

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50159110 (1-(3-(4-(piperidin-1-ylmethyl)phenoxy)propyl)piper...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C. Curated by ChEMBL | Assay Description Displacement of [125I]iodoproxyfan from human recombinant histamine H3 receptor expressed in human SK-N-MC cells | Bioorg Med Chem Lett 18: 5796-9 (2009) Article DOI: 10.1016/j.bmcl.2008.09.077 BindingDB Entry DOI: 10.7270/Q2154H28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50293202 ((S)-4-cyclopentyl-2-((4-(morpholinomethyl)phenoxy)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C. Curated by ChEMBL | Assay Description Displacement of [125I]iodoproxyfan from human recombinant histamine H3 receptor expressed in human SK-N-MC cells | Bioorg Med Chem Lett 18: 5796-9 (2009) Article DOI: 10.1016/j.bmcl.2008.09.077 BindingDB Entry DOI: 10.7270/Q2154H28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50293199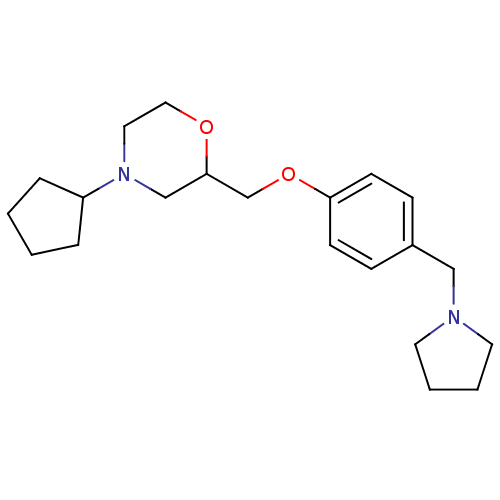 (4-cyclopentyl-2-((4-(pyrrolidin-1-ylmethyl)phenoxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C. Curated by ChEMBL | Assay Description Displacement of [125I]iodoproxyfan from human recombinant histamine H3 receptor expressed in human SK-N-MC cells | Bioorg Med Chem Lett 18: 5796-9 (2009) Article DOI: 10.1016/j.bmcl.2008.09.077 BindingDB Entry DOI: 10.7270/Q2154H28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50293200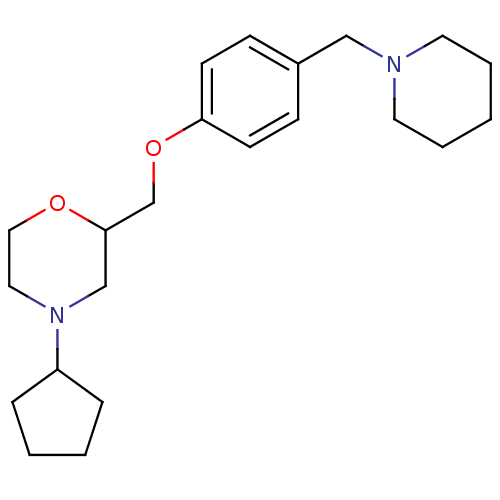 (4-cyclopentyl-2-((4-(piperidin-1-ylmethyl)phenoxy)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C. Curated by ChEMBL | Assay Description Displacement of [125I]iodoproxyfan from human recombinant histamine H3 receptor expressed in human SK-N-MC cells | Bioorg Med Chem Lett 18: 5796-9 (2009) Article DOI: 10.1016/j.bmcl.2008.09.077 BindingDB Entry DOI: 10.7270/Q2154H28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50293189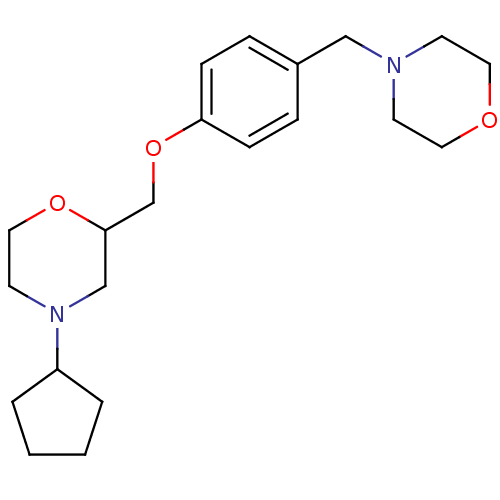 (4-cyclopentyl-2-((4-(morpholinomethyl)phenoxy)meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C. Curated by ChEMBL | Assay Description Displacement of [125I]iodoproxyfan from human recombinant histamine H3 receptor expressed in human SK-N-MC cells | Bioorg Med Chem Lett 18: 5796-9 (2009) Article DOI: 10.1016/j.bmcl.2008.09.077 BindingDB Entry DOI: 10.7270/Q2154H28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50412863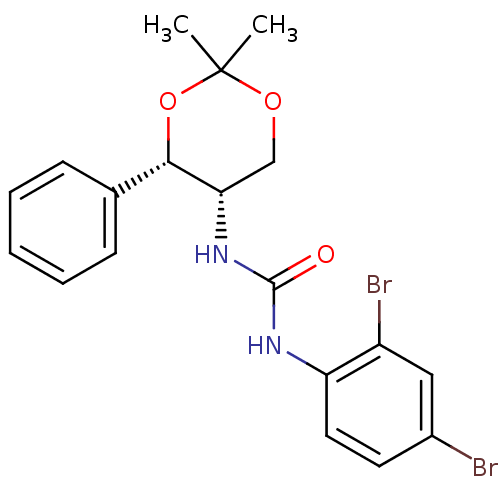 (CHEMBL359632 | JNJ-10397049) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human orexin receptor type 2 was determined using [125I]-Orexin A as radio ligand | Bioorg Med Chem Lett 14: 4225-9 (2004) Article DOI: 10.1016/j.bmcl.2004.06.032 BindingDB Entry DOI: 10.7270/Q2K64MV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50412861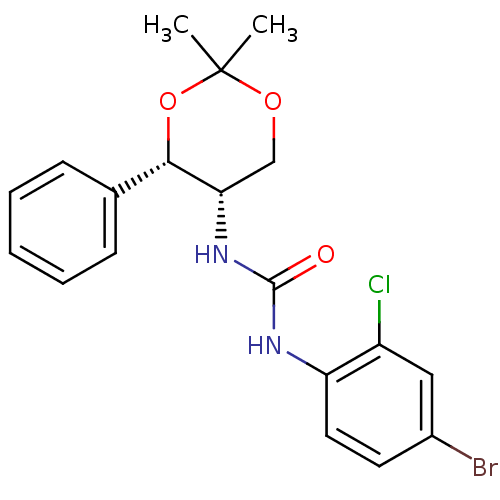 (CHEMBL185088) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human orexin receptor type 2 was determined using [125I]-Orexin A as radio ligand | Bioorg Med Chem Lett 14: 4225-9 (2004) Article DOI: 10.1016/j.bmcl.2004.06.032 BindingDB Entry DOI: 10.7270/Q2K64MV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50293202 ((S)-4-cyclopentyl-2-((4-(morpholinomethyl)phenoxy)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C. Curated by ChEMBL | Assay Description Displacement of [125I]iodoproxyfan from rat recombinant histamine H3 receptor expressed in human SK-N-MC cells | Bioorg Med Chem Lett 18: 5796-9 (2009) Article DOI: 10.1016/j.bmcl.2008.09.077 BindingDB Entry DOI: 10.7270/Q2154H28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50293199 (4-cyclopentyl-2-((4-(pyrrolidin-1-ylmethyl)phenoxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C. Curated by ChEMBL | Assay Description Displacement of [125I]iodoproxyfan from rat recombinant histamine H3 receptor expressed in human SK-N-MC cells | Bioorg Med Chem Lett 18: 5796-9 (2009) Article DOI: 10.1016/j.bmcl.2008.09.077 BindingDB Entry DOI: 10.7270/Q2154H28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50293200 (4-cyclopentyl-2-((4-(piperidin-1-ylmethyl)phenoxy)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C. Curated by ChEMBL | Assay Description Displacement of [125I]iodoproxyfan from rat recombinant histamine H3 receptor expressed in human SK-N-MC cells | Bioorg Med Chem Lett 18: 5796-9 (2009) Article DOI: 10.1016/j.bmcl.2008.09.077 BindingDB Entry DOI: 10.7270/Q2154H28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50293188 (4-cyclobutyl-2-((4-(morpholinomethyl)phenoxy)methy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C. Curated by ChEMBL | Assay Description Displacement of [125I]iodoproxyfan from human recombinant histamine H3 receptor expressed in human SK-N-MC cells | Bioorg Med Chem Lett 18: 5796-9 (2009) Article DOI: 10.1016/j.bmcl.2008.09.077 BindingDB Entry DOI: 10.7270/Q2154H28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50293189 (4-cyclopentyl-2-((4-(morpholinomethyl)phenoxy)meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C. Curated by ChEMBL | Assay Description Displacement of [125I]iodoproxyfan from rat recombinant histamine H3 receptor expressed in human SK-N-MC cells | Bioorg Med Chem Lett 18: 5796-9 (2009) Article DOI: 10.1016/j.bmcl.2008.09.077 BindingDB Entry DOI: 10.7270/Q2154H28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50412862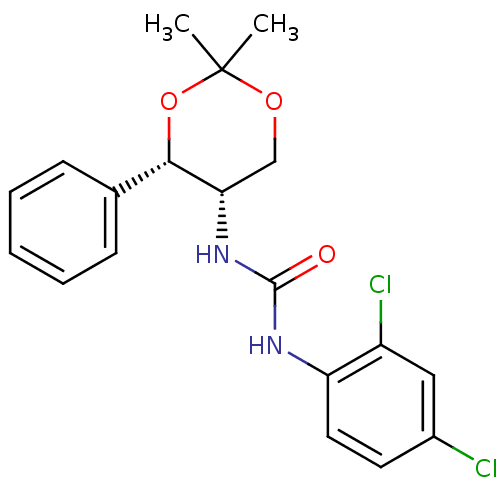 (CHEMBL185136) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human orexin receptor type 2 was determined using [125I]-Orexin A as radio ligand | Bioorg Med Chem Lett 14: 4225-9 (2004) Article DOI: 10.1016/j.bmcl.2004.06.032 BindingDB Entry DOI: 10.7270/Q2K64MV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50474855 (CHEMBL364814) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human orexin receptor type 2 was determined using [125I]-Orexin A as radio ligand | Bioorg Med Chem Lett 14: 4225-9 (2004) Article DOI: 10.1016/j.bmcl.2004.06.032 BindingDB Entry DOI: 10.7270/Q2K64MV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50293201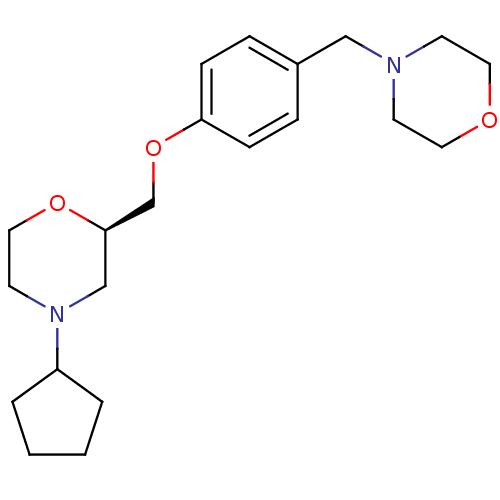 ((R)-4-cyclopentyl-2-((4-(morpholinomethyl)phenoxy)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C. Curated by ChEMBL | Assay Description Displacement of [125I]iodoproxyfan from human recombinant histamine H3 receptor expressed in human SK-N-MC cells | Bioorg Med Chem Lett 18: 5796-9 (2009) Article DOI: 10.1016/j.bmcl.2008.09.077 BindingDB Entry DOI: 10.7270/Q2154H28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50293192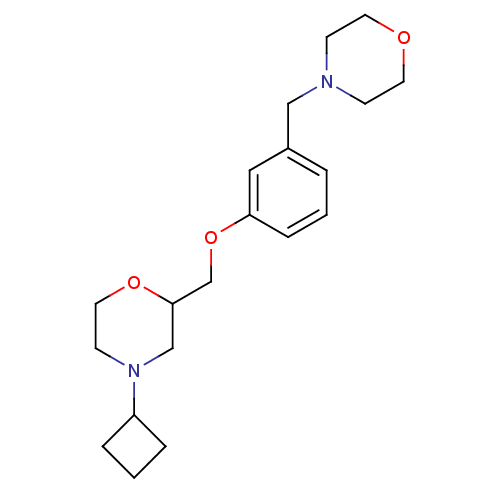 (4-cyclobutyl-2-((3-(morpholinomethyl)phenoxy)methy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C. Curated by ChEMBL | Assay Description Displacement of [125I]iodoproxyfan from human recombinant histamine H3 receptor expressed in human SK-N-MC cells | Bioorg Med Chem Lett 18: 5796-9 (2009) Article DOI: 10.1016/j.bmcl.2008.09.077 BindingDB Entry DOI: 10.7270/Q2154H28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50412860 (CHEMBL185093) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human orexin receptor type 2 was determined using [125I]-Orexin A as radio ligand | Bioorg Med Chem Lett 14: 4225-9 (2004) Article DOI: 10.1016/j.bmcl.2004.06.032 BindingDB Entry DOI: 10.7270/Q2K64MV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50293187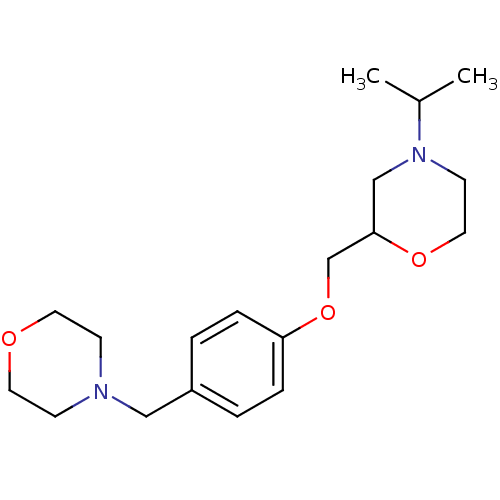 (4-isopropyl-2-((4-(morpholinomethyl)phenoxy)methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C. Curated by ChEMBL | Assay Description Displacement of [125I]iodoproxyfan from human recombinant histamine H3 receptor expressed in human SK-N-MC cells | Bioorg Med Chem Lett 18: 5796-9 (2009) Article DOI: 10.1016/j.bmcl.2008.09.077 BindingDB Entry DOI: 10.7270/Q2154H28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50474849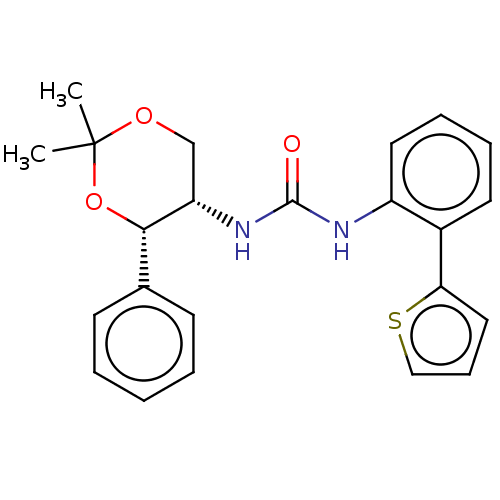 (CHEMBL185424) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human orexin receptor type 2 was determined using [125I]-Orexin A as radio ligand | Bioorg Med Chem Lett 14: 4225-9 (2004) Article DOI: 10.1016/j.bmcl.2004.06.032 BindingDB Entry DOI: 10.7270/Q2K64MV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50412859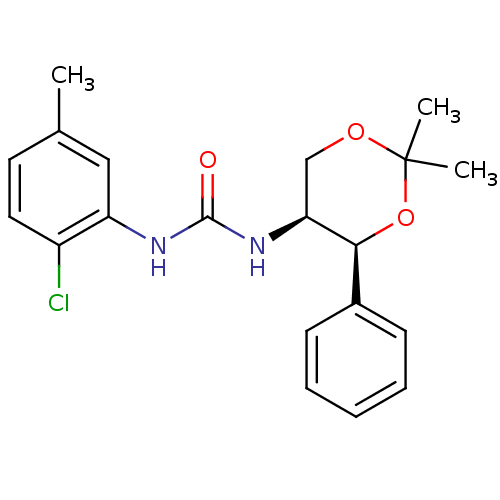 (CHEMBL182473) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human orexin receptor type 2 was determined using [125I]-Orexin A as radio ligand | Bioorg Med Chem Lett 14: 4225-9 (2004) Article DOI: 10.1016/j.bmcl.2004.06.032 BindingDB Entry DOI: 10.7270/Q2K64MV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50412857 (CHEMBL361716) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human orexin receptor type 2 was determined using [125I]-Orexin A as radio ligand | Bioorg Med Chem Lett 14: 4225-9 (2004) Article DOI: 10.1016/j.bmcl.2004.06.032 BindingDB Entry DOI: 10.7270/Q2K64MV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50293188 (4-cyclobutyl-2-((4-(morpholinomethyl)phenoxy)methy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C. Curated by ChEMBL | Assay Description Displacement of [125I]iodoproxyfan from rat recombinant histamine H3 receptor expressed in human SK-N-MC cells | Bioorg Med Chem Lett 18: 5796-9 (2009) Article DOI: 10.1016/j.bmcl.2008.09.077 BindingDB Entry DOI: 10.7270/Q2154H28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50412855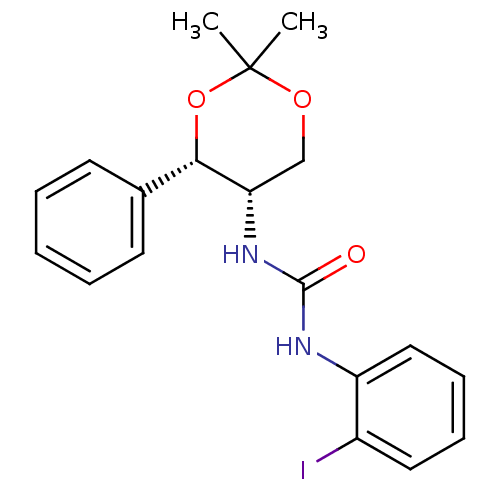 (CHEMBL185382) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human orexin receptor type 2 was determined using [125I]-Orexin A as radio ligand | Bioorg Med Chem Lett 14: 4225-9 (2004) Article DOI: 10.1016/j.bmcl.2004.06.032 BindingDB Entry DOI: 10.7270/Q2K64MV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50474846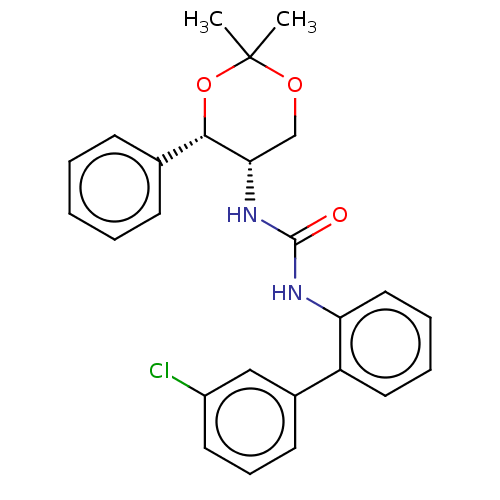 (CHEMBL185131) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human orexin receptor type 2 was determined using [125I]-Orexin A as radio ligand | Bioorg Med Chem Lett 14: 4225-9 (2004) Article DOI: 10.1016/j.bmcl.2004.06.032 BindingDB Entry DOI: 10.7270/Q2K64MV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50412854 (CHEMBL185435) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human orexin receptor type 2 was determined using [125I]-Orexin A as radio ligand | Bioorg Med Chem Lett 14: 4225-9 (2004) Article DOI: 10.1016/j.bmcl.2004.06.032 BindingDB Entry DOI: 10.7270/Q2K64MV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50474848 (CHEMBL184596) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human orexin receptor type 2 was determined using [125I]-Orexin A as radio ligand | Bioorg Med Chem Lett 14: 4225-9 (2004) Article DOI: 10.1016/j.bmcl.2004.06.032 BindingDB Entry DOI: 10.7270/Q2K64MV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50293187 (4-isopropyl-2-((4-(morpholinomethyl)phenoxy)methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 92 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C. Curated by ChEMBL | Assay Description Displacement of [125I]iodoproxyfan from rat recombinant histamine H3 receptor expressed in human SK-N-MC cells | Bioorg Med Chem Lett 18: 5796-9 (2009) Article DOI: 10.1016/j.bmcl.2008.09.077 BindingDB Entry DOI: 10.7270/Q2154H28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50412851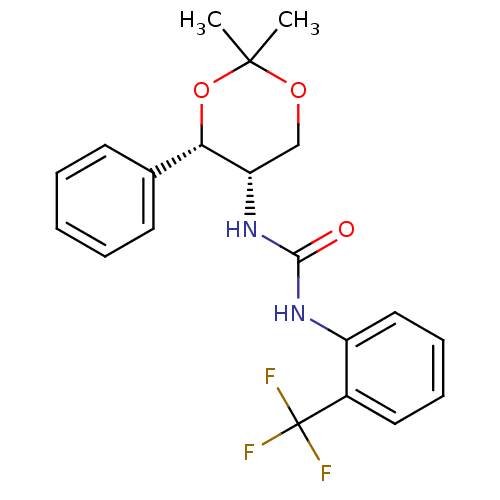 (CHEMBL363951) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human orexin receptor type 2 was determined using [125I]-Orexin A as radio ligand | Bioorg Med Chem Lett 14: 4225-9 (2004) Article DOI: 10.1016/j.bmcl.2004.06.032 BindingDB Entry DOI: 10.7270/Q2K64MV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50474847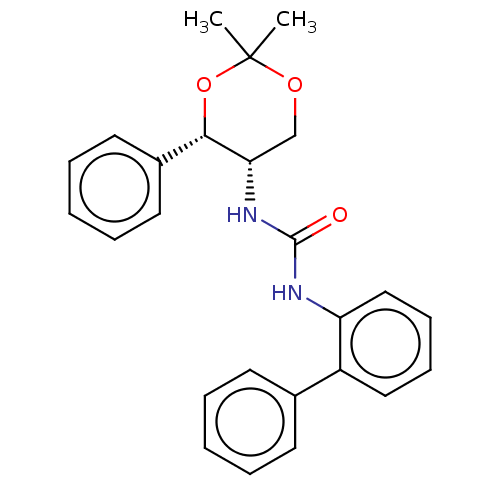 (CHEMBL183421) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human orexin receptor type 2 was determined using [125I]-Orexin A as radio ligand | Bioorg Med Chem Lett 14: 4225-9 (2004) Article DOI: 10.1016/j.bmcl.2004.06.032 BindingDB Entry DOI: 10.7270/Q2K64MV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50412849 (CHEMBL363743) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human orexin receptor type 2 was determined using [125I]-Orexin A as radio ligand | Bioorg Med Chem Lett 14: 4225-9 (2004) Article DOI: 10.1016/j.bmcl.2004.06.032 BindingDB Entry DOI: 10.7270/Q2K64MV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50293191 (4-isopropyl-2-((3-(morpholinomethyl)phenoxy)methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 102 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C. Curated by ChEMBL | Assay Description Displacement of [125I]iodoproxyfan from human recombinant histamine H3 receptor expressed in human SK-N-MC cells | Bioorg Med Chem Lett 18: 5796-9 (2009) Article DOI: 10.1016/j.bmcl.2008.09.077 BindingDB Entry DOI: 10.7270/Q2154H28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50293185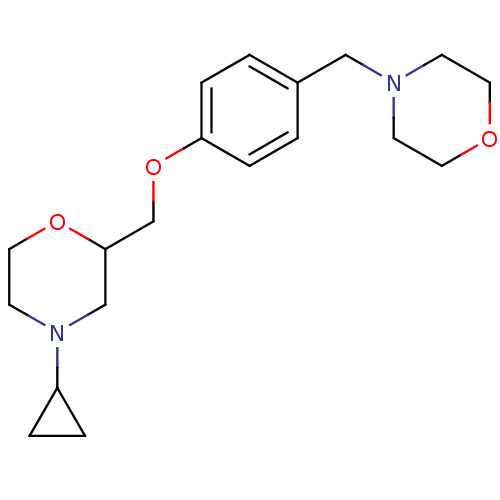 (4-cyclopropyl-2-((4-(morpholinomethyl)phenoxy)meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 106 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C. Curated by ChEMBL | Assay Description Displacement of [125I]iodoproxyfan from human recombinant histamine H3 receptor expressed in human SK-N-MC cells | Bioorg Med Chem Lett 18: 5796-9 (2009) Article DOI: 10.1016/j.bmcl.2008.09.077 BindingDB Entry DOI: 10.7270/Q2154H28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50293201 ((R)-4-cyclopentyl-2-((4-(morpholinomethyl)phenoxy)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 121 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C. Curated by ChEMBL | Assay Description Displacement of [125I]iodoproxyfan from rat recombinant histamine H3 receptor expressed in human SK-N-MC cells | Bioorg Med Chem Lett 18: 5796-9 (2009) Article DOI: 10.1016/j.bmcl.2008.09.077 BindingDB Entry DOI: 10.7270/Q2154H28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50412858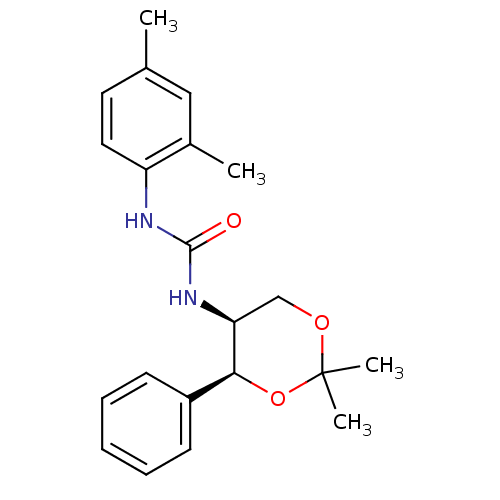 (CHEMBL183576) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human orexin receptor type 2 was determined using [125I]-Orexin A as radio ligand | Bioorg Med Chem Lett 14: 4225-9 (2004) Article DOI: 10.1016/j.bmcl.2004.06.032 BindingDB Entry DOI: 10.7270/Q2K64MV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50474855 (CHEMBL364814) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human orexin receptor type 1 was determined using [125I]-Orexin A as radio ligand | Bioorg Med Chem Lett 14: 4225-9 (2004) Article DOI: 10.1016/j.bmcl.2004.06.032 BindingDB Entry DOI: 10.7270/Q2K64MV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50293184 (4-ethyl-2-((4-(morpholinomethyl)phenoxy)methyl)mor...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 165 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C. Curated by ChEMBL | Assay Description Displacement of [125I]iodoproxyfan from human recombinant histamine H3 receptor expressed in human SK-N-MC cells | Bioorg Med Chem Lett 18: 5796-9 (2009) Article DOI: 10.1016/j.bmcl.2008.09.077 BindingDB Entry DOI: 10.7270/Q2154H28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50474850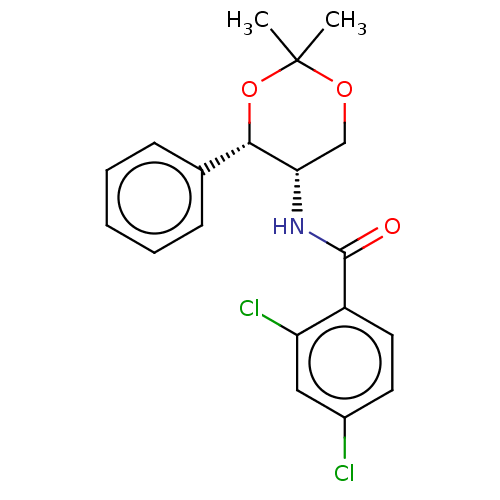 (CHEMBL185324) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human orexin receptor type 2 was determined using [125I]-Orexin A as radio ligand | Bioorg Med Chem Lett 14: 4225-9 (2004) Article DOI: 10.1016/j.bmcl.2004.06.032 BindingDB Entry DOI: 10.7270/Q2K64MV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50474844 (CHEMBL183734) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human orexin receptor type 2 was determined using [125I]-Orexin A as radio ligand | Bioorg Med Chem Lett 14: 4225-9 (2004) Article DOI: 10.1016/j.bmcl.2004.06.032 BindingDB Entry DOI: 10.7270/Q2K64MV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50412864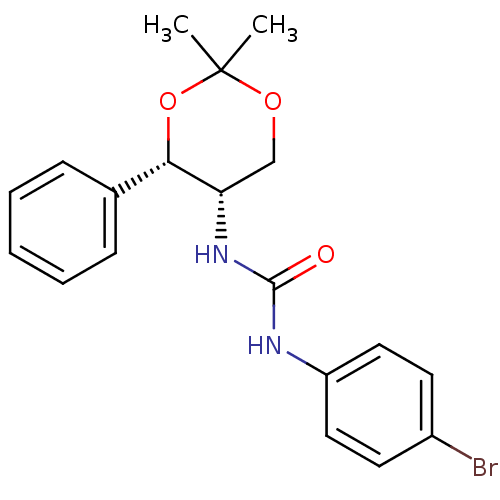 (CHEMBL184936) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human orexin receptor type 2 was determined using [125I]-Orexin A as radio ligand | Bioorg Med Chem Lett 14: 4225-9 (2004) Article DOI: 10.1016/j.bmcl.2004.06.032 BindingDB Entry DOI: 10.7270/Q2K64MV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50293192 (4-cyclobutyl-2-((3-(morpholinomethyl)phenoxy)methy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 277 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C. Curated by ChEMBL | Assay Description Displacement of [125I]iodoproxyfan from rat recombinant histamine H3 receptor expressed in human SK-N-MC cells | Bioorg Med Chem Lett 18: 5796-9 (2009) Article DOI: 10.1016/j.bmcl.2008.09.077 BindingDB Entry DOI: 10.7270/Q2154H28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50474845 (CHEMBL182269) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human orexin receptor type 2 was determined using [125I]-Orexin A as radio ligand | Bioorg Med Chem Lett 14: 4225-9 (2004) Article DOI: 10.1016/j.bmcl.2004.06.032 BindingDB Entry DOI: 10.7270/Q2K64MV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50474854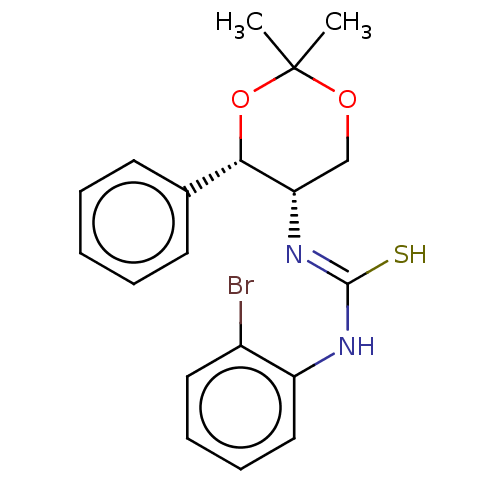 (CHEMBL184303) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human orexin receptor type 2 was determined using [125I]-Orexin A as radio ligand | Bioorg Med Chem Lett 14: 4225-9 (2004) Article DOI: 10.1016/j.bmcl.2004.06.032 BindingDB Entry DOI: 10.7270/Q2K64MV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50412853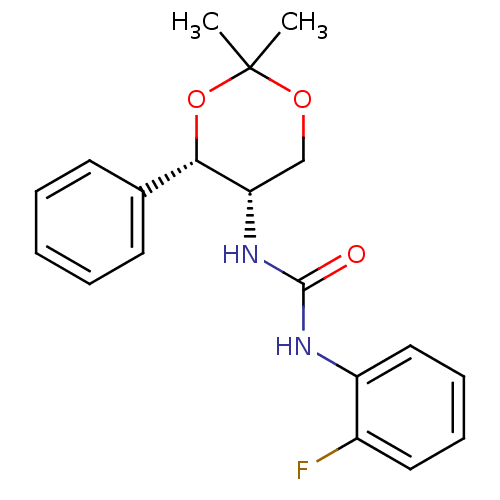 (CHEMBL361748) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human orexin receptor type 2 was determined using [125I]-Orexin A as radio ligand | Bioorg Med Chem Lett 14: 4225-9 (2004) Article DOI: 10.1016/j.bmcl.2004.06.032 BindingDB Entry DOI: 10.7270/Q2K64MV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50412866 (CHEMBL360377) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human orexin receptor type 2 was determined using [125I]-Orexin A as radio ligand | Bioorg Med Chem Lett 14: 4225-9 (2004) Article DOI: 10.1016/j.bmcl.2004.06.032 BindingDB Entry DOI: 10.7270/Q2K64MV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50474847 (CHEMBL183421) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human orexin receptor type 1 was determined using [125I]-Orexin A as radio ligand | Bioorg Med Chem Lett 14: 4225-9 (2004) Article DOI: 10.1016/j.bmcl.2004.06.032 BindingDB Entry DOI: 10.7270/Q2K64MV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50474846 (CHEMBL185131) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human orexin receptor type 1 was determined using [125I]-Orexin A as radio ligand | Bioorg Med Chem Lett 14: 4225-9 (2004) Article DOI: 10.1016/j.bmcl.2004.06.032 BindingDB Entry DOI: 10.7270/Q2K64MV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50293191 (4-isopropyl-2-((3-(morpholinomethyl)phenoxy)methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 562 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C. Curated by ChEMBL | Assay Description Displacement of [125I]iodoproxyfan from rat recombinant histamine H3 receptor expressed in human SK-N-MC cells | Bioorg Med Chem Lett 18: 5796-9 (2009) Article DOI: 10.1016/j.bmcl.2008.09.077 BindingDB Entry DOI: 10.7270/Q2154H28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50293190 (4-cyclopropyl-2-((3-(morpholinomethyl)phenoxy)meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 566 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C. Curated by ChEMBL | Assay Description Displacement of [125I]iodoproxyfan from human recombinant histamine H3 receptor expressed in human SK-N-MC cells | Bioorg Med Chem Lett 18: 5796-9 (2009) Article DOI: 10.1016/j.bmcl.2008.09.077 BindingDB Entry DOI: 10.7270/Q2154H28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50412865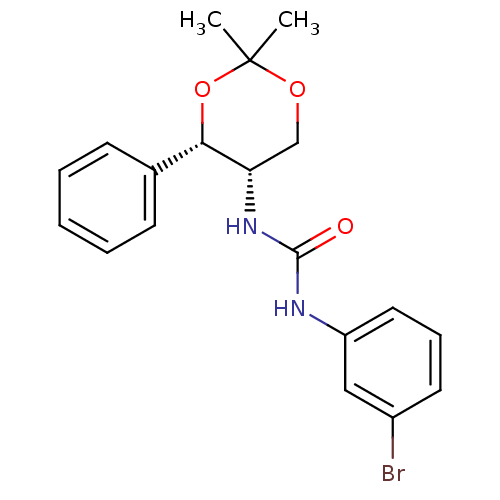 (CHEMBL185080) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human orexin receptor type 2 was determined using [125I]-Orexin A as radio ligand | Bioorg Med Chem Lett 14: 4225-9 (2004) Article DOI: 10.1016/j.bmcl.2004.06.032 BindingDB Entry DOI: 10.7270/Q2K64MV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50474849 (CHEMBL185424) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human orexin receptor type 1 was determined using [125I]-Orexin A as radio ligand | Bioorg Med Chem Lett 14: 4225-9 (2004) Article DOI: 10.1016/j.bmcl.2004.06.032 BindingDB Entry DOI: 10.7270/Q2K64MV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 128 total ) | Next | Last >> |