

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prostaglandin D2 receptor 2 (Homo sapiens (Human)) | BDBM111610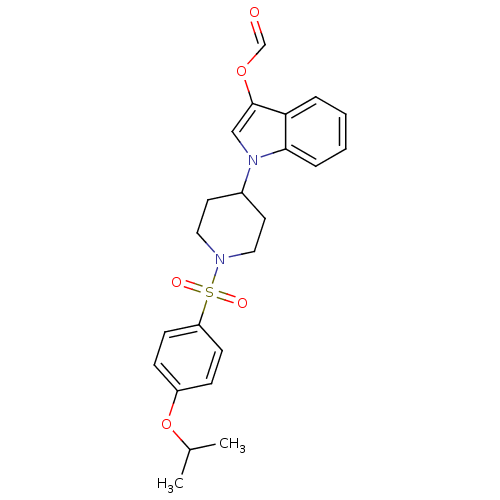 (US8623903, I-23) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.40 | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. US Patent | Assay Description A prepared WP was homogenated and a membrane fraction was collected with high-speed centrifugation. A compound of the present invention was added to ... | US Patent US8623903 (2014) BindingDB Entry DOI: 10.7270/Q2KP80TK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor 2 (Homo sapiens (Human)) | BDBM111612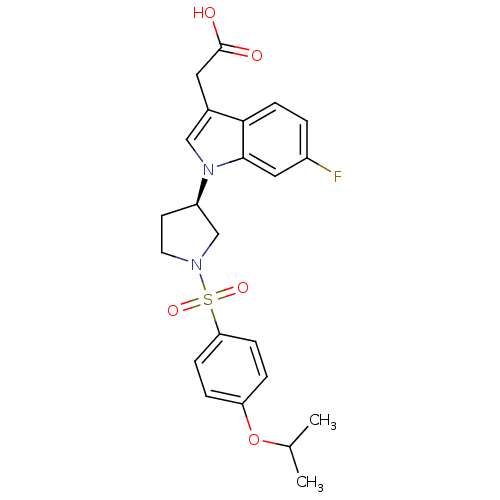 (US8623903, I-31) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 4.30 | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. US Patent | Assay Description A prepared WP was homogenated and a membrane fraction was collected with high-speed centrifugation. A compound of the present invention was added to ... | US Patent US8623903 (2014) BindingDB Entry DOI: 10.7270/Q2KP80TK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor 2 (Homo sapiens (Human)) | BDBM111611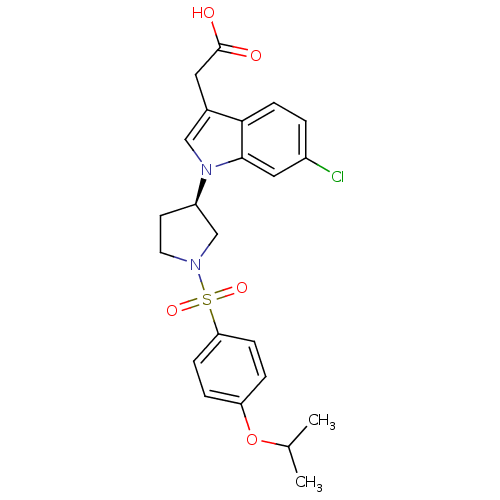 (US8623903, I-30) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 5.40 | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. US Patent | Assay Description A prepared WP was homogenated and a membrane fraction was collected with high-speed centrifugation. A compound of the present invention was added to ... | US Patent US8623903 (2014) BindingDB Entry DOI: 10.7270/Q2KP80TK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor 2 (Homo sapiens (Human)) | BDBM111606 (US8623903, I-14) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 5.60 | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. US Patent | Assay Description A prepared WP was homogenated and a membrane fraction was collected with high-speed centrifugation. A compound of the present invention was added to ... | US Patent US8623903 (2014) BindingDB Entry DOI: 10.7270/Q2KP80TK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor 2 (Homo sapiens (Human)) | BDBM111597 (US8623903, I-1) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 7.70 | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. US Patent | Assay Description A prepared WP was homogenated and a membrane fraction was collected with high-speed centrifugation. A compound of the present invention was added to ... | US Patent US8623903 (2014) BindingDB Entry DOI: 10.7270/Q2KP80TK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor 2 (Homo sapiens (Human)) | BDBM111598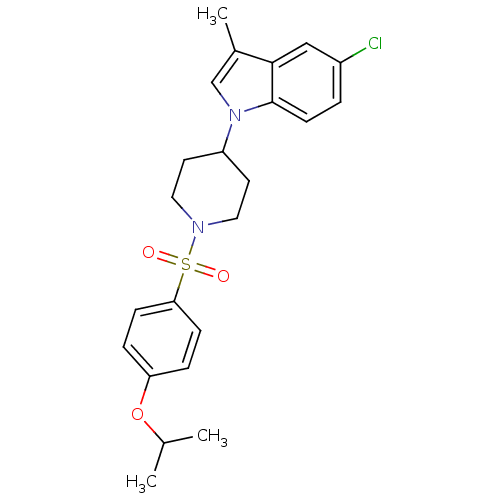 (US8623903, I-5) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 22 | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. US Patent | Assay Description A prepared WP was homogenated and a membrane fraction was collected with high-speed centrifugation. A compound of the present invention was added to ... | US Patent US8623903 (2014) BindingDB Entry DOI: 10.7270/Q2KP80TK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50181395 (1-benzyl-N-[3-spiro(2,3-dihydro-1H-indene-1,4'-pip...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Antagonist activity on nociceptin-induced [35S]GTPgammaS binding to ORL1 expressed in CHO cells | J Med Chem 49: 847-9 (2006) Article DOI: 10.1021/jm0509851 BindingDB Entry DOI: 10.7270/Q2DJ5F78 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50181392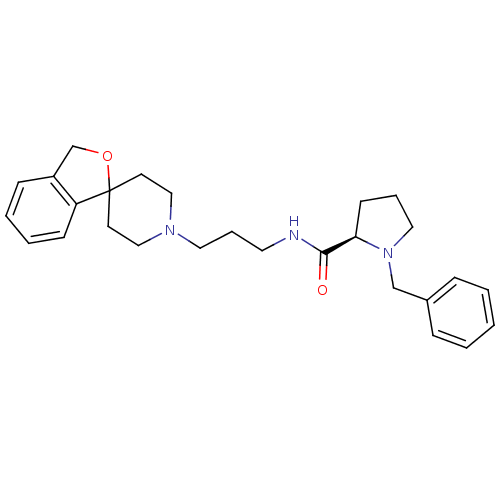 ((R)-N-(3-(3H-spiro[isobenzofuran-1,4'-piperidine]-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Antagonist activity on nociceptin-induced [35S]GTPgammaS binding to ORL1 expressed in CHO cells | J Med Chem 49: 847-9 (2006) Article DOI: 10.1021/jm0509851 BindingDB Entry DOI: 10.7270/Q2DJ5F78 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50181395 (1-benzyl-N-[3-spiro(2,3-dihydro-1H-indene-1,4'-pip...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-nociceptin from cloned human ORL1 expressed in CHO cells | J Med Chem 49: 847-9 (2006) Article DOI: 10.1021/jm0509851 BindingDB Entry DOI: 10.7270/Q2DJ5F78 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50181392 ((R)-N-(3-(3H-spiro[isobenzofuran-1,4'-piperidine]-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-nociceptin from cloned human ORL1 expressed in CHO cells | J Med Chem 49: 847-9 (2006) Article DOI: 10.1021/jm0509851 BindingDB Entry DOI: 10.7270/Q2DJ5F78 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nicotinamide N-methyltransferase (Homo sapiens (Human)) | BDBM50598881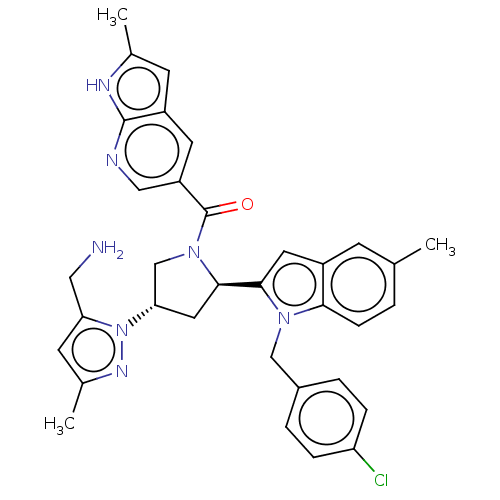 (CHEMBL5194905) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00919 BindingDB Entry DOI: 10.7270/Q2KS6WJ1 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor 2 (Homo sapiens (Human)) | BDBM111607 (US8623903, I-16) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. US Patent | Assay Description A prepared WP was homogenated and a membrane fraction was collected with high-speed centrifugation. A compound of the present invention was added to ... | US Patent US8623903 (2014) BindingDB Entry DOI: 10.7270/Q2KP80TK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50083230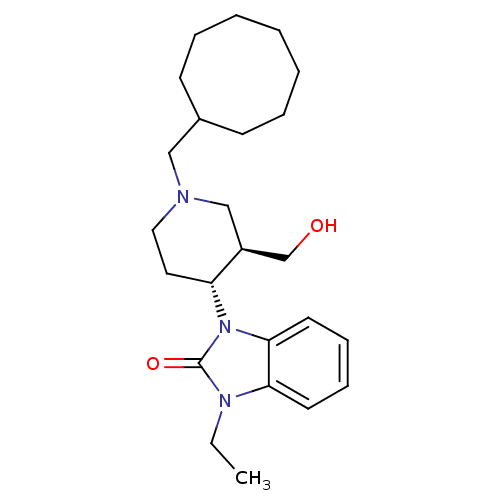 (1-((3R,4R)-1-Cyclooctylmethyl-3-hydroxymethyl-pipe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Antagonist activity on nociceptin-induced [35S]GTPgammaS binding to ORL1 expressed in CHO cells | J Med Chem 49: 847-9 (2006) Article DOI: 10.1021/jm0509851 BindingDB Entry DOI: 10.7270/Q2DJ5F78 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM50386816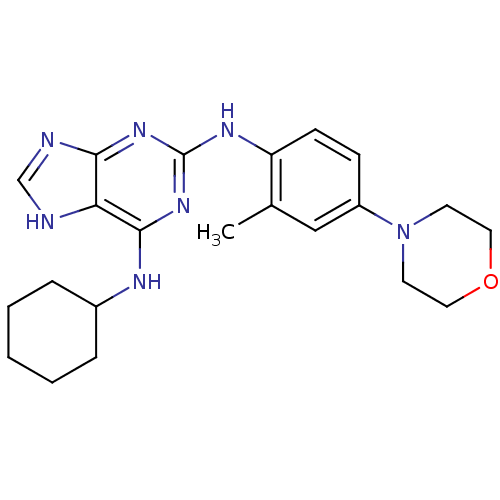 (CHEMBL2047943) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi Pharmaceutical Research Center Curated by ChEMBL | Assay Description Inhibition of human MPS1 expressed in Escherichia coli | J Med Chem 56: 4343-56 (2013) Article DOI: 10.1021/jm4000215 BindingDB Entry DOI: 10.7270/Q2SF2XJB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin D2 receptor 2 (Homo sapiens (Human)) | BDBM111600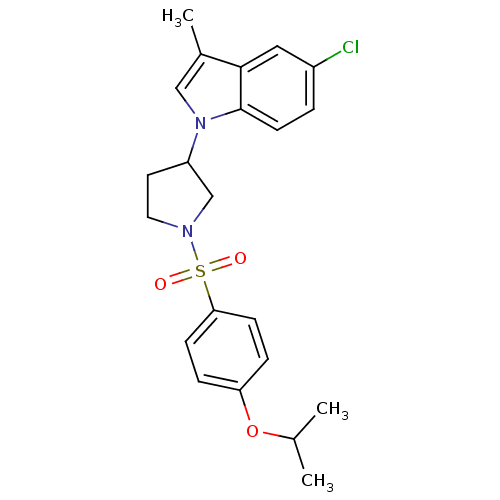 (US8623903, I-7) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. US Patent | Assay Description A prepared WP was homogenated and a membrane fraction was collected with high-speed centrifugation. A compound of the present invention was added to ... | US Patent US8623903 (2014) BindingDB Entry DOI: 10.7270/Q2KP80TK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor 2 (Homo sapiens (Human)) | BDBM111602 (US8623903, I-9) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. US Patent | Assay Description A prepared WP was homogenated and a membrane fraction was collected with high-speed centrifugation. A compound of the present invention was added to ... | US Patent US8623903 (2014) BindingDB Entry DOI: 10.7270/Q2KP80TK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50083230 (1-((3R,4R)-1-Cyclooctylmethyl-3-hydroxymethyl-pipe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-nociceptin from cloned human ORL1 expressed in CHO cells | J Med Chem 49: 847-9 (2006) Article DOI: 10.1021/jm0509851 BindingDB Entry DOI: 10.7270/Q2DJ5F78 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor 2 (Homo sapiens (Human)) | BDBM111609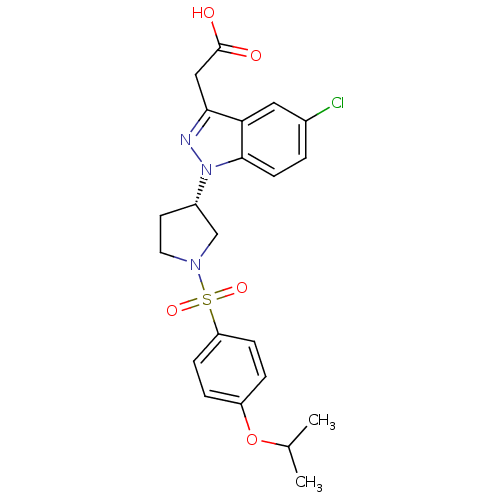 (US8623903, I-21) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. US Patent | Assay Description A prepared WP was homogenated and a membrane fraction was collected with high-speed centrifugation. A compound of the present invention was added to ... | US Patent US8623903 (2014) BindingDB Entry DOI: 10.7270/Q2KP80TK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM50433907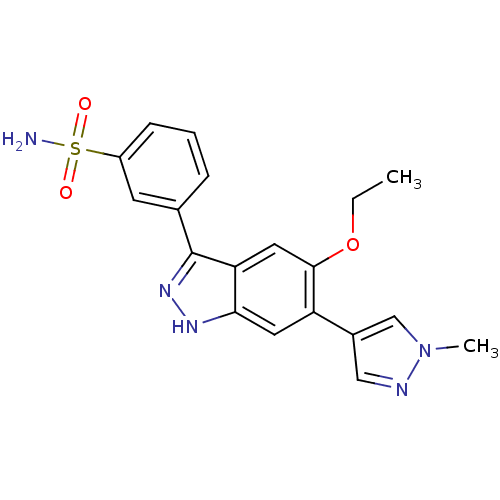 (CHEMBL2380582) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi Pharmaceutical Research Center Curated by ChEMBL | Assay Description Inhibition of MPS1 (unknown origin) using biotin-labeled AGAGLARHTDDEMTGYVA as substrate after 90 mins by DELFIA | J Med Chem 56: 4343-56 (2013) Article DOI: 10.1021/jm4000215 BindingDB Entry DOI: 10.7270/Q2SF2XJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM50433906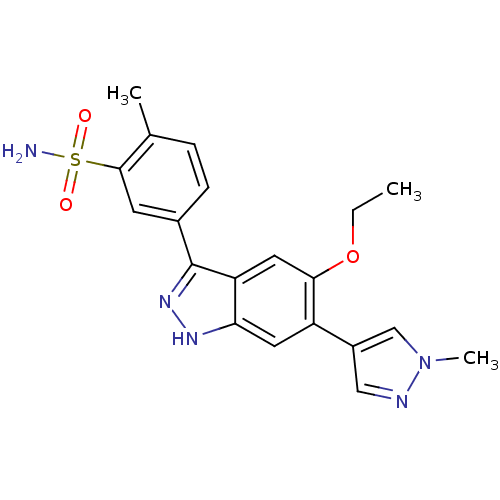 (CHEMBL2380583) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi Pharmaceutical Research Center Curated by ChEMBL | Assay Description Inhibition of MPS1 (unknown origin) using biotin-labeled AGAGLARHTDDEMTGYVA as substrate after 90 mins by DELFIA | J Med Chem 56: 4343-56 (2013) Article DOI: 10.1021/jm4000215 BindingDB Entry DOI: 10.7270/Q2SF2XJB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin D2 receptor 2 (Homo sapiens (Human)) | BDBM111608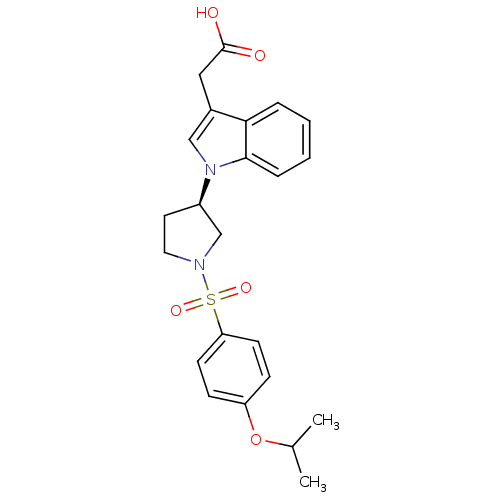 (US8623903, I-20) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. US Patent | Assay Description A prepared WP was homogenated and a membrane fraction was collected with high-speed centrifugation. A compound of the present invention was added to ... | US Patent US8623903 (2014) BindingDB Entry DOI: 10.7270/Q2KP80TK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50181374 (2-phenoxy-N-[3-spiro(2,3-dihydro-1H-indene-1,4'-pi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Antagonist activity on nociceptin-induced [35S]GTPgammaS binding to ORL1 expressed in CHO cells | J Med Chem 49: 847-9 (2006) Article DOI: 10.1021/jm0509851 BindingDB Entry DOI: 10.7270/Q2DJ5F78 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 8 (Homo sapiens (Human)) | BDBM15913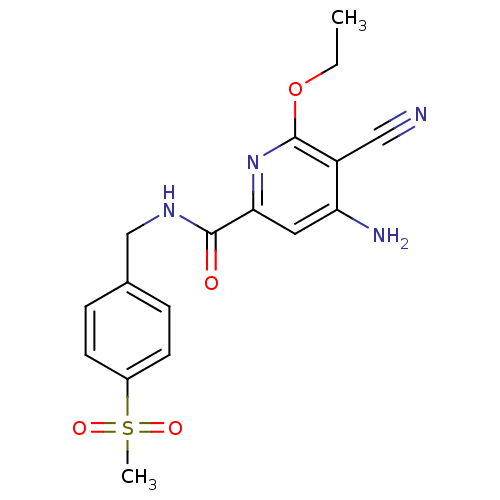 (2-pyridinecarboxamide deriv. 8c | 4-Amino-5-cyano-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of JNK1-mediated ATF2 phosphorylation after 1 hr by ELISA | ACS Med Chem Lett 3: 560-564 (2012) Article DOI: 10.1021/ml3000879 BindingDB Entry DOI: 10.7270/Q2RV0PZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor 2 (Homo sapiens (Human)) | BDBM111599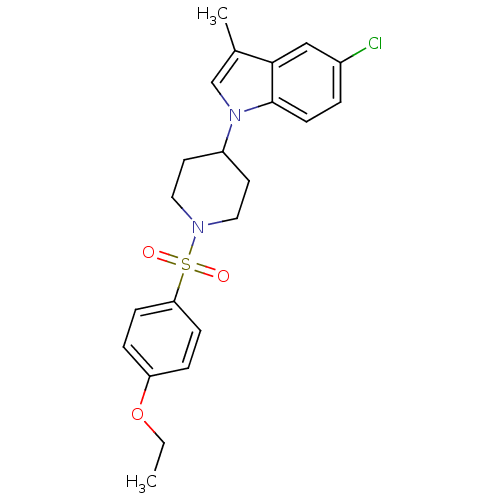 (US8623903, I-6) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. US Patent | Assay Description A prepared WP was homogenated and a membrane fraction was collected with high-speed centrifugation. A compound of the present invention was added to ... | US Patent US8623903 (2014) BindingDB Entry DOI: 10.7270/Q2KP80TK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor 2 (Homo sapiens (Human)) | BDBM111604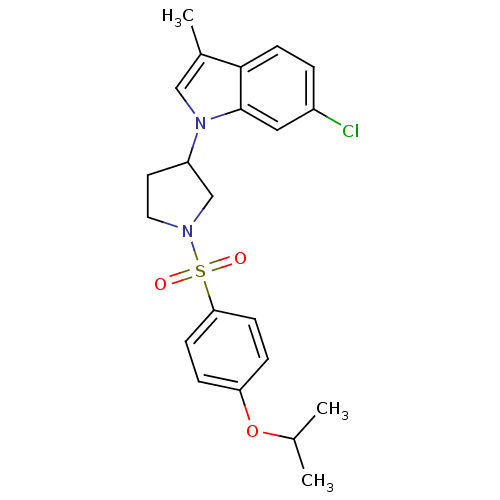 (US8623903, I-11) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. US Patent | Assay Description A prepared WP was homogenated and a membrane fraction was collected with high-speed centrifugation. A compound of the present invention was added to ... | US Patent US8623903 (2014) BindingDB Entry DOI: 10.7270/Q2KP80TK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor 2 (Homo sapiens (Human)) | BDBM111601 (US8623903, I-8) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. US Patent | Assay Description A prepared WP was homogenated and a membrane fraction was collected with high-speed centrifugation. A compound of the present invention was added to ... | US Patent US8623903 (2014) BindingDB Entry DOI: 10.7270/Q2KP80TK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 40S ribosomal protein S27 (Homo sapiens (Human)) | BDBM50420401 (CHEMBL2089255 | US11208696, Example 31) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Mps1-mediated p38 MAPK phosphorylation after 90 mins by DELFIA assay | ACS Med Chem Lett 3: 560-564 (2012) Article DOI: 10.1021/ml3000879 BindingDB Entry DOI: 10.7270/Q2RV0PZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor 2 (Homo sapiens (Human)) | BDBM111605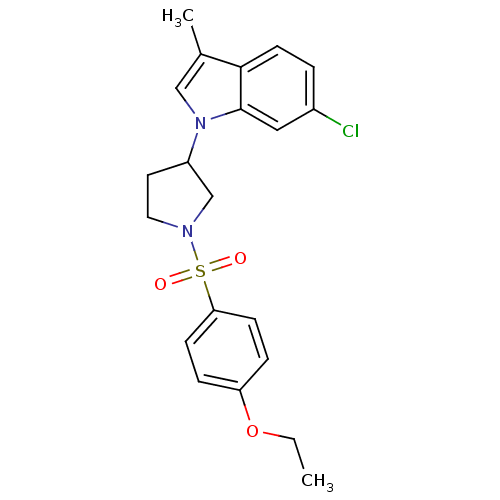 (US8623903, I-12) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. US Patent | Assay Description A prepared WP was homogenated and a membrane fraction was collected with high-speed centrifugation. A compound of the present invention was added to ... | US Patent US8623903 (2014) BindingDB Entry DOI: 10.7270/Q2KP80TK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor 2 (Homo sapiens (Human)) | BDBM111603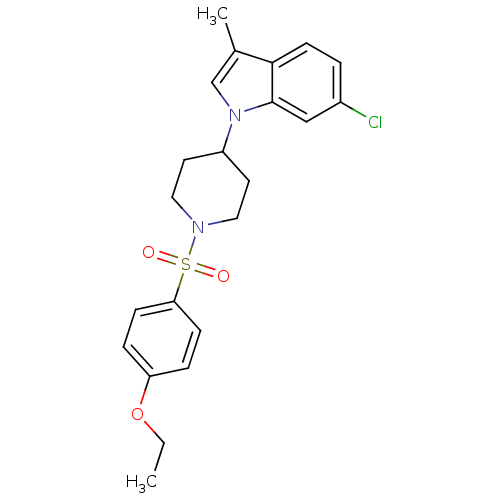 (US8623903, I-10) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. US Patent | Assay Description A prepared WP was homogenated and a membrane fraction was collected with high-speed centrifugation. A compound of the present invention was added to ... | US Patent US8623903 (2014) BindingDB Entry DOI: 10.7270/Q2KP80TK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor 2 (Homo sapiens (Human)) | BDBM111613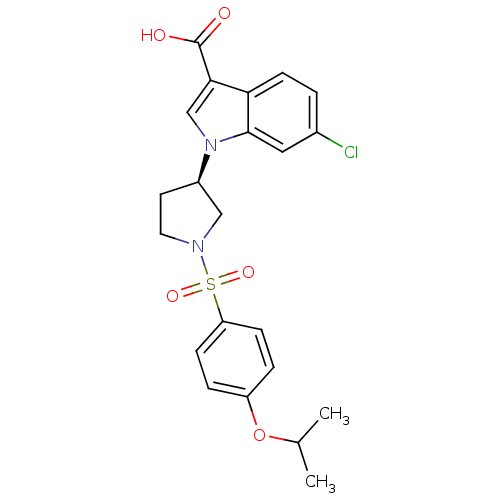 (US8623903, I-32) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. US Patent | Assay Description A prepared WP was homogenated and a membrane fraction was collected with high-speed centrifugation. A compound of the present invention was added to ... | US Patent US8623903 (2014) BindingDB Entry DOI: 10.7270/Q2KP80TK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50605058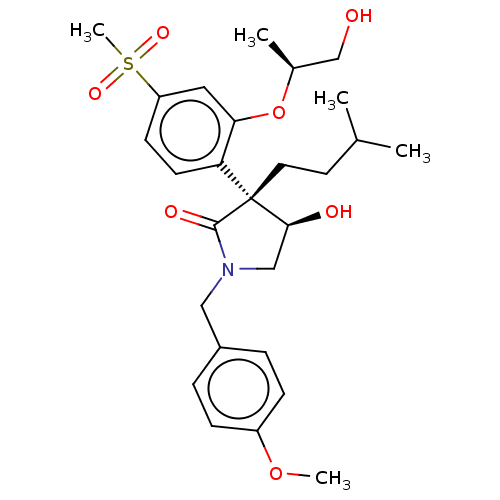 (CHEMBL5208218) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c02217 BindingDB Entry DOI: 10.7270/Q2C53QZ4 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50181382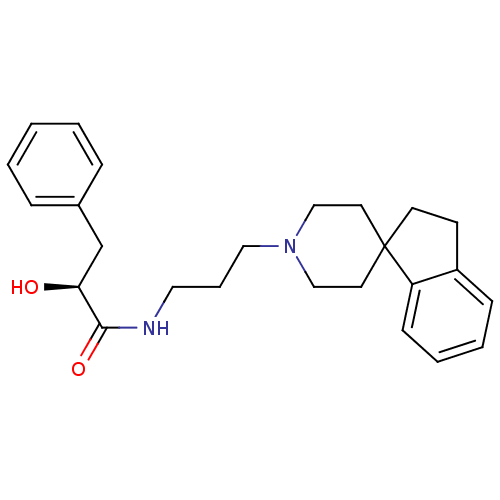 (CHEMBL199639 | N-[3-spiro(2,3-dihydro-1H-indene-1,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Antagonist activity on nociceptin-induced [35S]GTPgammaS binding to ORL1 expressed in CHO cells | J Med Chem 49: 847-9 (2006) Article DOI: 10.1021/jm0509851 BindingDB Entry DOI: 10.7270/Q2DJ5F78 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM50433904 (CHEMBL2380585) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi Pharmaceutical Research Center Curated by ChEMBL | Assay Description Inhibition of MPS1 (unknown origin) using biotin-labeled AGAGLARHTDDEMTGYVA as substrate after 90 mins by DELFIA | J Med Chem 56: 4343-56 (2013) Article DOI: 10.1021/jm4000215 BindingDB Entry DOI: 10.7270/Q2SF2XJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM50349102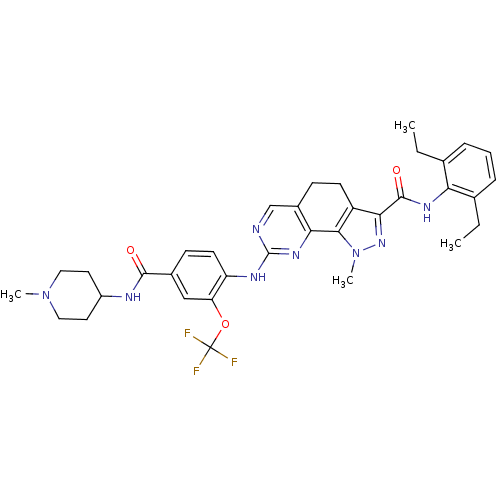 (CHEMBL1236095 | US11208696, Example 3) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi Pharmaceutical Research Center Curated by ChEMBL | Assay Description Inhibition of MPS1 (unknown origin) | J Med Chem 56: 4343-56 (2013) Article DOI: 10.1021/jm4000215 BindingDB Entry DOI: 10.7270/Q2SF2XJB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50181374 (2-phenoxy-N-[3-spiro(2,3-dihydro-1H-indene-1,4'-pi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-nociceptin from cloned human ORL1 expressed in CHO cells | J Med Chem 49: 847-9 (2006) Article DOI: 10.1021/jm0509851 BindingDB Entry DOI: 10.7270/Q2DJ5F78 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50181380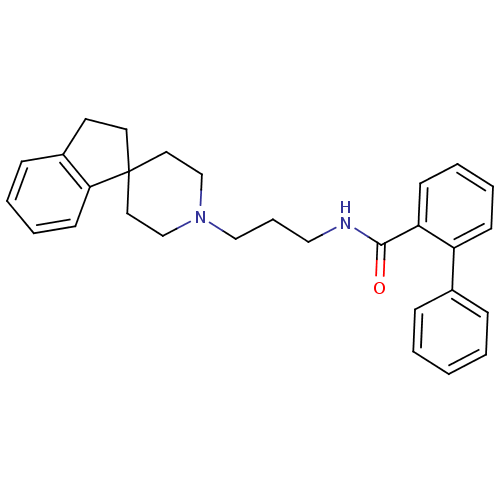 (CHEMBL413571 | N-[3-spiro(2,3-dihydro-1H-indene-1,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Antagonist activity on nociceptin-induced [35S]GTPgammaS binding to ORL1 expressed in CHO cells | J Med Chem 49: 847-9 (2006) Article DOI: 10.1021/jm0509851 BindingDB Entry DOI: 10.7270/Q2DJ5F78 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM50433908 (CHEMBL2380581) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi Pharmaceutical Research Center Curated by ChEMBL | Assay Description Inhibition of MPS1 (unknown origin) using biotin-labeled AGAGLARHTDDEMTGYVA as substrate after 90 mins by DELFIA | J Med Chem 56: 4343-56 (2013) Article DOI: 10.1021/jm4000215 BindingDB Entry DOI: 10.7270/Q2SF2XJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM50433903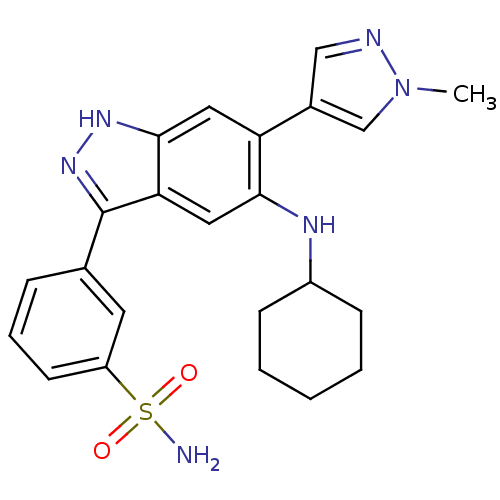 (CHEMBL2380586) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi Pharmaceutical Research Center Curated by ChEMBL | Assay Description Inhibition of MPS1 (unknown origin) using biotin-labeled AGAGLARHTDDEMTGYVA as substrate after 90 mins by DELFIA | J Med Chem 56: 4343-56 (2013) Article DOI: 10.1021/jm4000215 BindingDB Entry DOI: 10.7270/Q2SF2XJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50181380 (CHEMBL413571 | N-[3-spiro(2,3-dihydro-1H-indene-1,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-nociceptin from cloned human ORL1 expressed in CHO cells | J Med Chem 49: 847-9 (2006) Article DOI: 10.1021/jm0509851 BindingDB Entry DOI: 10.7270/Q2DJ5F78 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM50433902 (CHEMBL2380587) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi Pharmaceutical Research Center Curated by ChEMBL | Assay Description Inhibition of MPS1 (unknown origin) using biotin-labeled AGAGLARHTDDEMTGYVA as substrate after 90 mins by DELFIA | J Med Chem 56: 4343-56 (2013) Article DOI: 10.1021/jm4000215 BindingDB Entry DOI: 10.7270/Q2SF2XJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50181382 (CHEMBL199639 | N-[3-spiro(2,3-dihydro-1H-indene-1,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-nociceptin from cloned human ORL1 expressed in CHO cells | J Med Chem 49: 847-9 (2006) Article DOI: 10.1021/jm0509851 BindingDB Entry DOI: 10.7270/Q2DJ5F78 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM513874 (bioRxiv20220126.477782, S-217622 | bioRxiv20220126...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00117 BindingDB Entry DOI: 10.7270/Q2TB1C0F | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM513874 (bioRxiv20220126.477782, S-217622 | bioRxiv20220126...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description The 3CL protease inhibition assay was conducted in 384-well plates (Corning 3702). The substance solution (10 mM dimethyl sulfoxide [DMSO] solution) ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2QJ7MFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide N-methyltransferase (Homo sapiens (Human)) | BDBM50598880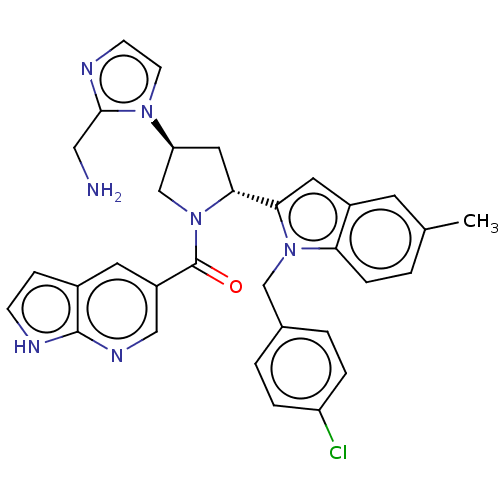 (CHEMBL5187248) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00919 BindingDB Entry DOI: 10.7270/Q2KS6WJ1 | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM50433911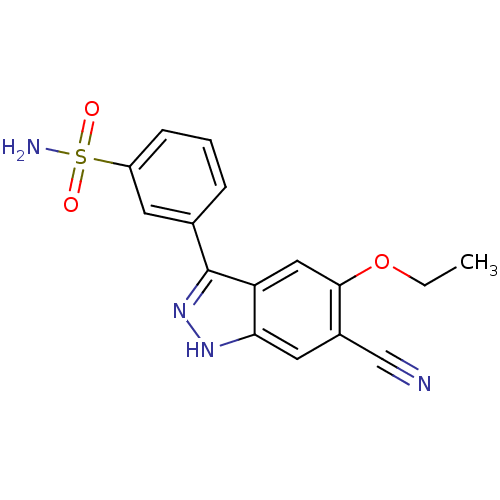 (CHEMBL2380578) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi Pharmaceutical Research Center Curated by ChEMBL | Assay Description Inhibition of MPS1 (unknown origin) using biotin-labeled AGAGLARHTDDEMTGYVA as substrate after 90 mins by DELFIA | J Med Chem 56: 4343-56 (2013) Article DOI: 10.1021/jm4000215 BindingDB Entry DOI: 10.7270/Q2SF2XJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50181366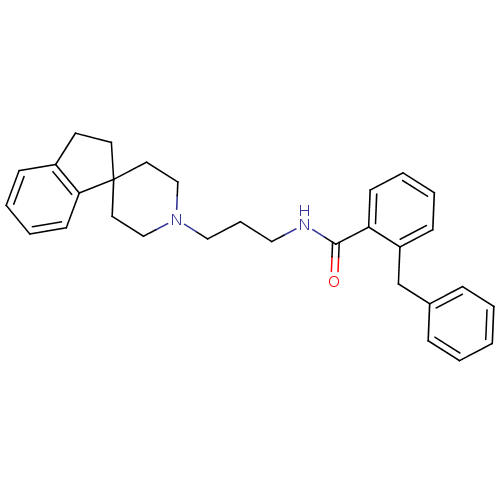 (2-benzyl-N-(3-{2,3-dihydrospiro[indene-1,4'-piperi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-nociceptin from cloned human ORL1 expressed in CHO cells | J Med Chem 49: 847-9 (2006) Article DOI: 10.1021/jm0509851 BindingDB Entry DOI: 10.7270/Q2DJ5F78 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM50433903 (CHEMBL2380586) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi Pharmaceutical Research Center Curated by ChEMBL | Assay Description Inhibition of FLAG-tagged MPS1 phosphorylation in human RERF-LC-AI Tet-off cells after 3 hrs | J Med Chem 56: 4343-56 (2013) Article DOI: 10.1021/jm4000215 BindingDB Entry DOI: 10.7270/Q2SF2XJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50181378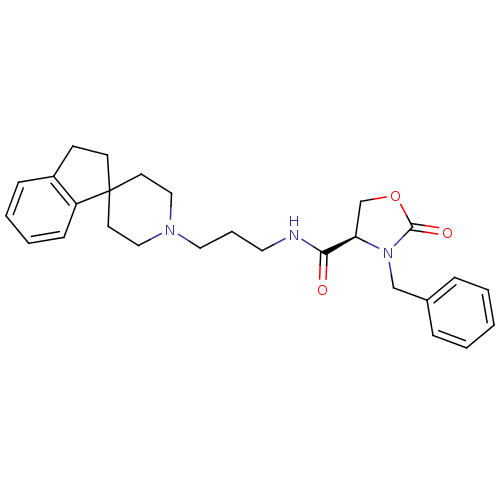 ((4R)-3-benzyl-N-(3-{2,3-dihydrospiro[indene-1,4'-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-nociceptin from cloned human ORL1 expressed in CHO cells | J Med Chem 49: 847-9 (2006) Article DOI: 10.1021/jm0509851 BindingDB Entry DOI: 10.7270/Q2DJ5F78 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM50433912 (CHEMBL2380577) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi Pharmaceutical Research Center Curated by ChEMBL | Assay Description Inhibition of MPS1 (unknown origin) using biotin-labeled AGAGLARHTDDEMTGYVA as substrate after 90 mins by DELFIA | J Med Chem 56: 4343-56 (2013) Article DOI: 10.1021/jm4000215 BindingDB Entry DOI: 10.7270/Q2SF2XJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50181402 ((4S,5S)-4-benzyl-N-(3-{2,3-dihydrospiro[indene-1,4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-nociceptin from cloned human ORL1 expressed in CHO cells | J Med Chem 49: 847-9 (2006) Article DOI: 10.1021/jm0509851 BindingDB Entry DOI: 10.7270/Q2DJ5F78 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 286 total ) | Next | Last >> |