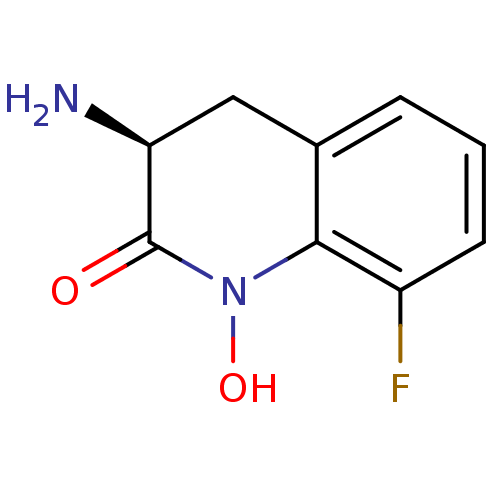
(CHEMBL2047856)Show InChI InChI=1S/C9H9FN2O2/c10-6-3-1-2-5-4-7(11)9(13)12(14)8(5)6/h1-3,7,14H,4,11H2/t7-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant KAT2 assessed as conversion of L-kynurenine into kynurenic acid after 15 to 20 hrs |
ACS Med Chem Lett 3: 187-192 (2012)
Article DOI: 10.1021/ml200204m
BindingDB Entry DOI: 10.7270/Q2MC9135 |
More data for this
Ligand-Target Pair | |
D-amino-acid oxidase
(Rattus norvegicus (rat)) | BDBM31156

(3-hydroxyquinolin-2(1H)-one, 10)Show InChI InChI=1S/C9H6ClNO2/c10-6-2-1-3-7-5(6)4-8(12)9(13)11-7/h1-4,12H,(H,11,13) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer
| Assay Description
Inhibitory effect of compounds was determined in a cell free fluorescence assay. The H2O2 generated from the degradation of D-serine was linked to ox... |
J Med Chem 52: 3576-85 (2009)
Article DOI: 10.1021/jm900128w
BindingDB Entry DOI: 10.7270/Q2CZ35HM |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50386295
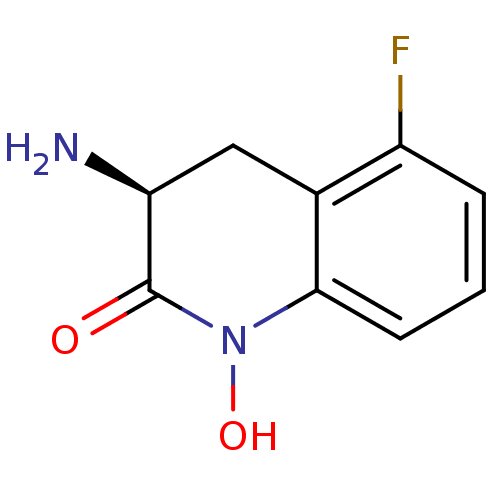
(CHEMBL2049096)Show InChI InChI=1S/C9H9FN2O2/c10-6-2-1-3-8-5(6)4-7(11)9(13)12(8)14/h1-3,7,14H,4,11H2/t7-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant KAT2 assessed as conversion of L-kynurenine into kynurenic acid after 15 to 20 hrs |
ACS Med Chem Lett 3: 187-192 (2012)
Article DOI: 10.1021/ml200204m
BindingDB Entry DOI: 10.7270/Q2MC9135 |
More data for this
Ligand-Target Pair | |
D-amino-acid oxidase
(Rattus norvegicus (rat)) | BDBM31172

(3-hydroxyquinolin-2(1H)-one, 26)Show InChI InChI=1S/C9H5ClFNO2/c10-8-4-3-7(13)9(14)12-6(4)2-1-5(8)11/h1-3,13H,(H,12,14) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer
| Assay Description
Inhibitory effect of compounds was determined in a cell free fluorescence assay. The H2O2 generated from the degradation of D-serine was linked to ox... |
J Med Chem 52: 3576-85 (2009)
Article DOI: 10.1021/jm900128w
BindingDB Entry DOI: 10.7270/Q2CZ35HM |
More data for this
Ligand-Target Pair | |
D-amino-acid oxidase
(Homo sapiens (Human)) | BDBM31154
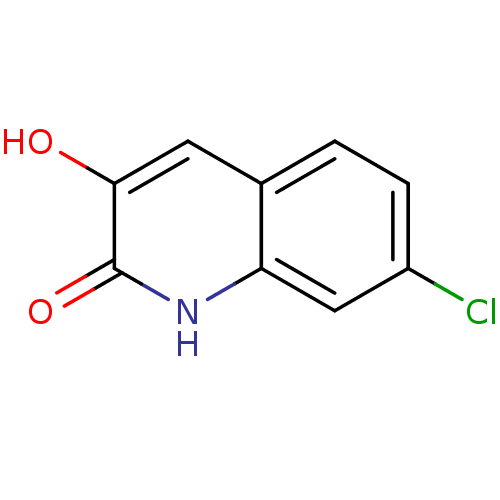
(3-hydroxyquinolin-2(1H)-one, 8)Show InChI InChI=1S/C9H6ClNO2/c10-6-2-1-5-3-8(12)9(13)11-7(5)4-6/h1-4,12H,(H,11,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | 8.5 | 22 |
Pfizer
| Assay Description
Inhibitory effect of compounds was determined in a cell free fluorescence assay. The H2O2 generated from the degradation of D-serine was linked to ox... |
J Med Chem 52: 3576-85 (2009)
Article DOI: 10.1021/jm900128w
BindingDB Entry DOI: 10.7270/Q2CZ35HM |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Rattus norvegicus) | BDBM50386308

(CHEMBL2047861)Show InChI InChI=1S/C9H9ClN2O2/c10-6-2-1-5-3-7(11)9(13)12(14)8(5)4-6/h1-2,4,7,14H,3,11H2/t7-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 118 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of rat recombinant KAT2 assessed as conversion of L-kynurenine into kynurenic acid after 15 to 20 hrs |
ACS Med Chem Lett 3: 187-192 (2012)
Article DOI: 10.1021/ml200204m
BindingDB Entry DOI: 10.7270/Q2MC9135 |
More data for this
Ligand-Target Pair | |
D-amino-acid oxidase
(Homo sapiens (Human)) | BDBM31167
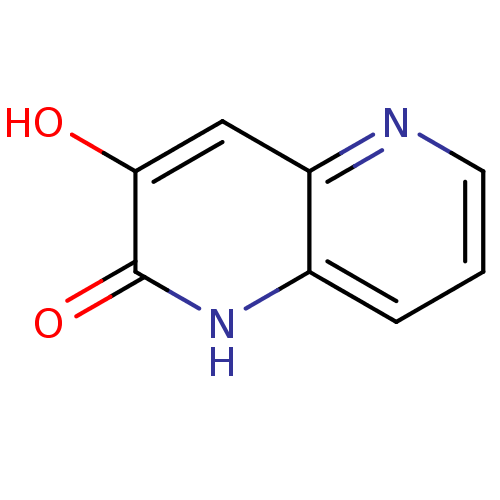
(naphthyridinone analog.,21)Show InChI InChI=1S/C8H6N2O2/c11-7-4-6-5(10-8(7)12)2-1-3-9-6/h1-4,11H,(H,10,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 128 | n/a | n/a | n/a | n/a | 8.5 | 22 |
Pfizer
| Assay Description
Inhibitory effect of compounds was determined in a cell free fluorescence assay. The H2O2 generated from the degradation of D-serine was linked to ox... |
J Med Chem 52: 3576-85 (2009)
Article DOI: 10.1021/jm900128w
BindingDB Entry DOI: 10.7270/Q2CZ35HM |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Rattus norvegicus) | BDBM50386310

(CHEMBL2049092)Show InChI InChI=1S/C10H12N2O3/c1-15-7-3-2-6-4-8(11)10(13)12(14)9(6)5-7/h2-3,5,8,14H,4,11H2,1H3/t8-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 137 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of rat recombinant KAT2 assessed as conversion of L-kynurenine into kynurenic acid after 15 to 20 hrs |
ACS Med Chem Lett 3: 187-192 (2012)
Article DOI: 10.1021/ml200204m
BindingDB Entry DOI: 10.7270/Q2MC9135 |
More data for this
Ligand-Target Pair | |
D-amino-acid oxidase
(Homo sapiens (Human)) | BDBM31155

(3-hydroxyquinolin-2(1H)-one, 9)Show InChI InChI=1S/C9H6ClNO2/c10-6-1-2-7-5(3-6)4-8(12)9(13)11-7/h1-4,12H,(H,11,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 155 | n/a | n/a | n/a | n/a | 8.5 | 22 |
Pfizer
| Assay Description
Inhibitory effect of compounds was determined in a cell free fluorescence assay. The H2O2 generated from the degradation of D-serine was linked to ox... |
J Med Chem 52: 3576-85 (2009)
Article DOI: 10.1021/jm900128w
BindingDB Entry DOI: 10.7270/Q2CZ35HM |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50386307
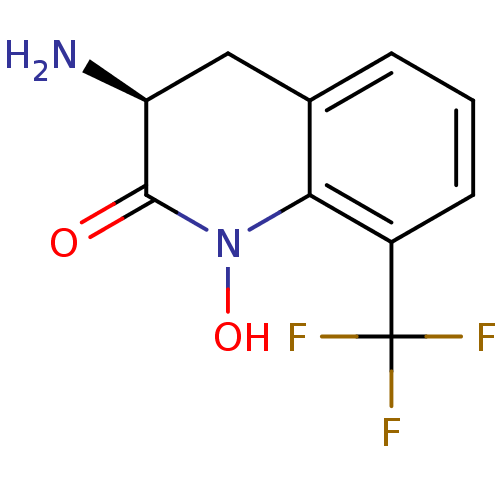
(CHEMBL2047860)Show InChI InChI=1S/C10H9F3N2O2/c11-10(12,13)6-3-1-2-5-4-7(14)9(16)15(17)8(5)6/h1-3,7,17H,4,14H2/t7-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 174 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant KAT2 assessed as conversion of L-kynurenine into kynurenic acid after 15 to 20 hrs |
ACS Med Chem Lett 3: 187-192 (2012)
Article DOI: 10.1021/ml200204m
BindingDB Entry DOI: 10.7270/Q2MC9135 |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50386298

(CHEMBL2049099)Show InChI InChI=1S/C10H12N2O3/c1-15-9-4-2-3-8-6(9)5-7(11)10(13)12(8)14/h2-4,7,14H,5,11H2,1H3/t7-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 179 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant KAT2 assessed as conversion of L-kynurenine into kynurenic acid after 15 to 20 hrs |
ACS Med Chem Lett 3: 187-192 (2012)
Article DOI: 10.1021/ml200204m
BindingDB Entry DOI: 10.7270/Q2MC9135 |
More data for this
Ligand-Target Pair | |
D-amino-acid oxidase
(Rattus norvegicus (rat)) | BDBM31149

(3-hydroxyquinolin-2(1H)-one, 3)Show InChI InChI=1S/C9H6FNO2/c10-6-3-1-2-5-4-7(12)9(13)11-8(5)6/h1-4,12H,(H,11,13) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer
| Assay Description
Inhibitory effect of compounds was determined in a cell free fluorescence assay. The H2O2 generated from the degradation of D-serine was linked to ox... |
J Med Chem 52: 3576-85 (2009)
Article DOI: 10.1021/jm900128w
BindingDB Entry DOI: 10.7270/Q2CZ35HM |
More data for this
Ligand-Target Pair | |
D-amino-acid oxidase
(Rattus norvegicus (rat)) | BDBM31152

(3-hydroxyquinolin-2(1H)-one, 6)Show InChI InChI=1S/C9H6FNO2/c10-6-2-1-3-7-5(6)4-8(12)9(13)11-7/h1-4,12H,(H,11,13) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 196 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer
| Assay Description
Inhibitory effect of compounds was determined in a cell free fluorescence assay. The H2O2 generated from the degradation of D-serine was linked to ox... |
J Med Chem 52: 3576-85 (2009)
Article DOI: 10.1021/jm900128w
BindingDB Entry DOI: 10.7270/Q2CZ35HM |
More data for this
Ligand-Target Pair | |
D-amino-acid oxidase
(Homo sapiens (Human)) | BDBM31158
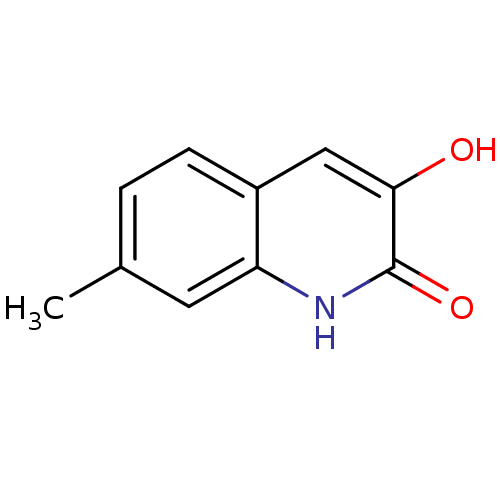
(3-hydroxyquinolin-2(1H)-one, 12)Show InChI InChI=1S/C10H9NO2/c1-6-2-3-7-5-9(12)10(13)11-8(7)4-6/h2-5,12H,1H3,(H,11,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 197 | n/a | n/a | n/a | n/a | 8.5 | 22 |
Pfizer
| Assay Description
Inhibitory effect of compounds was determined in a cell free fluorescence assay. The H2O2 generated from the degradation of D-serine was linked to ox... |
J Med Chem 52: 3576-85 (2009)
Article DOI: 10.1021/jm900128w
BindingDB Entry DOI: 10.7270/Q2CZ35HM |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
D-amino-acid oxidase
(Rattus norvegicus (rat)) | BDBM31164

(naphthyridinone analog.,18)Show InChI InChI=1S/C8H6N2O2/c11-6-4-5-2-1-3-9-7(5)10-8(6)12/h1-4,11H,(H,9,10,12) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer
| Assay Description
Inhibitory effect of compounds was determined in a cell free fluorescence assay. The H2O2 generated from the degradation of D-serine was linked to ox... |
J Med Chem 52: 3576-85 (2009)
Article DOI: 10.1021/jm900128w
BindingDB Entry DOI: 10.7270/Q2CZ35HM |
More data for this
Ligand-Target Pair | |
D-amino-acid oxidase
(Rattus norvegicus (rat)) | BDBM31148

(3-hydroxyquinolin-2(1H)-one, 2 | US9701638, 1)Show InChI InChI=1S/C9H7NO2/c11-8-5-6-3-1-2-4-7(6)10-9(8)12/h1-5,11H,(H,10,12) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 215 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer
| Assay Description
Inhibitory effect of compounds was determined in a cell free fluorescence assay. The H2O2 generated from the degradation of D-serine was linked to ox... |
J Med Chem 52: 3576-85 (2009)
Article DOI: 10.1021/jm900128w
BindingDB Entry DOI: 10.7270/Q2CZ35HM |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50386299
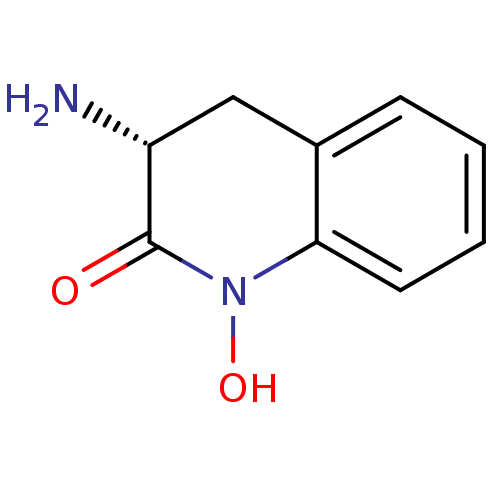
(CHEMBL2047852)Show InChI InChI=1S/C9H10N2O2/c10-7-5-6-3-1-2-4-8(6)11(13)9(7)12/h1-4,7,13H,5,10H2/t7-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 219 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant KAT2 assessed as conversion of L-kynurenine into kynurenic acid after 15 to 20 hrs |
ACS Med Chem Lett 3: 187-192 (2012)
Article DOI: 10.1021/ml200204m
BindingDB Entry DOI: 10.7270/Q2MC9135 |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
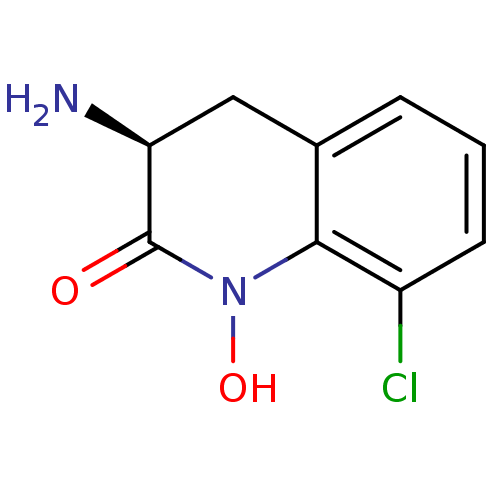
(Homo sapiens (Human)) | BDBM50386305
(CHEMBL2047858)Show InChI InChI=1S/C9H9ClN2O2/c10-6-3-1-2-5-4-7(11)9(13)12(14)8(5)6/h1-3,7,14H,4,11H2/t7-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 252 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant KAT2 assessed as conversion of L-kynurenine into kynurenic acid after 15 to 20 hrs |
ACS Med Chem Lett 3: 187-192 (2012)
Article DOI: 10.1021/ml200204m
BindingDB Entry DOI: 10.7270/Q2MC9135 |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Rattus norvegicus) | BDBM50386311

(CHEMBL2049093)Show InChI InChI=1S/C9H9ClN2O2/c10-6-1-2-8-5(3-6)4-7(11)9(13)12(8)14/h1-3,7,14H,4,11H2/t7-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 258 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of rat recombinant KAT2 assessed as conversion of L-kynurenine into kynurenic acid after 15 to 20 hrs |
ACS Med Chem Lett 3: 187-192 (2012)
Article DOI: 10.1021/ml200204m
BindingDB Entry DOI: 10.7270/Q2MC9135 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data